Abstract
Objectives
This study aimed to provide guidance for clinical treatment and increase public confidence in COVID-19 vaccines.
Methods
The Cochrane Library, Embase, PubMed, Web of Science, ClinicalKey, and other COVID-19 datasets were searched from December 2019 to May 2022. Case-control studies and prospective cohort studies of COVID-19 vaccine effectiveness and safety in pregnant women were included.
Results
From day 11 to day 13, after the first dose of the COVID-19 messenger RNA vaccine, the effectiveness was 54% (95% confidence interval: 0.33-0.69). On days 14 to 27, the effectiveness was 59%. There was a 14% increase in vaccine effectiveness 28 days after the first dose was given. The inactivated vaccines showed similar effectiveness. The proportions of placental abruptions, postpartum hemorrhages, miscarriages, stillbirths, premature births, and small for gestational age infants were not significantly different between vaccinated and nonvaccinated pregnant women. Fatigue and fever were also not associated with pregnancy.
Conclusion
Our findings affirm that the effectiveness varies for different types of vaccines and is significantly and positively correlated with time in the pregnant population. COVID-19 vaccines have also been deemed safe for pregnant women. Thus, we developed a comprehensive understanding of the role of vaccines in pregnant women.
Keywords: COVID-19, Vaccine, Effectiveness, Safety, Obstetrics
Introduction
From December 30, 2019, to May 14, 2022, 517,648,631 people were infected with COVID-19, and 6,261,708 people died in total. The male-to-female ratio was 0.9 among infected people and 1.29 in deceased people [1]. According to the United States Centers for Disease Control and Prevention (https://www.cdc.gov/, last accessed 05/09/22), 207,793 pregnant women in the United States were infected, and 296 died.
Research shows that pregnant women are a high-risk group for COVID-19 infection [2]. Physiological changes during pregnancy often increase susceptibility to infection. For instance, the maternal immune system is biased toward T helper 2 cells (Th2) during pregnancy, which makes the mother more susceptible to COVID-19 [3].
Vaccination is currently considered the most effective intervention for the prevention of COVID-19. As of May 15, 2022, approximately 65.6% of the world's population had received a COVID-19 vaccine [1]. Of all countries, Cuba ranked first, where approximately 94.23% of residents have received at least one dose [4]. According to the World Health Organization, 142 COVID-19 vaccines have been in the clinical development phase, mainly including viral vector vaccines, inactivated vaccines, and RNA vaccines [5]. However, the acceptance of vaccination among pregnant women is relatively low [6], and there are no systematic evaluations of dose- and time-dependent vaccine effectiveness (VE) in pregnant women.
To better help with COVID‐19 prevention and control in gravida, we combined the random forest predictive model with classical statistical methods to identify and compare the effectiveness of different COVID-19 vaccines in pregnant women. Time-effectiveness charts were also constructed for the temporal dynamics of VE. In addition, we analyzed both the obstetric and neonatal outcomes as well as local and systemic reactogenicity. We hope that our research can provide guidance for clinical treatment and increase public confidence in COVID-19 vaccines.
Materials and methods
Literature search strategy
We identified records by searching the Cochrane Library, Embase, Web of Science, PubMed, ClinicalKey, COVID-19 Research Database, COVID-19 Open Research Dataset Challenge (CORD-19), COVID-19 Intelligent Insight and COVID-19 Public Media Dataset for “(SARS-CoV-2 OR COVID-19 OR 2019-nCoV) AND pregnancy AND vaccine” (up to May 12, 2022). Clinical trial registries such as ClinicalTrials.gov were also searched. We also checked the reference lists of the included studies on the topic. Only articles written in English were included in our meta-analysis, and other types of papers and language articles were excluded. A complete description of the initial and supplementary search strategies is available in Table S1.
Eligibility criteria
We included randomized controlled trials and observational studies of COVID-19 VE and safety in pregnant women published from December 2019 onward.
Studies were eligible for inclusion if their subjects included pregnant women, regardless of nationality and race; research contents contained the effectiveness of and adverse reactions to COVID-19 vaccines among pregnant women.
We excluded case reports, cross-sectional studies, review articles, conference abstracts, and editorials due to their low levels of evidence. In addition, males and any other nonpregnant females were not included in this study.
In this study, all participants received both doses of the vaccine, and no participant received a booster dose.
Study selection and data extraction
References were imported into EndNote (version X9), and duplicates were removed. We completed the screening in two stages. The titles and abstracts of the studies were initially screened for eligibility. Afterward, the full texts of the studies assessed as potentially relevant for the review were retrieved and checked against our inclusion and exclusion criteria. Two investigators independently performed abstract screening, full-text screening, and data extraction. Disagreements between reviewers were resolved by discussion or, if needed, by consultation with a third review author.
Data were recorded using the predesigned PROforma method and managed on a Microsoft Excel spreadsheet. We extracted the primary and secondary outcomes from appropriate randomized controlled trials and nonrandomized studies of interventions. Other relevant data included study names, basic information from the studies, study population characteristics, and types of SARS-CoV-2 vaccines.
Risk of bias assessment
The risk of bias was evaluated using the Cochrane risk of bias (RoB) tool [7] for randomized trials and the Risk Of Bias In Nonrandomized Studies of Interventions (ROBINS-I) tool [8] for nonrandomized studies. We included randomized and nonrandomized trials; however, we found no randomized trials. For nonrandomized studies, we judged the RoB in seven domains and differentiated bias due to confounding, the selection of study participants, the classification of interventions, deviations from intended interventions, missing data, the measurement of outcomes, and the selection of reported results. Disagreements were resolved by discussion with a third investigator.
Outcomes of interest
The primary outcome was the VE of COVID-19 vaccines. VE is a measure of how well vaccines work in the real world. In this research, VE reflects protection from infection. We estimated the effectiveness of vaccines against SARS-CoV-2 infection as follows: VE = 100% × (1 - adjusted odds ratio [OR] of completing COVID-19 vaccination during pregnancy among mothers in the case and control groups) [9,10]. The case group was defined as vaccinated pregnant women, and the control group was defined as unvaccinated pregnant women in this meta-analysis.
The secondary outcomes were obstetric and neonatal outcomes, as well as local and systemic reactogenicity. The obstetric and neonatal outcomes mainly included postpartum hemorrhage, placental abruption, miscarriage, premature birth, stillbirth, and small for gestational age (SGA) infant. The local and systemic reactogenicity included pain, fever, and fatigue.
Statistical analysis
Our primary outcome was the dose- and time-dependent VE of COVID-19 vaccines, expressed by how well the messenger RNA (mRNA) and inactivated vaccines worked to protect communities as a whole and stratified according to time. Table 1 reports the respective data for the vaccines included in the study. The VE of the COVID-19 vaccines is defined as the relative reduction in the risk of SARS-CoV-2 infection after vaccination. VE was used to evaluate the vaccines, and we can easily find the VE and both the upper and lower limits of the 95% confidence interval (CI) from the articles. We performed separate meta-analyses for the VE of different types of vaccines and different times. Spearman correlation was used to assess correlation. The statistical significance of the VE on different days was assessed by the nonparametric Mann–Whitney U test.
Table 1.
Overview of the included vaccines.
| Research contents | Type | Number of studies | Vaccine name | Type of ingredient (in one dose) | Study ID | ||
|---|---|---|---|---|---|---|---|
| Vaccine effectiveness | mRNA vaccine | 3 | Pfizer-BioNTech | Active ingredient | Adjuvant | Other excipients | Dagan et al.[11]; Goldshtein et al.[12]; Butt et al.[13] |
|
mRN: Tozinameran is a single-stranded, 5’-capped mRNA produced using a cell-free in vitro transcription from the corresponding DNA templates, encoding the viral spike protein of SARS-CoV-2. |
/ | -Lipids (fats)*: ‧2[(polyethylene glycol (PEG))-2000]-N,N-ditetradecylacetamide ‧1,2-distearoyl-sn-glycero-3-phosphocholine ‧Cholesterol (plant derived) ‧((4-hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis(2-hexyldecanoate) -Sugar and acid stabilizers: ‧ Sucrose (table sugar) ‧ Tromethamine ‧Tromethamine hydrochloride |
|||||
| One vial (0.45 ml) contains six doses of 0.3 ml after dilution. One dose (0.3 ml) contains 30 micrograms of tozinameran, a COVID-19 mRNA vaccine (embedded in lipid nanoparticles). * Lipid nanoparticle formulation has intrinsic adjuvant activity. | |||||||
| Moderna | Active ingredient | Adjuvant | Other excipients | Butt et al.[13] | |||
| -mRNA: Elasomeran is a single-stranded, 5’-capped mRNA produced using a cell-free in vitro transcription from the corresponding DNA templates, encoding the viral spike protein of SARS-CoV-2 |
/ | -Lipids (fats)*: ‧ PEG2000-DMG: 1,2-dimyristoyl-rac-glycerol, methoxypolyethylene glycol ‧1,2-distearoyl-sn-glycero-3-phosphocholine ‧ BotaniChol® (nonanimal origin cholesterol) ‧ SM-102: heptadecane-9-yl 8-((2-hydroxyethyl) (6-oxo-6-(undecyloxy) hexyl) amino) octanoate -Salt, sugar, acid stabilizers, and acid: ‧ Sodium acetate ‧ Sucrose (basic table sugar) ‧ Tromethamine ‧Tromethamine hydrochloride ‧ Acetic acid (the main ingredient in white household vinegar) |
|||||
| This is a multidose vial that contains 10 doses of 0.5 ml each or a maximum of 20 doses of 0.25 ml each. One dose (0.5 ml) contains 100 micrograms of elasomeran, a COVID-19 mRNA vaccine (embedded in SM-102 lipid nanoparticles). One dose (0.25 ml) contains 50 micrograms of elasomeran, a COVID-19 mRNA vaccine (embedded in SM-102 lipid nanoparticles). * Lipid nanoparticle formulation has intrinsic adjuvant activity. |
|||||||
| Inactivated vaccines | 1 | CoronaVac | Active ingredient | Adjuvant | Other excipients | Paixao et al.[14] | |
| -3 μg of inactivated SARS-CoV-2 virus |
-Aluminum hydroxide: 0.5 ml of aluminum hydroxide diluent per dose |
- Salt, sugar, acid stabilizers, and acid: disodium hydrogen phosphate, sodium dihydrogen phosphate, sodium, chloride, and water for injection |
|||||
| One dose (0.5 ml) is composed of 3 μg of inactivated SARS-CoV-2 virus | |||||||
| Research contents | Type | Number of studies | Vaccine name |
Type of ingredient (in one dose) |
Study ID | ||
| Vaccine Safety | mRNA vaccine | 13 | Pfizer-BioNTech | Active ingredient | Adjuvant | Other excipients | Theiler et al.[16]; Wainstock et al.[17]; Goldshtein et al.[12]; Rottenstreich et al.[18]; Bookstein Peretz et al.[21]; Blakeway et al.[19]; Lipkind et al.[20]; Shimabukuro et al.[26]; Kachikis et al.[22]; Trostle et al.[23]; Zauche et al.[24]; Kharbanda et al.[25]; Magnus et al.[15] |
| ‑Messenger ribonucleic acid (mRNA) | / | -Lipids (fats)* -Sugar and acid stabilizers |
|||||
| One vial (0.45 ml) contains six doses of 0.3 ml after dilution. One dose (0.3 ml) contains 30 micrograms of tozinameran, a COVID-19 mRNA vaccine (embedded in lipid nanoparticles). * Lipid nanoparticle formulation has intrinsic adjuvant activity. | |||||||
| Moderna | Active ingredient | Adjuvant | Other excipients | Theiler et al.[16]; Blakeway et al.[19]; Lipkind et al.[20]; Shimabukuro et al.[26]; Kachikis et al.[22]; Trostle et al.[23]; Kharbanda et al.[25]; Magnus et al.[15] |
|||
| -Messenger ribonucleic acid (mRNA) | -Lipids (fats)* -Salt, sugar, acid stabilizers, and acid |
||||||
| One dose (0.5 ml) contains 100 micrograms of elasomeran, a COVID-19 mRNA vaccine (embedded in SM-102 lipid nanoparticles). One dose (0.25 ml) contains 50 micrograms of elasomeran, a COVID-19 mRNA vaccine (embedded in SM-102 lipid nanoparticles). * Lipid nanoparticle formulation has intrinsic adjuvant activity. | |||||||
| Viral vector vaccine | Janssen | Active ingredient | Adjuvant | Other excipients | Theiler et al.[16]; Lipkind et al.[20]; [21] |
||
| -A harmless version of a virus unrelated to the COVID-19 virus: Recombinant, replication-incompetent Ad26 vector, encoding a stabilized variant of the SARS-CoV-2 Spike (S) protein |
/ | -Excipients with known effect: Each dose (0.5 ml) contains approximately 2 mg of ethanol. -Sugars, salts, acid, and acid stabilizer: ‧ Polysorbate-80 ‧ 2-hydroxypropyl-β-cyclodextrin ‧ Trisodium citrate dihydrate ‧ Sodium chloride (basic table salt) ‧ Citric acid monohydrate (closely related to lemon juice) |
|||||
| This is a multidose vial which contains five doses of 0.5 ml. One dose (0.5 ml) contains: Adenovirus type 26 encoding the SARS-CoV-2 spike glycoprotein* (Ad26.COV2-S), no less than 8.92 log10 infectious units. Produced in the PER.C6 TetR Cell Line and by recombinant DNA technology. | |||||||
| Oxford-AstraZeneca | Active ingredient | Adjuvant | Other excipients | Blakeway et al.[19]; Magnus et al.[15] |
|||
| -COVID-19 Vaccine (ChAdOx1-S* recombinant), not less than 2.5 × 108 infectious units -Recombinant, replication-deficient chimpanzee adenovirus vector encoding the SARS-CoV-2 spike glycoprotein: Produced in genetically modified human embryonic kidney 293 cells. |
/ | - Sugars, salts, acid, and acid stabilizer: L-Histidine, L-Histidine hydrochloride monohydrate, magnesium chloride hexahydrate, polysorbate 80 (E 433), ethanol, sucrose, Sodium chloride, disodium edetate dihydrate, and water for injections |
|||||
| One dose (0.5 ml) contains no less than 2.5 × 108 infectious units. | |||||||
mRNA, messenger RNA.
Our secondary outcomes were obstetric and neonatal outcomes as well as local and systemic reactogenicity. The ORs and corresponding 95% CIs were estimated. A hazard ratio of less than 1 indicates a reduced risk for adverse outcomes. We also calculated and reported the averaged data of the adverse outcomes as the means ± standard errors (SEs).
We analyzed the data with RevMan 5.3 and StataMP 16. At least two studies were required for each meta-analysis. Random effects models were selected to calculate effect sizes because they represented a more conservative estimate of the mean prevalence. Assessing heterogeneity was necessary, and it was assessed using the I2 statistic. Heterogeneity was categorized as 25% (low heterogeneity), 50% (moderate heterogeneity), or 75% (high heterogeneity). A sensitivity analysis was also conducted to determine the possible cause of high heterogeneity. Publication bias was assessed using a funnel plot and both Egger's test and Begg's test for funnel plot asymmetry. A P-value < 0.05 indicated statistical significance.
Results
Study selection
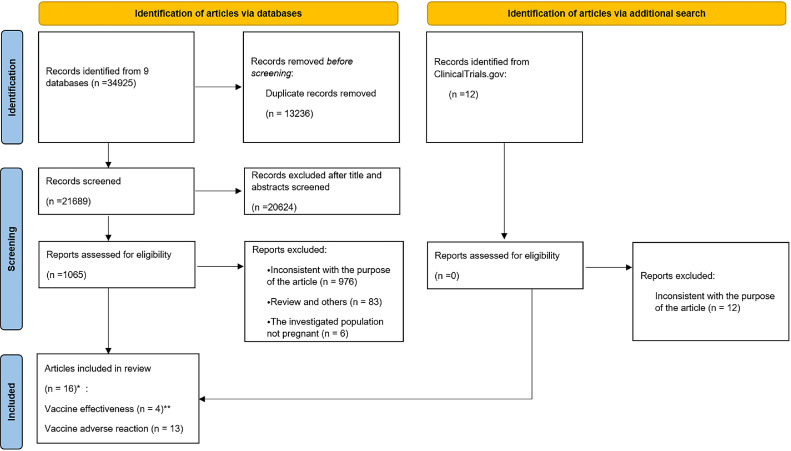
Our research identified 34,925 records from nine databases. After removing duplicates, 21,689 records remained. We excluded another 20,624 articles after reading the titles and abstracts. A total of 1065 articles remained for further evaluation after reading the full texts. A total of 591 works were excluded for irrelevance to our purpose, and 106 works were also removed as they were reviews. Finally, 16 articles that met the inclusion criteria were included [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26]. Four of the articles were about VE [11], [12], [13], [14]. Thirteen articles were about adverse reactions to vaccines, including obstetric and neonatal outcomes, as well as local and systemic reactogenicity [12,[15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26]]. Among these studies, the study conducted by Goldshtein et al. [12] involved both types of outcomes. Figure 1 shows a flowchart describing the study selection process.
Figure 1.
Flowchart of the study selection process.
*The study conducted by Goldshtein et al.[12] included both vaccine effectiveness and adverse reactions to vaccines.
**The four included articles contained six studies. The article by Dagan et al. included two studies: Dagan et al.[11] (documented infection) and Dagan et al.[11] (symptomatic infection). The article by Paixao et al.[14] also included two studies: Paixao et al.[14] (symptomatic infection) and Paixao et al.[14] (severe infection).
Descriptive characteristics
Overall, this meta-analysis was based on a pooled sample of 196,609 women. We included six studies [11], [12], [13], [14] from four articles about COVID-19 VE and 13 studies from 13 articles about adverse reactions to vaccines [12,[15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26]]. Trials primarily included women who were vaccinated at any time during pregnancy. Of the 16 included studies, two different kinds of vaccines were involved: mRNA vaccines and inactivated vaccines. All included studies were nonrandomized trials. Their characteristics are summarized in Table S2 and Table S3.
Risk of bias assessment
Based on the ROBINS-I tool, four studies were rated as having moderate RoB, and 15 studies were deemed to have low RoB. The RoB assessments are shown in Table S4.
Dose- and time-dependent COVID-19 vaccine effectiveness for pregnant women
Regarding COVID-19 VE, a total of six studies from four articles, Dagan et al. [11] (documented infection), Dagan et al. [11] (symptomatic infection), Goldshtein et al. [12], Butt et al. [13], Paixao et al. [14] (symptomatic infection) and Paixao et al. [14] (severe infection), were included. The average VE over a period of time (first dose: within 10 days, from 11 to 13 days, from 14 to 20 days, from 21 to 27 days, after 28 days; second dose: from 7 to 13 days, from 14 to 56 days) in these studies was included in our research (Table S5). The main results on the relationship between VE and days of vaccination are presented in Table 2 .
Table 2.
Summary of the effectiveness of the different types of vaccines.
| Type |
The effectiveness of messenger RNA-based vaccines against COVID-19 on different days |
Study ID |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| First dosea |
Second doseb |
||||||||
| ≤10 days | 11-13 days | 14-20 days | 21-27 days | ≥28 days | 7-13 days | 14-56 days | |||
| Messenger RNA vaccine | 4% (95% CI: -0.33-0.31) | 54% (95% CI: 0.33-0.69) | 59% (95% CI: 0.47-0.71) | 59% (95% CI:0.46-0.72) | 64% (95% CI: 0.29-0.99) | 97% (95% CI: 0.93-1.00) | 96% (95% CI: 0.93-1.00) | Dagan et al.[11]; Goldshtein et al.[12]; Butt et al.[13] |
|
| Inactivated vaccine | Paixao et al.[14] (Symptomatic infection)c | / | / | 5.02% (95% CI: -0.1822-0.2369) | 5.02% (95% CI: -0.1822-0.2369) | 5.02% (95% CI: -0.1822-0.2369) | / | 40.97% (0.2707-0.5222) | Enny Paixao et al.[14] |
| Paixao et al.[14] (Severe infection)c | / | / | 67.74% (95% CI: 0.20-0.87) | 67.74% (95% CI: 0.20-0.87) | 67.74% (95% CI: 0.20-0.87) | / | 85.39% (0.5944-0.9480) | ||
| Overall | / | / | 60% (95% CI: 0.49-0.71) | 60% (95% CI: 0.48-0.73) | 69% (95% CI: 0.50-0.88) | / | 96% (0.93-0.99) | Dagan et al.[11]; Goldshtein et al.[12]; Butt et al.[13] |
|
Different days after the first dose of vaccination.
Different days after the second dose of vaccination.
The Paixao et al.[14] (symptomatic infection) study included pregnant women who had symptomatic infections after vaccination and did not have a positive reverse transcription-polymerase chain reaction test result within the previous 90 days. The Paixao et al.[14] (severe infection) study included pregnant women with severe infections who did not have a positive reverse transcription-polymerase chain reaction test result within the previous 90 days.
The effectiveness of messenger RNA-based vaccines against COVID-19 on different days
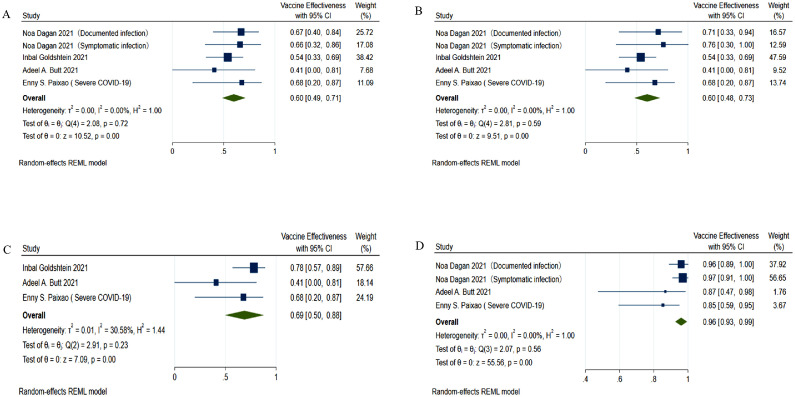
A study conducted by Goldshtein et al. [12] indicated that within 10 days after the first dose of the Pfizer-BioNTech mRNA COVID-19 vaccine, the VE was 4% (95% CI: -0.33-0.31). However, from the 11th to the 13th day after the first dose, the VE increased to 54% (95% CI: 0.33-0.69).
The mRNA-based VE in pregnant women was the lowest from 14 to 20 days after the first dose (VE = 59%, 95% CI: 0.47-0.71) (Figure 2 a). It was almost the same from 21 to 27 days after the first dose (VE = 59%, 95% CI: 0.46-0.72) (Figure 2b). At ≥28 days after the first dose, the VE was 64% (95% CI: 0.29-0.99) in pregnant women (Figure 2c).
Figure 2.
Forest plots of the VE of mRNA-based vaccines.
(a) VE at 14 to 20 days after the first dose of mRNA vaccines; (b) VE at 21 to 27 days after the first dose of mRNA vaccines; (c) VE at ≥28 days after the first dose of mRNA vaccine; (d) VE from 7 to 13 days after the second dose of mRNA vaccines; (e) VE from 14 to 56 days after the second dose of mRNA vaccines.
CI, confidence interval; mRNA, messenger RNA; VE, vaccine effectiveness.
From 7 to 13 days after the second dose of an mRNA vaccine, the VE in pregnant women was much higher (VE = 97%, 95% CI: 0.93-1.00) (Figure 2d). However, since only Dagan et al.’s [11] data were available for these days, the result was not representative. The VE was 96% (95% CI: 0.93-1.00) 14 to 56 days after the second dose (Figure 2e).
The effectiveness of the inactivated vaccine against COVID-19 on different days
The relevant data were compiled from the study by the Paixao et al. [14] team. The VE of CoronaVac on different days is also shown in Table S5. Due to high heterogeneity (I2 >75%), it was not possible to obtain the overall effectiveness of an inactivated vaccine (Table S6).
Overall vaccine effectiveness of COVID-19 vaccines on different days
The overall VE of different kinds of COVID-19 vaccines is shown in Figure S1. Due to high statistical heterogeneity, the results could not be pooled.
A funnel plot was applied to determine if there was any publication bias. However, due to the small number of included studies, the funnel chart had no obvious significance (Figure S2). Egger's test and Begg's test showed no evidence of publication bias for each parameter included in the study (Table S7).
To evaluate the stability of the pooled results of our meta-analysis, we performed a sensitivity analysis by sequentially excluding each study. Paixao et al. [14] (symptomatic infection) was the main source of heterogeneity. This might be due to the nonsignificant VE in this study (VE = 5.02%, 95% CI: -18.22-23.69).
With this study removed, the overall VE was 0.60 from days 14-28 after the first dose (Figure 3 a-b). It rose significantly after the 28th day of the first vaccine dose (Figure 3c, VE = 69%, 95% CI: 0.50-0.88) and increased to 96% after the second dose (Figure 3d, 95% CI: 0.93-0.99).
Figure 3.
Adjusted overall VE.
The study by Paixao et al.[14] (symptomatic infection) was the source of heterogeneity and was removed. (a) Overall VE at 14 to 20 days after the first dose; (b) Overall VE at 21 to 27 days after the first dose; (c) Overall VE at ≥28 days after the first dose of mRNA vaccine; (d) Overall VE at ≥14 days after two doses.
CI, confidence interval; mRNA, messenger RNA; VE, vaccine effectiveness.
Correlation between vaccine effectiveness and different days after vaccination
Spearman correlation was used to assess the correlation between VE and time since vaccination. This resulted in a highly significant positive correlation (P <0.005) for each dose. The VE of each dose gradually increased as the average number of days since vaccination increased (Figure S3). The vaccine was more effective after the second dose than after the first dose alone (Figure S3A). Likewise, the overall effectiveness of various kinds of vaccines (with the study that was the source of heterogeneity removed) also showed the same trend (Figure S3B). However, the results revealed that the mRNA-based COVID-19 vaccine was more effective at 7-13 days than at 14 days after the second dose (97% vs 96%), but the change was not statistically significant (P >0.05 by the nonparametric Mann–Whitney U test).
Safety of the COVID-19 vaccines in pregnant women
Thirteen studies evaluated the safety of COVID-19 vaccines. All 13 studies reported obstetric and neonatal outcomes [12,[15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26]], and three also involved local and systemic reactogenicity [21,22,26]. There were seven case-control studies, and the other studies were prospective cohort studies.
Major obstetric and neonatal outcomes
For the 13 studies reviewed, comparisons were made for a range of major obstetric and neonatal outcomes associated with COVID-19 vaccination, including miscarriage, SGA, premature delivery, stillbirth, placental abruption, and postpartum hemorrhage (Table S8).
The proportions of major obstetric and neonatal outcomes in vaccinated and unvaccinated pregnant women are shown in Figure S4. No significant differences were found in the average between the two groups.
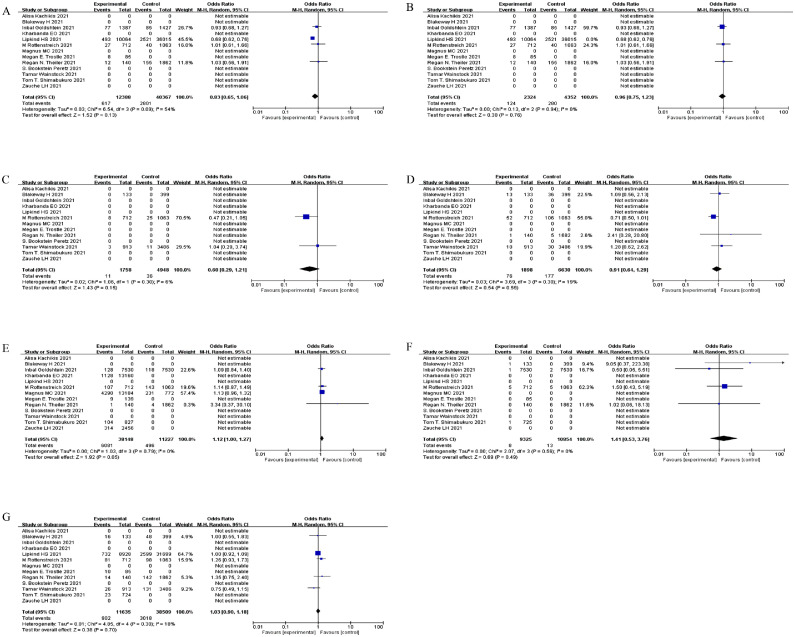
There was a pooled OR of 0.83 (Figure 4 a, 95% CI: 0.65-1.06, P = 0.13, I2 = 54%) in the premature delivery group, which included vaccinated and unvaccinated controls. Therefore, we concluded that the probability of premature delivery was not significantly associated with vaccination. The analysis method, detection method, and research center might contribute to the heterogeneity (Figure 4b).
Figure 4.
Forest plots of obstetric and neonatal outcomes (vaccinated pregnant women vs unvaccinated pregnant women).
(a) The risk of premature delivery in vaccinated pregnant women vs unvaccinated pregnant women (OR = 0.83, 95% CI: 0.65-1.06, P = 0.13, I2 = 54%); (b) Subgroup analysis; (c) The odds of placental abruption (OR = 0.60, 95% CI: 0.29-1.21, P = 0.15, I2 = 6%); (d) The odds of postpartum hemorrhage (OR = 0.91, 95% CI: 0.64-1.29, P = 0.59, I2 = 19%); (e) The odds of miscarriage (OR = 1.12, 95% CI: 1.00-1.27, P = 0.05, I2 = 0%); (f) The odds of stillbirth (OR = 1.42, 95% CI: 0.53-3.76, P = 0.49, I2 = 0%); (g) The odds of SGA (OR = 1.03, 95% CI: 0.90-1.18, P = 0.70, I2 = 18%).
CI, confidence interval; OR, odds ratio.
The odds of placental abruption (Figure 4c, P = 0.15), postpartum hemorrhage (Figure 4d, P = 0.59), miscarriage (Figure 4e, P = 0.05), stillbirth (Figure 4f, P = 0.49), and SGA (Figure 4g, P = 0.70) among pregnant women were not associated with COVID-19 vaccination.
These results confirmed our conjecture that obstetric and neonatal outcomes were not associated with COVID-19 vaccination. Thus, the safety of COVID-19 vaccines was partially proven.
Local and systemic reactogenicity
Local and systemic reactogenicity included muscle pain, fever (>38°C), and fatigue. The number of individuals with data was 35,157, including pregnant women and 9976 nonpregnant women. Data for 6074 pregnant women and 1105 nonpregnant women were missing for the second dose (Table S9).
Compared with that in nonpregnant women after vaccination, the OR of muscle pain was 0.45 in pregnant women (95% CI: 0.22-0.93, P = 0.03) (Figure 5 a). However, there were too few included studies in these comparisons for these data to be representative. Muscle pain was not associated with pregnancy after receipt of the second dose of the COVID-19 vaccine (P = 0.39) (Figure 5b). The data regarding fatigue were not statistically significant (Figure 5c–d).
Figure 5.
Forest plots of the ORs for local and systemic reactogenicity (pregnant women vs nonpregnant women).
(a) OR for muscle pain in pregnant women after the first dose (OR = 0.45, 95% CI: 0.22-0.93, P = 0.03); (b) Muscle pain after receipt of the second dose of a COVID-19 vaccine (OR comparing pregnant to nonpregnant women 1.28, 95% CI: 0.73-2.23, P = 0.39); (c) Fatigue in pregnant women compared with nonpregnant women (first dose: OR = 0.90, 95% CI: 0.63-1.28, P = 0.56); (d) Fatigue in pregnant women compared with nonpregnant women (second dose: OR = 0.89, 95% CI: 0.65-1.22, P = 0.48); (e) Risk of developing a fever in pregnant women compared with nonpregnant women (random effect models, first dose: OR = 1.02, 95% CI: 0.12-8.61, P = 0.98); (f) Risk of developing a fever in pregnant women compared with nonpregnant women (random effect models, second dose: OR = 0.65, 95% CI: 0.42-1.01, P = 0.05).
CI, confidence interval; OR, odds ratio.
Random effect models were used to compare the risk of developing a fever in pregnant and nonpregnant women due to high statistical heterogeneity (Figure 5e–f). The risk after vaccination was proven not to be related to pregnancy. However, it varied by different doses. The risk of fever in pregnant women after the second dose was almost 12.30 times higher than that in those who received the first dose (Figure S5A, 95% CI: 8.76-17.27, P <0.01). The different methodologies used in the study by Shimabukuro et al. [26] might contribute to the high heterogeneity (Figure S5B). However, among nonpregnant women, the OR was 10.28 (Figure S5C, 95% CI: 4.88-21.65, P <0.01, I2 = 23%). This illustrated that different doses of COVID-19 vaccines led to no significant difference in fever in pregnant and nonpregnant women.
In general, all local and systemic reactions were considered unrelated to pregnancy in vaccinated women (Table 3 ).
Table 3.
Summary of vaccine safety.
| Safety of the COVID-19 vaccines | Odds ratio | 95% confidence interval | P-value | Study ID |
|---|---|---|---|---|
| Obstetric and neonatal outcomes (vaccinated pregnant women vs unvaccinated pregnant women) | Theiler et al.[16];Wainstock et al.[17]; Goldshtein et al.[12]; Rottenstreich et al.[18]; Bookstein Peretz et al.[21]; Blakeway et al.[19]; Lipkind et al.[20]; Shimabukuro et al.[26]; Kachikis et al.[22]; Trostle et al.[23]; Zauche et al.[24]; Kharbanda et al.[25]; Magnus et al.[15] |
|||
| Premature delivery | 0.83 | 0.65-1.06 | 0.13 | |
| Placental abruption | 0.60 | 0.29-1.21 | 0.15 | |
| Postpartum hemorrhage | 0.91 | 0.64-1.29 | 0.59 | |
| Miscarriage | 1.12 | 1.00-1.27 | 0.05 | |
| Stillbirth | 1.42 | 0.53-3.76 | 0.49 | |
| Small for gestational age infant | 1.03 | 0.90-1.18 | 0.70 | |
| Local and systemic reactogenicity (pregnant women vs nonpregnant women) | ||||
| Muscle pain (first dose) | 0.45 | 0.22-0.93 | 0.03 | |
| Muscle pain (second dose) | 1.28 | 0.73-2.23 | 0.39 | |
| Fatigue (first dose) | 0.90 | 0.63-1.28 | 0.56 | |
| Fatigue (second dose) | 0.89 | 0.65-1.22 | 0.48 | |
| Fever (first dose) | 1.02 | 0.12-8.61 | 0.98 | |
| Fever (second dose) | 0.65 | 0.42-1.01 | 0.05 |
Discussion
Principal findings
To better help with COVID-19 prevention and control, we conducted a systematic review and meta-analysis to assess COVID-19 vaccines within the pregnant population. This meta-analysis evaluated 19 studies on the dose- and time-dependent effectiveness and safety of COVID-19 vaccines from 16 included articles based on data from six countries. The article by Dagan et al. [11] included two studies: Dagan et al. [11] (documented infection) and Dagan et al. [11] (symptomatic infection). The article by Paixao et al. [14] also included two studies: Paixao et al. [14] (symptomatic infection) and Paixao et al. [14] (severe infection).
Our main finding is that vaccination is an effective and safe strategy for acquiring immunity to COVID-19 among pregnant women. We had certainty of the dose- and time-dependent effectiveness of COVID-19 vaccines in pregnant women. A comprehensive review also similarly showed an encouraging safety profile of COVID-19 vaccines in pregnant women.
Comparison with literature
Six items were included to evaluate COVID-19 VE in our study. The overall VE in pregnant women after two doses was approximately 96% (95% CI: 0.93-0.99). Among them, the effectiveness of mRNA-based vaccines against COVID-19 was 96% (two doses >14, 95% CI: 0.93-1.00). Published studies on nonpregnant women have shown that the VE of all different kinds of COVID-19 vaccines is more than 70%, with RNA-based vaccines having the highest effectiveness, reaching 94.29% [27]. Our findings showed that the VE estimate for pregnant women is not lower than that for the general population. This is similar to that reported by Prasad et al. [28], with the VE reaching 89.5% (7 days after the second dose). However, in our meta-analysis, we further discussed the changes in COVID-19 VE based on dose and time and tried to find the trends of VE vs time. Our results suggested that the estimated dose- and time-dependent VE of future variants of vaccines in the general population can apply to the same variants in pregnant women, especially for mRNA vaccines.
Evidence shows that compared with that in nonpregnant women, Fc receptor binding to antibodies in pregnant and lactating women is delayed after the first dose of vaccine and returns to normal after the second dose [29]. This indicates that pregnancy promotes resistance to the production of proinflammatory antibodies. This was especially obvious for inactivated vaccines. Our study showed that the overall VE was 72% after the first dose and 96% after the second dose. However, the VE of the inactivated vaccines was 40.97% (one dose ≥14 days) and 85.39% after the second dose. Compared with other COVID-19 vaccines, inactivated vaccines such as CoronaVac are effective against COVID-19 only after a complete immunization scheme. Based on our observations and current available evidence, we have the following recommendation: Given the low response to vaccines in individuals who receive mRNA vaccines and inactivated vaccines after primary immunization, it is critically important that pregnant women receive booster vaccinations to optimize their immunity.
In addition, evidence shows that COVID-19 vaccines can not only affect the mother but also have a certain protective effect on the fetus [30]. However, pregnant women have shown a high rate of vaccine hesitancy due to safety considerations [31], [32], [33], especially for obstetric and neonatal outcomes. By integrating published clinical data, we also focused on miscarriages, placental abruptions, postpartum hemorrhage, premature deliveries, stillbirths, and SGA infants. The probabilities of these obstetric and neonatal outcomes are relatively low. We found no significant change in the probabilities of any of these conditions in vaccinated pregnant women compared with unvaccinated pregnant women. Vaccines are safe regarding obstetric and neonatal outcomes. This conclusion was supported by a similar finding by Prasad et al. [28].
Furthermore, there were also no adverse effects reported in the developmental and reproductive toxicology studies of the Pfizer-BioNTech vaccine in rats [34]. In research from Israel, the protective effect of COVID-19 vaccines was greater than their potential adverse pregnancy outcomes [35]. Furthermore, in a recent study, compared with the published incidence in pregnant populations before the appearance of COVID-19, the frequency of adverse pregnancy outcomes and neonatal outcomes after vaccination was found to be similar, although not directly comparable [26]. These findings indicate that COVID-19 vaccines during pregnancy are safe.
Regarding local and systemic reactogenicity, studies by both the Bookstein Peretz team and the Golan team have demonstrated that the COVID-19 mRNA vaccine has no severe local or systemic reactogenicity in pregnant women [21,36]. Taken together, our results are in agreement with these findings. We also compared the difference in local and systemic reactogenicity after the first and second doses of the vaccines. Reactogenicity was more likely to occur after the second dose than after the first dose in both pregnant and nonpregnant women. Our study demonstrated a similar response strength influenced by the COVID-19 vaccine dose in pregnant and nonpregnant women.
However, some evidence suggests stronger reactogenicity in pregnant women than in nonpregnant women after vaccination. For example, mucosal-associated invariant T (MAIT) cells from pregnant women were proven to balance immune tolerance and antimicrobial defense. They responded more strongly than cells in nonpregnant women after attack by microbial and inflammatory stimuli, which has been validated in studies on influenza A virus and group B Streptococcus [37]. Therefore, future studies are needed to further investigate whether the response to a COVID-19 vaccine dose in pregnant and nonpregnant women is similar.
Strengths and limitations
This was the first study to analyze the dose- and time-dependent effectiveness of COVID-19 vaccination. We also tried to provide a more comprehensive overview of vaccine safety, including obstetric and neonatal outcomes, as well as local and systemic reactogenicity. Nonetheless, our study still has some limitations. First, as in any observational study, there may still be residual confounding from unmeasured confounders, such as the timing of pregnancy and diseases during pregnancy. Thus, more randomized controlled trials are needed with representative samples of the population and a low risk for bias. Notably, only English articles were included in this study, which may lead to a potential selection bias. In addition, although our study provides a reliable estimate of VE in pregnant women, whether the effectiveness of the vaccine differs among pregnant women in the first, second, and third trimesters remains to be evaluated. At present, studies have shown that the effectiveness of a completed COVID-19 primary vaccine series in early and late pregnancy was 32% (95% CI: 43%-68%) and 80% (95% CI: 55%-91%), respectively, in 17 states from July 2021 to January 2022 [15]. However, further investigations are still needed in a broader range of populations. Finally, since vaccine safety is the priority consideration before vaccination, whether the currently approved mRNA vaccines and other kinds of vaccines have different effects on adverse pregnancy outcomes remains to be explored.
Clinical implications
This meta-analysis indicates that the effectiveness of COVID-19 vaccines for pregnant women is not lower than that for the general population. The frequency of obstetric and neonatal outcomes in pregnant women after vaccination was similar to that in unvaccinated pregnant women. The response strength influenced by doses of COVID-19 vaccines was not significantly different in pregnant and nonpregnant women. These findings suggest a high effectiveness and good safety of COVID-19 vaccines in pregnant women.
This study addresses the concerns and fears of pregnant women related to the COVID‐19 vaccines. This may have a role in reducing the rate of maternal COVID-19 infection.
Public health implications
Currently, COVID-19 is a major public health challenge worldwide, and COVID-19 vaccine development is occurring at an unprecedented pace. Research has shown that pregnant women are a high-risk group for COVID-19 infection [2]. Recently, many studies have also demonstrated the duration of effectiveness of the COVID-19 vaccine in the general population. Research in both Thailand [38] and England [39] has shown that as the vaccine dose increases, the effectiveness increases.
However, there was no relevant evidence in pregnant women, and actions may also be required to raise awareness and consensus about the benefits of vaccination and to increase the overall vaccine uptake in pregnant women.
This systematic review and meta-analysis were able to assess COVID-19 vaccines within the pregnant population.
Conclusion
In summary, our findings affirm the certainty of the effectiveness of COVID-19 vaccines in pregnant women. The effectiveness varies among different types of vaccines and has a significant positive correlation with time. Given the low response to vaccines after primary immunization, it is critically important for pregnant women to receive booster shots to optimize their immunity. COVID-19 vaccines were deemed safe.
Declaration of competing interest
The authors have no competing interests to declare.
Acknowledgments
Funding
The present work was supported in part by the National Key Research and Development Program (2022YFC2704600 and 2022YFC2704604); the National Natural Science Foundation of China (Nos. 82071678 and 82171683); the National Key R&D Program of China (Nos. 2021YFC2701601, 2021YFC2701603 and 2021YFC2701604); the Shanghai Municipal Science and Technology Commission Research Fund (No. 21140903800); Clinical Research Plan of SHDC (SHDC2020CR2059B); and the Key Research Project of Pudong New Area Population and Family Planning Commission (No. PW2020E-1). The funder had no role in the study design or the collection, analysis, or interpretation of data, the writing of the manuscript, or the decision to submit the article for publication.
Ethical approval
Not needed.
Acknowledgments
We thank the authors of the meta-analysis for providing the original data and clarifying the findings.
Author contributions
Contributors: Hao Ying conceived and supervised the study. Hao Ying, Han Xie, and Xiaohua Liu initiated and designed the study plan. Shengyu Wu, Luyao Wang, and Jiaqi Dong performed the search, independently screened the search results, and extracted data from eligible studies. Yirong Bao and Xiaohui Liu exchanged data extraction results and resolved disagreements through discussion. Yuhong Li performed the statistical analysis. Shengyu Wu wrote the manuscript. All authors contributed to data analysis and discussed the paper.
Registration and protocol
PROSPERO ID: CRD42022347621
Data availability statement
The raw data in this systematic review with a meta-analysis were extracted from published and preprint studies that are available on the internet.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2023.01.018.
Appendix. Supplementary materials
References
- 1.World Health Organization. WHO Coronavirus (COVID-19) Dashboard, https://covid19.who.int/; 2022 (accessed 14 May 2022).
- 2.Wastnedge EAN, Reynolds RM, van Boeckel SR, Stock SJ, Denison FC, Maybin JA, et al. Pregnancy and COVID-19. Physiol Rev. 2021;101:303–318. doi: 10.1152/physrev.00024.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dashraath P, Wong JLJ, Lim MXK, Lim LM, Li S, Biswas A, et al. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am J Obstet Gynecol. 2020;222:521–531. doi: 10.1016/j.ajog.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Our world in data. Cumulative number of vaccinations. 2022 https://ourworldindata.org/covid-vaccinations (accessed 15 May 2022) [Google Scholar]
- 5.World Health Organization. Draft landscape and tracker of COVID-19 candidate vaccines, https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines; 2022 (accessed 13 March 2022).
- 6.Skjefte M, Ngirbabul M, Akeju O, Escudero D, Hernandez-Diaz S, Wyszynski DF, et al. COVID-19 vaccine acceptance among pregnant women and mothers of young children: results of a survey in 16 countries. Eur J Epidemiol. 2021;36:197–211. doi: 10.1007/s10654-021-00728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 8.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. Robins-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Vaccine efficacy, effectiveness and protection, https://www.who.int/news-room/feature-stories/detail/vaccine-efficacy-effectiveness-and-protection; 2022 (accessed 12 May 2022).
- 10.Halasa NB, Olson SM, Staat MA, Newhams MM, Price AM, Boom JA, et al. Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19-associated hospitalization in infants aged <6 months - 17 states, July 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:264–270. doi: 10.15585/mmwr.mm7107e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dagan N, Barda N, Biron-Shental T, Makov-Assif M, Key C, Kohane IS, et al. Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnancy. Nat Med. 2021;27:1693–1695. doi: 10.1038/s41591-021-01490-8. [DOI] [PubMed] [Google Scholar]
- 12.Goldshtein I, Nevo D, Steinberg DM, Rotem RS, Gorfine M, Chodick G, et al. Association between BNT162b2 vaccination and incidence of SARS-CoV-2 infection in pregnant women. JAMA. 2021;326:728–735. doi: 10.1001/jama.2021.11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butt AA, Chemaitelly H, Al Khal A, Coyle PV, Saleh H, Kaleeckal AH, et al. SARS-CoV-2 vaccine effectiveness in preventing confirmed infection in pregnant women. J Clin Invest. 2021;131 doi: 10.1172/JCI153662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paixao ES, Wong KLM, Alves FJO, de Araújo Oliveira V, Cerqueira-Silva T, Júnior JB, et al. CoronaVac vaccine is effective in preventing symptomatic and severe COVID-19 in pregnant women in Brazil: a test-negative case-control study. BMC Med. 2022;20:146. doi: 10.1186/s12916-022-02353-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magnus MC, Gjessing HK, Eide HN, Wilcox AJ, Fell DB, Håberg SE. Covid-19 vaccination during pregnancy and first-trimester miscarriage. N Engl J Med. 2021;385:2008–2010. doi: 10.1056/NEJMc2114466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Theiler RN, Wick M, Mehta R, Weaver AL, Virk A, Swift M. Pregnancy and birth outcomes after SARS-CoV-2 vaccination in pregnancy. Am J Obstet Gynecol MFM. 2021;3 doi: 10.1016/j.ajogmf.2021.100467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wainstock T, Yoles I, Sergienko R, Sheiner E. Prenatal maternal COVID-19 vaccination and pregnancy outcomes. Vaccine. 2021;39:6037–6040. doi: 10.1016/j.vaccine.2021.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rottenstreich M, Sela HY, Rotem R, Kadish E, Wiener-Well Y, Grisaru-Granovsky S. Covid-19 vaccination during the third trimester of pregnancy: rate of vaccination and maternal and neonatal outcomes, a multicentre retrospective cohort study. BJOG. 2022;129:248–255. doi: 10.1111/1471-0528.16941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blakeway H, Prasad S, Kalafat E, Heath PT, Ladhani SN, Le Doare K, et al. COVID-19 vaccination during pregnancy: coverage and safety. Am J Obstet Gynecol. 2022;226 doi: 10.1016/j.ajog.2021.08.007. 236.e1–236.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipkind HS, Vazquez-Benitez G, DeSilva M, Vesco KK, Ackerman-Banks C, Zhu J, et al. Receipt of COVID-19 vaccine during pregnancy and preterm or small-for-gestational-age at birth - eight integrated health care organizations, United States, December 15, 2020–July 22, 2021. MMWR Morb Mortal Wkly Rep. 2022;71:26–30. doi: 10.15585/mmwr.mm7101e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bookstein Peretz S, Regev N, Novick L, Nachshol M, Goffer E, Ben-David A, et al. Short-term outcome of pregnant women vaccinated with BNT162b2 mRNA COVID-19 vaccine. Ultrasound Obstet Gynecol. 2021;58:450–456. doi: 10.1002/uog.23729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kachikis A, Englund JA, Singleton M, Covelli I, Drake AL, Eckert LO. Short-term reactions among pregnant and lactating individuals in the first wave of the COVID-19 vaccine rollout. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.21310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trostle ME, Limaye MA, Avtushka V, Lighter JL, Penfield CA, Roman AS. COVID-19 vaccination in pregnancy: early experience from a single institution. Am J Obstet Gynecol MFM. 2021;3 doi: 10.1016/j.ajogmf.2021.100464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zauche LH, Wallace B, Smoots AN, Olson CK, Oduyebo T, Kim SY, et al. Receipt of mRNA COVID-19 vaccines preconception and during pregnancy and risk of self-reported spontaneous abortions. CDC v-safe COVID-19 Vaccine Pregnancy Registry 2020-21. Research Square. 09 August 2021. https://www.researchsquare.com/article/rs-798175/v1 [accessed 15 May 2022].
- 25.Kharbanda EO, Haapala J, DeSilva M, Vazquez-Benitez G, Vesco KK, Naleway AL, et al. Spontaneous abortion following COVID-19 vaccination during pregnancy. JAMA. 2021;326:1629–1631. doi: 10.1001/jama.2021.15494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimabukuro TT, Kim SY, Myers TR, Moro PL, Oduyebo T, Panagiotakopoulos L, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384:2273–2282. doi: 10.1056/NEJMoa2104983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai C, Peng Y, Shen E, Huang Q, Chen Y, Liu P, et al. A comprehensive analysis of the efficacy and safety of COVID-19 vaccines. Mol Ther. 2021;29:2794–2805. doi: 10.1016/j.ymthe.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prasad S, Kalafat E, Blakeway H, Townsend R, O'Brien P, Morris E, et al. Systematic review and meta-analysis of the effectiveness and perinatal outcomes of COVID-19 vaccination in pregnancy. Nat Commun. 2022;13:2414. doi: 10.1038/s41467-022-30052-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Atyeo C, DeRiso EA, Davis C, Bordt EA, De Guzman RM, Shook LL, et al. COVID-19 mRNA vaccines drive differential antibody Fc-functional profiles in pregnant, lactating, and nonpregnant women. Sci Transl Med. 2021;13:eabi8631. doi: 10.1126/scitranslmed.abi8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burd I, Kino T, Segars J. The Israeli study of Pfizer BNT162b2 vaccine in pregnancy: considering maternal and neonatal benefits. J Clin Invest. 2021;131 doi: 10.1172/JCI150790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tao L, Wang R, Han N, Liu J, Yuan C, Deng L, et al. Acceptance of a COVID-19 vaccine and associated factors among pregnant women in China: a multi-center cross-sectional study based on health belief model. Hum Vaccin Immunother. 2021;17:2378–2388. doi: 10.1080/21645515.2021.1892432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goncu Ayhan S, Oluklu D, Atalay A, Menekse Beser D, Tanacan A, Moraloglu Tekin O, et al. COVID-19 vaccine acceptance in pregnant women. Int J Gynaecol Obstet. 2021;154:291–296. doi: 10.1002/ijgo.13713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riad A, Jouzová A, Üstün B, Lagová E, Hruban L, Janků P, et al. COVID-19 vaccine acceptance of pregnant and lactating women (PLW) in Czechia: an analytical cross-sectional study. Int J Environ Res Public Health. 2021;18:13373. doi: 10.3390/ijerph182413373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bowman CJ, Bouressam M, Campion SN, Cappon GD, Catlin NR, Cutler MW, et al. Lack of effects on female fertility and prenatal and postnatal offspring development in rats with BNT162b2, a mRNA-based COVID-19 vaccine. Reprod Toxicol. 2021;103:28–35. doi: 10.1016/j.reprotox.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hadar E, Dollinger S, Wiznitzer A. Coronavirus disease and vaccination during pregnancy and childbirth: a review of the Israeli perspective and experience. J Matern Fetal Neonatal Med. 2022;35:7794–7805. doi: 10.1080/14767058.2021.1937110. [DOI] [PubMed] [Google Scholar]
- 36.Golan Y, Prahl M, Cassidy AG, Gay C, Wu AHB, Jigmeddagva U, et al. COVID-19 mRNA Vaccination in Lactation: assessment of adverse events and vaccibe related antibodies in mother-infant dyads. Front Immunol. 2022;12 doi: 10.3389/fimmu.2021.777103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raffetseder J, Lindau R, van der Veen S, Berg G, Larsson M, Ernerudh J. MAIT cells balance the requirements for immune tolerance and anti-microbial defense during pregnancy. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.718168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sritipsukho P, Khawcharoenporn T, Siribumrungwong B, Damronglerd P, Suwantarat N, Satdhabudha A, et al. Comparing real-life effectiveness of various COVID-19 vaccine regimens during the delta variant-dominant pandemic: a test-negative case-control study. Emerg Microbes Infect. 2022;11:585–592. doi: 10.1080/22221751.2022.2037398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paranthaman K, Subbarao S, Andrews N, Kirsebom F, Gower C, Lopez-Bernal J, et al. Effectiveness of BNT162b2 and ChAdOx-1 vaccines in residents of long-term care facilities in England using a time-varying proportional hazards model. Age Ageing. 2022;51 doi: 10.1093/ageing/afac115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data in this systematic review with a meta-analysis were extracted from published and preprint studies that are available on the internet.