Abstract
Background.
The lead (Pb) exposure crisis in Flint, Michigan has passed from well-publicized event to a footnote, while its biological and social impact will linger for lifetimes. Interest in the “water crisis” has dropped to preevent levels, which is neither appropriate nor safe. Flint’s exposure was severe, but it was not unique. Problematic Pb levels have also been found in schools and daycares in 42 states in the USA. The enormity of Pb exposure via municipal water systems requires multiple responses. Herein, we focus on addressing a possible answer to long-term sequelae of Pb exposure. We propose “4R’s” (remediation, renovation, reallocation, and research) against the Pb crisis that goes beyond a short-term fix. Remediation for affected individuals must continue to provide clean water and deal with both short and long-term effects of Pb exposure. Renovation of current water delivery systems, at both system-wide and individual site levels, is necessary. Reallocation of resources is needed to ensure these two responses occur and to get communities ready for potential sequelae of Pb exposure. Finally, properly focused research can track exposed individuals and illuminate latent (presumably epigenetic) results of Pb exposure and inform further resource reallocation.
Conclusion.
Motivation to act by not only the general public but also by scientific and medical leaders must be maintained beyond initial news cycle spikes and an annual follow-up story. Environmental impact of Pb contamination of drinking water goes beyond one exposure incident in an impoverished and forgotten Michigan city. Population effects must be addressed long-term and nationwide.
Keywords: Alzheimer’s, Brain, CNS, Dementia, Environment, Early-life, Epigenetics, Heavy metals, Latent-effects, Lead, Neurodegeneration, Pollution, Safety, Toxicity
1. Introduction
In 2014, over 100,000 residents of the city of Flint, Michigan, were potentially exposed to high levels of lead (Pb) via drinking water from a contaminated water source, their own water system’s pipes, leached out by improperly-treated source water. This contamination led to declaration of a federal state of emergency by President Barack Obama in 2016 and widespread attention to this state and national public health catastrophe. Pb is a known neurotoxin that adversely affects neurodevelopment and cognition (Santa Maria et al., 2018). In addition, prolonged Pb exposure can cause digestive problems, renal issues, as well as anemia (Nemsadze et al., 2009; Yilmaz et al., 2012). Pb can substitute for calcium ions (Ca++), which makes it a disruptor of Ca++ homeostasis. This can leads to an accumulation of Ca++ in cells, affecting the activity of secondary messengers (Bressler et al., 1999). This disruption can cause apoptosis and changes in excitotoxicity and neurotransmitter release and storage. The incidence of Pb poisoning from cumulative Pb exposure shows a dose-response relationship (Wu et al., 2016). In short, the toxicity of acute and chronic Pb exposure are dramatic and devastating. However, these strictly “toxic” responses are not the sum total of the dangerous sequelae of Pb exposure and may not even be the most serious long-term consequences.
2. Pb exposure causes epigenetic changes
These long-term consequences are likely to be maintained within the body as changes in epigenetic markers. In the present day, “epigenetics” has at times been broadly defined, perhaps beyond meaning. Herein, we restrict it to “phenomena and mechanisms that cause chromosome-bound, potentially heritable changes in gene expression that are not changes in the DNA sequence” (Deans and Maggert, 2015). Biochemically, this would include DNA oxidation (Zawia et al., 2009), methylation (Sen et al., 2015b), and hydroxymethylation (Sen et al., 2015a) patterns, and histone modifications (such as acetylation) (Xu et al., 2015c). It is likely that such epigenetic “lesions” are responsible for a large number of the latent sequelae to Pb exposure, resulting in genome-wide changes of DNA methylation and expression profiles (Dosunmu et al., 2009). The contribution of epigenetic dysfunction to late-life neurodegeneration is well-attested (Maloney and Lahiri, 2016). In the realm of DNA methylation, this damage may occur due to disruption of the methionine-homocysteine cycle, wherein Pb exposure elevates levels of homocysteine (Brucker et al., 2015; Yakub and Iqbal, 2010) and by reprogramming expression levels of enzymes that regulate epigenetic DNA and histone modification (Eid et al., 2016). Homocysteine contributes both to aberrant DNA methylation (Lin et al., 2014; Perng et al., 2014; Yang et al., 2014) and to altered histone acetylation (Xu et al., 2015b). Furthermore, early-life Pb exposure may be linked to Alzheimer’s disease (AD) (Wu et al., 2008). In particular, early-life exposure to low (sub-toxic) levels of Pb can induce late-life aberrations in expression of AD-associated proteins and peptides, even though late-life Pb levels were the same as for un-exposed individuals (Basha et al., 2005b; Wu et al., 2008). These latent abnormalities are most likely preserved through epigenetic means (Bolin et al., 2006; Zawia et al., 2009), and later-life triggers interact with these altered epigenetics to produce clinical abnormalities (Maloney and Lahiri, 2016). In addition to altering DNA modification, Pb may increase histone acetylation in some tissues (Xu et al., 2015a). Also of great concern is that environmentally-induced epigenetic lesions may be passed across generations, leading to familial predisposition to neuropsychiatric disorders, as we have previously discussed in more detail (Lahiri et al., 2016). Finally, in addition to epigenetic factors, Pb exposure alters levels of microRNA (miRNA) species that target proteins associated with disease (Masoud et al., 2016), although this is not as well elucidated and may be due to epigenetic modification of the miRNA genes’ own regulatory sequences.
3. Chelation does not prevent long-term effects
An unfortunately popular response was to call for chelation therapy for all exposed children (Anon., 2016a, 2016b; Conat, 2016; Environews DC News Bureau, 2016; Trowbridge, 2014). Some press outlets did note that this approach previously proved ineffective (Lapook, 2016), and public health experts also counseled against this approach (Reilly, 2016; Wade, 2016; Young, 2016), Nevertheless, even some of these public statements were watered down by publishers, who thought it appropriate to “balance” legitimate experts with counter-statements from “chiropractic neurologists” (Young, 2016). Chelation might reduce levels of circulating Pb, but the reduction offers little to no benefit to any cognitive, neuromotor, or behavioral endpoints (Dietrich et al., 2004). In any case, even the legitimate public health response (e.g., high-ascorbate/calcium/iron diet) was geared toward preventing chronic accumulation (Reilly, 2016; USDA Office of Communications, 2016; Wade, 2016), not the sort of latent sequelae we describe. One might conclude that the results of Pb exposure would be, therefore, insuperable, since removal of the Pb from the body does not reverse outcomes of exposure.
4. Pb exposure requires timely intervention
There is, nevertheless, a viable response to long-term sequelae of Pb exposure, because there is a biochemical mechanism for this long-term, latent Pb damage, i. e., long-term effects of disruption of epigenetic markers. Thus, epigenetic intervention may be possible. Epigenetic drugs currently under human trial, such as resveratrol, a histone deacetylase inhibitor (HDACi) (Vahid et al., 2015), also regulate the homocysteine cycle (Koz et al., 2012) and may likewise have cognitive benefits (Koz et al., 2012). Resveratrol may be able to perform double-duty against Pb-induced latent hazards, given that it can regulate both homocysteine and histone acetylation. Dietary supplementation with vitamins B6, B12, and folate may reverse several epigenetic effects of homocysteine activity (Kok et al., 2015). In particular, such supplementation accompanies reduced decline or even improvement in neurological patient samples (Chan et al., 2010; Kim et al., 2014; Lahiri and Maloney, 2010; Reynolds, 2014), although it should be cautioned that response to B vitamin supplementation may be subject to significant genetic influence (Chhillar et al., 2014).
5. Consequences of environmental catastrophes have complex health consequences
Environmental exposures certainly affect subgroups differently, and the disparity among those groups is notable. A life course model proposes that the high infant mortality rates of Blacks vs non-Hispanic whites is due to a higher number of multifactorial risk factors from conception to death (Lu and Halfon, 2003).
While Pb exposure is classically associated with learning difficulties, hyperactivity, and developmental delays, some of which may be irreversible, and current public concern was and remains over these specific pediatric and later behavioral difficulties (Ungar, 2016), which were recognized decades ago (De la Burde and Choate, 1972). There are far deeper and longer-lasting effects of Pb exposure. Multiple latent sequelae exist of Pb exposure to sub-toxic levels (Bakulski, 2013; Basha et al., 2005a, b; Bihaqi et al., 2014; Bolin et al., 2006; Chiu et al., 2014; Eid et al., 2016; Masoud et al., 2016; Sen et al., 2015a, b; Wu et al., 2008). Pb exposure can instill latent epigenetic modifications (Zawia et al., 2009) that occur in the absence of toxicity symptoms. This is the basis of the Latent Early-Life Regulation (LEARn) model that has been expanded recently (Maloney and Lahiri, 2016). Briefly, LEARn posits that individual “hits” can occur early in life. These can take the form of exposure to toxic materials (such as Pb), to environmental stress (such as poverty or racial disparity), to cultural stress (such as warfare), or other negative impacts. Each hit has the potential to impose epigenetic changes, such as DNA methylation (Maloney and Lahiri, 2016) or proliferation of long, interspersed nuclear element (LINE)1 transposons within brain regions such as the hippocampus (Bedrosian et al., 2018; Lahiri et al., 2018). If sufficient hits are suffered before a critical age threshold, risk of developing a later-life disorder, such as AD, greatly increases (Fig. 1A). Multiple dementias have strong epigenetic connections, both abnormal histone modification and DNA methylation (Maloney and Lahiri, 2016). Several specific loci differences in DNA methylation associate with AD (De Jager et al., 2014). Epigenetic connections also exist to Parkinson’s disease (Feng et al., 2015), frontotemporal dementia/other tauopathies (Li et al., 2014), and overall mortality (Marioni et al., 2015).
Fig. 1.
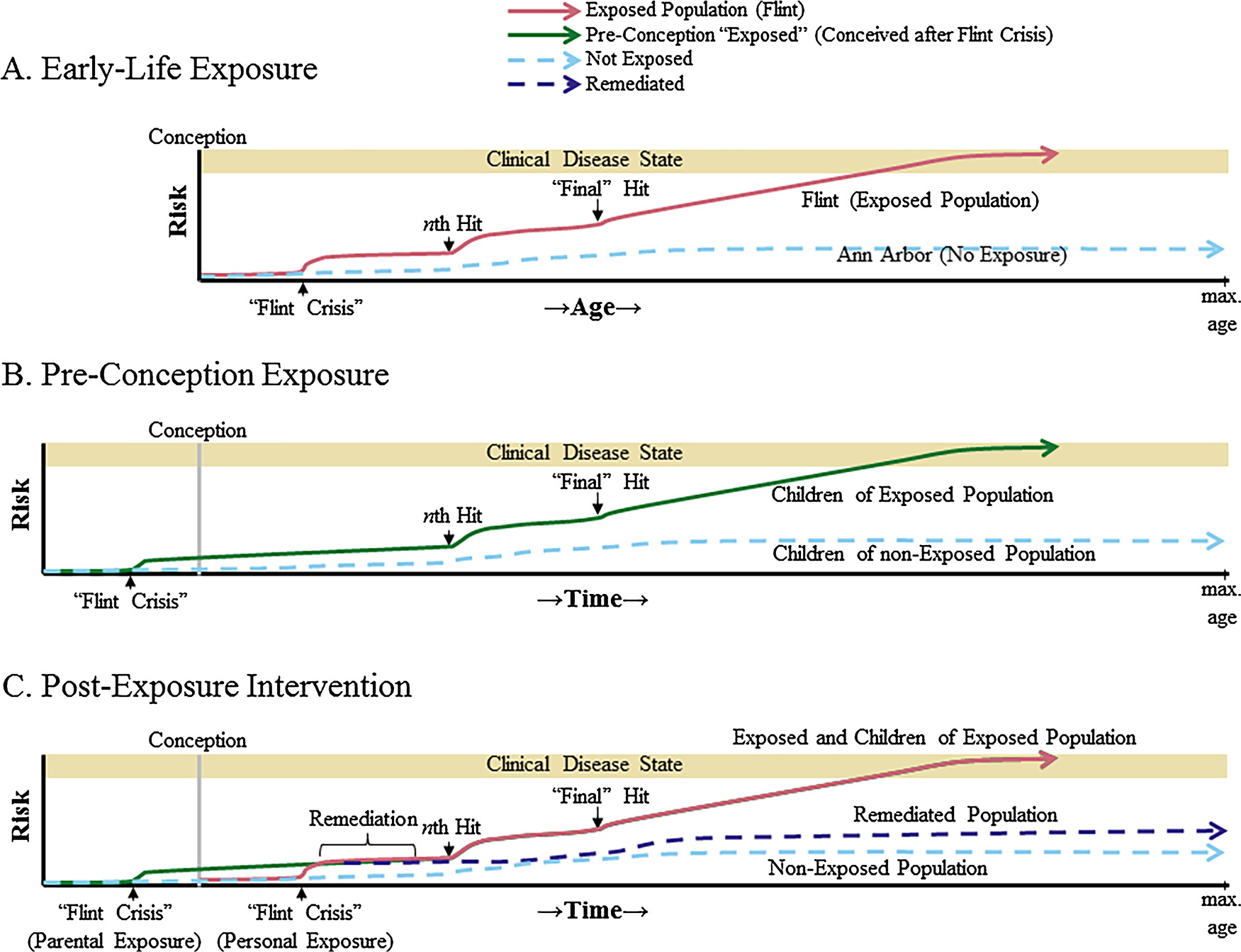
The LEARn Models and Flint. LEARn explains idiopathic disorders in a testable manner, on the basis of accumulation of “hits” through an organism’s lifespan. Hits can be environmental, genetic, or epigenetic. A) An event such as a Pb/water exposure crisis would be the critical “initial hit” that sets up latent increased risk (red line) for late-life neurodegenerative disorders. As additional hits are received, people who had the primary hit progress to clinical neurodegeneration. People who did not have the critical early-life hit (un-exposed, dashed blue line) do not progress to disease, even though they also might bear later hits. B) The critical hit may occur before conception. Environmentally-induced epigenetic changes can be inherited, thus, even though individuals may be born after an acute “water crisis” has passed and suffer no direct exposure, the results of that exposure could still be passed on epigenetically and act as the critical primary hit (green line). C) In either case, appropriate remediation at an early enough interval may reverse epigenetic lesion in either primary-exposed individuals or their descendants (violet line). However, finding such lesions and appropriate remediation will require longitudinal research of affected individuals and their offspring.
These epigenomic effects of environmental hazards are not limited to a single generation. Epigenetic changes, including those with behavioral effects, can be inherited (Anway et al., 2005; Bygren, 2013; Dias and Ressler, 2014; Stegemann and Buchner, 2015; Vassoler and Sadri-Vakili, 2014; Vassoler et al., 2013; Wei et al., 2014; Xie et al., 2018). Combining this possibility with LEARn produces t-LEARn (transgenerational LEARn) (Lahiri et al., 2016). In essence, there may be a significant risk of passing down the latent consequences of “sub-toxic” Pb exposure to future generations (Fig. 1B), even though the future generations are not exposed to elevated Pb (Lahiri et al., 2016). Multigenerational effects are not like simple prenatal exposure. A Detroit population was surveyed for aberrant DNA methylation. Subjects were grandmothers, mothers, and grandchildren. A woman exposed to Pb was more likely to have aberrant DNA methylation for specific genes in her, in her daughter’s, and in her grandchild’s DNA. These genes included ninjurin2 (NINJ2) and N-myc downstream-regulated gene 4 (NDRG4) (Sen et al., 2015c), both of which are associated with AD (Lin et al., 2011; Zhou et al., 2001), as well as apolipoprotein A5 (APOA5), which is associated with hypertriglyceridemia, a cardiovascular risk factor (Caussy et al., 2014).
In addition to general considerations, it is important to continue to consider the unique impact the Flint water crisis will continue to have on a predominantly African-American population, given particular risk associations that exist between this group and later-life neurodegeneration (Barnes and Bennett, 2014), and investigate risk factor disparities vs. other ethnic and racial groups. Furthermore, it will be key to explore the range of long term mental health effects of chronic Pb exposure combined with the stress of poverty and other environmental factors on the people of and from Flint. Future outcomes of unemployment and unstable family structures could be associated with poor mental health among those children not only exposed to high Pb levels for extended periods of time, but exacerbated by limited social support and institutionalized stigma. These risk factors contribute to poor mental health outcomes in youth affected by other traumatic experiences such as war (Sharma et al., 2017). Coping strategies and mechanisms employed by those who have suffered crises such as famine, war, and forced migration may be useful in building adaptive and prosocial behaviors in Flint youth in order to recover from such a devastating early life exposure. However, behavioral therapy would only have limited effect in the face of neurobiological alterations brought about by Pb exposure.
6. Flint’s Water problem was not unique
The Centers for Disease Control (CDC) sets 5 μg/dl blood Pb as the actionable value. By one estimate, frequency of children in Flint under six years of age with blood Pb levels above 5 μg/dl fell from 16.2% in 2005 to 3.6% in 2013, at which point it was on par with the USA as a whole. Upon switching to the more corrosive water from the Flint River, which leached Pb from the water system, levels rose to 6.4% by 2015 (Drum, 2016). More in-depth investigation established pre- and post-source change levels at 2.4% vs. 4.9% in Flint in general, with high Pb level areas of Flint starting from 4% in 2013 and elevated to 10.6% in 2015 (Hanna-Attisha et al., 2016).
Even though these shocking levels received widespread attention at the time, the majority of the world has simply moved on. This is neither ethical nor safe. Flint’s exposure was severe, but it is not unique. Troubling Pb levels were recently found in drinking water sources in schools and daycares in 42 states in the USA (Ungar, 2016). The current public health goal, regardless of official standards, is no Pb content: No level of Pb exposure is non-toxic (Environmental Protection Agency USA, 2016; Ungar, 2016). Testing the water at sinks and water fountains revealed multiple instances at more than 100 ppb, with the highest being a 5000 ppb sample from the restroom sink at Caroline Elementary School in Ithaca, NY (Ungar, 2016). Putting this situation into even bleaker perspective, no national mandate in the U.S. exists for Pb testing specifically at schools or daycares (Environmental Protection Agency USA, 2016), and not all states require such testing. While city-wide “water systems” are required to test for Pb at the tap, and if more than 10% of samples fall above 15 ppb, a water system is required to take action, a system that serves 100,000 or more people need test at no more than 100 locations, even if the service area actually applies to millions of people (Environmental Protection Agency USA, 2016). To exacerbate this issue, multiple US cities have used testing “cheats” that conceal dangerous levels of Pb (Gajanan, 2016). These “cheats” include pre-flushing pipes before testing, removal of aerators, and running water slowly for test purposes.
7. Beyond water: Pb exposure originates from various sources
Pb exposure through drinking water is far from the only significant source of exposure to environmental Pb. Although leaded gasoline and paint have now been eliminated from the US domestic market, paint that contains Pb is not routinely removed from older dwellings. Pb also adheres to soil and persists for extended periods. Such soil-bound Pb may be a significant source of exposure for children in many urban areas (Frazer, 2008). Measurement of Pb exposure in several urban areas at least a decade after the bans on leaded gasoline and paint were enacted showed effects of temperature and soil moisture on pediatric blood Pb levels. Inner-city children had higher blood Pb levels in late summer months and lower levels in winter (Laidlaw et al., 2005). Soil Pb vs. pediatric blood Pb levels were explicitly modeled for New Orleans, and an extrapolation of changing soil Pb limits from 400 mg/kg to 100 mg/kg predicted an economic benefit of approximate $4700 to $12,500 per child (year 2000 dollars) (Zahran et al., 2011). A neighborhood’s past can continue to haunt for decades. For example, the Indiana city of South Bend repeatedly shows high levels of Pb in tests of children from the northwest side of the city. This area has a concentration of homes with Pb-containing paint (Associated Press, 2018; Booker, 2018). According to the CDC, other sources of Pb include toys, candy imported from Mexico and artificial turf (Centers for Disease Control and Prevention USA, 2015).
8. Developing a larger solution: remediation, renovation, reallocation and research
The Pb exposure crisis in Flint has passed from an initial outcry, a feeling of “crisis” to a mere footnote. A crisis must, after all, be dramatic and isn’t expected to last for years; however, in the case of Pb, effects last long after feelings of urgency pass. Of course, the short memory of the general public does not mean that the scientific community has any obligation to go with such a flow.
We propose “4R’s” (Fig. 2) against the Pb crisis that goes beyond any quick fix. Acute remediation must, of course, be done first to address acute symptoms. Flint residents need to have clean water. In parallel, water systems need to be renovated, including adding anti-corrosive agents to water. Pb-containing components would be removed and replaced, possibly on a home-by-home basis. Steps to reduce overall corrosiveness of water supplies could reduce risk in systems while they are being updated. All of these measures require reallocation of considerable resources. Administrators of water systems need to be made aware not only of regulatory requirements but how regulation may fall short of the needs of their community. Bare adherence to the letter of the law does not guarantee safety from dangerous levels of Pb. Willingness to accurately measure Pb concentration and exposure is necessary. Proper Pb level quantification and monitoring need to be modernized to account for water systems that far exceed the capacity and volumes envisioned when rules were originally formulated. Methods must be standardized and standards enforced to limit Pb exposures (Rosen et al., 2017). All of these measures may be inadequate to the so-called epigenetic “time bomb” that was set in Flint. It will not be enough to clean up Flint’s water. In addition to acute “remediation”, attention must be paid to discovering and possibly reversing these latent epigenetic lesions, which may consist of aberrant DNA methylation, disruption of histone modification, and other markers. While there is research on promising epigenetic remediation (Fig. 1C), such as resveratrol (Wang et al., 2016) or dietary supplementation with S-adenosylmethionine (Chan and Shea, 2006) or folic acid (Bae et al., 2014; Kok et al., 2015), it is still far from conclusive.
Fig. 2.
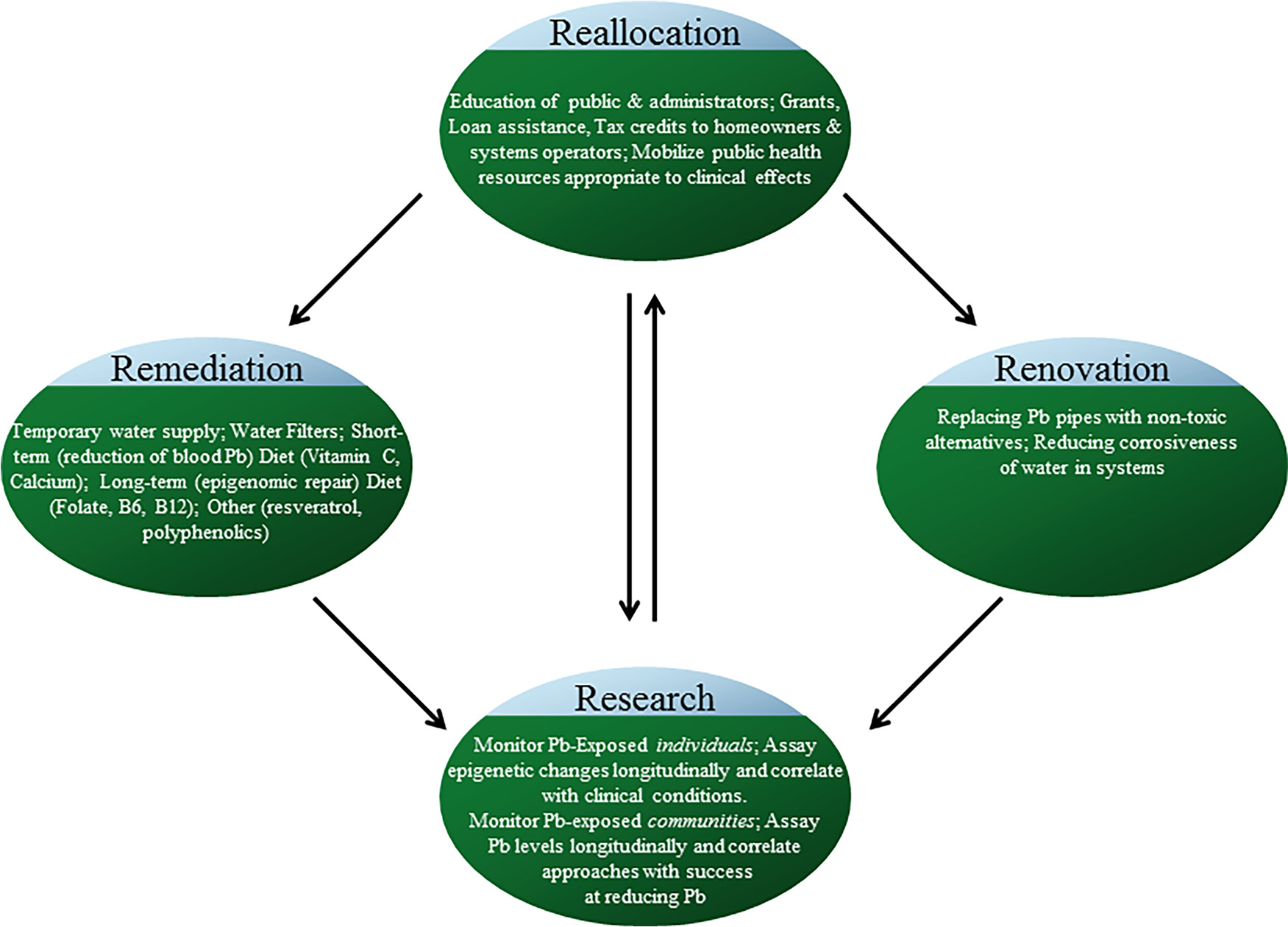
Proposal of four R’s of response to widespread Pb contamination. The enormity of Pb exposure via municipal water systems requires multiple responses. Immediate Remediation must be done for affected individuals, in terms of providing clean water and dealing with both short and long-term effects of Pb exposure. Renovation of current water delivery systems, at both system-wide and individual site levels, is necessary. Reallocation of resources is necessary to ensure these two responses occur and to get communities ready for potential sequelae of Pb exposure. Research, properly focused can track exposed individuals and illuminate latent (presumably epigenetic) results of Pb exposure and inform further resource Reallocation.
Providing ‘Remediation’ and ‘Renovation’ will require money, be it by tax credits, loans, or outright grants to water system operators, municipalities, school systems, or individual homeowners. Fortunately, some movement has occurred. Millions of dollars of grants have been allocated to research and renovation for Flint (Community Foundation Greater Flint, 2018; Environmental Protection Agency USA, 2017; Mott Foundation, 2016) and South Bend (Booker, 2017). However, this is still a piecemeal approach to what appears to be a much larger, if latent, problem.
Once potential “Pb hotspots” have been identified, public health and educational resources may need to be mobilized to deal with long-term effects of exposure. Underpinning and supporting all of these efforts would be focused research. It should not be limited to a single approach or discipline. Epigenetic monitoring of exposed individuals is certainly important, particularly in correlation with development of later-life neurological conditions, potential dietary modification of their epigenomes, etc. However, monitoring exposed communities is also necessary, not only to ensure regulatory compliance, but to track which local policy decisions end up significantly preventing Pb exposure over the long term. These research results, of course, could then be used to inform future resource reallocation decisions. This research would need to be conducted in a culturally sensitive manner, considering the vulnerability of the population and justifiable public mistrust, further exacerbated by an unresponsive government that has devastated so many. In any case, an integrated approach of all of these factors will likely be necessary to meet not only the challenges posed by Pb exposure, but by the ever-increasing number of man-made environmental challenges (e.g., pesticides) that also have been found to exert a significant long-term public health and epigenomic effect.
Flint is a city of nearly 100,000 with a majority of African-American citizens, making up nearly 57% of its population (US Census Bureau, 2017). Complaints from its residents concerning skin problems and changes in the appearance of their water at most evoked boil water advisories (Lin, 2016) until Pb levels in Flint’s tap water were tested and found to be more than twice the maximum contaminant level classification of the EPA (Craft-Blacksheare, 2017). Socioeconomic disparity is a fundamental element in this crisis. Even though Flint residents, on average, pay more than other citizens in the United States for their water (Lynch, 2016), they have essentially been poisoned by it, further emphasizing disparity on outcome vs. prices assessed to individual residents. Institutional problems are still entrenched in Flint governance. Flint’s water situation is neither strictly biomedical nor limited to a single city. It is a large-scale public health issue that particularly infests low-income, low-status communities. The scientific community could take a lead in addressing such issues.
We hope that we have provided a useful model to transcend this sole event and that can be applied to other sources of intoxication, other toxicants, and other mechanisms of action. We wish for readers to consider the full dimensions of the problem and seriously consider the long-term socioeconomic consequences of a presumably isolated episode of intoxication. Such awareness deserves to be known worldwide, not only to the research community (who takes part in one R) but also to regulatory agencies and decision makers who are involved with the other 3 Rs.
Funding
Funding was through grant support from the National Institute on Aging (US NIA)R01-AG051086, 1R41AG053117-01 and P30AG010133, Indiana Alzheimer Disesae Center and Indiana Clinical & Translational Sciences Institute (ICTSI) and ISDH Spinal Cord and Brain Injury Board Fund to DKL. Data cited in this work can be obtained through the cited sources.
Abbreviations:
- CDC
Centers for Disease Control (USA)
- DNA
deoxyribonucleic acid
- EPA
Environmental Protection Agency (USA)
- GCF
Great Chinese Famine
- HDACi
histone deacetylase inhibitors
- miRNA
microRNA
- Pb
lead
- ppb
parts per billion
- RNA
ribonucleic acid
- tLEARn
Transgenerational Latent Early-Life Regulation
Footnotes
Competing interests
The authors declare that they have no competing interests.
Declarations
The authors declare they have no competing financial interests, have contributed equally to the article.
References
- Help for Flint water crisis: Chelation therapy. 2016. http://whitakerwellness.com/2016/08/help-flint-water-crisis-chelation-therapy/ (Accessed January 23 2018).
- The Flint water crisis and the role of chelation therapy. 2016. http://chelation.me/the-flint-water-crisis-and-the-role-of-chelation-therapy/. (Accessed January 23 2018).
- Anway MD, Cupp AS, Uzumcu M, Skinner MK, 2005. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 308 (5727), 1466–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Associated Press, 2018. South Bend Primary Schools to Offer Free Lead Tests. (Accessed May 1 2018). https://www.usnews.com/news/best-states/indiana/articles/2018-03-19/south-bend-primary-schools-to-offer-free-lead-tests.
- Bae S, Ulrich CM, Bailey LB, Malysheva O, Brown EC, Maneval DR, Neuhouser ML, Cheng TY, Miller JW, Zheng Y, Xiao L, Hou L, Song X, Buck K, Beresford SA, Caudill MA, 2014. Impact of folic acid fortification on global DNA methylation and one-carbon biomarkers in the women’s health initiative observational study cohort. Epigenetics 9 (3), 396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakulski KM, 2013. Lead Exposure, Homocysteine, DNA Methylation and Late-Onset Alzheimer’s Disease., Environmental Health Sciences. University of Michigan, Ann Arbor, MI, USA, pp. 198. [Google Scholar]
- Barnes LL, Bennett DA, 2014. Alzheimer’s disease in African americans: risk factors and challenges for the future. Health Aff. (Millwood) 33 (4), 580–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basha MR, Murali M, Siddiqi HK, Ghosal K, Siddiqi OK, Lashuel HA, Ge YW, Lahiri DK, Zawia NH, 2005a. Lead (Pb) exposure and its effect on APP proteolysis and abeta aggregation. FASEB J. 19 (14), 2083–2084. [DOI] [PubMed] [Google Scholar]
- Basha MR, Wei W, Bakheet SA, Benitez N, Siddiqi HK, Ge YW, Lahiri DK, Zawia NH, 2005b. The fetal basis of amyloidogenesis: exposure to lead and latent overexpression of amyloid precursor protein and beta-amyloid in the aging brain. J. Neurosci. 25 (4), 823–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedrosian TA, Quayle C, Novaresi N, Gage FH, 2018. Early life experience drives structural variation of neural genomes in mice. Science 359 (6382), 1395–1399. [DOI] [PubMed] [Google Scholar]
- Bihaqi SW, Bahmani A, Adem A, Zawia NH, 2014. Infantile postnatal exposure to lead (Pb) enhances tau expression in the cerebral cortex of aged mice: relevance to AD. Neurotoxicology 44, 114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolin CM, Basha R, Cox D, Zawia NH, Maloney B, Lahiri DK, Cardozo-Pelaez F, 2006. Exposure to lead and the developmental origin of oxidative DNA damage in the aging brain. FASEB J. 20 (6), 788–790. [DOI] [PubMed] [Google Scholar]
- Booker T, 2017. Grant Will Put Boots on Ground to Combat South Bend’S Lead Problem. (Accessed May 1 2018). https://socialconcerns.nd.edu/news/grant-will-put-boots-ground-combat-south-bends-lead-problem. [Google Scholar]
- Booker T, 2018. Testing for Lead Poisoning Climbs by 40 Percent in St. Joseph County. (Accessed May 1 2018). https://www.southbendtribune.com/news/local/testing-for-lead-poisoning-climbs-by-percent-in-st-joseph/article_4e38a507-caac-52c1-997f-64384dd13878.html.
- Bressler J, Kim KA, Chakraborti T, Goldstein G, 1999. Molecular mechanisms of lead neurotoxicity. Neurochem. Res. 24 (4), 595–600. [DOI] [PubMed] [Google Scholar]
- Brucker N, Moro A, Charao M, Bubols G, Nascimento S, Goethel G, Barth A, Prohmann AC, Rocha R, Moresco R, Sangoi M, Hausen BS, Saint’Pierre T, Gioda A, Duarte M, Castro I, Saldiva PH, Garcia SC, 2015. Relationship between blood metals and inflammation in taxi drivers. Clin. Chim. Acta 444, 176–181. [DOI] [PubMed] [Google Scholar]
- Bygren LO, 2013. Intergenerational health responses to adverse and enriched environments. Annu. Rev. Public Health 34, 49–60. [DOI] [PubMed] [Google Scholar]
- Caussy C, Charriere S, Marcais C, Di Filippo M, Sassolas A, Delay M, Euthine V, Jalabert A, Lefai E, Rome S, Moulin P, 2014. An APOA5 3’ UTR variant associated with plasma triglycerides triggers APOA5 downregulation by creating a functional miR-485–5p binding site. Am. J. Hum. Genet. 94 (1), 129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention USA, 2015. Sources of Lead. (Accessed December 13 2017). https://www.cdc.gov/nceh/lead/tips/sources.htm.
- Chan A, Shea TB, 2006. Supplementation with apple juice attenuates presenilin-1 overexpression during dietary and genetically-induced oxidative stress. J. Alzheimers Dis. 10 (4), 353–358. [DOI] [PubMed] [Google Scholar]
- Chan A, Remington R, Kotyla E, Lepore A, Zemianek J, Shea TB, 2010. A vitamin/nutriceutical formulation improves memory and cognitive performance in community-dwelling adults without dementia. J. Nutr. Health Aging 14 (3), 224–230. [DOI] [PubMed] [Google Scholar]
- Chhillar N, Singh NK, Banerjee BD, Bala K, Basu M, Sharma D, 2014. Intergenotypic variation of Vitamin B12 and Folate in AD: in North Indian population. Ann. Indian Acad. Neurol. 17 (3), 308–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S, Woodbury-Farina MA, Shad MU, Husni M, Copen J, Bureau Y, Cernovsky Z, Hou JJ, Raheb H, Terpstra K, Sanchez V, Hategan A, Kaushal M, Campbell R, 2014. The role of nutrient-based epigenetic changes in buffering against stress, aging, and Alzheimer’s disease. Psychiatr. Clin. North Am. 37 (4), 591–623. [DOI] [PubMed] [Google Scholar]
- Community Foundation Greater Flint, 2018. Flint Child Health and Development Fund. (Accessed May 1 2018). https://www.cfgf.org/cfgf/GoodWork/FlintArea/FlintKids/tabid/855/Default.aspx.
- Conat R, 2016. Chelation Could Be One Answer to Flint’S Lead Problem. (Accessed June 6 2017). http://www.wndu.com/content/news?article=367645701.
- Craft-Blacksheare MG, 2017. Lessons learned from the crisis in Flint, Michigan regarding the effects of contaminated Water on maternal and child health. J. Obstet. Gynecol. Neonatal. Nurs. 46 (2), 258–266. [DOI] [PubMed] [Google Scholar]
- De Jager PL, Srivastava G, Lunnon K, Burgess J, Schalkwyk LC, Yu L, Eaton ML, Keenan BT, Ernst J, McCabe C, Tang A, Raj T, Replogle J, Brodeur W, Gabriel S, Chai HS, Younkin C, Younkin SG, Zou F, Szyf M, Epstein CB, Schneider JA, Bernstein BE, Meissner A, Ertekin-Taner N, Chibnik LB, Kellis M, Mill J, Bennett DA, 2014. Alzheimer’s disease: early alterations in brain DNA methylation at ANK1, BIN1, RHBDF2 and other loci. Nat. Neurosci. 17 (9), 1156–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Burde B, Choate MS Jr., 1972. Does asymptomatic lead exposure in children have latent sequelae? J. Pediatr. 81 (6), 1088–1091. [DOI] [PubMed] [Google Scholar]
- Deans C, Maggert KA, 2015. What do you mean, “epigenetic”? Genetics 199 (4), 887–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias BG, Ressler KJ, 2014. Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat. Neurosci. 17 (1), 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich KN, Ware JH, Salganik M, Radcliffe J, Rogan WJ, Rhoads GG, Fay ME, Davoli CT, Denckla MB, Bornschein RL, Schwarz D, Dockery DW, Adubato S, Jones RL, 2004. Effect of chelation therapy on the neuropsychological and behavioral development of lead-exposed children after school entry. Pediatrics 114 (1), 19–26. [DOI] [PubMed] [Google Scholar]
- Dosunmu R, Wu J, Adwan L, Maloney B, Basha MR, McPherson CA, Harry GJ, Rice DC, Zawia NH, Lahiri DK, 2009. Lifespan profiles of alzheimer’s disease-associated genes and products in monkeys and mice. J. Alzheimers Dis. 18 (1), 211–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drum K, 2016. Raw Data: Lead Poisoning of Kids in Flint. (Accessed June 6 2017). http://www.motherjones.com/kevin-drum/2016/01/raw-data-lead-poisoning-kids-flint.
- Eid A, Bihaqi SW, Renehan WE, Zawia NH, 2016. Developmental lead exposure and lifespan alterations in epigenetic regulators and their correspondence to biomarkers of alzheimer’s disease. Alzheimers Dement. 2, 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Environews DC News Bureau, 2016. Media Should Discuss EDTA and Other Chelation Therapies for Children of Flint. (Accessed January 23 2018). https://www.environews.tv/012516-editorial-media-should-promote-edtazeolite-detoxification-for-children-in-flint/.
- Environmental Protection Agency USA, 2016. Control of Lead and Copper (Title 40, Chapter I, Subchapter D, Part 141, Subpart I). (Accessed June 6 2017). http://www.ecfr.gov/cgi-bin/text-idx?SID=531617f923c3de2cbf5d12ae4663f56d&mc=true&node=sp40.23.141.i&rgn=div6.
- Environmental Protection Agency USA, 2017. EPA Awards $100 Million to Michigan for Flint Water Infrastructure Upgrades. (Accessed May 1 2018). https://www.epa.gov/newsreleases/epa-awards-100-million-michigan-flint-water-infrastructure-upgrades.
- Feng Y, Jankovic J, Wu YC, 2015. Epigenetic mechanisms in Parkinson’s disease. J. Neurol. Sci. 349 (1–2), 3–9. [DOI] [PubMed] [Google Scholar]
- Frazer L, 2008. Soil in the city: a prime source of lead. Environ. Health Perspect. 116 (12), A522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajanan M, 2016. At Least 33 US Cities Used Water Testing’ Cheats’ Over Lead Concerns. (Accessed June 6 2017). https://www.theguardian.com/environment/2016/jun/02/lead-water-testing-cheats-chicago-boston-philadelphia.
- Hanna-Attisha M, LaChance J, Sadler RC, Champney Schnepp A, 2016. Elevated blood lead levels in children associated with the flint drinking water crisis: a spatial analysis of risk and public health response. Am. J. Public Health 106 (2), 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Kim G, Jang W, Kim SY, Chang N, 2014. Association between intake of B vitamins and cognitive function in elderly Koreans with cognitive impairment. Nutr. J. 13 (1), 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok DE, Dhonukshe-Rutten RA, Lute C, Heil SG, Uitterlinden AG, van der Velde N, van Meurs JB, van Schoor NM, Hooiveld GJ, de Groot LC, Kampman E, Steegenga WT, 2015. The effects of long-term daily folic acid and vitamin B12 supplementation on genome-wide DNA methylation in elderly subjects. Clin. Epigenetics 7, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koz ST, Etem EO, Baydas G, Yuce H, Ozercan HI, Kuloglu T, Koz S, Etem A, Demir N, 2012. Effects of resveratrol on blood homocysteine level, on homocysteine induced oxidative stress, apoptosis and cognitive dysfunctions in rats. Brain Res. 1484, 29–38. [DOI] [PubMed] [Google Scholar]
- Lahiri DK, Maloney B, 2010. The “LEARn” (latent early-life associated regulation) model integrates environmental risk factors and the developmental basis of Alzheimer’s disease, and proposes remedial steps. Exp. Gerontol. 45 (4), 291–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri DK, Maloney B, Bayon BL, Chopra N, White FA, Greig NH, Nurnberger JI, 2016. Transgenerational latent early-life associated regulation unites environment and genetics across generations. Epigenomics 8 (3), 373–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri DK, Zawia N, Maloney B, 2018. LINEing up With a LEARn Approach: Somatic Mosaicism Fits into Epigenetic Models of Neurodegenerative Disorders. (Accessed May 1 2018). http://science.sciencemag.org/content/359/6382/1395/tab-e-letters.
- Laidlaw MA, Mielke HW, Filippelli GM, Johnson DL, Gonzales CR, 2005. Seasonality and children’s blood lead levels: developing a predictive model using climatic variables and blood lead data from Indianapolis, Indiana, Syracuse, New York, and New orleans, Louisiana (USA). Environ. Health Perspect. 113 (6), 793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapook J, 2016. Doctors Explain the Long-Term Health Effects of Flint Water Crisis. (Accessed June 6 2017). http://www.cbsnews.com/news/doctors-explain-the-long-term-health-effects-of-flint-water-crisis/.
- Li Y, Chen JA, Sears RL, Gao F, Klein ED, Karydas A, Geschwind MD, Rosen HJ, Boxer AL, Guo W, Pellegrini M, Horvath S, Miller BL, Geschwind DH, Coppola G, 2014. An epigenetic signature in peripheral blood associated with the haplotype on 17q21.31, a risk factor for neurodegenerative tauopathy. PLoS Genet. 10 (3), e1004211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JCF, 2016. Events That Led to Flint’s Water Crisis. (Accessed June 6 2017). https://www.nytimes.com/interactive/2016/01/21/us/flint-lead-water-timeline.html.
- Lin KP, Chen SY, Lai LC, Huang YL, Chen JH, Chen TF, Sun Y, Wen LL, Yip PK, Chu YM, Chen WJ, Chen YC, 2011. Genetic polymorphisms of a novel vascular susceptibility gene, Ninjurin2 (NINJ2), are associated with a decreased risk of Alzheimer’s disease. PLoS One 6 (6), e20573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin N, Qin S, Luo S, Cui S, Huang G, Zhang X, 2014. Homocysteine induces cytotoxicity and proliferation inhibition in neural stem cells via DNA methylation in vitro. FEBS J. 281 (8), 2088–2096. [DOI] [PubMed] [Google Scholar]
- Lu MC, Halfon N, 2003. Racial and ethnic disparities in birth outcomes: a life-course perspective. Matern. Child Health J. 7 (1), 13–30. [DOI] [PubMed] [Google Scholar]
- Lynch J, 2016. Officials: Flint Water Rates Could Double in Five Years, The Detroit News. [Google Scholar]
- Maloney B, Lahiri DK, 2016. Epigenetics of dementia: understanding the disease as a transformation rather than a state. Lancet Neurol. 15 (7), 760–774. [DOI] [PubMed] [Google Scholar]
- Marioni RE, Shah S, McRae AF, Chen BH, Colicino E, Harris SE, Gibson J, Henders AK, Redmond P, Cox SR, Pattie A, Corley J, Murphy L, Martin NG, Montgomery GW, Feinberg AP, Fallin MD, Multhaup ML, Jaffe AE, Joehanes R, Schwartz J, Just AC, Lunetta KL, Murabito JM, Starr JM, Horvath S, Baccarelli AA, Levy D, Visscher PM, Wray NR, Deary IJ, 2015. DNA methylation age of blood predicts all-cause mortality in later life. Genome Biol. 16, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoud AM, Bihaqi SW, Machan JT, Zawia NH, Renehan WE, 2016. Early-life exposure to lead (Pb) alters the expression of microRNA that target proteins associated with Alzheimer’s disease. J. Alzheimers Dis. 51 (4), 1257–1264. [DOI] [PubMed] [Google Scholar]
- Mott Foundation, 2016. Mott Foundation Grants Related to the Flint Water Crisis. (Accessed May 1 2018). https://www.mott.org/work/flint/flint-water-crisis/all-grants/.
- Nemsadze K, Sanikidze T, Ratiani L, Gabunia L, Sharashenidze T, 2009. Mechanisms of lead-induced poisoning. Georgian Med. News 172–173, 92–96. [PubMed] [Google Scholar]
- Perng W, Villamor E, Shroff MR, Nettleton JA, Pilsner JR, Liu Y, Diez-Roux AV, 2014. Dietary intake, plasma homocysteine, and repetitive element DNA methylation in the multi-ethnic study of atherosclerosis (MESA). Nutr. Metab. Cardiovasc. Dis. 24 (6), 614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly M, 2016. Can the Lead Poisoining in Flint Be Fixed? (Accessed January 23 2018). https://www.technologyreview.com/s/546121/can-the-lead-poisoning-in-flint-be-fixed/.
- Reynolds EH, 2014. The neurology of folic acid deficiency. Handb. Clin. Neurol. 120, 927–943. [DOI] [PubMed] [Google Scholar]
- Rosen MB, Pokhrel LR, Weir MH, 2017. A discussion about public health, lead and Legionella pneumophila in drinking water supplies in the United States. Sci. Total Environ. 590—591, 843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santa Maria MP, Hill BD, Kline J, 2018. Lead (Pb) neurotoxicology and cognition. Appl. Neuropsychol. Child 1–22. [DOI] [PubMed] [Google Scholar]
- Sen A, Cingolani P, Senut MC, Land S, Mercado-Garcia A, Tellez-Rojo MM, Baccarelli AA, Wright RO, Ruden DM, 2015a. Lead exposure induces changes in 5-hydroxymethylcytosine clusters in CpG islands in human embryonic stem cells and umbilical cord blood. Epigenetics 10 (7), 607–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen A, Heredia N, Senut MC, Hess M, Land S, Qu W, Hollacher K, Dereski MO, Ruden DM, 2015b. Early life lead exposure causes gender-specific changes in the DNA methylation profile of DNA extracted from dried blood spots. Epigenomics 7 (3), 379–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen A, Heredia N, Senut MC, Land S, Hollocher K, Lu X, Dereski MO, Ruden DM, 2015c. Multigenerational epigenetic inheritance in humans: DNA methylation changes associated with maternal exposure to lead can be transmitted to the grandchildren. Sci. Rep. 5, 14466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Fine SL, Brennan RT, Betancourt TS, 2017. Coping and mental health outcomes among Sierra Leonean war-affected youth: results from a longitudinal study. Dev. Psychopathol. 29 (1), 11–23. [DOI] [PubMed] [Google Scholar]
- Stegemann R, Buchner DA, 2015. Transgenerational inheritance of metabolic disease. Semin. Cell Dev. Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge JP, 2014. Tragedy of the Flint Michigan Contamination Lead in the Water – Is It Time to Freak-Out? (Accessed January 23 2018). http://www.drjonathancollin.com/chelation_flintlead.html.
- Ungar L, 2016. Lead Taints Drinking Water in Hundreds of Schools, Day Cares Across USA. (Accessed June 6 2017). http://www.usatoday.com/story/news/nation/2016/03/17/drinking-water-lead-schools-day-cares/81220916/.
- US Census Bureau, 2017. American FactFinder. (Accessed June 6 2017). https://factfinder.census.gov/faces/nav/jsf/pages/index.xhtml.
- USDA Office of Communications, 2016. Fact Sheet: USDA Assistance to Residents Affected by the Water Emergency in Flint, Michigan. (Accessed January 23 2018). https://www.fns.usda.gov/pressrelease/2016/003816.
- Vahid F, Zand H, Nosrat-Mirshekarlou E, Najafi R, Hekmatdoost A, 2015. The role dietary of bioactive compounds on the regulation of histone acetylases and deacetylases: a review. Gene 562 (1), 8–15. [DOI] [PubMed] [Google Scholar]
- Vassoler FM, Sadri-Vakili G, 2014. Mechanisms of transgenerational inheritance of addictive-like behaviors. Neuroscience 264, 198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassoler FM, White SL, Schmidt HD, Sadri-Vakili G, Pierce RC, 2013. Epigenetic inheritance of a cocaine-resistance phenotype. Nat. Neurosci. 16 (1), 42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade L, 2016. Flint’S High Lead Levels Have Doctors Struggling for Answers. (Accessed June 6, 2017). http://www.wired.com/2016/01/flints-high-lead-levels-have-doctors-struggling-for-answers/.
- Wang G, Chen L, Pan X, Chen J, Wang L, Wang W, Cheng R, Wu F, Feng X, Yu Y, Zhang HT, O’Donnell JM, Xu Y, 2016. The effect of resveratrol on beta amyloid-induced memory impairment involves inhibition of phosphodiesterase-4 related signaling. Oncotarget 7 (14), 17380–17392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Yang CR, Wei YP, Zhao ZA, Hou Y, Schatten H, Sun QY, 2014. Paternally induced transgenerational inheritance of susceptibility to diabetes in mammals. Proc. Acad. Sci. U. S. A. 111 (5), 1873–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Basha MR, Brock B, Cox DP, Cardozo-Pelaez F, McPherson CA, Harry J, Rice DC, Maloney B, Chen D, Lahiri DK, Zawia NH, 2008. Alzheimer’s disease (AD)-like pathology in aged monkeys after infantile exposure to environmental metal lead (Pb): evidence for a developmental origin and environmental link for AD. J. Neurosci. 28 (1), 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Gu JM, Huang Y, Duan YY, Huang RX, Hu JA, 2016. Dose-response relationship between cumulative occupational lead exposure and the associated health damages: a 20-year cohort study of a smelter in China. Int. J. Environ. Res. Public Health 13 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie K, Ryan DP, Pearson BL, Henzel KS, Neff F, Vidal RO, Hennion M, Lehmann I, Schleif M, Schroder S, Adler T, Rathkolb B, Rozman J, Schutz AL, Prehn C, Mickael ME, Weiergraber M, Adamski J, Busch DH, Ehninger G, Matynia A, Jackson WS, Wolf E, Fuchs H, Gailus-Durner V, Bonn S, Hrabe de Angelis M, Ehninger D, 2018. Epigenetic alterations in longevity regulators, reduced life span, and exacerbated aging-related pathology in old father offspring mice. Proc. Acad. Sci. U. S. A. 115 (10), E2348–E2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Li-Hui, Mu F-F, Zhao Jian-Hong, He Qiang, Cao Cui-Li, Yang Hui, Liu Qi, Liu Xue-Hui, Sun Su-Ju, 2015a. Lead induces apoptosis and histone hyperacetylation in rat cardiovascular tissues. PLoS One 10 (16), e0129091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Chen J, Gao J, Yu H, Yang P, 2015b. Crosstalk of homocysteinylation, methylation and acetylation on histone H3. Analyst 140 (9), 3057–3063. [DOI] [PubMed] [Google Scholar]
- Xu LH, Mu FF, Zhao JH, He Q, Cao CL, Yang H, Liu Q, Liu XH, Sun SJ, 2015c. Lead induces apoptosis and histone hyperacetylation in rat cardiovascular tissues. PLoS One 10 (6), e0129091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakub M, Iqbal MP, 2010. Association of blood lead (Pb) and plasma homocysteine: a cross sectional survey in Karachi, Pakistan. PLoS One 5 (7), e11706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XL, Tian J, Liang Y, Ma CJ, Yang AN, Wang J, Ma SC, Cheng Y, Hua X, Jiang YD, 2014. Homocysteine induces blood vessel global hypomethylation mediated by LOX-1. Genet. Mol. Res. 13 (2), 3787–3799. [DOI] [PubMed] [Google Scholar]
- Yilmaz H, Keten A, Karacaoglu E, Tutkun E, Akcan R, 2012. Analysis of the hematological and biochemical parameters related to lead intoxication. J. Forensic Leg. Med. 19 (8), 452–454. [DOI] [PubMed] [Google Scholar]
- Young M, 2016. ‘No Magic Pill’ for Flint Lead Poisoning, Whistleblowing Doctor Says. (Accessed January 23 2018). http://www.mlive.com/news/flint/index.ssf/2016/03/hurley_doctor_says_no_magic_pi.html.
- Zahran S, Mielke HW, Weiler S, Gonzales CR, 2011. Nonlinear associations between blood lead in children, age of child, and quantity of soil lead in metropolitan New orleans. Sci. Total Environ. 409 (7), 1211–1218. [DOI] [PubMed] [Google Scholar]
- Zawia NH, Lahiri DK, Cardozo-Pelaez F, 2009. Epigenetics, oxidative stress, and Alzheimer disease. Free Radic. Biol. Med. 46 (9), 1241–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou RH, Kokame K, Tsukamoto Y, Yutani C, Kato H, Miyata T, 2001. Characterization of the human NDRG gene family: a newly identified member, NDRG4, is specifically expressed in brain and heart. Genomics 73 (1), 86–97. [DOI] [PubMed] [Google Scholar]


