Abstract
Background
Non-clinical evidence and a few human studies with short follow-ups suggest increased risk of dyslipidaemia in the post-acute phase of COVID-19 (ie, >30 days after SARS-CoV-2 infection). However, detailed large-scale controlled studies with longer follow-ups and in-depth assessment of the risks and burdens of incident dyslipidaemia in the post-acute phase of COVID-19 are not yet available. We, therefore, aimed to examine the risks and 1-year burdens of incident dyslipidaemia in the post-acute phase of COVID-19 among people who survive the first 30 days of SARS-CoV-2 infection.
Methods
In this cohort study, we used the national health-care databases of the US Department of Veterans Affairs to build a cohort of 51 919 participants who had a positive COVID-19 test and survived the first 30 days of infection between March 1, 2020, and Jan 15, 2021; a non-infected contemporary control group (n=2 647 654) that enrolled patients between March 1, 2020, and Jan 15, 2021; and a historical control group (n=2 539 941) that enrolled patients between March 1, 2018, and Jan 15, 2019. Control groups had no evidence of SARS-CoV-2 infection, and participants in all three cohorts were free of dyslipidaemia before cohort enrolment. We then used inverse probability weighting using predefined and algorithmically-selected high dimensional variables to estimate the risks and 1-year burdens of incident dyslipidaemia, lipid-lowering medications use, and a composite of these outcomes. We reported two measures of risk: hazard ratios (HRs) and burden per 1000 people at 12 months. Additionally, we estimated the risks and burdens of incident dyslipidaemia outcomes in mutually exclusive groups based on the care setting of the acute infection (ie, participants who were non-hospitalised, hospitalised, or admitted to intensive care during the acute phase of SARS-CoV-2 infection).
Findings
In the post-acute phase of the SARS-CoV-2 infection, compared with the non-infected contemporary control group, those in the COVID-19 group had higher risks and burdens of incident dyslipidaemia, including total cholesterol greater than 200 mg/dL (hazard ratio [HR] 1·26, 95% CI 1·22–1·29; burden 22·46, 95% CI 19·14–25·87 per 1000 people at 1 year), triglycerides greater than 150 mg/dL (1·27, 1·23–1·31; 22·03, 18·85–25·30), LDL cholesterol greater than 130 mg/dL (1·24, 1·20–1·29; 18·00, 14·98–21·11), and HDL cholesterol lower than 40 mg/dL (1·20, 1·16–1·25; 15·58, 12·52–18·73). The risk and burden of a composite of these abnormal lipid laboratory outcomes were 1·24 (95% CI 1·21–1·27) and 39·19 (95% CI 34·71–43·73), respectively. There was also increased risk and burden of incident lipid-lowering medications use (HR 1·54, 95% CI 1·48–1·61; burden 25·50, 95% CI 22·61–28·50). A composite of any dyslipidaemia outcome (laboratory abnormality or lipid-lowering medications use) yielded an HR of 1·31 (95% CI 1·28–1·34) and a burden of 54·03 (95% CI 49·21–58·92). The risks and burdens of these post-acute outcomes increased in a graded fashion corresponding to the severity of the acute phase of COVID-19 infection (ie, whether patients were non-hospitalised, hospitalised, or admitted to intensive care). The results were consistent in analyses comparing the COVID-19 group to the non-infected historical control group.
Interpretation
Our findings suggest increased risks and 1-year burdens of incident dyslipidaemia and incident lipid-lowering medications use in the post-acute phase of COVID-19 infection. Post-acute care for those with COVID-19 should involve attention to dyslipidaemia as a potential post-acute sequela of SARS-CoV-2 infection.
Funding
US Department of Veterans Affairs.
Introduction
Evidence suggests substantial alterations in metabolomic and proteomic profiles, oral and gut microbiome, and lipid metabolism following SARS-CoV-2 infection in previously healthy individuals,1, 2, 3, 4 with some of these abnormalities persisting even in the post-acute stage (ie, >30 days after SARS-CoV-2 infection) of COVID-19.5 Although total cholesterol, LDL cholesterol, and HDL cholesterol are often reduced during the acute phase of SARS-CoV-2 infection, emerging evidence from small observational studies with short follow-ups (of up to 6 months) suggest increased risk of dyslipidaemia in the post-acute phase of COVID-19.4, 5, 6, 7, 8, 9 A study of 501 young adults (aged 18–30 days) of the Swiss Armed Forces showed that compared with non-infected controls, those with COVID-19 had a higher blood cholesterol and LDL concentrations 180 days after their first positive PCR test.8 A large observational study of more than 2 million people of all ages with COVID-19 from FAIR Health, with no control group, reported that approximately 3% developed dyslipidaemia after the first 30 days of infection.9 Together, these observations suggest the hypothesis that SARS-CoV-2 infection is associated with increased risk of post-acute dyslipidaemia. However, large-scale controlled studies addressing this hypothesis and evaluating the risks of incident dyslipidaemia as a potential post-acute sequela of SARS-CoV-2 infection are not yet available. Addressing the question of whether survivors of the acute phase of COVID-19 are at increased risk of incident dyslipidaemia is important to deepen our understanding of the scope and scale of post-acute sequelae of SARS-CoV-2 infection and could help inform post-acute COVID-19 care strategies.
Research in context.
Evidence before this study
We searched PubMed for human studies published between Dec 1, 2019, and Dec 31, 2021, using the search terms “COVID-19”, “SARS CoV-2” or “Long COVID”, and “dyslipidaemia”, with no language restrictions. Experimental evidence suggests substantial alterations in lipid metabolism following SARS-CoV-2 infection that could persist in the post-acute phase. A study of 501 young adults (aged 18–30 years) from the Swiss Armed Forces suggested that compared with a non-infected serologically negative control group, those with COVID-19 had an increased risk of developing metabolic disorders, including dyslipidaemia 180 days after SARS-CoV-2 diagnosis. An uncontrolled study from the USA that evaluated records of more than 2 million people reported that approximately 3% of those with COVID-19 developed dyslipidaemia after the first 30 days of infection. A large-scale controlled study with long-term follow-up and in-depth assessment of the risks and burdens of incident dyslipidaemia in people who survive the acute phase of COVID-19 has not been done, to the best of our knowledge. In this study, we aimed to examine the 1-year post-acute risk and burden of incident dyslipidaemia in people who survived the first 30 days of SARS-CoV-2 infection.
Added value of this study
In this national study involving 5 239 514 participants, we provide evidence that compared with non-infected controls, and beyond the first 30 days of infection, COVID-19 survivors exhibited increased risks and burdens of incident dyslipidaemia outcomes at 12 months, including increased risks of elevated total cholesterol, triglycerides, and LDL cholesterol and reduced levels of HDL cholesterol.
The risks were significant among those who were non-hospitalised and increased in a graded fashion according to the care setting of the acute phase of the disease (ie, whether people were non-hospitalised, hospitalised, or admitted to intensive care during the acute phase of COVID-19). Altogether, the findings suggest that SARS-CoV-2 infection might be associated with higher risk of dyslipidaemia in the post-acute phase of COVID-19.
Implications of all the available evidence
Our study provides evidence that dyslipidaemia is a facet of the multifaceted long COVID. Woven together with the body of evidence that has emerged thus far suggesting increased risk of diabetes, cardiovascular disease, and kidney disease in the post-acute phase of COVID-19, the totality of evidence suggests cardiometabolic disease as a long-term consequence of SARS-CoV-2 infection. Post-acute care strategies of people with COVID-19 should include attention to dyslipidaemia and more broadly cardiometabolic disease potential facets of long COVID.
In our study, we aimed to estimate the risks and 1-year burdens of incident dyslipidaemia, incident lipid-lowering medication use, and a composite outcome of these endpoints in individuals who survived the first 30 days of COVID-19 infection. We also estimated the risk and burden according to care setting of the acute infection (non-hospitalised, hospitalised, and admitted to intensive care).
Methods
Study design and participants
In this cohort study, we used the US Department of Veterans Affairs (VA) national health-care databases to build a cohort of US veterans who survived the first 30 days of COVID-19 infection and two control groups: a contemporary cohort consisting of users of the US Department of Veterans Health Care System (VHA) with no evidence of SARS-CoV-2 infection and a historical cohort consisting of VHA users during 2017. We identified 169 476 users of the VHA in 2019 who had a positive COVID-19 test between March 1, 2020, and Jan 15, 2021. Those who were alive 30 days after the positive test result and did not have any history of abnormal lipid laboratory results or lipid-lowering medications prescriptions in the year before having a positive COVID-19 test were further enrolled to the COVID-19 cohort. For each participant, T0 was set as the date of the first positive COVID-19 test; participants were followed-up until Dec 31, 2021.
A contemporary cohort and a historical cohort were then constructed. The contemporary cohort was comprised of veterans who used the VHA from Jan 1, 2019, to Dec 31, 2019; who were alive by March 1, 2020; and who were not already part of the COVID-19 cohort. The historical control cohort consisted of users of the VHA from Jan 1, 2017, to Dec 31, 2017; who were alive on March 1, 2018; and who were not already in the COVID-19 cohort.
The beginning of follow-up for participants in both control groups were assigned randomly and corresponded to the same date distribution of the first positive COVID-19 test result in the COVID-19 cohort to ensure that the proportion of participants with a start of follow-up on a certain date was identical in both groups. This ensured that the COVID-19 and the control cohorts had a similar distribution of follow-up. Participants with any history of abnormal lipid laboratory results or lipid-lowering medications prescriptions in the year prior to T0 were excluded. The follow-up data was collected until Dec 31, 2021, for the contemporary control group and until Dec 31, 2019, for the historical control group.
The COVID-19 group was further categorised into those who were not hospitalised (n=46 568) hospitalised for COVID-19 (n=4169), or admitted to an intensive care unit during the acute phase of the disease (n=1182).
This study was approved by the Institutional Review Board of the VA St. Louis Health Care System, which granted a waiver of informed consent (protocol number 1606333).
Data sources
Data from the US Department of VA Veterans Health Administration were used to build datasets for this study. The VA Corporate Data Warehouse (CDW) provided demographic and clinical information.10, 11, 12, 13, 14, 15, 16, 17, 18, 19 Diagnoses were obtained from VA CDW inpatient and outpatient encounters domains, corresponding to clinical information gathered during hospitalisations and outpatient visits. The CDW outpatient pharmacy domain and CDW bar code medication administration domain provided medication use information and laboratory measurements were collected from the CDW laboratory results domain.10, 11, 12, 13, 14, 15, 16, 17, 18, 19 The VA COVID-19 shared data resource provided information on COVID-19 test results.20 The exposure was defined as the first positive SARS-CoV-2 test result based on PCR tests done between March 1, 2020, and Jan 15, 2021.
We used the area deprivation index as a composite measure of contextual disadvantage at participants' residential locations; the area deprivation index is composed of income, education, employment, and housing data for a specific residential district.21
Outcomes
Dyslipidaemia outcomes consisted of either incident abnormal lipid laboratory results (comprised of total cholesterol >200 mg/dL, triglycerides >150 mg/dL, LDL >130 mg/dL, or HDL <40 mg/dL) or incident lipid-lowering medications prescriptions (consisting of prescription of statins, bile acid resins, and fibrates). All abnormal lipid laboratory results and all prescription outcomes were aggregated into composite outcomes called “any abnormal lipid laboratory result” and “lipid-lowering medications prescription”, respectively. Furthermore, we specified the composite of any dyslipidaemia outcome as the first incident occurrence of any of the predefined dyslipidaemia outcomes (abnormal lipid laboratory results or lipid-lowering medications prescription) examined during this study.
We also examined additional outcomes, including the association between COVID-19 and various thresholds of lipid levels (including total cholesterol >210 mg/dL, >220 mg/dL, and >230 mg/dL; triglycerides >160 mg/dL, >170 mg/dL, and >180 mg/dL; LDL >140 mg/dL, >150 mg/dL, and >160 mg/dL; and HDL <35 mg/dL, <30 mg/dL, and <25 mg/dL). In consideration of the relationship between dyslipidaemia and BMI, we also examined the association between COVID-19 and an increase in BMI greater than 3%, 5%, and 10%.
Post-acute COVID-19 outcomes were examined in the period following the first 30 days after T0 until the end of follow-up.
Covariates
In recognition that our knowledge about COVID-19 and long COVID is still evolving, we used a two-pronged strategy to select covariates for this study. Pre-defined covariates were selected based on previous knowledge and algorithmically-selected covariates were used during modelling, both were assessed in the year before T0.
Pre-defined covariates consisted of four groups composed of demographic characteristics, clinical characteristics, comorbidities, and medication usage.22, 23, 24, 25, 26 Demographic characteristics included age, race (White, Black, and other [Asian or Pacific Islander; Hispanic or Latino; or Native American or multiracial]), sex (male or female), BMI category (underweight, normal, overweight, or obese), smoking status (never, former, or current), and area deprivation index. Clinical characteristics were composed of the number of outpatient encounters (zero, one, and two or more), long-term care (assessed 1 year before enrolment and was defined as admission to a nursing home or assisted living facility), estimated glomerular filtration rate, and systolic and diastolic blood pressure readings. Comorbidities consisted of cancer, cardiovascular disease, cerebrovascular disease, chronic kidney disease, chronic lung disease, type 2 diabetes, and hypertension. Prescription medications that could affect the risk of dyslipidaemia included anti-epileptic drugs, anti-psychotics, β-blockers, corticosteroids, diuretics, HIV protease inhibitors, immunosuppressants, hormone therapeutics, and SGLT-2 inhibitors. Continuous variables were transformed into restricted cubic spline functions to account for potential non-linear relationships.
Because our understanding of the covariates that could confound the relationship between COVID-19 and post-acute health outcomes is still developing, we also used algorithmically-selected covariates from several data domains, including comorbidities, medications, and laboratory test results.27 We achieved this by categorising all comorbidities, prescriptions, and laboratory data into 540 comorbidities groups, 543 medication classes, and 62 laboratory test abnormalities, respectively.28, 29 For all participants in our cohort, we selected variables from these three data domains that occurred in at least 100 participants within each of the groups. This was done in acknowledgement that rare variables (eg, alkaptonuria) occurred in fewer than 100 participants in these large cohorts (smallest cohort was comprised of 51 919 participants) and they might not sufficiently describe the characteristics of the cohort or materially influence the examined associations. All cohort participants (COVID-19, contemporary control, or historical control) were used to quantify the prevalence of each variable within the group. The univariate relative risk between each variable and the exposure (defined as the first positive SARS-CoV-2 test result based on PCR tests done between March 1, 2020, and Jan 15, 2021) was then estimated based on the prevalence of the variables in each group from all participants within the group and the top 100 variables with the highest relative risk were selected.22, 30 This algorithmic selection process for high dimensional covariates was done independently for each comparison (eg, the COVID-19 vs contemporary control analysis and the COVID-19 vs historical control analysis). The algorithmically selected variables were used along with the predefined variables in the propensity score models.
Statistical analyses
Baseline characteristics and standardised mean differences of the COVID-19, contemporary, and historical groups were reported. Logistic regression was built for the COVID-19, contemporary, and historical control groups conditional on all predefined variables and algorithmically-selected variables to estimate the probabilities of belonging to the target population of VHA users in 2019 (equivalent to the combination of the COVID-19 and contemporary control group). Pre-defined and comparison-specific algorithmically-selected high dimensional variables were used to estimate the probabilities, which were subsequently used as the propensity score. This propensity score was then used in the calculation of the inverse probability weight (given as the propensity score divided by 1 minus the propensity score).31 After the application of weighting, covariate balance was assessed by standardised mean differences. For all covariates, a standardised mean difference of less than 0·15 was considered good balance.32
Once inverse probability weights were applied, cause-specific hazard models with death considered as competing risk were used to estimate the hazard ratios (HRs) of incident dyslipidaemia outcomes between both the COVID-19 and contemporary groups and the COVID-19 and historical control groups. Estimates of burdens per 1000 participants at 1 year of follow-up in the COVID-19 and control groups were calculated using survival probability at 1 year. The survival probability at 1 year was defined as the cumulative probability of those that did not experience the event or were not censored until 1 year given the exposure group (S(t,A)=∏t 0(PR(D t=0|D t–1=0,A))) where D is the event, A is the exposure group, and t is the time. Differences between the estimated burdens in the COVID-19 and control groups was used to compute the excess burdens per 1000 participants at 1 year.
Furthermore, subgroup analyses consisting of age (≤65 years or >65 years), race (White or Black), sex (male or female), obesity (<30 kg/m2 or ≥30kg/m2), smoking, diabetes, cardiovascular disease, chronic kidney disease, hypertension, and dyslipidaemia risk score (based on risk quartiles) were done. The dyslipidaemia risk score to quantify the baseline risk (before exposure to COVID-19) of having dyslipidaemia was defined as the probability of developing any dyslipidaemia outcome within 1 year, and was estimated from the logistic regression, which was calculated within control groups and included previously recognised dyslipidaemia risk factors, including age, race, sex, BMI, cardiovascular disease, type 2 diabetes, and hypertension.33 The parameter estimated from the logistic regression was then applied to the COVID-19 group to generate the risk score given the baseline covariates before COVID-19 exposure. We then categorized the risk into quartiles and examined the association between COVID-19 and risk of developing incident dyslipidaemia in each risk quartile.
To further understand the association between COVID-19 and risks of post-acute dyslipidaemia outcomes, the COVID-19 cohort was stratified into mutually exclusive groups based on each participants' care setting during the first 30 days of COVID-19 infection (ie, whether participants were non-hospitalised, hospitalised, or admitted to intensive care). Inverse probability weights were estimated for each care setting group using the approach outlined in the previous paragraph. Cause-specific hazard models with inverse probability weighting were applied and HRs, burdens, and excess burdens were calculated.
We then subjected our study design to multiple sensitivity analyses to test the robustness of our results. First, during construction of inverse probability weights, our covariate selection was restricted to only pre-defined variables (ie, we did not include any algorithmically selected covariates). Second, when constructing the inverse probability weights, our covariate selection was expanded to 300 algorithmically-selected variables (instead of the 100 used in the original analysis). Third, alternatively, we applied a doubly robust approach, where the associations were estimated by additionally adjusting for covariates in the inverse probability weighted survival models.34
Robust sandwich variance estimators were used to provide estimation of variance in models with application of weights. Evidence of statistical significance was determined by a 95% CI that excluded unity for all analyses. All analyses were done using SAS Enterprise Guide (version 8.2) and results were visualised using R (version 4.04).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
There were 51 919 participants in the COVID-19 group (enrolled between March 1, 2020, and Jan 15, 2021), 2 647 654 participants in the contemporary group (enrolled between March 1, 2020, and Jan 15, 2021), and 2 539 941 participants in the historical control group (enrolled between March 1, 2018, and Jan 15, 2019; (appendix p 3). The median follow-up time was 408 (IQR 378–500) days in the COVID-19 group, 409 (379–506) days in the contemporary control group, and 409 (379–506) days in the historical control group, corresponding to 62 715 person-years, 3 184 253 person-years, and 3 070 573 person-years of follow-up, respectively; altogether corresponding to 6 317 541 person-years of follow-up.
The demographic and clinical characteristics of the COVID-19, contemporary control, and historical control groups before weighting are presented in the appendix (pp 8–9).
The COVID-19 and contemporary control groups were balanced using the inverse probability weighting method. The demographic and clinical characteristics of the COVID-19 and contemporary control groups after weighting are presented in the appendix table (appendix pp 10–11). Evaluation of standardised mean differences of these characteristics after weighting showed differences of less than 0·15, suggesting good balance (appendix pp 4, 10–11).
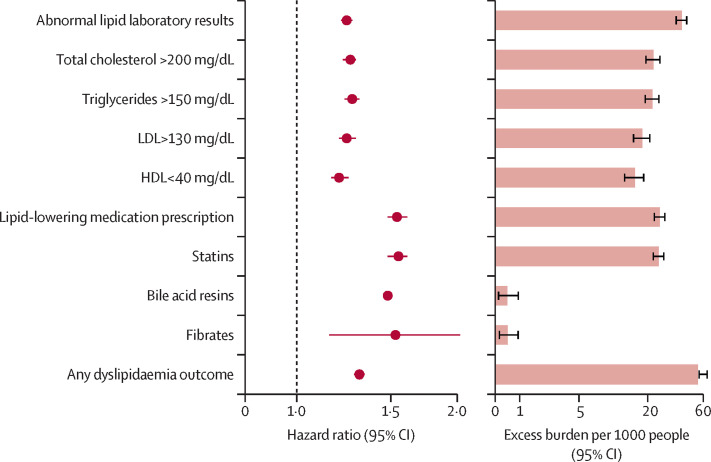
The risks of a set of pre-specified dyslipidaemia outcomes were estimated in the COVID-19 versus contemporary control groups. Additionally, the adjusted excess burden of dyslipidaemia outcomes due to COVID-19 per 1000 people at 12 months was estimated based on the difference between the estimated incidence rate in participants with COVID-19 and the contemporary control group. The risks and burdens of individual dyslipidaemia outcomes are presented in figure 1 and the appendix (p 12).
Figure 1.
Risks and 1-year burdens of incident post-acute COVID-19 dyslipidaemia outcomes compared with the contemporary control cohort
Outcomes were ascertained 30 days after the COVID-19-positive test until the end of follow-up. Adjusted HRs and 95% CIs are presented. The length of the bar represents the excess burden per 1000 people at 1 year and associated 95% CIs are also shown.
Those who survived the first 30 days of COVID-19 exhibited an increased risk of incident total cholesterol greater than 200 mg/dL (hazard ratio [HR] 1·26, 95% CI 1·22–1·29; burden 22·46, 95% CI 19·14–25·87), triglycerides greater than 150 mg/dL (1·27, 1·23–1·31; 22·03, 18·85–25·30), LDL greater than 130 mg/dL (1·24, 1·20–1·29; 18·00, 14·98–21·11), and HDL lower than 40 mg/dL (1·20, 1·16–1·25; 15·58, 12·52–18·73). The risk and burden of a composite of these abnormal lipid laboratory results were 1·24 (1·21–1·27) and 39·19 (34·71–43·73), respectively (appendix p 12).
Those who survived the first 30 days of COVID-19 exhibited increased risk of incident use of statins (HR 1·55, 95% CI 1·48–1·61; burden 24·94, 95 % CI 22·09–27·91 per 1000 people at 12 months), bile acid resins (1·48, 1·11–1·97; 0·48, 0·11–0·97), and fibrates (1·53, 1·15–2·02; 0·50, 0·14–0·96). The risk and burden of a composite of these lipid-lowering medications were 1·54 (95% CI 1·48–1·61) and 25·50 (95% CI 22·61–28·50), respectively (appendix p 12).
We examined the risk and burden of developing any dyslipidaemia outcome (defined as the occurrence of any incident prespecified dyslipidaemia outcome included in this study). Compared with the contemporary control group, there was an increased risk and burden of any dyslipidaemia outcome (HR 1·31, 95% CI 1·28–1·34; burden 54·03, 95% CI 49·21–58·92; appendix p 12).
We examined the association between COVID-19 and various thresholds of lipid levels. COVID-19 was associated with increased risk of total cholesterol greater than 210 mg/d, 220 mg/dL, and 230 mg/dL; triglycerides greater than 160 mg/dL, 170 mg/dL, and 180 mg/dL; LDL greater than 140 mg/dL, 150 mg/dL, and 160 mg/dL; and HDL lower than 35 mg/dL, 30 mg/dL, and 25 mg/dL. COVID-19 was also associated with a higher risk of more than 3%, 5%, and 10% increase in BMI (appendix p 13).
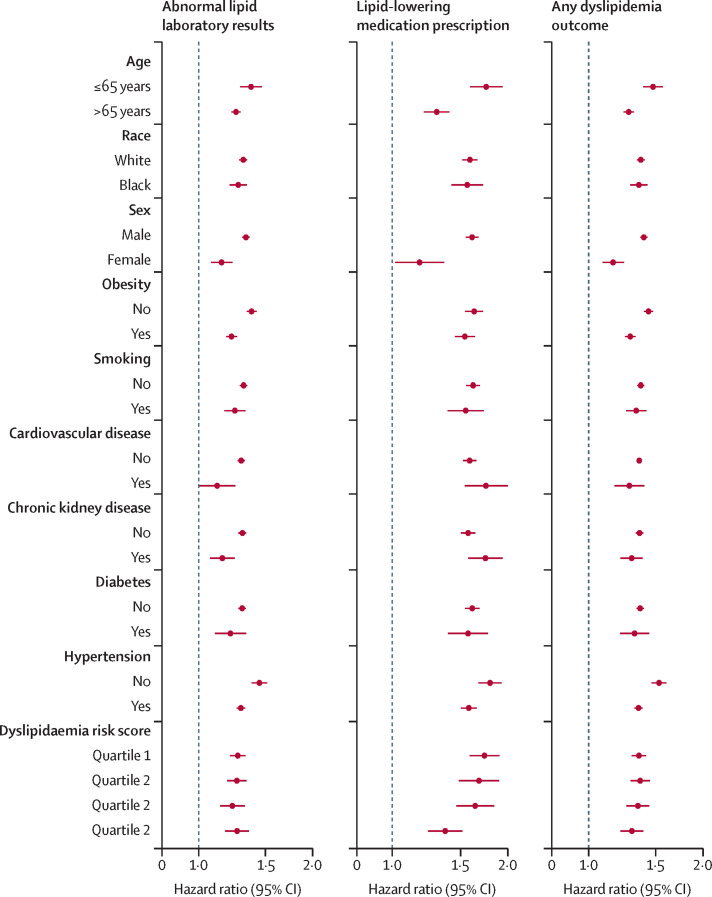
The risks of incident composite dyslipidaemia outcomes were evident in subgroups based on age, race, sex, obesity, smoking, diabetes, chronic kidney disease, hypertension, and dyslipidaemia risk score (figure 2 and appendix pp 14–16). Risks of incident composite dyslipidaemic outcomes were evident in all subgroups based on age, race, sex obesity, smoking, cardiovascular disease, chronic kidney disease, diabetes, hypertension, and dyslipidaemia risk score.
Figure 2.
Subgroup analyses of the risks of incident post-acute COVID-19 composite dyslipidaemia outcomes compared with the contemporary control cohort
Composite outcomes consisted of lipid laboratory abnormalities (total cholesterol >200 mg/dL, triglycerides >150 mg/dL, LDL >130 mg/dL, and HDL <40 mg/dL), lipid-lowering medication prescription (statins, bile acid resins, and fibrates), and any dyslipidaemia outcome (incident occurrence of any dyslipidaemia outcome studied). Outcomes were ascertained 30 days after the COVID-19 positive test until the end of follow-up. Adjusted hazard ratios and 95% CIs are presented.
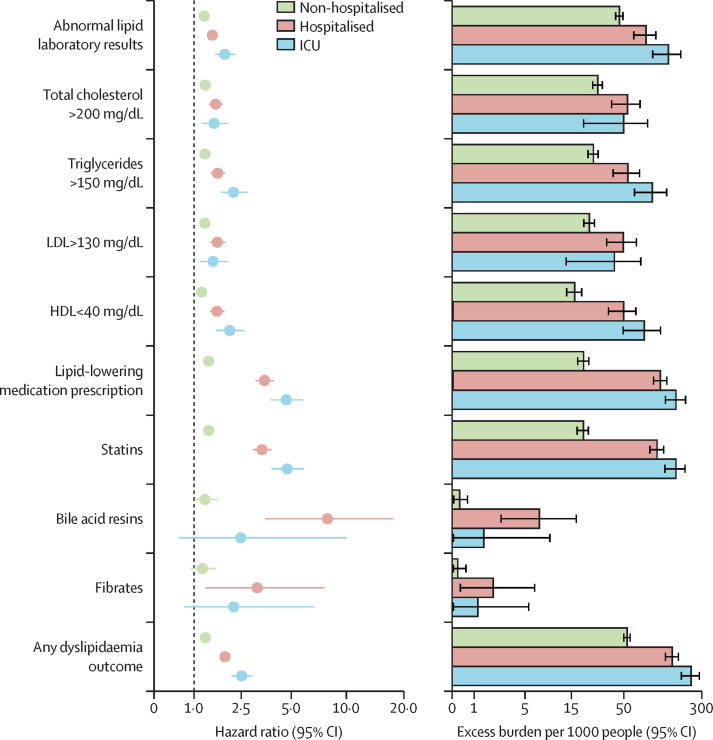
The risks and burdens of incident dyslipidaemia outcomes in mutually exclusive groups were based on the care setting of the acute infection (participants who were non-hospitalised [n=46 568], hospitalised [n=4169], or admitted to intensive care [n=1182]). Demographic and clinical characteristics of these three groups before and after weighting are presented in the appendix (pp 17–22). Standardised mean differences after application of inverse probability weighting showed diferences of less than 0·15, suggesting that the covariates were well balanced (appendix p 4).
Compared with the contemporary control group, the risks and burdens of the pre-specified dyslipidaemia outcomes were evident even among those who were not hospitalised during the acute phase of COVID-19 infection. The risks and burdens increased in a graded manner in accordance with the severity of the acute infection from non-hospitalised to hospitalised, to those admitted to intensive care (figure 3 and appendix pp 23–25).
Figure 3.
Risks and 1-year burdens of incident post-acute COVID-19 dyslipidaemia outcomes compared with the contemporary control cohort by care setting of the acute infection
Risks and burdens were assessed at 1 year in mutually exclusive groups comprising non-hospitalised individuals with COVID-19, individuals hospitalised for COVID-19, and individuals admitted to intensive care for COVID-19 during the acute phase (first 30 days) of COVID-19. Outcomes were ascertained 30 days after the COVID-19-positive test until the end of follow-up. The contemporary control cohort served as the reference category. Within the COVID-19 cohort, there were those who were non-hospitalised, hospitalised, and admitted to intensive care. Adjusted hazard ratios and 95% CIs are presented. The length of the bar represents the excess burden per 1000 people at 1 year and related 95% CIs are also presented.
We tested the reliability of our study design by evaluating the associations between COVID-19 and the prespecified dyslipidaemia outcomes in analyses using a historical control group (from an era predating the COVID-19 pandemic) as the reference category. Demographic and health characteristics before weighting and after weighting are available in the appendix (pp 8–11). Covariate balance was assessed through standardised mean differences and suggested that covariates were balanced after application of inverse probability weighting (appendix p 4). Our results showed increased risks and associated burdens of the dyslipidaemia outcomes in comparisons of COVID-19 versus the historical control group (appendix p 31), in subgroup analyses (appendix pp 33–35), and by care setting of the acute phase of COVID-19 infection (appendix pp 36–37). The direction and magnitude of risks were consistent with analyses using the contemporary control as the reference category.
The robustness of our results was challenged in multiple sensitivity analyses (including varying the covariate specification approach and modeling strategy as described in the methods section) and the results were consistent with those generated using the primary approach. All sensitivity analyses provided results consistent with those generated using the primary approach (appendix pp 38–39).
Discussion
In this study involving participants with COVID-19, contemporary controls, and historical controls (altogether contributing to 6 317 541 person-years), we found that people who survive the first 30 days of SARS-CoV-2 infection exhibited increased risk and 1-year burden of incident dyslipidaemia (including elevated total cholesterol >200 mg/dL, triglycerides >150mg/dL, LDL >130 mg/dL, and HDL <40 mg/dL) and incident use of lipid-lowering agents (including statins, bile acid resins, and fibrates). The increased risks and burdens were evident in people whose acute disease did not necessitate hospitalisation and increased in a graded fashion according to the care setting of the acute phase of the disease (lowest in non-hospitalised, higher in hospitalised, and highest in those who needed intensive care). The results were consistent in analyses considering varying levels of lipid parameters to define the outcomes and in analyses using a contemporary and historical control groups as the reference category. The results were robust to challenge in multiple sensitivity analyses.
Over the past 2 years, it has become increasingly clear that SARS-CoV-2 infection could lead to post-acute sequelae in several organ systems—collectively referred to as long COVID. We and others have previously reported increased risk of cardiometabolic disease (including increased risk of cardiovascular disease, diabetes, and kidney disease) in the year following acute SARS-CoV-2 infection.22, 23, 25, 30, 35, 36 Our current study adds to that body of evidence suggesting increased risk of both dyslipidaemia and lipid-lowering medication use. Woven altogether, the evidence suggests that people with COVID-19 are at increased risk of developing cardiometabolic disorders. Whether and to what extent this increased risk will influence the global burden of cardiometabolic disease and how will it affect health systems and health-care costs will need to be examined in future studies.
The possible mechanisms driving the increase in dyslipidaemia are unclear. Experimental evidence suggests that the immune and inflammatory response following the initial infection could alter hepatic lipoprotein metabolism, which might transiently result in depressed levels during the acute phase with putative over compensatory rebound in the post-acute phase.37 Evidence from studies of patients with MERS-CoV infections suggests abnormalities in sterol regulatory element-binding proteins.38 Studies also suggest substantial changes in oral and gut microbiome and proteomic and metabolomic profiles of individuals infected with SARS-CoV-2 that could last well beyond the acute phase and contribute to changes in lipid profiles.1, 2, 3, 4, 5, 37, 38, 39, 40 Behavioural changes, such as a change in diet and exercise, other COVID-19 pandemic stressors (including lockdowns, social isolation, and loneliness), grief, and other stressors might have differentially affected people with COVID-19 and could also be responsible for some of the effect seen here. We observed a higher risk of an increase in BMI in people with COVID-19 versus both the contemporary and historical controls; the extent to which this increase in BMI following SARS-CoV-2 infection might mediate incident dyslipidaemia or whether, conversely, occurrence of dyslipidaemia following infection might mediate the increase in BMI will need to be examined in future studies. Regardless of whether the increased risk of dyslipidaemia is a direct result of the viral infection or driven by indirect effects of the infection or the COVID-19 pandemic, the risks and burdens noted in people with COVID-19 reflects the realised excess burden of disease that will require care and attention by health-care systems.
Disorders of lipid metabolism have been reported to be associated with both incidence and severity of acute SARS-CoV-2 infection.3, 4, 41, 42, 43, 44 Our results suggest that in people who do not have any previous history of dyslipidaemia, SARS-CoV-2 infection is associated with increased risk of incident dyslipidaemia. These observations suggest a likely bidirectional link that is consistent with the broader observation that cardiometabolic disease is both a risk factor and a sequelae of SARS-CoV-2.25, 26, 35, 36
The constellation of findings from this report and previous research suggests that SARS-CoV-2 infection is associated with post-acute and long-term risks of not only dyslipidaemia, but other metabolic abnormalities, including diabetes, cardiovascular disease, kidney disease, neurological, and other disorders.22, 23, 25, 26, 30, 35, 36, 45, 46 Collectively, this evidence suggests the need to address cardiometabolic health in people with previous infection with SARS-CoV-2.47 It is also important to contextualise these findings within the broader spectrum of infection-associated chronic illnesses; these risks are probably not unique to SARS-CoV-2 and could reflect a much broader connection between infections (viral and non-viral) and post-acute and chronic illnesses.48 The COVID-19 global pandemic represents a historical opportunity (a natural experiment) to investigate and deepen our understanding of the post-acute and chronic consequences of viral (and other infections). This improved understanding will unlock mysteries surrounding other infection-associated chronic illnesses and position us to be better prepared to address the challenges posed by future pandemics.49, 50
This study has several strengths. We leveraged the national health-care databases of the US Department of VA to build a cohort of 51 919 people who had COVID-19 and two control groups of more than 5 million individuals each. The use of two control groups allowed us to verify that the association between COVID-19 and the risk of dyslipidaemia was evident in comparisons involving a contemporary control group of those without COVID-19 but exposed to the broader contextual forces of the COVID-19 pandemic, and in comparisons involving a pre-pandemic era historical control group that is undisturbed by the pandemic. We leveraged advances in causal interference and used advanced statistical methodologies to balance the cohorts and estimate the risks of both abnormal lipid laboratory results and lipid lowering medication prescriptions.
This study has several limitations. Our cohort was predominately White and male, which could limit the generalisability of our findings. National electronic health-care databases were used to construct out cohort, and although pre-defined outcomes were carefully selected and our analyses were adjusted for a large set of pre-defined and algorithmically selected variables, we cannot completely rule out misclassification bias and residual confounding. For example, potential confounding might exist if there are substantial differences in unknown or unmeasured characteristics that might be associated with risk of SARS-CoV-2 infection and dyslipidaemia that were not accounted for in this study, including potential genetic susceptibility, environmental exposures, or other factors. A positive COVID-19 test was a requirement for enrolment in the COVID-19 group, yet we cannot rule out the possibility that those enrolled in the contemporary cohort might have contracted COVID-19 and did not test for it; if these people were present in large numbers in the contemporary control group, this could have biased the results. Although we balanced characteristics at baseline, low lipid levels as a risk or as a consequence of SARS-CoV-2 infection might be associated with short term acute mortality, and our cohort evaluating post-acute outcomes could have selected survivors who have higher lipid levels. Because we needed to follow-up people for at least 1 year to characterise risks and burdens of dyslipidaemia at 1 year, the study enrolled people with SARS-CoV-2 infection between March 1, 2020, and Jan 15, 2021. Although genotyping data was not available, the period of enrolment in this study corresponded to the era of the COVID-19 pandemic predominated by the ancestral wild type SARS-CoV-2,51 therefore the risk estimates in this study might not necessarily represent the outcomes associated with infections due to subsequent and future variants. Finally, as the COVID-19 pandemic continues to evolve, the SARS-CoV-2 virus continues to mutate and new variants or subvariants emerge. Therefore vaccine and antiviral use continues to expand and it is possible that the epidemiology of post-acute COVID-19 sequelae, including dyslipidaemia, will continue to evolve and change over time.
Altogether, our results suggest that patients who survive the first 30 days of COVID-19 infection exhibit increased risk and burden of incident dyslipidaemia and incident lipid-lowering medications use. These risks and burdens were evident among those who were non-hospitalised during the acute phase of infection and increased in a graded fashion based on the severity of the acute infection (ie, those who were non-hospitalised, hospitalised, and admitted to intensive care). The body of evidence suggests that dyslipidaemia should be considered as a component of the multifaceted long COVID. Post-acute care strategies of people with COVID-19 should integrate screening and management of dyslipidaemia.
Data sharing
The data that support the findings of this study are available from the US Department of Veterans Affairs (VA), Office of Research and Development, VA Information Resource Center by emailing VIReC@va.gov.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
This study used data from the VA COVID-19 shared data resource. This research was funded by the US Department of Veterans Affairs to ZA-A. The contents of this manuscript do not represent the views of the US Department of Veterans Affairs or the US Government.
Contributors
ZA-A was responsible for the research and study design. EX acquired the data. EX and YX did the statistical analysis. All authors analysed the data, interpreted the data, critically revised the manuscript, and provided administrative, technical, or material support. EX and ZA-A drafted the manuscript. ZA-A provided supervision and mentorship. All authors contributed important intellectual content during manuscript drafting or revision and accept accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. ZAA takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted, and that any discrepancies from the study as planned have been explained. All authors had full access to all the data, have verified the accuracy of all underlying data, and had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Zhang S, Luo P, Xu J, et al. Plasma metabolomic profiles in recovered COVID-19 patients without previous underlying diseases 3 months after discharge. J Inflamm Res. 2021;14:4485–4501. doi: 10.2147/JIR.S325853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sorokin AV, Karathanasis SK, Yang ZH, Freeman L, Kotani K, Remaley AT. COVID-19-associated dyslipidemia: implications for mechanism of impaired resolution and novel therapeutic approaches. FASEB J. 2020;34:9843–9853. doi: 10.1096/fj.202001451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roccaforte V, Daves M, Lippi G, Spreafico M, Bonato C. Altered lipid profile in patients with COVID-19 infection. J Lab Precis Med. 2021;6:2. [Google Scholar]
- 4.Ren Z, Wang H, Cui G, et al. Alterations in the human oral and gut microbiomes and lipidomics in COVID-19. Gut. 2021;70:1253–1265. doi: 10.1136/gutjnl-2020-323826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Yao H, Zhang N, et al. Proteomic analysis identifies prolonged disturbances in pathways related to cholesterol metabolism and myocardium function in the COVID-19 recovery stage. J Proteome Res. 2021;20:3463–3474. doi: 10.1021/acs.jproteome.1c00054. [DOI] [PubMed] [Google Scholar]
- 6.Feingold KR. In: Endotext. Anawalt B, Boyce A, Chrousos G, et al., editors. MDText.com; South Dartmouth, MA: 2000. Lipid and lipoprotein levels in patients with COVID-19 infections. [Google Scholar]
- 7.Fijen LM, Grefhorst A, Levels JHM, Cohn DM. Severe acquired hypertriglyceridemia following COVID-19. BMJ Case Rep. 2021;14:e246698. doi: 10.1136/bcr-2021-246698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deuel JW, Lauria E, Lovey T, et al. Persistence, prevalence, and polymorphism of sequelae after COVID-19 in unvaccinated, young adults of the Swiss Armed Forces: a longitudinal, cohort study (LoCoMo) Lancet Infect Dis. 2022;22:1694–1702. doi: 10.1016/S1473-3099(22)00449-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.FAIR Health A detailed study of patients with long-haul COVID. An analysis of private healthcare claims. 2021. https://s3.amazonaws.com/media2.fairhealth.org/whitepaper/asset/A%20Detailed%20Study%20of%20Patients%20with%20Long-Haul%20COVID--An%20Analysis%20of%20Private%20Healthcare%20Claims--A%20FAIR%20Health%20White%20Paper.pdf
- 10.Bowe B, Xie Y, Xian H, Lian M, Al-Aly Z. Geographic variation and US county characteristics associated with rapid kidney function decline. Kidney Int Rep. 2016;2:5–17. doi: 10.1016/j.ekir.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie Y, Bowe B, Li T, Xian H, Yan Y, Al-Aly Z. Higher blood urea nitrogen is associated with increased risk of incident diabetes mellitus. Kidney Int. 2018;93:741–752. doi: 10.1016/j.kint.2017.08.033. [DOI] [PubMed] [Google Scholar]
- 12.Bowe B, Xie Y, Yan Y, Al-Aly Z. Burden of cause-specific mortality associated with PM2.5 air pollution in the United States. JAMA Netw Open. 2019;2:e1915834. doi: 10.1001/jamanetworkopen.2019.15834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie Y, Bowe B, Yan Y, Xian H, Li T, Al-Aly Z. Estimates of all cause mortality and cause specific mortality associated with proton pump inhibitors among US veterans: cohort study. BMJ. 2019;365:l1580. doi: 10.1136/bmj.l1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bowe B, Xie Y, Xian H, Li T, Al-Aly Z. Association between monocyte count and risk of incident CKD and progression to ESRD. Clin J Am Soc Nephrol. 2017;12:603–613. doi: 10.2215/CJN.09710916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie Y, Bowe B, Gibson A, McGill J, Yan Y, Maddukuri G, Al-Aly Z. Comparative effectiveness of the sodium-glucose co-transporter-2 inhibitor empagliflozin vs. other antihyperglycemics on risk of major adverse kidney events. Diabetes Care. 2020;43:2785–2795. doi: 10.2337/dc20-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie Y, Bowe B, Gibson AK, McGill JB, Maddukuri G, Al-Aly Z. Comparative effectiveness of sodium-glucose cotransporter 2 inhibitors vs sulfonylureas in patients with type 2 diabetes. JAMA Intern Med. 2021;181:1043–1053. doi: 10.1001/jamainternmed.2021.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowe B, Cai M, Xie Y, Gibson AK, Maddukuri G, Al-Aly Z. Acute kidney injury in a national cohort of hospitalized US veterans with COVID-19. Clin J Am Soc Nephrol. 2020;16:14–25. doi: 10.2215/CJN.09610620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie Y, Bowe B, Gibson AK, et al. Comparative effectiveness of SGLT2 inhibitors, GLP-1 receptor agonists, DPP-4 inhibitors, and sulfonylureas on risk of kidney outcomes: emulation of a target trial using health care databases. Diabetes Care. 2020;43:2859–2869. doi: 10.2337/dc20-1890. [DOI] [PubMed] [Google Scholar]
- 19.Xie Y, Bowe B, Gibson AK, McGill JB, Maddukuri G, Al-Aly Z. Clinical Implications of estimated glomerular filtration rate dip following sodium-glucose cotransporter-2 inhibitor initiation on cardiovascular and kidney outcomes. J Am Heart Assoc. 2021;10:e020237. doi: 10.1161/JAHA.120.020237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Department of Veterans Affairs COVID-19: shared data resource. 2020. https://vhacdwdwhweb100.vha.med.va.gov/phenotype/index.php/COVID-19:Shared_Data_Resource#Acknowledgements_COVID-19_Shared_Data_Resource
- 21.Kind AJH, Buckingham WR. Making Neighborhood-disadvantage metrics accessible—the neighborhood atlas. N Engl J Med. 2018;378:2456–2458. doi: 10.1056/NEJMp1802313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259–264. doi: 10.1038/s41586-021-03553-9. [DOI] [PubMed] [Google Scholar]
- 23.Xie Y, Bowe B, Al-Aly Z. Burdens of post-acute sequelae of COVID-19 by severity of acute infection, demographics and health status. Nat Commun. 2021;12:6571. doi: 10.1038/s41467-021-26513-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowe B, Xie Y, Xu E, Al-Aly Z. Kidney outcomes in long COVID. J Am Soc Nephrol. 2021;32:2851–2862. doi: 10.1681/ASN.2021060734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583–590. doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie Y, Xu E, Al-Aly Z. Risks of mental health outcomes in people with COVID-19: cohort study. BMJ. 2022;376:e068993. doi: 10.1136/bmj-2021-068993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schneeweiss S, Rassen JA, Glynn RJ, Avorn J, Mogun H, Brookhart MA. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology. 2009;20:512–522. doi: 10.1097/EDE.0b013e3181a663cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olvey EL, Clauschee S, Malone DC. Comparison of critical drug-drug interaction listings: the Department of Veterans Affairs medical system and standard reference compendia. Clin Pharmacol Ther. 2010;87:48–51. doi: 10.1038/clpt.2009.198. [DOI] [PubMed] [Google Scholar]
- 29.Greene M, Steinman MA, McNicholl IR, Valcour V. Polypharmacy, drug-drug interactions, and potentially inappropriate medications in older adults with human immunodeficiency virus infection. J Am Geriatr Soc. 2014;62:447–453. doi: 10.1111/jgs.12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bowe B, Xie Y, Xu E, Al-Aly Z. Kidney outcomes in long COVID. J Am Soc Nephrol. 2021;32:2851–2862. doi: 10.1681/ASN.2021060734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rubin DB. Using propensity scores to help design observational studies: application to the tobacco litigation. Health Serv Outcomes Res Methodol. 2001;2:169–188. [Google Scholar]
- 33.Lloyd-Jones DM, Braun LT, Ndumele CE, et al. Use of risk assessment tools to guide decision-making in the primary prevention of atherosclerotic cardiovascular disease: a special report from the American Heart Association and American College of Cardiology. J Am Coll Cardiol. 2019;73:3153–3167. doi: 10.1016/j.jacc.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 34.Funk MJ, Westreich D, Wiesen C, Stürmer T, Brookhart MA, Davidian M. Doubly robust estimation of causal effects. Am J Epidemiol. 2011;173:761–767. doi: 10.1093/aje/kwq439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al-Aly Z, Bowe B, Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med. 2022;28:1461–1467. doi: 10.1038/s41591-022-01840-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xie Y, Al-Aly Z. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol. 2022;10:311–321. doi: 10.1016/S2213-8587(22)00044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han CY, Chiba T, Campbell JS, et al. Reciprocal and coordinate regulation of serum amyloid A versus apolipoprotein A-I and paraoxonase-1 by inflammation in murine hepatocytes. Arterioscler Thromb Vasc Biol. 2006;26:1806–1813. doi: 10.1161/01.ATV.0000227472.70734.ad. [DOI] [PubMed] [Google Scholar]
- 38.Yuan S, Chu H, Chan JF, et al. SREBP-dependent lipidomic reprogramming as a broad-spectrum antiviral target. Nat Commun. 2019;10:120. doi: 10.1038/s41467-018-08015-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merad M, Blish CA, Sallusto F, Iwasaki A. The immunology and immunopathology of COVID-19. Science. 2022;375:1122–1127. doi: 10.1126/science.abm8108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farley SE, Kyle JE, Leier HC, et al. A global lipid map reveals host dependency factors conserved across SARS-CoV-2 variants. Nat Commun. 2022;13:3487. doi: 10.1038/s41467-022-31097-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Masana L, Correig E, Ibarretxe D, et al. Low HDL and high triglycerides predict COVID-19 severity. Sci Rep. 2021;11:7217. doi: 10.1038/s41598-021-86747-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.López-Reyes A, Martinez-Armenta C, Espinosa-Velázquez R, et al. NLRP3 inflammasome: the stormy link between obesity and COVID-19. Front Immunol. 2020;11:570251. doi: 10.3389/fimmu.2020.570251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Y, Pan Y, Yin Y, Chen W, Li X. Association of dyslipidemia with the severity and mortality of coronavirus disease 2019 (COVID-19): a meta-analysis. Virol J. 2021;18:157. doi: 10.1186/s12985-021-01604-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Atmosudigdo IS, Lim MA, Radi B, et al. Dyslipidemia increases the risk of severe COVID-19: a systematic review, meta-analysis, and meta-regression. Clin Med Insights Endocrinol Diabetes. 2021;14 doi: 10.1177/1179551421990675. 1179551421990675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu E, Xie Y, Al-Aly Z. Long-term neurologic outcomes of COVID-19. Nat Med. 2022;28:2406–2415. doi: 10.1038/s41591-022-02001-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bowe B, Xie Y, Al-Aly Z. Acute and postacute sequelae associated with SARS-CoV-2 reinfection. Nat Med. 2022;28:2398–2405. doi: 10.1038/s41591-022-02051-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iqbal Z, Ho JH, Adam S, et al. Managing hyperlipidaemia in patients with COVID-19 and during its pandemic: an expert panel position statement from HEART UK. Atherosclerosis. 2020;313:126–136. doi: 10.1016/j.atherosclerosis.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xie Y, Bowe B, Maddukuri G, Al-Aly Z. Comparative evaluation of clinical manifestations and risk of death in patients admitted to hospital with covid-19 and seasonal influenza: cohort study. BMJ. 2021;371:m4677. doi: 10.1136/bmj.m4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Al-Aly Z, Agarwal A, Alwan N, Luyckx VA. Long COVID: long-term health outcomes and implications for policy and research. Nat Rev Nephrol. 2022 doi: 10.1038/s41581-022-00652-2. https://doi.org/10.1038%2Fs41581-022-00652-2 published online Nov 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Al-Aly Z. Diabetes after SARS-Co-V 2 infection. Lancet Diabetes Endocrinol. 2023;11:11–13. doi: 10.1016/S2213-8587(22)00324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Centers for Disease Control and Prevention COVID data tracker. 2023. https://covid.cdc.gov/covid-data-tracker/#variant-proportions
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the US Department of Veterans Affairs (VA), Office of Research and Development, VA Information Resource Center by emailing VIReC@va.gov.