Abstract
Purpose.
The association of recreational physical activity (RPA) with mortality is well established only for breast and colon cancers and few studies have evaluated relationships for exercising before and after diagnosis, across multiple disease sites. We examined the joint associations of pre- and post-diagnosis RPA with mortality in a cohort of 5,807 patients enrolled in the Data Bank and BioRepository at Roswell Park.
Methods.
Patients were classified into one of four activity categories (habitually active, increased activity after diagnosis, decreased activity after diagnosis, habitually inactive). Cox proportional hazards models were used to estimate the associations of activity status with mortality.
Results.
In comparison to patients who were habitually inactive, habitually active patients experienced a 39% decreased hazard of all-cause mortality (HR=0.61, 95% CI 0.54–0.69) and a 36% decreased hazard of cancer-specific mortality (HR=0.64, 95% CI 0.56–0.73). Previously inactive patients who began exercising after diagnosis experienced a 28% decreased hazard of all-cause (HR=0.72, 95% CI 0.59–0.89) and cancer-specific mortality (HR=0.72, 95% CI 0.57–0.91) in comparison to patients who remained inactive. Patients engaging in 3–4 sessions/week experienced the greatest survival advantages, but 1–2 sessions/week also yielded significant survival advantages in comparison to inactivity.
Conclusion.
Low-to-moderate frequency pre-and-post diagnosis RPA was associated with significantly decreased mortality in patients diagnosed with a variety of malignancies. These observations solidify the clinical and public health importance of the message that some regular activity is better than inactivity, which is particularly encouraging, given that cancer survivors can be overwhelmed by current daily physical activity recommendations.
Keywords: physical activity, survival, mortality
Introduction
As the number of cancer survivors in the US increases, their physical activity patterns are becoming an important focus of scientific inquiry, because maintenance or adoption of an active lifestyle has the potential to reduce morbidity and mortality and dramatically improve quality of life among this population [1]. Yet, inverse associations between pre- and post-diagnosis recreational physical activity (RPA) with mortality have only been well established for breast and colorectal cancer [2, 3], with mounting evidence for prostate cancer.
To date, surprisingly few reports have described the associations of RPA with cancer outcomes across multiple disease sites among men and women in the United States [4–11]. Furthermore, few studies have examined associations for both pre- and post-diagnosis RPA, and little is known about how changes in RPA relative to cancer diagnosis may be associated with survival. Given the growing burden of cancer in the aging population and the prevalence of physical inactivity among cancer patients and survivors, we sought to examine the associations of pre- and post-diagnosis RPA with mortality in a large cohort of men and women diagnosed with primary incident cancer at Roswell Park Comprehensive Cancer Center.
Methods
Study Population
We conducted the current analyses using patient data from the Roswell Park Data Bank and BioRepository (DBBR), an NCI cancer center support grant shared resource. Established in 2003, the DBBR obtains informed consent from newly diagnosed adult patients for the completion of an extensive epidemiological questionnaire, collection of a blood specimen, and permission to link epidemiological and bio-specimen data to additional clinical data. Herein, we included 5,807 participants diagnosed with a malignant tumor who enrolled in the DBBR between 2003 and 2016, and who completed their epidemiological questionnaire within 1 year after diagnosis (median time between diagnosis and questionnaire completion was 53 days). The protocols for the DBBR and the current analyses were approved by the Roswell Park Institutional Review Board and all DBBR participants provided informed consent.
Epidemiological Questionnaire
The DBBR questionnaire is an extensive, self-administered epidemiological survey designed to ascertain patient demographic and lifestyle factors, including RPA. The questionnaire includes a detailed assessment of medical history and RPA prior to enrollment (i.e., frequency, intensity, duration, and mode of RPA performed in the decade prior to study enrollment) as well as a brief assessment of “Current Lifestyle” and RPA at the time of questionnaire completion. Upon submission of the DBBR questionnaire, each completed survey is reviewed by a data manager and a dedicated research associate who re-contacts patients via telephone to clarify any data discrepancies prior to scanning data for storage. During the time of enrollment for the current study (1 January 2003 through 31 December 2016), 77.68% of all patients approached consented to enrollment in the DBBR.
Recreational Physical Activity
In the current analysis, we examined moderate-to-vigorous intensity aerobic recreational physical activities in association with cancer outcomes. Three activity exposures of interest were examined, including RPA in the decade prior to DBBR enrollment (i.e., pre-diagnosis RPA), RPA within one year after diagnosis (i.e., post-diagnosis RPA), and a derived joint exposure variable (i.e., habitual RPA), our primary analytic exposure of interest.
Pre-diagnosis RPA.
DBBR questionnaire items pertaining to pre-diagnosis RPA assessed frequency of exercise (days/week), duration of exercise (minutes/session), length of time exercising (years), and mode of exercise, including walking for exercise or other moderate or vigorous intensity physical activities (MVPA) such as “running, aerobics, swimming, or cycling.”
Post-diagnosis RPA.
Post-diagnosis RPA was queried in the “Current Lifestyle” section of the DBBR questionnaire; these data were less detailed than those provided for pre-diagnostic RPA. Respondents indicated how many days/week they performed at least 20 minutes/session of MVPA. Because all respondents included in the current analysis completed their questionnaire within one year after diagnosis, these data reflect post-diagnosis RPA.
Habitual RPA.
Information on days/week (frequency) of performing MVPA was available for both pre- and post-diagnosis RPA, thus, habitual RPA was developed as a joint-exposure variable from self-reported frequency of pre- and post-diagnosis RPA. To derive this variable, patients were first categorized as engaging in regular, weekly, pre- or post-diagnosis RPA (inactive/active). “Regular” pre-diagnosis RPA was defined as a minimum of 1–2 sessions per/week for a minimum of 1–3 years; regular post-diagnosis RPA was defined as a minimum of 1–2 sessions per/week. Next, patients were categorized into one of four joint-exposure groups: inactive pre- and post-diagnosis (habitually inactive), inactive pre-and active post-diagnosis (increased activity), active pre- and inactive post-diagnosis (decreased activity), or active pre- and post-diagnosis (habitually active).
Clinical Outcomes
We examined associations of RPA with all-cause and cancer-specific mortality. For all-cause mortality, survival time was calculated from the date of diagnosis until the date of death from any cause; patients not deceased were censored at the date of last clinical contact. For cancer mortality, survival time was measured from the date of diagnosis until the date of death from cancer or complications from cancer. Patients with death from non-cancer causes were censored at the date of death from other causes or date of last clinical contact. Vital status was obtained from Roswell Park Cancer Registry, with updates obtained in January 2018. Because death certificate verification is only conducted for patients dying at Roswell, all-cause mortality is the primary outcome of interest.
Statistical Analyses
We used Cox-proportional hazards models to estimate the associations of pre-diagnosis, post-diagnosis, and habitual RPA with mortality. For all multivariable analyses, we a priori defined age at diagnosis, sex, tumor stage, and smoking status as important covariates. We examined additional epidemiological and clinical variables for confounding using the ten percent change-in-estimate method [12]. Additional variables tested included Body Mass Index (BMI), race, education, alcohol consumption, tumor grade, type of treatment completed (surgery only, adjuvant only, adjuvant plus post-adjuvant, neoadjuvant plus adjuvant, unknown), occupational activity, and comorbidities (i.e., diabetes, asthma, emphysema, COPD, rheumatoid arthritis and osteoarthritis). For female participants, we also assessed the potential confounding role of reproductive variables such as menopausal status and hormone replacement use. Importantly, treatment regimen, BMI, and education did not meet the definition of a confounder according to our a priori criteria, and were not included in final multivariable models. Furthermore, although detailed exercise duration, intensity, and mode information was not available for post-diagnosis RPA, associations for frequencies, durations, minutes, and intensity of pre-diagnosis RPA were investigated in exploratory analyses.
In a series of subgroup analyses according to varying clinical and epidemiological characteristics among patients, we examined the association of habitual RPA with mortality according to strata by disease site, tumor stage, treatment type, BMI, smoking status, sex, and age of diagnosis. For BMI analyses, we excluded underweight patients due to a strong correlation with cancer cachexia and mortality, and because we were primarily interested in examining whether associations of RPA with mortality were independent of overweight and/or obesity. For all subgroup analyses aside from disease site, if we observed evidence that HRs varied considerably across strata, we evaluated the potential for statistical interaction via the inclusion of a cross-product term in multivariable models and declared significance at p<0.01.
To estimate associations of habitual RPA with mortality while accounting for statistical heterogeneity by disease site, we applied the DerSimonian and Laird random effects meta-analytic technique [13]. Disease sites with less than 30 events were excluded from the meta-analysis and heterogeneity by disease site was evaluated by the Cochran Q-statistic (p<0.05) and I-squared statistic (>50%) [14].
In sensitivity analyses, to minimize the chance that our observed results were due to a healthy survivor bias, we excluded patients with < 3 years of follow-up. We further excluded patients diagnosed with breast, colorectal, and prostate cancer to examine whether the association between RPA and mortality could be observed among less common tumors for which a well-established inverse association between physical activity and mortality has not been apparent in the scientific literature. Lastly, we conducted additional sensitivity analyses designed to examine potential biases associated with the variability in length of time between the date of diagnosis and the date of questionnaire completion. To accomplish this, we stratified analyses according to sub-groups of patients who completed questionnaires within 6, 12, 18, 24, and 30 months of diagnosis.
Proportional hazards assumptions were tested for all RPA exposure variables and covariates via visual inspection of log-log plots and by assessing a time*covariate cross-product interaction term for statistical significance (p<0.01) in multivariable models. Statistical analyses were performed using IBM SPSS Statistics 21.0, and were independently confirmed by a second data analyst. Meta-analysis was conducted utilizing Comprehensive Meta-analysis Software. All statistical tests were two-tailed and considered statistically significant at p<0.05 unless otherwise noted.
Results
Our cohort consisted of 5,807 patients diagnosed with a variety of malignancies including breast, prostate, hematological, lung, colorectal, kidney, esophageal, bladder, gynecological, pancreatic, liver, stomach, sarcoma, head and neck, cervical, thyroid, testicular, brain, and skin cancer, of which 95% were melanomas. The study population included slightly more females than males (55% vs. 45%) and was primarily white. Collectively, 1,390 patients (24.4%) reported no regular pre-diagnosis RPA and 2,400 participants (41.9%) reported no regular post-diagnosis RPA. When pre- and post-diagnosis RPA were considered as a joint exposure, 1,056 participants (18.7%) were habitually inactive, 323 patients (5.7%) increased activity after diagnosis, 1,309 patients (23.2%) decreased activity, and 2,951 participants (52.3%) were habitually active. We identified 1,956 deaths through 31 January 2018; median follow-up time was 52.7 months (Table 1).
Table 1.
Demographic and clinical characteristics of the Data Bank and Biorepository cancer patient cohort from Roswell Park Comprehensive Cancer Center (n=5,807)
| Characteristic | na | Mean (SD) or % |
|---|---|---|
| Age at Diagnosis | 5,807 | 60.63 (11.99) |
| Sex | ||
| Female | 3,180 | 54.80% |
| Male | 2,627 | 45.20% |
| Race | ||
| White | 5,398 | 93.00% |
| Black | 203 | 3.50% |
| Other | 73 | 1.30% |
| Unknown | 133 | 2.30% |
| Education | ||
| <High school | 396 | 6.90% |
| High school/GED | 1,680 | 29.40% |
| Some college or tech school | 1,870 | 32.80% |
| 4-year college degree or higher | 1,763 | 30.90% |
| Pre-diagnostic RPA | ||
| Yes | 4,417 | 75.60% |
| No | 1,390 | 24.40% |
| Pre-diagnostic RPA Frequency | ||
| None | 1,390 | 24.80% |
| 1–2 days per week | 985 | 17.60% |
| 3–4 days per week | 1,912 | 34.10% |
| 5 or more days per week | 1,319 | 23.40% |
| Post-diagnostic RPA | ||
| Yes | 3,332 | 58.10% |
| No | 2,400 | 41.90% |
| Post-diagnostic RPA Frequency | ||
| None | 2,400 | 41.90% |
| 1–2 days per week | 1,301 | 22.70% |
| 3–4 days per week | 1,173 | 20.50% |
| 5 or more days per week | 858 | 15.00% |
| Habitual RPA Status | ||
| Inactive pre & post (habitual inactivity) | 1,056 | 18.70% |
| Inactive pre; active post (increased activity) | 323 | 5.70% |
| Active pre; inactive post (decreased activity) | 1,309 | 23.20% |
| Active pre & post (habitual activity) | 2,951 | 52.30% |
| Body Mass Index (kg/m2) | ||
| Underweight (BMI <18.5) | 94 | 1.60% |
| Normal Weight (BMI 18.5–24.99) | 1,539 | 26.50% |
| Overweight (BMI 25–29.99) | 1,932 | 33.30% |
| Obese (BMI ≥30) | 1,991 | 34.30% |
| Smoking Status | ||
| Never | 2,426 | 42.60% |
| Former | 2,594 | 45.50% |
| Current | 680 | 11.90% |
| Tumor Stage | ||
| Stage I | 1,723 | 29.70% |
| Stage II | 1,393 | 24.00% |
| Stage III | 1,024 | 17.60% |
| Stage IV | 979 | 16.90% |
| Unknown | 688 | 11.80% |
| Treatment Type | ||
| Surgery only | 813 | 14.00% |
| Adjuvant only | 3,768 | 64.90% |
| Adjuvant and post-adjuvant | 367 | 6.30% |
| Neoadjuvant and adjuvant | 830 | 14.30% |
| Unknown | 29 | 0.50% |
| Disease Site | ||
| Bladder | 123 | 2.10% |
| Brain | 18 | 0.30% |
| Breast | 1,232 | 21.20% |
| Cervical | 48 | 0.80% |
| Colorectal | 345 | 5.90% |
| Esophageal | 173 | 3.00% |
| Head and neck | 74 | 1.30% |
| Hematological | 486 | 8.40% |
| Kidney | 342 | 5.90% |
| Liver | 107 | 1.80% |
| Lower GI | 72 | 1.20% |
| Lung | 555 | 9.60% |
| Other | 156 | 2.70% |
| Other Gynecological | 45 | 0.80% |
| Ovarian | 151 | 2.60% |
| Pancreas | 127 | 2.20% |
| Prostate | 833 | 14.30% |
| Sarcoma | 84 | 1.40% |
| Skin (95% Melanomas) | 347 | 6.00% |
| Stomach | 107 | 1.80% |
| Testicular | 9 | 0.20% |
| Thyroid | 57 | 1.00% |
| Endometrial | 316 | 5.40% |
| Follow-up Duration (Months) | 5,807 | 59.06 (0.56) |
| All-cause Mortality | ||
| Alive | 3,851 | 66.30% |
| Deceased | 1,956 | 33.70% |
| Cancer-Specific Mortality | ||
| Alive | 3,851 | 66.30% |
| Deceased from cancer | 1,570 | 27.00% |
| Deceased from non-cancer cause | 229 | 3.90% |
| Deceased from unknown cause | 157 | 2.70% |
Columns may not sum to total due to missing data
Table 2 shows the age- and multivariable-adjusted models representing the associations of pre-diagnosis RPA with mortality. In comparison to inactive patients, patients reporting any amount of pre-diagnosis RPA experienced a 26% and 22% reduced hazard of all-cause (HR=0.74, 95% CI 0.70–0.81) and cancer-specific mortality (HR=0.78, 95% CI 0.70–0.87), respectively. Notably, patients reporting 3–4 days/week experienced the greatest survival advantage in comparison to inactive patients, with a 34% reduced hazard of all-cause mortality (HR=0.66, 95% CI 0.59–0.74) and 32% reduced hazard of cancer-specific mortality (HR=0.68, 95% CI 0.60–0.78). More detailed exploratory dose-response analyses of pre-diagnosis RPA are provided in Supplemental Table 1. In these analyses, walking pace was the only parameter for which a linear, dose-response association was apparent (p-for-trend <0.001), with patients reporting the most brisk pace (≤19 min/mile) experiencing the greatest survival advantage (HR=0.64, 95% CI 0.52–0.78, Supplemental Table 1).
Table 2.
Hazard ratios and 95% confidence intervals representing the associations of pre-diagnostic recreational physical activity with all-cause and cancer-specific mortality (n=5,807)
| Clinical Outcome | Pre-diagnostic RPA | Category of RPA | Age-adjusted models | Multi-variable modelsa | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | |||||||||
| HR | Lower | Upper | p-value | HR | Lower | Upper | p-value | |||
| All-cause mortality | Any regular/weekly pre-diagnostic MVPA RPA | No | Ref | Ref | ||||||
| Yes | 0.66 | 0.60 | 0.72 | <0.001 | 0.74 | 0.70 | 0.81 | <0.001 | ||
| Frequency of MVPA pre-diagnostic RPA | None | Ref | Ref | |||||||
| 1–2 days | 0.67 | 0.58 | 0.77 | <0.001 | 0.76 | 0.66 | 0.87 | <0.001 | ||
| 3–4 days | 0.58 | 0.52 | 0.66 | <0.001 | 0.66 | 0.59 | 0.74 | <0.001 | ||
| 5–7 days | 0.71 | 0.62 | 0.80 | <0.001 | 0.78 | 0.69 | 0.88 | <0.001 | ||
| Cancer-specific mortality | Any regular/weekly pre-diagnostic MVPA RPA | No | Ref | Ref | ||||||
| Yes | 0.68 | 0.61 | 0.75 | <0.001 | 0.78 | 0.70 | 0.87 | <0.001 | ||
| Frequency of MVPA pre-diagnostic RPA | None | Ref | Ref | |||||||
| 1–2 days | 0.70 | 0.60 | 0.82 | <0.001 | 0.81 | 0.69 | 0.95 | <0.008 | ||
| 3–4 days | 0.59 | 0.52 | 0.67 | <0.001 | 0.68 | 0.60 | 0.78 | <0.001 | ||
| 5–7 days | 0.75 | 0.65 | 0.86 | <0.001 | 0.85 | 0.74 | 0.98 | 0.022 | ||
All multivariable models adjusted for age, sex, tumor stage, smoking status
The multivariable associations of post-diagnosis RPA with mortality are presented in Table 3. In comparison to inactive patients, patients reporting any amount of post-diagnosis RPA experienced a 32% reduced hazard of all-cause (HR=0.68, 95% CI 0.62–0.75) and cancer-specific mortality (HR=0.68, 95% CI 0.61–0.75). As was observed with pre-diagnosis RPA, patients reporting 3–4 days/week experienced the greatest survival advantage in comparison to inactive patients, with a 40% reduced hazard of all-cause mortality (HR=0.60, 95% CI 0.52–0.68) and 39% reduced hazard of cancer-specific mortality (HR=0.61, 95% CI 0.52–0.70).
Table 3.
Hazard ratios and 95% confidence intervals representing the associations of post-diagnostic recreational physical activity with all-cause and cancer-specific mortality (n=5,807)
| Clinical Outcome | Post-diagnostic RPA | Category of RPA | Age-adjusted models | Multi-variable modelsa | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | |||||||||
| HR | Lower | Upper | p-value | HR | Lower | Upper | p-value | |||
| All-cause mortality | Any regular/weekly post-diagnostic RPA | No | Ref | Ref | ||||||
| Yes | 0.62 | 0.57 | 0.68 | <0.001 | 0.68 | 0.62 | 0.75 | <0.001 | ||
| Frequency of post-diagnostic RPA | None | Ref | Ref | |||||||
| 1–2 days | 0.66 | 0.58 | 0.74 | <0.001 | 0.71 | 0.63 | 0.80 | <0.001 | ||
| 3–4 days | 0.53 | 0.46 | 0.60 | <0.001 | 0.60 | 0.52 | 0.68 | <0.001 | ||
| 5–7 days | 0.69 | 0.60 | 0.79 | <0.001 | 0.76 | 0.66 | 0.87 | <0.001 | ||
| Cancer-specific mortality | Any post-diagnostic RPA | No | Ref | Ref | ||||||
| Yes | 0.61 | 0.55 | 0.67 | <0.001 | 0.68 | 0.61 | 0.75 | <0.001 | ||
| Frequency of post-diagnostic RPA | None | Ref | Ref | |||||||
| 1–2 days | 0.66 | 0.57 | 0.75 | <0.001 | 0.71 | 0.62 | 0.82 | <0.001 | ||
| 3–4 days | 0.52 | 0.45 | 0.60 | <0.001 | 0.61 | 0.52 | 0.70 | <0.001 | ||
| 5–7 days | 0.65 | 0.56 | 0.76 | <0.001 | 0.73 | 0.63 | 0.86 | <0.001 | ||
All multivariable models adjusted for age, sex, tumor stage, smoking status
Habitual RPA, our primary exposure of interest, was also inversely associated with mortality. As shown in Table 4, habitually active patients experienced a 39% decreased hazard of all-cause mortality (HR=0.61, 95% CI 0.54–0.69) and a 36% decreased hazard of cancer-specific mortality (HR=0.64, 95% CI 0.56–0.73) in comparison to habitually inactive patients. Further adjustment for type of treatment did not appreciably change estimates for all-cause (HR=0.63, 95% CI 0.56–0.71) or cancer mortality (HR=0.66, 95% CI 0.58–0.75, data not shown). Nor did adjustment for BMI and education substantively change estimates (HR=0.64, 95% CI 0.57–0.73 and HR=0.67, 95% CI 0.59–0.77) for all-cause or cancer mortality, respectively (data not shown).
Table 4.
Hazard ratios and 95% confidence intervals representing the associations of habitual recreational physical activity with all-cause and cancer mortality (n=5,807)
| Clinical Outcome | Habitual Recreational Physical (In)activity Status | Age-adjusted models | Multivariable-adjusted modelsa | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | ||||
| CI lower | CI upper | CI lower | CI upper | ||||||
| All-cause mortality | Habitually inactive | Ref | Ref | ||||||
| Inactive pre; active post (increased RPA) | 0.72 | 0.59 | 0.88 | 0.002 | 0.72 | 0.59 | 0.89 | 0.002 | |
| Active pre; inactive post (decreased RPA) | 0.79 | 0.70 | 0.89 | <0.001 | 0.83 | 0.74 | 0.95 | 0.005 | |
| Habitually active | 0.53 | 0.47 | 0.59 | <0.001 | 0.61 | 0.54 | 0.69 | <0.001 | |
| Cancer-specific mortality | Habitually inactive | Ref | Ref | ||||||
| Inactive pre; active post (increased RPA) | 0.70 | 0.55 | 0.88 | 0.002 | 0.72 | 0.57 | 0.91 | 0.006 | |
| Active pre; inactive post (decreased RPA) | 0.84 | 0.73 | 0.96 | 0.012 | 0.90 | 0.79 | 1.04 | 0.161 | |
| Habitually active | 0.54 | 0.47 | 0.61 | <0.001 | 0.64 | 0.56 | 0.73 | <0.001 | |
All multivariable models in primary analyses are adjusted for age, sex, tumor stage, smoking status
Importantly, formerly inactive patients who reported beginning RPA after diagnosis experienced a 28% reduced hazard of all-cause (HR=0.72, 95% CI 0.59–0.89) and cancer mortality (HR=0.72, 95% CI 0.57–0.91) in comparison to patients who remained inactive. Additional adjustment for treatment did not appreciably change hazard estimates for all-case (HR=0.76, 95% CI 0.62–0.93) or cancer mortality (HR=0.75, 95% CI 0.59–0.95) among this group of patients (data not shown). Likewise, adjustment for BMI and education did not substantially change estimates (HR=0.77, 95% CI 0.63–0.95 and HR=0.77, 95% CI 0.61–0.98) for all-cause and cancer-specific mortality, respectively.
In sub-group analyses by disease site, significant associations between habitual RPA and all-cause mortality were observed for bladder, breast, colorectal, esophageal, prostate, skin, endometrial (uterine), and ovarian cancers (Figure 1a). Because significant heterogeneity in the association of RPA with mortality was noted between disease sites (Q-statistic =34.30, p=0.002 and I2=59.18%), we present the random-effects summary estimate representing the association of habitual RPA with all-cause mortality in the overall study population (HR=0.60, 95% CI 0.49–0.73, Figure 1a). This estimate is nearly identical to the HR previously presented in Table 4 (HR=0.61, 95% CI 0.54–0.69). Similarly, for cancer-specific mortality, the random-effects summary estimate was HR=0.62 (95% CI 0.50–0.76), and significant associations were observed for bladder, breast, colorectal, ovarian, skin, and endometrial (uterine) cancer, with borderline significance for prostate and stomach cancer (Figure 1b).
Figure 1.
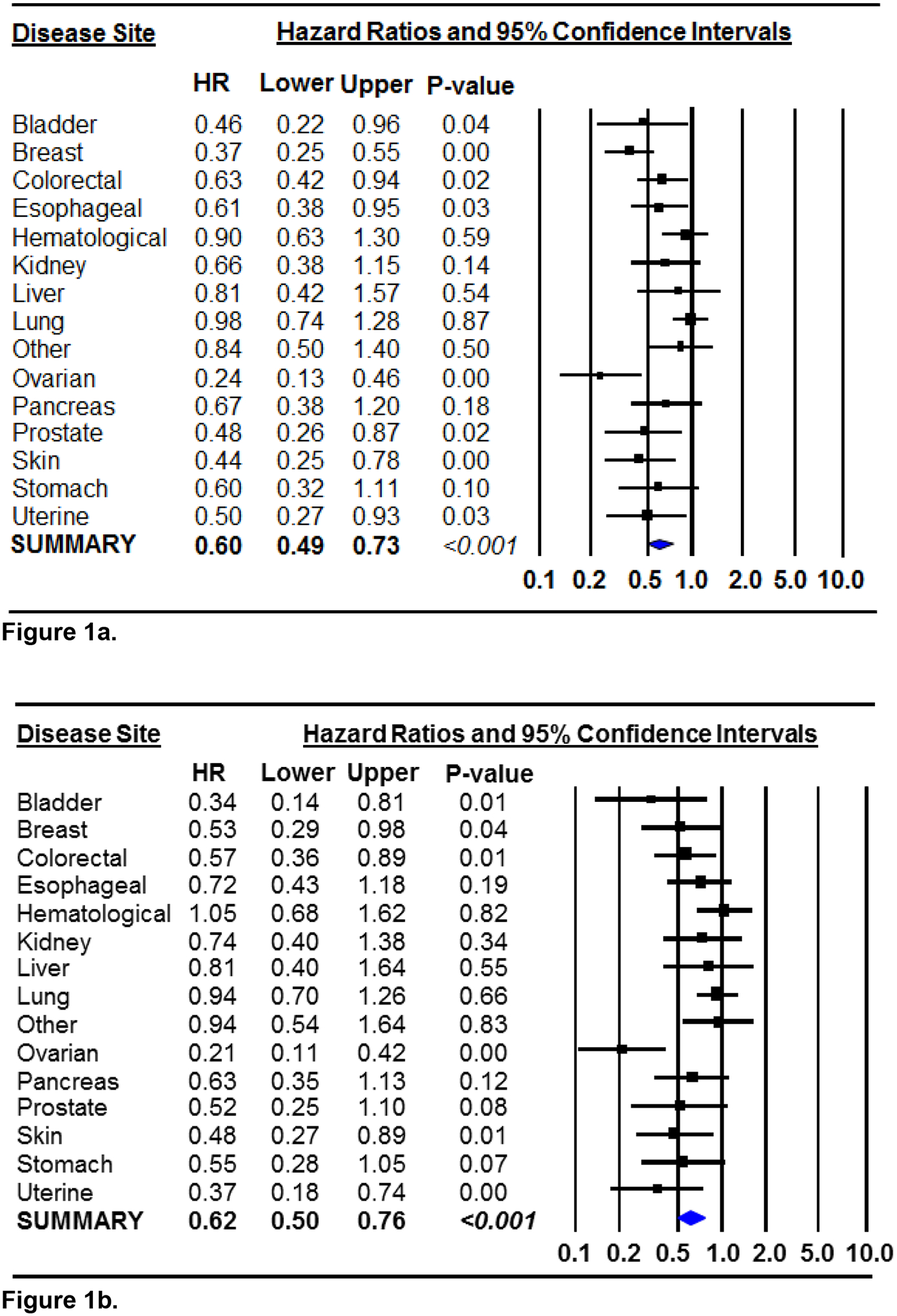
Forest plots of the hazard ratios and 95% confidence intervals representing the associations of habitual recreational physical activity, in comparison to habitual inactivity, with all-cause mortality (Figure 1a) and cancer-specific mortality (Figure 1b) by disease site. Multivariable models were adjusted for age of diagnosis, sex, tumor stage and smoking. The random-effects summary estimates are presented in both figures because significant heterogeneity was noted. Each square represents the disease site-specific HRs and the corresponding horizontal lines represent the width of the 95% CIs. The weighted, summary HR and its 95% CI is represented by the diamond in each figure.
In additional subgroup analyses by clinical characteristics such as tumor stage, we observed significant inverse associations between habitual activity and mortality among patients diagnosed with Stage I, II, III, and IV disease (Table 5). However, the association was somewhat attenuated among patients with Stage IV disease; among patients with unknown staging and/or among patients in which staging was not applicable (i.e., hematological cancer), the association between habitual activity and mortality was not significant (p-for-interaction=0.051 for all-cause and 0.227 for cancer-specific mortality, Table 5).
Table 5.
Hazard ratios and 95% confidence intervals representing the associations of habitual recreational physical activity with all-cause and cancer mortality by tumor stage
| Tumor Stage | Habitual Recreational Physical (in)activity Status | All-cause Mortality | Cancer-specific Mortality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | ||||
| CI lower | CI upper | CI lower | CI upper | ||||||
| Stage I | Habitually inactive | Ref | Ref | ||||||
| Inactive pre; active post (increased RPA) | 0.77 | 0.42 | 1.40 | 0.389 | 0.64 | 0.28 | 1.44 | 0.280 | |
| Active pre; inactive post (decreased RPA) | 0.97 | 0.67 | 1.40 | 0.872 | 0.99 | 0.61 | 1.60 | 0.969 | |
| Habitually active | 0.63 | 0.45 | 0.87 | 0.005 | 0.56 | 0.36 | 0.87 | 0.011 | |
| Stage II | Habitually inactive | Ref | Ref | ||||||
| Inactive pre; active post (increased RPA) | 0.71 | 0.40 | 1.28 | 0.253 | 0.42 | 0.16 | 1.06 | 0.067 | |
| Active pre; inactive post (decreased RPA) | 0.67 | 0.47 | 0.95 | 0.023 | 0.85 | 0.55 | 1.31 | 0.451 | |
| Habitually active | 0.47 | 0.34 | 0.64 | <0.001 | 0.56 | 0.37 | 0.83 | 0.004 | |
| Stage III | Habitually inactive | Ref | Ref | ||||||
| Inactive pre; active post (increased RPA) | 0.76 | 0.50 | 1.15 | 0.193 | 0.73 | 0.45 | 1.17 | 0.186 | |
| Active pre; inactive post (decreased RPA) | 0.85 | 0.65 | 1.11 | 0.234 | 0.96 | 0.72 | 1.28 | 0.768 | |
| Habitually active | 0.53 | 0.42 | 0.68 | <0.001 | 0.56 | 0.43 | 0.73 | <0.001 | |
| Stage IV | Habitually inactive | Ref | Ref | ||||||
| Inactive pre; active post (increased RPA) | 0.78 | 0.55 | 1.09 | 0.145 | 0.77 | 0.54 | 1.10 | 0.152 | |
| Active pre; inactive post (decreased RPA) | 0.95 | 0.77 | 1.17 | 0.602 | 0.96 | 0.77 | 1.19 | 0.690 | |
| Habitually active | 0.83 | 0.68 | 1.00 | 0.051 | 0.81 | 0.67 | 0.99 | 0.040 | |
| Unstaged (unknown or N/A) | Habitually inactive | Ref | Ref | ||||||
| Inactive pre; active post (increased RPA) | 0.74 | 0.42 | 1.31 | 0.302 | 1.02 | 0.56 | 1.88 | 0.940 | |
| Active pre; inactive post (decreased RPA) | 0.76 | 0.54 | 1.07 | 0.120 | 0.80 | 0.53 | 1.19 | 0.270 | |
| Habitually active | 0.75 | 0.56 | 1.02 | 0.066 | 0.82 | 0.58 | 1.16 | 0.256 | |
| Habitual RPA × tumor stage | p-for-interaction = 0.051 | p-for-interaction = 0.227 | |||||||
All multivariable models in primary analyses are adjusted for age, sex, tumor stage, smoking status; tumor stage × habitual RPA
We further examined the associations between habitual RPA and mortality according to treatment regimen (Supplemental Table 2). We observed inverse associations between habitual activity and mortality across all treatment types, but the association was not significant among patients receiving neoadjuvant treatment. In these analyses, we observed some evidence of a treatment*activity interaction for all-cause mortality (p-for-interaction<0.001), but not for cancer-specific mortality (p-for-interaction=0.036). Additional evaluation of the associations of habitual RPA with mortality stratified by epidemiological characteristics including age, sex, smoking status, and BMI classification did not support effect modification by any of these factors (Supplemental Table 3).
Lastly, in sensitivity analyses, when patients with < 3 years of follow-up were excluded, habitually active patients experienced a 47% decreased hazard of all-cause mortality (HR=0.53, 95% CI 0.43–0.65) and a 44% decreased hazard of cancer mortality (HR=0.56, 95% 0.43–0.73, data not shown). Further, when breast and colorectal cancer patients were excluded, habitually active participants experienced a 34% and 33% reduction in all-cause and cancer mortality, respectively (HR=0.66, 95% CI 58–0.75 and HR=0.67, 95% CI 0.59–0.77, data not shown). Associations remained significant and similar in magnitude upon further exclusion of prostate cancer patients for all-cause mortality (HR= 0.72, 95% CI 0.63–0.82) and cancer-specific mortality (HR= 0.73, 95% CI 0.64–0.84). Finally, in sensitivity analyses designed to identify potential bias resulting from variations in the length of time between date of diagnosis and date of questionnaire completion, we observed virtually no differences in associations among patients completing their questionnaires within 6, 12, 18, 24, or 30 months of diagnosis (Supplementary Table 4).
Discussion
In our study of the Roswell Park DBBR cancer patient cohort, we made four principle observations that expand the current knowledge regarding the associations of pre- and post-diagnosis RPA with mortality, each having important implications in the context of cancer survivorship. First, in comparison to patients who were habitually inactive, we observed a marked survival advantage among patients who reported regular RPA before and after diagnosis in the overall study population and in eight disease sites including breast, colon, prostate, ovarian, bladder, endometrial, esophageal, and skin cancer. Previous reports depicting the associations of pre- and post-diagnosis RPA with cancer mortality are sparse, [2, 15–22], and to our knowledge, none have examined associations with cancer mortality across multiple disease sites. We observed the expected associations for breast and colon cancer [2] and our findings of an inverse association for prostate cancer are consistent with one previous report demonstrating that men who were active before and after diagnosis experienced the lowest risk of mortality [16]. Our findings are congruent with previous reports showing that pre- or post-diagnosis RPA was associated with ovarian [23–26], bladder [27], and esophageal cancer mortality [28]. Lastly, our observation that RPA is inversely associated with endometrial cancer mortality coincides with one previous report [29] and conflicts with another that found no association [30]. Collectively, these data extend our current knowledge base by providing evidence that RPA is associated with improved outcomes among patients diagnosed with a variety of less common tumors.
Second, one of the most striking observations in the present study was that previously inactive patients in the decade prior to diagnosis, who reported engaging in regular, weekly RPA after diagnosis experienced a significantly reduced hazard of mortality in comparison to patients who remained inactive. These findings have important implications in the oncology setting because they suggest that a cancer diagnosis serves as an impetus for healthy behavior change in some patients, and among these patients, beginning an exercise program after diagnosis resulted in a significant survival advantage.
A third principle finding was that patients reporting 3–4 days/week of pre or post-diagnosis RPA experienced the best survival, while patients reporting1–2 days of regular, weekly, pre- and post-diagnosis RPA experienced similar survival to patients reporting 5–7 days per/week. These findings extend our knowledge about the benefits of low-frequency RPA and are consistent with two recently published reports demonstrating that 1–2 days/week of RPA was associated with a 17–21% reduced hazard of cancer-specific mortality in comparison to inactive participants [10] and that even the smallest amounts of daily physical activity associated with decreased all-cause mortality in post-menopausal women [31]. These data are also consistent with previously published meta-analyses showing that the association of RPA with mortality is non-linear [2, 32, 33]. To this end, it has been consistently reported in the exercise science literature that the association between physical activity and health benefits is curvilinear [34], with the steepest curve (i.e., the most significant health or survival benefits) occurring at the lower ends of the activity continuum and benefits plateauing [34] or decreasing [35, 36] at higher ends of the activity continuum. Collectively, these observations solidify the clinical and public health importance of the message that any amount of regular, weekly activity is better than inactivity, which is particularly encouraging given that cancer patients and survivors can be overwhelmed by the current physical activity recommendations of 30 minutes per day of moderate-intensity physical activity.
Fourth, we examined the association between habitual RPA and mortality in subgroups according to tumor stage, BMI classification, smoking status, sex and age, and found no support for effect modification by any of these factors. In fact, the significant inverse association between habitual RPA and mortality was consistently observed across all tumor stages, all smoking status categories, and among normal-weight and overweight/obese patients, demonstrating that habitual RPA is an independent, modifiable predictor of mortality, even among patients diagnosed with advanced disease and among patients who were obese and persistent smokers.
In subgroup analyses stratified by treatment regimen, we observed some evidence that the association between habitual activity and mortality was attenuated as treatment regimens intensified. For example, among patients receiving neoadjuvant therapy, the association between habitual activity and mortality was not significant. However, upon further examination of these data, we noted that the neoadjuvant treatment group was comprised mostly of patients diagnosed with hematological and lung malignancies for which we observed no associations in sub-group analyses by disease site. Furthermore, patients undergoing neoadjuvant therapy also experienced the greatest percentage of events and were more likely to become inactive after diagnosis (29% versus 22% for patients undergoing other treatment regimens). To this end, we acknowledge that the patients who stopped exercising after diagnosis were possibly the sickest patients and/or were undergoing the most difficult treatments, and subgroup analyses by treatment regimen support this assertion. However, we also observed a significant inverse association between habitual activity and mortality among patients diagnosed with stage IV disease, which may argue against a reverse-causation bias. Importantly, our method of comparing mortality in patients who were active as a lifestyle, in reference to those who were inactive as a lifestyle, potentially reduced the impact of a reverse causation bias that might be observed if the sickest patients were active before diagnosis but stopped exercising after diagnosis.
The most commonly cited mechanisms explaining the associations between RPA and cancer mortality include a decrease in circulating levels of sex hormones, decreased chronic inflammation, improved insulin sensitivity, improved immune surveillance, improved adipokine milieu (i.e., decreased leptin and increased adiponectin), and decreased adiposity [37, 38]. While it has long been hypothesized that physical activity reduces cancer risk and decreases cancer mortality primarily by lowering body weight [5, 37, 38], there has been an increasing recognition that RPA may be associated with cancer endpoints through pathways that are, at least, in part, independent from obesity-related pathways [39–41].
In our analyses, the association of habitual RPA with mortality was not confounded by BMI, nor did we observe evidence of effect modification across subgroups of BMI, suggesting that the observed association of habitual RPA with mortality is independent of obesity. Furthermore, emerging data from animal models suggests that voluntary aerobic exercise in tumor-bearing mice enhances sensitivity to chemotherapy by way of decreasing hypoxia and directly suppresses tumor growth and progression by enhancing the immune response to transformed cells, which is subsequently accompanied by more than a 60% reduction in tumor growth across several mouse models [42, 43]. Additional mechanistic studies have demonstrated that myokines secreted by contracting skeletal muscle, such as secreted protein acidic and rich cysteine (SPARC) and calprotectin, likely prevent carcinogenesis through the promotion of autophagy, apoptosis, and anti-tumor immunity, while preventing invasion and metastases [41].
The primary strength of our study was the availability of epidemiological data for 5,807 participants, including detailed clinical follow-up data spanning 14 years, and RPA data encompassing pre- and post-diagnosis exposure windows. That is, reliance solely upon pre-diagnosis RPA does not account for changes in activity throughout follow-up and reliance upon post-diagnosis RPA cannot rule out reverse causation bias. We contend that deriving a joint pre- and post-diagnosis RPA exposure and identifying habitual RPA as the primary exposure of interest potentially reduced the impact of these biases by identifying and comparing patients who were (in)active as a lifestyle.
A primary limitation of our study remains the reliance upon self-reported RPA data, which may be subject to recall error and misclassification and this can be especially true for the exposure window spanning the decade prior to diagnosis. Furthermore, unlike the pre-diagnosis DBBR RPA questionnaire items, the post-diagnosis RPA survey items did not yield detailed information about the mode, intensity, or specific duration of RPA. Thus, the habitual physical activity variable is based primarily upon weekly frequency of RPA. Despite this limitation, the DBBR questionnaire yielded the expected prevalence of pre- and post-diagnosis physical inactivity, and we observed the anticipated associations between activity and mortality for breast and colorectal cancer [2]. In fact, the inactivity prevalence data reported herein are nearly identical to recently reported national statistics suggesting that between 24.0 and 25.4% of the general population are inactive [44] and between 38.3 and 42.0% of adult cancer patients are inactive [1], thus bolstering our confidence in the generalizability and validity of these data.
An additional limitation of the current analysis was a lack of continued follow-up of RPA collected across multiple time points after study enrollment. As such, if post-diagnostic RPA levels changed after questionnaire completion and throughout the follow-up period, our observed estimates could be biased. Further, although we assessed the role of several potential confounders, we cannot entirely rule out the possibility that residual confounding by measured or unmeasured factors influenced our results. We also cannot rule out the possibility that our findings are due to a healthy survivor effect. We attempted to minimize the possibility of a healthy survivor bias by conducting sensitivity analyses to exclude participants who died within three years of diagnosis and the point estimates in our primary analyses were strengthened.
Additional limitations include a lack of detailed treatment data and the reliance upon Roswell Park Cancer Registry data for cause-specific death; verification by death certificate was only available for patients dying at Roswell Park. We also acknowledge that the heterogeneity of the DBBR patient cohort is a limitation of the current analysis. Yet, from a public health and survivorship standpoint, it is imperative to demonstrate that the inverse association between physical activity and mortality was observed among patients diagnosed with a variety of malignancies. Lastly, despite the overall size of the DBBR patient cohort, we lacked sufficient statistical power to conduct meaningful multivariable and subgroup analyses for more rare disease sites for which more research is needed regarding the associations of RPA with clinical outcomes.
Conclusion
Regular participation in RPA before and after a cancer diagnosis was associated with decreased mortality in the overall study population and in patients diagnosed with 8 specific tumors. Importantly, the current study suggests that beginning a regular RPA program after a cancer diagnosis yields a significant survival advantage in comparison to patients who remained inactive, and that as little as 1–2 days per/week of regular, weekly RPA associated with significant reductions in mortality. These data demonstrate the potential value of implementing exercise into the supportive care continuum of cancer patients and can inform targeted intervention trials designed to improve clinical outcomes among patients diagnosed with a variety of malignancies.
Supplementary Material
Funding:
The study was funded in part by The Roswell Park Databank and Biorepository (DBBR) is a Cancer Center Support Grant Shared Resource, supported by the National Cancer Institute grant P30CA016056; Arinden Sen is supported by NIH R21CA194634 (AS).
List of abbreviations
- RPA
recreational physical activity
- MVPA
moderate to vigorous intensity physical activity
- BMI
body mass index
- DBBR
DataBank and BioRepository
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
Ethical approval: The research described herein was approved by Roswell Park’s IRB. Thus, all procedures and analyses performed were in accordance with the ethical standards of Roswell Park and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: Informed consent was obtained from all individuals participants included in the DBBR study.
References
- 1.NCI. Cancer Survivors and Physical Activity: U.S. Department of Health and Human Services; 2018. [updated February 2018; cited 2018 March 5, 2018]. Available from: https://progressreport.cancer.gov/after/physical_activity.
- 2.Li T, Wei S, Shi Y, Pang S, Qin Q, Yin J, et al. The dose-response effect of physical activity on cancer mortality: findings from 71 prospective cohort studies. British journal of sports medicine. 2016;50(6):339–45. Epub 2015/09/20. doi: 10.1136/bjsports-2015-094927. [DOI] [PubMed] [Google Scholar]
- 3.Schmid D, Leitzmann MF. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta-analysis. Annals of oncology : official journal of the European Society for Medical Oncology. 2014;25(7):1293–311. Epub 2014/03/20. doi: 10.1093/annonc/mdu012. [DOI] [PubMed] [Google Scholar]
- 4.Alfano CM, Klesges RC, Murray DM, Bowen DJ, McTiernan A, Vander Weg MW, et al. Physical activity in relation to all-site and lung cancer incidence and mortality in current and former smokers. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2004;13(12):2233–41. Epub 2004/12/16. [PubMed] [Google Scholar]
- 5.Arem H, Moore SC, Park Y, Ballard-Barbash R, Hollenbeck A, Leitzmann M, et al. Physical activity and cancer-specific mortality in the NIH-AARP Diet and Health Study cohort. International journal of cancer. 2014;135(2):423–31. Epub 2013/12/07. doi: 10.1002/ijc.28659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hastert TA, Beresford SA, Sheppard L, White E. Adherence to the WCRF/AICR cancer prevention recommendations and cancer-specific mortality: results from the Vitamins and Lifestyle (VITAL) Study. Cancer causes & control : CCC. 2014;25(5):541–52. Epub 2014/02/22. doi: 10.1007/s10552-014-0358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kampert JB, Blair SN, Barlow CE, Kohl HW 3rd. Physical activity, physical fitness, and all-cause and cancer mortality: a prospective study of men and women. Annals of epidemiology. 1996;6(5):452–7. Epub 1996/09/01. [DOI] [PubMed] [Google Scholar]
- 8.Lee JY, Ryu S, Cheong E, Sung KC. Association of Physical Activity and Inflammation With All-Cause, Cardiovascular-Related, and Cancer-Related Mortality. Mayo Clinic proceedings. 2016;91(12):1706–16. Epub 2016/10/26. doi: 10.1016/j.mayocp.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 9.McCullough ML, Patel AV, Kushi LH, Patel R, Willett WC, Doyle C, et al. Following cancer prevention guidelines reduces risk of cancer, cardiovascular disease, and all-cause mortality. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2011;20(6):1089–97. Epub 2011/04/07. doi: 10.1158/1055-9965.epi-10-1173. [DOI] [PubMed] [Google Scholar]
- 10.O’Donovan G, Lee IM, Hamer M, Stamatakis E. Association of “Weekend Warrior” and Other Leisure Time Physical Activity Patterns With Risks for All-Cause, Cardiovascular Disease, and Cancer Mortality. JAMA internal medicine. 2017;177(3):335–42. Epub 2017/01/18. doi: 10.1001/jamainternmed.2016.8014. [DOI] [PubMed] [Google Scholar]
- 11.Parekh N, Lin Y, Craft LL, Vadiveloo M, Lu-Yao GL. Longitudinal associations of leisure-time physical activity and cancer mortality in the Third National Health and Nutrition Examination Survey (1986–2006). Journal of obesity. 2012;2012:518358. Epub 2012/06/08. doi: 10.1155/2012/518358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. American journal of epidemiology. 1993;138(11):923–36. Epub 1993/12/01. [DOI] [PubMed] [Google Scholar]
- 13.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled clinical trials. 1986;7(3):177–88. Epub 1986/09/01. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed). 2003;327(7414):557–60. Epub 2003/09/06. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borch KB, Braaten T, Lund E, Weiderpass E. Physical activity before and after breast cancer diagnosis and survival - the Norwegian women and cancer cohort study. BMC cancer. 2015;15:967. Epub 2015/12/18. doi: 10.1186/s12885-015-1971-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedenreich CM, Wang Q, Neilson HK, Kopciuk KA, McGregor SE, Courneya KS. Physical Activity and Survival After Prostate Cancer. European urology. 2016;70(4):576–85. Epub 2016/01/18. doi: 10.1016/j.eururo.2015.12.032. [DOI] [PubMed] [Google Scholar]
- 17.Irwin ML, Crumley D, McTiernan A, Bernstein L, Baumgartner R, Gilliland FD, et al. Physical activity levels before and after a diagnosis of breast carcinoma: the Health, Eating, Activity, and Lifestyle (HEAL) study. Cancer. 2003;97(7):1746–57. Epub 2003/03/26. doi: 10.1002/cncr.11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irwin ML, McTiernan A, Manson JE, Thomson CA, Sternfeld B, Stefanick ML, et al. Physical activity and survival in postmenopausal women with breast cancer: results from the women’s health initiative. Cancer prevention research (Philadelphia, Pa). 2011;4(4):522–9. Epub 2011/04/06. doi: 10.1158/1940-6207.capr-10-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campbell PT, Patel AV, Newton CC, Jacobs EJ, Gapstur SM. Associations of recreational physical activity and leisure time spent sitting with colorectal cancer survival. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(7):876–85. Epub 2013/01/24. doi: 10.1200/jco.2012.45.9735. [DOI] [PubMed] [Google Scholar]
- 20.Kuiper JG, Phipps AI, Neuhouser ML, Chlebowski RT, Thomson CA, Irwin ML, et al. Recreational physical activity, body mass index, and survival in women with colorectal cancer. Cancer causes & control : CCC. 2012;23(12):1939–48. Epub 2012/10/12. doi: 10.1007/s10552-012-0071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyerhardt JA, Giovannucci EL, Holmes MD, Chan AT, Chan JA, Colditz GA, et al. Physical activity and survival after colorectal cancer diagnosis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(22):3527–34. Epub 2006/07/11. doi: 10.1200/jco.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- 22.Schmid D, Behrens G, Arem H, Hart C, Herr W, Jochem C, et al. Pre- and post-diagnosis physical activity, television viewing, and mortality among hematologic cancer survivors. PloS one. 2018;13(1):e0192078. Epub 2018/02/01. doi: 10.1371/journal.pone.0192078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cannioto RA, LaMonte MJ, Kelemen LE, Risch HA, Eng KH, Minlikeeva AN, et al. Recreational physical inactivity and mortality in women with invasive epithelial ovarian cancer: evidence from the Ovarian Cancer Association Consortium. British journal of cancer. 2016;115(1):95–101. Epub 2016/06/15. doi: 10.1038/bjc.2016.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cannioto RA, Moysich KB. Epithelial Ovarian Cancer and Recreational Physical Activity: A Review of the Epidemiological Literature and Implications for Exercise Prescription. Gynecologic oncology. 2015. Epub 2015/03/24. doi: 10.1016/j.ygyno.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moorman PG, Jones LW, Akushevich L, Schildkraut JM. Recreational physical activity and ovarian cancer risk and survival. Annals of epidemiology. 2011;21(3):178–87. Epub 2011/02/08. doi: 10.1016/j.annepidem.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou Y, Chlebowski R, LaMonte MJ, Bea JW, Qi L, Wallace R, et al. Body mass index, physical activity, and mortality in women diagnosed with ovarian cancer: results from the Women’s Health Initiative. Gynecologic oncology. 2014;133(1):4–10. Epub 2014/04/01. doi: 10.1016/j.ygyno.2014.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liss MA, White M, Natarajan L, Parsons JK. Exercise Decreases and Smoking Increases Bladder Cancer Mortality. Clinical genitourinary cancer. 2017;15(3):391–5. Epub 2016/12/23. doi: 10.1016/j.clgc.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang L, Wang C, Guan S, Cheng Y. Impacts of physically active and under-active on clinical outcomes of esophageal cancer patients undergoing esophagectomy. American journal of cancer research. 2016;6(7):1572–81. Epub 2016/08/11. [PMC free article] [PubMed] [Google Scholar]
- 29.Arem H, Park Y, Pelser C, Ballard-Barbash R, Irwin ML, Hollenbeck A, et al. Prediagnosis body mass index, physical activity, and mortality in endometrial cancer patients. Journal of the National Cancer Institute. 2013;105(5):342–9. Epub 2013/01/09. doi: 10.1093/jnci/djs530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arem H, Chlebowski R, Stefanick ML, Anderson G, Wactawski-Wende J, Sims S, et al. Body mass index, physical activity, and survival after endometrial cancer diagnosis: results from the Women’s Health Initiative. Gynecologic oncology. 2013;128(2):181–6. Epub 2012/11/07. doi: 10.1016/j.ygyno.2012.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LaMonte MJ, Buchner DM, Rillamas-Sun E, Di C, Evenson KR, Bellettiere J, et al. Accelerometer-Measured Physical Activity and Mortality in Women Aged 63 to 99. J Am Geriatr Soc. 2017. Epub 2017/11/17. doi: 10.1111/jgs.15201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhong S, Jiang T, Ma T, Zhang X, Tang J, Chen W, et al. Association between physical activity and mortality in breast cancer: a meta-analysis of cohort studies. European journal of epidemiology. 2014;29(6):391–404. Epub 2014/05/24. doi: 10.1007/s10654-014-9916-1. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Gu M, Jing F, Cai S, Bao C, Wang J, et al. Association between physical activity and all cancer mortality: Dose-response meta-analysis of cohort studies. International journal of cancer Journal international du cancer. 2016;138(4):818–32. Epub 2015/09/01. doi: 10.1002/ijc.29828. [DOI] [PubMed] [Google Scholar]
- 34.Blair SN, Cheng Y, Holder JS. Is physical activity or physical fitness more important in defining health benefits? Medicine and science in sports and exercise. 2001;33(6 Suppl):S379–99; discussion S419–20. Epub 2001/06/28. [DOI] [PubMed] [Google Scholar]
- 35.Janssen I, Jolliffe CJ. Influence of physical activity on mortality in elderly with coronary artery disease. Medicine and science in sports and exercise. 2006;38(3):418–7. Epub 2006/03/17. doi: 10.1249/01.mss.0000191185.58467.be. [DOI] [PubMed] [Google Scholar]
- 36.Sundquist K, Qvist J, Sundquist J, Johansson SE. Frequent and occasional physical activity in the elderly: a 12-year follow-up study of mortality. American journal of preventive medicine. 2004;27(1):22–7. Epub 2004/06/24. doi: 10.1016/j.amepre.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 37.Berger N, editor. Cancer and Energy Balance, Epidemiology and Overview: Springer; 2010. [Google Scholar]
- 38.McTiernan A Mechanisms linking physical activity with cancer. Nature reviews Cancer. 2008;8(3):205–11. Epub 2008/02/01. doi: 10.1038/nrc2325. [DOI] [PubMed] [Google Scholar]
- 39.Byers T Physical activity and gastric cancer: so what? An epidemiologist’s confession. Cancer prevention research (Philadelphia, Pa). 2014;7(1):9–11. Epub 2013/12/19. doi: 10.1158/1940-6207.capr-13-0400. [DOI] [PubMed] [Google Scholar]
- 40.Hildebrand JS, Gapstur SM, Gaudet MM, Campbell PT, Patel AV. Moderate-to-vigorous physical activity and leisure-time sitting in relation to ovarian cancer risk in a large prospective US cohort. Cancer causes & control : CCC. 2015. Epub 2015/09/04. doi: 10.1007/s10552-015-0656-7. [DOI] [PubMed] [Google Scholar]
- 41.Sanchis-Gomar F, Lucia A, Yvert T, Ruiz-Casado A, Pareja-Galeano H, Santos-Lozano A, et al. Physical inactivity and low fitness deserve more attention to alter cancer risk and prognosis. Cancer prevention research (Philadelphia, Pa). 2015;8(2):105–10. Epub 2014/11/25. doi: 10.1158/1940-6207.capr-14-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pedersen L, Idorn M, Olofsson GH, Lauenborg B, Nookaew I, Hansen RH, et al. Voluntary Running Suppresses Tumor Growth through Epinephrine- and IL-6-Dependent NK Cell Mobilization and Redistribution. Cell metabolism. 2016;23(3):554–62. Epub 2016/02/21. doi: 10.1016/j.cmet.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 43.Betof AS, Lascola CD, Weitzel D, Landon C, Scarbrough PM, Devi GR, et al. Modulation of murine breast tumor vascularity, hypoxia and chemotherapeutic response by exercise. Journal of the National Cancer Institute. 2015;107(5). Epub 2015/03/18. doi: 10.1093/jnci/djv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moore LV, Harris CD, Carlson SA, Kruger J, Fulton JE. Trends in no leisure-time physical activity--United States, 1988–2010. Research quarterly for exercise and sport. 2012;83(4):587–91. Epub 2013/02/02. doi: 10.1080/02701367.2012.10599884. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


