Abstract
Background:
Chorioamnionitis from ascending bacterial infection through the endocervix is a potential risk factor for cerebral palsy. Tetrahydrobiopterin, an essential cofactor for nitric oxide synthase (NOS) and amino acid hydroxylases, when augmented in fetal brain, prevents some of the cerebral palsy-like deficits in a rabbit hypoxia-ischemia model.
Objectives:
Study the effect of LPS-induced intrauterine inflammation in preterm gestation on motor deficits in newborn and whether biosynthesis of tetrahydrobiopterin or inflammatory mediators is affected in fetal brain.
Methods:
Pregnant rabbits at 28 days gestation (89% term) were administered either saline or lipopolysaccharide in both endocervical openings. One group underwent spontaneous delivery and neurobehavior tests were performed at postnatal days 1 and 11 (P1, P11) with some kits being sacrificed at P1 for histological analysis. Another group received C-section at 24 hours post-lipopolysaccharide. Gene sequences for rabbit biosynthetic enzymes of tetrahydrobiopterin pathways were determined and analyzed in addition to cytokines using quantitative RT-PCR.
Results:
Lipopolysaccharide 200 μg/kg/ml exposure caused a locomotion deficit but mild hypertonia at P1. By P11, most animals turned to normal-appearing kits. There was no difference in neuronal cell death in the caudate between hypertonic and non-hypertonic kits at P1 (n=3–5 in each group). Fetal brain GTP cyclohydrolase 1 was increased, whereas sepiapterin reductase and pyruvoyl-tetrahydropterin synthase decreased 24 hours post-lipopolysaccharide. Neuronal NOS was also increased. Regardless of position in the uterus or brain region, expression of TNFα and TGFβ decreased, whereas IL1β, IL6, and IL8 increased (n=3–4 in each group).
Conclusions:
This is a first study using a lipopolysaccharide-induced ascending-intrauterine inflammation model in rabbit, showing mostly transient hypertonia and mainly locomotor deficits in kits. Not all pro-inflammatory cytokines increase in fetal brain following LPS. The changes of key tetrahydrobiopterin biosynthetic enzymes possibly indicates the different effects from the inflammatory insult.
Keywords: LPS, cerebral palsy, neurobehavioral assessment, cytokine, tetrahydrobiopterin
Introduction
Cerebral palsy (CP) is the most common motor disability in childhood [1]. The characteristic signs of CP include hypertonia, movement disorders, muscle weakness, ataxia, and rigidity [2]. Intrauterine infection has been linked to CP [3]. Motor deficits have been reported in a rabbit model using a gram-negative bacterial endotoxin, lipopolysaccharides (LPS), injected into the myometrium [4]. The suspected route of involvement in this model could be directly from myometritis to eventual endometritis or through the circulation to the placenta. In humans, the route of infection and inflammation is from vagina to endocervix to endometrium. However, it is unclear whether ascending cervical inflammation would cause increased inflammation in fetal brain and result in motor deficits in an antenatal rabbit model as occurs in humans.
Tetrahydrobiopterin (BH4) is an essential cofactor for nitric oxide (NO) formation as well as for dopamine and serotonin synthesis. BH4 levels in fetal rabbit brains are at very low levels at premature gestation and decrease after hypoxia-ischemia (H-I) injury [5]. In our H-I CP model, we found that inhibition of neuronal nitric oxide synthase (nNOS) [6] and supplementation of BH4 analog prevented CP in rabbits [7]. BH4 plays a role in fetal neuronal survival and function in rabbits following H-I [5]. Inflammatory mediators such as LPS and cytokines are known to alter BH4 synthesis in a cell-specific manner [8–10]. Not much is known about the involvement of BH4 pathway in developing brain and inflammation during the perinatal period.
Herein we show that using a rabbit model of ascending intrauterine inflammation caused by intracervical LPS injection, newborn kits present with mainly locomotor deficits but hypertonia is mostly transient. We show most pro-inflammatory cytokines increased in different fetal brain regions, but not all. For this study, we sequenced the rabbit genes for the enzymes of the de novo BH4 biosynthetic pathways. We show that inflammatory changes are accompanied by changes in expression of different biosynthetic enzymes indicating that LPS is sufficient to alter fetal brain gene expression which may be linked to motor mechanisms.
Materials and Methods
All surgical procedures were performed under the permission of IACUC of Northshore University Healthsystem, the former place of employment for three of the authors (ZS, KL, ST).
LPS injection
Near term pregnant New Zealand White rabbits at 28 days gestation (E28, 89% term) were anesthetized and using a hysteroscope introduced into the vagina, the cervical os was visualized and a flexible cannula inserted via the hysteroscope 1–2 cm into the cervix. Penetration of a dye such as 1% fast green showed that any fluid injected via the flexible cannula could reach all the fetuses. The LPS powder (L4524, Escherichia coli 055:B5, Sigma) was dissolved in sterile saline and filtered through a 0.22-micron filter before administration. Under direct visualization, 0.5 ml of LPS or 0.9% sterile saline (control) was injected in each cervical opening at a rate of 0.2 ml/min of 200 to 1000 μg/kg/ml. We did a dose-response study and investigated mortality and maternal fever in a dose-range from 0.2–1 mg/kg/dose. To minimize adverse effects, we selected 0.2 mg/kg/dose for subsequent studies. The cannula remained in the cervix for 5 min before it was removed, and the dam rested for another 30 min in the supine position to avoid leaking of the LPS solution.
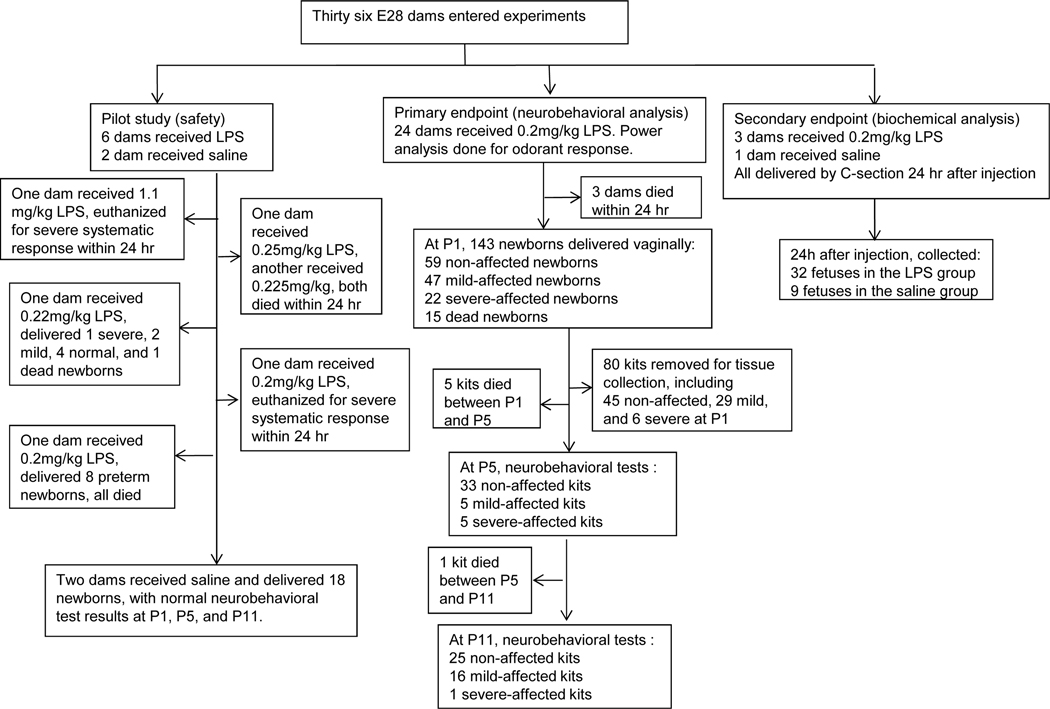
The number of animals involved in this study is summarized in Fig 1.
Figure 1.
Experimental flow chart showing breakdown of number and outcome of dams and fetuses/kits.
Neurobehavioral assessment
For primary endpoint experiments, the dams were allowed to recover and deliver at term gestation (E31.5). Neurobehavioral assessment was done on postnatal days 1, 5, 11 (P1, P5, P11) using a protocol that was published before [11]. We noticed more of a dissociation of hypertonia from locomotor deficits following LPS administration, compared to our H-I models. Categorization of overall neurobehavioral status was as follows:
Severe: presence of a) moderate hypertonia (defined as tone≥3.0 in forelimb or ≥2.0 in hind limb, or combined hypertonia in both fore- and hind limbs (tone=2.5 in forelimb AND 1.5 in hind limb), or b) postural changes,
Mild: a) Mild hypertonia in one limb (defined as tone=2.5 in forelimb or 1.5 in hind limb) by itself or accompanied by minor locomotor deficit, b) locomotor deficit without hypertonia.
Normal: No locomotor deficit.
For each animal, the testing was videotaped and scored on a scale of 0–4 (0=worst and 4=best) by two blinded observers. Olfaction was tested by aversive response to a cotton swab soaked with peppermint, amyl acetate, or pure ethanol. Locomotion on a flat surface was assessed by grading the amount of spontaneous movement of head, trunk and limbs. Tone was assessed by active flexion and extension of the fore and hind limbs and scored (0–4) according to the Ashworth scale [12]. The righting reflex was assessed when the pups were placed on their backs. Suck and swallow were assessed, by introduction of infant milk formula into the pup’s mouth with a plastic pipette.
Swimming Test
Swimming tests were used to investigate the subtle locomotor deficits as published before [13]. Kits were marked with water resistant markers on their toes, ankle, knee, hip, shoulder, elbow, wrist, and finger tips. Swimming movements were videotaped in a fish tank filled with 33–35°C water. The video was recorded for approximately 60 seconds. Kits were then dried and returned to their dam or the neonatal incubator. Kits were swum on P1. 80–100 frames (30 frames per second) of the video digitized. The digitized video was assessed using a custom motion analysis program developed using MATLAB software. Each frame was assessed by selecting markers of the position of desired joints manually. The marked positions were then tracked throughout the videos (4–5 swimming cycles) and the locations were scatter-plotted to identify the center and range of the motion for both animal groups. The total coverage area was calculated by using distance regularized level set evolution [14]. The mean and 95% confidence intervals of all the kits in the group was then calculated (n=3/group).
Primary endpoints were death or neurobehavioral assessments based on locomotion, motor ability and muscle tone.
Tissue collection
Brains from some P1 kits after neurobehavioral tests were collected after perfusion fixation, which would be 4 days after the LPS insult. Briefly, the kit was anesthetized, and the chest was opened to expose the chest cavity. A 22g needle was inserted into the left ventricle of the heart and a cut into the right atrium was made. Circulating blood was replaced by PBS perfusion, with infusion of 10 ml; followed by perfusing with 100 ml of PFA (10 ml/min). The brain was then removed and post-fixed by immersion in 4% PFA for 24 hr, followed by 30% sucrose in PBS to cryoprotect the tissue. Perfusion fixed brains were frozen at −30°C in isopentane, and cut in the coronal plane at 50 μ on a freezing microtome. Sections spaced 1200 μ apart were mounted and stained with cresyl violet. This process yielded > 3 sections from the caudate. Three sections, 1 each from anterior, mid, and posterior sections of the caudate were analyzed. Under oil (1000x magnification), each cell in the caudate was examined. Neurons exhibiting any of the phenotypic features of cell death along the apoptotic-necrotic continuum [15] were counted. Counts at 3 sections were compared between hypertonic and non-hypertonic kits.
For secondary endpoint experiments, Caesarean sections (C-sections) were done at 24 hours after LPS or saline injection in another experiment. The time point was chosen because the maximum effect on cytokines in a previous rabbit model using LPS myometritis is at 24 hours, E29 [16]. Fetal cortex (CO), basal ganglia (BG), thalamus (TH), and cerebellum (CE) from 3 fetuses at the base (close to the cervix) and 3 at the pole of uterus (farthest from the cervix) were collected, snap-frozen in liquid nitrogen, and kept at −80°C until ready for analysis by biochemical and gene expression assays.
Rabbit gene sequencing done first time
Previously unknown rabbit genes were cloned using RACE-based techniques (SMARTer RACE 5’/3’ Kit, Clontech Laboratories) by lab-designed gene-specific primers, sequenced, and confirmed by alignment with the predicted sequences at GenBank (Table 1). Primers were designed using Primer-Blast (http://www.ncbi.nlm.nih.gov/tools/primer-blast/).
Table 1.
List of novel rabbit genes sequenced.
Quantitative transcription analysis with real time polymerase chain reaction (qRT-PCR)
Total RNA was purified from corresponding brain tissues (CO, BG, TH, and CE) using PureLink RNA Mini Kit (Life Technologies, Grand Island, NY) according to the manufacturer’s protocol. High capacity RNA-to-DNA Kit (Applied Biosystems) was used for reverse transcription according to the manufacturer’s protocol. The RT-PCR was performed in a StepOnePlus real-time PCR System (Applied Biosystems) using the 2x SYBR Green qPCR Master Mix (Biotool). Reactions were performed in duplicates in MicroAmp optical 96-well plates in a total volume of 10 μL comprising the final concentration of 1× SYBR Green PCR Master mix, cDNA (10 ng), and primers (75 nM, forward and reverse primers listed in Table 2). The following thermal protocol was used: initial incubation at 95°C for 10 min, 40 cycles of 15 s at 95°C, and 1 min at 60°C. Cycle threshold (ct) values of individual genes were subtracted from Ct values for hypoxanthine phosphoribosyltransferase 1 (HPRT1, a hypoxia-stable housekeeping gene), and were then used to calculate fold change in relative gene expression (2−ΔΔCT). At least 3 animals were tested in each group. Results were compared to saline controls in each batch of samples analyzed.
Table 2.
List of qRT-PCR primers.
| Gene | Forward (5’−3’) | Reverse (5’−3’) |
|---|---|---|
| TGF-β | CAGTGGAAAGACCCCACATCTC | GACGCAGGCAGCAATTATCC |
| IL-1β | TTGAAGAAGAACCCGTCCTCTG | CTCATACGTGCCAGACAACACC |
| IL-2 | GCCCAAGAAGGTCACAGAATTG | CCCCCATGAGAGTTTTTGCCT |
| IL-6 | CTACCGCTTTCCCCACTTCAG | TCCTCAGCTCCTTGATGGTCTC |
| IL-8 | CCACACCTTTCCATCCCAAAT | CTTCTGCACCCACTTTTCCTTG |
| IL-10 | CTTTGGCAGGGTGAAGACTTTC | AACTGGATCATCTCCGACAAGG |
| TNF-α | CTGCACTTCAGGGTGATCG | CTACGTGGGCTAGAGGCTTG |
| nNOS | CAGAGACCACTTTGAGAGCGC | ACGGAGAACCTCACATTGGC |
| PTPS | CCGTTACGGGAATGGTTATGA | TGAAGTACGGCACATCCAGG |
| GTPCH | AGTTGGGGTGGTGGTTGAAG | TCTTCCCGAGTCTTGGGGTC |
| SPR | TGAACATCTCGTCGCTGTGT | AGCTTCTGGGCTGACTCCTT |
| DHPR | TTGGATGGGACTCCTGGGAT | GTATCCAGGGTAACCGGCAG |
| DHFR | caCAACGTCGTCAGTGGAAGG | ccTTGTGGCGGTTCCTTGAG |
| HPRT | TGGATACAGGCCAGACTTTGTTG | TCGCAGTTTCATCTTAGGCTTTG |
Statistics
Measurement data were expressed as the mean ± standard error of the mean (SEM). Student’s t test was used to compare differences between corresponding groups. Power analysis for number of dams was done with assuming moderate effect for odorant response at P1, α error=0.05 and β error=0.2, power 80%. For primary endpoints of individual neurobehavioral battery of tests, statistical significance was tested by two tailed sample t-test with Bonferroni correction for multiple comparisons (p < 0.0038). For secondary endpoints, α error=0.05.
Results
Thirty three dams received intracervical LPS injection, and 3 dams received saline as control. The distribution of various dams in different experiments and breakdown of behavioral grouping of kits are provided in Figure 1. When monitoring temperature, sixteen out of eighteen of the LPS-receiving dams (89%) had increased body temperature (1–2 °C, Tmax=40 °C) at 4 hr after LPS injection. None of the saline control kits (18 total) or dams (3 total) had any change in vital signs.
Neurobehavioral Deficits-Overall
Twenty four dams received 0.2 mg/kg LPS. Death occurred in 3 dams before E30 (12.5%). The rest of the dams delivered 143 kits, of which 15/143 were fetal/newborn deaths (10%). Of the survivors, 22/128 (17%) kits had severe, and 47/128 (37%) kits had mild motor deficits from the neurobehavioral battery of tests at P1. We did follow up of 16 severe kits. One died immediately after P1, one died after P5, five improved to mild at P11, and nine improved to normal at P11. We also did follow up on 18 mild kits. Three died before P5, ten were still mild at P11, and the rest 5 became normal at P11. On follow up of 14 normal kits at P1, one died before P5, eleven were still normal at P11, one became severe and one became mild at P11.
Neurobehavioral Deficits-Individual Parameters
As a population, the LPS group showed significant increase in tone in forelimbs and hind limbs (Fig 2), decrease in locomotion for forelimbs and hind limbs, decrease in trunk, neck and limb movements, and decrease in righting reflex (Fig 3), compared to saline group at P1 (alpha error with Bonferroni correction, Table 3). Incidence of any hypertonia was 27% at P1 (35 out of 128), most of them mild. The locomotor abnormalities were mostly subtle to mild, 36% for hind limb movement (46 out of 128). All individual parameters had the same median indicating that less than half of the kits were different (Table 3).
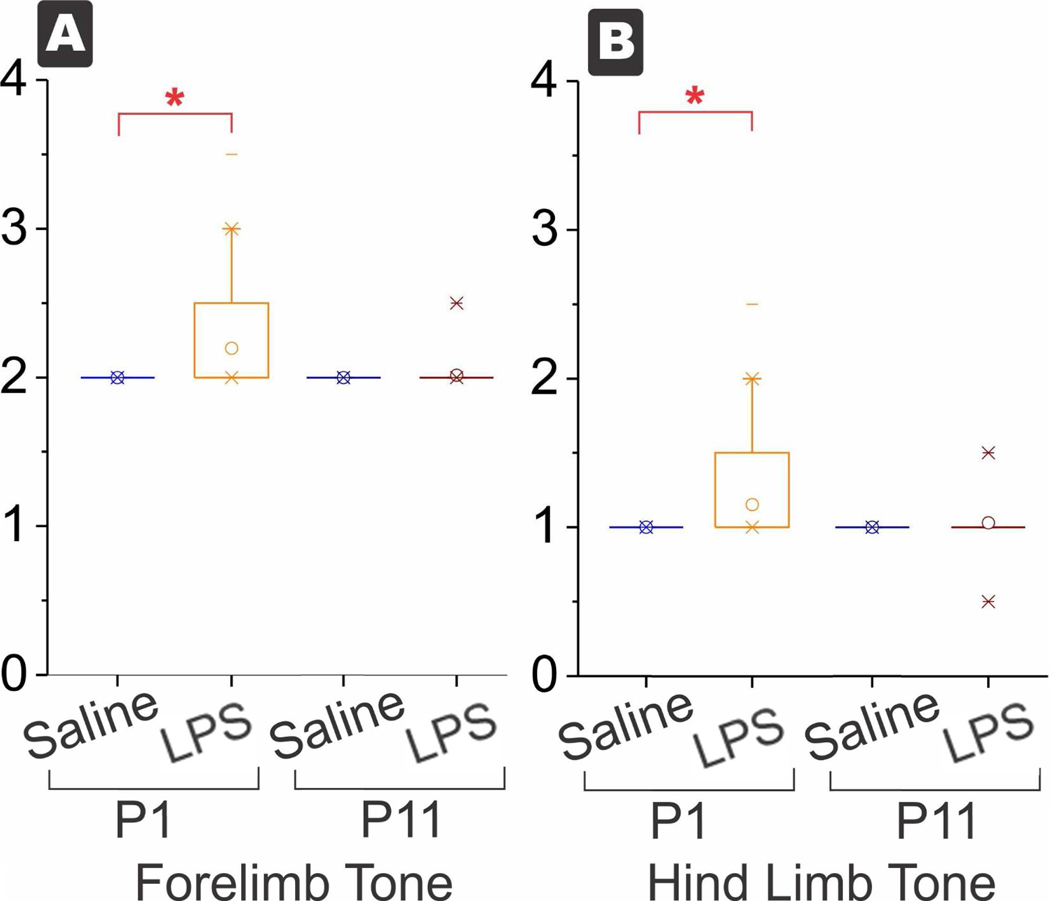
Figure 2.
Box and whisker plots of tone. Increased tone in forelimbs (A) and hind limbs (B) at P1 in the LPS group but the survivors at P11 were not significantly different between saline and LPS. *p<0.0038, with Bonferroni correction for multiple tests.
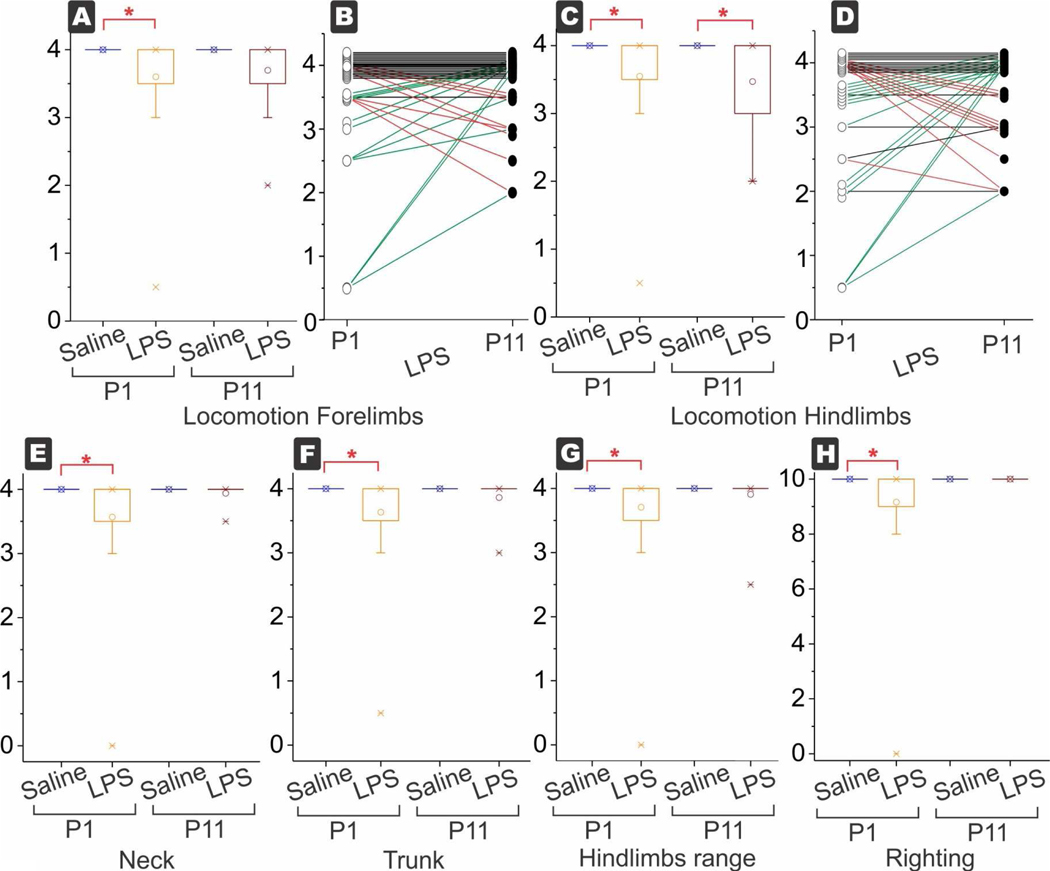
Figure 3.
Box and whisker plots of each variable. Decreased motor function in locomotion of forelimbs (A) and hind limbs (C) at P1 in the LPS group. The locomotion of hind limbs was persistently low at P11, the only motor test to be significant at P11. In the LPS group, change in each kit is shown for forelimb in (B) and hind limb in (D). Arrows and symbols have been offset a little artificially to show change for each kit. Red lines show worsening and green lines show improvement from P1 to P11. The number of worsening kits equaled roughly the number of improving kits both in forelimbs and hind limbs, while the biggest proportion was in the kits that were unchanged. Motor function of neck (E), trunk (F), range of hind limbs (G) and righting (H) were all decreased at P1 and recovered to normal at P11. The forelimb range was not shown for brevity but was also decreased at P1. *p<0.0038, with Bonferroni correction for multiple tests.
Table 3.
Neurobehavioral battery of tests.
| Neurobehavioral test | Saline P1 | LPS P1 | Saline P11 | LPS P11 |
|---|---|---|---|---|
| n=18 | n=128 | 16 | n=33 | |
| Tone, forelimbs | 2±0, (2) | 2.2±0.0, (2)** | 2±0, (2) | 2±0, (2)§§ |
| Tone, hind limbs | 1±0, (1) | 1.2±0.0, (1)** | 1±0, (1) | 1±0, (1)§ |
| Righting Reflex | 10±0, (10) | 9.2±0.2, (10)** | 10±0, (10) | 10±0, (10)§§ |
| Locomotion Time Forelimbs | 4±0, (4) | 3.6±0.1, (4)** | 4±0, (4) | 3.7±0.1, (4) |
| Locomotion Time Hind Limbs | 4±0, (4) | 3.5±0.1, (4)** | 4±0, (4) | 3.5±0.1, (4)## |
| Trunk | 4±0, (4) | 3.6±0.1, (4)** | 4±0, (4) | 3.9±0.1, (4) |
| Neck | 4±0, (4) | 3.6±0.1, (4)** | 4±0, (4) | 3.9±0.0, (4)§§ |
| Range of Motion Forelimb | 4±0, (4) | 3.8±0.1, (4)** | 4±0, (4) | 3.9±0.0, (4) |
| Range of Motion Hind Limb | 4±0, (4) | 3.7±0.1, (4)** | 4±0, (4) | 3.9±0.1, (4) |
| Smell response to peppermint | 1.0±0.1, (1.0) | 1.4±0.1, (1.0) | 1.5±0.3, (1.0) | 1.4±0.2, (1.0) |
| Smell response to alcohol | 0.9±0.2, (0.5) | 1.2±0.1, (1.0)* | 1.3±0.3, (1.0) | 0.9±0.1, (1.0) |
| Smell response to amyl acetate | 1.4±0.2, (1.0) | 1.8±0.2, (1.5) | 2.6±0.2, (2.3) | 1.2±0.2, (1.0)## |
| Tactile response to face | 1.8±0.2, (2.0) | 1.9±0.1, (1.5) | 3.3±0.1, (3.3) | 3.1±0.1, (3.5)§§ |
P1 LPS compared to P1 Saline if significant is depicted by
P11 LPS compared to P11 Saline is depicted by
P1 LPS compared to P11 LPS is depicted by
Mean+SEM, (Median).
p<0.0038 (alpha error taking into account Bonferroni correction for multiple comparisons),
p<0.00038, two sample t-test.
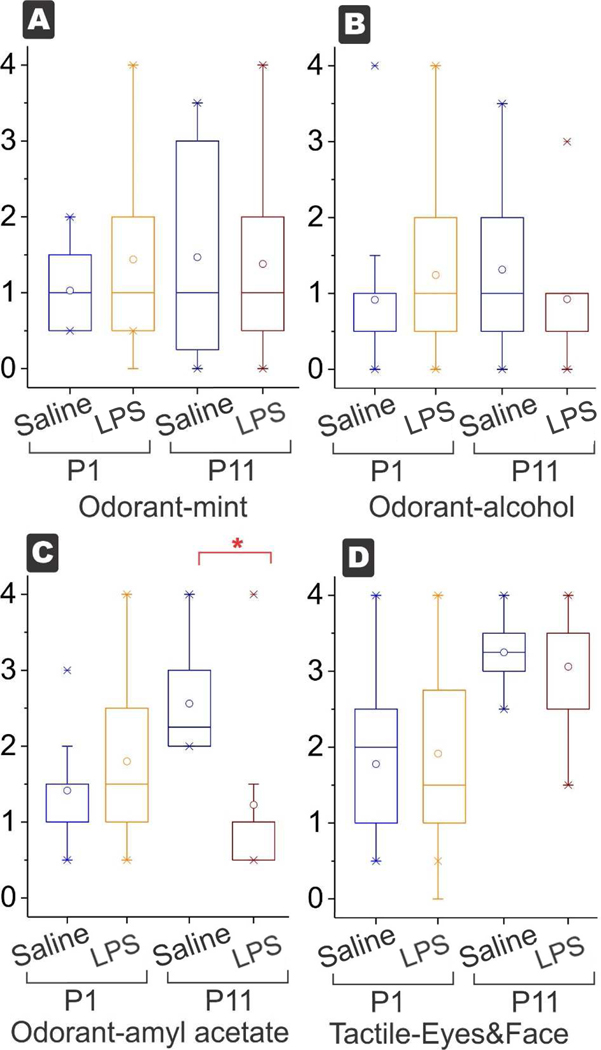
Regarding the change in motor deficits, P11 kits were different from P1 kits, and recovered to the level of controls in almost all parameters except for spontaneous movement of hind limbs. The locomotor deficits of hindlimbs was worse at P11 despite improvement of hypertonia, found in 46% of P11 kits. Of the survivors, the incidence of any hypertonia at P11 was 9%, but there may have been a selection bias because some severe P1 were sacrificed for histological analysis. The sensory aspects of odorant response and tactile response to face was significantly different only for amyl acetate at P11, but not different for peppermint and alcohol between the groups at P1 or at P11 (Fig 4).
Figure 4.
Box and whisker plots for sensory variables. Odorant response to peppermint (A) and alcohol (B) were not different at P1 or P11 (power=71% with moderate sized effect and α error=0.05). The only decrease was observed in odorant amyl acetate (C) at P11 but not at P1. (*p<0.0038, with Bonferroni correction for multiple tests). There was no difference between the tactile response to eye and face (D) at P1 and P11 (power=71% with moderate sized effect and α error=0.05).
Neurobehavioral Deficits-Swimming Test
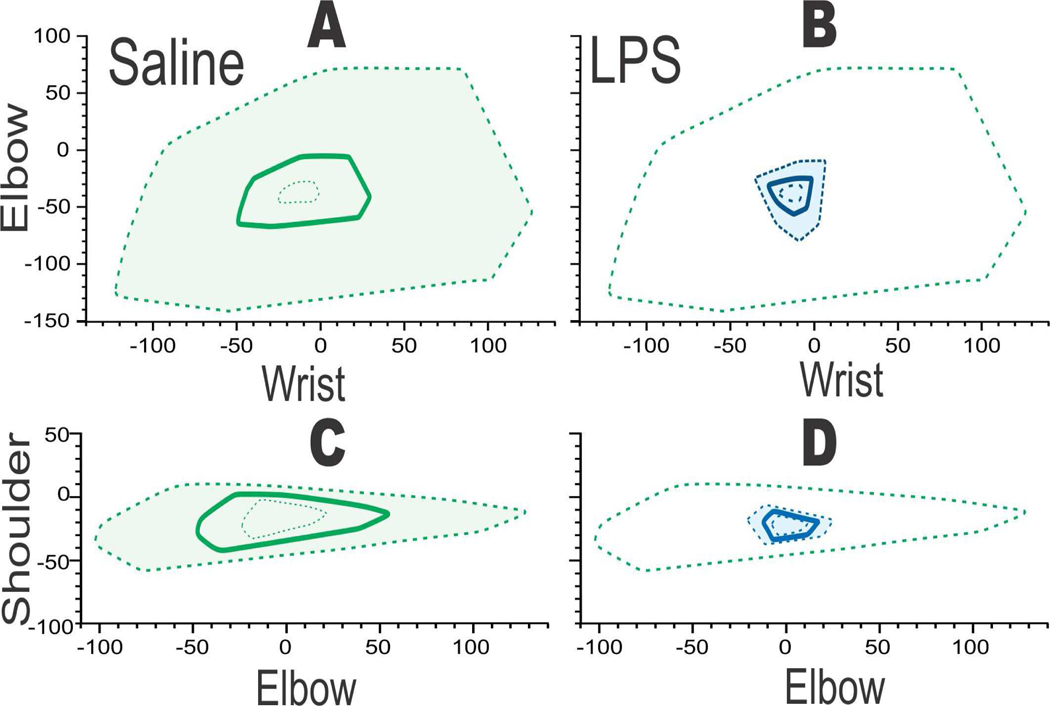
Confirmation of the locomotor deficits were obtained by doing two-joint analysis on a forced swim test, which has previously been shown to be sensitive to picking up subtle locomotor deficits [13]. At P1, one can easily visualize the differences between saline and LPS groups in Figure 5.
Figure 5.
Two joint analysis showing deficit in wrist-elbow and elbow-shoulder range of motion when saline (A,C green) was compared to LPS (B, D blue, n=3/group). Saline or LPS 0.2 mg/kg administered intracervically at E28 (89% gestation. After delivery, subtle locomotor deficits noticed at P1. Polygons showing mean (bold) with 95% confidence intervals in dashed lines. Green dashed line in B,D for reference of upper 95%tile of saline.
Hypertonia and Histopathological Change
Neurons exhibiting continuum cell death were not different between Hypertonic kits (two severe, one mild) vs Non-hypertonic kits (4 normal, one mild) at any of the 3 levels of the caudate (Table 4).
Table 4.
Caudate counts of neurons with cell death continuum in LPS kits at P1.
| LPS Group | Anterior | Mid | Posterior |
|---|---|---|---|
| Non-hypertonic | 54±14 (45) | 47±12 (34) | 39±8 (35) |
| Hypertonic | 28±5 (29) | 31±11 (34) | 26±11 (25) |
Legend: Caudate counts of neurons with cell death continuum in LPS kits at P1. Comparison of Hypertonic kits (n=3, including two severe and one mild) with Non-hypertonic kits (n=5, including one mild and 4 normal). Mean+SEM, (Median). No significant difference with two sample t-test. Power not calculated because Hypertonic kits showed a trend for less cell death.
Expression of inflammatory cytokines
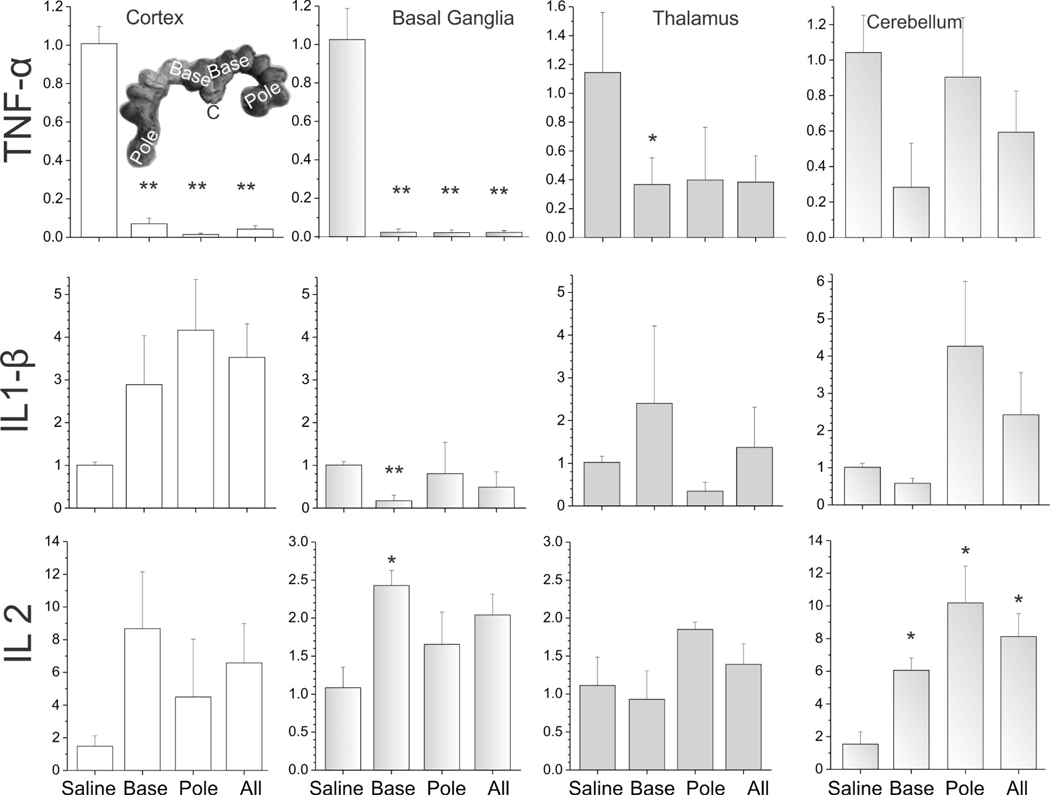
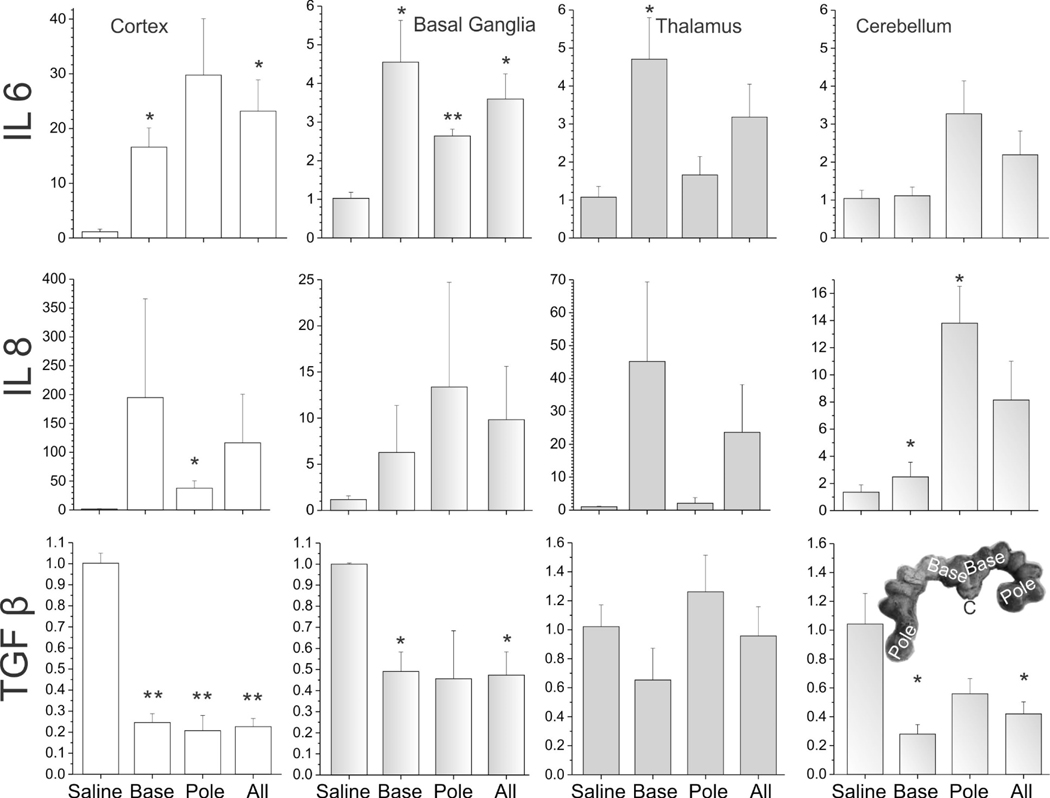
Compared to the saline group at E29, the LPS group showed a significant increase in IL-2 in cerebellum, IL-6 in all regions, and IL-8 in cerebellum. In contrast, a decrease of TNF-α in cortex, basal ganglia and thalamus, and IL-1β in basal ganglia was observed. Similarly, there was also a decrease in TGF-β in cortex, basal ganglia and cerebellum (Figures 6,7).
Figure 6.
The expression of cytokines in different brain regions of E29 fetus. TNF-α, IL-1 β, IL-2. The unit of Y-axis is fold change, with n=3–4 in each group. The 4 columns represent the saline group, the base group, the pole group, and LPS group (all=base + pole), respectively. *p<0.05; **p<0.01, compared with the saline group.
Figure 7.
The expression of cytokines in different brain regions of E29 fetus. IL-6, IL-8 and TGF-β. The unit of Y-axis is fold change, with n=3–4 in each group. The 4 columns represent the saline group, the base group, the pole group, and LPS group (all=base + pole), respectively. *p<0.05; **p<0.01, compared with the saline group.
In order to find out whether there were differences in the spread of LPS, we compared fetuses collected from cervical end or the “base” with those collected from the farthest point away from the cervix or “pole” of the uterus (see insets in Figure 6–9). Most of the regions showed no significant differences between the base and pole fetuses in all the cytokines tested except for a relative increase in cerebellum for IL-6 in pole fetuses (Figure 7), indicating that the LPS injection had reached the farthest fetuses and caused inflammation in all fetal brains irrespective of position in the uterus.
Figure 9.
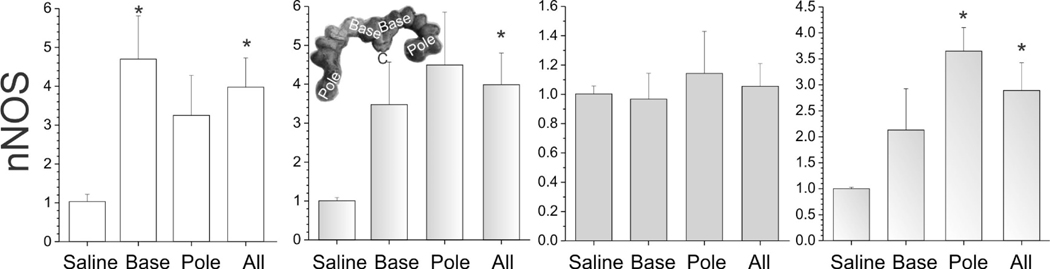
The expression of nNOS in different brain regions of E29 fetus. The unit of Y-axis is fold change, with n=3–4 in each group. The 4 columns represent the saline group, the base group, the pole group, and LPS group (all=base + pole), respectively. *p<0.05; **p<0.01, compared with the saline group.
Expression of perinatal BH4 biosynthesis enzymes
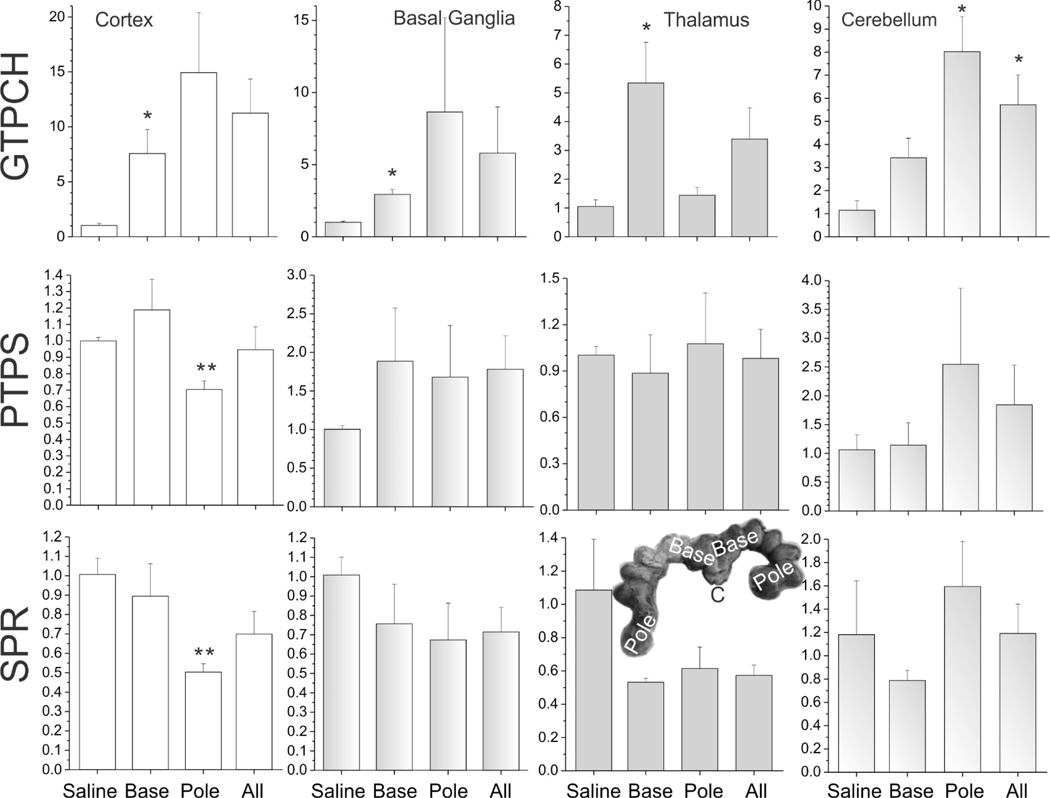
We compared expression levels between LPS and saline-treated groups for BH4 biosynthetic enzymes and nitric oxide synthase. Specifically, we analyzed GTP cyclohydrolase I (GTPCH), 6-pyruvoyltetrahydropterin synthase (PTS), quinoid dihydropteridine reductase (QDPR/DHPR), dihydrofolate reductase (DHFR) and sepiapterin reductase (SPR), using primers in Table 2. A significant increase in GTPCH (Figure 8) and nNOS was observed (Figure 9) in all brain regions in LPS group fetuses, except for thalamus for nNOS. There was a decrease in SPR expression in cortex of the LPS group fetuses (Figure 8). PTPS was decreased in the pole fetus cortex similar to SPR (Figure 8). We found no change in either QDPR/DHPR, or DHFR expression (data not shown).
Figure 8.
The expression of BH4 biosynthesis enzymes in different brain regions of E29 fetus: GTPCH, PTPT, and SPR. The unit of Y-axis is fold change, with n=3–4 in each group. The 4 columns represent the saline group, the base group, the pole group, and LPS group (all=base + pole), respectively. *p<0.05; **p<0.01, compared with the saline group.
Discussion
This was the first study to show a unique pattern of locomotor deficits following inflammation arising from endocervicitis in a pregnant rabbit model. Following intrauterine inflammation at near-term, P1 kits exhibited locomotor deficits, which was more common than hypertonia. At P11, locomotor abnormalities persisted with hypertonia improving in two-thirds of the kits. Sensory deficits tested by odorant response or tactile stimulation of eyes and face showed decreased response only amyl acetate at P11 in the LPS group. This was also the first study to show that LPS exposure in fetal brain affects gene expression of biosynthetic enzymes of BH4 in different ways.
In contrast to our previous H-I model at E22 and E25, the present LPS model showed less severe hypertonia and predominant locomotor abnormalities, and over time having persistent locomotor abnormalities but with improvement in hypertonia. In an attempt to clarify whether this transient nature of hypertonia was related to differences in amplitude of insult, we tested LPS greater than 0.2 mg/kg/ml, which resulted in numerous complications to the pregnant dam resulting in forced euthanasia and death, making it impossible to know the fate of the kits. It is possible that the advanced gestational age (E28 for the LPS model) is another possible explanation for the transient nature of hypertonia. Even in an HI model at E29, we observed about half the number of kits to improve in locomotor deficits with time in pilot data. Unlike the well-known model of myometrial injection of LPS, which results in a CP phenotype, the current chorioamnionitis LPS model caused less severe hypertonia. The clinical phenotype in most kits was more subtle with locomotor abnormalities predominating instead of clear cut postural changes accompanied by hypertonia.
This study also validated a fetal animal model for chorioamnionitis from ascending bacterial infection through endocervix, allowing a detailed investigation into the causal links between gram-negative bacterial infection and development of motor disorder. However, in humans, chorioamnionitis is not necessarily associated with white matter injury, in contrast to neonatal sepsis [17]. The evidence for a causal role of chorioamnionitis in CP is weak (recently reviewed by us), especially when the confounding factor of preterm delivery is excluded [18].
We were unable to find an etiological link between histopathological changes and severely affected kits in this model, as the caudate counts of neurons exhibiting continuum cell death were not significantly different at P1. There may have been Type II error but the trend showed that the numbers were lower in hypertonic group, which raises the possibility that the timing of peak cell death may be earlier or later. These studies investigating etiological relationship of cell death to motor deficits suffers from the drawback of having to sacrifice the animals before establishment of the phenotype of persistent hypertonia.
There are many animal models investigating both the effects of bacteria and LPS in ascending infection, and the role of cytokines produced by the immune system [19]. Inflammation in rodents also causes little hypertonia or postural deficits [20,21]. We chose rabbits because they are perinatal brain developers similar to humans [22,23]. Most perinatal studies have been done in rodents where development is postnatal. In contrast, higher mammals including non-human primates have prenatal development with motor function adequate at time of birth or very soon after. Rabbits also have low circulating oxidant-generating systems, such as xanthine oxidase in contrast to rodents.
The present LPS-chorioamnionitis model indicated a widespread inflammatory reaction in brains from both the base and the pole fetuses, allaying ab initio concern of possible absence of spread of LPS. Dye injection through the cannula showed that dye could reach the pole (Figure not shown for brevity). We also showed that most inflammatory cytokines increased in fetal brain regions including IL-2, IL-6, and IL-8, which was expected after LPS injection. IL-1β showed mixed results. Interestingly, TNF-α and TGF-β showed a decrease. We have no exact explanation for the decrease. Given the limited n, the non-significant differences could have been prone to Type II error. Also, because we replicated the 24 hour time point from the previous rabbit myometritis study, we could not be sure whether these inflammatory changes could be linked to neurobehavioral deficits at 4 days post-insult (P1). There may be greater involvement in the base but the fact that cytokines did increase in the pole fetuses suggested inflammatory changes were widespread in the uterus. The overall trend was that cytokines did change in LPS fetuses compared to saline.
This was the first time rabbit gene sequences of the BH4 biosynthetic enzymes were successfully employed in a translational rabbit model. Investigating the BH4 pathway, there was an increase in GTPCH but possibly a decrease in SPR and PTPS. Further studies need to be done to understand the significance of these changes in terms of neuronal activity and survival next.
Source and dose of LPS
In previous studies using LPS, the range of dose was wide and there were differences in bacterial origin, subtype, batch, and method of preparation of LPS. We have identified a need for comparison between animal species and other laboratories (see review [24]). The dose of endocervical LPS in rabbits has not been published before and effective dose for desired effect may need to be tested with every protocol. Previous publications in rabbits have used from 0.02–0.04 mg/kg [25] to 2 mg/kg [26] by myometrial injection. Rodents are less sensitive to LPS than rabbits and doses of 10 mg/kg have been used for intrauterine injection [27].
In our study, we used LPS produced from Escherichia coli O22:B55 (catalog # L4524), which is purified by ion exchange chromatography, and TLR ligand tested. It contains ≤1% protein, ≤1% RNA, and has a potency of ≥5×105 EU/mg. The induced recognition is predominantly mediated by TLR4 pathways without affecting TLR-2 pathways [24], which might partly explain high IL-6 and low TNF-α found in our present model.
The use of LPS as a source of inflammation allowed for investigating a Gram negative bacterial cell wall component as a pathogenic source. There is controversy whether whole bacteria are preferable to LPS. If whole bacteria were to be used, then probably the best technique would be to use attenuated bacteria or dead bacteria because there would be no risk of live bacterial proliferation and systemic complications secondary to infection.
Inflammation and Tetrahydrobiopterin Pathway
Tetrahydrobiopterin is also a co-factor for enzymes involved in the phenylalanine, dopamine, and serotonin pathways in addition to NOS. A low baseline BH4 expression is observed in normal premature fetal brains [7]. Cytokines have been shown to up-regulate BH4 synthesis [28]. However, this study showed that the interaction of BH4 pathway with LPS may be quite complicated on many levels. Increased GTPCH expression and low PTS expression may actually only increase levels of neopterin [8], which has pro-oxidant activity [29]. The consequences of oxidant activity is increased by the increase of nNOS expression and hence increased nitric oxide production. This could result in deleterious effects on fetal brain from reactive nitrogen species. Supposed inhibition of nNOS by 7-nitroindazole ameliorates the effect of LPS on spatial memory and excitatory post-synaptic potential [30]. Interestingly, the absence of nNOS does not change the response of LPS in knockout mice [31]. Overlaid on this complex situation is the effect on tryptophan metabolism. Kyneurenine metabolites from tryptophan are upregulated by cytokines and one of the metabolites, xanthurenic acid, inhibits SPR and subsequent BH4 biosynthesis [32]. We did observe a direct down-regulation of expression of SPR in fetal brains by LPS, which has not been reported before.
Conclusions
This is the first study to show that an ascending chorioamnionitis model could elicit motor deficits with little sensory deficits to odorants or to tactile stimuli to eyes or face. Most of the motor deficits were locomotor deficits which persisted, but hypertonia was mostly transient at the 200 μg/kg/ml dose of LPS. Higher dose of 1000 μg/kg/ml caused fetal death. This is the first study demonstrating the gene expression of biosynthetic enzymes of BH4 in the rabbit. The cytokine results showed that this model of ascending inflammation was valid as the inflammatory changes were widespread. Different biosynthetic enzymes of the tetrahydrobiopterin pathway responded differently to an inflammatory insult with opposing results. We did not find any particular pattern identifying a particular regional involvement in either cytokines or BH4. Further studies are needed to characterize the different pathways stemming from BH4 in response to LPS.
Acknowledgments
This study was supported in part by NINDS NIH R01 NS081936 (ST).
Footnotes
Conflict of Interest
The authors declare no conflicts of interest.
References
- 1.Graham HK, Rosenbaum P, Paneth N, Dan B, Lin JP, Damiano DL, Becher JG, Gaebler-Spira D, Colver A, Reddihough DS, Crompton KE, Lieber RL. Cerebral palsy. Nat Rev Dis Primers 2016;2:15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koman LA, Smith BP, Shilt JS: Cerebral palsy. Lancet 2004;363:1619–1631. [DOI] [PubMed] [Google Scholar]
- 3.Yoon BH, Park CW, Chaiworapongsa T: Intrauterine infection and the development of cerebral palsy. BJOG 2003;110 Suppl 20:124–127. [DOI] [PubMed] [Google Scholar]
- 4.Saadani-Makki F, Kannan S, Lu X, Janisse J, Dawe E, Edwin S, Romero R, Chugani D : Intrauterine administration of endotoxin leads to motor deficits in a rabbit model: a link between prenatal infection and cerebral palsy. Am J Obstet Gynecol 2008;199:651.e1-e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu L, Vásquez-Vivar J, Jiang R, Luo K, Derrick M, Tan S: Developmental susceptibility of neurons to transient tetrahydrobiopterin insufficiency and antenatal hypoxia-ischemia in fetal rabbits. Free Radic Biol Med 2014;67:426–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu L, Derrick M, Ji H, Silverman RB, Whitsett J, Vásquez-Vivar J, Tan S: Neuronal nitric oxide synthase inhibition prevents cerebral palsy following hypoxia-ischemia infetal rabbits: comparison between JI-8 and 7-nitroindazole. Dev Neurosci 2011;33:312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vasquez-Vivar J, Whitsett J, Derrick M, Ji X, Yu L, Tan S: Tetrahydrobiopterin in the prevention of hypertonia in hypoxic fetal brain. Ann Neurol 2009;66:323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Werner ER, Werner-Felmayer G, Fuchs D, Hausen A, Reibnegger G, Yim JJ, Pfleiderer W, Wachter H: Tetrahydrobiopterin biosynthetic activities in human macrophages, fibroblasts, thp-1, and t 24 cells. Gtp-cyclohydrolase i is stimulated by interferon-gamma, and 6-pyruvoyl tetrahydropterin synthase and sepiapterin reductase are constitutively present. J Biol Chem 1990;265:3189–3192. [PubMed] [Google Scholar]
- 9.Oxenkrug GF: Interferon-gamma-inducible kynurenines/pteridines inflammation cascade: Implications for aging and aging-associated psychiatric and medical disorders. J Neural Transm 2011;118:75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ota A, Kaneko YS, Mori K, Nakashima A, Nagatsu I, Nagatsu T: Effect of peripherally administered lipopolysaccharide (LPS) on GTP cyclohydrolase I, tetrahydrobiopterin and norepinephrine in the locus coeruleus in mice. Stress 2007;10:131–136. [DOI] [PubMed] [Google Scholar]
- 11.Derrick M, Luo NL, Bregman JC, Jilling T, Ji X, Fisher K, Gladson CL, Beardsley DJ, Murdoch G, Back SA, Tan S: Preterm fetal hypoxia-ischemia causes hypertonia and motor deficits in the neonatal rabbit: a model for human cerebral palsy? J Neurosci 2004;24:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Damiano DL, Quinlivan JM, Owen BF, Payne P, Nelson KC, Abel MF: What does the Ashworth scale really measure and are instrumented measures more valid and precise? Dev Med Child Neurol 2002;44:112–118. [DOI] [PubMed] [Google Scholar]
- 13.Derrick M, Drobyshevsky A, Ji X, Chen L, Yang Y, Ji H, Silverman RB, Tan S: Hypoxia-ischemia causes persistent movement deficits in a perinatal rabbit model of cerebral palsy: assessed by a new swim test. Int J Dev Neurosci 2009;27:549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li C, Xu C, Gui C, Fox MD. Distance regularized level set evolution and its application to image segmentation. IEEE Trans Image Process 2010;19:3243–3254. [DOI] [PubMed] [Google Scholar]
- 15.Northington FJ, Zelaya ME, O’Riordan DP, Blomgren K, Flock DL, Hagberg H, Ferriero DM, Martin LJ: Failure to complete apoptosis following neonatal hypoxia-ischemia manifests as “continuum” phenotype of cell death and occurs with multiple manifestations of mitochondrial dysfunction in rodent forebrain. Neuroscience 2007;149:822–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Z, Jyoti A, Balakrishnan B, Williams M, Singh S, Chugani DC, Kannan S: Trajectory of inflammatory and microglial activation markers in the postnatal rabbit brain following intrauterine endotoxin exposure. Neurobiol Dis 2018;111:153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chau V, McFadden DE, Poskitt KJ, Miller SP. Chorioamnionitis in the pathogenesis of brain injury in preterm infants. Clin Perinatol 2014;41:83–103. [DOI] [PubMed] [Google Scholar]
- 18.Shi Z, Ma L, Luo K, Bajaj M, Chawla S, Natarajan G, Hagberg H, Tan S. Chorioamnionitis in the Development of Cerebral Palsy: A Meta-analysis and Systematic Review. Pediatrics 2017;139. pii: e20163781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDuffie RS, Gibbs RS: Animal models of ascending genital-tract infection in pregnancy. Infect Dis Obstet Gynecol 1994; 2:60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harre EM, Galic MA, Mouihate A, Noorbakhsh F, Pittman QJ: Neonatal inflammation produces selective behavioural deficits and alters N-methyl-D-aspartate receptor subunit mRNA in the adult rat brain. Eur J Neurosci 2008;27:644–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Girard S, Kadhim H, Beaudet N, Sarret P, Sebire G: Developmental motor deficits induced by combined fetal exposure to lipopolysaccharide and early neonatal hypoxia/ischemia: a novel animal model for cerebral palsy in very premature infants. Neuroscience 2009;158:673–682. [DOI] [PubMed] [Google Scholar]
- 22.Harel S, Watanabe K, Linke I, Schain RJ: Growth and development of the rabbit brain. Biol Neonate 1972;21:381–399. [DOI] [PubMed] [Google Scholar]
- 23.Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev 1979;3:79–83. [DOI] [PubMed] [Google Scholar]
- 24.Dean JM, Shi Z, Fleiss B, Gunn KC, Groenendaal F, van Bel F, Derrick M, Juul SE, Tan S, Gressens P, Mallard C, Bennet L, Gunn AJ: A Critical Review of Models of Perinatal Infection. Dev Neurosci 2015;37:289–304. [DOI] [PubMed] [Google Scholar]
- 25.Kannan S, Saadani-Makki F, Muzik O, Chakraborty P, Mangner TJ, Janisse J, Romero R, Chugani DC: Microglial activation in perinatal rabbit brain induced by intrauterine inflammation: detection with 11C-(R)-PK11195 and small-animal PET. J Nucl Med 2007;48:946–954. [DOI] [PubMed] [Google Scholar]
- 26.Rygg M: Cytokine-induced differential expression of serum amyloid A genes in fetal and neonatal rabbits. Clin Exp Immunol 1996;103:335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Breen K, Brown A, Burd I, Chai J, Friedman A, Elovitz MA: TLR-4-dependent and - independent mechanisms of fetal brain injury in the setting of preterm birth. Reprod Sci 2012;19:839–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Werner ER, Werner-Felmayer G, Mayer B: Tetrahydrobiopterin, cytokines, and nitric oxide synthesis. Proc Soc Exp Biol Med 1998;219:171–182. [DOI] [PubMed] [Google Scholar]
- 29.Fuchs D, Avanzas P, Arroyo-Espliguero R, Jenny M, Consuegra-Sanchez L, Kaski JC: The role of neopterin in atherogenesis and cardiovascular risk assessment. Curr Med Chem 2009;16:4644–4653. [DOI] [PubMed] [Google Scholar]
- 30.Anaeigoudari A, Soukhtanloo M, Shafei MN, Sadeghnia HR, Reisi P, Beheshti F, Behradnia S, Mousavi SM, Hosseini M: Neuronal nitric oxide synthase has a role in the detrimental effects of lipopolysaccharide on spatial memory and synaptic plasticity in rats. Pharmacol Rep 2016;68:243–249. [DOI] [PubMed] [Google Scholar]
- 31.Garcia JA, Ortiz F, Miana J, Doerrier C, Fernandez-Ortiz M, Rusanova I, Escames G, Garcia JJ, Acuna-Castroviejo D: Contribution of inducible and neuronal nitric oxide synthases to mitochondrial damage and melatonin rescue in lps-treated mice. J Physiol Biochem 2017. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 32.Haruki H, Hovius R, Pedersen MG, Johnsson K: Tetrahydrobiopterin biosynthesis as a potential target of the kynurenine pathway metabolite xanthurenic acid. J Biol Chem 2016;291:652–657 [DOI] [PMC free article] [PubMed] [Google Scholar]