Abstract
Nitric oxide (NO) has been shown to have antimicrobial activity in vitro and in some in vivo models, while the virucidal activity of NO remains elusive. Some studies using NO donors have suggested that NO could be a potential candidate to treat SARS-CoV infection. The Covid-19 pandemic raised the hypothesis that NO gas might have an impact on Sars-CoV-2 replication cycle and might be considered as a candidate therapy to treat COVID-19. To our knowledge, there are no in vitro preclinical studies demonstrating a virucidal effect of gaseous NO on SARS-CoV-2.
This study aims to determine whether gaseous NO has an impact on the replication cycle of SARS-CoV-2 in vitro. To that end, SARS-CoV-2 infected epithelial (VeroE6) and pulmonary (A549-hACE2) cells were treated with repeated doses of gaseous NO at different concentrations known to be efficient against bacteria. Our results show that exposing SARS-CoV-2 infected-cells to NO gas even at high doses (160 ppm, 6 h) does not influence the replication cycle of the virus in vitro.
We report here that NO gas has no antiviral properties in vitro on SARS-COV-2. Therefore, there is no rationale for its usage in clinical settings to treat COVID-19 patients for direct antiviral purposes, which does not exclude other potential physiological benefits of this gas.
Keywords: Gaseous nitric oxide, COVID-19, SARS-CoV-2
Abbreviations: NO, Nitric Oxide
1. Introduction
Nitric oxide (NO) is a free radical gas, which functions as a key signaling molecule in animals. NO is ubiquitously produced by the different types of Nitric Oxide Synthase (NOS) enzymes, differently regulated depending on cell type, which catalyzes the conversion of l-arginine to NO and l-Citrulline. NO is involved in a wide array of physiological functions: vascular processes such as vasodilation, systemic circulation, hemodynamics; neuronal functions such as neurotransmission, neuroprotection or memory; and immune response such as innate immunity or inflammation. As an example, macrophage cells of the immune system produce NO locally (through iNOS) in order to eliminate pathogenic bacteria [[1], [2], [3]].
Medicinal gaseous Nitric oxide is a well known intensive care therapy, delivered by inhalation, used at very low dosages (∼20 ppm) as a rapid onset of action vasodilator treatment for pulmonary hypertension for adults and even for newborns. In this clinical setting, gaseous NO is continuously administered through a ventilation system during hours up to few days, depending on patient needs. Over the past few years, studies have shed light on direct antimicrobial properties of NO. This molecule is a bactericidal compound active against a large spectrum of microorganisms: gram-positive and gram-negative bacteria, yeast, mycobacteria … [4]. At high dosages (160 ppm), NO gas has been considered as a potent bactericidal agent to treat a broad array of pulmonary infections, including nosocomial pneumonia or infections caused by multi-drug resistant bacterial strains [5,6]. In a study led by Miller, pulmonary cells infected with different bacterial strains (S. aureus, P. aeruginosa, E. coli.) were exposed to high (160 ppm) intermittent (30min) gNO doses. It resulted in a total extinction of the bacterial load [7]. Another study also demonstrated the bactericidal capacity of high doses of NO gas for the treatment of infections (S. aureus) in non-healing wounds in rabbits. On the other hand, it seems clear that gaseous NO does not have bactericidal effects at low dosages, less than 80 ppm [8]. It has been established that NO effect on cell signaling has no specificity and no evidence of possible resistance development. Finally, it has been shown that NO was safe to be inhaled even at a high concentration required for the antimicrobial effect (160 ppm) used for short repeated periods of 30 min 3 times daily. This was demonstrated in a phase I clinical trial on young cystic fibrosis patients [[9], [10], [11]]. Despite this, the antibacterial effect of NO has not yet reached the stage of a clinical demonstration of efficacy in a randomized clinical study, and is not used to treat patients apart from compassionate use in a few case reports such as on this cystic fibrosis patient infected with non-tuberculosis mycobacteria [12].
Such promising effects led several research teams to focus their attention on other potential pathogen targets, such as viruses. In vitro studies, noted an antiviral effect of NO molecule on various types of viruses like Herpes Simplex Virus (HSV1), influenza A (responsible for the flu), or mouse hepatitis virus (MHV) [[13], [14], [15]]. Regev-Shoshani and colleagues exposed a saline suspension of influenza A virions to NO gas (80 and 160 ppm) and observed a decrease of their ability to infect kidney epithelial (MDKC) cells, this antiviral effect was enhanced at higher doses (160 ppm) and with increased duration of exposure (tested up to 180 min). This effect was considerably lower when the treatment was performed on cells after infection. They suggested that NO gas has a dose- and time-dependent antiviral activity on influenza A virus [16]. Free virions may be more vulnerable than replication within a host cell. However, Darwish and colleagues failed to demonstrate any impact of inhaled nitric oxide therapy on the viral load in mice infected with influenza A, the only published in vivo study exploring this antiviral hypothesis [17].
Two in vitro studies assessed NO virucidal activity on Severe Acute Respiratory Syndrome Coronavirus-1 (SARS-CoV-1) at the time of SARS epidemia during the first decade of 2000. To that end, African green monkey kidney epithelial (VeroE6) cells were infected and then treated with chemical NO donors at very high concentrations. “NO donors” are pharmacologically active compounds that spontaneously generate, or are metabolized into NO or NO-related species in vitro and in vivo. S-nitroso-N-acetylpenicillamine (SNAP) or sodium nitroprusside (SNP) are commonly used as NO donors in preclinical studies [18]. Both studies concluded that NO inhibits SARS-coronavirus replication cycle in a dose dependent manner [19,20]. Akaberi led the same type of experimentation on cells infected with SARS-CoV-2 and reached the same conclusion [21].
It is difficult to quantify accurately the real amount of NO delivered into cells when treated with gaseous NO or NO donors. To that end, we have compiled a comparative table of NO doses used in the mentioned articles to understand the discrepancies observed between the publications and to better appreciate the conclusions of each study regarding the antiviral effect of NO (Table 1 ).
Table 1.
Comparative analysis of NO doses used in the studies on the antimicrobial effect of nitric oxide
A search of the scientific database was conducted to identify published articles containing relevant information on the antimicrobial effects of inhaled nitric oxide therapy. We focus our attention on the effect of NO on bacteria or viruses. The search included articles published between 2000 and April 2022.
| Publication | Doses and time exposure | Equivalent (g) | |
|---|---|---|---|
| NO donors | [13] (in vitro, HSV 1) | SNAP 500 μM, 5 h | 6.3 |
| [20] (cells) | SNAP the NO concentration released by 222 μM SNAP is between 30 and 55 μM NO | ||
| [19] (cells in vitro, Sars-Cov1) | SNAP, 400 μM, One shot, 1 h | 1 | |
| [21] (cells, Sars-CoV-2) | SNAP 200–500 μM, 30min every 4 h for 36 h period | 5.7 | |
| Gaseous NO in vitro | [6] (Bacteria suspension) | Over 6 h, 200 ppm NO gas | 1.70E-01 |
| [22] (testing device, fibroblast, in vitro) | maximum 400 ppm NO gas for 48 h | 6.8 | |
| [9] (bacteria in vitro) | 4 cycles of 160 ppm NO gas, 30 min every 4 h | 4.50E-02 | |
| [16] (in vitro, influenza virus) | 80 and 160 ppm NO gas, 2 h | 2.30E-01 | |
| This study Protocol 1 | 80 ppm or 160 ppm of NO gas for 15 min, two times daily, for two consecutive days | 4.5e-2 | |
| This study Protocol 2 | 10 ppm or 40 ppm of NO gas for 6 h, two times daily, for two consecutive days | 3.4e-3 | |
| This study Protocol 3 | 160 ppm of NO gas for 6 h, two times daily, for two consecutive days | 1.4e-2 | |
| Gaseous NO in vivo (animal or human patients) | [17] (in vivo mice) | 160 ppm, 30 min, twice per day, one shot | 1.40E-01 |
| [11] (human, cystic fibrosis) | 160 ppm for 30min, 3 times daily for two periods of 5 days | ||
| [23] (human, infants, acute bronchiolitis) | 160 ppm for 30min, 5 times a day for 5 days | 1.40E-01 | |
| [24] (human, Covid 19) | 160 ppm 30 min twice per day (max 9 days) | ||
| [25] (Pregnant patients) | 160–220 ppm twice a day A total of 39 treatments were administered. | ||
| [26] (in vivo, healthy Pig) | 160 ppm, 6 h, one shot |
Overall, there are very few in vivo studies that reported a viral load reduction after NO treatment (regardless of the treatment with gaseous NO or NO donors, or the virus studied), but while the antiviral activity of gaseous NO remains elusive, the emergence of coronavirus disease in 2019 (COVID-19) and the search for an effective treatment for patients who become critically ill has put the spotlight back on the search for potential antiviral properties of gaseous NO.
COVID-19 is an infectious respiratory disease caused by SARS-CoV-2. Individuals with COVID-19 show a variety of symptoms, including in the respiratory tract and vascular system that in most severe cases may progress to acute respiratory distress syndrome (ARDS). Most usage of gaseous NO during the pandemic was made in a clinical context of COVID-19 induced ARDS, in emergency conditions to attempt to improve oxygenation by way of the known NO-vasodilation properties. Although this usage is not approved by regulatory authorities, it is a common off-label use in intensive care units for ARDS patients. Others considered the use of high doses of inhaled NO for its potential, virucidal effect, and the antibacterial effect demonstrated during its early development to treat cystic fibrosis superinfections. Several clinical trials have been launched, with no efficacy data reported so far. A retrospective study reported that high-dose inhaled NO (160 ppm) for 30 min as rescue therapy in non-intubated patients was well tolerated and improved the respiratory effort [24]. A preliminary clinical report of spontaneously breathing pregnant patients with severe COVID-19 showed a benefit on their condition after high doses of NO therapy (160–200 ppm) without adverse effects for patients or newborns [25]. Despite the feasibility and safety of this approach has been established, to date, preclinical evidence of efficacy is still lacking, as no preclinical study has formally demonstrated an antiviral outcome of gaseous NO on the SARS-CoV-2 replication.
To investigate the potential therapeutic value of NO gas as an antiviral agent against COVID-19, we set up a simple in vitro experiment in which epithelial cells infected with SARS-CoV-2 were exposed to high doses of gaseous NO. We also performed a comparative analysis of NO doses used in previous studies evaluating the potential antiviral activity of NO.
2. Material and methods
2.1. Cell culture and infection with SARS-CoV-2
African green monkey kidney epithelial cells (Vero E6 cells) and human lung carcinoma (A549) cells overexpressing human ACE2 (angiotensin I-2 converting enzyme) (A549-hACE2 cells) were used for this study.
VeroE6 or A549-hACE2 cells were seeded in 96-well plates (4.104 cells/well) at day −1 in DMEM (Gibco, 31966047) 10% FBS (Gibco, 10270106), 1% P/S (Life Technologies, 11548876). The next day (day 0), cells were moved to the Biosafety level 3 facility (BSL-3) for infection with a low passage isolate of SARS-CoV-2 (hCoV-19/France/GES-1973/2020) carrying the D614G mutation in the spike (Pango lineage B.1). The complete media was removed and replaced with an inoculum of SARS-CoV-2 (Multiplicity of Infection 0.1) in DMEM 0% FBS and incubated at 37 °C/5% CO2 for 1 h. After 1 h, the inoculum was removed and replaced with 100 μL of DMEM 2% FBS, 1% P/S. Cell death was induced by treating the cells with the apoptosis inducing drug camptothecin (10 μM, Sigma Aldrich, PHL89593).
2.2. Nitric oxide gas exposure
Exposure of cell to gasses has been designed inspired from previously described methods of NO administration [7] integrating lessons learnt from our models of in vitro gas exposure [27]. Infected cells were placed within hermetically sealed Plexiglas incubation chambers (220 × 220 × 194mm) with separate gas entry and exit ports allowing a continuous flow of gaseous NO through the chamber. The incubation chamber was then placed in a conventional incubator at 37 °C for incubation times indicated below.
Gases were supplied from pressurized premixed gas cylinders (Air Liquide Santé France): Kinox 450 ppm, N2, O2 and CO2 Gas concentrations were controlled using manual flowmeters for NO and a Gasmix with its software (Alytech, Juvisy/Orge, France) for N2, O2 and CO2 (4l/min for shorts exposures, 0,1l/min for long exposure). Atmosphere was maintained standard for cell culture during NO exposure (74% N2, 21% O2, 5% CO2), while NO ppm were adjusted as indicated in each experiment).
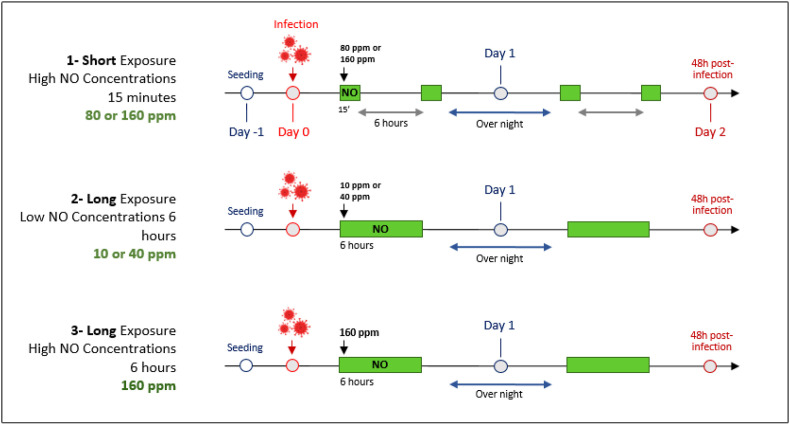
Control cells were infected at the same time as NO treated cells but the plates remained in a regular incubator (37 °C/ 5% CO2) during the whole experiment. Three protocols with different NO concentrations and durations of exposure were performed, as described below and in Fig. 1 . At 48 h post-infection, supernatants were harvested, centrifuged 5 min at 450 g, transferred to fresh tubes and stored at −80 °C before being processed.
Fig. 1.
Schematic representation of experimental protocols for NO gas exposure.
VeroE6 and A549-hACE2 cells were plated at day −1 (24 h before the infection). On day 0, cells were infected with SARS-CoV-2 (MOI 0.1). Cells were exposed to gaseous NO right after the infection (Day 0 and Day 1). 48 h post infection, viral loads were tittered on cells by plaque forming assay. Green rectangles represent NO gas exposures, durations and concentrations are indicated on the schematic. Control cells were infected at the same time as gaseous NO treated cells; plates remained in a regular incubator (37 °C 5%CO2) during the whole experiment.
Ppm: Part per million, NO: Nitric Oxide, MOI: Multiplicity of infection.
Protocol 1- Short exposure – High NO concentrations: Two doses of NO gas were tested, 80 ppm and 160 ppm, considered as high concentrations. At Day 0, both cell types were exposed to 80 ppm or 160 ppm of NO gas for 15 min (flow rate 4 l/min). Cells were returned to the regular incubator for 6 h and exposed again to NO gas for 15 min. Cells were placed back in the regular incubator overnight. At day 1, the same exposition cycle as on day 0 was performed.
Protocol 2- Long exposure – Low NO concentrations: Two doses of NO gas were tested (10 ppm and 40 ppm), considered as low concentrations. At Day 0, both cell types were exposed to 10 ppm or 40 ppm of NO gas for 6 h (flow rate 100 mL/min) then cells were placed back to the regular incubator overnight. At day 1, the same exposition cycle as on day 0 was performed.
Protocol 3- Long exposure – High NO concentration: Cells were exposed to gaseous NO at 160 ppm for 6 h (flow rate 100 mL/min), two times daily, for two consecutive days.
2.3. Viral load measurement by plaque forming assay
Viral progeny production in supernatants from cells treated with gaseous NO was quantified by plaque-forming assay and compared to control. Briefly, 10-fold serial dilutions of the cells supernatants (in triplicates) were performed in DMEM+1% P/S and adsorbed onto a monolayer of VeroE6 or A549-hACE2 cells (1.5 × 10^5 cells per well of a 24-well plates, plated on day −1). After 1 h of adsorption the inoculum were removed and a semi-solid overlay medium (DMEM + 2%FBS + P/S 1% + CMC 1% (VWR, 22525.296) was applied to the cell monolayer. 72 h after infection the overlay medium was removed and the cells were stained with a crystal violet solution containing 4% of PFA (Sigma Aldrich, 15,512-1l-R/94,448-2.5l-F) for 20 min. Crystal violet solution was then removed and the cells were allowed to dry before plaques enumeration by eye. The calculated titers are expressed in Plaque Forming Units per milliliter (PFU/ml).
For protocols 1 and 2, cytotoxicity was measured on non-infected cells in a BSL2 environment prior to infection experiments. Cells were subjected to protocol 1 and protocol 2 to determine the potential cell toxicity of the NO gas treatments and the cytotoxicity was measured using the CellTiter-Glo® 2.0 Cell Viability Assay according to the manufacturer's protocol. For protocol 3 the experiment was conducted directly in a BSL3 environment and the cell death was monitored by visual assessment of the monolayer under a microscope. GraphPad Prism 9 was used to generate graphs and perform statistical analysis. For all analyses, the two-tailed Student's t-test was used to compare groups.
2.4. NO dosing quantification in preclinical and clinical studies
In general the key parameter to quantify the dose of an inhaled gas is the concentration because it is the concentration that will determine the saturated blood level (based on the solubility of the gas species). The duration of administration also needs to be considered. So the total dose administered (related to the area under the curve (AUC) of pharmacokinetics) is:
| (1) |
where C is concentration, Q is the inhalation flow rate, and Tin is the time for inhalation. Note that Q and Tin are difficult to monitor continuously in a clinical setting so a more practical approach is to use the minute ventilation (MV) over the duration (D) of the treatment period:
| (2) |
Minute ventilation (or respiratory minute volume or minute volume) is the volume of gas inhaled (inhaled minute volume) and is equal to the tidal volume times the breathing frequency. Of primary importance is the fact that in the clinical setting, to maintain the inhaled gas concentration a specialized device used with a mechanical ventilator is required. However, simple flow mixing is possible for in vitro administration chambers.
This delivered dose is not the same as the gas uptake. For example, in general there is only gas uptake in the alveolar gas exchange region of the lung. Typically, 65% of the inhaled gas reaches this region during normal breathing. It has been found that when administered at low doses (up to 40 ppm) practically all NO delivered to the alveolar space is taken up (e.g., see [28]). We do not know of any studies of uptake at higher antiviral doses. However, as the target is not only the vasculature but also viral load in the airways, uptake to blood may be less pertinent.
For preclinical in vitro experiments with cell plates equation (1) applies for the gas entering the administration chamber. Clearly, only a small percentage of this NO will be available to cells. In particular, the solubility of NO in the cell medium (the Ostwald solubility coefficient in water for NO is 0.04128 [29] (Table 1, pg 229) is a ceiling on the potential exposed concentration [27].
NO can be generated by creating a spark in air. The concentration of NO will depend on the generation rate of the particular device used and the gas flow rate. NO gas can also be created through chemical reactions in solution. For NO creation using these methods the concentration is complicated to determine. However, production or production rate are provided in terms of mass (μg) or moles (μM). Thus, to compare to direct gas administration consider the gas concentration in ppm. For a perfect gas there are 22.4 l/M at 0 °C. At 37 °C this increases to 25.4 l/M. In terms of concentration this is the inverse, 0.039 M/l. Thus, for each ppm the concentration is 0.039 μM/l (because one million is 106). Therefore, the typical antiviral dose of 160 ppm = 6.3 μM/l. The molecular weight of NO is 30.01 g/M, so this is 189 μg/l. This form of concentration can be used with equations (1), (2)) to determine the quantity of NO delivered. For the NO donor S-nitroso-N-acetylpenicillamine (SNAP) (Sigma-Aldrich, product number N3398) or its non S-nitrosated version NAP (Sigma-Aldrich, product number 01423), the product data sheet states that 100 μM of SNAP can produce 1.4 μM NO/min. In effect this approach bubbles pure NO in the cell media.
When we speak of the quantity of NO delivered it must also be determined what is the quantity of the volume of tissue (cells, blood, etc.) that is to be saturated to properly understand the dose. In other words, this is a fundamental concept of physiologically based pharmacokinetics.
For every case of NO application also to be considered is the reaction of NO with oxygen to form NO2, thus reducing the NO concentration and creating a toxic species for cells or for the patient clinically.
3. Results and discussion
3.1. Evaluation of gaseous NO cytotoxicity
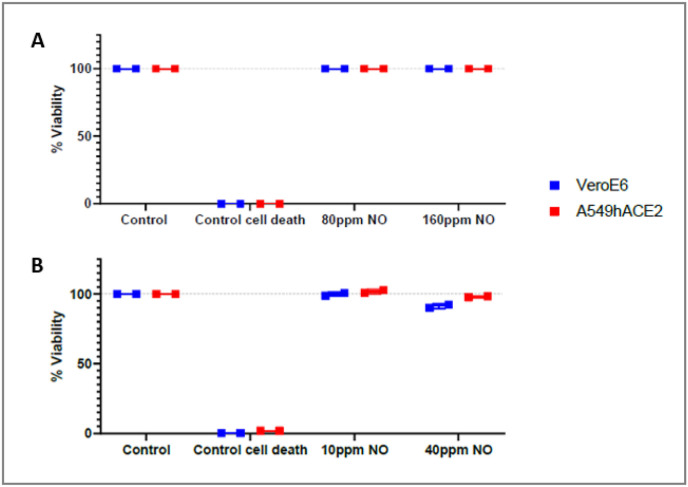
To ensure the lack of interference with viral titer measurement, we assessed in vitro gaseous NO cytotoxicity at basal conditions on non-infected VeroE6 and A549-hACE2 cells upon the three tested protocols. No measurable cell death was observed upon gaseous NO treatments at concentrations up to 160 ppm on both tested cell types (Fig. 2 ).
Fig. 2.
Cytotoxicity evaluation upon NO gas exposure on non-infected cells.
Cytotoxicity measurement was performed on Vero E6 (blue squares) and A549-hACE2 (red squares) after high (A) or low (B) exposure to gaseous NO, using CellTiter-Glo® 2.0 Cell Viability Assay kit in duplicates. The control cell death wells were treated with 10 μM camptothecin for 24 h. NB: For the protocol 3, 160 ppm NO gas exposure during 6 h, lack of cytotoxicity was evaluated empirically by eye evaluation through a microscope.
3.2. Assessment of gaseous NO effect on SARS-CoV-2 replication cycle
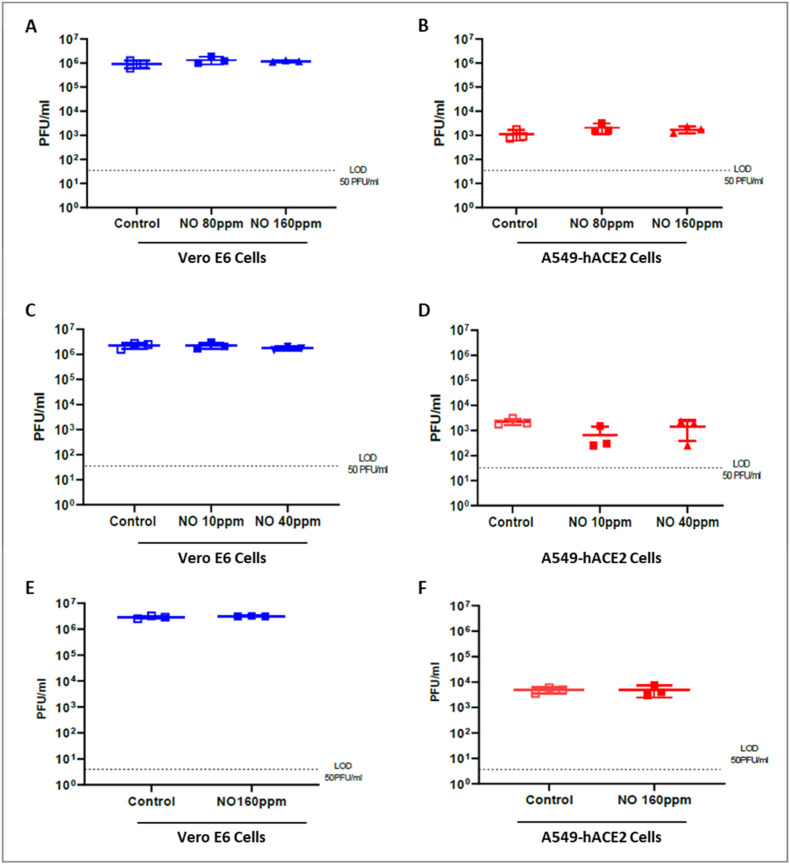
In order to evaluate if exposure to gaseous NO has an antiviral effect on SARS-CoV-2, VeroE6 and A549-hACE2 cells were infected with a SARS-CoV-2 isolate, using a previously described approach [30]. In the absence of treatment, we observed a lower viral load in A549-hACE2 cells supernatant as compared to VeroE6 cells (Fig. 3 ). Vero E6 cells have been shown to be more permissive for SARS-CoV-2 infection than airways epithelial cells or other cell types [[31], [32]].
Fig. 3.
No effect of gaseous NO Exposure on SARS-CoV-2 replication in vitro
Viral loads (PFU/ml) were measured 48 h post infection in supernatants of non-treated cells (control) or cells exposed to various dosages of NO gas (VeroE6 cells (A) (C) (E) and A549-hACE2 cells (B) (D) (F)). Panels A and B present the results of short exposure with high NO concentrations (80 or 160 ppm for 15 min). Panels C and D show results for long exposure with low NO gas Concentrations (10 or 40 ppm for 6 h). Panels (E) and (F) show results for long exposure with high gaseous NO concentrations. Data present a representative experiment (from three independent assays) Bars indicate S.D., and no significant difference was noted between treatment and controls.
Gaseous NO treatment on SARS-CoV-2 infected cells did not induce any effect on viral load as compared to control regardless of the type of cell used and the treatment performed (Fig. 3). Our experiments did not reveal any antiviral effect on SARS-CoV-2 replication activity after in vitro exposure to gaseous nitric oxide. While our system has been validated using compounds with in vitro effect against SARS-CoV-2, no effective gaseous treatment was available as control.
Inhaled NO has been reported as a promising strategy to treat bacterial lung infections developed up to clinical stage in the context of cystic fibrosis [9,11], and a candidate to treat viral infections (as demonstrated in vitro using very high doses on viruses). Antibacterial effect of NO is explained by its nitrosative and oxidative action that form reactive species that may then interact with microbial proteins, DNA and metabolic enzymes, ultimately disrupting vital cellular functions and structures leading to potent antimicrobial efficacy. The molecular basis of the antibacterial action is therefore not transposable to a putative antiviral action.
NO gas had never been tested on SARS-CoV-2 in vitro or in vivo, although it was assessed in patients during the pandemic to treat COVID-19. The antiviral effect has never been clearly evaluated by the measurement of viral load on upper airways after inhaled NO treatment. Most usage of inhaled NO during pandemic were made in a clinical context of COVID-19 induced ARDS, in emergency conditions to attempt to improve oxygenation using known vasodilation properties of NO, although this usage is not approved. Its efficacy in this context still remains to be evaluated in clinical trials. Our results suggest that NO administration has no antiviral effect (at least at dosages that could be safely used in humans). Any beneficial outcome observed in NO-treated COVID-19 patients might be due to NO vasodilator, bronchodilator and hemodynamic beneficial effects on upper airways rather than a potential antiviral impact [33]. The molecule basis of vasodilator action of NO is different from the antibacterial effect, and requires lower concentrations. NO diffuses into vascular smooth muscle cells and reacts with the iron of soluble guanylate cyclase resulting in the production of cyclic guanosine monophosphate (cGMP), leading to relaxation of the smooth muscle cells and an overall dilation of blood vessels.
Nitric Oxide is currently undergoing clinical trials in order to treat COVID-19, either as a gas or with NO-donors. Rather than evaluating the viral load of SARS-CoV-2 in upper airways, most of the studies involving NO gas focus their attention on pulmonary hemodynamics and the improvement of oxygenation in COVID-19-induced ARDS patients in order to avoid an evolution to severe ARDS. A retrospective study of COVID-19 patients who received 20 ppm of gaseous NO for 30 min showed an improvement in oxygenation and pulmonary hemodynamics [34].
In this study, the prophylactic effect of gaseous NO on cells was not assessed (i.e., by exposing cells to gaseous NO before infection). Darwish and colleagues exposed mice for 1 h to continuous gaseous NO (160 ppm) prior to infection with influenza virus. They did not observe any difference in the outcome as compared to control.
To our knowledge, the publications showing a positive in vitro outcome on SARS-CoV strains viral load were only those using NO donors [19,21]. To evaluate the amount of NO delivered into cells when treated with gaseous NO or NO donors, several parameters come into play such as pressure, flow rate, incubation time, pH and even the composition of the culture media [35,36]. We performed a comparative analysis of NO dose when using NO donors and gaseous NO (Table 1). The difficulty to estimate the amount of NO that is delivered to cells is a general limitation of any in vitro experiment with gases, however, we can reasonably estimate that NO donors induce at least 500 times more NO than when using NO gas (Table 1), dosages that are reasonably not achievable in vivo, especially if it is meant for human therapy. Indeed, at high concentrations NO induces the accumulation of methemoglobin and NO2 that are both known to be sources of toxicity. Ghaffari and colleagues designed an NO gas delivery device for cell cultures and tested its efficiency on a fibroblast cell culture with various concentrations of gaseous NO. They observed that NO was well tolerated by fibroblasts over a 48 h period at 200 ppm [22,37]. More recently, a safety preclinical study delivered gaseous NO at 160 ppm to pigs. NO2 levels remained below the safety threshold (5 ppm) during the entire experiment. Nevertheless, to maintain methemoglobin levels at safety thresholds they needed to co-administer methylene blue [26].
We could have evaluated cytotoxicity and assessed antimicrobial activity at higher gaseous NO doses however, we remained in the admitted highest safe concentrations range in vivo where no deleterious effects of NO2 and methemoglobin accumulation on tissues have been demonstrated. To date, more studies of NO gas-based antiviral therapy with a systemic approach that would include the assessment of pulmonary hemodynamic function, of ventilatory function and including the measurement of viral load in the upper airways, as well as safety parameters are needed.
4. Conclusion
The COVID-19 pandemic has had a major impact on public health. NO has an essential function in lungs, it improves tissue oxygenation and positively influences pulmonary hemodynamics. Due to its known effects on bacterial pulmonary infections, and on the oxygenation in distress conditions, nitric oxide has been considered as a worthy candidate for COVID-19 treatment. The current study is the first of its kind, by continuously exposing infected cells to high doses of NO gas, we demonstrated that NO does not directly affect SARS-CoV-2 replication. Therefore, there is no rationale for its antiviral usage to treat COVID-19 patients with high doses. Its clinical evaluation in COVID-19 patients should rather be limited to standard dosages to evaluate hemodynamic effects associated with improved oxygenation.
Declaration of competing interest
The study was funded by Air Liquide. AR, IK, JFRG and GF are employees of Air Liquide.
Data availability
Data will be made available on request.
References
- 1.Billack B. Macrophage activation: role of toll-like receptors, nitric oxide, and nuclear factor kappa B. Am. J. Pharmaceut. Educ. 2006;70:102. doi: 10.5688/aj7005102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Curvello V., Pastor P., Hekierski H., Armstead W.M. Inhaled nitric oxide protects cerebral autoregulation and reduces hippocampal necrosis after traumatic brain injury through inhibition of ET-1, ERK MAPK and IL-6 upregulation in pigs. Neurocritical Care. 2019;30:467–477. doi: 10.1007/s12028-018-0638-1. [DOI] [PubMed] [Google Scholar]
- 3.Liu P., Li Y.-S., Quartermain D., Boutajangout A., Ji Y. Inhaled nitric oxide improves short term memory and reduces the inflammatory reaction in a mouse model of mild traumatic brain injury. Brain Res. 2013;1522:67–75. doi: 10.1016/j.brainres.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 4.Bath P.M., Coleman C.M., Gordon A.L., Lim W.S., Webb A.J. 2021. Nitric Oxide for the Prevention and Treatment of Viral, Bacterial, Protozoal and Fungal Infections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jensen J., Packert D., Miller C., Packert G., Hanft J., Jensen S. Discovery and development of gaseous nitric oxide under increased atmospheric pressure as an antimicrobial: in vitro and in vivo testing of nitric oxide against multidrug-resistant organisms. Clin. Podiatr. Med. Surg. 2020;37:231–246. doi: 10.1016/j.cpm.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 6.McMullin B.B., Chittock D.R., Roscoe D.L., Garcha H., Wang L., Miller C.C. The antimicrobial effect of nitric oxide on the bacteria that cause nosocomial pneumonia in mechanically ventilated patients in the intensive care unit. Respir. Care. 2005;50:1451–1456. [PubMed] [Google Scholar]
- 7.Miller C., McMullin B., Ghaffari A., Stenzler A., Pick N., Roscoe D., Ghahary A., Road J., Av-Gay Y. Gaseous nitric oxide bactericidal activity retained during intermittent high-dose short duration exposure. Nitric Oxide Biol. Chem. 2009;20:16–23. doi: 10.1016/j.niox.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Ghaffari A., Jalili R., Ghaffari M., Miller C., Ghahary A. Efficacy of gaseous nitric oxide in the treatment of skin and soft tissue infections. Wound Repair Regen. 2007;15:368–377. doi: 10.1111/j.1524-475X.2007.00239.x. [DOI] [PubMed] [Google Scholar]
- 9.Bentur L., Gur M., Ashkenazi M., Livnat-Levanon G., Mizrahi M., Tal A., Ghaffari A., Geffen Y., Aviram M., Efrati O. Pilot study to test inhaled nitric oxide in cystic fibrosis patients with refractory Mycobacterium abscessus lung infection. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2020;19:225–231. doi: 10.1016/j.jcf.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Bentur L., Masarweh K., Livnat-Levanon G., Ashkenazi M., Dagan A., Mizrahi M., Av-Gay Y., Aviram M., Efrati O. Nitric oxide inhalations in CF patients infected with Mycobacterium abscessus complex: a prospective, open-labeled, multi-center pilot study. Am. J. Respir. Crit. Care Med., Am. Thorac. Soc. Int. Conf. Abstr. 2018;197:A5919. doi: 10.1164/ajrccm-conference.2018.197.1_MeetingAbstracts.A5919. –A5919. [DOI] [Google Scholar]
- 11.Deppisch C., Herrmann G., Graepler-Mainka U., Wirtz H., Heyder S., Engel C., Marschal M., Miller C.C., Riethmüller J. Gaseous nitric oxide to treat antibiotic resistant bacterial and fungal lung infections in patients with cystic fibrosis: a phase I clinical study. Infection. 2016;44:513–520. doi: 10.1007/s15010-016-0879-x. [DOI] [PubMed] [Google Scholar]
- 12.Goldbart A., Gatt D., Golan Tripto I. Non-tuberculous mycobacteria infection treated with intermittently inhaled high-dose nitric oxide. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-243979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Croen K.D. Evidence for antiviral effect of nitric oxide. Inhibition of herpes simplex virus type 1 replication. J. Clin. Invest. 1993;91:2446–2452. doi: 10.1172/JCI116479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lane T.E., Paoletti A.D., Buchmeier M.J. Disassociation between the in vitro and in vivo effects of nitric oxide on a neurotropic murine coronavirus. J. Virol. 1997;71:2202–2210. doi: 10.1128/JVI.71.3.2202-2210.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Powell K.L., Baylis S.A. The antiviral effects of nitric oxide. Trends Microbiol. 1995;3:81–82. doi: 10.1016/s0966-842x(00)88884-8. [DOI] [PubMed] [Google Scholar]
- 16.Regev-Shoshani G., Vimalanathan S., McMullin B., Road J., Av-Gay Y., Miller C. Gaseous nitric oxide reduces influenza infectivity in vitro. Nitric Oxide Biol. Chem. 2013;31:48–53. doi: 10.1016/j.niox.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darwish I., Miller C., Kain K.C., Liles W.C. Inhaled nitric oxide therapy fails to improve outcome in experimental severe influenza. Int. J. Med. Sci. 2012;9:157–162. doi: 10.7150/ijms.3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller M.R., Megson I.L. Recent developments in nitric oxide donor drugs. Br. J. Pharmacol. 2007;151:305–321. doi: 10.1038/sj.bjp.0707224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akerström S., Mousavi-Jazi M., Klingström J., Leijon M., Lundkvist A., Mirazimi A. Nitric oxide inhibits the replication cycle of severe acute respiratory syndrome coronavirus. J. Virol. 2005;79:1966–1969. doi: 10.1128/JVI.79.3.1966-1969.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keyaerts E., Vijgen L., Chen L., Maes P., Hedenstierna G., Van Ranst M. Inhibition of SARS-coronavirus infection in vitro by S-nitroso-N-acetylpenicillamine, a nitric oxide donor compound. Int. J. Infect. Dis. 2004;8:223–226. doi: 10.1016/j.ijid.2004.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akaberi D., Krambrich J., Ling J., Luni C., Hedenstierna G., Järhult J.D., Lennerstrand J., Lundkvist Å. Mitigation of the replication of SARS-CoV-2 by nitric oxide in vitro. Redox Biol. 2020;37 doi: 10.1016/j.redox.2020.101734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghaffari A., Neil D.H., Ardakani A., Road J., Ghahary A., Miller C.C. A direct nitric oxide gas delivery system for bacterial and mammalian cell cultures. Nitric Oxide Biol. Chem. 2005;12:129–140. doi: 10.1016/j.niox.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Goldbart A, Golan-Tripto I, Pillar G, Livnat-Levanon G, Efrati O, Spiegel R, Lubetzky R, Lavie M, Carmon L, Ghaffari A, Nahum A. Inhaled nitric oxide therapy in acute bronchiolitis: A multicenter randomized clinical trial. Sci. Rep. 2020 Jun 15;10(1) doi: 10.1038/s41598-020-66433-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiegand S.B., Safaee Fakhr B., Carroll R.W., Zapol W.M., Kacmarek R.M., Berra L. Rescue treatment with high-dose gaseous nitric oxide in spontaneously breathing patients with severe coronavirus disease 2019. Crit. Care Explor. 2020;2 doi: 10.1097/CCE.0000000000000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Safaee Fakhr B., Wiegand S.B., Pinciroli R., Gianni S., Morais C.C.A., Ikeda T., Miyazaki Y., Marutani E., Di Fenza R., Larson G.M., Parcha V., Gibson L.E., Chang M.G., Arora P., Carroll R.W., Kacmarek R.M., Ichinose F., Barth W.H., Kaimal A., Hohmann E.L., Zapol W.M., Berra L. High concentrations of nitric oxide inhalation therapy in pregnant patients with severe coronavirus disease 2019 (COVID-19) Obstet. Gynecol. 2020;136:1109–1113. doi: 10.1097/AOG.0000000000004128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michaelsen V.S., Ribeiro R.V.P., Brambate E., Ali A., Wang A., Pires L., Kawashima M., Zhang Y., Gazzalle A., Keshavjee S., Del Sorbo L., Cypel M. A novel pre-clinical strategy to deliver antimicrobial doses of inhaled nitric oxide. PLoS One. 2021;16 doi: 10.1371/journal.pone.0258368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katz I., Palgen M., Murdock J., Martin A.R., Farjot G., Caillibotte G. Gas transport during in vitro and in vivo preclinical testing of inert gas therapies. Med. Gas Res. 2016;6:14–19. doi: 10.4103/2045-9912.179342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Westfelt U.N., Lundin S., Stenqvist O. Uptake of inhaled nitric oxide in acute lung injury. Acta Anaesthesiol. Scand. 1997;41:818–823. doi: 10.1111/j.1399-6576.1997.tb04794.x. [DOI] [PubMed] [Google Scholar]
- 29.Wilhelm E., Battino R., Wilcock R.J. Low-pressure solubility of gases in liquid water. Chem. Rev. 1977;77:219–262. [Google Scholar]
- 30.Plaze M., Attali D., Prot M., Petit A.C., Blatzer M., Vinckier F., Levillayer L., Chiaravalli J., Perin-Dureau F., Cachia A., Friedlander G., Chrétien F., Simon-Loriere E., Gaillard R. Inhibition of the replication of SARS-CoV-2 in human cells by the FDA-approved drug chlorpromazine. Int. J. Antimicrob. Agents. 2021 Mar;57(3) doi: 10.1016/j.ijantimicag.2020.106274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Essaidi-Laziosi M, Perez Rodriguez FJ, Hulo N, Jacquerioz F, Kaiser L, Eckerle I. Estimating clinical SARS-CoV-2 infectiousness in Vero E6 and primary airway epithelial cells. Lancet Microbe. 2021 Nov;2(11):e571. doi: 10.1016/S2666-5247(21)00216-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pommerenke Claudia, Rand Ulfert, Uphoff Cord C, Nagel Stefan, Zaborski Margarete, Hauer Vivien, Kaufmann Maren, Meyer Corinna, Denkmann Sabine A, Riese Peggy, Eschke Kathrin, Kim Yeonsu, Safranko Zeljka Macak, Kurolt Ivan-Christian, Markotic Alemka, Cicin-Sain Luka, Steenpass Laura. Identification of cell lines CL-14, CL-40 and CAL-51 as suitable models for SARS-CoV-2 infection studies. PLoS One. 2021 Aug 2;16(8) doi: 10.1371/journal.pone.0255622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maniscalco M., Sofia M., Pelaia G. Nitric oxide in upper airways inflammatory diseases. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. Al. 2007;56:58–69. doi: 10.1007/s00011-006-6111-1. [DOI] [PubMed] [Google Scholar]
- 34.Lotz C., Muellenbach R.M., Meybohm P., Mutlak H., Lepper P.M., Rolfes C.-B., Peivandi A., Stumpner J., Kredel M., Kranke P., Torje I., Reyher C. Effects of inhaled nitric oxide in COVID-19-induced ARDS - is it worthwhile? Acta Anaesthesiol. Scand. 2021;65:629–632. doi: 10.1111/aas.13757. [DOI] [PubMed] [Google Scholar]
- 35.Hall J.R., Rouillard K.R., Suchyta D.J., Brown M.D., Ahonen M.J.R., Schoenfisc M.H. Mode of nitric oxide delivery affects antibacterial action. ACS Biomater. Sci. Eng. 2020;6:433–441. doi: 10.1021/acsbiomaterials.9b01384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jensen B., Fago A. A novel possible role for met hemoglobin as carrier of hydrogen sulfide in the blood. Antioxidants Redox Signal. 2020;32:258–265. doi: 10.1089/ars.2019.7877. [DOI] [PubMed] [Google Scholar]
- 37.Ghaffari A., Miller C.C., McMullin B., Ghahary A. Potential application of gaseous nitric oxide as a topical antimicrobial agent. Nitric Oxide Biol. Chem. 2006;14:21–29. doi: 10.1016/j.niox.2005.08.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.