Abstract
Ischemic stroke is one of the major disabling health‐care problem and multiple different approaches are needed to enhance rehabilitation, in which neural repair is the structural basement. Remote ischemic conditioning (RIC) is a strategy to trigger endogenous protect. RIC has been reported to play neuroprotective role in acute stage of stroke, but the effect of RIC on repair process remaining unclear. Several studies have discovered some overlapped mechanisms RIC and neural repair performs. This review provides a hypothesis that RIC is a potential therapeutic strategy on stroke rehabilitation by evaluating the existing evidence and puts forward some remaining questions to clarify and future researches to be performed in the field.
Keywords: ischemic stroke, remote ischemic conditioning, repair
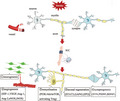
The figure shows the speculation that chronic RIC may enhance neural repair after ischemic stroke by improving neurogenesis, angiogenesis, axonal regeneration, synaptogenesis and remyelination.
Abbreviations
- AIS
Acute ischemic stroke
- Ang
Angiotensin
- BDNF
brain‐derived neurotrophic factor
- CNS
central nervous system
- eNOS
endothelial nitric oxide synthase
- EPO
erythropoietin
- GAP‐43
growth‐associated protein 43
- HIF‐1
Hypoxia inducible factor‐1
- iNOS
inducible nitric oxide synthase
- LTD
long‐term depression
- LTP
long‐term potentiation
- mTOR
mammalian target of rapamycin
- Ngb
neuroglobin
- NO
nitric oxide
- OPC
oligodendrocyte precursor cell
- PSD95
Postsynaptic density protein‐95
- p‐STAT3
signal transducer and activator of transcription 3 phosphorylation
- RIC
Remote ischemic condition
- STAT3
signal transducer and activator of transcription 3
- SYN
Synaptophysin
- Treg
regulatory T cell
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor 2
1. INTRODUCTION
Stroke is still one of the leading causes of disability and death in the world. 1 Acute ischemic stroke (AIS) is a disease caused by the occlusion of blood vessel, which results in brain tissue necrosis. Recanalization, including intravenous thrombolysis and endovascular therapy, is the proven treatment for AIS that can reduce mortality. However, there are still a large number of patients having mild to moderate functional deficits at chronic stage after stroke and the burden of stroke is still increasing. This is partly due to the limited treatment option for stroke deficits, which provides incomplete recovery. It is urgent to find effective treatment methods to further improve the functional recovery and reduce the burden of disease. Different from the neuroprotection strategies, which protect brain from ischemia and/or reperfusion injury in the acute stage of stroke (i.e., oxidative stress, excitotoxicity, inflammation), 2 the targets of neural repair are axonal sprouting, dendritic branching, neurogenesis, axon preservation, remyelination and glial scar reversing. 3 Therefore, it is critical to develop approaches to augment neural repair to enhance the rate of poststroke functional recovery. Seeking the treatment to combine with rehabilitation will be an effective way. Recent therapeutic strategies under study focus on pharmacotherapy or growth factor that combine with rehabilitation treatment to improve long‐term outcome after stroke. 4 In addition, physical and non‐invasive strategy are also considered as a promising adjunctive therapy. 5 , 6
Remote ischemic condition (RIC) is a non‐invasive procedure by which several episodes of sublethal ischemia and reperfusion in distant organs are applied to trigger endogenous protection of important organs. In the application of RIC, it is common practice to occlude blood flow repetitively of upper limbs in human or hind limbs in rodent bilaterally. The principle of RIC is developed from ischemic conditioning in situ, which was first described by Murry et al. that cycles of myocardial ischemia and reperfusion could protect the heart from a subsequent sustained ischemic injury. 7 The similar protective effect was subsequently reported in hypoxic conditioning that includes intermittent moderately severe hypoxia or single exposure to acute hypoxia. 8 The conditioning procedures mentioned above share similar endogenous mechanisms of protection and repair, among which RIC has attracted much attention and emerged as a promising treatment for stroke as it is much safer and more convenient to operate.
RIC has shown an impressive neuroprotective function in ischemic stroke in the past few years. It has been reported that among patients treated with recombinant tissue plasmin, those who received RIC had better prognosis for 90 days than their counterparts who did not. 9 For patients who failed to receive revascularization therapy within 24 hours after onset, a single episode of RIC provided long‐term protection that significantly reduced 90‐days National Institutes of Health Stroke Scale scores. 10 Numerous preclinical investigations have also systematically studied the molecular pathways and potential benefits of RIC with promising results. It has been suggested that RIC can significantly reduce the infarct volume, reduce brain edema and improve neurological function. 11 , 12 , 13 , 14 , 15 However, the effects of RIC on neural repair after ischemic stroke in the adult brain have yet to be elucidated. In this review, we propose a hypothesis that RIC may be a potential feasible approach for neural repair after ischemic stroke.
2. BACKGROUND AND THEORY OF THE HYPOTHESIS
Neural repair after stroke is a dynamic and complex process, wherein a variety of mechanisms are involved. After suffering a vascular event, focal cerebral ischemia will induce neurogenesis, angiogenesis and glial cell activation, thereby creating a favorable environment for endogenous neural repair. However, this endogenous repair in the brain is short‐lasting and light, so it is insufficient to restore neurological function. Therefore, interventions that facilitate neural plasticity by amplifying endogenous mechanisms may accelerate recovery, and this is the principle of most the current complementary treatments in stroke rehabilitation. For instance, physical exercise training, which is considered as an effective therapeutic intervention in stroke recovery, can attenuate neuronal apoptosis and promote neurogenesis, 16 as well as increase the expression of growth factors such as brain‐derived neurotrophic factor (BDNF), 17 nerve growth factor, 18 insulin‐like growth factor‐1 and neurotrophin 4. 19 Physical training can also alleviate blood–brain barrier dysfunction, 20 promote angiogenesis and increase cerebral blood flow, 21 , 22 increase the density and reuptake ability of astrocytes 23 , 24 and promote motor map reorganization, 25 thus exerting a neural repair effect. Interestingly, RIC has similar underlying mechanisms to physical exercise and may mimic regular exercise. 26 A number of studies have reported that RIC could amplify neurogenesis and angiogenesis in peri‐infarct area 13 , 27 , 28 , 29 , 30 and regulate glial cell function 31 during stroke recovery. Significantly, early RIC followed by exercise increases mRNA and protein expression of neuroplasticity, synaptogenesis, and angiogenesis related molecules. 32 In clinical practice, the administration of chronic RIC improved long‐term recovery (modified Rankin Scale score 0–1) and reduced white matter damage in patients with symptomatic atherosclerotic intracranial arterial stenosis (including ischemic stroke and transit ischemic attack). 33 , 34 Moreover, RIC could significantly facilitate motor learning in healthy adults, which indicated that RIC might induce neural plasticity. 35 As of June 13th, 2022, there are two clinical trials at ClinicalTrials.gov investigating the effect of RIC on central nervous system (CNS) rehabilitation are now ongoing (see Table 1). Based on these evidences, we speculate that RIC causes neural repair effect poststroke.
TABLE 1.
Representative Clinical Trials of Remote Ischemic Conditioning for rehabilitation in ClinicalTrials.gov
| Study | N | Participants | RIC protocol | Primary outcome | Status | ClinicalTrials.gov Identifier |
|---|---|---|---|---|---|---|
| Remote Ischemic Conditioning, Bimanual Skill Learning, and Corticospinal Excitability | 30 | Children with unilateral cerebral palsy | 5 × 5 min inflation/deflation of cuff Cuff pressure: 20 mmHg above systolic blood pressure to 250 mmHg | Change in Assisting Hand Assessment, Bimanual Task Performance, Resting and Active Motor Thresholds and Stimulus–response curves | Recruiting | NCT05355883 |
| Remote Ischemic Conditioning for Motor Recovery After Acute Ischemic Stroke | 20 | Patients with AIS | 5 × 5 min inflation/deflation of cuff Cuff pressure: 200 mmHg | Changes in Fugl‐Meyer score | Recruiting | NCT05263531 |
Abbreviations: AIS, acute ischemic conditioning; RIC, remote ischemic conditioning.
3. EVALUATION OF THE HYPOTHESIS
There are numerous mechanisms involved in neural repair to improve recovery poststroke, and some of them also exist in the potential mechanisms of RIC. Here, these overlapping ones are presented to support the hypothesis, in which both direct evidence and indirect evidence are contained.
3.1. Neurogenesis
Neurogenesis after stroke is a process by which neural progenitor cells generate and differentiate into mature neurons, migrate to infarct area and integrate to existing neuronal circuits, 36 which significantly contributes to the brain function recovery poststroke. It is unclear whether RIC promote neurogenesis after stroke. Esposito et al. reported that ischemic postconditioning in situ significantly increased the number of newborn neurons and improved neurological outcomes. 27 Furthermore, trophic factors enhance neurogenesis in the stroke brain. 37 hypoxic conditioning could promote the proliferation and migration of endogenous neuronal precursor in rodents by increasing the expression of BDNF 38 and regulating metabolism of neural stem cells (decreasing fatty acid oxidation and increasing glycolysis). 39 Hypoxia inducible factor‐1 (HIF‐1) is well known to stimulate neurogenesis and neuronal differentiation, and one important pathway contributing to the neurogenesis effect of HIF‐1 involves induction of neuroglobin (Ngb). 40 Ngb has a function of elevating oxygen distribution to the neurons. It promotes both proliferation and neuronal differentiation of neural progenitor cells by enhancement of Wnt signaling, thereby promoting neurogenesis after stroke. 41 Ren et al. found that a single episode of RIC at onset of cerebral ischemia (per‐conditioning) combined with daily RIC initiated after reperfusion (postconditioning) during the 14 days could significantly increase Ngb expression in the peri‐infarct region. 13 Therefore, we assume that RIC could amplify and provide long‐term induction of neurogenesis.
3.2. Angiogenesis
It has been reported that blood vessels could create a microenvironment, which is suitable for neurogenesis by supplying oxygen, nutrients, and soluble factors. 42 It acts as scaffold for neural progenitor cells to migrate to damaged region. 43 Functional recovery after stroke is accompanied by revascularization in perilesional areas. 44 Therefore, treatments that promote angiogenesis play a critical role in the neural repair process. RIC has been found to promote angiogenesis after stroke. Ren et al. demonstrated that remote ischemic per‐conditioning followed by postconditioning for 7 or 14 days could promote arteriogenesis and increase cerebral blood flow and collateral circulation after ischemic stroke in the rat through increasing vascular Notch signaling activity. 29 In addition, they reported that RIC significantly increased the expression of endothelial nitric oxide synthase (eNOS) and regulated angiogenesis by eNOS/nitric oxide (NO) pathway after chronic cerebral hypoperfusion. 30 Consistently, Khan et al. found that long‐term daily RIC therapy (1 or 4 months) significantly enhanced both angiogenesis and arteriogenesis in mouse bilateral carotid artery stenosis model. 45 , 46 Moreover, RIC could enhance the expression of angiogenesis related factors. For instance, vascular endothelial growth factor (VEGF) is the key factor to proangiogenic activity. It could increase the vascular permeability, promote endothelial cell migration and survival when binding to VEGF receptor 2 (VEGFR2). 47 For in vivo experiment, the expression of HIF‐1α and VEGF was significantly increased by RIC at the mRNA and protein levels. 32 , 48 , 49 , 50 Additionally, exosomes derived from RIC could increase the expression of eNOS, inducible nitric oxide synthase (iNOS), HIF‐1α, Angiotensin 1 (Ang‐1), and VEGF in rats. 51 Ischemic postconditioning in situ also upregulate the VEGF within peri‐infarct regions. 52 Hence, RIC facilitates recovery possibly by enhancing angiogenesis.
3.3. Axonal regeneration
Neurogenesis and angiogenesis are the structural basis of neural repair, while functional connections between newborn and surviving neurons need to be established to realize effective neurological function recovery. The action potentials between neurons are conducted by axons, therefore, axonal regeneration and reconnection play a central role in poststroke recovery. In the central nervous system, the influence of RIC on axonal regeneration has not been tested yet, but encouraging results confirmed that hypoxic conditioning significantly increased the numbers of thinly myelinated regenerating axons in surgically repaired peripheral nerves. 53 Axon sprouting can be enhanced or limited by several transcription factors, cytokines, chemokines, growth factors, and other molecules. Signal transducer and activator of transcription 3 (STAT3) is a transcription factor, which promotes the regrowth of damaged axons in adult central nervous system. 54 Many cytokines and neurotrophic factors play a role in promoting neurite extension through activation of STAT3. 55 Hildebrandt et al. recruited healthy volunteers to receive RIC and collected their venous blood to obtain dialysates before and after conditioning. The analysis result suggested that RIC significantly increased the level of STAT3 phosphorylation (p‐STAT3) compared to baseline. 56 Youn et al. also reported that p‐STAT3 were increased by RIC, 57 which indicated that RIC may activate STAT3. Growth‐associated protein 43 (GAP‐43) is also an important marker related to the structural integrity of neurons, which is known to promote axonal regeneration by guiding growth cones, 58 , 59 , 60 It was reported that mRNA and protein expressions of GAP‐43 could be increased by RIC in rats after cerebral ischemia. 61 , 62 Similarly, intermittent hypoxia (6 min FiO2 = 6% with 6 min normoxia, 8 h per day) applied for 4 weeks increased the expression of GAP‐43 in young rats. 63 Additionally, prior studies suggest that erythropoietin (EPO) induces axonal sprouting via activating cellular JAK2 and PI3K signaling. 64 Hypoxic conditioning has been discovered to increase EPO levels in healthy individuals serum 65 and rat brains. 66 Mammalian target of rapamycin (mTOR) is a protein kinase, which regulate cell growth, survival and metabolism. In the CNS, mTOR also act as a nutrient sensor that support neuronal growth and plasticity. 67 mTOR pathway is a crucial signaling pathway, which can promote axon regeneration, 68 as the activation of mTOR may promote the synthesis of the raw materials for axon extension. 69 However, RIC has been proved to downregulate mTOR activity and upregulate miRNAs, which repress mTOR expression and signaling. 70 , 71 In this regard, the effect of RIC on axon regeneration need to be further explored.
3.4. Synaptogenesis
Neuronal synapse formation and maturation are complex and regulated through multiple steps. In recent years, RIC has been discovered to improve synaptogenesis in stroke model. Wang et al. found that 3 cycles of RIC after cerebral artery occlusion could improve synaptogenesis in ischemic penumbra. 61 Moreover, there are several markers that monitor synaptogenesis. It was reported that serine 41 on GAP43 could regulate synaptic plasticity when it was phosphorylated. 72 Postsynaptic density protein‐95 (PSD95) is a major synaptic scaffolding molecule, which controls synaptic transmission 73 and can be used to label postsynaptic terminals. Synaptophysin(SYN), a protein located in neurotransmitter vesicles, can identify presynaptic terminals. Geng et al. reported that RIC significantly increased mRNA and protein expressions of GAP43, SYN and PSD‐95 compared to stroke‐only rats, indicating a potential role in promoting synaptogenesis. 62 Dendritic spines can respond to many events with rapid changes as long‐term potentiation (LTP) and long‐term depression (LTD). Neurotrophic factors play critical roles in LTP thus enhancing synaptic plasticity. Among all neurotrophins, BDNF distribute most widely in mammalian brain and modulate broad range of process including synaptic plasticity and remodeling. 74 RIC has shown to upregulate BDNF significantly after ischemic reperfusion injury. 75 In addition to RIC, similar protective effects of hypoxic conditioning were also described in synaptic connection. Tsai et al. demonstrated that hypoxic conditioning applied after cerebral ischemia increased hippocampal functional synaptogenesis via BDNF expression. 76 The studies mentioned above may support the hypothesis that RIC is an adjunct treatment in synaptic alteration.
3.5. Remyelination
Damaged oligodendrocytes in the acute phase cause myelin loss, which is the leading cause of white matter dysfunction. Remyelination is one of the key processes in neural repair after ischemic stroke, 77 which is mediated by oligodendrocyte precursor cells (OPC) proliferation and differentiation at sites of infarct with demyelination. 78 Oligodendrogenesis can occur in peri‐infarct areas in repair processes companied with angiogenesis and neurogenesis. So far, no studies have investigated the influence of RIC on poststroke remyelination. Nevertheless, OPCs communicated closely with endothelial cells in the oligovascular niche, wherein endothelial cells support the survival and proliferation of OPC. 79 , 80 RIC is proven to promote angiogenesis poststroke, therefore, RIC may be beneficial to remyelination. Notably, in chronic cerebral hypoperfusion rats, Li et al. showed that RIC for 28 days promoted myelination by inhibiting oligodendrocytes apoptosis in the corpus callosum. 81 Daily RIC for 2 weeks in mice also ameliorated the loss of myelin basic protein caused by chronic cerebral hypoperfusion. 46 The above results suggest that RIC has a potential positive influence on poststroke remyelination. Furthermore, infiltration of peripheral immune cells into ischemic area also contribute to poststroke repair. 82 Over the last decade, several studies have described the infiltration of T cells in CNS after stroke, 83 , 84 demonstrating that regulatory T cells (Treg) promote oligodendrocyte differentiation and remyelination. 85 Shi et al. reported that the function of Treg cells in facilitating oligodendrogenesis and remyelination was mediated by promoting tissue‐reparative microglia responses following crosstalk with microglia at the chronic stage of stroke. 86 It was reported that ischemic postconditioning (reperfusion for 10 min after which the middle cerebral artery was reoccluded for 10 min) for one episode can facilitate to shift microglia into tissue repairing forms, 52 which actively participate in myelin repair. 87 , 88 In addition, a recent study confirmed the contribution of RIC is linked to Treg cells thriving. The increased quantity of Treg cells by 3 cycles of RIC immediately after middle cerebral artery reperfusion was showed in mice and directly linked to improvement of neurological function. 89 Hence, we suggest that RIC can enhance poststroke remyelination through directly acting on oligodendrocytes and modulating microglial response.
4. CHALLENGES AND FUTURE DIRECTIONS FOR THE STUDY OF RIC IN POSTSTROKE REHABILITATION
After summarizing the evidence that RIC can enhance neural repair (see Table 2), the positive effects of RIC on stroke rehabilitation were put forward, but the hypothesis is needed to be test in stroke models. To clarify whether RIC facilities the process of neural repair, there are still some questions and perspectives need to be noted in further studies.
TABLE 2.
The possibly overlapping mechanism between RIC and neural repair in preclinical studies.
| Study | Type of animal | Model | Protocol of RIC or other form of conditioning | Possible mechanism | Direct/indirect evidence |
|---|---|---|---|---|---|
| Neurogenesis | |||||
| Esposito 27 | Male Sprague–Dawley rats | MCAO | 100‐minute middle cerebral artery occlusion plus 10‐minute reperfusion plus 10‐minute reocclusion | NR | indirect |
| Zhu 38 | Male Sprague–Dawley rats | ‐ | Expose to pressure equivalent to an altitude of 5000 m for 4 h, then reach the normal level in 15 min, once daily for 14 days | BDNF–TrkB signaling | indirect |
| Li 39 | Male C57BL/6 mice | MCAO | After reperfusion, 8% O2 for 3 h, once daily until sacrifice | decreased FAO and increased glycolysis | indirect |
| Ren 13 | Male Sprague Dawley rats | MCAO | 3 × 10 min occlusion or release, immediately following ischemia and once daily for 14 days | promotes neuroglobin expression | indirect |
| Angiogenesis | |||||
| Ren 30 | Male Sprague–Dawley rats | 2VO | 3 × 10 min occlusion or release, once daily for 28 days | eNOS/NO pathway | direct |
| Ren 29 | Male Sprague–Dawley rats | MCAO | 3 × 10 min occlusion or release, immediately following ischemia and once daily for 7 or 14 days | Notch 1 signaling | direct |
| Khan 45 | Male C57BL/6 mice | BCAS | 4 × 5 min occlusion or release, once daily for 1 or 4 months | NR | direct |
| Khan 46 | Male C57BL/6 mice | BCAS | 4 × 5 min occlusion or release, once daily for 2 weeks | NR | direct |
| Wang 32 | Male Sprague–Dawley rats | MCAO | 6 h after reperfusion, 3 × 10 min occlusion or release, once daily for 28 days | promotes VEGF, Ang‐1, Ang‐2 expression | direct |
| Chen 51 | Male Sprague–Dawley rats | myocardial infarction | NR | Increases eNOS, iNOS, HIF‐1α, Ang‐1, and VEGF expression by exosomes | indirect |
| Esposito 52 | Male Sprague–Dawley rats | MCAO | 100‐minute middle cerebral artery occlusion plus 10‐minute reperfusion plus 10‐minute reocclusion | promotes VEGF expression in peri‐infarct area | direct |
| Axonal regeneration | |||||
| Huang 94 | Male Sprague–Dawley rats | Hemorrhagic shock | 4 × 5 min occlusion or release, | increases the phosphorylation levels of STAT3 | indirect |
| Wang 61 | Male Sprague–Dawley rats | MCAO | 3 × 10 min occlusion or release, once after reperfusion | Promotes GAP43 expression | direct |
| Geng 62 | Male Sprague–Dawley rats | MCAO | 3 × 10 min occlusion or release | Promotes GAP43 expression | direct |
| Chen 63 | Male Sprague–Dawley rats | dMCAO | 3 cycles of 30 s reperfusions and 10 s occlusions of the bilateral CCAs, once after reperfusion | Promotes GAP43 expression | indirect |
| Coimbra‐Costa 66 | Male Sprague–Dawley rats | Exposure to pressure equivalent to an altitude of 4000 m for 4 h, once daily for 8 days | increase EPO levels in the brain | indirect | |
| Synaptogenesis | |||||
| Wang 61 | Male Sprague–Dawley rats | MCAO | 3 × 10 min occlusion or release, once after reperfusion | Promotes SYN, PSD95 expression | direct |
| Geng 62 | Male Sprague–Dawley rats | MCAO | 3 × 10 min occlusion or release | Promotes SYN, PSD95 expression | direct |
| Tsai 76 | Male Sprague–Dawley rats | MCAO | 12% O2 for 4 hours, once daily for 7 days | promotes BDNF expression | indirect |
| Remyelination | |||||
| Li 81 | Male Sprague–Dawley rats | 2VO | 3 × 10 min occlusion or release, once daily for 28 days | PI3K/Akt/mTOR pathway | direct |
| Khan 45 | Male C57BL/6 mice | BCAS | 4 × 5 min occlusion or release, once daily for 2 weeks | NR | direct |
| Yu 89 | Male C57BL/6 mice | MCAO | 3 × 10 min occlusion or release, once after reperfusion | activating and maintaining the Tregs | indirect |
Note: Direct evidence: the intervention is RIC instead of ischemic conditioning in situ or hypoxic conditioning, and the result can indicate the effect of RIC on neural repair processes directly instead of related processes. Indirect evidence: studies that do not meet the definition of direct evidence.
Abbreviations: 2VO, 2‐vessel occlusion; Ang, angiotensin; BCAS, bilateral carotid artery stenosis; BDNF, brain‐derived neurotrophic factor; eNOS/NO, endothelial nitric oxide synthase /nitric oxide; EPO, erythropoietin; FAO, fatty acid oxidation; GAP43, growth‐associated protein 43; MCAO, middle cerebral artery occlusion; NR, not reported; PSD95, postsynaptic density 95; RIC, remote ischemic conditioning; STAT3, signal transducer and activator of transcription 3; YN, synaptophysin; Treg, regulatory T cells; TrkB, tropomyosin related kinase B; VEGF, vascular endothelial growth factor.
Firstly, it is important to identify suitable time window for RIC in stroke rehabilitation, such as when to begin treatment. It is worth noting that four‐week chronic RIC initiated on the fifth day of cerebral ischemia has failed to improve the neurobehavioral deficit in rats endothelin‐1 stroke model. 90 Thus RIC initiated at chronic stage seems to have no advantage in function improvement. Meanwhile, acute stage application of RIC is proved to be effective, so it may be beneficial to apply RIC as early as possible and provide treatment until the chronic phase of stroke in further animal studies.
Secondly, how long RIC treatment should last in stroke rehabilitation needed to be considered as well. As a non‐invasive and effective conditioning strategy, RIC has been widely investigated clinically in the acute phase of ischemic stroke. The results of these studies emphasized the neuroprotection role of short‐term RIC at the beginning of stroke onset, but the effect of chronic RIC application on stroke patients remain unclear. Poalelungi et al. evaluated the effect of RIC applied in first 5 days after onset of patients, and results suggested that RIC partly improved disability and cognition without statistical difference. 91 Similarly, there are no significant improvements in infarct size or clinical outcome in patients who receive RIC for 4 days after stroke. 92 Conversely, 1‐year application of RIC significantly improved cerebral blood flow and slowed the arterial progression of the stenotic‐occlusive lesions in patient with Moyamoya disease. 93 These findings suggest that for the long‐lasting process like vascular remodeling in Moyamoya disease and neural repair, chronic daily RIC may be a more appropriate strategy in further clinical studies. Additionally, besides the assessment of function, neuroimaging is needed during recovery to evaluate whether distributed structural and functional connectivity can be changed by RIC.
5. CONCLUSION
More and more evidence has emerged to identify the influence of RIC on stroke recovery. Although there is still a lack of direct evidence to support the repair effects of RIC on ischemic stroke, these preclinical results have given the insight that the potential positive effects of RIC on neural repair, including neurogenesis, angiogenesis, axon regeneration, synaptogenesis and remyelination. More preclinical and clinical research work is needed to support the use of RIC as a promising therapy in stroke rehabilitation.
CONFLICT OF INTEREST
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ACKNOWLEDGMENT
This work was supported by the Natural Science Foundation of China (No. 81971114, No. 81801313). We thank Junkai Jia for the illustration of the manuscript.
Yu W, Ren C, Ji X. A review of remote ischemic conditioning as a potential strategy for neural repair poststroke. CNS Neurosci Ther. 2023;29:516‐524. doi: 10.1111/cns.14064
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1. Collaborators GBDRF . Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990‐2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392(10159):1923‐1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chamorro A, Dirnagl U, Urra X, Planas AM. Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016;15(8):869‐881. [DOI] [PubMed] [Google Scholar]
- 3. Regenhardt RW, Takase H, Lo EH, Lin DJ. Translating concepts of neural repair after stroke: structural and functional targets for recovery. Restor Neurol Neurosci. 2020;38(1):67‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mala H, Rasmussen CP. The effect of combined therapies on recovery after acquired brain injury: systematic review of preclinical studies combining enriched environment, exercise, or task‐specific training with other therapies. Restor Neurol Neurosci. 2017;35(1):25‐64. [DOI] [PubMed] [Google Scholar]
- 5. Wessel MJ, Zimerman M, Hummel FC. Non‐invasive brain stimulation: an interventional tool for enhancing behavioral training after stroke. Front Hum Neurosci. 2015;9:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kelly LP, Basset FA, McCarthy J, Ploughman M. Normobaric hypoxia exposure during treadmill aerobic exercise after stroke: a safety and feasibility study. Front Physiol. 2021;12:702439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74(5):1124‐1136. [DOI] [PubMed] [Google Scholar]
- 8. Shizukuda Y, Mallet RT, Lee SC, Downey HF. Hypoxic preconditioning of ischaemic canine myocardium. Cardiovasc Res. 1992;26(5):534‐542. [DOI] [PubMed] [Google Scholar]
- 9. An JQ, Cheng YW, Guo YC, et al. Safety and efficacy of remote ischemic postconditioning after thrombolysis in patients with stroke. Neurology. 2020;95(24):e3355‐e3363. [DOI] [PubMed] [Google Scholar]
- 10. England TJ, Hedstrom A, O'Sullivan S, et al. RECAST (remote ischemic conditioning after stroke trial): a pilot randomized placebo controlled phase II trial in acute ischemic stroke. Stroke. 2017;48(5):1412‐1415. [DOI] [PubMed] [Google Scholar]
- 11. Basalay MV, Wiart M, Chauveau F, et al. Neuroprotection by remote ischemic conditioning in the setting of acute ischemic stroke: a preclinical two‐Centre study. Sci Rep. 2020;10(1):16874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hahn CD, Manlhiot C, Schmidt MR, Nielsen TT, Redington AN. Remote ischemic per‐conditioning: a novel therapy for acute stroke? Stroke. 2011;42(10):2960‐2962. [DOI] [PubMed] [Google Scholar]
- 13. Ren C, Wang P, Wang B, et al. Limb remote ischemic per‐conditioning in combination with post‐conditioning reduces brain damage and promotes neuroglobin expression in the rat brain after ischemic stroke. Restor Neurol Neurosci. 2015;33(3):369‐379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ren C, Gao M, Dornbos D 3rd, et al. Remote ischemic post‐conditioning reduced brain damage in experimental ischemia/reperfusion injury. Neurol Res. 2011;33(5):514‐519. [DOI] [PubMed] [Google Scholar]
- 15. Wang L, Ren C, Li Y, et al. Remote ischemic conditioning enhances oxygen supply to ischemic brain tissue in a mouse model of stroke: role of elevated 2,3‐biphosphoglycerate in erythrocytes. J Cereb Blood Flow Metab. 2021;41(6):1277‐1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang L, Hu X, Luo J, et al. Physical exercise improves functional recovery through mitigation of autophagy, attenuation of apoptosis and enhancement of neurogenesis after MCAO in rats. BMC Neurosci. 2013;14:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lan X, Zhang M, Yang W, et al. Effect of treadmill exercise on 5‐HT, 5‐HT1A receptor and brain derived neurophic factor in rats after permanent middle cerebral artery occlusion. Neurol Sci. 2014;35(5):761‐766. [DOI] [PubMed] [Google Scholar]
- 18. Mizutani K, Sonoda S, Yamada K, Beppu H, Shimpo K. Alteration of protein expression profile following voluntary exercise in the perilesional cortex of rats with focal cerebral infarction. Brain Res. 2011;1416:61‐68. [DOI] [PubMed] [Google Scholar]
- 19. Chung JY, Kim MW, Bang MS, Kim M. Increased expression of neurotrophin 4 following focal cerebral ischemia in adult rat brain with treadmill exercise. PLoS One. 2013;8(3):e52461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang P, Zhang Q, Pu H, et al. Very early‐initiated physical rehabilitation protects against ischemic brain injury. Front Biosci (Elite Ed). 2012;4(7):2476‐2489. [DOI] [PubMed] [Google Scholar]
- 21. Yang YR, Chang HC, Wang PS, Wang RY. Motor performance improved by exercises in cerebral ischemic rats. J Mot Behav. 2012;44(2):97‐103. [DOI] [PubMed] [Google Scholar]
- 22. Ma Y, Qiang L, He M. Exercise therapy augments the ischemia‐induced proangiogenic state and results in sustained improvement after stroke. Int J Mol Sci. 2013;14(4):8570‐8584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Senna PN, Bagatini PB, Galland F, et al. Physical exercise reverses spatial memory deficit and induces hippocampal astrocyte plasticity in diabetic rats. Brain Res. 2017;1655:242‐251. [DOI] [PubMed] [Google Scholar]
- 24. Wang X, Zhang M, Feng R, et al. Physical exercise training and neurovascular unit in ischemic stroke. Neuroscience. 2014;271:99‐107. [DOI] [PubMed] [Google Scholar]
- 25. Shiromoto T, Okabe N, Lu F, et al. The role of endogenous neurogenesis in functional recovery and motor map reorganization induced by rehabilitative therapy after stroke in rats. J Stroke Cerebrovasc Dis. 2017;26(2):260‐272. [DOI] [PubMed] [Google Scholar]
- 26. Zhao W, Li S, Ren C, Meng R, Ji X. Chronic remote ischemic conditioning may mimic regular exercise:perspective from clinical studies. Aging Dis. 2018;9(1):165‐171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Esposito E, Hayakawa K, Maki T, Arai K, Lo EH. Effects of Postconditioning on neurogenesis and angiogenesis during the recovery phase after focal cerebral ischemia. Stroke. 2015;46(9):2691‐2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huang D, Liu H, Qu Y, Wang P. Non‐invasive remote ischemic postconditioning stimulates neurogenesis during the recovery phase after cerebral ischemia. Metab Brain Dis. 2017;32(6):1805‐1818. [DOI] [PubMed] [Google Scholar]
- 29. Ren C, Li S, Wang B, et al. Limb remote ischemic conditioning increases notch signaling activity and promotes arteriogenesis in the ischemic rat brain. Behav Brain Res. 2018;340:87‐93. [DOI] [PubMed] [Google Scholar]
- 30. Ren C, Li N, Li S, et al. Limb ischemic conditioning improved cognitive deficits via eNOS‐dependent augmentation of angiogenesis after chronic cerebral Hypoperfusion in rats. Aging Dis. 2018;9(5):869‐879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li S, Hu X, Zhang M, et al. Remote ischemic post‐conditioning improves neurological function by AQP4 down‐regulation in astrocytes. Behav Brain Res. 2015;289:1‐8. [DOI] [PubMed] [Google Scholar]
- 32. Wang Q, Wills M, Li F, Geng X, Ding Y. Remote ischemic conditioning with exercise (RICE) promotes functional rehabilitation following ischemic stroke. Neurol Res. 2021;43(11):874‐883. [DOI] [PubMed] [Google Scholar]
- 33. Meng R, Asmaro K, Meng L, et al. Upper limb ischemic preconditioning prevents recurrent stroke in intracranial arterial stenosis. Neurology. 2012;79(18):1853‐1861. [DOI] [PubMed] [Google Scholar]
- 34. Zhou D, Ding J, Ya J, et al. Efficacy of remote ischemic conditioning on improving WMHs and cognition in very elderly patients with intracranial atherosclerotic stenosis. Aging (Albany NY). 2019;11(2):634‐648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cherry‐Allen KM, Gidday JM, Lee JM, Hershey T, Lang CE. Remote limb ischemic conditioning enhances motor learning in healthy humans. J Neurophysiol. 2015;113(10):3708‐3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70(4):687‐702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kawauchi S, Yasuhara T, Kin K, et al. Transplantation of modified human bone marrow‐derived stromal cells affords therapeutic effects on cerebral ischemia in rats. CNS Neurosci Ther. 2022;28(12):1974‐1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhu XH, Yan HC, Zhang J, et al. Intermittent hypoxia promotes hippocampal neurogenesis and produces antidepressant‐like effects in adult rats. J Neurosci. 2010;30(38):12653‐12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li H, Li S, Ren C, et al. Hypoxic postconditioning promotes neurogenesis by modulating the metabolism of neural stem cells after cerebral ischemia. Exp Neurol. 2022;347:113871. [DOI] [PubMed] [Google Scholar]
- 40. Haines B, Demaria M, Mao X, et al. Hypoxia‐inducible factor‐1 and neuroglobin expression. Neurosci Lett. 2012;514(2):137‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yu Z, Cheng C, Liu Y, Liu N, Lo EH, Wang X. Neuroglobin promotes neurogenesis through Wnt signaling pathway. Cell Death Dis. 2018;9(10):945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hatakeyama M, Ninomiya I, Kanazawa M. Angiogenesis and neuronal remodeling after ischemic stroke. Neural Regen Res. 2020;15(1):16‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Font MA, Arboix A, Krupinski J. Angiogenesis, neurogenesis and neuroplasticity in ischemic stroke. Curr Cardiol Rev. 2010;6(3):238‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yanev P, van Tilborg GA, van der Toorn A, Kong X, Stowe AM, Dijkhuizen RM. Prolonged release of VEGF and Ang1 from intralesionally implanted hydrogel promotes perilesional vascularization and functional recovery after experimental ischemic stroke. J Cereb Blood Flow Metab. 2022;42(6):1033‐1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Khan MB, Hafez S, Hoda MN, et al. Chronic remote ischemic conditioning is Cerebroprotective and induces vascular remodeling in a VCID model. Transl Stroke Res. 2018;9(1):51‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Khan MB, Hoda MN, Vaibhav K, et al. Remote ischemic postconditioning: harnessing endogenous protection in a murine model of vascular cognitive impairment. Transl Stroke Res. 2015;6(1):69‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Melincovici CS, Bosca AB, Susman S, et al. Vascular endothelial growth factor (VEGF) ‐ key factor in normal and pathological angiogenesis. Rom J Morphol Embryol. 2018;59(2):455‐467. [PubMed] [Google Scholar]
- 48. Zheng Y, Lu X, Li J, Zhang Q, Reinhardt JD. Impact of remote physiological ischemic training on vascular endothelial growth factor, endothelial progenitor cells and coronary angiogenesis after myocardial ischemia. Int J Cardiol. 2014;177(3):894‐901. [DOI] [PubMed] [Google Scholar]
- 49. Zhou M, Lu S, Lu G, et al. Effects of remote ischemic postconditioning on fracture healing in rats. Mol Med Rep. 2017;15(5):3186‐3192. [DOI] [PubMed] [Google Scholar]
- 50. Homme RP, Zheng Y, Smolenkova I, Singh M, Tyagi SC. Remote hind‐limb ischemia mechanism of preserved ejection fraction during heart failure. Front Physiol. 2021;12:745328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen Q, Huang M, Wu J, Jiang Q, Zheng X. Exosomes isolated from the plasma of remote ischemic conditioning rats improved cardiac function and angiogenesis after myocardial infarction through targeting Hsp70. Aging (Albany NY). 2020;12(4):3682‐3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Esposito E, Hayakawa K, Ahn BJ, et al. Effects of ischemic post‐conditioning on neuronal VEGF regulation and microglial polarization in a rat model of focal cerebral ischemia. J Neurochem. 2018;146(2):160‐172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nadeau JR, Arnold BM, Johnston JM, Muir GD, Verge VMK. Acute intermittent hypoxia enhances regeneration of surgically repaired peripheral nerves in a manner akin to electrical stimulation. Exp Neurol. 2021;341:113671. [DOI] [PubMed] [Google Scholar]
- 54. Luo X, Ribeiro M, Bray ER, et al. Enhanced transcriptional activity and mitochondrial localization of STAT3 Co‐induce axon regrowth in the adult central nervous system. Cell Rep. 2016;15(2):398‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Conway G. STAT3‐dependent pathfinding and control of axonal branching and target selection. Dev Biol. 2006;296(1):119‐136. [DOI] [PubMed] [Google Scholar]
- 56. Hildebrandt HA, Kreienkamp V, Gent S, Kahlert P, Heusch G, Kleinbongard P. Kinetics and signal activation properties of circulating factor(s) from healthy volunteers undergoing remote ischemic pre‐conditioning. JACC Basic Transl Sci. 2016;1(1–2):3‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Youn YJ, Yoo BS, Son JW, et al. Remote ischemic conditioning by effluent collected from a novel isolated Hindlimb model reduces infarct size in an isolated heart model. Korean Circ J. 2017;47(5):714‐726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Granziera C, D'Arceuil H, Zai L, Magistretti PJ, Sorensen AG, de Crespigny AJ. Long‐term monitoring of post‐stroke plasticity after transient cerebral ischemia in mice using in vivo and ex vivo diffusion tensor MRI. Open Neuroimag J. 2007;1:10‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Strata P, Buffo A, Rossi F. Mechanisms of axonal plasticity. Arch Ital Biol. 1999;137(2–3):181‐192. [PubMed] [Google Scholar]
- 60. Coley AA, Gao WJ. PSD95: a synaptic protein implicated in schizophrenia or autism? Prog Neuropsychopharmacol Biol Psychiatry. 2018;82:187‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wang Y, Zhang Z, Zhang L, Yang H, Shen Z. RLIPostC protects against cerebral ischemia through improved synaptogenesis in rats. Brain Inj. 2018;32(11):1429‐1436. [DOI] [PubMed] [Google Scholar]
- 62. Geng X, Wang Q, Lee H, et al. Remote ischemic Postconditioning vs. physical exercise after stroke: an alternative rehabilitation strategy? Mol Neurobiol. 2021;58(7):3141‐3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chen Y, Zhao C, Zhang C, Luo L, Yu G. Influence of chronic intermittent hypoxia on growth associated protein 43 expression in the hippocampus of young rats. Neural Regen Res. 2012;7(16):1241‐1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Li SJ, Cui KF, Fu JJ, et al. EPO promotes axonal sprouting via upregulating GDF10. Neurosci Lett. 2019;711:134412. [DOI] [PubMed] [Google Scholar]
- 65. Wojan F, Stray‐Gundersen S, Nagel MJ, Lalande S. Short exposure to intermittent hypoxia increases erythropoietin levels in healthy individuals. J Appl Physiol (1985). 2021;130(6):1955‐1960. [DOI] [PubMed] [Google Scholar]
- 66. Coimbra‐Costa D, Garzon F, Alva N, et al. Intermittent hypobaric hypoxic preconditioning provides neuroprotection by increasing antioxidant activity, erythropoietin expression and preventing apoptosis and Astrogliosis in the brain of adult rats exposed to acute severe hypoxia. Int J Mol Sci. 2021;22(10):5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Querfurth H, Lee HK. Mammalian/mechanistic target of rapamycin (mTOR) complexes in neurodegeneration. Mol Neurodegener. 2021;16(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Park KK, Liu K, Hu Y, et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322(5903):963‐966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Park KK, Liu K, Hu Y, Kanter JL, He Z. PTEN/mTOR and axon regeneration. Exp Neurol. 2010;223(1):45‐50. [DOI] [PubMed] [Google Scholar]
- 70. Rohailla S, Clarizia N, Sourour M, et al. Acute, delayed and chronic remote ischemic conditioning is associated with downregulation of mTOR and enhanced autophagy signaling. PLoS One. 2014;9(10):e111291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lassen TR, Just J, Hjortbak MV, et al. Cardioprotection by remote ischemic conditioning is transferable by plasma and mediated by extracellular vesicles. Basic Res Cardiol. 2021;116(1):16. [DOI] [PubMed] [Google Scholar]
- 72. Norden JJ, Lettes A, Costello B, et al. Possible role of GAP‐43 in calcium regulation/neurotransmitter release. Ann N Y Acad Sci. 1991;627:75‐93. [DOI] [PubMed] [Google Scholar]
- 73. Xu W, Schluter OM, Steiner P, Czervionke BL, Sabatini B, Malenka RC. Molecular dissociation of the role of PSD‐95 in regulating synaptic strength and LTD. Neuron. 2008;57(2):248‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Phillips C. Brain‐derived neurotrophic factor, depression, and physical activity: making the Neuroplastic connection. Neural Plast. 2017;2017:7260130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ramagiri S, Taliyan R. Protective effect of remote limb post conditioning via upregulation of heme oxygenase‐1/BDNF pathway in rat model of cerebral ischemic reperfusion injury. Brain Res. 2017;1669:44‐54. [DOI] [PubMed] [Google Scholar]
- 76. Tsai YW, Yang YR, Sun SH, Liang KC, Wang RY. Post ischemia intermittent hypoxia induces hippocampal neurogenesis and synaptic alterations and alleviates long‐term memory impairment. J Cereb Blood Flow Metab. 2013;33(5):764‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Khodanovich MY, Gubskiy IL, Kudabaeva MS, et al. Long‐term monitoring of chronic demyelination and remyelination in a rat ischemic stroke model using macromolecular proton fraction mapping. J Cereb Blood Flow Metab. 2021;41(11):2856‐2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Itoh K, Maki T, Lok J, Arai K. Mechanisms of cell‐cell interaction in oligodendrogenesis and remyelination after stroke. Brain Res. 2015;1623:135‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Arai K, Lo EH. Wiring and plumbing: oligodendrocyte precursors and angiogenesis in the oligovascular niche. J Cereb Blood Flow Metab. 2021;41(8):2132‐2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Arai K, Lo EH. An oligovascular niche: cerebral endothelial cells promote the survival and proliferation of oligodendrocyte precursor cells. J Neurosci. 2009;29(14):4351‐4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Li X, Ren C, Li S, et al. Limb remote ischemic conditioning promotes myelination by upregulating PTEN/Akt/mTOR signaling activities after chronic cerebral Hypoperfusion. Aging Dis. 2017;8(4):392‐401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Park KW, Ju H, Kim ID, et al. Delayed infiltration of peripheral monocyte contributes to phagocytosis and Transneuronal degeneration in chronic stroke. Stroke. 2022;53(7):2377‐2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Liesz A, Suri‐Payer E, Veltkamp C, et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009;15(2):192‐199. [DOI] [PubMed] [Google Scholar]
- 84. Wang HY, Ye JR, Cui LY, Chu SF, Chen NH. Regulatory T cells in ischemic stroke. Acta Pharmacol Sin. 2022;43(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Dombrowski Y, O'Hagan T, Dittmer M, et al. Regulatory T cells promote myelin regeneration in the central nervous system. Nat Neurosci. 2017;20(5):674‐680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Shi L, Sun Z, Su W, et al. Treg cell‐derived osteopontin promotes microglia‐mediated white matter repair after ischemic stroke. Immunity. 2021;54(7):1527‐1542 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Yu F, Wang Y, Stetler AR, Leak RK, Hu X, Chen J. Phagocytic microglia and macrophages in brain injury and repair. CNS Neurosci Ther. 2022;28(9):1279‐1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lyu J, Xie D, Bhatia TN, Leak RK, Hu X, Jiang X. Microglial/macrophage polarization and function in brain injury and repair after stroke. CNS Neurosci Ther. 2021;27(5):515‐527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Yu HH, Ma XT, Ma X, et al. Remote limb ischemic Postconditioning protects against ischemic stroke by promoting regulatory T cells thriving. J Am Heart Assoc. 2021;10(22):e023077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. McDonald MW, Dykes A, Jeffers MS, et al. Remote ischemic conditioning and stroke recovery. Neurorehabil Neural Repair. 2021;35(6):545‐549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Poalelungi A, Tulba D, Turiac E, Stoian D, Popescu BO. Remote ischemic conditioning may improve disability and cognition after acute ischemic stroke: a pilot randomized clinical trial. Front Neurol. 2021;12:663400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Landman TRJ, Schoon Y, Warle MC, Meijer FJA, de Leeuw FE, Thijssen DHJ. The effect of repeated remote ischemic postconditioning after an ischemic stroke (REPOST): a randomized controlled trial. Int J Stroke. 2022;14:17474930221104710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Xu J, Zhang Q, Rajah GB, et al. Daily remote ischemic conditioning can improve cerebral perfusion and slow arterial progression of adult Moyamoya disease‐a randomized controlled study. Front Neurol. 2021;12:811854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Huang J, Xu D, Guo Q, et al. Remote ischemic Postconditioning improves myocardial dysfunction via the risk and safe pathways in a rat model of severe hemorrhagic shock. Shock. 2018;49(4):460‐465. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.


