Abstract
We previously demonstrated that the principal component of capsular material of Cryptococcus neoformans, glucuronoxylomannan (GXM), induces interleukin-10 (IL-10) secretion from human monocytes. Here we report that encapsulation of the yeast with GXM is able to down-regulate interleukin-12 (IL-12) production by monocytes that would normally occur in the absence of encapsulation. This phenomenon appeared to be the result of inhibition of the phagocytic process by encapsulation with GXM as well as of negative signals such as IL-10 secretion produced by interaction of GXM with leukocytes. Decreased secretion of IL-12 correlated with decreased release of gamma interferon (IFN-γ) from T cells, suggesting a role for encapsulation with GXM in hindering a T helper type 1 (Th1) response. This is supported by the ability of encapsulation with GXM to limit increased expression of B7-1 costimulatory molecules that otherwise might limit IL-10 secretion. Endogenous IL-10 played a critical role in modulatory activity associated with encapsulation with GXM. Blocking IL-10 with monoclonal antibody to IL-10 resulted in increased (i) IL-12 secretion, (ii) IFN-γ release from T cells, and (iii) killing of C. neoformans by monocytes. These results suggest that encapsulation with GXM limits development of a protective Th1-type response, an inhibitory process in which IL-10 plays a critical role. Scavengers of GXM and/or IL-10 could be useful in a protective Th1-type response in patients with cryptococcosis.
Cryptococcus neoformans is an opportunistic pathogen that causes serious life-threatening disease in patients with impaired cell-mediated immunity, particularly in patients with AIDS (8, 10, 31). The infectious particle is believed to enter the host through the respiratory tract and reach the lung, where primary infection appears to be restricted in the immunocompetent host. In contrast, the ability to control infection is severely hampered in immunosuppressed subjects, causing fungal cells to disseminate, eventually producing a life-threatening meningitis (6).
While clinical and experimental data convincingly demonstrate that cell-mediated immunity is crucial in host defense against C. neoformans, the specific mechanisms by which an intact cell-mediated immune response results in protection are poorly defined. Induction of proinflammatory cytokines, which recruit and activate leukocytes to inhibit and kill invading fungi (15), is central to the cell-mediated immune response that protects the host against fungal infections. Important among the cytokines involved in a protective response to C. neoformans are interleukin-12 (IL-12) and gamma interferon (IFN-γ). Several studies demonstrated that IL-12 is an important cytokine in host defense against C. neoformans that induces the generation of IFN-γ (12, 24). IFN-γ in turn stimulates macrophage anticryptococcal activity (22). Previous studies found that treatment with IL-12 in combination with conventional antifungal therapy reduces the fungal load and prolongs survival of mice infected with C. neoformans (9, 23, 24).
Recently we described biphasic secretion of IL-12 by monocytes exposed to C. neoformans. Early production is a consequence of direct interaction with the fungal cells, while late secretion involves CD40-CD40 ligand interaction as well as the presence of IFN-γ, suggesting that late secretion of IL-12 is primarily through a T-cell-dependent pathway (35). Monocytes alone are poor producers of IL-12 when stimulated with encapsulated C. neoformans but produce a significant amount of interleukin-10 (IL-10) (42), a potent biological inhibitor of IL-12.
We previously demonstrated that encapsulation of C. neoformans promotes IL-10 release from human monocytes (42). In contrast, encapsulation appeared to suppress release of IL-12 from monocytes. The suppressive effect of encapsulation was observed in both the early T-cell-independent release of IL-12 from monocytes that were directly exposed to cryptococci and in a delayed T-cell-dependent pathway (35). Previous studies have shown that IL-10 is a potent biological inhibitor of IL-12 synthesis by macrophages that acts at both the protein and mRNA levels (36, 38). Since encapsulation of C. neoformans, and in particular encapsulation with glucuronoxylomannan (GXM), stimulates IL-10 secretion (42), we examined the possibility that suppression of IL-12 secretion, associated with encapsulation, could be a consequence of the action of IL-10.
Thus, we hypothesized that encapsulation of cryptococci with the major capsular polysaccharide (GXM) could influence IL-12 release and that IL-12 secretion may be limited by the presence of IL-10 (32). The aim of our study was to determine the regulatory effect of endogenous IL-10 on IL-12 secretion and the phases and mechanisms involved in this interdependency.
MATERIALS AND METHODS
Reagents and media.
RPMI 1640 medium and fetal calf serum (FCS) were obtained from Gibco BRL (Milan, Italy). Human serum (HS) was obtained from Biosource International (Camarillo, Calif.). GXM was isolated from culture supernatant fluid of a serotype A strain (ATCC 24064) grown in liquid synthetic medium in a gyratory shaker for 4 days at 30°C (5). GXM was isolated by differential precipitation with ethanol and hexadecyltrimethyl ammonium bromide (CTAB) (Sigma Chemical Co., St. Louis, Mo.) (4). The isolation procedure has been described in detail (21). Anti-IL-10 monoclonal antibody (MAb) was obtained from Genzyme Corporation (Boston, Mass). Mouse monoclonal anti-human CD80 (B7-1; immunoglobulin [IgM]) fluorescein isothiocyanate (FITC) conjugates were purchased from Calbiochem-Novabiochem Corporation (La Jolla, Calif.). Mouse monoclonal anti-human CD80 (B7-1; IgM) and mouse monoclonal anti-human CD86 (B7-2; IgG1) were purchased from Ancell Corporation, (Bayport, Maine). Human recombinant IL-10 was from EuroClone (Devon, United Kingdom). Human recombinant IFN-γ was obtained from Life Technologies (Paisley, Scotland). Cytochalasin B and FITC-conjugated MAb to human CD4 (IgG1) were purchased from Sigma. Lipopolysaccharide (LPS) from Escherichia coli O55:135 was obtained from Difco (Detroit, Mich.). RPMI 1640, FCS, C. neoformans (approximately 5 × 107 cells), and HS were tested for endotoxin contamination by Limulus amebocyte lysate assay (Sigma) which had a sensitivity of approximately 0.05 to 0.1 ng of E. coli LPS/ml. All reagents tested negative.
Preparation of PBM and lymphocytes.
Heparinized venous blood, obtained from healthy donors, was diluted with RPMI 1640 plus 5% FCS (cRPMI), and the mononuclear cells were separated by density gradient centrifugation on Ficoll-Hypaque (39). Peripheral blood mononuclear cells (PBMC) were used unfractionated or were washed twice in cRPMI and incubated for 1 h at a concentration of 2 × 106 to 3 × 106/ml in cell culture petri dishes (Nunc Inter Med, Roskilde, Denmark). Adherent cells were carefully recovered with a rubber policeman. The adherent cells (peripheral blood monocytes [PBM]) were >98% viable as evaluated by trypan blue dye exclusion. Nonadherent cells were E rosetted as previously described (41). The cells recovered were T lymphocyte T(E+) cells, >98% CD3+ as evaluated by flow cytometry analysis.
Microorganisms.
The two strains of C. neoformans used in this study were obtained from J. Orendi (Central Bureau Schimmel Cultures [CBS], Delft, The Netherlands). C. neoformans var. neoformans 6995 (CBS 6995, also known as NIH 37) is a thinly encapsulated isolate of serotype A. C. neoformans var. neoformans 7698 (CBS 7698, also known as NIH B-4131) is an acapsular mutant. The morphological characteristics and growth conditions of the two strains of C. neoformans have been described previously (42). The cultures were maintained by serial passage on Sabouraud agar (Bio Merieux, Lyon, France) and harvested by suspending a single colony in RPMI 1640. The cells were washed twice, counted on a hematocytometer, and adjusted to the desired concentration. Cells of C. neoformans 6995 and 7698 were killed by autoclaving for those experiments that required heat-inactivated yeast cells.
Killing of cryptococci by monocytes.
Killing activity was evaluated by CFU inhibition assay. Briefly, PBM (105) in 0.1 ml of suspension per well were incubated in flat-bottom 96-well microtiter tissue culture plates (Falcon) with live encapsulated (6995) or acapsular (7698) C. neoformans (104) in 0.1 ml of RPMI plus 10% HS. Monocytes were incubated with C. neoformans (6995 or 7698) for 3 h. After incubation at 37°C in 5% CO2, the plates were vigorously shaken, monolayers were lysed by adding 0.1% Triton X-100 in distilled water (final concentration in the well, 0.01%), and serial dilutions were prepared in distilled water from each well. Plates (triplicate samples) were made by spreading each sample on Sabouraud dextrose agar, and CFU were evaluated after 72 h of incubation at 28°C. Uninhibited controls consisted of C. neoformans (6995 or 7698) incubated without effector cells in HS. Killing activity versus C. neoformans (6995 or 7698) was expressed as the percentage of CFU inhibition according to the following formula: % killing activity = 100 − (CFU in experimental group/CFU in control cultures) × 100.
Coculture of monocytes and T lymphocytes.
PBMC (2 × 106/ml) or PBM (2 × 106/ml), in flat-bottom 96-well plates, were incubated with or without heat-inactivated C. neoformans (4 × 106). Supernatant fluids were harvested after various numbers of days of culture for cytokine determination.
Flow cytometry analysis.
PBM untreated or treated with indicated stimuli and cultured with 10% HS were harvested by scraping into phosphate-buffered saline (PBS) containing 0.5% bovine serum albumin and 0.4% sodium azide. A total of 106 cells in 50 μl was mixed with 10 μl of a FITC-conjugated mouse MAb specific for human B7-1. FITC-conjugated mouse MAb specific for human immunoglobulin IgM (Sigma) was used as a negative control. After 45 min of incubation, the cells were washed three times and stained with phycoerythrin-conjugated anti-human CD14. Phycoerythrin-conjugated mouse monoclonal anti-human CD14 (IgG2a) was purchased from Ancell Corporation. CD14-positive cells were gated before quantitation; 5,000 events were counted. For each sample, B7-1 expression was measured on the surface of CD14 positive cells using a fluorescence activated cell sorter (Becton Dickinson, San Jose, Calif.).
Cytokine determination.
IL-10, IL-12, and IFN-γ were determined with human cytokine enzyme-linked immunosorbent assay (ELISA) kits purchased from Biosource. The IL-12 ELISA kit is a solid-phase ELISA based on the antibody sandwich principle; its sensitivity is <0.8 pg/ml. This assay recognizes both natural and recombinant human IL-12, as well as the free p40 subunit. IL-12 p70 was determined with a human cytokine ELISA kit from Genzyme.
Viability of cells treated with cytochalasin B or GXM.
The viability of monocytes treated with cytochalasin B cells was measured with a colorimetric reaction that is based on the capacity of mitochondrial dehydrogenase of living cells to reduce MTT (3-[4,5-dimethylthiazol-2-yi]-2,5-diphenyl tetrazolium bromide; Aldrich Chemical, Milan, Italy) into formazan. The quantity of the formazan produced and measured at an optical density of 540 nm in a microplate reader (Sorin Biomedica, Saluggia, Italy) correlated with the number of living cells (35).
Statistical analysis.
Statistical analysis was calculated using analysis of variance with Bonferroni's post-test analysis, which gave the significant α level at 0.025. A 6.12 version of the SAS software package was used for all statistical analysis.
RESULTS
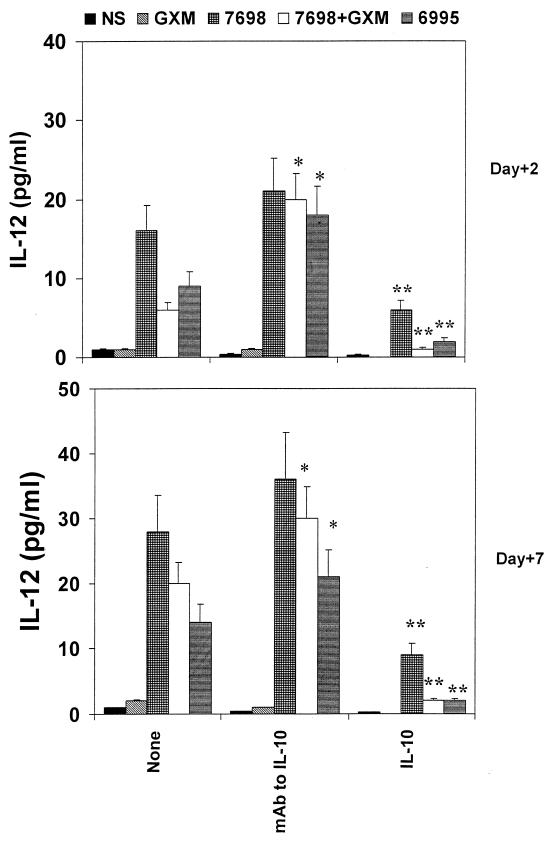
We utilized a model system in which monocytes or PBMC were stimulated with encapsulated cryptococci, acapsular cryptococci, or acapsular cryptococci plus purified GXM. GXM confers an experimentally constructed capsule when mixed with the acapsular strain (26); consequently, use of acapsular cryptococci in the presence of GXM allows an evaluation of the effect of the GXM component of the capsular polysaccharide in the absence of any other capsular constituents. The use of a given dose of GXM, LPS, or other reagents for these experiments was based on preliminary experiments or prior studies of dose- and time-dependent responses (42, 43). IL-12 was determined in supernatant fluids of both monocytes cultured for 48 h or PBMC cultured for 7 days to determine early and late IL-12 production, respectively. The contribution of IL-10 to IL-12 secretion in response to stimulation with cryptococci was assessed in two ways. First, MAb to IL-10 was added to block any contribution by IL-10. Second, exogenous IL-10 (25 ng/ml) was added to assess its effect in the system. Confirming our earlier report (35), reduced secretion of IL-12 in response to encapsulation occurred in both the T-cell-independent and T-cell-dependent pathways (P < 0.025). Results from the addition of anti-IL-10 MAb to the system suggest that IL-10 is at least partially responsible for the reduced secretion of IL-12. There was no significant difference between T-cell-independent secretion in response to acapsular cryptococci, acapsular cryptococci plus GXM, or encapsulated cryptococci (P > 0.025) in the presence of anti-IL-10. Addition of anti-IL-10 MAb did not restore T-cell-dependent IL-12 secretion in response to encapsulated cryptococci (strain 6995 or strain 7698 plus GXM) to the level observed with acapsular cells alone; however, the level of IL-12 secretion in response to stimulation with encapsulated cryptococci was significantly higher than levels observed in the absence of anti-IL-10 (P < 0.025 for each pairwise comparison). Finally, addition of exogenous IL-10 suppressed both T-cell-independent and-dependent secretion of IL-12 in response to encapsulated and acapsular cryptococci, demonstrating the potentially suppressive role of IL-10 in this system.
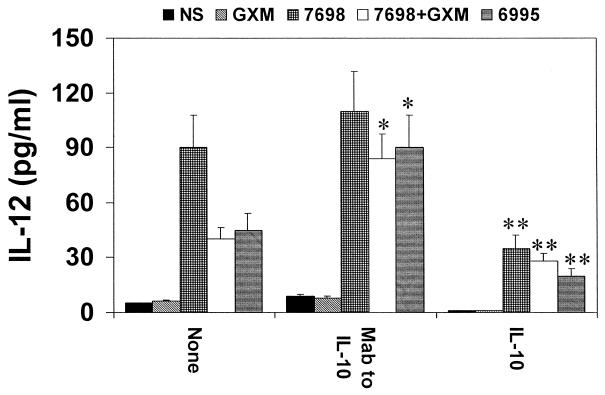
IL-12 secretion is highly dependent on the presence of IFN-γ. Consequently, an experiment was done that was similar to the experiment shown in Fig. 1; however, the monocytes were first primed for 18 h with IFN-γ (100 U/ml). Additionally, the assay for IL-12 in supernatant fluids was specific for biologically active IL-12 p70. The results (Fig. 2) show that IFN-γ priming produces an appreciable release of IL-12 p70 production; that is, IL-12 as measured in an assay specific for IL-12 p70 was increased when monocytes were stimulated with encapsulated cryptococci in the presence of MAb to IL-10, and there was a suppression of IL-12 secretion when exogenous IL-10 was added to the system.
FIG. 1.
IL-12 levels in supernatant fluids of monocytes (day +2) or PBMC (day +7) stimulated with encapsulated (6995) or acapsular (7698) C. neoformans, or with GXM (250 μg/ml), or with 7698 plus GXM in the presence or absence of MAb to IL-10 (5 μg/ml) or human recombinant IL-10 (25 ng/ml). The determination was performed with supernatant fluids harvested from cells after 2 or 7 days of incubation. Irrelevant MAb (2.5 μg/ml) did not affect IL-12 release in our experimental system. The results reported are the means + SD (error bars) of four experiments with samples from four different donors (each donor sample was done in duplicate). NS, not stimulated; ∗, P < 0.025 (MAb to IL-10-treated versus untreated cells); ∗∗, P < 0.025 (IL-10-treated versus untreated cells).
FIG. 2.
IL-12 levels in supernatant fluids of monocytes treated for 18 h with IFN-γ (100 U/ml) and then washed and stimulated for 48 h with encapsulated (6995) or acapsular (7698) C. neoformans, or with GXM (250 μg/ml), or with 7698 plus GXM in the presence or absence of MAb to IL-10 (5 μg/ml) or human recombinant IL-10 (25 ng/ml). The determination was performed with supernatant fluids harvested from cells after 2 days of incubation using an assay specific for IL-12 p70. Irrelevant MAb (2.5 μg/ml) did not affect IL-12 release in our experimental system. The results reported are the means + SD (error bars) of four experiments with samples from four different donors (each donor sample was done in duplicate). NS, not stimulated; ∗, P < 0.025 (MAb to IL-10-treated versus untreated cells); ∗∗, P < 0.025 (IL-10-treated versus untreated cells).
The suppressive effect of encapsulation could be due to GXM-mediated inhibition of phagocytosis or to GXM delivering inhibitory signals independently of the presence of C. neoformans. Both possibilities were investigated. Cytochalasin B (5 μg/ml), an inhibitor of phagocytosis (34), was added to our coculture of monocytes and acapsular cryptococci to determine whether the GXM-mediated inhibitory effect was comparable to an inhibitory effect that could be achieved by direct blockade of phagocytosis. The results (Fig. 3) showed that addition of cytochalasin B significantly reduced IL-12 secretion by monocytes in response to 7698 (P < 0.025). Moreover, the reduced response produced by cytochalasin B was comparable to that produced by addition of GXM to the system (P > 0.025 for 7698 plus cytochalasin B versus 7698 plus GXM). Cytochalasin at 5 μg/ml inhibits the phagocytic process by approximately 60% but had no effect on cell viability as determined by MTT assay (data not shown). As further evidence that cytochalasin B is not toxic for monocytes under the conditions used in these experiments, cytochalasin B did not produce significant changes in LPS-induced IL-12 secretion (data not shown)
FIG. 3.
IL-12 levels in supernatant fluids from monocytes stimulated with acapsular (7698) C. neoformans or with 7698 plus GXM (250 μg/ml) in the presence or absence of cytochalasin B (Cytoch. B) (5 μg/ml). The determination was performed with supernatant fluids harvested from cells after 2 days of incubation. The results reported are the means + SD (error bars) of three experiments with samples from three different donors (each donor sample was done in duplicate). NS, not stimulated; ∗, P < 0.025 (7698-plus-cytochalasin B-treated versus 7698-treated cells).
LPS, a known stimulant of IL-12 released by monocytes (11, 20), was used to determine whether GXM directly suppresses IL-12 secretion. Monocytes were incubated for 48 h with LPS (2 μg/ml) alone or in the presence of GXM (500 μg/ml). The results showed a strong secretory response to LPS alone (150 ± 19 pg/ml; mean ± standard deviation [SD] for three independent experiments). Addition of GXM to the system prevented increase (P < 0.025) of IL-12 (105 ± 18 pg/ml; mean ± SD for three independent experiments). An examination of the dose-response effect produced by addition of GXM to the system showed a moderate prevention of IL-12 secretion in response to LPS at the dose of 250 μg of GXM/ml (120 ± 18 pg/ml; mean ± SD for three independent experiments). Lower doses (25 to 0.25 μg/ml) of GXM did not affect LPS-induced IL-12 release. In a control experiment relevant to this and other experiments in the present report incubation of monocytes for 18, 72, or 96 h with GXM at concentrations that ranged from 1 to 500 μg/ml had no effect on cell viability as determined by the MTT assay (data not shown).
To assess the role of GXM in the failure of encapsulated cryptococci to stimulate increased B7-1 expression in our experimental system and the potential involvement of B7 molecules in regulating the IL-10/IL-12 loop, GXM was added to the acapsular strain and B7-1 expression was determined. The results (Fig. 4) showed that GXM alone did not influence B7-1 expression; however, a significant failure to enhance B7-1 expression was observed when GXM was used in combination with cells of the acapsular strain compared to the increased expression observed after stimulation with acapsular cryptococci alone.
FIG. 4.
B7-1 expression on monocytes treated with acapsular (7698) C. neoformans, or with GXM (250 μg/ml), or with 7698 plus GXM. B7-1 expression was assessed after 24 h of incubation. (a) Dot plots are shown. (b) Percentage of positive cells. The results are reported as mean fluorescence intensity and represent the means + SD (error bars) of four experiments with samples from four different donors (each donor sample was done in duplicate). NS, not stimulated; ∗, P < 0.025 (7698 + GXM-treated versus 7698-treated cells).
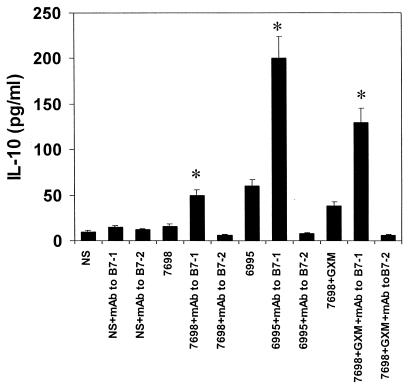
GXM on the C. neoformans surface limits B7-1 expression in response to stimulation with the yeast; consequently, we evaluated the influence of B7-1 expression on induction of IL-10 secretion. Blocking experiments were performed using MAb to B7-1 (2 μg/ml) or MAb to B7-2 (2 μg/ml) molecules, and IL-10 levels were tested in supernatant fluids of PBMC cultured for 7 days. The results (Fig. 5) show that blockade of B7-1 had little or no effect on IL-10 secretion in the absence of stimulation with cryptococcal cells but produced a significant (P < 0.025) increase in the IL-10 secretory response to encapsulated cryptococci, acapsular cryptococci, or acapsular cryptococci plus GXM. In contrast, anti-B7-2 had no effect on IL-10 secretion.
FIG. 5.
IL-10 levels in supernatant fluids from Cryptococcus-laden monocytes cocultured with autologous T cells in the presence or absence of MAb to B7-1 (2 μg/ml) or MAb to B7-2 (2 μg/ml). The results are the means + SD (error bars) of three experiments with samples from three different donors (each donor sample was done in duplicate). Irrelevant MAb (IgM[2 μg/ml] or [IgG1 2 μg/ml]) did not produce changes in proliferative response. NS, not stimulated. ∗, P < 0.025 (C. neoformans + MAb to B7-1-treated versus respective C. neoformans-treated cells alone).
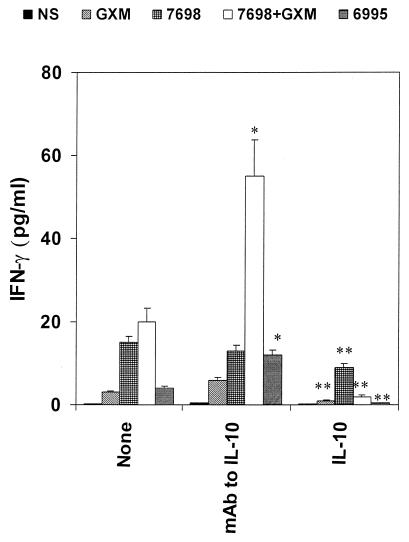
Given the fact that MAb to IL-10 augments IL-12 release by antigen-presenting cells and that IL-12 production is directly related to IFN-γ production (37), we considered the possibility that up-regulation of IL-12, by blocking IL-10, could influence IFN-γ release. MAb to IL-10 was added to our cells at the time of coculture of PBMC and cryptococcal cells, and IFN-γ production was determined after 7 days of incubation. The results (Fig. 6) showed that MAb to IL-10 had no effect on IFN-γ secreted in response to acapsular cryptococci, in agreement with results presented in Fig. 1 which showed no significant effect of anti-IL-10 on IL-12 secretion. In contrast, MAb to IL-10 produced a striking increase in IFN-γ secreted in response to acapsular cryptococci plus GXM (P < 0.025) or encapsulated cryptococci (P < 0.025). Indeed, the effect of anti-IL-10 on IFN-γ secretion appeared to be greater than the effect on IL-12 secretion (Fig. 1). Addition of recombinant IL-10 to the system had the opposite effect of that produced by anti-IL-10 MAb; a significant decrease in the IFN-γ response was observed for all stimuli (Fig. 6).
FIG. 6.
IFN-γ levels in supernatant fluids of PBMC stimulated with encapsulated (6995) or acapsular (7698) C. neoformans, or with GXM (250 μg/ml), or with 7698 plus GXM in the presence or absence of MAb to IL-10 (5 μg/ml) or human recombinant IL-10 (25 ng/ml). The determination was performed with supernatant fluids harvested from cells after 7 days of incubation. Irrelevant MAb (5 μg/ml) did not affect IFN-γ release in our experimental system. The results reported are the means + SD (error bars) of four experiments with samples from four different donors (each donor sample was done in duplicate). NS, not stimulated; ∗, P < 0.025 (MAb to IL-10-treated versus untreated cells); ∗∗, P < 0.025 (IL-10-treated versus untreated cells).
IFN-γ is a potent inducer of antimicrobial activity of macrophages (44); therefore, the neutralization of IL-10 which enhanced IFN-γ availability may potentiate phagocytic cell function (37). To this end, monocytes were treated with autologous T cells responding to encapsulated or acapsular C. neoformans in the presence or absence of MAb to IL-10 or recombinant IL-10. The results (Table 1) showed that the addition of T cells, previously activated by coculture with cryptococci, to the monocytes significantly increased the killing of encapsulated (P < 0.025) and acapsular (P < 0.025) cryptococci. No increase in killing was found when T lymphocytes were previously cocultured with autologous unstimulated monocytes. In every case, the addition of exogenous IL-10 significantly impaired killing of C. neoformans by monocytes. Addition of MAb to IL-10 had no effect on killing of cryptococci cultured with monocytes alone or monocytes cultured with inactivated T cells. In contrast, anti-IL-10 significantly increased killing of encapsulated cryptococci incubated with monocytes and activated T cells (P < 0.025) but had no effect (P > 0.025) on killing of acapsular cryptococci incubated with monocytes and activated T cells.
TABLE 1.
Effect of MAb to IL-10 or the addition of human recombinant IL-10 on anticryptococcal activity of monocytes cocultured with T cells responding to C. neoformansa
| Treatment | % Killing activity against C. neoformans strains
|
|||||
|---|---|---|---|---|---|---|
| Not stimulated
|
Treated with MAb to IL-10
|
Treated with IL-10
|
||||
| 7698 | 6995 | 7698 | 6995 | 7698 | 6995 | |
| Monocytes alone | 21 ± 3 | 9 ± 1 | 25 ± 7 | 13 ± 3 | 10 ± 3b | 2 ± 1b |
| Monocytes + unstimulated T cells | 22 ± 3 | 7 ± 4 | 24 ± 3 | 10 ± 3 | 11 ± 3b | 3 ± 1b |
| Monocytes + T cells prestimulated with 7698 | 43 ± 6c | NDd | 49 ± 7 | ND | 20 ± 6b | ND |
| Monocytes + T cells prestimulated with 6995 | ND | 18 ± 3e | ND | 29 ± 4f | ND | 10 ± 3b |
Unstimulated T cells were T lymphocytes cocultured with autologous unstimulated monocytes for 4 days. Activated T cells prestimulated with 7698 or 6995 were T lymphocytes cocultured with autologous monocytes treated with 7698 or 6995 for 4 days. Monocytes (106) were cultured alone for 4 days and unexposed or exposed for 2 days to inactivated or activated T cells (106) in the presence or absence of MAb to IL-10 (5 μg/ml) or irrelevant MAb (5 μg/ml) or in the presence or absence of IL-10. The addition of an irrelevant MAb (5 μg/ml) did not affect the killing activity of monocytes. Killing activity was determined by CFU inhibition at an E/T ratio of 10:1. Results represent the means ± SD of three separate experiments with samples from three different donors. Activated T cells exposed to 7698 or 6995 were unable to kill 7698 or 6995 at an E/T ratio of 10:1.
P < 0.025 (IL-10-treated versus untreated monocytes).
P < 0.025 (7698-stimulated T cells versus unstimulated T cells).
ND, not determined.
P < 0.025 (6995-stimulated T cells versus unstimulated T cells).
P < 0.025 (MAb-to-IL-10-treated versus untreated monocytes).
In a variation in the experiment reported in Table 1, we determined whether priming with IFN-γ might amplify the differences between treatment groups. Experiments were performed in which monocytes were primed with IFN-γ (100 U/ml) for 18 h before the killing assay against the encapsulated strain (6995). The results showed the following killing activities: for monocytes alone, 22% ± 2% monocytes treated with unstimulated T cells in the absence of MAb to IL-10, 25% ± 3%; and for monocytes in the presence of MAb to IL-10, 28% ± 3%. When monocytes were exposed to T cells primed with 6995 the killing activities were 49% ± 3% and 61% ± 4% in the presence of MAb to IL-10. These results indicate that priming with IFN-γ produces a general increase in the level of killing, but there was no amplification of differences between groups.
DISCUSSION
In the present study we provide evidence that differentiation of a T-cell response and anticryptococcal activity of monocytes are regulated in part by a balance between IL-10 and IL-12. Encapsulation of the yeast, either naturally in the form of encapsulated yeast cells or experimentally by the addition of purified GXM to acapsular cells (26) is associated with suppression of IL-12 and IFN-γ release from monocytes and T cells, respectively, and influences B7-1 expression, which plays a role in IL-10 regulation. We also show that endogenous IL-10 is a relevant mechanism involved in the suppressive effect mediated by encapsulated yeast cells. This was demonstrated by results showing that neutralization of endogenous IL-10 by an anti-IL-10 MAb (i) augments IL-12 release from monocytes, (ii) enhances IFN-γ production by T cells, and (iii) interferes with the anticryptococcal activity of monocytes. The data of the present study show a clear link between the presence of endogenous IL-10 and the synthesis of IL-12 by monocytes in response to C. neoformans. Negative regulation by IL-10 occurs in both the early and late phases of IL-12 secretion, suggesting that IL-12 production by monocytes is regulated by the presence of IL-10. This phenomenon could reflect the ability of encapsulated cryptococci to dampen a dominant Th1-type response by suppressing IL-12 production.
The mechanisms involved in inhibition of IL-12 secreted in response to encapsulated cryptococci or to the acapsular strain plus GXM may include simple inhibition of phagocytosis as well as a direct negative impact on IL-12 secretion. Our data support both possibilities. In contrast, studies in other systems found phagocytosis alone to be a sufficient stimulus for IL-12 secretion (7, 17).
With regard to inhibition of phagocytosis, addition of purified GXM to acapsular cryptococci produces an experimentally generated capsule that is marginally visible by use of the India ink stain but is readily visible by immunofluorescence (26). Moreover, the experimentally generated capsule is sufficient to completely inhibit phagocytosis of the yeast. Importantly, addition of GXM to acapsular cryptococci mimicked results observed with fully encapsulated cryptococci that were resistant to phagocytosis. The importance of phagocytosis in influencing IL-12 secretion is further suggested by blocking internalization of acapsular C. neoformans with cytochalasin B. Addition of cytochalasin B to the system produced a decrease in IL-12 secretion comparable to the decrease observed by addition of GXM to acapsular cryptococci. We cannot exclude the possibility that cytochalasin B may have subtle toxic effects that fall short of death of the cells. Similarly, soluble GXM has effects on monocytes that are independent of inhibition of phagocytosis. However, the congruence of effects of the known phagocytosis inhibiting action of cytochalasin B and the antiphagocytic action of GXM strongly suggest that inhibition of phagocytosis is one mechanism by which GXM prevented an increase in IL-12 secretion.
Direct negative impact by GXM on monocytes could include (i) induction of IL-10 secretion, (ii) down-regulation of B7-1 expression with a consequent increase in IL-10 secretion, or (iii) a decrease of IL-12 secretion induced by an unrelated stimulus such as LPS. With regard to the effect of GXM on IL-12 secretion in response to LPS, CD14, a receptor found on myeloid cells, mediates binding of LPS with phagocytic cells (16). The mechanism leading to a proinflammatory cytokine response via CD14 signaling is mediated by NFκB activation (19). One possible effect of GXM is interference in the interaction between LPS and its cellular receptor CD14. Results from our studies suggest that this is not the case, because GXM differentially regulates LPS-induced secretion of IL-12 and IL-1. In the present study, we found that treatment of monocytes with GXM suppressed LPS-induced release of IL-12. In contrast, we previously demonstrated that treatment of monocytes with GXM fails to influence IL-1β secretion in response to LPS (43).
We observed a suppression of IL-12 secretion in response to stimulation with LPS at 500 and 250 μg of GXM/ml but not at lower doses such as 25 μg/ml. The remainder of our studies were done with GXM at a concentration of 250 μg/ml. Since the effects of GXM may be due to both the direct effect of soluble GXM on monocytes and the indirect effect of GXM in creating an experimental capsule on the surface of acapsular cells, this raises a question as to what constitutes a biologically relevant concentration of GXM. Eng et al. reported a latex agglutination titer of 1/2,000,000 for GXM in serum of an AIDS patient with cryptococcosis (14). Assuming that the limit of sensitivity of the latex agglutination titer for GXM is approximately 10 ng/ml (18), this titer would correspond to 20 mg of GXM/ml. Chuck and Sande found that 68% of AIDS patients with cryptococcosis had serum cryptococcal antigen titers of at least 1/1,024, and 21% had serum titers of 1/10,000 or higher (8). These titers would correspond to approximately 10 and 100 μg of GXM per ml, respectively. More importantly, the concentration of GXM in focal sites of infection where immune effectors must function are likely to be much higher than reported concentrations of GXM in serum. As a consequence, the concentrations of GXM used in our experiments fell within the upper range of concentrations observed clinically in serum and may be well below concentrations found in infected tissue.
Our experiments were performed using 10% normal HS; thus, the generation of C3a and C5a following activation of the alternative complement pathway by GXM or encapsulated C. neoformans should be considered. Previous studies have shown that incubation of encapsulated cryptococci in HS leads to the release of biologically active fragments of complement proteins (reviewed in reference 27). A recent study found that C5a suppresses the ability of LPS to induce synthesis and secretion of IL-12 by monocytes (46). However, the relatively weak ability of purified GXM to activate the complement system (30), in contrast to the remarkably potent activation of the complement system by encapsulated cryptococci (28), would argue against a role for complement activation by purified GXM as a mechanism for suppression of LPS-induced secretion of IL-12 by monocytes.
We previously demonstrated that IL-12 is secreted early in response to C. neoformans in a T-cell-independent manner and late in a T-cell-dependent manner that involves the presence of IFN-γ and CD40-CD40L interaction. Here, we show that upregulation of early IL-12 production, by blocking IL-10, results in increased secretion of IFN-γ. Thus, the absence of IL-10 improves early IL-12 secretion and stimulates a more prompt and efficient Th1 response. In fact, the commitment to a Th1 or Th2 phenotype appears to occur early after antigen stimulation (33), thus blocking IL-10 results in a change in the cytokine milieu and gives rise to an efficient Th1 response. This is consistent with the fact that early IL-12 secretion regulates IFN-γ production that in turn regulates late IL-12 secretion, suggesting an interdependency between early and late IL-12 production. In addition, we previously demonstrated that IL-12 p40 gene expression was not found in monocytes after 6 h of incubation with C. neoformans (34). This correlates with late secretion of IL-12 (35). Given that it is well known that IL-10 is a late-produced cytokine (13), it is possible that the late appearance of IL-12 gene expression represents an optimal condition for favoring the inhibitory effect of IL-10.
B7-1 overexpression is known to correlate with induction of a dominant Th1 response, and anti-B7-1 antibodies drive the immune response along a Th2 pathway (29). In contrast, anti-B7-2 favors Th1 development. Results from previous studies distinguishing between the effects of anti-B7-1 and anti-B7-2 on Th1 and Th2 development (29) agree well with our observation that anti-B7-1 stimulated increased secretion of IL-10 in response to encapsulated cryptococci, acapsular cryptococci, or acapsular cryptococci plus GXM, whereas anti-B7-2 had no effect on IL-10 secretion. It is also possible that late IL-10 secretion may be produced by monocytes influenced by cell-to-cell contact or T-cell products. Consistent with this hypothesis, it has recently been reported that soluble factors such as tumor necrosis factor or CD40 ligation regulate IL-10 secretion by antigen-presenting cells (2).
Encapsulation of C. neoformans limits B7-1 molecule expression on monocytes that would normally occur in response to stimulation with acapsular yeast cells (40). Here, we report that a similar reduction in B7-1 expression occurs when monocytes are exposed to acapsular cryptococci in the presence of purified GXM. This result demonstrates that GXM is the component of the capsule that accounts for the limited expression of B7-1 when monocytes are stimulated with encapsulated cryptococci. This phenomenon may have biological relevance because the limited expression of B7-1 seen with monocytes stimulated with acapsular cryptococci plus GXM (Fig. 4) correlated well with the increased secretion of IL-10 observed after stimulation of monocytes with encapsulated cryptococci or acapsular cryptococci plus GXM compared to stimulation with acapsular cryptococci alone (Fig. 5). The presence of IL-10 limits IL-12 production and may be an adjunctive strategy by GXM to elude the host immune response. This hypothesis is consistent with a recent report by Blackstock et al. (1) that found a direct relation between the virulence of a C. neoformans strain and IL-10 production in vivo.
The potentially protective Th1 response to C. neoformans could be promoted in the absence of endogenous IL-10. Activation of a Th1 response following IL-10 depletion mirrored enhanced secretion of endogenous IFN-γ (Fig. 6) that, in turn, correlated with stimulated anticryptococcal activity of macrophages (Table 1). In contrast, the addition of exogenous IL-10 significantly suppressed the killing activity of macrophages, suggesting that IL-10 has a negative effect on effector function of macrophages directly by inhibiting killing activity and indirectly by suppressing IFN-γ production.
It is conceivable that the cytokine profiles reported here may occur during induction of the immune response, as a consequence of the direct interaction of C. neoformans with immune cells. This is supported by the fact that IL-10 is promptly secreted by monocytes after the addition of GXM or encapsulated C. neoformans (42). Given that late IL-12 is strictly dependent on the presence of IFN-γ it is possible that the inhibition of IL-10-induced IFN-γ (Fig. 6) greatly influences late IL-12 production. However, the complexity of the interaction and the effector-to-target (E/T) ratio required in vitro to elicit this effect do not necessarily depict only the early immune events. Given that IL-10 is implicated in blocking the Th1 protective response, it is possible that it contributes to the induction of anergy, as suggested by Blackstock et al. (1). The inhibitory mechanism induced by IL-10 may be a consequence of the decreased phagocytic process. This is consistent with inhibition of the IL-10-mediated inhibition of the phagocytic process observed for other microorganisms (45). Alternatively, IL-10 may affect microbicidal machinery. We cannot exclude the possibility that multiple mechanisms cooperate in producing the observed effect; indeed, our results emphasize the complexity of the interaction between C. neoformans and the immune system. The cytokine response to C. neoformans is influenced by encapsulation with GXM and is profoundly affected by a GXM-dependent inappropriate response. Cross-regulation of IL-10, IL-12, and IFN-γ plays an important part in driving the Th response and in modulating the effector function of macrophages and the inflammatory process. The involvement of IL-10 as a means to dampen a protective response to C. neoformans raises the possibility of new therapeutic approaches in cryptococcosis aimed at depleting IL-10 or removing GXM that promotes IL-10 induction.
ACKNOWLEDGMENTS
We thank Eileen Mahoney Zannetti for excellent and dedicated editorial and secretarial support.
This study was supported by a grant from the National Research Program on AIDS, “Opportunistic Infections and Tuberculosis,” contract n.50A.0.35, Italy, and by National Institutes of Health grant AI 14209 (T.R.K).
REFERENCES
- 1.Blackstock R, Buchanan K L, Adesina A M, Murphy J W. Differential regulation of immune responses by highly and weakly virulent Cryptococcus neoformans isolates. Infect Immun. 1999;67:3601–3609. doi: 10.1128/iai.67.7.3601-3609.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brossart P, Zobywalski A, Grunebach F, Behnke L, Stuhler G, Reichardt V L, Kanz L, Brugger W. Tumor necrosis factor alpha and CD40 ligand antagonize the inhibitory effects of interleukin 10 on T-cell stimulatory capacity of dendritic cells. Cancer Res. 2000;60:4485–4492. [PubMed] [Google Scholar]
- 3.Chang J T, Segal B M, Shevach E M. Role of costimulation in the induction of the IL-12/IL-12 receptor pathway and the development of autoimmunity. J Immunol. 2000;164:100–106. doi: 10.4049/jimmunol.164.1.100. [DOI] [PubMed] [Google Scholar]
- 4.Cherniak R, Reiss E, Slodki M E, Plattner R D, Blumer S O. Structure and antigenic activity of the capsular polysaccharide of Cryptococcus neoformans. Mol Immunol. 1980;17:1025–1032. doi: 10.1016/0161-5890(80)90096-6. [DOI] [PubMed] [Google Scholar]
- 5.Cherniak R, Reiss E, Turner S H. A galactoxylomannan antigen of Cryptococcus neoformans serotype A. Carbohydr Res. 1982;103:239–250. [Google Scholar]
- 6.Cherniak R, Sundstrom J B. Polysaccharide antigens of the capsule of Cryptococcus neoformans. Infect Immun. 1994;62:1507–1512. doi: 10.1128/iai.62.5.1507-1512.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiani P, Bromuro C, Torosantucci A. Defective induction of IL-12 in human monocytes by germ-tube forms of Candida albicans. Infect Immun. 2000;68:5628–5634. doi: 10.1128/iai.68.10.5628-5634.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chuck S L, Sande M A. Infections with Cryptococcus neoformans in the acquired immunodeficiency syndrome. N Engl J Med. 1989;321:794–799. doi: 10.1056/NEJM198909213211205. [DOI] [PubMed] [Google Scholar]
- 9.Clemons K V, Brummer E, Stevens D A. Cytokine treatment of central nervous system infection: efficacy of interleukin-12 alone and synergy with conventional antifungal therapy in experimental cryptococcosis. Antimicrob Agents Chemother. 1994;38:460–464. doi: 10.1128/aac.38.3.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Currie B P, Casadevall A. Estimation of the prevalence of cryptococcal infection among patients infected with the human immunodeficiency virus in New York City. Clin Infect Dis. 1994;19:1029–1033. doi: 10.1093/clinids/19.6.1029. [DOI] [PubMed] [Google Scholar]
- 11.D'Andrea A, Rengaraju M, Valiante N M, Chehimi J, Kubin M, Aste M, Chan S H, Kobayashi M, Young D, Nickbarg E, Chizzonite R, Wolf S F, Trinchieri G. Production of natural killer cell stimulatory factor (interleukin-12) by peripheral blood mononuclear cells. J Exp Med. 1992;176:1387–1398. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Decken K, Kohler G, Palmer-Lehmann K, Wunderlin A, Mattner F, Magram J, Gately M K, Alber G. Interleukin-12 is essential for a protective Th1 response in mice infected with Cryptococcus neoformans. Infect Immun. 1998;66:4994–5000. doi: 10.1128/iai.66.10.4994-5000.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Waal Malefyt R, Yssel H, Roncarolo M G, Spits H, de Vries J E. Interleukin-10. Curr Opin Immunol. 1992;4:314–320. doi: 10.1016/0952-7915(92)90082-p. [DOI] [PubMed] [Google Scholar]
- 14.Eng R H K, Bishburg E, Smith S M, Kapila R. Cryptococcal infections in patients with acquired immunodeficiency syndrome. Am J Med. 1986;81:19–23. doi: 10.1016/0002-9343(86)90176-2. [DOI] [PubMed] [Google Scholar]
- 15.Feldmesser M, Casadevall A. Effect of serum IgG1 to Cryptococcus neoformans glucuronoxylomannan on murine pulmonary infection. J Immunol. 1997;158:790–799. [PubMed] [Google Scholar]
- 16.Frevert C W, Matute-Bello G, Skerrett S J, Goodman R B, Kajikawa O, Sittipunt C, Martin T R. Effect of CD14 blockade in rabbits with Escherichia coli pneumonia and sepsis. J Immunol. 2000;164:5439–5445. doi: 10.4049/jimmunol.164.10.5439. [DOI] [PubMed] [Google Scholar]
- 17.Fulton S A, Jahnsen J M, Wolf S F, Sieburth D S, Boom W H. Interleukin-12 production by human monocytes infected with Mycobacterium tubercolosis: role of phagocytosis. Infect Immun. 1996;64:2523–2531. doi: 10.1128/iai.64.7.2523-2531.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gade W, Hinnefeld S W, Babcock L S, Gilligan P, Kelly W, Wait K, Greer D, Pinilla M, Kaplan R L. Comparison of the PREMIER cryptococcal enzyme immunoassay and the latex agglutination assay for detection of cryptococcal antigens. J Clin Microbiol. 1991;29:1616–1619. doi: 10.1128/jcm.29.8.1616-1619.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gregory C D. CD14-dependent clearance of apoptotic cells: relevance to the immune system. Curr Opin Immunol. 2000;12:27–34. doi: 10.1016/s0952-7915(99)00047-3. [DOI] [PubMed] [Google Scholar]
- 20.Heinzel F P, Rerko M, Ling P, Hakimi J, Schoenhaut D S. Interleukin 12 is produced in vivo during endotoxemia and stimulates synthesis of gamma interferon. Infect Immun. 1994;62:4244–4249. doi: 10.1128/iai.62.10.4244-4249.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houpt D C, Pfrommer G S T, Young B J, Larson T A, Kozel T R. Occurrences, immunoglobulin classes, and biological activities of antibodies in normal human serum that are reactive with Cryptococcus neoformans glucuronoxylomannan. Infect Immun. 1994;62:2857–2864. doi: 10.1128/iai.62.7.2857-2864.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawakami K, Kono S, Kadota J, Tohyama M, Teruya K, Kudeken N, Saito A, Hara K. T cell-dependent activation of macrophages and enhancement of their phagocytic activity in the lungs of mice inoculated with heat-killed Cryptococcus neoformans: involvement of IFN-γ and its protective effect against cryptococcal infection. Microbiol Immunol. 1995;39:135–143. doi: 10.1111/j.1348-0421.1995.tb02180.x. [DOI] [PubMed] [Google Scholar]
- 23.Kawakami K, Tohyama M, Qifeng X, Saito A. Expression of cytokines and inducible nitric oxide synthase mRNA in the lungs of mice infected with Cryptococcus neoformans: effects of IL-12. Infect Immun. 1997;65:1307–1312. doi: 10.1128/iai.65.4.1307-1312.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawakami K, Tohyama M, Xie Q, Saito A. IL-12 protects mice against pulmonary and disseminated infection caused by Cryptococcus neoformans. Clin Exp Immun. 1996;104:208–214. doi: 10.1046/j.1365-2249.1996.14723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelleher P, Knight S C. IL-12 increases CD80 expression and the stimulatory capacity of bone marrow-derived dendritic cells. Int Immunol. 1998;10:749–755. doi: 10.1093/intimm/10.6.749. [DOI] [PubMed] [Google Scholar]
- 26.Kozel T R. Nonencapsulated variant of Cryptococcus neoformans. II. Surface receptors of cryptococcal polysaccharide and their role in inhibition of phagocytosis by polysaccharide. Infect Immun. 1977;16:99–106. doi: 10.1128/iai.16.1.99-106.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kozel T R. Activation of the complement system by pathogenic fungi. Clin Microbiol Rev. 1996;9:34–46. doi: 10.1128/cmr.9.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kozel T R, Tabuni A, Young B J, Levitz S M. Influence of opsonization conditions on C3 deposition and phagocyte binding of large- and small-capsule Cryptococcus neoformans cells. Infect Immun. 1996;64:2336–2338. doi: 10.1128/iai.64.6.2336-2338.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuchroo V K, Das M P, Brown J A, Ranger A M, Zamvil S S, Sobel R A, Weiner H L, Nabavi N, Glimcher L H. B7–1 and B7–2 costimulatory molecules activate differentially the Th1/Th2 development pathways: application to autoimmune disease therapy. Cell. 1995;80:707–718. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 30.Laxalt K A, Kozel T R. Chemotaxigenesis and activation of the alternative complement pathway by encapsulated and nonencapsulated Cryptococcus neoformans. Infect Immun. 1979;26:435–440. doi: 10.1128/iai.26.2.435-440.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levitz S M. The ecology of Cryptococcus neoformans and the epidemiology of cryptococcosis. Rev Infect Dis. 1994;13:1163–1169. doi: 10.1093/clinids/13.6.1163. [DOI] [PubMed] [Google Scholar]
- 32.Meyaard L, Hovenkamp E, Otto S A, Miedema F. IL-12 induced IL-10 production by human T cells as negative feedback for IL-12-induced immune responses. J Immunol. 1996;156:2776–2782. [PubMed] [Google Scholar]
- 33.Nakamura T, Kamogawa Y, Bottomly K, Flavell R A. Polarization of IL-4- and IFN-γ-producing CD4+ T cells following activation of naive CD4+ T cells. J Immunol. 1997;158:1085–1094. [PubMed] [Google Scholar]
- 34.Pitzurra L, Cherniak R, Giammarioli M, Perito S, Bistoni F, Vecchiarelli A. Early induction of IL-12 by human monocytes exposed to Cryptococcus neoformans mannoproteins. Infect Immun. 2000;68:558–563. doi: 10.1128/iai.68.2.558-563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Retini C, Casadevall A, Pietrella D, Monari C, Palazzetti B, Vecchiarelli A. Specific activated T cells regulate IL-12 production by human monocytes stimulated with Cryptococcus neoformans. J Immunol. 1999;162:1618–1623. [PubMed] [Google Scholar]
- 36.Snijders A, Hilkens C M, van der Pouw Kraan T C, Engel M, Aarden L A, Kapsenberg M L. Regulation of bioactive IL-12 production in lipolysaccharide-stimulated human monocytes is determined by the expression of the p35 subunit. J Immunol. 1996;156:1207–1212. [PubMed] [Google Scholar]
- 37.Trinchieri G. Cytokines acting on or secreted by macrophages during intracellular infection (IL-10, IL-12, IFN-γ) Curr Opin Immunol. 1997;9:17–23. doi: 10.1016/s0952-7915(97)80154-9. [DOI] [PubMed] [Google Scholar]
- 38.van der Pouw Kraan T C, Boeije L C, Snijders A, Smeenk R J, Wijdenes J, Aarden L A. Regulation of IL-12 production by human monocytes and the influence of prostaglandin E2. Ann N Y Acad Sci. 1996;795:147–157. doi: 10.1111/j.1749-6632.1996.tb52663.x. [DOI] [PubMed] [Google Scholar]
- 39.Vecchiarelli A, Dottorini M, Pietrella D, Monari C, Retini C, Todisco T, Bistoni F. Role of alveolar macrophages as antigen presenting cells in Cryptococcus neoformans infection. Am J Respir Cell Mol Biol. 1994;11:130–137. doi: 10.1165/ajrcmb.11.2.8049074. [DOI] [PubMed] [Google Scholar]
- 40.Vecchiarelli A, Monari C, Retini C, Pietrella D, Palazzetti B, Pitzurra L, Casadevall A. Cryptococcus neoformans differently regulates B7–1 (CD80) and B7–2 (CD86) expression on human monocytes. Eur J Immunol. 1998;28:114–121. doi: 10.1002/(SICI)1521-4141(199801)28:01<114::AID-IMMU114>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 41.Vecchiarelli A, Pietrella D, Dottorini M, Monari C, Retini C, Todisco T, Bistoni F. Encapsulation of Cryptococcus neoformans regulates fungicidal activity and antigen presentation process in human alveolar macrophages. Clin Exp Immunol. 1994;98:217–223. doi: 10.1111/j.1365-2249.1994.tb06128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vecchiarelli A, Retini C, Monari C, Tascini C, Bistoni F, Kozel T R. Purified capsular polysaccharide of Cryptococcus neoformans induces interleukin-10 secretion by human monocytes. Infect Immun. 1996;64:2846–2849. doi: 10.1128/iai.64.7.2846-2849.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vecchiarelli A, Retini C, Pietrella D, Monari C, Tascini C, Beccari T, Kozel T R. Down-regulation of cryptococcal polysaccharide of tumor necrosis factor-α and interleukin-1β secretion from human monocytes. Infect Immun. 1995;63:2919–2923. doi: 10.1128/iai.63.8.2919-2923.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vecchiarelli A, Todisco T, Puliti M, Dottorini M, Bistoni F. Modulation of anti-Candida activity of human alveolar macrophages by interferon-γ or interleukin-1β. Am J Respir Cell Mol Biol. 1989;1:49–55. doi: 10.1165/ajrcmb/1.1.49. [DOI] [PubMed] [Google Scholar]
- 45.Vieth M, Will A, Schroppel K, Rollinghoff M, Gessner A. Interleukin-10 inhibits antimicrobial activity against Leishmania major in murine macrophages. Scan J Immunol. 1994;40:403–409. doi: 10.1111/j.1365-3083.1994.tb03481.x. [DOI] [PubMed] [Google Scholar]
- 46.Wittmann M, Zwizner J, Larsson V A, Kirchhoff K, Begemann G, Kappa A, Gotze O, Werfel T. C5a suppresses the production of IL-12 by IFN-γ-primed and lipopolysaccharide-challenged human monocytes. J Immunol. 1999;162:6763–6769. [PubMed] [Google Scholar]