Abstract
Objective:
Recent clinical practice guidelines recommend the delivery of evidence-based psychotherapies for both substance use disorder (SUD) and posttraumatic stress disorder (PTSD) within the same treatment episode for patients with SUD/PTSD comorbidity. This randomized clinical trial evaluated the comparative effectiveness of integrating versus phasing evidence-based psychotherapies for SUD and PTSD among veterans with co-occurring SUD/PTSD.
Method:
183 veterans with DSM-IV PTSD and SUD at two VA Medical Centers were randomized to one of two psychotherapies during which Motivational Enhancement Therapy [MET] for SUD and Prolonged Exposure [PE] for PTSD were either phased or integrated throughout treatment. Primary outcomes as evaluated by blinded assessors were percent days with drug use or heavy drinking and PTSD symptomology. We hypothesized integrated MET/PE (n = 95) would yield better SUD and PTSD-related outcomes at posttreatment than phased MET/PE (n = 88).
Results:
In intent-to-treat analyses (n=183), both treatment groups achieved clinically (d=0.46 – 1.06) and statistically significant reductions in SUD (p < 0.01) and PTSD (p < 0.01) symptomology; the time by treatment interactions were not significant. Post-hoc analyses could not confirm statistical non-inferiority; between-group effect sizes suggest a lack of clinically-meaningful differences between the two treatment approaches (d=0.08 – 0.27).
Conclusions:
Our hypothesis that integrated MET/PE would result in better outcomes than phased MET/PE across a range of PTSD and SUD measures was not supported; both strategies for combining two single-disorder treatments for co-occurring SUD/PTSD yielded significant symptom reduction.
Keywords: Substance use disorders, Posttraumatic stress disorder, Veterans, Psychotherapy
1. Introduction
Substance use disorders (SUD) and posttraumatic stress disorder (PTSD) are two of the most commonly diagnosed mental health conditions within the Department of Veterans Affairs (VA) and rates of comorbidity between the two conditions are high (Seal et al., 2007; Watkins et al., 2011). Approximately 25% of veterans entering out-patient VA PTSD clinics have a SUD diagnosis and about one-third of those in SUD clinics meet criteria for PTSD (National Center for PTSD, 2010; VA Northeast Program Evaluation Center (NEPEC), 2013). Evidence-based psychotherapies (EBPs) exist for both conditions (VA/Department of Defense [DoD], 2015; 2017). Motivational Enhancement Therapy (MET) has strong evidence for its utility and has been found to be as effective as more complex SUD interventions (Project MATCH Research Group1, 1998; VA/DoD, 2015). Prolonged exposure (PE) has demonstrated large, clinically-meaningful reductions in PTSD symptomology (Steenkamp et al., 2015). As such, both MET for SUD and PE for PTSD have been disseminated throughout VA (Drapkin et al., 2016; Karlin et al., 2010).
Questions remain regarding how to apply these single-disorder EBPs to those with co-occurring SUD/PTSD. The VA/DoD SUD and PTSD Clinical Practice Guidelines explicitly discourage treating SUD at the exclusion of comorbidities such as PTSD or delaying PTSD treatment due to the presence of SUD. Rather, they recommend the delivery of EBPs for both conditions within the same treatment episode (VA/DoD, 2017, 2015). Integrated psychotherapies that include elements of EBPs for both PTSD and SUD show promise for concurrently treating both conditions (Roberts et al., 2015; Simpson et al., 2017); however, these treatment have not yet been broadly disseminated. Thus, guidance regarding how to most effectively combine the more widely disseminated single-disorder EBPs is urgently needed.
The literature points to two strategies for delivering multiple EBPs for patients with comorbidities: a phased approach (i.e., augmented or sequential) in which single-condition EBPs are delivered consecutively within the same treatment episode, or an integrated approach in which single-condition EBPs are either combined into one treatment or delivered concurrently. Phased applications have demonstrated mixed effects, with some finding no benefit for either diagnosis as compared to single-disorder treatments (e.g., Galovski et al., 2016; Haller et al., 2016) and others demonstrating improved outcomes for both conditions (e.g., Angelakis and Nixon, 2013). Integrated approaches have been more widely applied, both for the treatment of comorbid SUD/PTSD and other frequently co-occurring conditions (e.g., Acierno et al., 2016). Overall, integrated treatments have yielded better outcomes for at least one of the two co-occurring conditions than single-disorder treatments (e.g., Back et al., 2019; Wolitzky-Taylor et al., 2018). Two recent systematic reviews specifically examining integrated treatment for PTSD and SUD both concluded that trauma-focused integrated treatments result in greater reductions in PTSD and SUD symptoms at follow-up than SUD-only treatments (Roberts et al., 2015; Simpson et al., 2017); further, trauma-focused integrated approaches yield better PTSD-related outcomes than non-trauma-focused therapies (e.g., skills-based approaches; Norman et al., 2019; Roberts et al., 2015) The comparative effectiveness of phasing versus integrating two single-disorder EBPs for SUD and PTSD has not previously been studied.
We conducted a randomized controlled trial (RCT) to evaluate the effectiveness of integrating versus phasing two single-disorder EBPs for treating veterans with co-occurring SUD/PTSD. Veterans were randomized to one of two psychotherapy conditions that included the same treatments for SUD and PTSD (MET and PE respectively) but differed by whether the components were phased in delivery or integrated such that PTSD and SUD symptoms were both addressed in each session. We hypothesized that veterans who received integrated MET/PE would demonstrate greater improvements in SUD and self-reported PTSD symptomology than veterans assigned to phased MET/PE. We further hypothesized that veterans assigned to integrated MET/PE would demonstrate greater reductions in a range of secondary outcomes (e.g., depression, quality of life, and alternative drinking measures). Finally, given evidence suggesting patients’ preference for targeting SUD/PTSD symptoms together in treatment (Back et al., 2014, 2006a), we expected that higher levels of veteran retention in the integrated MET/PE condition would contribute to superior outcomes.
2. Methods
2.1. Design
This was a two-arm parallel group RCT conducted at two US VA Medical Centers and registered at ClinicalTrials.gov (NCT01211106). Procedures were approved by the Institutional Review Boards of the Minneapolis and Philadelphia VA Medical Centers and all participants provided written informed consent.
2.2. Participants
Participants were 183 veterans recruited from February 2011 to June 2015 through a variety of channels, including provider referrals and advertisements in VA medical centers. Inclusion criteria included (a) DSM-IV (American Psychiatric Association, 2000) diagnosed PTSD, (b) DSM-IV diagnosis of a current substance use disorder other than nicotine and marijuana dependence, (c) PTSD Checklist for DSM-IV (PCL; (Weathers et al., 1993) score ≥ 50, and (d) alcohol or drug use at least 10 out of 30 days prior to enrollment. Exclusion criteria included (a) imminent suicidal or homicidal ideation, (b) current diagnosis of bipolar affective disorder or psychotic disorder, (c) unstable or serious medical illness, (d) treatment interfering cognitive impairments, (e) participation in PE in the past 6 months, (f) psychotherapy program initiation in the past two months, (g) current participation in a formal addiction treatment program, (h) past month change in psychotropic medication, and (i) use of a benzodiazepine greater than the equivalent of 40 mg of diazepam.
2.3. Procedures
The Substance Use Disorders and Posttraumatic Stress Disorder Modules of the Structured Clinical Interview for DSM-IV (First et al., 2002), Timeline Follow Back Interview (TLFB; Sobell and Sobell, 1992), and PCL were administered during in-person screening to determine study eligibility. The Mini International Neuropsychiatric Interview (MINI) assessed for comorbid mental health diagnoses (Lecrubier et al., 1997). Within 30 days of screening, eligible participants attended a second visit at to complete additional baseline assessments and be randomized to treatment condition. Randomization was allocated at a 1:1 ratio and stratified by site, illicit drug use, severity of PTSD symptoms, and veteran service era. Immediately following randomization, veterans met with their therapist for their first treatment session. Assessments occurred at baseline; treatments weeks 4, 8, and 12; posttreatment; and six-months posttreatment. Unless otherwise noted, all assessments were administered at each time point by study staff blinded to intervention condition. Three clinical psychologists, one masters level social worker, and one registered nurse with extensive mental health research experience administered diagnostic interviews; bachelors and masters level study staff delivered the TLFB. All assessors received initial and ongoing training on administration from study investigators.
2.4. Measures
2.4.1. Primary outcomes
The TLFB interview was used as the primary SUD outcome. The TLFB was used to calculate the percentage of days over the past twenty-eight with either illicit drug use or heavy drinking (five or more standard drinks in a single day for men, four or more for women). Prescription drugs used longer, more often, or at a higher dose than prescribed were recorded as illicit. The PCL was the primary PTSD outcome measure. The PCL is a self-report measure that evaluates DSM-IV PTSD symptom severity in the prior month, with higher scores indicating greater severity.
2.4.2. Secondary outcomes
The Short Inventory of Problems (SIP-R; Bender et al., 2007), a measure of adverse interpersonal, physical, social, impulsive, and intrapersonal consequences of substance abuse, was administered at the baseline, posttreatment, and six-month follow-up. PTSD symptoms were also assessed at baseline and posttreatment by the PTSD Symptom Scale-Interview Version (PSS-I; Foa et al., 1993), a clinician-administered interview that corresponds to DSM-IV PTSD symptom criteria. Depressive symptoms were assessed with the Patient Health Questionnaire – 9 (PHQ-9; (Kroenke and Spitzer, 2002), mental health quality of life was measured via the Mental Component subscale (MCS) of the Medical Outcomes Study Short Form (SF-12; Gandek et al., 1998; Jenkinson et al., 1997), and state anxiety was assessed by the State-Trait Anxiety Inventory–State Subscale (STAI; (Spielberger, 2010).
2.5. Treatments
2.5.1. Treatment overview
Both conditions consisted of sixteen 90-minute psychotherapy sessions during which full courses of MET and PE were delivered. PE is an evidence-based psychotherapy for PTSD that includes in vivo (exposure to trauma-related safe situations, objects, or people that cause distress and are avoided) and imaginal (revisiting and processing of the trauma memory) exposure (Foa et al., 2007). MET is an evidence-based, structured adaptation of motivational interviewing that encourages change through the structured assessment and feedback of SUD behaviors (Miller, 1995). VA’s online MET Assessment & Feedback Tool was used to facilitate assessment and feedback. In both conditions, MET and PE were provided by the same therapist. Treatment was to be completed within 20 weeks of randomization, although protocol violations were allowed; 17 participants attended one, and one participant attended two sessions beyond the 20-week mark.
MET training included either a one and one-half day in person or one-on-one remote training that included videotaped practice with a member of the Motivational Interviewing Network of Trainers. Therapists also completed four days of PE training and supervision of two cases. Weekly case consultation was led by experts in PE and MET. Treatment sessions were videotaped and 107 tapes (7.4%) were coded to ensure adherence to the treatment conditions and delivery competence; at least 25 sessions were randomly selected from each of the following groups: sessions 1–4, session 5, sessions 6–9, and sessions 10 and above. Sessions were rated by experienced PE fidelity assessors at the Center for the Treatment and Study of Anxiety and a study investigator (DVH) using established methods for assessing PE fidelity and the Yale Adherence and Competence Scale for assessing MET fidelity (Carroll, 2000). Three sessions were rated by all assessors and discussed until consensus was reached to establish interrater reliability.
2.5.2. Phased MET/PE
Treatment began with four 45-minute weekly MET sessions. Health education content was also presented for 45 min in the first four sessions to ensure equal therapist contact in the two conditions. A 12-session course of PE began at session five and continued through the end of treatment. During PE, participants received a brief SUD-check in at the beginning of each treatment session. If the check-in indicated that in the clinicians’ judgement additional attention to SUD-related symptoms was required, those issues were addressed within a MET framework (occurred in 10.6% of rated therapy sessions) before continuing with the PE components. If the SUD check-in did not indicate additional attention was needed, addiction was discussed only as it related to PE delivery.
2.5.3. Integrated MET/PE
All integrated MET/PE sessions included MET and PE components. Sessions one through four included the same MET content as in the phased MET/PE condition. Starting with session five, the MET portion focused on current substance use and progress towards goals; during the final sessions, posttreatment plans were discussed. PE components were delivered as outlined in the PE treatment manual (including initiation of imaginal exposure and processing in session three). PTSD and SUD were conceptually linked during the treatment process (e.g., veterans reflected on how their use related to their PTSD; processing included discussion of the relationship between trauma and use).
2.6. Data analysis
To test our primary hypotheses, we performed separate mixed-effects regression analyses, including time, treatment group, time by treatment groups interaction, outcome measured at baseline, and site as predictors. The two measures were analyzed using an information-criterion-selected covariance matrix for the repeated measures within subject. We then tested for group by time interactions. To address missing data, we computed the probability that participants provided complete data for the outcome of interest and then used those predicted probabilities as inverse probability weights in reruns of the analyses for the main hypotheses (Fitzmaurice et al., 2012). These models did not significantly change the results for the primary outcomes; therefore, we present findings from the original models. We had over 80% power to detect a standardized main effect of d = 0.40 for either outcome and a standardized linear group by time interaction effect of d = 0.45.
To assess differences at six-months posttreatment, the analyses described above extended the treatment phase mixed effects models for PTSD and SUD to include the six-month follow-up and an inflection point at the end of treatment. Models similar to those described for the primary outcomes and six-month follow-up assessment were used to examine the secondary outcomes administered at all assessment time-points. Generalized linear mixed effects models were chosen according to the response distributions. We compared the groups on posttreatment clinician-assessed PTSD using a linear mixed model, with baseline and posttreatment PSS-I score as a repeated response and included time and intervention group as binary factors, their interaction as a predictor, and site as a covariate. To test the secondary anxiety measure, we first used the strategy employed to test clinician-assessed PTSD symptomology; to examine the six-month follow-up, the analyses were rerun with the baseline scores included as a covariate and time treated as a categorical variable. Finally, within and between group standardized effect sizes were calculated for all outcome measures at posttreatment as six-month follow-up for the full sample and for treatment completers (attended at least twelve treatment sessions and thus received at least eight PE sessions in both conditions).
3. Results
3.1. Sample description and patient flow
368 veterans consented and attended the baseline assessment; 183 (49.7%) were randomized and attended at least one treatment session (see Fig. 1). Table 1 describes the two groups’ demographic and baseline characteristics. Most (85.2%) of participants were diagnosed with alcohol dependence, 8.7% met criteria for alcohol abuse but not dependence, and 6.0% were not diagnosed with an alcohol use diagnosis (e.g., met criteria for drug dependence only). No participants met criteria for drug abuse; 18% were diagnosed with drug dependence (stimulant [n=26] and opioid use [n = 8] disorders were most common).
Fig. 1.
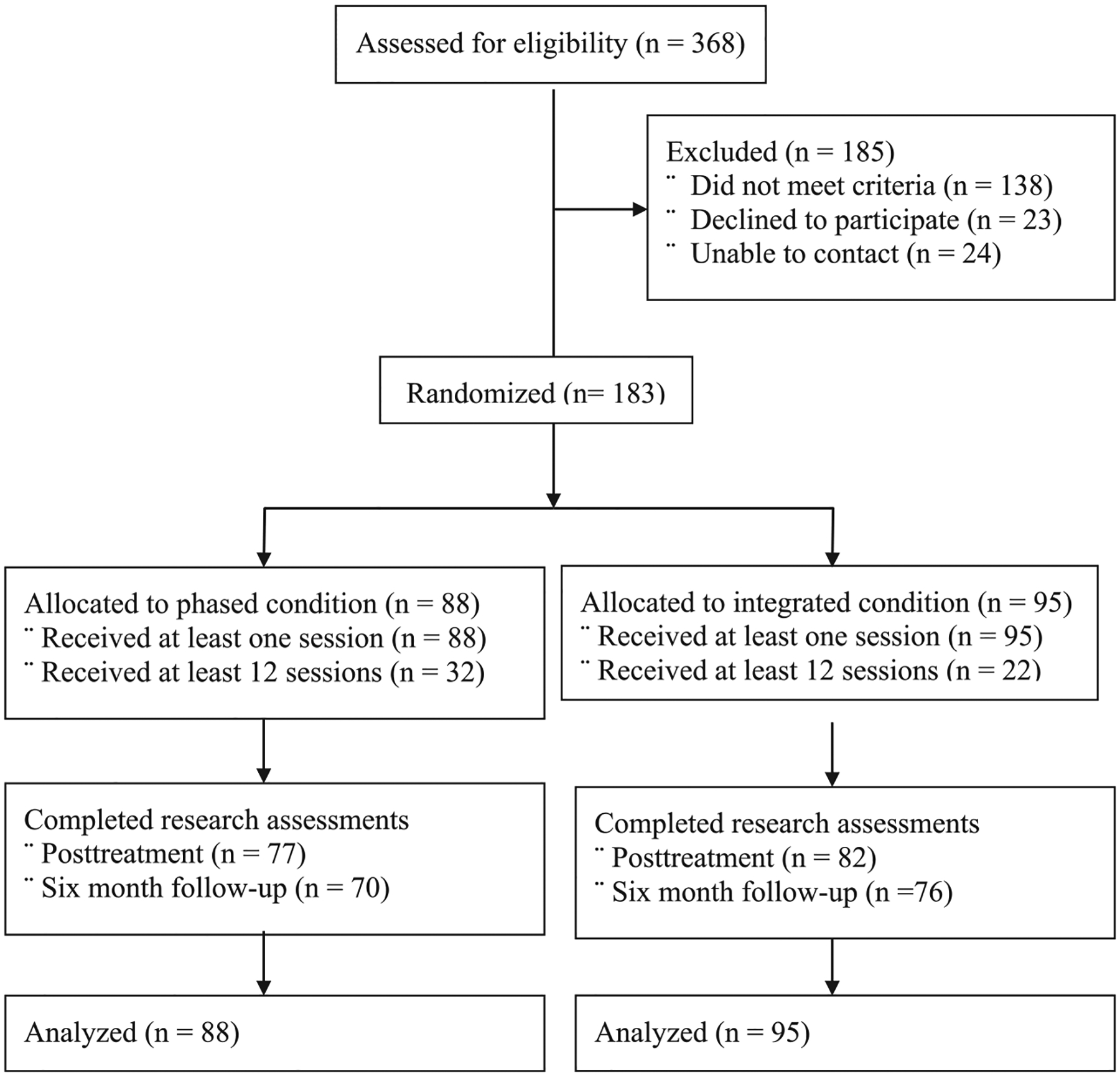
Flow of participants through the study.
Table 1.
Sample characteristics by treatment condition.
| Integrated Condition | Phased Condition | |||||
|---|---|---|---|---|---|---|
| Variable | N or M | % or SD | n or M | % or SD | Test Statistic | P |
| Age | 44.4 | 13.1 | 43.8 | 13.0 | t(181) = 0.35 | 0.73 |
| Gender, Male | 90 | 94.7 | 79 | 89.8 | χ2 = 1.59 | 0.207 |
| Race | χ2 = 0.34 | 0.561 | ||||
| White | 37 | 38.9 | 38 | 43.2 | ||
| Black / African American | 43 | 45.2 | 45 | 51.1 | ||
| Native Hawaiian / Pacific Islander | 1 | 1.1 | 1 | 1.1 | ||
| Multiracial | 2 | 2.1 | 0 | 0.0 | ||
| Ethnicity, Hispanic | 7 | 7.4 | 2 | 2.3 | χ2 = 2.54 | 0.111 |
| Marital Status, Married | 44 | 46.3 | 23 | 26.1 | χ2 = 8.02 | 0.005 |
| Service Era, Iraq or Afghanistan | 64 | 67.3 | 58 | 65.9 | χ2 = 0.04 | 0.834 |
| PTSD Checklist | 62.5 | 11.3 | 63.6 | 10.3 | t(181) = −0.66 | 0.513 |
| % Days Drug Use or Heavy Drinking* | 56.8 | 27.3 | 59.7 | 27.7 | t(181) = −0.70 | 0.483 |
| PTSD Symptom Scale Interview | 35.8 | 6.9 | 36.9 | 6.4 | t(181) = −1.12 | 0.263 |
| Patient Health Questionnaire - 9 | 14.7 | 5.8 | 16.1 | 5.5 | t(181) = −1.75 | 0.083 |
| SF-12 Mental Component | 30.9 | 9.4 | 27.3 | 9.8 | t(181) = 2.52 | 0.013 |
| Short Inventory of Problems - Revised** | 16.4 | 10.6 | 19.5 | 10.6 | t(180) = −2.00 | 0.047 |
| State Trait Anxiety Inventory – State | 51.67 | 10.69 | 51.82 | 11.62 | t(180) = 0.09 | 0.931 |
Over prior 28 days; heavy drinking defined as five or more drinks in a single day for men, four or more for women;
n = 182 for this variable.
3.2. Treatment adherence
Therapists delivered PE in 94.8% of rated sessions required to have PE components and MET in 100% of rated sessions required to include MET delivery. PE adherence/competence was rated at 2.56 (0.71) on a 0–3 Likert scale, indicating good to excellent overall adherence/competence. MET competence was rated at 5.73 (0.64) on a 1–7 Likert scale, indicating good to very good MET skill. Ratings did not differ by treatment arm. There was excellent differentiation between the two treatment approaches. Both PE and MET components were delivered in 93.8% of integrated sessions one through four; conversely, in phased MET/PE sessions one through four, 100% of sessions included MET and none included the assessment or treatment of PTSD.
Participants completed an average of 8.73 (4.35) therapy sessions. The integrated (M=8.35 [4.15]) and phased (M = 9.16 [4.55]) conditions did not differ in the number of treatment sessions attended (p = 0.21). An equivalent percentage of veterans in the integrated (23.2%) and phased (36.4%) conditions completed treatment, although there was a trend toward higher rates of completion in the phased condition (p=0.05).
3.3. Treatment outcomes
3.3.1. Primary outcomes
There was a time-dependent decrease in percentage days with drug use or heavy drinking for all groups (p < 0.01); however, there group by time interaction was not significant (see Fig. 2). Across both conditions, the mean percentage days with drug use or heavy drinking decreased by 25.76 (34.07). Few veterans in phased MET/PE reported at least two weeks of abstinence prior to the start of PE (e.g., immediately prior to session five; 8.3%) and the percentage who achieved abstinence during that time did not differ by treatment group (p=0.890). A similar pattern emerged for PCL-assessed PTSD symptomology; symptoms decreased for all groups (p < 0.01), but there was no significant group by time interactions (see Fig. 2). Across both conditions, PCL scores decreased 8.11 (14.74) points from pre- to posttreatment.
Fig. 2.
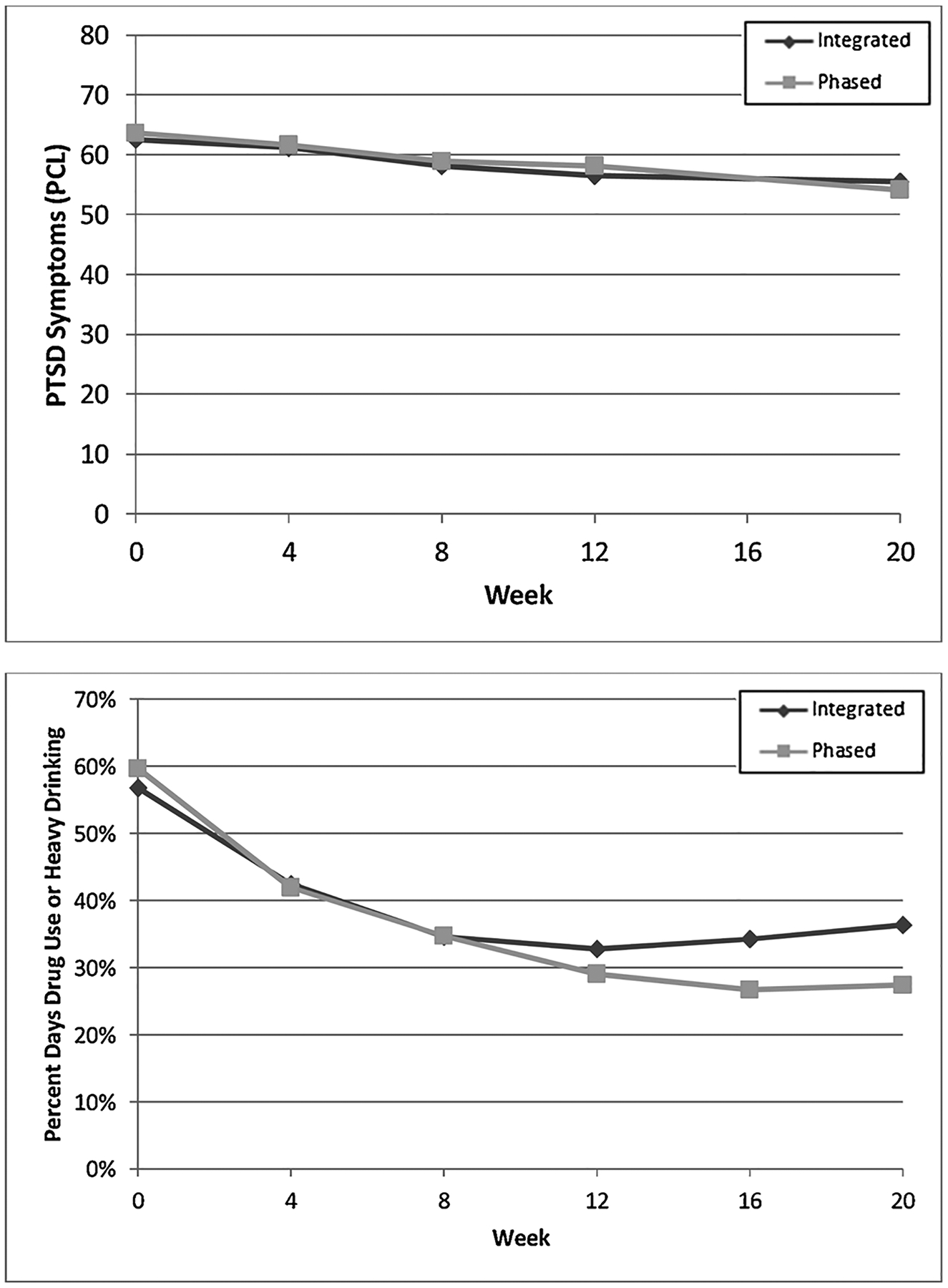
PTSD and SUD symptomology for the integrated and phased treatment groups throughout treatment.
3.3.2. Six-month follow-up
Percent days with drug use or heavy drinking had a significant inflection point after end of treatment (p < 0.01), and there was a change in percent days with drug use or heavy drinking between end of treatment and the six-month follow-up (p = 0.02). Thus, veterans showed continued improvement (although at a slower rate than during treatment) in the primary SUD outcome from posttreatment to six-month follow-up. There was no significant interaction between time and treatment (p = 0.20). The PCL had a significant inflection point after end of treatment (p < 0.01), demonstrating there was no change in the PCL between end of treatment and the six-month follow-up (p = 0.45). The time by treatment condition interaction was not significant (p = 0.69).
3.3.3. Secondary outcomes
While there was a decrease in clinician assessed PTSD symptomology (PSS-I) from pre- to post-treatment (p < 0.01), there was not a difference in the rate of decrease between the treatment groups (p = .76). From baseline to posttreatment there was a decreasing time trend for depression (PHQ-9), mental health functioning (SF-12 MCS), consequences of substance use (SIP-R), and anxiety (STAI; p < 0.01 for all analyses). There were no significant group by time interactions for these outcomes. Adding the six-month follow-up time point to the models, there was a significant inflection point at end of treatment for the SF-12 MCS (p < 0.01). There was no interaction between time and treatment after end of treatment for any of these secondary outcomes.
3.3.4. Clinically significant change
In the intent-to-treat sample, there were few clinically-significant differences between the two conditions (using d=0.25 as a minimum clinically-significant difference), and those that did exist were small in magnitude and did not consistently favor one condition over the other (Table 2). We also examined Cohen’s ds for integrated and phased MET/PE treatment completers. Within group effect sizes were larger for all outcomes, including posttreatment PCL-measured PTSD symptomology (d = 0.77 – 0.80) and percent days with heavy drinking or drug use (d=1.01–1.20). There were no clinically-significant between group differences in the completers’ sample for the primary outcomes; several small to moderate differences in secondary outcomes consistently favored integrated MET/PE.
Table 2.
Within and Between Condition Effect Sizes for Primary and Secondary Treatment Outcomes for Intent-to-Treat Sample.
| Integrated Condition | Phased Condition | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Posttreatment | Six Month Follow-Up | Posttreatment | Six Month Follow-Up | |||||||||||
| Variable | n | M (SD) | d | n | M (SD) | d | n | M (SD) | d | n | M (SD) | d | Between Group Posttreatment d | Between Group Six Month Follow-Up d |
| PTSD Checklist | 82 | 55.50 (17.57) | 0.46 | 76 | 56.57 (17.96) | 0.38 | 71 | 54.12 (17.86) | 0.64 | 70 | 53.39 (17.22) | 0.71 | 0.08 | 0.18 |
| % Days Drug Use or Heavy Drinking* | 82 | 36.4 (34.5) | 0.67 | 75 | 28.14 (34.10) | 0.93 | 77 | 27.41 (29.9) | 1.06 | 70 | 22.2 (28.3) | 1.25 | 0.27 | 0.20 |
| PTSD Symptom Scale Interview | 80 | 26.01 (12.79) | 0.99 | – | – | – | 77 | 26.43 (12.70) | 1.05 | – | – | – | −0.03 | – |
| Patient Health Questionnaire - 9 | 82 | 12.62 (6.29) | 0.30 | 76 | 12.83 (6.73) | 0.26 | 77 | 13.94 (6.85) | 0.37 | 69 | 13.25 (7.15) | 0.42 | −0.20 | −0.06 |
| Medical Outcomes Study Short Form (SF-12) Mental Component | 81 | 35.93 (13.67) | −0.38 | 76 | 34.82 (13.78) | −0.29 | 77 | 32.43 (12.85) | −0.46 | 70 | 33.38 (14.29) | −0.50 | 0.26 | 0.10 |
| Short Inventory of Problems - Revised | 81 | 18.35 (13.34) | −0.16 | 76 | 17.88 (13.92) | −0.13 | 76 | 19.26 (12.08) | 0.00 | 69 | 14.13 (12.48) | 0.50 | −0.07 | 0.28 |
| State Trait Anxiety Inventory – State Subscale | 81 | 48.49 (15.23) | 0.30 | 76 | 48.64 (15.25) | 0.30 | 76 | 48.87 (14.75) | 0.20 | 59 | 46.41 (15.29) | 0.48 | −0.03 | 0.15 |
Over prior 28 days; heavy drinking defined as five or more drinks in a single day for men, four or more for women.
3.3.5. Post-hoc analyses
We performed post-hoc non-inferiority tests of integrated MET/PE versus phased MET/PE on posttreatment TLFB and PCL. To account for performing two tests, we used a 2.5% significance level for both analyses. Since higher scores indicate poorer outcomes, the test of non-inferiority corresponds to testing whether the upper limit of a 95% confidence interval on the group difference is below a threshold value (Piaggio et al., 2006). For interpretability, we set a value of d = 0.3 for the between group effect. For the (square-root) of the TLFB response, the upper limit of the confidence interval was d = 0.50, and for the PCL response was d = 0.34. Thus, we cannot reject the inferiority hypothesis for either primary outcome.
4. Discussion
Contrary to our hypotheses, integrated MET/PE did not yield greater reductions in SUD and PTSD symptomology than phased MET/PE. Further, between-group effect sizes suggest a lack of clinically-meaningful differences between the two approaches. To the best of our knowledge, this is the first examination of how to best combine two single-disorder EBPs for individuals with comorbid disorders. Our findings suggest that the two most common approaches to combining EBPs for individuals with comorbidity yield similar – although not necessarily equivalent - results in the case of delivery of PE and MET to patients with SUD/PTSD.
Unexpectedly, we did not find a difference in the number of sessions attended in the integrated and phased conditions. Although the difference in dropout between the two conditions fell just under the threshold of statistical significance, there was a 50% higher completion rate for the phased group. This signal should be examined in future research; it is possible that patients in phased treatment may have greater self-efficacy or a stronger therapeutic alliance prior to approaching trauma content, facilitating greater completion. Rates of treatment dropout in both conditions were high. Similarly high levels have been observed among other trauma-focused treatments for SUD/PTSD (Brady et al., 2001; Mills et al., 2012). Since treatment effects were larger among treatment completers, improving patient engagement must be a priority. Applying contingency management to PE adherence in patients with comorbid SUD/PTSD (Schacht et al., 2017) and delivering PE in massed formats (Foa et al., 2018; Norman et al., 2016) hold promise for facilitating completion.
Participants in both conditions achieved moderate to large improvements in PTSD and SUD symptomology. The 25% reduction achieved in the primary SUD outcome compared favorably with other recent studies integrating evidence-based treatments for SUD/PTSD (Back et al., 2019; Norman et al., 2019). Studies examining dual-focus SUD/PTSD treatments have consistently found trauma-focused interventions to have similar posttreatment SUD outcomes as SUD-only treatment, suggesting that shared focus during the treatment episode does not negatively impact SUD outcomes (Back et al., 2019; Norman et al., 2019; Roberts et al., 2015). Relatedly, in the current study, the initiation of imaginal exposure did not appear to impact progress on SUD-related goals; only a small minority of participants achieved abstinence prior to initiating imaginal exposure and SUD symptoms continued to improve following its initiation.
Clinician-assessed improvement of PTSD was comparable to prior trials in veteran samples (e.g., Schnurr et al., 2007); however, decreases in self-reported PTSD symptoms were more modest than have been previously seen in veterans without co-occurring SUD (e.g., Eftekhari et al., 2013). Effect sizes in the intent-to-treat sample were likely impacted by the high level of treatment dropout. PE is intended to be of variable length, with eight sessions suggested as the minimum (Foa et al., 2007); thus, relatively few patients, particularly in the phased condition, received a full course. However, within group treatment effects for self-reported PTSD symptoms among treatment completers were also smaller than reported among non-substance dependent completers (e.g., Eftekhari et al., 2013). Data regarding the effectiveness of PE among those with SUD diagnoses are not yet conclusive as to whether the effects of PE are attenuated in this complex population. For example, in a trial examining PE combined with naltrexone among individuals with PTSD and alcohol use disorders, PE un-characteristically did not differ from supportive counseling in its effects on posttreatment PTSD symptoms, although it did result in large magnitude improvements (Foa et al., 2013). Conversely, several studies utilizing PE in patients with SUD/PTSD comorbidity have demonstrated effects that mirror those found in non-SUD PTSD samples (e.g., Back et al., 2019; Coffey et al., 2016). Continued research examining the effectiveness of PE among patients with comorbid PTSD/SUD, and potential moderators of effectiveness, is warranted.
We recommend that future research examine the effectiveness of other frontline single-disorder EBPs used in combination for the treatment of SUD/PTSD. PE and MET were selected due to their inclusion in the VA/DoD Clinical Practice Guidelines and their dissemination within VA; however, other single-disorder EBPs may be as or more effective among those with SUD/PTSD. Further, MET with a flexible number of sessions designed to reflect patients’ progress (rather than the standard four session protocol) may enhance outcomes in this complex population. We also recommend comparing the effectiveness of integrated treatments specifically developed for the comorbidity to single-disorder EBPs combined for use such as in the current study. Given the low level of treatment completion in ours and other studies of SUD/PTSD, having multiple treatment options to facilitate shared decision making may be important in improving outcomes. Finally, research designed to understand patients’ reasons for early treatment discontinuation and continued study of adherence interventions is needed.
The strengths and weaknesses of our study design have been outlined elsewhere (Kehle-Forbes et al., 2016); however, several limitations warrant additional consideration. We had few women veterans, Hispanic veterans, and drug-only users in our sample; caution should be applied in generalizing our findings to these populations. The high treatment dropout rate must be considered a study limitation and caution must be exercised in interpreting findings from the completer subsample. Given that MET encompasses a style of interaction in addition to specific treatment elements, it is possible that the MET style was present during the phased condition’s brief SUD check-in. Finally, because we did not have a control group, we cannot be certain that the observed improvements were not due to outside factors.
5. Conclusions
Our hypotheses that integrated MET/PE would result in better outcomes than phased MET/PE across a range of PTSD and SUD measures were not supported. Further, between group effect sizes suggest a lack of clinically-important differences between the two approaches. We recommend that clinicians seeking to deliver care consistent with the VA/DoD PTSD and SUD Clinical Practice Guidelines by delivering single-disorder EBPs within the same treatment episode make the decision as to whether to phase or integrate individual EBPs in collaboration with their patients.
Acknowledgements
This material is based upon work supported by Merit Review Award # ZDA1-03-W10 from the United States (U.S.) Department of Veterans Affairs, Clinical Science Research & Development. Dr. Kehle-Forbes was supported by a Health Services Research & Development Career Development Award (09-020). This material is the result of work supported with resources and the use of facilities at the Mental Illness Research, Education, and Clinical Center at the Corporal Michael J. Crescenz VA Medical Center (CMCVAMC) and the Minneapolis VA Healthcare System. The funder had no involvement in the study design; collection, analysis, and interpretation of data; writing the report; or the decision to submit this article for publication. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Footnotes
Declaration of Competing Interest
Dr. Foa receives royalties from the sale of Prolonged Exposure Therapy for PTSD: Emotional Processing of Traumatic Experiences Therapist Guide, and Reclaiming your Life from a Traumatic Experience Workbook by Oxford University Press. She also receives payment for training she conducts on prolonged exposure therapy.
The remaining authors wish to confirm that there are no known conflicts of interest associated with this publication.
References
- Acierno R, Gros DF, Ruggiero KJ, Hernandez-Tejada MA, Knapp RG, Lejuez CW, Muzzy W, Frueh CB, Egede LE, Tuerk PW, 2016. Behavioral activation and therapeutic exposure for posttraumatic stress disorder: a noninferiority trials of treatment delivered in person versus home-based telehealth. Depress. Anxiety 33, 415–423. 10.1002/da.22476. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, 2000. Diagnostic and Statistical Manual of Mental Disorders, 4th edition - Text Revision (DSM-IV-TR). American Psychiatric Association, Washington, DC. [Google Scholar]
- Angelakis S, Nixon R, 2013. The treatment of posttraumatic stress disorder and major depressive disorder: the utility of a combined treatment approach. In: Presented at the 29th Annual Meeting of the International Society for Traumatic Stress Studies. Philadelphia, PA. [Google Scholar]
- Back SE, Brady KT, Jaanimägi U, Jackson JL, 2006. Cocaine dependence and PTSD: a pilot study of symptom interplay and treatment preferences. Addict. Behav 31, 351–354. 10.1016/j.addbeh.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Back SE, Killeen T, Badour CL, Flanagan JC, Allan NP, Santa Ana E, Lozano B, Korte KJ, Foa EB, Brady KT, 2019. Concurrent treatment of substance use disorders and PTSD using prolonged exposure: a randomized clinical trial in military veterans. Addict. Behav 90, 369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SE, Killeen TK, Teer AP, Hartwell EE, Federline A, Beylotte F, Cox E, 2014. Substance use disorders and PTSD: an exploratory study of treatment preferences among military veterans. Addict. Behav 39, 369–373. 10.1016/j.addbeh.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender RE, Griffin ML, Gallop RJ, Weiss RD, 2007. Assessing negative consequences in patients with substance use and bipolar disorders: psychometric properties of the short inventory of problems (SIP). Am. J. Addict 16, 503–509. 10.1080/10550490701641058. [DOI] [PubMed] [Google Scholar]
- Carroll K, 2000. A general system for evaluating therapist adherence and competence in psychotherapy research in the addictions. Drug Alcohol Depend. 57, 225–238. 10.1016/S0376-8716(99)00049-6. [DOI] [PubMed] [Google Scholar]
- Department of Veterans Affairs / Department of Defense, 2017. VA/DoD Clinical Practice Guidelines: Management of Posttraumatic Stress Disorder and Acute Stress Reaction. Department of Veterans Affairs and Department of Defense, Washington, D.C. [Google Scholar]
- Department of Veterans Affairs / Department of Defense, 2015. VA/DOD Clincal Practice Guideline for the Management of Substance Use Disorders, 3rd ed. Department of Veterans Affairs, Washington, D.C. [Google Scholar]
- Drapkin ML, Wilbourne P, Manuel JK, Baer J, Karlin B, Raffa S, 2016. National dissemination of motivation enhancement therapy in the Veterans Health Administration: training program design and initial outcomes. J. Subst. Abuse Treat 65, 83–87. 10.1016/j.jsat.2016.02.002. [DOI] [PubMed] [Google Scholar]
- Eftekhari A, Ruzek JI, Crowley JJ, Rosen CS, Greenbaum MA, Karlin BE, 2013. Effectiveness of national implementation of prolonged exposure therapy in veterans affairs care. JAMA Psychiatry 70, 949. 10.1001/jamapsychiatry.2013.36. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB, 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. New York, NY. [Google Scholar]
- Fitzmaurice GM, Laird NM, Ware JH, 2012. Applied Longitudinal Analysis. Wiley, Hoboken. [Google Scholar]
- Foa EB, Hembree E, Rothbaum BO, 2007. Prolonged Exposure Therapy for PTSD: Emotional Processing of Traumatic Experiences. Oxford University Press, Inc., New York, NY. [Google Scholar]
- Foa EB, McLean CP, Zang Y, Rosenfield D, Yadin E, Yarvis JS, Mintz J, Young-McCaughan S, Borah EV, Dondanville KA, et al. , 2018. Effect of prolonged exposure therapy delivered over 2 weeks vs 8 weeks vs present-centered therapy on PTSD symptom severity in military personnel: a randomized clinical trial. JAMA 319, 354–364. 10.1001/jama.2017.21242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa EB, Riggs DS, Dancu CV, Rothbaum BO, 1993. Reliability and validity of a brief instrument for assessing post-traumatic stress disorder. J. Trauma Stress 6, 459–473. 10.1002/jts.2490060405. [DOI] [Google Scholar]
- Foa EB, Yusko DA, McLean CP, Suvak MK, Bux DA, Oslin D, et al. , 2013. Concurrent naltrexone and prolonged exposure therapy for patients with comorbid alcohol dependence and PTSD: a randomized clinical trial. JAMA 310, 488–495. 10.1001/jama.2013.8268. [DOI] [PubMed] [Google Scholar]
- Galovski TE, Harik JM, Blain LM, Elwood L, Gloth C, Fletcher TD, 2016. Augmenting cognitive processing therapy to improve sleep impairment in PTSD: a randomized controlled trial. J. Consult. Clin. Psychol 84, 167–177. 10.1037/ccp0000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandek B, Ware JE, Aaronson NK, Apolone G, Bjorner JB, Brazier JE, Bullinger M, Kaasa S, Leplege A, Prieto L, Sullivan M, 1998. Cross-validation of item selection and scoring for the SF-12 health survey in nine countries. J. Clin. Epidemiol 51, 1171–1178. 10.1016/S0895-4356(98)00109-7. [DOI] [PubMed] [Google Scholar]
- Haller M, Norman SB, Cummins K, Trim RS, Xu X, Cui R, Allard CB, Brown SA, Tate SR, 2016. Integrated cognitive behavioral therapy versus cognitive processing therapy for adults with depression, substance use disorder, and trauma. J. Subst. Abuse Treat 62, 38–48. 10.1016/j.jsat.2015.11.005. [DOI] [PubMed] [Google Scholar]
- Jenkinson C, Layte R, Jenkinson D, Lawrence K, Petersen S, Paice C, Stradling J, 1997. A shorter form health survey: can the SF-12 replicate results from the SF-36 in longitudinal studies? J. Public Health 19, 179–186. [DOI] [PubMed] [Google Scholar]
- Karlin BE, Ruzek JI, Chard KM, Eftekhari A, Monson CM, Hembree EA, Resick PA, Foa EB, 2010. Dissemination of evidence-based psychological treatments for posttraumatic stress disorder in the Veterans Health Administration. J. Trauma Stress 23, 663–673. 10.1002/jts.20588. [DOI] [PubMed] [Google Scholar]
- Kehle-Forbes SM, Drapkin ML, Foa EB, Koffel E, Lynch KG, Polusny MA, Van Horn DHA, Yusko DA, Charlesworth M, Blasco M, Oslin DW, 2016. Study design, interventions, and baseline characteristics for the Substance use and TRauma Intervention for VEterans (STRIVE) trial. Contemp. Clin. Trials 50, 45–53. 10.1016/j.cct.2016.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, 2002. The PHQ-9: a new depression diagnostic and severity measure. Psychiatr. Ann 32, 509–515. 10.3928/0048-5713-20020901-06. [DOI] [Google Scholar]
- Lecrubier Y, Sheehan DV, Weiller E, Amorim P, Bonora I, Sheehan KH, Janavs J, Dunbar GC, 1997. The Mini International Neuropsychiatric Interview (MINI). A short diagnostic structured interview: reliability and validity according to the CIDI. Eur. Psychiatry 12 (5), 224–231. 10.1016/S0924-9338(97)83296-8. [DOI] [Google Scholar]
- Miller W, 1995. Motivational Enhancement Therapy Manual: A Clinical Research Guide for Therapists Treating Individuals With Alcohol Abuse and Dependence. DIANE Publishing, Coolingdale, PA. [Google Scholar]
- Mills KL, Teesson M, Back SE, Brady KT, Baker AL, Hopwood S, Sannibale C, Barrett EL, Merz S, Rosenfeld J, et al. , 2012. Integrated exposure-based therapy for co-occurring posttraumatic stress disorder and substance dependence: a randomized controlled trial. JAMA 308, 690–699. [DOI] [PubMed] [Google Scholar]
- National Center for PTSD, 2010. Report of (VA) Consensus Conference: Practice Recommendations for Treatment of Veterans With Comorbid Substance Abuse and PTSD.
- Norman SB, Davis BC, Colvonen PJ, Haller M, Myers US, Trim RS, Bogner R, Robinson SK, 2016. Prolonged exposure with veterans in a residential substance use treatment program. Cogn. Behav. Pract 23 (2), 162–172. 10.1016/j.cbpra.2015.08.002. [DOI] [Google Scholar]
- Norman SB, Trim R, Haller M, Davis BC, Myers US, Colvonen PJ, Blanes E, Lyons R, Siegel EY, Angkaw AC, et al. , 2019. Efficacy of integrated exposure therapy vs integrated coping skills therapy for comorbid posttraumatic stress disorder and alcohol use disorder: a randomized clinical trial. JAMA Psychiatry 76 (8), 791–799. 10.1001/jamapsychiatry.2019.0638. Published online April 24, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piaggio G, Elbourne DR, Altman DG, Pocock SJ, Evans SJW, CONSORT Group, for the, 2006. Reporting of noninferiority and equivalence randomized trials: an extension of the CONSORT statement. JAMA 295, 1152. 10.1001/jama.295.10.1152. [DOI] [PubMed] [Google Scholar]
- Project MATCH Research Group1, 1998. Matching alcoholism treatments to client heterogeneity: project MATCH three-year drinking outcomes. Alcohol. Clin. Exp. Res 22, 1300–1311. 10.1111/j.1530-0277.1998.tb03912.x. [DOI] [PubMed] [Google Scholar]
- Roberts NP, Roberts PA, Jones N, Bisson JI, 2015. Psychological interventions for post-traumatic stress disorder and comorbid substance use disorder: a systematic review and meta-analysis. Clin. Psychol. Rev 38, 25–38. 10.1016/j.cpr.2015.02.007. [DOI] [PubMed] [Google Scholar]
- Schacht RL, Brooner RK, King VL, Kidorf MS, Peirce JM, 2017. Incentivizing attendance to prolonged exposure for PTSD with opioid use disorder patients. J. Consult. Clin. Psychol 85, 689–701. 10.1037/ccp0000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnurr PP, Friedman MJ, Engel CC, Foa EB, Shea MT, Chow BK, Resick PA, Thurston V, Orsillo SM, Haug R, Turner C, 2007. Cognitive behavioral therapy for posttraumatic stress disorder in women: a randomized controlled trial. JAMA 297, 820–830. 10.1001/jama.297.8.820. [DOI] [PubMed] [Google Scholar]
- Seal KH, Bertenthal D, Miner CR, Sen S, Marmar C, 2007. Bringing the war back home: mental health disorders among 103 788 US veterans returning from Iraq and Afghanistan seen at Department of Veterans Affairs Facilities. Arch. Intern. Med 167, 476–482. [DOI] [PubMed] [Google Scholar]
- Simpson TL, Lehavot K, Petrakis IL, 2017. No wrong doors: findings from a critical review of behavioral randomized clinical trials for individuals with co-occurring alcohol/drug problems and posttraumatic stress disorder. Alcohol. Clin. Exp. Res 41, 681–702. 10.1111/acer.13325. [DOI] [PubMed] [Google Scholar]
- Sobell L, Sobell M, 1992. Timeline Follow-back in Measuring Alcohol Consumption. Springer, New York, NY. [Google Scholar]
- Spielberger C, 2010. State-trait Anxiety Inventory. Wiley Online Library. [Google Scholar]
- Steenkamp MM, Litz BT, Hoge CW, Marmar CR, 2015. Psychotherapy for military-related PTSD: a review of randomized clinical trials. JAMA 314, 489. 10.1001/jama.2015.8370. [DOI] [PubMed] [Google Scholar]
- VA Northeast Program Evaluation Center (NEPEC), 2013. The Long Journal Home XXII: Progress Report on Specialty PTSD Treatment FY 2013.
- Watkins KE, Pincus HA, Smith B, Paddock SM, Mannle TE Jr, Woodroffe A, Call C, 2011. Veterans Health Administration Mental Health Program Evaluation. St. Monica CA RAND Corp. TR-956-VHA Novemb. 19, 2012 [Google Scholar]
- Weathers F, Litz B, Herman D, Huska J, Keane T, 1993. The PTSD checklist (PCL): reliability, validity, and diagnostic utility. In: Presented at the Annual Meeting of the International Society for Traumatic Stress Studies. San Antonio, TX. [Google Scholar]
- Wolitzky-Taylor K, Krull J, Rawson R, Roy-Byrne P, Ries R, Craske MG, 2018. Randomized clinical trial evaluating the preliminary effectiveness of an integrated anxiety disorder treatment in substance use disorder specialty clinics. J. Consult. Clin. Psychol 86, 81–88. 10.1037/ccp0000276. [DOI] [PMC free article] [PubMed] [Google Scholar]


