Abstract
Background
Although the COVID-19 pandemic has persisted for more than two years with the evident excess mortality from diabetes, few studies have investigated its temporal patterns. This study aims to estimate the excess deaths from diabetes in the United States (US) during the COVID-19 pandemic and evaluate the excess deaths by spatiotemporal pattern, age groups, sex, and race/ethnicity.
Methods
Diabetes as one of multiple causes of death or an underlying cause of death were both considered into analyses. The Poisson log-linear regression model was used to estimate weekly expected counts of deaths during the pandemic with adjustments for long-term trend and seasonality. Excess deaths were measured by the difference between observed and expected death counts, including weekly average excess deaths, excess death rate, and excess risk. We calculated the excess estimates by pandemic wave, US state, and demographic characteristic.
Results
From March 2020 to March 2022, deaths that diabetes as one of multiple causes of death and an underlying cause of death were about 47.6 % and 18.4 % higher than the expected. The excess deaths of diabetes had evident temporal patterns with two large percentage increases observed during March 2020, to June 2020, and June 2021 to November 2021. The regional heterogeneity and underlying age and racial/ethnic disparities of the excess deaths were also clearly observed.
Conclusions
This study highlighted the increased risks of diabetes mortality, heterogeneous spatiotemporal patterns, and associated demographic disparities during the pandemic. Practical actions are warranted to monitor disease progression, and lessen health disparities in patients with diabetes during the COVID-19 pandemic.
Keywords: COVID-19, Pandemic waves, Excess deaths, Demographic analyses, Temporal pattern
Introduction
Diabetes mellitus is a metabolic disease that needs continuing management, close monitoring, and medication. It is one of the leading causes of mortality, with more than one million deaths worldwide per year [1]. In the United States (US), death rate of diabetes has declined steadily between 2000 and 2019, according to Global Burden of Disease Study 2019 [2]. However, large increases in deaths associated with diabetes has been observed in the early stage of the COVID-19 pandemic [3], [4]. It could attribute to detected or undetected COVID-19 cases from patients with diabetes, varied restrictions imposed by the pandemic and reductions in healthcare delivery for acute emergencies or chronic disease management during the pandemic [5], [6], [7]. Timely monitoring of excess deaths from diabetes during the COVID-19 pandemic is necessary to adjust public health strategies to address the large and growing burden of diabetes.
Since 2020, the continuingly mutated SARS-CoV-2 has caused multiple pandemic waves in the US, which might lead to temporal disparity in excess mortality from diabetes. Across different pandemic waves, number of COVID-19 cases and deaths vary widely [8]. Population vaccination rates and strict restrictions imposed by the government (e.g., stay-at-home orders) are constantly changing as well. The negative impact of different pandemic waves on the people of diabetes may therefore differ, resulting in temporal disparity in excess mortality from diabetes. To our knowledge, this is the first study evaluating the temporal pattern of excess mortality from diabetes by COVID-19 pandemic waves. Furthermore, comparisons of excess mortality from diabetes by population subgroups and states allow for a complete assessment of the differential impact of the pandemic on diabetes mortality in different subgroups.
In this study we aimed to estimate the excess deaths from diabetes between March 2020 and March 2022 in the US, investigate the pattern of these deaths across multiple pandemic waves, and evaluate the deaths by age, sex, race/ethnicity, and state.
Methods
We obtained weekly death data between January 2018 and July 2022 from the Wide-ranging Online Data for Epidemiologic Research database (WONDER) of the Centers for Disease Control and Prevention (CDC) [9]. Since diabetes are usually associated with other medical conditions and disabilities (i.e., ischemic heart disease, renal or visual impairment etc.,) and sometimes was not be listed as the primary cause of death [10], we considered two kinds of death data in our analysis: 1) deaths associated with diabetes when diabetes was listed as one of multiple causes on the death certificate, and 2) deaths that diabetes was listed as the single underlying cause of death. Those deaths from diabetes were coded as E10-E14 according to the tenth revision of the International Classification of Diseases. The coding procedure for the underlying or multiple causes of death can be found in National Vital Statistics System from CDC [11], [12]. Death counts across US states and by age, sex, and race/ethnicity were also retrieved. Considering the potential reporting lag bias, we excluded the most recent data after March 2022. Census data by states between 2015 and 2020 were obtained from the US Census Bureau [13]. Population information in 2021 and 2022 was projected according to the baseline population from 2015 to 2020. Because the data used was de-identified and publicly available, this study was exempted from institutional review board approval.
The weekly number of excess deaths was calculated by the observed deaths subtracting the expected deaths. The death sequence of diabetes from January 2018 to February 2020 was fitted with the Poisson log-linear regression model and controlled for time-varying patterns. Then, the fitted model was adopted to project the expected deaths from March 2020 to March 2022 [14]. The quasi-Poisson family was adopted in the model prediction since the overdispersion feature of the data was detected. The time-varying pattern included temporal trend and seasonality components. The temporal trend was controlled with a linear term for the calendar year (2018–2020 in the model fitting, 2020–2022 in the prediction). The seasonality was parameterized using a natural spline function for epidemiological weeks (from week 1 to week 52). Nine candidate models (natural spline function with the degree of freedom from 4 to 12) were tested through the maximum likelihood approach. The natural spline function with degree of freedom of 9 was used in the analyses based on the lowest Akaike information criterion (AIC) score. The subgroup data was fitted its own Poisson regression model with the same degree of freedom for the natural spline function. For the overall and subgroup estimates, we calculated the following estimates with 95 % confidence interval (CI) measured by mean estimate± 1.96 standard error (SE): excess death number (observed death counts subtracted expected death counts), excess death number per week (weekly average excess death number within specific periods), excess death rates (excess death number per million persons) and excess risk (the ratio between excess death number and expected death number × 100 %), respectively. Subgroup analyses were performed by age (20–64 years, 65–84 years, ≥ 85 years), sex (male and female), race/ethnicity (non-Hispanic White, non-Hispanic Black, non-Hispanic Asian, and Hispanic), pandemic years (March 2020 - February 2021 vs March 2021 - March 2022), and pandemic waves. The pandemic waves were identified according to the COVID mortality surveillance data from CDC, where showed five COVID-19 death waves by the end of March 2022 [8]. Thus, the pandemic waves from Wave I to V were defined as follows: March 1 2020 to June 6 2020 (Wave I), June 7 2020 to October 3 2020 (Wave II), October 4 2020 to June 26 2021 (Wave III), June 27 2021 to November 27 2021 (Wave IV), and November 28 2021 to March 26 2022 (Wave V).
Sensitivity analyses were operated to verify the robustness of fitting for seasonal effect of baseline curve. First, the nature splines with degrees of freedom of 6 and 12 (degree of freedom of 9 was used in the main analysis) were utilized in the model. Second, the p-spline with degree of freedom of 6 was used (degrees of freedom from 4 to 16 were tested, and 6 was then chosen according to the lowest AIC value). This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines. All analyses were done with R statistical software (version 3.6.1).
Results
Between March 2020, and March 2022, there were a 47.6 % (95 % CI, 41.7–53.1) increase in deaths associated with diabetes that diabetes was shown as one of multiple causes of death on the death certificate. The increased percentage projected to 270,591 (95 % CI, 237, 294–302,016) excess deaths in the US. Among the excess death number, there were 35,093, 36,185, 98,263, 55,857, and 45,193 deaths occurred in Waves I to V, separately ( Table 1). For the deaths that diabetes was shown as the underlying causes of death, there were a 18.4 % (95 % CI 15.1 %−21.7 %) increase, which projected to 33,603 (95 % CI, 27,514–39,508) excess deaths. Among them, there were 4653, 5153, 10,907, 7269, and 5621 deaths occurred in Waves I to V, separately ( Table 2).
Table 1.
Excess mortality associated with diabetes in the United States from March 2020, to March 2022.
| Subgroups | Observed death number | Excess death number (95 % CI) |
Excess death number per week (95 % CI) |
Excess death rates per million persons (95 % CI) | Excess risk % (95 % CI) |
|---|---|---|---|---|---|
| Overalla | 839,591 | 270,591 (237,294, 302,016) | 2505 (2197, 2796) | 828.6 (726.6, 924.8) | 47.6 (41.7, 53.1) |
| Age groups | |||||
| Age 20–64 | 207,208 | 70,397 (60,661, 79,466) | 652 (562, 736) | 366.1 (315.5, 413.3) | 51.5 (44.3, 58.1) |
| Age 65–84 | 453,199 | 143,899 (125,604, 161,153) | 1332 (1163, 1492) | 3146.0 (2746.0, 3523.2) | 46.5 (40.6, 52.1) |
| Age 85 + | 178,217 | 50,139 (42,466, 57,373) | 464 (393, 531) | 7571.8 (6413.0, 8664.2) | 39.1 (33.2, 44.8) |
| Sex | |||||
| Female | 366,615 | 115,938 (101,774, 129,327) | 1074 (942, 1197) | 699.5 (614.0, 780.2) | 46.2 (40.6, 51.6) |
| Male | 472,976 | 154,670 (135,168, 173,030) | 1432 (1252, 1602) | 961.8 (840.5, 1075.9) | 48.6 (42.5, 54.4) |
| Race/ethnicity | |||||
| NH-White | 548,412 | 130,032 (109,664, 149,432) | 1204 (1015, 1384) | 662.6 (558.8, 761.4) | 31.1 (26.2, 35.7) |
| NH-Black | 134,498 | 57,360 (50,594, 63,571) | 531 (468, 589) | 1434.2 (1265, 1589.5) | 74.4 (65.6, 82.4) |
| NH-Asian | 29,268 | 12,500 (10,774, 14,054) | 116 (100, 130) | 687.4 (592.5, 772.9) | 74.5 (64.3, 83.8) |
| Hispanic | 107,358 | 60,499 (54,722, 65,636) | 560 (507, 608) | 1019.2 (921.9, 1105.7) | 129.1 (116.8, 140.1) |
| Pandemic year | |||||
| Mar 2020 - Feb 2021 | 416,668 | 140,110 (126,094, 153,450) | 2694 (2425, 2951) | 429.0 (386.1, 469.9) | 50.7 (45.6, 55.5) |
| Mar 2021 – Mar 2022 | 422,923 | 130,481 (111,200, 148,566) | 2330 (1986, 2653) | 399.6 (340.5, 454.9) | 44.6 (38.0, 50.8) |
| Pandemic wavesb | |||||
| Wave I | 105,817 | 35,093 (31,624, 38,401) | 2507 (2259, 2743) | 107.5 (96.8, 117.6) | 49.6 (44.7, 54.3) |
| Wave II | 119,659 | 36,185 (32,023, 40,150) | 2129 (1884, 2362) | 110.8 (98.1, 122.9) | 43.3 (38.4, 48.1) |
| Wave III | 304,875 | 98,263 (86,555, 109,333) | 2586 (2278, 2877) | 300.9 (265.0, 334.8) | 47.6 (41.9, 52.9) |
| Wave IV | 165,447 | 55,857 (48,765, 62,520) | 2539 (2217, 2842) | 171.0 (149.3, 191.4) | 51.0 (44.5, 57.0) |
| Wave V | 143,793 | 45,193 (38,327, 51,612) | 2658 (2255, 3036) | 138.4 (117.4, 158.0) | 45.8 (38.9, 52.3) |
Abbreviation: CI, confidence interval.
Excess estimates in this table were measured according to the multiple causes of death that diabetes was listed anywhere on the death certificate.
Wave I was from Week 10 March 1, 2020, to Week 23 June 6 2020, Wave II was from Week 24 June 7 2020 to Week 40 October 3 2020, Wave III was from Week 41 October 4 2020 to Week 25 June 26 2021, Wave IV was from Week 26 June 27 2021 to Week 47 November 27 2021, and Wave V was from Week 48 November 28 2021 to Week 12 March 26 2022, according to the daily surveillance of COVID-19 deaths in the US reported to CDC (shown at https://covid.cdc.gov/covid-data-tracker/#trends_dailydeaths).
Table 2.
Excess mortality of diabetes in the United States from March 2020, to March 2022, when diabetes was the underlying cause of death.
| Subgroups | Observed death number | Excess death number (95 % CI) |
Excess death number per week (95 % CI) |
Excess death rates per million persons (95 % CI) |
Excess risk % (95 % CI) |
|---|---|---|---|---|---|
| Overalla | 216,071 | 33,603 (27,514, 39,508) | 311 (255, 366) | 102.9 (84.3, 121.0) | 18.4 (15.1, 21.7) |
| Age groups | |||||
| Age 20–64 | 62,717 | 11,788 (9376, 14,088) | 109 (87, 130) | 61.3 (48.8, 73.3) | 23.1 (18.4, 27.7) |
| Age 65–84 | 112,229 | 15,501 (12,164, 18,727) | 144 (113, 173) | 338.9 (265.9, 409.4) | 16.0 (12.6, 19.4) |
| Age 85 + | 39,336 | 4858 (3226, 6411) | 45 (30, 59) | 733.6 (487.2, 968.2) | 14.1 (9.4, 18.6) |
| Sex | |||||
| Female | 93,689 | 15,675 (12,809, 18,441) | 145 (119, 171) | 94.6 (77.3, 111.3) | 20.1 (16.4, 23.6) |
| Male | 122,382 | 17,918 (14,070, 21,626) | 166 (130, 200) | 111.4 (87.5, 134.5) | 17.2 (13.5, 20.7) |
| Race/ethnicity | |||||
| NH-White | 136,302 | 15,298 (11,581, 18,900) | 142 (107, 175) | 78.0 (59.0, 96.3) | 12.6 (9.6, 15.6) |
| NH-Black | 40,351 | 9837 (7893, 11,660) | 91 (73, 108) | 246.0 (197.4, 291.5) | 32.2 (25.9, 38.2) |
| NH-Asian | 7758 | 1880 (1289, 2423) | 17 (12, 22) | 103.4 (70.9, 133.2) | 32.0 (21.9, 41.2) |
| Hispanic | 25,762 | 5905 (4545, 7192) | 55 (42, 67) | 99.5 (76.6, 121.2) | 29.7 (22.9, 36.2) |
| Pandemic year | |||||
| Mar 2020 - Feb 2021 | 104,657 | 16,972 (14,438, 19,439) | 326 (278, 374) | 52.0 (44.2, 59.5) | 19.4 (16.5, 22.2) |
| Mar 2021 - Mar 2022 | 111,414 | 16,631 (13,076, 20,069) | 297 (234, 358) | 50.9 (40.0, 61.5) | 17.5 (13.8, 21.2) |
| Pandemic wavesb | |||||
| Wave I | 27,715 | 4653 (4013, 5277) | 332 (287, 377) | 14.2 (12.3, 16.2) | 20.2 (17.4, 22.9) |
| Wave II | 31,827 | 5153 (4395, 5890) | 303 (259, 346) | 15.8 (13.5, 18.0) | 19.3 (16.5, 22.1) |
| Wave III | 76,840 | 10,907 (8769, 12,982) | 287 (231, 342) | 33.4 (26.9, 39.8) | 16.5 (13.3, 19.7) |
| Wave IV | 42,751 | 7269 (5963, 8531) | 330 (271, 388) | 22.3 (18.3, 26.1) | 20.5 (16.8, 24.0) |
| Wave V | 36,938 | 5621 (4374, 6828) | 331 (257, 402) | 17.2 (13.4, 20.9) | 17.9 (14.0, 21.8) |
Abbreviation: CI, confidence interval.
Excess estimates in this table were measured according to the single underlying cause of death shown on the death certificate.
Wave I was from Week 10 March 1, 2020, to Week 23 June 6 2020, Wave II was from Week 24 June 7 2020 to Week 40 October 3 2020, Wave III was from Week 41 October 4 2020 to Week 25 June 26 2021, Wave IV was from Week 26 June 27 2021 to Week 47 November 27 2021, and Wave V was from Week 48 November 28 2021 to Week 12 March 26 2022, according to the daily surveillance of COVID-19 deaths in the US reported to CDC (shown at https://covid.cdc.gov/covid-data-tracker/#trends_dailydeaths).
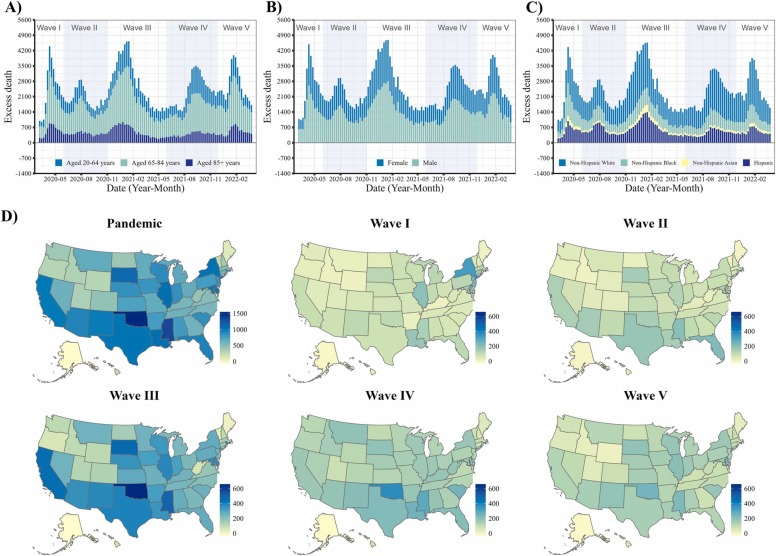
The increased deaths associated with diabetes were more pronounced in Wave I (March 1, 2020, to June 6, 2020) and Wave IV (June 27, 2021 to November 27, 2021) with excess risks of 49.6 % (95 % CI, 44.7–54.3 %) in Wave I and 51.0 % (95 % CI, 44.5–57.0 %) in Wave IV, respectively. Wave III (October 4 2020 to June 26 2021) and Wave IV (June 27, 2021 to November 27, 2021) showed larger excess death rates than other waves (Table 1 and Fig. 1). The excess risks from March 2021 to March 2022 was slightly attenuated than that of March 2020 to February 2021 (excess risk, 50.7 % [95 % CI, 45.6–55.5 %] and 44.6 % [95 % CI, 38.0–50.8 %]) (Table 1). The excess death estimates that diabetes was the underlying cause of death showed consistent temporal patterns, while the magnitude of excess death rate and excess risk were about one-eighth to one-third than the estimates associated with diabetes by the multiple causes of death among the whole US population (Table 2 and Fig. S1-S3). The results were similar in the sensitivity analyses of robustness.
Fig. 1.
Excess death rates of diabetes per million in the United States from March 2020 to March 2022, when diabetes was one of the multiple causes of death. Weekly number of excess deaths by A) age group (20–64 years, 65–84 years and 85 + years); B) sex (male and female); C) race/ethnicity (non-Hispanic White, non-Hispanic Black, non-Hispanic Asian, and Hispanic); D) excess death rates per million across US states by the COVID-19 death waves.
The excess deaths associated with diabetes disproportionately affected people of various races/ethnicities. The excess risks were about 2–4 times higher in Hispanic, non-Hispanic Black, and non-Hispanic Asian people than non-Hispanic White people (129.1 % [95 % CI, 116.8–140.1 %] in Hispanic, 74.4 % [95 % CI, 65.6–82.4 %] in Black, 74.5 % [95 % CI, 64.3–83.8 %] in Asian, and 31.1 % [95 % CI, 26.2–35.7 %] in White people) (Table 1). The elderly and males had higher excess death rates than others (7571.8 per million persons [95 % CI, 6413.0 – 8664.2] in people aged 85 and above, and 961.8 per million persons [95 % CI, 840.5 – 1075.9] in males). Of note, there was a surge in mortality in people aged between 20 and 64 years, as indicated by the highest excess risk of death (51.5 % [95 % CI, 44.3–58.1 %]). The excess estimates of diabetes by demographic subgroup when diabetes was the underlying cause of death was shown in Table 2. The state-level estimates of excess deaths, excess death rates and excess risk across different pandemic periods were shown in Table S1-S4 for diabetes as the multiple causes of death, and Table S5-S8 for diabetes as the underlying cause of death. Although some states in the east (i.e., New York, New Jersey, Connecticut, Louisiana, Rhode Island, etc.) severely suffered from the excess deaths associated with diabetes at the early pandemic stage, they were attenuated later. On the contrary, some states (i.e., Arkansas, Kansas, Montana, etc.) showed worsen diabetes-associated excess deaths over time. The rest states showed mild fluctuation of excess risks associated with diabetes over time.
Discussion
We observed that substantial excess deaths of diabetes existed throughout the COVID-19 pandemic, in most U.S. states, and the temporal patterns were along with the COVID-19 mortality trajectory. Given the large sample available, we were able to demonstrate disparities in the risks of excess deaths across subgroups defined by demographic features, including age, sex, and race/ethnicity. Additionally, the temporal patterns of excess deaths associated with diabetes showed regional heterogeneity.
The finding of increase in diabetes mortality during the COVID-19 pandemic is consistent with a nationwide study in the UK, which indicated that at the early stage of the pandemic in 2020, the mortality rate in patients with diabetes increased by 0.19 (95 % CI, 0.14 – 0.23) in England and by 0.13 (95 % CI, 0.08 – 0.16) in other UK nations [15]. Herein, we add to the evidence by showing the long-term trajectory of excess diabetes mortality throughout the COVID-19 pandemic in the US, and disparities in mortality across age, sex, and racial/ethnic groups. Compared with several studies that estimated excess deaths when diabetes was the underlying causes of death, we also estimated excess deaths wherein diabetes was one of multiple causes of death, which could avoid underestimation when patients with diabetes died and their underlying cause of death was assigned to other causes, such as cardiovascular diseases, cancer, etc [10], [16]. The deaths classified by multiple causes of death also included individuals with co-occurrence of diabetes and COVID-19. As reported by the US CDC, for about 95 % deaths when COVID-19 was listed on the death certificate, their underlying cause of death was most likely to be coded to COVID-19 [17]. In turn, if diabetes was selected as the underlying causes, it’s unlikely those patients had detected COVID-19 infections. Thus, the estimates by using the underlying causes of death might represent an indirect impact of COVID-19 on patients with diabetes or misclassified COVID-19 deaths. Whereas the estimates by using diabetes as one of multiple causes of deaths could show the overall impact of the COVID-19 pandemic on patients with diabetes, included those infected with COVID-19 and those not.
There are several potential explanations for our findings. Previous evidence found there were many direct associations between the metabolic and endocrine systems and COVID-19 adverse outcomes. Patients with metabolic dysfunction showed a higher risk of COVID-19 infection and death. Whereas infection with SARS-CoV-2 might induce new-onset diabetes or exacerbate metabolic disorders [18]. Taken together, the COVID-19 infections resulted in substantial deaths co-occurring with diabetes. For the indirect impact of COVID-19, the pandemic induced frequent disruptions and severe delay of most routine care for many non-COVID-19-related conditions [15], [19]. Reductions in routine care delivery, diabetes care in particular, might contribute to higher non-COVID-19-related mortality in people with diabetes [20]. The reduction in HbA1c testing is another crucial risk factor of the excess death, because the absence of HbA1c data may pose challenges for physicians making appropriate treatment decisions [15]. Plausible explanations of excess deaths associated with diabetes also include the enormous effect of COVID-19 on social determinants of health (e.g. income, jobs and food security) and psychological distress.
No previous study has investigated the temporal pattern of excess deaths due to diabetes by COVID-19 pandemic waves. We found the temporal patterns of excess estimates by the underlying cause of death and multiple causes of deaths were similar and they were also synchronous with the temporal variation of COVID-19 mortality in the US [8]. This study observed two sharp increases in excess mortality associated with diabetes in Waves I and IV, and two large excess mortality waves in Waves III and IV. The temporal pattern could be explained that considerable proportion of diabetes-related deaths were attributed to COVID-19 because individuals with diabetes were vulnerable to COVID-19 infections, thus the temporal trend of excess diabetes associated deaths were partly and proportionally in line with that of COVID-19 deaths. In addition, the healthcare systems were unprepared for the sudden outbreaks during the initial wave and set strict restriction for pandemic control, which might lead to patients with other illness hard to access the emergency healthcare services [21]. With the development of the ongoing pandemic, the overwhelmed healthcare systems lead to interruptions on routine service delivery for chronic diseases such as diabetes. Part of patients might fail to maintain glycemic control levels in absence of the professional guidance and experience deterioration in diabetes progression [18], [22]. The large excess mortality increases in subsequent waves might be associated with substantial COVID-19 incidences which led to the sustained interruption of healthcare services. Since the initial wave, many expert concerns, guidelines, and modified treatment procedures have been raised to combat the pandemic and minimized the indirect impact to other chronic illness [23], [24], [25]. Thus, despite the infection rates of COVID-19 in the later pandemic waves were much higher than the initial wave, the excess diabetes mortality did not increase proportionally and there was slightly lower excess mortality in the second pandemic year.
Our results of racial/ethnic disparities in excess deaths of diabetes were consistent of early studies which demonstrated similar disparities in non-COVID-19 excess deaths [26], [27]. Those studies showed that the excess deaths in the early stage of the pandemic were two to four times higher in the non-white ethnic group, compared with the white ethnic group. Our study extends findings from prior work by providing a systematic overview of diabetes-specific deaths throughout the COVID-19 pandemic. The relative higher risk of deaths among racial/ethnic minorities during the pandemic could be attributed to disadvantaged socioeconomic position, more occupational exposures to COVID-19, living in more densely populated communities, and less access to health care facilities and private transportation.
We also captured the disproportionate excess deaths associated with diabetes among different age groups. Although older people suffered from substantial excess mortality burden of diabetes, the steepest rise in the excess death risk was seen in people aged between 20 and 64. Our findings are consistent with recent nationwide studies in England, suggesting a higher excess death risk in younger diabetics compared with older diabetics [5], [28], [29]. Young adults are less likely to meet the target of blood glucose control, having poorer adherence to diabetes management, and more likely to delay medical care during the pandemic than the elderly.
The potential regional heterogeneity in excess death due to diabetes is generally in line with previous studies in the early stage of COVID-19 pandemic [3]. The regional difference could be explained by different age, sex, race/ethnical structures, socioeconomic characteristics, healthcare coverage, access to health care services [30], [31], [32], [33]. Our study provided additional information on the time-varying regional patterns. The excess mortalities showed attenuated, worsen, or mildly variations over time in different states. The temporal disparities may be explained by the differences in scale and timing of the pandemic in each wave, dominant SARS-CoV-2 strains with different virulent profile, the progress of COVID-19 vaccination across regions, state-specific non-pharmaceutical interventions, efficiency of movement restrictions, and different magnitudes of the interruption of healthcare delivery [34], [35], [36], [37].
Several studies showed some demographic, lifestyle-related and clinical risk factors for COVID-19 deaths were also associated with non-COVID-19 deaths [38], [39]. While COVID-19 may multiply some risks, such as older age, male sex, ethnicity, deprivation, obesity, uncontrolled diabetes, etc. In addition, the long-term effects of post-COVID-19 sequelae may also contribute to increased mortality burden among patients with diabetes. A US veterans reported increased risks (hazard ratio, HR 1.4) and excess burdens of incident diabetes at 12 months (13.5 per 1000 persons) among COVID-19 survivors [40]. Such risks and burdens were higher in older people than adults younger than 65 years (HR 1.4), higher in Black people than White people (HR 1.6), higher in those with cardiovascular conditions than those without (HR 1.9). However, our estimates were limited by using aggregated death count data that could not explore associations of increased mortality risks with any baseline conditions or risk factors among patients with diabetes. Further studies with individual clinical data are warranted to quantify risk factors associated with adverse diabetic outcomes attributed to COVID-19 infection, progressed underlying condition, and long-term sequelae during the pandemic.
This study had some limitations, including using provisional data to ascertain diabetes mortality and the inability to generalize study findings to those states with insufficient data. Second, a regional comparison of excess mortality could be biased since the age-structure across states could be different. However, a comparison by excess risk could fix the problem since it showed relative ratios between excess deaths and expected deaths within states. Although the precision of comparison on absolute estimates across states might be limited without age standardization, the findings are still important for stakeholders to prioritize efforts for diabetes management during the COVID-19 pandemic. Third, the weekly death data from WONDER was limited to a short reference period of two years which might not sufficiently capture a long-term variation. We compared the temporal trends with alternative dataset from National Center for Health Statistics (NCHS), CDC [17], which provided national weekly death counts of diabetes classified by the underlying cause of death. We also did a sensitivity analysis with NCHS dataset from 2017 to 2022. The results suggested the temporal patterns of excess deaths from our main estimates were generally robust.
Conclusions
This study highlighted the increased diabetes mortality, spatiotemporal pattern, and associated demographic disparities during the pandemic. Our results should motivate pertinent stakeholders to monitor the progression of diabetes for patients. Racial/ethnic disparities in excess deaths of diabetes demonstrated the necessity to address the health inequalities. More attention should be given to vulnerable populations to minimize unexpected deaths during the pandemic. Since we may live with COVID-19 for a longer period, sustainable and effective policies should be developed to maintain qualified and timely diabetes management and tailored to decrease health disparities, especially in areas most severely affected by the COVID-19 pandemic.
Funding/Support
This work was supported by the National Natural Science Foundation of China, grant number 82103909 (Dr. Yao); the Natural Science Foundation of Guangdong Province, grant number 2021A1515220132 (Dr. Yao); Shanghai Science and Technology Development Foundation, grant number 22YF1421100 (Dr. Ran); Shenzhen Science and Technology Program, grant number JCYJ20220530144403007 (Dr. Yao) and Sci-Tech Innovation Programme 2022 at School of Global Health, SHUMU, grant number: SGHKJCX2022-02 (Dr. Han).
Ethics approval and consent to participate
The study was exempted from ethical review because the data were deidentified and publicly available.
Duality of interest
No potential conflicts of interest relevant to this article were reported.
Role of the funder/sponsor
The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Authors' contributions
J.R. and X.I.Y. designed the study. L.H. and J.R. contributed research data. J.R. and S.Z. contributed to data analysis. X.I.Y. and L.H. contributed to interpretation and manuscript writing. J.R. and X.I.Y. and Y.S. and D.H. contributed to supervision and manuscript revision. All authors gave final approval for publication.
Conflict of interests
All authors declare that they have no conflict of interest or financial conflicts to disclose. The funding agencies have no role in the study design, in the collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Acknowledgements
Not applicable.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jiph.2023.01.018.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.GBD 2017 Causes of Death Collaborators Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2019 Diabetes in the Americas Collaborators Burden of diabetes and hyperglycaemia in adults in the Americas, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Diabetes Endocrinol. 2022;10(9):655–667. doi: 10.1016/S2213-8587(22)00186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ran J., Zhao S., Han L., et al. Increase in diabetes mortality associated with COVID-19 pandemic in the U.S. Diabetes Care. 2021;44(7):e146–e147. doi: 10.2337/dc21-0213. [DOI] [PubMed] [Google Scholar]
- 4.Woolf S.H., Chapman D.A., Sabo R.T., Weinberger D.M., Hill L. Excess deaths from COVID-19 and other causes, March-April 2020. JAMA. 2020;324(5):510–513. doi: 10.1001/jama.2020.11787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGovern A.P., Thomas N.J., Vollmer S.J., Hattersley A.T., Mateen B.A., Dennis J.M. The disproportionate excess mortality risk of COVID-19 in younger people with diabetes warrants vaccination prioritisation. Diabetologia. 2021;64(5):1184–1186. doi: 10.1007/s00125-021-05404-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rathmann W., Kuss O., Kostev K. Incidence of newly diagnosed diabetes after Covid-19. Diabetologia. 2022;65(6):949–954. doi: 10.1007/s00125-022-05670-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J.L., Krupp G.R., Lo J.Y. The COVID-19 pandemic and changes in health care utilization among patients with Type 2 diabetes. Diabetes Care. 2022;45(4):e74–e76. doi: 10.2337/dc21-2248. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Trends in Number of COVID-19 Cases and Deaths in the US Reported to CDC, by State/Territory; 2022. 〈https://covid.cdc.gov/covid-data-tracker/#trends_weeklydeaths_select_00〉 (Accessed 15 August 2022).
- 9.Centers for Disease Control and Prevention. National Center for Health Statistics Mortality Data on CDC WONDER. 2022; 〈https://wonder.cdc.gov/mcd.html〉. Accessed July 20, 2022.
- 10.Stokes A., Preston S.H. Deaths attributable to diabetes in the United States: comparison of data sources and estimation approaches. PLoS One. 2017;12(1) doi: 10.1371/journal.pone.0170219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Center for Health Statistics. Instructions for Classification of Underlying and Multiple Causes of Death – 2022; 2022; 〈https://www.cdc.gov/nchs/nvss/manuals/2022/2a-2022.htm〉 (Accessed 30 November 2022).
- 12.National Center for Health Statistics. Instructions for Classifying the Multiple Causes of Death, 2022; 2022; 〈https://www.cdc.gov/nchs/nvss/manuals/2022/2b-2022.htm〉. Accessed November 30, 2022.
- 13.The United States Census Bureau. Census Data; 2022. 〈https://data.census.gov/cedsci/〉. (Accessed 20 July 2022).
- 14.Woolf S.H., Chapman D.A., Sabo R.T., Zimmerman E.B. Excess Deaths From COVID-19 and Other Causes in the US, March 1, 2020, to January 2, 2021. JAMA. 2021;325(17):1786–1789. doi: 10.1001/jama.2021.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carr M.J., Wright A.K., Leelarathna L., et al. Impact of COVID-19 on diagnoses, monitoring, and mortality in people with type 2 diabetes in the UK. Lancet Diabetes Endocrinol. 2021;9(7):413–415. doi: 10.1016/S2213-8587(21)00116-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim D., Li A.A., Cholankeril G., et al. Trends in overall, cardiovascular and cancer-related mortality among individuals with diabetes reported on death certificates in the United States between 2007 and 2017. Diabetologia. 2019;62(7):1185–1194. doi: 10.1007/s00125-019-4870-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Excess Deaths Associated with COVID-19; 2022; 〈https://www.cdc.gov/nchs/nvss/vsrr/covid19/excess_deaths.htm〉 (Accessed 20 July 2022).
- 18.Steenblock C., Schwarz P.E.H., Ludwig B., et al. COVID-19 and metabolic disease: mechanisms and clinical management. Lancet Diabetes Endocrinol. 2021;9(11):786–798. doi: 10.1016/S2213-8587(21)00244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carr M.J., Wright A.K., Leelarathna L., et al. Impact of COVID-19 restrictions on diabetes health checks and prescribing for people with type 2 diabetes: a UK-wide cohort study involving 618 161 people in primary care. BMJ Qual Saf. 2022;31(7):503–514. doi: 10.1136/bmjqs-2021-013613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valabhji J., Barron E., Gorton T., et al. Associations between reductions in routine care delivery and non-COVID-19-related mortality in people with diabetes in England during the COVID-19 pandemic: a population-based parallel cohort study. Lancet Diabetes Endocrinol. 2022;S2213–8587(22) doi: 10.1016/S2213-8587(22)00131-0. 00131-00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Handberry M., Bull-Otterson L., Dai M., et al. Changes in emergency medical services before and during the COVID-19 pandemic in the United States, January 2018-December 2020. Clin Infect Dis. 2021;73(Suppl 1):S84–S91. doi: 10.1093/cid/ciab373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montefusco L., Ben Nasr M., D'Addio F., et al. Acute and long-term disruption of glycometabolic control after SARS-CoV-2 infection. Nat Metab. 2021;3(6):774–785. doi: 10.1038/s42255-021-00407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Food and Drug Administration. Enforcement Policy for Non-Invasive Remote Monitoring Devices Used to Support Patient Monitoring During the Coronavirus Disease 2019 (COVID-19) Public Health Emergency (Revised); 2020. 〈https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enforcement-policy-non-invasive-remote-monitoring-devices-used-support-patient-monitoring-during〉. (Accessed 10 October 2022).
- 24.American Diabetes Association. COVID-19, diabetes and coronavirus; 2022; 〈https://diabetes.org/coronavirus-covid-19〉 (Accessed 10 October 2022).
- 25.Pasquel F.J., Lansang M.C., Dhatariya K., Umpierrez G.E. Management of diabetes and hyperglycaemia in the hospital. Lancet Diabetes Endocrinol. 2021;9(3):174–188. doi: 10.1016/S2213-8587(20)30381-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiels M.S., Haque A.T., Haozous E.A., et al. Racial and ethnic disparities in excess deaths during the COVID-19 Pandemic, March to December 2020. Ann Intern Med. 2021;174(12):1693–1699. doi: 10.7326/M21-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lv F., Gao X., Huang A.H., et al. Excess diabetes mellitus-related deaths during the COVID-19 pandemic in the United States. EClinicalMedicine. 2022;54 doi: 10.1016/j.eclinm.2022.101671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clift A.K., Coupland C.A.C., Keogh R.H., et al. Living risk prediction algorithm (QCOVID) for risk of hospital admission and mortality from coronavirus 19 in adults: national derivation and validation cohort study. BMJ. 2020;371:m3731. doi: 10.1136/bmj.m3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dennis J.M., Mateen B.A., Sonabend R., et al. Type 2 Diabetes and COVID-19-related mortality in the critical care setting: a national cohort study in England, March-July 2020. Diabetes Care. 2021;44(1):50–57. doi: 10.2337/dc20-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alva M.L., Hoerger T.J., Zhang P., Cheng Y.J. State-level diabetes-attributable mortality and years of life lost in the United States. Ann Epidemiol. 2018;28(11):790–795. doi: 10.1016/j.annepidem.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y., Rolka D., Xie H., Saydah S. Imputed state-level prevalence of achieving goals to prevent complications of diabetes in adults with self-reported diabetes - United States, 2017-2018. MMWR Morb Mortal Wkly Rep. 2020;69(45):1665–1670. doi: 10.15585/mmwr.mm6945a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X., Bullard K.M., Gregg E.W., et al. Access to health care and control of ABCs of diabetes. Diabetes Care. 2012;35(7):1566–1571. doi: 10.2337/dc12-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan A., Chen Z., Wang M., Mendez C.E., Egede L.E. Accessibility of medicare diabetes prevention programs and variation by State, Race, and Ethnicity. JAMA Netw Open. 2021;4(10) doi: 10.1001/jamanetworkopen.2021.28797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Czeisler M., Barrett C.E., Siegel K.R., et al. Health care access and use among adults with diabetes during the COVID-19 pandemic - United States, February-March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(46):1597–1602. doi: 10.15585/mmwr.mm7046a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ge Y., Zhang W.B., Liu H., et al. Impacts of worldwide individual non-pharmaceutical interventions on COVID-19 transmission across waves and space. Int J Appl Earth Obs Geoinf: ITC J. 2022;106 doi: 10.1016/j.jag.2021.102649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pan Y., Zhang L., Unwin J., Skibniewski M.J. Discovering spatial-temporal patterns via complex networks in investigating COVID-19 pandemic in the United States. Sustain Cities Soc. 2022;77 doi: 10.1016/j.scs.2021.103508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.IHME COVID-19 Forecasting Team Modeling COVID-19 scenarios for the United States. Nat Med. 2021;27(1):94–105. doi: 10.1038/s41591-020-1132-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhaskaran K., Bacon S., Evans S.J., et al. Factors associated with deaths due to COVID-19 versus other causes: population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet Reg Health Eur. 2021;6 doi: 10.1016/j.lanepe.2021.100109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hippisley-Cox J., Coupland C.A., Mehta N., et al. Risk prediction of covid-19 related death and hospital admission in adults after covid-19 vaccination: national prospective cohort study. BMJ. 2021;374:n2244. doi: 10.1136/bmj.n2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xie Y., Al-Aly Z. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol. 2022;10(5):311–321. doi: 10.1016/S2213-8587(22)00044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material