Abstract
Pertussis toxin (PT) and filamentous hemagglutinin (FHA) are two major virulence factors of Bordetella pertussis. FHA is the main adhesin, whereas PT is a toxin with an A-B structure, in which the A protomer expresses ADP-ribosyltransferase activity and the B moiety is responsible for binding to the target cells. Here, we show redundancy of FHA and PT during infection. Whereas PT-deficient and FHA-deficient mutants colonized the mouse respiratory tract nearly as efficiently as did the isogenic parent strain, a mutant deficient for both factors colonized substantially less well. This was not due to redundant functions of PT and FHA as adhesins, since in vitro studies of epithelial cells and macrophages indicated that FHA, but not PT, acts as an adhesin. An FHA-deficient B. pertussis strain producing enzymatically inactive PT colonized as poorly as did the FHA-deficient, PT-deficient strain, indicating that the ADP-ribosyltransferase activity of PT is required for redundancy with FHA. Only strains producing active PT induced a local transient release of tumor necrosis factor alpha (TNF-α), suggesting that the pharmacological effects of PT are the basis of the redundancy with FHA, through the release of TNF-α. This may lead to damage of the pulmonary epithelium, allowing the bacteria to colonize even in the absence of FHA.
Many bacterial pathogens produce adhesins and toxins to express their pathogenic potential. Although they are often studied as separate entities, it is possible that in some instances they act in a synergistic or in a redundant way. Redundancy among adhesins has previously been shown (40); however, redundancy between a toxin and an adhesin has not yet been demonstrated. Bordetella pertussis is a human pathogen that causes whooping cough after adherence to and colonization of the respiratory tract. B. pertussis synthesizes a number of virulence factors (16), whose production is under the control of a regulatory locus named bvg. Among them, filamentous hemagglutinin (FHA) and pertussis toxin (PT) have been proven to protect against bacterial infection (4, 31, 35) and are part of the present acellular vaccines. FHA is a monomeric protein of 220 kDa and has a filamentous structure (20). It is both surface exposed and secreted by B. pertussis. FHA is considered to be the main adhesin of B. pertussis (18) and contains at least three distinct binding sites: a glycosaminoglycan and a carbohydrate binding sites (10, 27) and an arginine-glycine-aspartate (RGD) sequence involved in binding to the CR3 integrin of macrophages (28). PT is an ADP-ribosylating toxin, belonging to the AB5 family of toxins (17) and is secreted into the extracellular milieu. PT consists of five subunits, named S1 to S5, and can be divided into two functional moieties. The A protomer, corresponding to the S1 subunit, expresses the ADP-ribosyltransferase activity. The B oligomer, composed of subunits S2 to S5, mediates binding of PT to its target cell receptors through the carbohydrate recognition domains of the S2 and S3 subunits (43). Several cell types, including erythrocytes, leukocytes, macrophages, and ciliated cells (33, 41, 43), display PT receptors on their surface whose precise identity still remains unknown. Although the B oligomer is essentially involved in binding of PT to target cells prior to translocation of the enzymatic S1 subunit, it is by itself also responsible for several biological activities, such as hemagglutination, T-cell mitogenicity, and initiation of signal transduction in target cells (25, 30, 37).
Many reports have established that FHA and PT are necessary for an optimal colonization of the respiratory tract by B. pertussis. Synergy between FHA and PT has also been suspected and has been ascribed to adhesin activities of FHA and PT (40) on macrophages (28, 34) and/or epithelial cells (39, 41). However, redundancy between FHA and PT has not yet been investigated during infection in vivo.
In this study, we show that FHA and PT are redundant during B. pertussis colonization of the mouse respiratory tract. This redundancy between both factors requires the enzymatic activity of PT rather than its adhesin activity, showing for the first time redundancy of an adhesin and a toxin as such during bacterial infection in vivo.
MATERIALS AND METHODS
B. pertussis strains and growth conditions.
The B. pertussis strains used in this study are listed in Table 1. They all derived from the Tohama I strain (24) and were grown on Bordet-Gengou (BG) agar (Difco, Detroit, Mich.) supplemented with 1% glycerol, 20% defibrinated sheep blood, and 100 μg of streptomycin (Sigma Chemical Co., St Louis, Mo.)/ml, and, when appropriate, 20 μg of ampicillin (Sigma)/ml, at 37°C for 72 h. Liquid cultures of B. pertussis were performed as described previously (23) in Stainer-Scholte medium containing 1 g of heptakis(2,6-di-o-methyl) β-cyclodextrin (Sigma)/liter. For cell adherence and macrophage invasion assays, exponentially growing B. pertussis was inoculated at an optical density of 0.15 at 600 nm in 2.5 ml of Stainer-Scholte medium and grown for 24 h at 37°C. For adherence studies, the bacteria were grown in the presence of 65 μCi of l-[35S]methionine plus l-[35S]cysteine (NEN, Boston, Mass.)/ml. The bacteria were then washed three times in phosphate-buffered saline (PBS) and resuspended in RPMI 1640 (Gibco, Grand Island, N.Y.) at the desired density.
TABLE 1.
B. pertussis strains used in this study
| B. pertussis strain | Relevant genotype | Reference |
|---|---|---|
| BPSM | Smr and Nalr Tohama I derivative | 23 |
| BPGR4 | FHA-deficient BPSM derivative | 19 |
| BPRA | PT-deficient BPSM derivative | 2 |
| BPDR | PT- and FHA-deficient BPSM derivative | This study |
| BPDR-RE | BPDR derivative producing mutant PT-RE | This study |
B. pertussis mutant constructs.
B. pertussis BPDR was obtained by double homologous recombination as described by Stibitz (36) using pGR5 (19) to delete the fhaB gene from the chromosome of PT-deficient B. pertussis BPRA (2). Consequently, this strain is PT and FHA deficient. B. pertussis BPDR-RE was constructed by introducing pPT-RE into the chromosome of BPDR by a single homologous recombination event. pPT-RE is a pPT2 derivative (1), containing the PT genes with mutations resulting in the R9K and E129G substitutions in the S1 subunit (15). The recombinant strain was selected on BG plates containing streptomycin and ampicillin. All strains produced normal amounts of fimbriae, as did the FHA-deficient strain BPGR4 (19).
Cells and growth conditions.
Human pulmonary (A549; ATCC no. CCL-185) and tracheal (HEp-2; ATCC no. CCL-23) epithelial cell lines were cultured in RPMI containing sodium penicillin G (1,000 U/ml) and streptomycin (50 μg/ml) (Gibco), 2 mM l-glutamine (Gibco), and 10% heat-inactivated fetal calf serum (Gibco) using uncoated tissue culture flasks and 24-well plates (Costar). The murine alveolar macrophage cell line MH-S (ATCC no. CRL-2019) was propagated in uncoated tissue culture flasks and 24-well plates (TPP) in the same RPMI-based medium as described above, supplemented with 1.5 g of sodium bicarbonate (Gibco)/liter, 4.5 g of glucose (Gibco)/liter, 10 mM HEPES (Gibco), 1 mM sodium pyruvate (Gibco), and 50 μM 2-β-mercaptoethanol (Gibco). Cells were detached mechanically by scraping. Dendritic cells were prepared from mouse spleen as described previously (21). Briefly, the mice received 10 μg of soluble flt3 ligand (Immunex, Seattle, Wash.) for 9 consecutive days, after which the spleen and mesenteric lymph nodes were harvested, treated with collagenase D (Boehringer Mannheim, Mannheim, Germany), and incubated with magnetic microbeads conjugated to hamster anti-mouse CD11c monoclonal antibodies (clone N418; Miltenyi Biotech, Auburn, Calif.). The CD11c+ dendritic cells were then purified over a magnetically activated cell sorter.
Cell adherence assay.
For 2 days in 24-well plates, 2 × 105 cells per well were cultured. Cells were washed once with RPMI medium before addition of 4 × 106 35S-labeled bacteria per well and incubation for 1 h 30 min at 37°C with 5% CO2. All bacterial strains were grown in parallel under the same conditions and harvested at the same growth phase. After three washes with RPMI to remove nonadherent bacteria, the mammalian cells were lysed with 0.5% sodium dodecyl sulfate. Whole lysates were quantified by liquid scintillation counting. The experiments were performed three times independently in triplicate.
Macrophage invasion assay.
Macrophages were grown and treated as described above. B. pertussis organisms were added at a multiplicity of infection of 20 and incubated for 1 h at 37°C with 5% CO2. After three washes in RPMI to remove nonadherent bacteria, RPMI containing 100 μg of gentamicin (Sigma)/ml was added to kill extracellular bacteria. After 2 h, macrophages were washed three times and lysed in 300 μl of sterile distilled water. Serial dilutions were then plated onto BG agar to determine the number of viable bacteria in each well. The experiments were performed twice independently in triplicate.
i.n. infection of mice.
B. pertussis grown on BG agar was suspended in sterile PBS and adjusted to a concentration of approximately 2.5 × 108 CFU/ml. Infections were performed by the intranasal (i.n.) route with 20 μl of the bacterial suspension deposited in the nostrils of 4-week-old female OF1 outbred mice (Iffa Credo, L'Arbresle, France), anesthetized (5) with a cocktail of physiological water containing 20% (vol/vol) Immalgen 1000 (Merial, Lyon, France), 10% atropine (Aguettant, Lyon, France), and 6% valium (Roche, Neuilly-sur-Seine, France), given intraperitoneally (150 μl per 20 g of body weight). At indicated time points, the lungs were aseptically removed and homogenized in PBS. Serial dilutions from individual lungs were plated onto BG agar, and the numbers of CFU were determined after 3 to 4 days of incubation at 37°C. The in vivo stability of pPT-RE in B. pertussis BPDR-RE was controlled at each time point by plating bacteria simultaneously onto BG agar containing streptomycin and onto BG agar containing streptomycin with ampicillin. The parent strain did not grow on 20 μg of ampicillin/ml after 5 days, whereas the recombinant strain formed distinct colonies after 3 days. Four OF1 mice per time point and per group of mice were assessed, and the experiments were performed five times independently. All animal studies were carried out under the guidelines of the Institut Pasteur animal study board.
TNF-α detection.
Bronchoalveolar lavage (BAL) fluids were recovered by a single lavage with 0.5 ml of sterile PBS and centrifuged for 30 s at 9,300 × g to remove cells. The BAL fluids were stored at −80°C until assayed for the presence of tumor necrosis factor alpha (TNF-α). TNF-α was quantified using the Quantikine M murine detection kit (R&D Systems, Wiesbaden, Germany). Results are expressed as picograms per milliliter. Experiments were done three times independently in triplicate.
Statistical analysis.
The results were analyzed using the unpaired Student t test. Differences were considered significant at a P value of <0.05.
RESULTS
Redundancy of FHA and PT in lung colonization by B. pertussis.
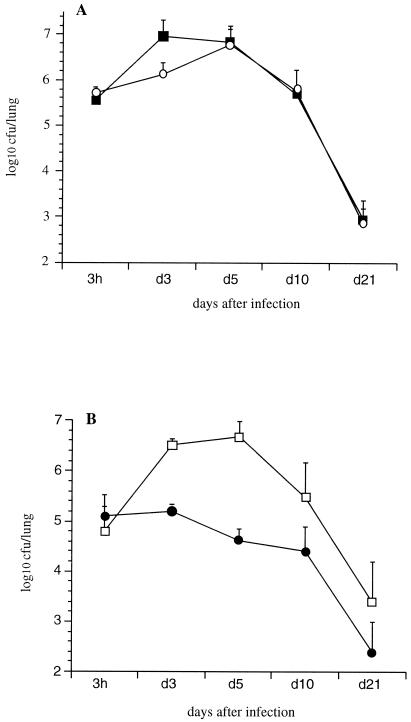
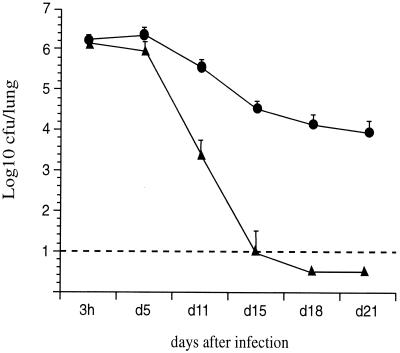
In order to determine the relative contributions of FHA and PT in lung colonization, mice were i.n. infected with B. pertussis BPSM or with its derivatives BPRA (ΔPT), BPGR4 (ΔFHA), or BPDR (ΔFHA ΔPT). BPSM showed a typical colonization profile with a multiplication peak 3 days after inoculation followed by a progressive clearance during the next 3 weeks (Fig. 1A). The colonization profiles obtained with BPRA (Fig. 1A) and BPGR4 (Fig. 1B) were similar to that of BPSM, indicating that the absence of either PT or FHA did not significantly affect the lung colonization.
FIG. 1.
Lung colonization by B. pertussis. OF1 mice were infected i.n. with B. pertussis BPSM (black squares) or BPRA (open circles) (A) or BPGR4 (open squares) or BPDR (black circles) (B). At the indicated time points, the mice were sacrificed, and the CFU present in the lungs were counted. Each curve represents an average of independent experiments. The results are expressed as the means ± the standard errors of the means. Four mice per time point and per group were assessed for each experiment.
When both PT and FHA were absent, such as in B. pertussis BPDR, no multiplication peak was observed (Fig. 1B), indicating that either PT or FHA is needed for optimal colonization of B. pertussis in mice.
In vitro adherence of B. pertussis strains.
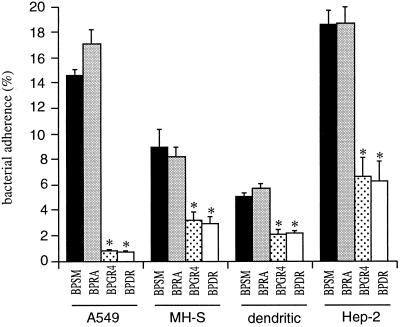
As PT has been proposed elsewhere to act as an adhesin (40, 41), we studied the adherence of BPSM and its derivatives BPRA, BPGR4, and BPDR to the following cell lines: the murine alveolar macrophages MH-S, the human tracheal epithelial cells HEp-2, and the human pulmonary epithelial cells A549. We also tested adherence to a primary culture of dendritic cells differentiated from murine splenocytes. The absence of PT did not affect adherence to either cell line (Fig. 2), whereas adherence of the FHA-deficient BPGR4 and BPDR strains, regardless of the presence of PT, was significantly reduced compared to that of the parent strain. Moreover, the addition of up to 25 μg of purified PT/ml to BPRA did not increase adherence to the MH-S cells (data not shown).
FIG. 2.
Adherence of B. pertussis to cell lines. Human pulmonary epithelial cells (A549), murine alveolar macrophages (MH-S), primary cultures of dendritic cells (dendritic), and human tracheal cells (HEp-2) were incubated with the indicated 35S-labeled B. pertussis strains for 1 h 30 min at a multiplicity of infection of 20. After washing, adherence was estimated by scintillation counting. The results are expressed as percentages of counts per minute relative to the counts per minute present in the inoculum. The data represent averages and standard deviations for quadruplicate experiments. ∗, P < 0.05.
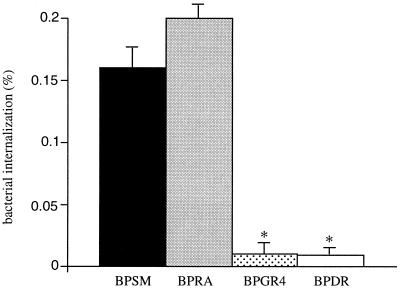
To test whether PT could affect the invasiveness of B. pertussis into macrophages, we compared the invasion by PT-deficient strains with that by PT-producing isogenic strains. As shown in Fig. 3, the PT-deficient BPRA invaded MH-S cells as efficiently as did BPSM. On the other hand, the FHA-deficient BPGR4 and BPDR strains were not internalized.
FIG. 3.
Internalization of B. pertussis into MH-S cell monolayers. MH-S cell monolayers were infected with the indicated B. pertussis strains at a multiplicity of infection of 20. After 1 h of incubation, the monolayers were treated with gentamicin and lysed, and the bacteria were plated onto BG agar. The results are expressed as percentages of internalized bacteria relative to the number of bacteria present in the inoculum. The data represent averages and standard deviations for triplicate experiments. ∗, P < 0.05.
These results indicate that FHA, but not PT, is involved in adherence to pulmonary and tracheal epithelial cell lines and in invasion of B. pertussis into macrophages.
Role of the enzymatic activity of PT in the lung colonization by FHA-deficient B. pertussis.
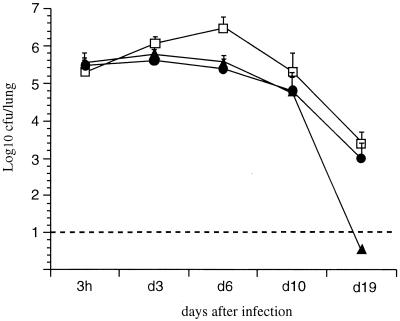
Since PT did not appear to act as an adhesin in the in vitro tests, we investigated the role of its ADP-ribosyltransferase activity in the redundancy with FHA in establishing colonization in vivo. An FHA-deficient B. pertussis strain producing an enzymatically inactive PT was therefore constructed. The ptx genes harboring mutations leading to the R9K and E129G substitutions in the S1 subunit of PT were introduced by homologous recombination into the chromosome of BPDR, resulting in the BPDR-RE strain. Both substitutions abolish the ADP-ribosyltransferase activity of PT (15). Immunoblot analysis of culture supernatants showed that PT-RE is efficiently produced and secreted by BPDR-RE (data not shown). The genetic constructs were stable even in the absence of selective pressure. No growth deficit was observed compared to the wild-type strain or the toxin-deletion mutant. As shown in Fig. 4, following an i.n. inoculation of BPDR-RE, no significant increase in the numbers of bacteria was observed in the lungs between days 3 and 5 compared to 3 h postinfection, similar to what was observed with the FHA- and PT-deficient BPDR strain. These results demonstrate that production of enzymatically active PT is important for cooperativity with FHA during B. pertussis infection of the mouse respiratory tract. Moreover, no necrotic lesions were observed in mouse lungs during the course of infection with BPDR-RE, in contrast to what is seen after infection with active toxin-producing B. pertussis strains, indicating that the ADP-ribosyltransferase activity of PT is responsible for the lung lesions observed after an i.n. infection with B. pertussis, consistent with earlier reports (12). Interestingly, we observed a rapid decrease of the number of BPDR-RE organisms beginning 10 days after the challenge, with a total clearance in less than 20 days. This was not seen in mice infected with BPDR, suggesting that the production of inactive PT reduces the persistence of B. pertussis in mouse lungs more than does the absence of PT. The bacteria isolated from the animals over the entire duration of the experiment remained resistant to ampicillin and produced PT-RE, indicating that the construct was stable in vivo.
FIG. 4.
Lung colonization by B. pertussis mutants. OF1 mice were infected i.n. with B. pertussis BPGR4 (open squares), BPDR (black circles), or BPDR-RE (black triangles), and colonization was monitored as described for Fig. 1. The stippled line represents the limit of detection of the number of CFU present in the lungs.
Local production of TNF-α.
Since the enzymatic ADP-ribosyltransferase activity of PT appears to be important for the observed redundancy with FHA in infection, we reasoned that this might be due to the pharmacological effects of PT on the host immune system rather than to its putative role as an adhesin. This prompted us to investigate the production of TNF-α, a cytokine transiently released into the BAL fluids early during B. pertussis infection (29) that induces inflammation which might facilitate infection even of FHA-deficient strains. Alternatively, it might up-regulate the immune response and facilitate clearance.
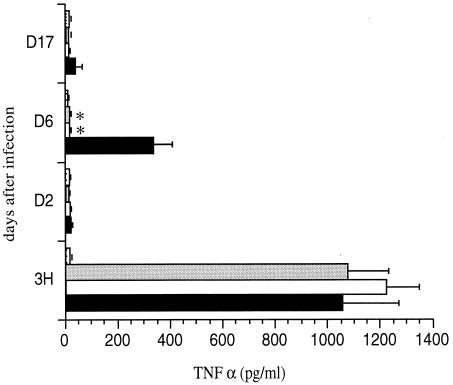
As shown in Fig. 5, after an initial burst of TNF-α in the BAL fluids of mice infected with any one of the three B. pertussis strains, only mice infected with BPGR4, producing active PT, showed a transiently increased level of TNF-α in BAL fluids 6 days after infection. In the mice infected with either BPDR or BPDR-RE producing inactive PT, the levels of TNF-α in the BAL fluids remained close to the baseline. The levels of TNF-α induced after infection with BPGR4 were similar to those observed previously with other PT-producing strains (29). These results indicate that the production of enzymatically active PT induces the release of TNF-α in the BAL fluids upon i.n. infection with B. pertussis and suggest that this may be the basis for the redundancy with FHA to optimize B. pertussis infection.
FIG. 5.
TNF-α production in BAL fluids of B. pertussis-infected mice. OF1 mice were infected i.n. with B. pertussis BPGR4 (black bars), BPDR (white bars), or BPDR-RE (gray bars) or received PBS (stippled bars). At indicated time points, the concentration of TNF-α was measured in the BAL fluids. Five mice were analyzed per time point for each group. ∗, P < 0.05.
Coinfection with BPDR and BPDR-RE.
Since the production of inactive toxin by B. pertussis results in faster clearing from the lungs than that in the absence of toxin, we wondered whether even enzymatically inactive toxin might induce general immunity against B. pertussis. This could conceivably occur via the binding activities of the B oligomer, since it has been shown elsewhere that the B oligomer expresses mitogenic activity on lymphocytes independently of the ADP-ribosyltransferase activity of PT (15, 25), which could of course increase the immune responses against B. pertussis. In that case, coinfection with BPDR and BPDR-RE would be expected to result in rapid clearing of both strains simultaneously. We therefore coinfected OF1 mice with both strains and monitored their presence in the lungs over time. The two strains could be distinguished because of their different antibiotic resistance patterns. As shown in Fig. 6, the course of colonization of BPDR was not affected by the presence of BPDR-RE in the lungs of the same mouse. Only BPDR-RE was rapidly cleared, and BPDR persisted longer. These results show that the production of enzymatically inactive yet mitogenic toxin does not induce general clearance but that the more rapid clearance of BPDR-RE than of BPDR is somehow due to the presence of enzymatically inactive PT on the surface of that particular B. pertussis organism.
FIG. 6.
Lung colonization after coinfection with B. pertussis BPDR and B. pertussis BPDR-RE. OF1 mice were infected i.n. with a mixture of B. pertussis BPDR (black circles) and B. pertussis BPDR-RE (black triangles). The results are the means ± standard errors of the means of four mice per time point.
DISCUSSION
The role of individual B. pertussis virulence factors in the mouse respiratory infection model has been intensively studied by a number of investigators (7, 9, 12–14, 32, 44, 45). Although FHA is an important adhesin, FHA-deficient B. pertussis strains have been reported previously to colonize the mouse respiratory tract nearly as efficiently as do the wild-type parent strains (9, 12–14, 44, 45), which has led to the assumption that several adhesins may have redundant functions (40). In this study, using isogenic B. pertussis strains we confirm that the mere absence of FHA does not affect the mouse colonization in any important way, since BPSM and BPGR4 appeared to colonize the mouse lungs equally well, at least at the high challenge doses used here. Furthermore, PT-deficient BPRA also colonized the mouse respiratory tract with the same efficiency as that of the wild-type parent strain BPSM. Previous studies have suggested that PT-deficient strains may sometimes be cleared somewhat faster than PT-producing strains (7, 12, 13, 45). These differences may be due to the doses of B. pertussis organisms used. Goodwin and Weiss (9) showed that, at low challenge doses (500 bacteria), PT-deficient B. pertussis was cleared much faster from the lungs of infant BALB/c mice than were PT-producing strains, whereas at higher doses (5 × 104 CFU) infection by the mutant persisted as long as that by the parental strain. Therefore, the doses used in this study (5 × 106) are probably too high to allow us to observe a difference of persistence between BPRA and BPSM.
Although the absence of either PT or FHA did not appear to affect colonization at the doses used in this study, the simultaneous absence of the two virulence factors affected colonization substantially, as after infection with BPDR the characteristic peak of multiplication seen at 3 to 6 days with wild-type B. pertussis was not observed. This suggests either that both factors express redundant adhesin functions, as previously suggested (28, 34, 39, 40), or that they are redundant by a different mechanism.
To test whether PT acts as an adhesin, adherence of FHA- and/or PT-deficient strains to several cell lines, including epithelial cells and macrophages, was investigated. In all cases, only FHA, not PT, was required for cell adherence of B. pertussis. These results are in agreement with several recent reports (3, 11, 42). However, PT-deficient B. pertussis has previously been shown to adhere less than normal B. pertussis to human and rabbit ciliated respiratory epithelial cells (40, 41), suggesting that it might act as an adhesin, an activity allocated to its carbohydrate binding sites located in the B moiety of the toxin (17, 33). Purified PT can indeed bind to the surface of bronchial and laryngeal cells in a dose-dependent fashion (42). However, preincubation of bronchial cells with purified PT inhibited the adherence of both PT-deficient and parental strains, and adherence of PT-deficient B. pertussis to both epithelial cell lines was similar to that of the parental strain (42), similar to what is observed in this study, suggesting that, unlike FHA, PT plays no significant role as an adhesin in vivo.
In this study, we also demonstrate that PT does not affect the invasiveness of B. pertussis into murine alveolar macrophages, although others (8, 28, 43) have suggested that PT mediates adherence and uptake of B. pertussis into human monocyte-derived macrophages through the carbohydrate recognition domains of both the S2 and S3 subunits. It is possible that differences in structure between the differentiated and specialized alveolar macrophages (22) and blood-derived mononuclear cells may explain these conflicting results.
Instead of being related to the adhesive properties of PT, we found that the redundancy with FHA during a B. pertussis infection is due to the enzymatic ADP-ribosyltransferase activity of PT, since an FHA-deficient mutant producing a correctly assembled toxin with alterations in the active site colonized as poorly as did a PT- and FHA-deficient strain. These results indicate that the redundancy between PT and FHA during colonization by B. pertussis depends on the pharmacological effects of PT, which are likely to be on the immune system of the host. This assumption is supported by the observation that only PT-producing strains induce the local release of proinflammatory cytokines such as TNF-α and that this release requires PT to be enzymatically active. Previous studies have shown that PT induces the production of TNF-α by monocytes in vitro (6, 26, 38). We therefore hypothesize that the release of TNF-α leading to inflammation in the lungs and to pulmonary necrotic lesions may favor colonization by B. pertussis in an FHA-independent manner and that, in the absence of such lesions, FHA is essential for colonization. Alternatively, TNF-α might up-regulate the immune response of the host and somehow facilitate clearance especially of FHA-deficient strains.
Interestingly, we observed that BPDR-RE producing inactive PT was cleared more rapidly from the lungs than was BPDR producing no toxin at all. This result demonstrates that the expression of an inactive PT is more detrimental to the bacterium than is the absence of PT. That this was not due to residual pharmacological effects of enzymatically inactive toxin on the immune system, perhaps via the adherence activities of the toxin, was demonstrated by the fact that, even during coinfection with BPDR and BPDR-RE, the strain producing inactive toxin was cleared faster than was the PT-deficient strain. The difference was also not due to a growth defect of BPDR-RE compared to BPDR, since it appeared only after at least 10 days of infection, at the time at which the immune response kicks in, and not early during infection, when a growth defect would be expected to have a major impact. In addition, BPDR and BPDR-RE showed the same growth rates in vitro. It is therefore likely that faster clearance of BPDR-RE than of BPDR is due to the presence of inactive toxin on the surface of B. pertussis BPDR-RE as a direct target for action of the immune system of the host.
ACKNOWLEDGMENTS
We gratefully acknowledge Christelle Faveeuw for the preparation of dendritic cells.
This work was supported by INSERM, Institut Pasteur de Lille, Région Nord-Pas de Calais, and the Ministère de l'Education Nationale et de la Recherche. S.A. holds a fellowship of Aventis-Pasteur, K.P. holds a fellowship of the Ministère de l'Education Nationale et de la Recherche, and N.M. holds a fellowship of the Fondation pour la Recherche Médicale.
REFERENCES
- 1.Antoine R, Locht C. Molecular studies on the interaction of the S1 subunit with the B oligomer of pertussis toxin. Zentbl Bakteriol Suppl. 1992;23:292–293. [Google Scholar]
- 2.Antoine R, Locht C. Roles of the disulfide bond and the carboxy-terminal region of the S1 subunit in the assembly and biosynthesis of pertussis toxin. Infect Immun. 1990;58:1518–1526. doi: 10.1128/iai.58.6.1518-1526.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bassinet L, Gueirard P, Maitre B, Housset B, Gounon P, Guiso N. Role of adhesins and toxins in invasion of human tracheal epithelial cells by Bordetella pertussis. Infect Immun. 2000;68:1934–1941. doi: 10.1128/iai.68.4.1934-1941.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cahill E S, O'Hagan D T, Illum L, Redhead K. Mice are protected against Bordetella pertussis infection by intra-nasal immunization with filamentous haemagglutinin. FEMS Microbiol Lett. 1993;107:211–216. doi: 10.1111/j.1574-6968.1993.tb06032.x. [DOI] [PubMed] [Google Scholar]
- 5.Dei-Cas E, Brun-Pascaud M, Bille-Hansen V, Allaert A, Aliouat E M. Animal models of pneumocystosis. FEMS Immunol Med Microbiol. 1998;22:163–168. doi: 10.1111/j.1574-695X.1998.tb01201.x. [DOI] [PubMed] [Google Scholar]
- 6.Ferrante A, Staugas R E M, Rowan-Kelly B, Bresatz S, Kumaratilake L, Rzepczyk C M, Adolf G R. Production of tumor necrosis factors alpha and beta by human mononuclear leukocytes stimulated with mitogens, bacteria, and malarial parasites. Infect Immun. 1990;58:3996–4003. doi: 10.1128/iai.58.12.3996-4003.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finn T M, Shahin R, Mekalanos J J. Characterization of vir-activated TnphoA gene fusions in Bordetella pertussis. Infect Immun. 1991;59:3273–3279. doi: 10.1128/iai.59.9.3273-3279.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedman R L, Nordensson K, Wilson L, Akporiaye E T, Yocum D E. Uptake and intracellular survival of Bordetella pertussis in human macrophages. Infect Immun. 1992;60:4578–4585. doi: 10.1128/iai.60.11.4578-4585.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodwin M S, Weiss A A. Adenylate cyclase toxin is critical for colonization and pertussis toxin is critical for lethal infection by Bordetella pertussis in infant mice. Infect Immun. 1990;58:3445–3447. doi: 10.1128/iai.58.10.3445-3447.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hannah J H, Menozzi F D, Renauld G, Locht C, Brennan M J. Sulfated glycoconjugate receptors for the Bordetella pertussis adhesin filamentous hemagglutinin (FHA) and mapping of the heparin-binding domain on FHA. Infect Immun. 1994;62:5010–5019. doi: 10.1128/iai.62.11.5010-5019.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hazenbos W L W, Van den Berg B, Van't Wout J W, Mooi F R, Van Furth R. Virulence factors determine attachment and ingestion of nonopsonized and opsonized Bordetella pertussis by human monocytes. Infect Immun. 1994;62:4818–4824. doi: 10.1128/iai.62.11.4818-4824.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khelef N, Bachelet C M, Vargaftig B B, Guiso N. Characterization of murine lung inflammation after infection with parental Bordetella pertussis and mutants deficient in adhesins or toxins. Infect Immun. 1994;62:2893–2900. doi: 10.1128/iai.62.7.2893-2900.1994. . (Erratum, 62:5707.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khelef N, Sakamoto H, Guiso N. Both adenylate cyclase and hemolytic activities are required by Bordetella pertussis to initiate infection. Microb Pathog. 1992;12:227–235. doi: 10.1016/0882-4010(92)90057-u. [DOI] [PubMed] [Google Scholar]
- 14.Kimura A, Mountzouros K T, Relman D A, Falkow S, Cowell J L. Bordetella pertussis filamentous hemagglutinin: evaluation as a protective antigen and colonization factor in a mouse respiratory infection model. Infect Immun. 1990;58:7–16. doi: 10.1128/iai.58.1.7-16.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lobet Y, Feron C, Dequesne G, Simoen E, Hauser P, Locht C. Site-specific alterations in the B oligomer that affect receptor-binding activities and mitogenicity of pertussis toxin. J Exp Med. 1993;177:79–87. doi: 10.1084/jem.177.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Locht C. Molecular aspects of Bordetella pertussis pathogenesis. Int Microbiol. 1999;2:137–144. [PubMed] [Google Scholar]
- 17.Locht C, Antoine R, Veithen A, Raze D. Pertussis toxin: structure-function relationship. Handb Exp Pharmacol. 2000;145:167–185. [Google Scholar]
- 18.Locht C, Bertin P, Menozzi F D, Renauld G. The filamentous haemagglutinin, a multifaceted adhesion produced by virulent Bordetella spp. Mol Microbiol. 1993;9:653–660. doi: 10.1111/j.1365-2958.1993.tb01725.x. [DOI] [PubMed] [Google Scholar]
- 19.Locht C, Geoffroy M C, Renauld G. Common accessory genes for the Bordetella pertussis filamentous hemagglutinin and fimbriae share sequence similarities with the papC and papD gene families. EMBO J. 1992;11:3175–3183. doi: 10.1002/j.1460-2075.1992.tb05394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makhov A M, Hannah J H, Brennan M J, Trus B L, Kocsis E, Conway J F, Wingfield P T, Simon M N, Steven A C. Filamentous hemagglutinin of Bordetella pertussis. A bacterial adhesin formed as a 50-nm monomeric rigid rod based on a 19-residue repeat motif rich in beta strands and turns. J Mol Biol. 1994;241:110–124. doi: 10.1006/jmbi.1994.1478. [DOI] [PubMed] [Google Scholar]
- 21.Marriott I, Hammond T G, Thomas E K, Bost K L. Salmonella efficiently enter and survive within cultured CD11c+ dendritic cells initiating cytokine expression. Eur J Immunol. 1999;29:1107–1115. doi: 10.1002/(SICI)1521-4141(199904)29:04<1107::AID-IMMU1107>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 22.Mbawuike I N, Herscowitz H B. MH-S, a murine alveolar macrophage cell line: morphological, cytochemical, and functional characteristics. J Leukoc Biol. 1989;46:119–127. doi: 10.1002/jlb.46.2.119. [DOI] [PubMed] [Google Scholar]
- 23.Menozzi F D, Gantiez C, Locht C. Identification and purification of transferrin- and lactoferrin-binding proteins of Bordetella pertussis and Bordetella bronchiseptica. Infect Immun. 1991;59:3982–3988. doi: 10.1128/iai.59.11.3982-3988.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menozzi F D, Mutombo R, Renauld G, Gantiez C, Hannah J H, Leininger E, Brennan M J, Locht C. Heparin-inhibitable lectin activity of the filamentous hemagglutinin adhesin of Bordetella pertussis. Infect Immun. 1994;62:769–778. doi: 10.1128/iai.62.3.769-778.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nencioni L, Pizza M G, Volpini G, De Magistris M T, Giovannoni F, Rappuoli R. Properties of the B oligomer of pertussis toxin. Infect Immun. 1991;59:4732–4734. doi: 10.1128/iai.59.12.4732-4734.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Njamkepo E, Pinot F, Francois D, Guiso N, Polla B S, Bachelet M. Adaptative responses of human monocytes infected by Bordetella pertussis: the role of adenylate cyclase hemolysin. J Cell Physiol. 2000;183:91–99. doi: 10.1002/(SICI)1097-4652(200004)183:1<91::AID-JCP11>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 27.Prasad S M, Yin Y, Rodzinski E, Tuomanen E I, Masure H R. Identification of a carbohydrate recognition domain in filamentous hemagglutinin from Bordetella pertussis. Infect Immun. 1993;61:2780–2785. doi: 10.1128/iai.61.7.2780-2785.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Relman D, Tuomanen E, Falkow S, Golenbock D T, Saukkonen K, Wright S D. Recognition of a bacterial adhesion by an integrin: macrophage CR3 (alpha M beta 2, CD11b/CD18) binds filamentous hemagglutinin of Bordetella pertussis. Cell. 1990;61:1375–1382. doi: 10.1016/0092-8674(90)90701-f. [DOI] [PubMed] [Google Scholar]
- 29.Remoue F, Poulain-Godefroy O, Mielcarek N, Pierce R, Capron A, Locht C, Riveau G. Local transient induction of inflammatory cytokines after intranasal administration of recombinant Bordetella pertussis. Microb Pathog. 1997;22:305–313. doi: 10.1006/mpat.1996.0130. [DOI] [PubMed] [Google Scholar]
- 30.Rosoff P M, Walker R, Winberry L. Pertussis toxin triggers rapid second messenger production in human T lymphocytes. J Immunol. 1987;139:2419–2423. [PubMed] [Google Scholar]
- 31.Sato H, Sato Y. Protective activities in mice of monoclonal antibodies against pertussis toxin. Infect Immun. 1990;58:3369–3374. doi: 10.1128/iai.58.10.3369-3374.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sato Y, Arai H. Leucocytosis-promoting factor of Bordetella pertussis. I. Purification and characterization. Infect Immun. 1972;6:899–904. doi: 10.1128/iai.6.6.899-904.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saukkonen K, Burnette W N, Mar V L, Masure H R, Tuomanen E I. Pertussis toxin has eukaryotic-like carbohydrate recognition domains. Proc Natl Acad Sci USA. 1992;89:118–122. doi: 10.1073/pnas.89.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saukkonen K, Cabellos C, Burroughs M, Prasad S, Tuomanen E. Integrin-mediated localization of Bordetella pertussis within macrophages: role in pulmonary colonization. J Exp Med. 1991;173:1143–1149. doi: 10.1084/jem.173.5.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shahin R D, Amsbaugh D F, Leef M F. Mucosal immunization with filamentous hemagglutinin protects against Bordetella pertussis respiratory infection. Infect Immun. 1992;60:1482–1488. doi: 10.1128/iai.60.4.1482-1488.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stibitz S. Use of conditionally counterselectable suicide vectors for allelic exchange. Methods Enzymol. 1994;235:458–465. doi: 10.1016/0076-6879(94)35161-9. [DOI] [PubMed] [Google Scholar]
- 37.Thom R E, Casnellie J E. Pertussis toxin activates protein kinase C and a tyrosine protein kinase in the human T cell line Jurkat. FEBS Lett. 1989;244:181–184. doi: 10.1016/0014-5793(89)81188-3. [DOI] [PubMed] [Google Scholar]
- 38.Torre D, Pugliese A, Tambini R, Speranza F, Zeroli C. Production and release of tumor necrosis factor alfa, interleukin-1B and interleukin-6 by human mononuclear leukocytes stimulated with pertussis toxin. New Microbiol. 1993;16:309–314. [PubMed] [Google Scholar]
- 39.Tuomanen E, Towbin H, Rosenfelder G, Braun D, Larson G, Hansson G C, Hill R. Receptor analogs and monoclonal antibodies that inhibit adherence of Bordetella pertussis to human ciliated respiratory epithelial cells. J Exp Med. 1988;168:267–277. doi: 10.1084/jem.168.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tuomanen E, Weiss A. Characterization of two adhesins of Bordetella pertussis for human ciliated respiratory-epithelial cells. J Infect Dis. 1985;152:118–125. doi: 10.1093/infdis/152.1.118. [DOI] [PubMed] [Google Scholar]
- 41.Tuomanen E, Weiss A, Rich R, Zak F, Zak O. Filamentous hemagglutinin and pertussis toxin promote adherence of Bordetella pertussis to cilia. Dev Biol Stand. 1985;61:197–204. [PubMed] [Google Scholar]
- 42.Van den Berg B M, Beekhuizen H, Willems R J L, Mooi F R, van Furth R. Role of Bordetella pertussis virulence factors in adherence to epithelial cell lines derived from the human respiratory tract. Infect Immun. 1999;67:1056–1062. doi: 10.1128/iai.67.3.1056-1062.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van't Wout J, Burnette N W, Mar V L, Rozdinski E, Wright S D, Tuomanen E I. Role of carbohydrate recognition domains of pertussis toxin in adherence of Bordetella pertussis to human macrophages. Infect Immun. 1992;60:3303–3308. doi: 10.1128/iai.60.8.3303-3308.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weiss A A, Goodwin M S. Lethal infection by Bordetella pertussis mutants in the infant mouse model. Infect Immun. 1989;57:3757–3764. doi: 10.1128/iai.57.12.3757-3764.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weiss A A, Hewlett E L, Myers G A, Falkow S. Pertussis toxin and extracytoplasmic adenylate cyclase as virulence factors of Bordetella pertussis. J Infect Dis. 1984;150:219–222. doi: 10.1093/infdis/150.2.219. [DOI] [PubMed] [Google Scholar]