Abstract
Background
Concerns have been raised regarding the reduced immunogenicity of vaccines against COVID-19 in patients with autoimmune diseases treated with rituximab. However, the incidence and severity of breakthrough infections in unbiased samples of patients with specific rheumatic and musculoskeletal diseases are largely unknown. We aimed to assess the incidence of breakthrough SARS-CoV-2 infection, compare rates of moderate-to-severe COVID-19 with any severe infection event, and evaluate predictors of moderate-to-severe COVID-19 outcomes in patients treated with rituximab.
Methods
We did a retrospective cohort study in all rituximab-treated patients with rheumatic and musculoskeletal diseases in a single centre in Leeds, UK between March 1, 2020 (the index date), and April 1, 2022. Adults aged 18 years and older, who fulfilled classification criteria for established rheumatic and musculoskeletal diseases, and received therapy with at least one rituximab infusion between Sept 1, 2019 (6 months before the pandemic in the UK), and April 1, 2022, were eligible for inclusion in the study. SARS-CoV-2 infection was defined by antigen test or PCR. COVID-19 outcomes were categorised as mild (from ambulatory to hospitalised but not requiring oxygen support) or moderate-to-severe (hospitalised and requiring oxygen support or death). The primary outcome was breakthrough COVID-19 infection, which was defined as an infection occurring 14 days or more after the second vaccine dose. Predictors of moderate-to-severe COVID-19 outcomes were analysed using Cox regression proportional hazards.
Findings
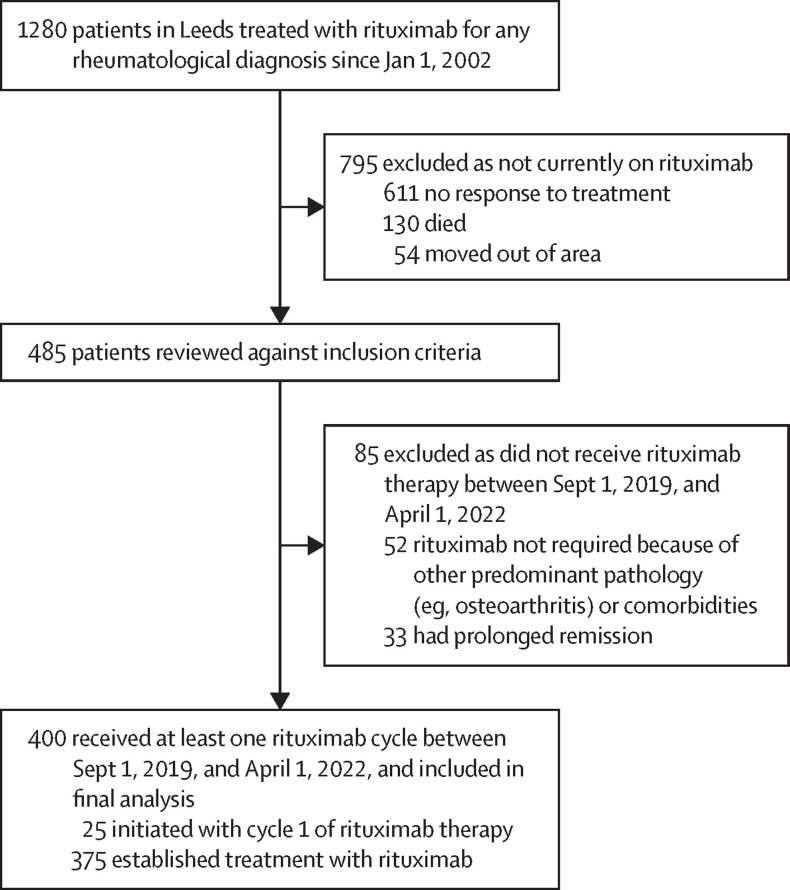
Of the 1280 patients who were treated with at least one cycle of rituximab since Jan 1, 2002, 485 (38%) remained on rituximab therapy on April 1, 2022. Of these patients, 400 fulfilled all inclusion criteria and were included in our final analysis. The mean age at the index date was 58·9 years (SD 14·6), 288 (72%) of 400 patients were female and 112 (28%) were male, 333 (83%) were White, and 110 (28%) had two or more comorbidities. 272 (68%) of 400 patients had rheumatoid arthritis, 48 (12%) had systemic lupus erythematosus, 48 (12%) had anti-neutrophil cytoplasmic antibody-associated vasculitis, and 46 (12%) had other rheumatic and musculoskeletal diseases. During the study, 798 rituximab cycles were administered. Of the 398 (>99%) of 400 patients with vaccine data, 372 (93%) were fully vaccinated. Over the 774·6 patient-years of follow-up, there was an incremental increase in all SARS-CoV-2 severity types over the three pandemic phases (wild-type or alpha, delta, and omicron), but most infections were mild. The rates of moderate-to-severe COVID-19 were broadly similar across these three variant phases. Of 370 patients who were fully vaccinated and with complete data, 110 (30%) had all severity type breakthrough COVID-19, 16 (4%) had moderate-to-severe breakthrough COVID-19, and one (<1%) died. In the post-vaccination phase (after Dec 18, 2020), the incidence rates of all severity type and moderate-to-severe COVID-19 were substantially lower in those who were fully vaccinated compared with unvaccinated or partially vaccinated individuals (22·83 per 100 person-years [95% CI 18·94–27·52] in those who were fully vaccinated vs 89·46 per 100 person-years [52·98–151·05] in those who were partially vaccinated or unvaccinated for infections of all severities, and 3·32 per 100 person-years [2·03–5·42] in those who were fully vaccinated vs 25·56 per 100 person-years [9·59–68·10] in those who were partially vaccinated or unvaccinated for moderate-to-severe infections). The rate of moderate-to-severe COVID-19 was broadly similar to other severe infection events in this cohort (5·68 per 100 person-years [95% CI 4·22–7·63]). In multivariable Cox regression analysis, factors associated with an increased risk of moderate-to-severe COVID-19 were the number of comorbidities (hazard ratio 1·46 [95% CI 1·13–1·89]; p=0·0037) and hypogammaglobulinaemia (defined by a pre-rituximab IgG concentration of <6 g/L; 3·22 [1·27–8·19]; p=0·014). This risk was reduced with each vaccine dose received (0·49 [0·37–0·65]; p<0·0001). Other factors, including concomitant prednisolone use, rituximab-associated factors (eg, rituximab dose and time to vaccination since last rituximab dose), and vaccine-associated factors (eg, vaccine type and peripheral B-cell depletion) were not predictive of moderate-to-severe COVID-19 outcomes.
Interpretation
This study presented detailed analyses of rituximab-treated patients during various phases of the COVID-19 pandemic. In later stages of the pandemic, the SARS-CoV-2 breakthrough infection rate was high but severe COVID-19 rates were similar to any severe infection event rate in patients who were vaccinated. The risk–benefit ratio might still favour rituximab in vaccinated patients with severe rheumatic and musculoskeletal diseases who have few other treatment options. Increased vigilance is needed in the presence of comorbidities and hypogammaglobulinaemia for all infection types.
Funding
Wellcome Trust and Eli Lilly.
Introduction
As rituximab is a B-cell depleting agent, there are concerns regarding safe use of this therapy in the context of the COVID-19 pandemic because of its effect on humoral immunity. Initial data have shown an increased risk of poor outcomes, including deaths, in rituximab-treated patients with rheumatic and musculoskeletal diseases compared with the general population1 and those treated with methotrexate monotherapy,2 tumour necrosis factor inhibitors,3 and other biological disease-modifying antirheumatic drugs (DMARDs) with or without with conventional synthetic DMARDs.4, 5 Furthermore, rituximab therapy is associated with a significantly reduced humoral response to COVID-19 vaccination,6, 7, 8 although the T-cell-mediated immune response is preserved in a majority of patients with rheumatic and musculoskeletal diseases, irrespective of the humoral response.9, 10, 11, 12 Nevertheless, the extent of protection conferred by the T-cell response in rituximab-treated patients with rheumatic and musculoskeletal diseases remains unclear.
It is important to note that the published registry studies mainly reported outcomes during the pre-vaccination phase of the COVID-19 pandemic. A limitation of registry data is reporting bias, which makes estimation of true incidence challenging.13 As rituximab is often used in people with refractory disease (eg, relapsing autoimmune connective tissue diseases or vasculitis) and multiple comorbidities, it is often not pragmatic to discontinue this therapy during an unexpected infection outbreak, particularly for patients with few alternative treatment options. In terms of practicality of rituximab use, there are few data on key rituximab-specific and vaccine-specific predictors of poor outcomes in rituximab-treated patients with rheumatic and musculoskeletal diseases and breakthrough infections after COVID-19 vaccination. A detailed large cohort study could overcome these issues. We aimed to assess the incidence of breakthrough SARS-CoV-2 infection, compare rates of moderate-to-severe COVID-19 with any severe infection event, and evaluate predictors of moderate-to-severe COVID-19 outcomes.
Research in context.
Evidence before this study
Although immunogenicity to COVID-19 vaccination is blunted in patients with rheumatic and musculoskeletal diseases who are treated with B-cell depleting therapy, there is a paucity of data on breakthrough infections in vaccinated patients. Most notably, little is known about the true incidence and nature of breakthrough infections in this clinically vulnerable population. We used COVID-19 filters in PubMed and searched the Cochrane Library and medRxiv for articles published in English between Jan 1, 2020, and Sept 1, 2022, using the following terms: (“rheumatic”) AND (COVID-19 OR SARS-CoV-2) AND (“breakthrough”), and identified 40 studies. Of these, only three studies assessed breakthrough infections in patients receiving immunosuppressive therapies including rituximab. No studies reported outcomes during the omicron wave of the pandemic.
Added value of this study
We captured data on a full unbiased cohort of patients treated with rituximab with details on specific rheumatic and musculoskeletal diseases, as well as multi-morbidities and with matched clinical, immunological, B-cell, and vaccination data. We also analysed data from various phases of the pandemic. We found that, despite a high uptake of vaccination, approximately a third of patients had breakthrough SARS-CoV-2 infections during therapy with rituximab. However, only a small proportion of patients had moderate-to-severe outcomes, with only one death. The incidence rates of poor COVID-19 outcomes were broadly similar across the three SARS-CoV-2 variant phases (wild-type or alpha, delta, and omicron), and were similar to other severe infection events recorded during our observation period. We also identified increasing numbers of comorbidities and hypogammaglobulinaemia as predictors of these moderate-to-severe outcomes, and this risk was reduced with each vaccine dose received.
Implications of all the available evidence
Our findings show that immunoglobulin concentrations should be monitored before and after rituximab therapy, and individualised risk-benefit assessment should be done in patients with rheumatic and musculoskeletal diseases with comorbidities and hypogammaglobulinaemia when making decisions about rituximab therapy, since both are consistent predictors for all infection types. This study also showed the clinical effectiveness of primary and booster COVID-19 vaccination and the need for additional mitigation strategies to overcome waning immunity after primary vaccination in this vulnerable population, emphasising that vaccination remains worthwhile despite B-cell depletion. We have quantified the risk of moderate-to-severe COVID-19 in patients treated with rituximab, and this risk should be balanced against the risk of under-treating patients with severe rheumatic and musculoskeletal diseases.
Methods
Study design and participants
This retrospective, observational, cohort study was done in all rituximab-treated patients with rheumatic and musculoskeletal diseases in a single centre in Leeds, UK. Adults aged 18 years and older, who fulfilled classification criteria for established rheumatic and musculoskeletal diseases, including rheumatoid arthritis, systemic lupus erythematosus (SLE), anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis, primary Sjogren's syndrome, inflammatory myopathy, systemic sclerosis, mixed connective tissue disease, undifferentiated connective tissue disease, IgG4-related disease and Behçet's disease, and who received therapy with at least one rituximab infusion between Sept 1, 2019 (6 months before the start of the COVID-19 pandemic in the UK), and April 1, 2022, were eligible for inclusion in the study. Data for the sex of participants were collected from self-report in electronic health records. The options were either female, male, or other.
Formal ethics approval was not required because all treatment decisions were made before evaluation of data, in accordance with the UK National Health Service (NHS) Research Ethics Committee guidelines. B-cell subset analysis was done in an accredited clinical diagnostic laboratory (Haematological Malignancy Diagnostic Service, Leeds Teaching Hospitals NHS Trust, Leeds, UK). This is a routine clinical test in patients who are receiving treatment with rituximab in this region. Participants provided written informed consent for the study.
Procedures
In patients who received a first cycle of rituximab, this regimen comprised 100 mg methylprednisolone and 1000 mg rituximab given intravenously on days 1 and 14. Further cycles comprising the same full-dose regimen or half-dose regimen (one 1000 mg rituximab infusion) were repeated on clinical relapse. In our department, most patients with rheumatoid arthritis received repeat cycles with a half-dose regimen in the 8 months after the beginning of the pandemic in the UK (March, 2020). A decision to continue therapy with a half-dose regimen or switch back to a full-dose regimen was made by the treating clinicians, depending on clinical response in the previous cycle and infection risk profile.
Salient clinical data, laboratory measures, and treatment characteristics were obtained from patient medical records. Comorbidity count was calculated using summation of the presence of 14 major medical conditions (hypertension, diabetes, ischaemic heart disease, stroke or transient ischaemic attacks, previous cancer, asthma, chronic obstructive pulmonary disease, interstitial lung disease, bronchiectasis, chronic kidney disease, epilepsy, multiple sclerosis, dementia, and depression) as previously described.14
SARS-CoV-2 infection was confirmed by antigen test or PCR test. Severity of COVID-19 was assessed and classified according to the WHO Working Group definition as follows: mild, which comprised no symptoms, requiring ambulatory care, or hospitalised but without the need for oxygen therapy (WHO grade 1–4); moderate, which required hospitalisation and oxygen therapy (WHO grade 5); and severe, which required ventilation support, admission to an intensive care unit (ICU), or led to death (WHO grade 6–10).15 Breakthrough SARS-CoV-2 infection was defined as infection that occurred 14 days or more after the second vaccine dose in a two-dose series (a fully vaccinated individual). Individuals who received only one vaccine dose in a two-dose series were classified as partially vaccinated.
Severe infection events were defined as non-SARS-CoV-2 infections resulting in hospitalisation for more than 24 h or requiring intravenous antibiotics (excluding concurrent admission for SARS-CoV-2-associated infection). Data for SARS-CoV-2 infection and severe infection events were obtained from electronic health records, pathology results servers, hospital discharge letters, clinic letters, correspondences from general practitioners, and medical case notes.
Full blood counts were processed at a single accredited diagnostic laboratory at Leeds Teaching Hospitals NHS Trust (Leeds, UK). Total serum immunoglobulin concentrations were measured by nephelometry before and around 4–6 months after each rituximab cycle (normal range for IgM 0·5–2·0 g/L; IgA 0·8–4·0 g/L; and IgG 6·0–16·0 g/L). Hypogammaglobulinaemia was defined as an IgG concentration of less than 6 g/L.
Peripheral blood B-cell subsets (naive, memory, and plasmablast cells) were enumerated using highly sensitive flow cytometry, as previously described,16 at week 0 and week 2, without knowledge of clinical status other than time since rituximab treatment. A six-colour flow cytometry protocol (CD3, CD14, CD19, CD27, CD38, and CD45) that counted 500 000 events was used. Naive (CD19+CD27–), memory (CD19++CD27+), and plasmablast (CD19+/–CD27++CD38++) counts were enumerated using CD45 to identify the total leukocyte population for calculation of absolute B-cell subset numbers, using CD3 and CD14 to exclude contaminating leukocyte populations. Complete B-cell depletion was defined as a total B-cell count less than 0·0001 × 109 cells/L at 2 weeks after rituximab treatment.
Outcomes
The primary outcome was breakthrough COVID-19 infections in patients who were fully vaccinated. Other outcomes included COVID-19 infections and other severe infection events recorded in all patients during our observation period.
Statistical analysis
Descriptive statistics were summarised using means with SDs or medians with IQRs or ranges for continuous variables and n (%) for categorical variables. Associations between categorical variables were tested by Fisher's exact test if the expected number was five or less, otherwise χ2 tests were done. Patients contributed patient-years of follow-up between the index date (March 1, 2020) in those with previous rituximab exposure or the start date of rituximab treatment in those who initiated the therapy and either the date of death, switch to different biological DMARDs or therapies, or final follow-up visit. Rates for each outcome (all severity type COVID-19, moderate-to-severe COVID-19, SARS-CoV-2 infections between three variant phases, pre-vaccination vs post-vaccination phases, and breakthrough infections in those who received two vaccine doses) were estimated by dividing the counts by their corresponding person-years of interest, with 95% CIs. Specific details of each estimation are described in the appendix (pp 2–3).
For survival analyses only, patients who had multiple SARS-CoV-2 infections were censored at the time of detection of their first infection. Survival analyses for categorically distributed variables were calculated using Kaplan-Meier plots and log-rank tests. For the survival prediction of moderate-to-severe COVID-19 outcomes, in multivariable analysis, only variables with p<0·20 in univariable analysis were included in the model. Missing data were estimated by multiple imputation by chained equations and 20 multiple imputation sets were used to provide stability of results. Sensitivity analysis was done on imputed data and inclusion of a variable of interest (ie, depth of B-cell depletion [complete vs incomplete]) in multivariable analysis. The proportional hazard assumption was tested by examining Schoenfeld residuals plots. Cox regression (proportional hazards) was done using backward elimination, with p<0·20 associated with the deviance used for exclusion from the model. All statistical analysis was done using Stata MP version 16 and IBM SPSS version 27 for Windows.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Of the 1280 patients who were treated with at least one cycle of rituximab since Jan 1, 2002, 485 (38%) remained on rituximab therapy at the date when the data were last updated (April 1, 2022; figure 1 ). Of these patients, 400 (82%) fulfilled all inclusion criteria and were included in the final analysis.
Figure 1.
Study flowchart
Clinical and treatment characteristics and laboratory measures at index date or rituximab initiation are described in table 1 . The mean age at the index date was 58·9 years (SD 14·6), 288 (72%) of 400 patients were female, 112 (28%) were male, and 333 (83%) were White. 272 (68%) of 400 patients had rheumatoid arthritis, 48 (12%) had SLE, 48 (12%) had ANCA-associated vasculitis, and 46 (12%) had other rheumatic and musculoskeletal diseases. 19 (5%) of 400 patients had pre-rituximab hypogammaglobulinaemia (IgG <6 g/L) and 110 (28%) had two or more comorbidities.
Table 1.
Clinical and treatment characteristics and laboratory measures at index date or rituximab initiation
| All patients (n=400) | Rheumatoid arthritis (n=272) | Other rheumatic and musculoskeletal diseases (n=128) | ||
|---|---|---|---|---|
| Age, years | 58·9 (14·6) | 62·4 (12·9) | 50·2 (14·5) | |
| Sex | ||||
| Female | 288 (72%) | 191 (70%) | 97 (76%) | |
| Male | 112 (28%) | 81 (30%) | 31 (24%) | |
| Ethnicity or race | ||||
| White | 333 (83%) | 239 (88%) | 94 (73%) | |
| South Asian | 43 (11%) | 25 (9%) | 18 (14%) | |
| Southeast Asian | 6 (2%) | 1 (<1%) | 5 (4%) | |
| Afro-Caribbean | 7 (2%) | 2 (1%) | 5 (4%) | |
| Mixed race | 11 (3%) | 5 (2%) | 6 (5%) | |
| Diagnosis | ||||
| Rheumatoid arthritis | 272 (68%) | 272 (100%) | .. | |
| Systemic lupus erythematosus | 48 (12%) | .. | 48 (38%) | |
| Antineutrophil cytoplasmic antibody-associated vasculitis | 48 (12%) | .. | 48 (38%) | |
| Inflammatory myopathies | 14 (4%) | .. | 14 (11%) | |
| Primary Sjogren's syndrome | 13 (3%) | .. | 13 (10%) | |
| Systemic sclerosis | 6 (2%) | .. | 6 (5%) | |
| IgG4-related disease | 5 (1%) | .. | 5 (4%) | |
| Undifferentiated CTD | 4 (1%) | .. | 4 (3%) | |
| Mixed CTD | 3 (1%) | .. | 3 (2%) | |
| Behçet's disease | 1 (<1%) | .. | 1 (1%) | |
| Disease duration, years | 11·7 (6·3–16·2) | 13·5 (8·2–16·6) | 7·2 (2·8–12·5) | |
| Comorbidities | ||||
| Diabetes | 45 (11%) | 24 (9%) | 21 (16%) | |
| Coronary artery disease | 34 (9%) | 30 (11%) | 4 (3%) | |
| Hypertension | 98 (25%) | 66 (24%) | 32 (25%) | |
| Heart failure | 16 (4%) | 8 (3%) | 8 (6%) | |
| Stroke | 12 (3%) | 7 (3%) | 5 (4%) | |
| Asthma | 29 (7%) | 18 (7%) | 11 (9%) | |
| Chronic obstructive pulmonary disease | 25 (6%) | 19 (7%) | 6 (5%) | |
| Interstitial lung disease | 37 (9%) | 22 (8%) | 15 (12%) | |
| Bronchiectasis | 25 (6%) | 22 (8%) | 3 (2%) | |
| Previous tuberculosis | 13 (3%) | 9 (3%) | 4 (3%) | |
| Previous cancer | 55 (14%) | 48 (18%) | 7 (5%) | |
| Chronic kidney disease | 24 (6%) | 10 (4%) | 14 (11%) | |
| Depression | 21 (5%) | 15 (6%) | 6 (5%) | |
| Comorbidity count* | 1 (0–6) | 1 (0–6) | 1 (0–5) | |
| Biological naive | 237 (59%) | 113 (42%) | 124 (97%) | |
| Previous cyclophosphamide | 57 (14%) | 5 (2%) | 52 (41%) | |
| Number of previous rituximab cycles* | 4 (0–19) | 6 (0–19) | 2 (0–18) | |
| Concomitant conventional synthetic DMARDs | 272 (68%) | 172 (64%) | 100 (78%) | |
| Methotrexate | 196 (49%) | 162 (60%) | 34 (27%) | |
| Mycophenolate mofetil | 41 (10%) | 2 (1%) | 39 (30%) | |
| Azathioprine | 23 (6%) | 2 (1%) | 21 (16%) | |
| Tacrolimus | 5 (1%) | 1 (<1%) | 4 (3%) | |
| Sulfasalazine | 4 (1%) | 4 (1%) | 0 | |
| Leflunomide | 3 (1%) | 1 (<1%) | 2 (2%) | |
| Concomitant antimalarials | 49 (12%) | 16 (6%) | 33 (26%) | |
| Concomitant prednisolone | 110 (28%) | 40 (15%) | 70 (55%) | |
| Daily prednisolone dose, mg | 2·8 (6·5) | 0·7 (1·8) | 7·3 (9·9) | |
| Immunoglobulin, g/L | ||||
| IgM (normal range 0·5–2·0) | 0·83 (1·61) | 0·78 (0·57) | 0·91 (1·88) | |
| IgA (normal range 0·8–4·0) | 2·72 (1·63) | 2·89 (1·53) | 2·36 (1·78) | |
| IgG (normal range 6·0–16·0) | 10·93 (4·17) | 10·68 (3·47) | 11·47 (5·34) | |
| Low IgG (<6 g/L) | 19 (5%) | 11 (4%) | 8 (6%) | |
| Lymphocyte count, ×109/L | 1·48 (1·03) | 1·52 (1·15) | 1·40 (0·75) | |
| Pre-rituximab peripheral B cells | ||||
| Total B cells, ×10*9/L | 0·0947 (0·13)† | 0·0858 (0·12)‡ | 0·1144 (0·15)§ | |
| Naive B cells, ×10*9/L | 0·0833 (0·12)† | 0·0776 (0·12)‡ | 0·0960 (0·13)§ | |
| Memory B cells, ×10*9/L | 0·0093 (0·03)† | 0·0067 (0·02)‡ | 0·0151 (0·03)§ | |
| Plasmablast cells, ×10*9/L | 0·0021 (0·01)† | 0·0016 (0·00)‡ | 0·0033 (0·01)§ | |
Data are n (%), mean (SD), or median (IQR), unless otherwise indicated. CTD=connective tissue disease. DMARDs=disease modifying anti-rheumatic drugs.
Data are median (range).
n=381.
n=263.
n=118.
The total follow-up time was 774·6 person-years. The mean follow-up per patient was 1·9 years (SD 0·4). 798 cycles of rituximab were administered during this study to 400 patients; the median number of rituximab cycles administered was two (range 1–5; IQR 1–2). In patients who received repeated cycles of rituximab, the median time to retreatment was 50 weeks for cycle 1 (IQR 35–65; n=272), 41 weeks for cycle 2 (33–52; n=101), and 35 weeks for cycle 3 (30–39; n=20). 81 (20%) patients were treated with MabThera, 315 (79%) with Truxima and four (1%) with Rixathon. In 349 patients for whom post-rituximab B-cell data were available, 255 (73%) had complete B-cell depletion (including plasmablast depletion) and 302 (87%) had complete CD20 cell depletion (naive and memory B cells) using highly sensitive flow cytometry.
At the end of follow-up, 27 (7%) of 400 patients discontinued rituximab (12 non-response and subsequently switched to different biological DMARDs or therapies, 14 deaths, and one escalation to intravenous cyclophosphamide for concurrent scleromalacia perforans). The causes of deaths were interstitial lung disease progression (four patients), COVID-19 (three patients), infective exacerbation of bronchiectasis (two patients), stroke (one patient), multi-organ failure (one patient), end-stage heart failure (one patient), cardiac tamponade (one patient), and upper gastrointestinal malignancy (one patient).
Vaccination data were available in 398 (>99%) of 400 patients. There was a high uptake of COVID-19 vaccination, and 372 (93%) patients were fully vaccinated (ie, completed both doses in a two-dose series). 21 (5%) of 400 patients were unvaccinated, five (1%) of 398 patients had received one vaccine dose, 47 (12%) had received two doses, 223 (56%) had received three, and 102 (26%) had received four vaccine doses. Of the 359 patients for whom the primary two-dose vaccine series was known, 243 (68%) received the ChAdOx1 nCoV-19 adenoviral vector vaccine (Oxford-AstraZeneca) as their primary vaccine and the remaining 116 (32%) had the BNT162b2 mRNA vaccine (Pfizer-BioNTech).
Of the 377 patients who received at least one vaccine dose, 25 (7%) initiated therapy with rituximab; all were vaccinated at least 6 weeks before therapy. In the 352 patients with previous rituximab exposure, 36 (10%) received their first vaccine dose within 3 months of previous rituximab treatment, 55 (16%) between 3–6 months of previous rituximab treatment, and 261 (74%) more than 6 months after rituximab treatment. The median time to vaccination from the last rituximab treatment was 47 weeks (IQR 25-62).
We assessed breakthrough SARS-CoV-2 infections in 370 patients who received at least two vaccine doses and with complete data. 110 (30%) patients had breakthrough COVID-19 (all severity), 16 (4%) had moderate-to-severe COVID-19, and there was 1 (<1%) death (table 2 ). The mean time to all severity COVID-19 and moderate-to-severe COVID-19 after the second vaccine dose was 32·2 weeks (SD 12·2) weeks and 28·5 weeks (12·4), respectively. Concomitant conventional synthetic DMARD treatment was associated with the frequency of breakthrough infections in fully vaccinated patients (85 [34%] of 252 patients who were treated with rituximab and concomintant DMARD vs 21 [18%] of 118 patients on rituximab monotherapy; p=0·0016), but not with the rate of moderate-to-severe breakthrough infections (ten [4%] of 252 patients who were treated with rituximab and concomitant DMARD vs six [5%] of 118 patients on rituximab monotherapy; p=0·79).
Table 2.
Rates of all severity and moderate-to-severe COVID-19 in rituximab treated patients pre-vaccination and post-vaccination
| Infections | Infections per 100 person-years (95% CI) | |
|---|---|---|
| All severity COVID-19 | ||
| Pre-vaccination programme (before Dec 18, 2020) | 16 in 16 patients | 5·59 (3·42–9·12) |
| Post-vaccination programme (after Dec 18, 2020), unvaccinated or partially vaccinated | 14 in 13 patients | 89·46 (52·98–151·05) |
| Post-vaccination programme (after Dec 18, 2020), fully vaccinated (breakthrough infections) | 110 in 100 patients | 22·83 (18·94–27·52) |
| Overall study follow-up | 140 in 129 patients | 18·07 (15·31–21·33) |
| Moderate-to-severe COVID-19 | ||
| Pre-vaccination programme (before Dec 18, 2020) | 7 in 7 patients | 2·44 (1·17–5·13) |
| Post-vaccination programme (after Dec 18, 2020), unvaccinated or partially vaccinated | 4 in 4 patients | 25·56 (9·59–68·10) |
| Post-vaccination programme (after Dec 18, 2020), fully vaccinated (breakthrough infections) | 16 in 16 patients | 3·32 (2·03–5·42) |
| Overall study follow-up | 27 in 27 patients | 3·49 (2·39–5·08) |
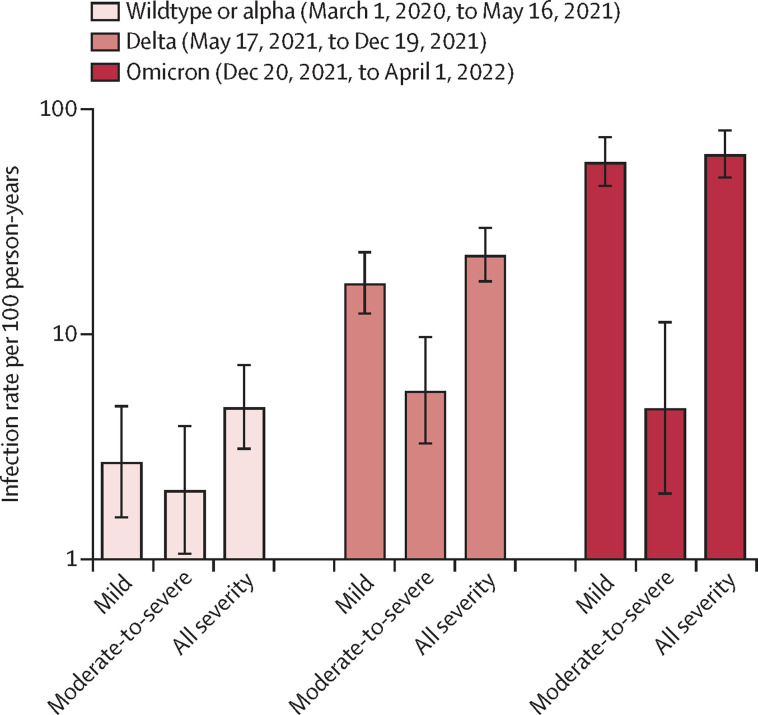
We compared SARS-CoV-2 infection rates between three variant phases (wild-type or alpha, delta, and omicron). We found an incremental increase in all severity types over the three phases; the highest rate during omicron was 63·21 infections per 100 person-years (95% CI 49·75–80·32). However, most cases were mild (58·50 mild infections per 100 person-years [95% CI 45·61–75·03] during omicron vs 16·93 mild infections per 100 person-years [12·37–23·17] for delta and 2·72 mild infections per 100 person-years [1·54-4·79] for wildtype or alpha). The rates of moderate-to-severe COVID-19 were broadly similar across the three variant phases (figure 2 ).
Figure 2.
Severity of COVID-19 across the three variants phases
Comparison of severity of COVID-19 (mild, moderate-to-severe, and all severities) between the three variant phases (wild-type or alpha, delta, and omicron). The y-axis is transformed to a log10 scale. Data are summarised as infection rate per 100 patient-years and the error bars denote 95% CIs.
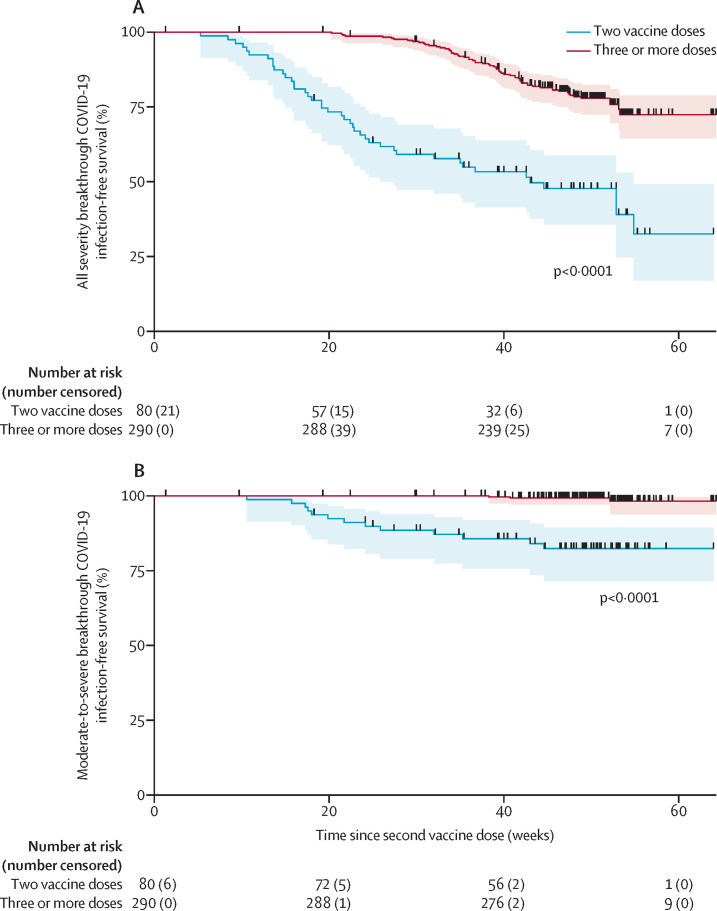
In the overall study follow-up, the rates for all severity types and moderate-to-severe COVID-19 were 18·07 per 100 person-years (95% CI 15·31–21·33) and 3·49 per 100 person-years (2·39–5·08), respectively (table 2). 11 (3%) of 400 patients had recurrent SARS-CoV-2 infection episodes (detected at least 3 months after their previous infection). Details of patients with moderate-to-severe infection and the treatments or interventions received are described in the appendix (pp 4–8). In our region, the COVID-19 vaccination programme commenced from Dec 18, 2020. In the post-vaccination phase, the rates of both all severity and moderate-to-severe infections were substantially lower in the fully vaccinated group than in those who were unvaccinated or partially vaccinated (22·83 per 100 person-years [95% CI 18·94–27·52] in those who were fully vaccinated vs 89·46 per 100 person-years [52·98–151·05] in those who were partially vaccinated or unvaccinated for infections of all severities, and 3·32 per 100 person-years [2·03–5·42] in those who were fully vaccinated vs 25·56 per 100 person-years [9·59–68·10] in those who were partially vaccinated or unvaccinated for moderate-to-severe infections; table 2). Moreover, those who received at least one additional vaccine dose (ie, three or more doses) were less likely to develop either all severity or moderate-to-severe COVID-19 compared with those who received two vaccine doses (Kaplan-Meier log-rank test, χ2 54·35; p<0·0001 and χ2 40·18; p<0·0001, respectively; figure 3 ).
Figure 3.
SARS-CoV-2 breakthrough infection according to the number of vaccine dose received
Kaplan-Meier survival graphs of breakthrough SARS-CoV-2 infection of any severity (A) and moderate-to-severe SARS-CoV-2 infection (B), as measured from the time of second vaccine dose. Survival was compared between patients who received two vaccine doses only versus those who received booster vaccination. Each curve is provided with number at risk and number of failures (ie, those who had infection between timepoints) in brackets. Each patient censored during follow-up is marked with vertical black line on the curve. Log-rank plot testing was used to test the equality of survivor function. The shaded areas represent 95% CIs.
We compared the rates of SARS-CoV-2 infection with any severe infection event. 44 severe infection events were recorded in 34 patients over 774·6 person-years of follow-up (5·68 per 100 person-years [95% CI 4·22–7·63]). The most common infections were lung and urinary tract infections. Sources of severe infection events are shown in the appendix (p 9). The rate of severe infection events in this cohort was similar to the rate of moderate-to-severe COVID-19 for the overall cohort (3·49 per 100 person-years [95% CI 2·39–5·08]) and in fully vaccinated patients (3·32 per 100 person-years [95% CI 2·03–5·42; table 2).
We evaluated key predictors of moderate-to-severe COVID-19 outcomes using Cox regression proportional hazards, including salient clinical and laboratory measures and treatment characteristics, key rituximab-specific variables (rituximab dose, number of previous rituximab cycles, low IgG before rituximab treatment, B-cell subsets, and depth of B-cell depletion), and vaccine-specific variables (vaccine type, time to vaccination from previous rituximab treatment, and number of vaccine doses received). In univariable analysis, previous history of cyclophosphamide therapy, number of comorbidities, concomitant oral prednisolone, concomitant oral prednisolone dose, and low IgG were associated with an increased risk of moderate-to-severe COVID-19 outcomes. By contrast, the number of vaccine doses received reduced this risk.
In multivariable analysis, only the number of comorbidities (hazard ratio 1·46 [95% CI 1·13–1·89]; p=0·0037) and low IgG before rituximab treatment (3·22 [1·27–8·19]; p=0·014) were associated with an increased risk of moderate-to-severe COVI-19 outcomes. This risk was reduced with each vaccine dose received (0·49 [0·37–0·65]; p<0·0001; table 3 ). Results for sensitivity analyses were similar (table 3).
Table 3.
Univariable and multivariable analyses (with multiple imputation) of risk factors for moderate-to-severe COVID-19 outcomes
| Univariable analysis, HR (95% CI); p value, N | Multivariable analysis, HR (95% CI); p value, N | Sensitivity analysis (with multiple imputation), multivariable analysis HR (95% CI); p value | |
|---|---|---|---|
| Age, per 10 years* | 0·95 (0·73–1·22); p=0·67, 400 | Not included in multivariable analysis | Not included in multivariable analysis |
| Female sex | 1·02 (0·43–2·42); p=0·96, 400 | Not included in multivariable analysis | Not included in multivariable analysis |
| Other ethnicities vs white | 1·20 (0·45–3·16); p=0·72, 400 | Not included in multivariable analysis | Not included in multivariable analysis |
| Disease duration, per year | 0·99 (0·95–1·04); p=0·78, 400 | Not included in multivariable analysis | Not included in multivariable analysis |
| Connective tissue disease diagnosis vs rheumatoid arthritis | 2·02 (0·94–4·31); p=0·070, 400 | Included in multivariable analysis but removed from final model as p<0·20 | Included in multivariable analysis but removed from final model as p<0·20 |
| Previous exposure to biological DMARDs | 0·64 (0·29–1·42); p=0·27, 400 | Not included in multivariable analysis | Not included in multivariable analysis |
| Previous therapy with cyclophosphamide | 3·20 (1·44–7·12); p=0·0044, 400 | Included in multivariable analysis but removed from final model as p<0·20 | Included in multivariable analysis but removed from final model as p<0·20 |
| Comorbidity count, per count | 1·69 (1·32–2·15); p<0·0001, 400 | 1·46 (1·13–1·89); p=0·0037, 398 | 1·46 (1·13–1·89); p=0·0037 |
| Number of previous rituximab cycles | 1·02 (0·95–1·11); p=0·56, 400 | Not included in multivariable analysis | Not included in multivariable analysis |
| Full-dose rituximab vs half-dose rituximab | 1·51 (0·62–3·64); p=0·36, 400 | Not included in multivariable analysis | Not included in multivariable analysis |
| Concomitant conventional synthetic DMARDs† | 1·12 (0·49–2·55); p=0·79, 400 | Not included in multivariable analysis | Not included in multivariable analysis |
| Concomitant daily oral prednisolone | 3·18 (1·50–6·78); p=0·0027, 400 | Not included in multivariable analysis because of collinearity with prednisolone dose | Not included in multivariable analysis because of collinearity with prednisolone dose |
| Concomitant daily oral prednisolone dose, mg | 1·06 (1·02–1·11); p=0·0079, 400 | 1·04 (0·99–1·10); p=0·13, 398 | 1·04 (0·99–1·10); p=0·13 |
| Naive B cells, ×109/L‡ | 1·00 (0·99–1·00); p=0·93, 381 | Not included in multivariable analysis | Not included in multivariable analysis |
| Memory B cells, ×109/L‡ | 0·99 (0·95–1·03); p=0·53, 381 | Not included in multivariable analysis | Not included in multivariable analysis |
| Plasmablasts, ×109/L‡ | 1·00 (0·94–1·06); p=0·90, 381 | Not included in multivariable analysis | Not included in multivariable analysis |
| Complete depletion at 2 weeks after rituximab treatment | 1·48 (0·50–4·36); p=0·47, 349 | Not included in multivariable analysis | Included in multivariable analysis but removed from final model as p<0·20 |
| Lymphocyte count, ×109/L | 0·97 (0·56–1·68); p=0·91, 400 | Not included in multivariable analysis | Not included in multivariable analysis |
| Low IgG (<6 g/L) | 7·19 (3·04–16·70); p<0·0001, 400 | 3·22 (1·27–8·19); p=0·014, 398 | 3·22 (1·27–8·19); p=0·014 |
| Number of COVID-19 vaccine doses received | 0·44 (0·33–0·58); p<0·0001, 398 | 0·49 (0·37–0·65); p<0·0001, 398 | 0·49 (0·37–0·65); p<0·0001 |
| mRNA vaccine vs vector vaccine | 0·68 (0·27–1·72); p=0·42, 380 | Not included in multivariable analysis | Not included in multivariable analysis |
| Timing of first vaccination since last rituximab dose, weeks§ | 1·00 (0·99–1·01); p=0·86, 352 | Not included in multivariable analysis | Not included in multivariable analysis |
| Timing of vaccination since last rituximab dose, >26 weeks vs ≤26 weeks§ | 2·04 (0·60–6·89); p=0·25, 352 | Not included in multivariable analysis | Not included in multivariable analysis |
Risk factors are clinical or serological characteristics at rituximab treatment index date or at rituximab initiation. DMARDs=disease modifying anti-rheumatic drugs.
Age was not a significant predictor even when dichotomised to either two groups (<65 years vs ≥65 years old) or three groups (0–50 years, 51–64 years, and ≥65 years.)
Concomitant DMARDs excluded those who were on anti-malarial monotherapy.
B-cell subset count is ×109 cells/L; for each subset multiply by 1000 before analysis.
The 25 patients who initiated therapy with rituximab during this study were excluded from these analyses.
Discussion
In this study, we have presented detailed analyses of a comprehensive cohort of patients with rheumatic and musculoskeletal diseases who were treated with rituximab during various phases of the COVID-19 pandemic. Our cohort is representative of patients with rheumatic and musculoskeletal diseases who are receiving rituximab in the UK and the salient clinical characteristics (age, sex distribution, disease duration, and comorbidites) of our cohort are comparable to real-world data of rituximab-treated patients from registries in Europe.17, 18, 19, 20 By capturing data from a broad spectrum of patients with rheumatic and musculoskeletal diseases with matched clinical, immunological, B-cell, and vaccination data, as well as medium-term follow-up, this study offers insights into the pragmatic use of rituximab in the context of a pandemic and a foundation for safe monitoring of rituximab therapy.
In this study, there was a high uptake of COVID-19 vaccination. However, the rate of breakthrough infections was high. A high breakthrough infection rate might be expected in immunosuppressed individuals and accordingly, the number of breakthrough infections in our study was higher than in the general population in England (9·8 per 100 person-years).21 Similarly, the rate of moderate-to-severe SARS-CoV-2 breakthrough infection in this cohort was higher than the general population in England (0·48 per 100 person-years), although the incidence was broadly similar to people with comorbidities, including end-stage kidney disease, haematological malignancy, and those who are immunocompromised (range 1·7 to 7·6 per 100 person-years).21 The rate of moderate-to-severe breakthrough infections in our cohort was slightly higher than in rituximab-treated patients with immune-mediated inflammatory diseases in the USA (29 [2%] of 1696).22 This difference could be due to the population studied (ie, the US study included patients with neuroinflammatory disease and fewer patients with multi-morbidity and concomitant DMARD use).
Strengths of this study include the rigorous methodology for data collection and extraction and the inclusion of all rituximab-treated patients with rheumatic and musculoskeletal diseases over various phases of the pandemic and SARS-CoV-2 variants (wild-type or alpha, delta, and omicron). In the later stages of the pandemic, the SARS-CoV-2 infection rate was high but the moderate-to-severe COVID-19 rate was broadly similar to the rate of moderate-to-severe COVID-19 in the pre-vaccination phase and the rate of any severe infection events in the vaccinated cohort. This finding was consistent with the findings of studies reporting that the cumulative incidences of both SARS-CoV-2 delta and omicron breakthrough infections were high in patients with immune-mediated inflammatory diseases on immunosuppressants, including rituximab, but these rates were similar to those reported in patients with immune-mediated inflammatory diseases who were not on immunosuppressants and those reported in healthy controls; furthermore, disease severity was mostly mild.23, 24 Meanwhile, another study that included rituximab-treated patients reported that SARS-CoV-2 infections in the initial omicron wave were associated with a 71% reduction in the risk of hospitalisation or death compared with the earliest time period of the pandemic.25 Moreover, the rate of moderate-to-severe SARS-CoV-2 breakthrough infections in our cohort was similar to the pre-pandemic severe infection event rate from global randomised trials and long-term extension studies of rituximab in patients with rheumatoid arthritis (3·94 per 100 person-years)26 and from registries of rituximab use in patients with rheumatoid arthritis and other rheumatic and musculoskeletal diseases (range 5·0–6·6 per 100 person-years).17, 27, 28 Thus, it is important to identify factors associated with poor COVID-19 outcomes and severe infection events to allow individualised risk–benefit assessment with regard to rituximab treatment decisions.
As expected, the risk of moderate-to-severe COVID-19 outcomes was increased with each comorbidity. In terms of rituximab-specific factors, pre-rituximab hypogammaglobulinaemia increased the risk of moderate-to-severe COVID-19 outcomes. This finding is concordant with a cohort study that identified lower IgG concentrations as a predictor of poor immunogenicity after vaccination with the BNT162b2 vaccine in patients with rheumatic and musculoskeletal diseases.29 We and others have shown that a low IgG concentration is an established predictor of severe infection events within the first 12 months of rituximab therapy and in repeated cycles in patients with rheumatic and musculoskeletal diseases.27, 30 Thus, immunoglobulin concentrations should be monitored at baseline and before each rituximab cycle, particularly in patients with comorbidities, to discern those at an increased risk of severe infection.
The continued effectiveness of B-cell depletion in patients with rheumatic and musculoskeletal diseases depends on repeated rituximab cycles to maintain depletion or low numbers of B cells, which sparks concerns in the context of a pandemic. Several studies reported poor immunogenicity to COVID-19 vaccination in patients with undetectable or low numbers of peripheral B cells.10, 31, 32 Nevertheless, similar to our previous findings for any severe infection event,30 in a fully adjusted model, this study showed that low B-cell numbers, the depth of depletion as measured using highly sensitive flow cytometry after rituximab therapy, and the number of previous rituximab cycles were not associated with an increased risk of moderate-to-severe COVID-19 outcomes. Therefore, the implications of absence of SARS-CoV-2 antibodies after vaccination must be weighed in view of other known risk factors for poor outcomes, as well as the changing pandemic burden and SARS-CoV-2 variants when informing patients and making clinical decisions regarding rituximab therapy.
Liew and colleagues33 reported a shorter time to breakthrough infection after the second vaccine dose in patients with rheumatic and musculoskeletal diseases treated with various therapies, including rituximab (mean 112 days, SD 60). By contrast, the median time to breakthrough infection in our cohort was over 30 weeks, which was consistent with the waning of humoral immunity 6 months after vaccination. Thus, our study provides support for the importance of booster COVID-19 vaccination and the development of new longer-lasting vaccines. Moreover, this study also provides assurance that risk of moderate-to-severe outcomes could be reduced with each vaccine dose received, particularly for patients who need repeated rituximab cycles and those who cannot access other newer and effective therapies, such as pre-exposure anti-SARS-CoV-2 monoclonal antibodies34 for infection prevention.
This study has some limitations. First, PCR and antigen testing were not widely available in the UK during the first four months of the COVID-19 pandemic. Hence, the rate of SARS-CoV-2 infection during the pre-vaccination period could be underestimated. Second, because of the single-centre nature of the study design, our findings might not be generalisable to other health-care services outside the UK, which might have different demographics and access to therapy. Moreover, most of our patients had rheumatoid arthritis and we mostly used rituximab retreatment on clinical relapse. Thus, our findings might not be extrapolatable to patients with specific rheumatic and musculoskeletal diseases, such as ANCA-associated vasculitis, in which a fixed retreatment strategy is frequently used. Third, the absence of a control group, the paucity of rituximab-treated patients who were unvaccinated, the small sample size, and insufficient power calculation for multivariable regression analysis limit our ability to interpret the effectiveness of COVID-19 vaccination. Our sample size was also insufficient to address other important questions, including factors associated with poor outcomes in different phases of the pandemic and in relation to different SARS-CoV-2 variants. Fourth, data for B-cell depletion at 2 weeks after rituximab treatment were missing for some patients at the beginning of the pandemic because of social restrictions that restricted hospital attendance for post-infusion blood testing (51 [13%] of 400). Multiple imputation with chained equations was used to reduce potential bias in parameter estimation. Finally, concomitant therapy with conventional synthetic DMARDs was used in roughly two-thirds of patients in our cohort, and daily oral corticosteroids were used in roughly a third; thus, the effectiveness or lack of effectiveness of COVID-19 vaccination should not be attributed to rituximab alone. Advice on withholding methotrexate or mycophenolate mofetil for 2 weeks after vaccination to improve vaccine immunogenicity was not protocolised during this study, although vaccination was avoided in first 3 months after rituximab treatment where possible.
In conclusion, breakthrough SARS-CoV-2 infection was common in rituximab-treated patients, but most COVID-19 cases were mild. Later in the pandemic, there have been less severe SARS-CoV-2 variants, increased use of vaccination, less social restrictions, and new therapies. The risk–benefit ratio might favour rituximab in vaccinated patients with severe rheumatic and musculoskeletal diseases who have few other treatment options. Increased vigilance is needed in the presence of comorbidities and low IgG concentrations for all infection types.
Data sharing
All data underlying this paper are available in the Article and online supplementary material. Upon justifiable request, de-identified data are available from the corresponding author.
Declaration of interests
MYMY has received consultancy fees from Aurinia Pharmaceuticals and UCB. SS has received consultancy fees from Novartis, Swedish Orphan Biovitrum, and Sire, and grant support from Novartis, Swedish Orphan Biovitrum, Octapharma, and CSL Behring. EMV has received consultancy fees from AstraZeneca, Aurinia Pharmaceuticals, GSK, Eli Lilly, Modus Therapeutics, Novartis, Ostuka, and UCB, and research grants paid to his employer by AstraZeneca, Roche, and Sandoz. PE has received consultancy fees from Abbvie, AstraZeneca, BMS, Boehringer Ingelheim, Eli Lilly, Galapagos, Gilead, Janssen, MSD, Novartis, Pfizer, Roche, and Samsung, and research grants paid to his employer from Abbvie, BMS, Eli Lilly, Pfizer, Novartis, Roche, and Samsung. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
This research was funded and supported by the Wellcome Trust Institutional Strategic Support Fund Fellowship to MYMY (204825/Z/16/Z) and an unrestricted grant by Eli Lilly to the University of Leeds. This Article also presents independent research funded and supported by the National Institute for Health and Care Research (NIHR) Leeds Biomedical Research Centre. The views expressed are those of the author(s) and not necessarily those of the NIHR or the UK Department of Health and Social Care. We would like to thank technicians at the Haematological Malignancy Diagnostic Service, Leeds Teaching Hospitals NHS Trust for acquisition of B-cell data, and patients, clinicians, and nurses at the Leeds Biologic Monitoring Clinic and Connective Tissue Disease Clinic, Leeds Teaching Hospitals NHS Trust for their substantial contributions to the acquisition of the data. PE is Versus Arthritis Professor of Rheumatology.
Contributors
MYMY, JA, and PE made substantial contributions to the conception and design of the work, the acquisition, analysis, and interpretation of data, drafting the work or revising it critically for important intellectual content, and final approval of the version to be published. BS, CV, SD, SS, and EMV made substantial contributions to the acquisition and interpretation of data, drafting the work or revising it critically for important intellectual content, and final approval of the version to be published. All authors had full access to all the data in the study. MYMY, JA, and PE have directly accessed and verified the underlying data in the manuscript. All authors have agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors had final responsibility for the decision to submit for publication. No other disclosures relevant to this Article were reported.
Supplementary Material
References
- 1.Patel NJ, D'Silva KM, Hsu TY, et al. Coronavirus disease 2019 outcomes among recipients of anti-CD20 monoclonal antibodies for immune-mediated diseases: a comparative cohort study. ACR Open Rheumatol. 2022;4:238–246. doi: 10.1002/acr2.11386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strangfeld A, Schäfer M, Gianfrancesco MA, et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2021;80:930–942. doi: 10.1136/annrheumdis-2020-219498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sparks JA, Wallace ZS, Seet AM, et al. Associations of baseline use of biologic or targeted synthetic DMARDs with COVID-19 severity in rheumatoid arthritis: results from the COVID-19 Global Rheumatology Alliance physician registry. Ann Rheum Dis. 2021;80:1137–1146. doi: 10.1136/annrheumdis-2021-220418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avouac J, Drumez E, Hachulla E, et al. COVID-19 outcomes in patients with inflammatory rheumatic and musculoskeletal diseases treated with rituximab: a cohort study. Lancet Rheumatol. 2021;3:e419–e426. doi: 10.1016/S2665-9913(21)00059-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Felten R, Duret PM, Bauer E, et al. B-cell targeted therapy is associated with severe COVID-19 among patients with inflammatory arthritides: a 1-year multicentre study in 1116 successive patients receiving intravenous biologics. Ann Rheum Dis. 2022;81:143–145. doi: 10.1136/annrheumdis-2021-220549. [DOI] [PubMed] [Google Scholar]
- 6.Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80:1330–1338. doi: 10.1136/annrheumdis-2021-220647. [DOI] [PubMed] [Google Scholar]
- 7.Schumacher F, Mrdenovic N, Scheicht D, Pons-Kühnemann J, Scheibelhut C, Strunk J. Humoral immunogenicity of COVID-19 vaccines in patients with inflammatory rheumatic diseases under treatment with rituximab: a case-control study (COVID-19VacRTX) Rheumatology (Oxford) 2022;61:3912–3918. doi: 10.1093/rheumatology/keac036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deepak P, Kim W, Paley MA, et al. Effect of immunosuppression on the immunogenicity of mRNA vaccines to SARS-CoV-2: a prospective cohort study. Ann Intern Med. 2021;174:1572–1585. doi: 10.7326/M21-1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bitoun S, Henry J, Desjardins D, et al. Rituximab impairs B cell response but not T cell response to COVID-19 vaccine in autoimmune diseases. Arthritis Rheumatol. 2022;74:927–933. doi: 10.1002/art.42058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mrak D, Tobudic S, Koblischke M, et al. SARS-CoV-2 vaccination in rituximab-treated patients: B cells promote humoral immune responses in the presence of T-cell-mediated immunity. Ann Rheum Dis. 2021;80:1345–1350. doi: 10.1136/annrheumdis-2021-220781. [DOI] [PubMed] [Google Scholar]
- 11.Prendecki M, Clarke C, Edwards H, et al. Humoral and T-cell responses to SARS-CoV-2 vaccination in patients receiving immunosuppression. Ann Rheum Dis. 2021;80:1322–1329. doi: 10.1136/annrheumdis-2021-220626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saleem B, Ross RL, Bissell LA, et al. Effectiveness of SARS-CoV-2 vaccination in patients with rheumatoid arthritis (RA) on DMARDs: as determined by antibody and T cell responses. RMD Open. 2022;8 doi: 10.1136/rmdopen-2021-002050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Courvoisier DS, Lauper K, Kedra J, et al. EULAR points to consider when analysing and reporting comparative effectiveness research using observational data in rheumatology. Ann Rheum Dis. 2022;81:780–785. doi: 10.1136/annrheumdis-2021-221307. [DOI] [PubMed] [Google Scholar]
- 14.Melville AR, Md Yusof MY, Fitton J, et al. Real-world experience of effectiveness of non-medical switch from originator to biosimilar rituximab in rheumatoid arthritis. Rheumatology (Oxford) 2021;60:3679–3688. doi: 10.1093/rheumatology/keaa834. [DOI] [PubMed] [Google Scholar]
- 15.Marshall JC, Murthy S, Diaz J, et al. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20:e192–e197. doi: 10.1016/S1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dass S, Rawstron AC, Vital EM, Henshaw K, McGonagle D, Emery P. Highly sensitive B cell analysis predicts response to rituximab therapy in rheumatoid arthritis. Arthritis Rheum. 2008;58:2993–2999. doi: 10.1002/art.23902. [DOI] [PubMed] [Google Scholar]
- 17.Silva-Fernández L, De Cock D, Lunt M, et al. Serious infection risk after 1 year between patients with rheumatoid arthritis treated with rituximab or with a second TNFi after initial TNFi failure: results from The British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Rheumatology (Oxford) 2018;57:1533–1540. doi: 10.1093/rheumatology/kex304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wendler J, Burmester GR, Sörensen H, et al. Rituximab in patients with rheumatoid arthritis in routine practice (GERINIS): six-year results from a prospective, multicentre, non-interventional study in 2,484 patients. Arthritis Res Ther. 2014;16:R80. doi: 10.1186/ar4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terrier B, Amoura Z, Ravaud P, et al. Safety and efficacy of rituximab in systemic lupus erythematosus: results from 136 patients from the French AutoImmunity and Rituximab registry. Arthritis Rheum. 2010;62:2458–2466. doi: 10.1002/art.27541. [DOI] [PubMed] [Google Scholar]
- 20.Cobo-Ibáñez T, Descalzo MÀ, Loza-Santamaría E, Carmona L, Muñoz-Fernández S. Serious infections in patients with rheumatoid arthritis and other immune-mediated connective tissue diseases exposed to anti-TNF or rituximab: data from the Spanish registry BIOBADASER 2.0. Rheumatol Int. 2014;34:953–961. doi: 10.1007/s00296-014-2945-y. [DOI] [PubMed] [Google Scholar]
- 21.Green A, Curtis H, Hulme W, et al. Describing the population experiencing COVID-19 vaccine breakthrough following second vaccination in England: a cohort study from OpenSAFELY. BMC Med. 2022;20:243. doi: 10.1186/s12916-022-02422-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calabrese CM, Kirchner E, Husni EM, et al. Breakthrough SARS-CoV-2 infections in patients with immune-mediated disease undergoing B cell-depleting therapy: a retrospective cohort analysis. Arthritis Rheumatol. 2022;74:1906–1915. doi: 10.1002/art.42287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boekel L, Stalman EW, Wieske L, et al. Breakthrough SARS-CoV-2 infections with the delta (B.1.617.2) variant in vaccinated patients with immune-mediated inflammatory diseases using immunosuppressants: a substudy of two prospective cohort studies. Lancet Rheumatol. 2022;4:e417–e429. doi: 10.1016/S2665-9913(22)00102-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stalman EW, Wieske L, van Dam KPJ, et al. Breakthrough infections with the SARS-CoV-2 omicron (B.1.1.529) variant in patients with immune-mediated inflammatory diseases. Ann Rheum Dis. 2022;81:1757–1766. doi: 10.1136/ard-2022-222904. [DOI] [PubMed] [Google Scholar]
- 25.Kawano Y, Patel NJ, Wang X, et al. Temporal trends in COVID-19 outcomes among patients with systemic autoimmune rheumatic diseases: from the first wave through the initial Omicron wave. Ann Rheum Dis. 2022;81:1742–1749. doi: 10.1136/ard-2022-222954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Vollenhoven RF, Fleischmann RM, Furst DE, Lacey S, Lehane PB. Longterm safety of rituximab: final report of the Rheumatoid Arthritis Global Clinical Trial Program over 11 years. J Rheumatol. 2015;42:1761–1766. doi: 10.3899/jrheum.150051. [DOI] [PubMed] [Google Scholar]
- 27.Gottenberg JE, Ravaud P, Bardin T, et al. Risk factors for severe infections in patients with rheumatoid arthritis treated with rituximab in the autoimmunity and rituximab registry. Arthritis Rheum. 2010;62:2625–2632. doi: 10.1002/art.27555. [DOI] [PubMed] [Google Scholar]
- 28.Tony HP, Burmester G, Schulze-Koops H, et al. Safety and clinical outcomes of rituximab therapy in patients with different autoimmune diseases: experience from a national registry (GRAID) Arthritis Res Ther. 2011;13:R75. doi: 10.1186/ar3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Furer V, Eviatar T, Zisman D, et al. Predictors of immunogenic response to the BNT162b2 mRNA COVID-19 vaccination in patients with autoimmune inflammatory rheumatic diseases treated with rituximab. Vaccines (Basel) 2022;10:901. doi: 10.3390/vaccines10060901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Md Yusof MY, Vital EM, McElvenny DM, et al. Predicting severe infection and effects of hypogammaglobulinemia during therapy with rituximab in rheumatic and musculoskeletal diseases. Arthritis Rheumatol. 2019;71:1812–1823. doi: 10.1002/art.40937. [DOI] [PubMed] [Google Scholar]
- 31.Avouac J, Miceli-Richard C, Combier A, et al. Risk factors of impaired humoral response to COVID-19 vaccination in rituximab-treated patients. Rheumatology (Oxford) 2022;61:SI163–SI178. doi: 10.1093/rheumatology/keab815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moor MB, Suter-Riniker F, Horn MP, et al. Humoral and cellular responses to mRNA vaccines against SARS-CoV-2 in patients with a history of CD20 B-cell-depleting therapy (RituxiVac): an investigator-initiated, single-centre, open-label study. Lancet Rheumatol. 2021;3:e789–e797. doi: 10.1016/S2665-9913(21)00251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liew J, Gianfrancesco M, Harrison C, et al. SARS-CoV-2 breakthrough infections among vaccinated individuals with rheumatic disease: results from the COVID-19 Global Rheumatology Alliance provider registry. RMD Open. 2022;8 doi: 10.1136/rmdopen-2021-002187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calabrese C, Kirchner E, Villa-Forte A, et al. Early experience with tixagevimab/cilgavimab pre-exposure prophylaxis in patients with immune-mediated inflammatory disease undergoing B cell depleting therapy and those with inborn errors of humoral immunity. RMD Open. 2022;8 doi: 10.1136/rmdopen-2022-002557. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data underlying this paper are available in the Article and online supplementary material. Upon justifiable request, de-identified data are available from the corresponding author.