Abstract
Purpose
To evaluate the impact of an optimal and reproducible cutoff value set according to a predefined lymphopenia scale as an early predictor of in-hospital mortality and other outcomes in patients hospitalized with pneumococcal pneumonia and positive urinary antigen at admission to the emergency department.
Methods
An observational cohort study was conducted based on analysis of a prospective registry of consecutive immunocompetent adults hospitalized for pneumococcal pneumonia in two tertiary hospitals. Generalized additive models were constructed to assess the smooth relationship between in-hospital mortality and lymphopenia.
Results
We included 1173 patients. Lymphopenia on admission was documented in 686 (58.4%). No significant differences were observed between groups regarding the presence of comorbidities. Overall, 299 (25.5%) patients were admitted to intensive care and 90 (7.6%) required invasive mechanical ventilation. Fifty-nine (5%) patients died, among them 23 (38.9%) in the first 72 h after admission. A lymphocyte count < 500/μL, documented in 282 (24%) patients, was the predefined cutoff point that best predicted in-hospital mortality. After adjustment, these patients had higher rates of intensive care admission (OR 2.9; 95% CI 1.9–4.3), invasive mechanical ventilation (OR 2.2; 95% CI 1.2–3.9), septic shock (OR 1.8; 95% CI 1.1–2.9), treatment failure (OR 2.1; 95% CI 1.2–3.5), and in-hospital mortality (OR 2.2; 95% 1.1–4.9). Severe lymphopenia outperformed PSI score in predicting early and 30-day mortality in patients classified in the higher-risk classes.
Conclusion
Lymphocyte count < 500/μL could be used as a reproducible predictor of complicated clinical course in patients with an early diagnosis of pneumococcal pneumonia.
Keywords: Early mortality, Lymphopenia, Pneumococcal pneumonia, Streptococcus pneumoniae, Community-acquired pneumonia
Introduction
Community-acquired pneumonia (CAP) remains one of the leading causes of hospitalization and places a burden on healthcare systems worldwide [1, 2]. It is currently the most common infectious cause of death in the developed world despite advances in antibiotic treatments [3]. Clinical deterioration occurs early after hospital admission with pneumonia [4]. For this reason, failure to triage the severity of illness and consequent allocation of an inappropriate site of care, including late ICU admission, may lead to worse outcomes [5]. Though current guidelines recommend routine risk stratification using various prediction rules, the detection of patients at high risk of early clinical deterioration by current scores remains suboptimal.
The prognosis of a patient with CAP depends on the virulence of the pathogen responsible, early administration of appropriate antibiotic therapy and the provision of adequate support measures, as well as the characteristics of the patient, among them, the host’s ability to respond to the infection. Recently, the concept of lymphopenic pneumonia has been described, referring to immunocompromised patients with pneumonia who have a low lymphocyte count in a complete blood count performed at the time of diagnosis. This subgroup of patients may have a poorer prognosis and higher mortality [6]. Nonetheless, studies on this topic have been based on the analysis of data from general series including patients with different etiologies of pneumonia [6–9]. Moreover, lymphocyte-count cutoffs defined previously have varied between the populations studied [6–9]. This makes it difficult to extrapolate the concept to other populations and apply it in clinical practice.
Streptococcus pneumoniae is the most commonly isolated pathogen, being responsible for the highest rates of hospital admission and mortality [10, 11]. The pneumococcal urinary antigen test (PUAT) is a non-invasive method with moderate sensitivity and high specificity, and its results are obtained in less than 1 h, helping to achieve an early diagnosis of pneumococcal CAP at hospital admission [12]. The prognostic ability of lymphocyte count on admission in this specific group, namely, patients with pneumococcal pneumonia, has not been investigated. At this point, it could be hypothesized that early information concerning pathogen–host immune system interaction could serve as a complementary tool in the risk stratification of these patients seeking to improve the process of care and outcome.
Given this, the objective of our study was to assess whether severe lymphopenia on admission (according to a predefined and standardized scale) is a reliable predictor of clinical outcomes in this subgroup of immunocompetent patients with an early diagnosis of pneumococcal CAP based on a positive PUAT at admission.
Material and methods
Study design and population
This was an observational study based on the analysis of a prospective registry of consecutive immunocompetent adults (age 18 years or more) hospitalized for pneumococcal pneumonia in two tertiary medical centers (Cruces and Galdakao Hospitals). The study was conducted between January 2002 and December 2020. The bacteriological diagnosis of pneumococcal pneumonia was based on the results of PUAT and/or blood culture. For the purpose of the study, we limited the analysis to consecutive patients who had a positive pneumococcal urinary antigen testing performed at admission to the emergency department. The PUAT was performed by analyzing concentrated urine samples with an immunochromatographic membrane assay (Binax Inc., Scarborough, ME) [13]. Participants were stratified according to the presence of lymphopenia in a blood sample drawn at the time of admission. Lymphopenia was defined as a peripheral blood lymphocyte cell count of less than 1000/μL measured using an automatic analyzer at our central laboratory. The grade of lymphopenia was evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE) [14]: (1) mild (1000–800 lymphocytes/μL); (2) moderate (799–500 lymphocytes/μL); (3) severe (< 500 lymphocytes/μL).
Patients were excluded if they had polymicrobial infection (detection of both S. pneumoniae and another pathogen), had been hospitalized at any point in the 14 days before the diagnosis of pneumonia, or had been diagnosed with pneumonia in the previous 3 months.
The study protocol was approved by the Drug Research Ethics Committee of the Basque Country (Comité de Etica de Investigación con Medicamentos de Euskadi, approval reference number: EPA2019043).
Data collection
Since 2002, there has been an ongoing standardized prospective registry of all patients hospitalized for pneumonia in our two hospitals. For eligible patients, we collected data on sociodemographic characteristics, comorbidities, influenza and pneumococcal vaccination status, vital signs, results of routine laboratory tests and radiological findings on admission. The severity of patients’ clinical condition was assessed on admission using the Pneumonia Severity Index (PSI) [15].
Measures of in-hospital clinical course and outcome included: (1) admission to the intensive care unit (ICU); (2) use of invasive mechanical ventilation (IMV); (3) septic shock; (4) treatment failure; and (5) in-hospital mortality. Patients were empirically treated in accordance with the current National Guidelines of the Spanish Society of Pulmonology (SEPAR) at the discretion of the attending doctor [16].
Objectives
The primary objective was to explore the existence of an optimal and reproducible cutoff value set according to a predefined lymphopenia scale as a reliable predictor of in-hospital course and outcome in a consecutive cohort of immunocompetent patients hospitalized with an early diagnosis of pneumococcal CAP based on urinary antigen testing at admission. The secondary objective was to evaluate the ability of severe lymphopenia in addition to PSI score to predict early mortality during hospitalization of this group of patients.
Other definitions
Pneumonia was defined as the presence of new pulmonary infiltrate on a chest X-ray together with signs and symptoms suggestive of lower respiratory tract infection. A patient was considered immunocompetent if they did not have a history of any of the following before hospital admission: primary immunodeficiencies, human immunodeficiency virus infection, leukemia, myeloproliferative or lymphoproliferative syndromes, monoclonal gammopathies, solid organ transplantation, treatment with > 10 mg/day of prednisone (for more than 2 weeks or equivalent in the 3 months before admission), chemotherapy during the last 3 months or other immunosuppressive drugs [17].
Septic shock was defined as systolic blood pressure of less than 90 mm Hg and a need for vasopressors for at least 4 h after fluid therapy [18].
Treatment was considered to have failed when patients’ clinical condition worsened during their hospital stay with: hemodynamic instability; appearance or worsening of respiratory failure; a need for IMV; progression of the pneumonia, as indicated by radiological findings, or the appearance of a new focus of infection; or persistence or reappearance of fever, if a change of treatment was required [19].
Early mortality was defined as death due to any cause in the first 72 h after hospitalization [20].
Statistical analysis
Descriptive analysis was undertaken, using frequencies and percentages, means and standard deviations (SDs) or medians and interquartile ranges (IQRs) depending on the distribution of the data. Shapiro–Wilks test and Q–Q plots were used to determine whether continuous variables were normally distributed. Statistical differences between lymphopenia groups were assessed using Student’s t test or one-way analysis of variance (ANOVA) in the case of normally distributed variables and Mann–Whitney or Kruskal–Wallis tests otherwise. The p values were adjusted for multiple testing using the Tukey method if data were normally distributed and the Benjamini and Hochberg method otherwise. Categorical variables were expressed as percentages. Comparisons were taken with Chi-square or Fisher’s exact tests as appropriate.
The smooth relationship between severity of lymphopenia, according to the CTCAE classification, and in-hospital mortality was analyzed using generalized additive models. This statistical technique allowed us to graphically display this relationship, and hence, a cutoff point that best predicted in-hospital mortality was selected considering the point at which the smooth curve crossed the x-axis.
Univariate logistic regression models were used to compare in-hospital course and clinical outcomes between the groups. Then, multivariate logistic regression models were built including clinically relevant variables with p < 0.05 in the univariate analysis as potential predictors. The results are reported as odds ratios (ORs) and 95% confidence intervals (CIs), taking patients with lymphocyte count ≥ 500/μL as the reference group.
Patient survival was analyzed using Kaplan–Meier curves. The log-rank test was used to compare survival between subgroups. Hazard ratios (HRs) and the corresponding 95% CIs for early mortality were estimated by multivariate Cox regression. The ability of lymphocyte count < 500/μL to predict early and 30-day mortality in patients with PSI score > 3 was assessed by calculating the area under the receiver operating characteristic curves (AUC).
All analyses were performed with the statistical software R (version 4.1.2): A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
Results
Over the study period, 1389 patients with pneumococcal pneumonia were assessed for eligibility. After applying the exclusion criteria, 1173 were eligible for the analysis. Overall, lymphopenia at admission was detected in 686 patients (58.4%) (Fig. 1).
Fig. 1.
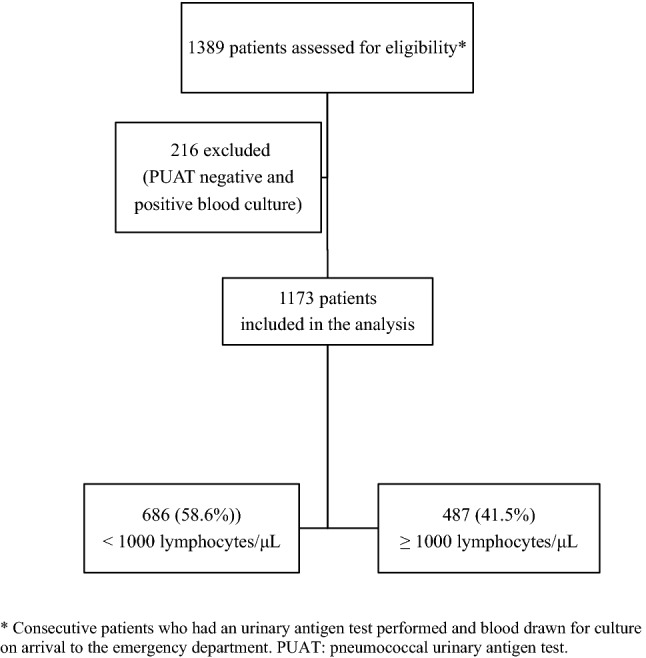
Flow of patients admitted with pneumococcal pneumonia through the study
The mean age of the entire cohort was 64.1 (17.3 SD) years, 655 patients (55.8. %) being ≥ 65 years. Table 1 summarizes the demographic and clinical features of the entire cohort by the presence or absence of lymphopenia, stratifying patients into three groups according to the lymphocyte count measured in a blood sample drawn at the time of admission. Severe lymphopenia was observed in 282 (24%) patients. These patients were more likely to be heavy drinkers, present multilobar involvement on chest X-ray and have higher levels of C-reactive protein and less likely to have received pneumococcal vaccination or antibiotic prescription before hospital admission. Overall, no statistically significant differences were observed between groups regarding either the presence of medical comorbidities or the mean number of days with symptoms prior to hospital admission. All patients received at least one dose of antibiotic treatment during their emergency department stay. A greater proportion of lymphopenic patients were classified in the higher-risk classes according to PSI score (P < 0.001).
Table 1.
Demographic and clinical characteristics of patients with pneumococcal pneumonia stratified by the presence or absence of lymphopenia (based on lymphocyte count on admission)
| Grade of lymphopenia | |||||||
|---|---|---|---|---|---|---|---|
| ≥ 1000 lymphocytes/μL (n = 487) |
< 1000 lymphocytes/μL (n = 686) |
p | Mild (1000–800 lymphocytes/μL) (n = 132) |
Moderate (799–500 lymphocytes/μL) (n = 272) |
Severe (< 500 lymphocytes/μL) (n = 282) |
p* | |
| Demographic variables | |||||||
| Male sex | 271 (57) | 427 (62.2) | 0.027 | 84 63.6.) | 164 (60.3) | 179 (63.5) | 0.119 |
| Age ≥ 65 years | 280 (57.5) | 375 (54.7) | 0.367 | 79 (59.8) | 157 (57.7) | 139 (49.3) | 0.082 |
| Nursing home resident | 14 (2.8) | 17 (2.4) | 0.816 | 3 (2.2) | 7 (2.6) | 7 (2.5) | 0.993 |
| Current smoker | 130 (26.8) | 171 (25.1) | 0.550 | 32 (24.6) | 66 (24.4) | 73 (26) | 0.885 |
| Heavy drinker | 47 (9.9) | 94 (14.2) | 0.004 | 14 (10.9) | 29 (11.1) | 51 (18.8) | 0.003 |
| Underlying conditions | |||||||
| Arterial hypertension | 193 (39.6) | 277 (40.5) | 0.812 | 65 (49.2) | 104 (38.2) | 108 (38.6) | 0.151 |
| Hyperlipidemia | 139 (28.5) | 139 (24.7) | 0.161 | 33 (25) | 77 (28.3) | 59 (21.1) | 0.117 |
| Chronic renal disease | 18 (3.7) | 37 (5.3) | 0.224 | 4 (3) | 18 (6.6) | 15 (5.3) | 0.221 |
| Chronic obstructive pulmonary disease | 99 (20.3) | 135 (19.7) | 0.841 | 28 (21.2) | 58 (21.3) | 49 (17.4) | 0.644 |
| Diabetes mellitus | 84 (17.2) | 114 (16.6) | 0.846 | 27 (20.5) | 45 (16.6) | 42 (14.9) | 0.561 |
| Cerebrovascular disease | 26 (5.3) | 44 (6.4) | 0.522 | 8 (6) | 17 (6.2) | 19 (6.7) | 0.877 |
| Congestive heart disease | 43 (8.8) | 72 (10.4) | 0.823 | 14 (10.6) | 31 (11.4) | 27 (9.5) | 0.705 |
| Cancer | 22 (4.5) | 33 (4.8) | 0.925 | 4 (3) | 15 (5.5) | 14 (4.9) | 0.727 |
| Liver disease | 16 (3.2) | 34 (4.9) | 0.214 | 3 (2.2) | 15 (5.5) | 16 (5.6) | 0.186 |
| Vaccination status | |||||||
| Influenza vaccine | 147 (31.5) | 186 (28.4) | 0.303 | 44 (34.4) | 75 (28.6) | 67 (25.4) | 0.208 |
| Pneumococcal vaccination | 74 (15.8) | 72 (11) | 0.022 | 17 (13.3) | 33 (12.6) | 22 (8.2) | 0.035 |
| Clinical characteristics at admission | |||||||
| Median number of days with symptoms prior to hospital admission (IQR) | 3 (2–5.7) | 3 (2–5) | 0.72 | 3 (2–5) | 4 (2–5) | 3 (2–5) | 0.97 |
| Prior antibiotic treatment | 71 (15) | 36 (5.3) | < 0.001 | 7 (5.3) | 15 (5.6) | 14 (5) | < 0.001 |
| Body temperature < 35 or > 40ºC | 7 (1.4) | 7 (1) | 0.708 | 2 (1.5) | 2 (0.7) | 3 (1.1) | 0.840 |
| Altered mental status | 42 (8.6) | 75 (10.9) | 0.230 | 14 (10.6) | 26 (9.5) | 35 (12.4) | 0.397 |
| Systolic blood pressure < 90 mm Hg | 33 (6.7) | 78 (11.4) | 0.011 | 19 (14.4) | 25 (9.2) | 34 (12.1) | 0.018 |
| Respiratory rate ≥ 30/minute | 87 (18) | 170 (25) | 0.006 | 29 (22.1) | 55 (20.4) | 86 (30.8) | 0.001 |
| Laboratory and radiological findings | |||||||
| BUN ≥ 30 mg/dL | 165 (33.9) | 303 (44.2) | < 0.001 | 53 (40.2) | 106 (39) | 144 (51.1) | < 0.001 |
| PaO2 < 60 mm Hg | 182 (45.6) | 335 (56.4) | 0.001 | 55 (50) | 125 (51.9) | 155 (63.8) | < 0.001 |
| Glucose > 250 mg/dL | 32 (6.5) | 58 (8.4) | 0.279 | 13 (9.8) | 21 (7.7) | 24 (8.5) | 0.573 |
| Hematocrit < 30% | 15 (3.1) | 30 (4.3) | 0.326 | 5 (3.8) | 9 (3.3) | 16 (5.6) | 0.315 |
| Blood pH < 7.35 | 22 (5.5) | 49 (8.2) | 0.137 | 8 (7.2) | 16 (6.7) | 25 (10.2) | 0.168 |
| Sodium < 130 MEq/L | 30 (6.1) | 58 (8.4) | 0.175 | 8 (6) | 21 (7.7) | 29 (10.3) | 0.185 |
| CRP ≥ 15 mg/dL | 226 (65) | 380 (75) | 0.002 | 76 (76) | 130 (65) | 174 (84.1) | < 0.001 |
| Multilobar pneumonia | 125 (25.7) | 238 (34.7) | 0.001 | 30 (22.7) | 88 (32.4) | 120 (42.7) | < 0.001 |
| Pleural effusion | 55 (11.3) | 75 (10.9) | 0.921 | 9 (6.8) | 25 (9.2) | 41 (14.5) | 0.076 |
| Severity of illness at admission | |||||||
| PSI risk class > 3 | 210 (43.1) | 381 (55.5) | < 0.001 | 70 (53) | 144 (52.9) | 167 (59.2) | < 0.001 |
IQR interquartile range, BUN blood urea nitrogen, CRP C-reactive protein, PSI Pneumonia Severity Index
p* lymphocytes count ≥ 1000/μL versus grade of lymphopenia.
Overall, 299 (25.5%) patients were admitted to an ICU and 90 (7.6%) required IMV. Further, 59 (5%) patients died, 23 (38.9%) in the first 72 h after admission.
Figure 2 shows the generalized additive models constructed to assess the smooth relationship between lymphopenia levels and in-hospital mortality. A lymphocyte count of less than 500/μL was the cutoff point, from the CTCAE classification, that best predicted in-hospital mortality.
Fig. 2.
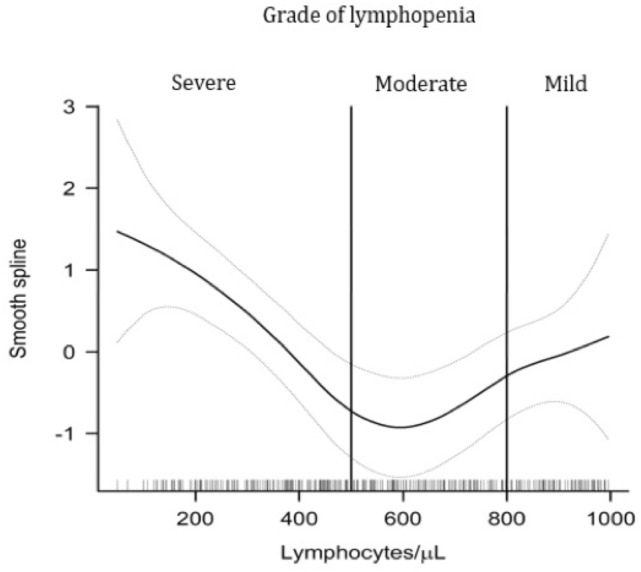
Plot of estimated smooth spline function with 95% confidence interval showing relationship between severe lymphopenia and in-hospital mortality
Table 2 presents the in-hospital mortality and other outcomes in the group of patients with severe lymphopenia. In the adjusted multivariate analysis, patients with a lymphocyte count of less than 500/μL on admission were more likely to be admitted to the ICU (OR 2.9; 95% CI 1.9–4.3; p < 0.001), require IMV (OR 2.2; 95% CI 1.2–3.9; p < 0.001), develop septic shock (OR 1.8; 95% CI 1.1–2.9; p < 0.001) and experience treatment failure (OR 2.1; 95% CI 1.2–3.5; p < 0.001) as well as having a higher in-hospital mortality rate (OR 2.2; 95% 1.1–4.9; p < 0.04).
Table 2.
In-hospital course and outcomes of patients with severe lymphopenia on admission
| ≥ 500 lymphocytes/μL (n = 891) |
< 500 lymphocytes/μL (n = 282) |
p | Unadjusted OR (95% CI) | p | Adjusted* OR (95% CI) | p | |
|---|---|---|---|---|---|---|---|
| ICU | 168 (18.9%) | 131 (46.6%) | < 0.001 | 3.7 (2.8–5) | < 0.001 | 2.9 (1.9–4.3) | < 0.001 |
| IMV | 39 (4.3%) | 51 (18.1%) | < 0.001 | 4.8 (3.1–7.5) | < 0.001 | 2.2 (1.2–3.9) | 0.008 |
| Septic shock | 78 (8.7%) | 66 (23.4%) | < 0.001 | 3.1 (2.2–4.5) | < 0.001 | 1.8 (1.1–2.9) | 0.012 |
| Treatment failure | 75 (8.6%) | 60 (21.7%) | < 0.001 | 2.9 (2–4.2) | < 0.001 | 2.1 (1.2–3.5) | 0.003 |
| In-hospital mortality | 29 (3.2%) | 30 (10.6%) | < 0.001 | 3.5 (2.1- 6) | < 0.001 | 2.2 (1.1–4.9) | 0.045 |
ICU intensive care unit, IMV invasive mechanical ventilation, OR odds ratio, CI confidence interval
*Adjusted by heavy drinker, pneumococcal vaccination, prior antibiotic treatment, PSI risk class > 3, CRP ≥ 15 mg/dL, multilobar pneumonia
Figure 3 shows Kaplan–Meier survival curves plotted as a function of lymphocyte count. These curves demonstrate marked differences in 30-day survival in patients with severe lymphopenia on admission (log-rank test < 0.001).
Fig. 3.
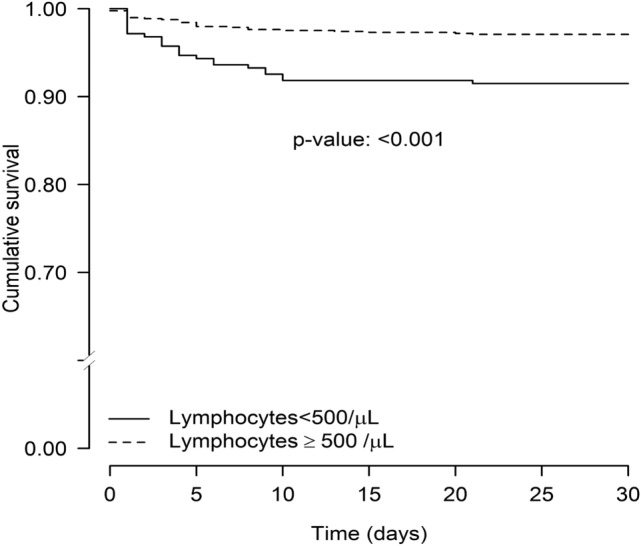
Kaplan–Meier survival curves for 30-day mortality in immunocompetent patients with pneumococcal pneumonia stratified by severe lymphopenia on admission
Figure 4 shows Kaplan–Meier survival curves plotted as a function of lymphocyte count (dichotomized to < 500/μL vs. ≥ 500/μL) and PSI score (dichotomized to ≤ 3 vs. > 3). Severe lymphopenia in patients with PSI score > 3 were significantly associated with higher early mortality (p = 0.02). Furthermore, patients with a lymphocyte count of less than 500/μL had significantly higher 30-day mortality in both low and high PSI risk classes (p = 0.004 and p = 0.005, respectively). Patients with lymphocyte count of less than 500/μL in addition to PSI score > 3 presented higher AUC for the prediction of both, early (0.793 vs 0.730) and 30-day mortality (0.790 vs 0.738) (Table 3).
Fig. 4.
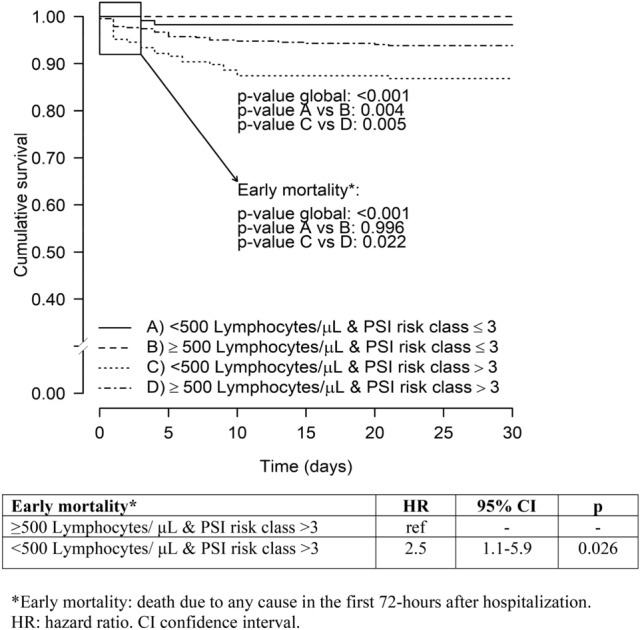
Kaplan–Meier survival curves for early and 30-day mortality plotted as a function lymphocyte count and PSI score on admission
Table 3.
Area under the receiver operating characteristics curve (AUC) for PSI score > 3 and PSI score > 3 in addition to lymphocyte count < 500/μL for predicting early and 30-day mortality
| Early mortality* AUC (95% CI) | 30-day mortality AUC (95% CI) | |
|---|---|---|
| PSI score > 3 | 0.730 (0.686–0.776) | 0.738 (0.708–0.769) |
| PSI score > 3 + lymphocytes < 500/μL | 0.793 (0.733–0.854) | 0.790 (0.750–0.832) |
CI confidence interval
*Early mortality: death due to any cause in the first 72 h after hospitalization
Discussion
This study provides a comprehensive evaluation of the role of lymphopenia as a prognostic factor in a consecutive series of immunocompetent hospitalized patients with an early diagnosis of pneumococcal pneumonia at the time of presentation to the emergency department. The main findings of this large prospective cohort study were: (1) nearly 25% of patients with pneumococcal pneumonia had severe lymphopenia on admission and were at increased risk of ICU admission, IMV, septic shock, treatment failure and in-hospital mortality. (2) A predefined cutoff point of 500/μL set according to a well-known classification could be used as a standardized value to predict mortality. (3) The finding of a lymphocyte count of less than 500/μL in addition to PSI score could reliably predict early mortality on admission.
The main strength of our study lies in the study population itself, namely, a large prospective sample of unselected immunocompetent patients and the reproducibility of the design, based on the current clinical management of these patients in the real world. Specifically, we only included immunocompetent patients with pneumococcal pneumonia diagnosed early based on testing positive in a urinary antigen test performed on emergency department arrival. In addition, we have evaluated the role of lymphopenia according to a predefined classification [14]. We consider that these factors enhance the reproducibility and strengthen the clinical applicability of our results.
Similar to other studies including patients with CAP in general, we have found that lymphopenia on admission is common in immunocompetent patients with pneumococcal pneumonia [6, 7, 9]. The role of lymphopenia as a predictor of mortality has been recognized in patients with SARS-CoV-2 pneumonia [21]. On the other hand, compared to patients with SARS-CoV-2 pneumonia, patients with bacteremic pneumococcal CAP were found to have a lower lymphocyte count on admission, even among those subsequently requiring ICU admission [22]. To our knowledge, this is the first study that has evaluated the importance of lymphopenia as a biomarker focusing on the subgroup of patients with an early diagnosis of pneumococcal pneumonia.
Over half of all CAP cases lack an etiological diagnosis [2]. Early identification of the etiological agent allows clinicians to adapt the antimicrobial management for individual patients enabling prompt adequate treatment and likely limiting the development of antibiotic resistance. Indications for microbiological testing in patients with CAP are controversial [23]. Considering that Streptococcus pneumoniae is the pathogen most frequently identified in patients requiring hospitalization, several national guidelines currently recommend UAT performance in this population [16, 24]. This diagnostic approach has been associated with lower mortality in patients in a more severe condition [25].
It remains unclear whether severe lymphopenia is a cause or an epiphenomenon secondary to the severity of the pneumonia [26]. Furthermore, patients with pneumococcal pneumonia have a higher level of proinflammatory cytokine production than those with CAP caused by other microorganisms [27]. In this context, our results could suggest that lymphopenia itself is a consequence of the inflammatory response associated with the severity of this clinical condition. This idea can be considered to be in line with a previous report showing that lymphopenic patients with severe CAP and a high inflammatory response present increases in lymphocyte count after receiving corticosteroid treatment [28]. On the other hand, we have observed that once severe lymphopenia has developed, patients have a higher probability of a poor clinical course regardless of the severity of their clinical condition as assessed by PSI score and other parameters. It could be hypothesized that lymphopenia may lead to dysregulation of the immune–host response to the infection. If so, severe lymphopenia may be interpreted as a surrogate marker of host–pathogen immune interaction that may have implications for use of immunomodulatory therapy. Future multicenter studies are required to clarify this issue.
In contrast to our study, other authors have evaluated the prognostic utility of this biomarker using different cutoff points based on the characteristics of the specific population under study [6–9]. Our study shows that a predefined lymphocyte count of less than 500/μL could be used as a reproducible value for predicting complicated clinical course. We consider that these results could facilitate the adoption of this parameter and even its incorporation into the risk stratification scales as an indicator of sepsis-related organ dysfunction.
Identification of patients with CAP at risk of early mortality continues to be a challenge in the initial management of this condition. Compared to later deaths, it has been classically accepted that early deaths are less dependent on antibiotic efficacy [29]. In line with this, our study population only included patients with a prompt etiological diagnosis which facilitated the prescription of guideline-concordant antibiotics shortly after admission. Furthermore, a lymphocyte count of less than 500/μL provides complementary information to that offered by PSI score and allows us to identify a subgroup of patients at high risk of death soon after admission. These observations are compatible with a possible role of immune system dysfunction in the prompt outcome of these patients. Interestingly, we found that patients with a normal lymphocyte count or less severe lymphopenia were at low risk of treatment failure. Further, treatment fails in 11–16% of cases and this has been associated with increased mortality in patients hospitalized for pneumonia [18, 30, 31]. Although leukopenia has been previously identified as a risk factor, our results highlight the potential role of lymphocytes in patients failing to respond adequately to treatment [19]. Lymphocyte count could represent an easy-to-perform and reproducible severity marker to alert clinicians to the need for close monitoring and intensive management.
We recognize that our study has some limitations: (1) We cannot exclude a lower sensitivity of conventional PUAT used in this study compared to serotype-specific urinary antigen detection assays for detecting pneumococcal CAP [32, 33]. However, this test is not available in the routine clinical practice. (2) This was an observational study and the study population was restricted to patients in which urinary antigen tests had been performed. On the other hand, this limitation could be considered a strength because this restricted group represents a well-defined cohort of patients and enhances the external validity of this study. (3) We have not evaluated lymphocyte subpopulations. Although such data could be useful for evaluating possible mechanisms of interaction between the host immune system and pneumococci, they are not routinely assessed in the real-world care of patients with CAP. (4) Finally, this study was conducted in two hospitals in the same geographical area and health system, and hence, it may not be possible to extrapolate the results to other areas with different diagnostic protocols.
Despite these limitations, our findings have important implications. Clinicians need to be aware of the importance of a simple biomarker such as lymphocyte count and its role in in-hospital outcome in these patients. A better knowledge of this biomarker may help identify patients at risk of a complicated clinical course warranting strict monitoring during their hospital stay.
In conclusion, early performance of pneumococcal urinary antigen and lymphocyte count in immunocompetent patients with CAP could help to stratify severity on emergency department arrival and could help to adjust the clinical management of this condition appropriately during hospitalization. Identifying a predefined cutoff value for lymphopenia with prognostic implications is important to underline the clinical applicability of this biomarker.
Author contributions
LAR and LS take equally the responsibility of the manuscript as a whole. LAR, LS, SP, SC, AU, AU, PPE and RZ conceived and designed the study. LAR, LS, SC, AU, AG, AU AA, enrolled patients and collected and compiled data. SP performed the statistical analysis. LAR, LS, SP, SC, AU, AA, AG, PPE and RZ analyzed and interpreted the data. LAR, LS, SP and RZ wrote the manuscript, which was critically reviewed and revised by SC, AU, AA, AG, AU and PPE. All authors have read and approved the final manuscript.
Funding
This research does not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Data availability statement
Data are available on reasonable request to the corresponding author.
Declarations
Conflict of interest
All authors have no conflict of interest related to this publication.
Ethics approval
The study protocol was approved by the Drug Research Ethics Committee of the Basque Country (Comité de Etica de Investigación con Medicamentos de Euskadi, approval reference number: EPA2019043).
Footnotes
Luis A. Ruiz and Leyre Serrano have contributed equally to this work.
References
- 1.Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax. 2012;67:71–79. doi: 10.1136/thx.2009.129502. [DOI] [PubMed] [Google Scholar]
- 2.Feldman C, Anderson R. Community-acquired pneumonia. Still a major burden of disease. Curr Opin Crit Care. 2016;22:477–484. doi: 10.1097/MCC.0000000000000340. [DOI] [PubMed] [Google Scholar]
- 3.GBD 2019 Diseases and Injuries Collaborators Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ewig S, Birkner N, Strauss R, et al. New perspectives on community-acquired pneumonia in 388.406 patients. Results from a nationwide mandatory performance measurement programme in healthcare quality. Thorax. 2009;64:1062–1069. doi: 10.1136/thx.2008.109785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phua J, Ngerng WJ, Lim TK. The impact of a delay in intensive care unit admission for community-acquired pneumonia. Eur Respir J. 2010;36:826–833. doi: 10.1183/09031936.00154209. [DOI] [PubMed] [Google Scholar]
- 6.Bermejo-Martin JF, Cilloniz C, Mendez R, for the NEUMONAC group et al. Lymphopenic community-acquired pneumonia (L-CAP), an immunological phenotype associated with higher risk of mortality. EBioMedicine. 2017;24:231–236. doi: 10.1016/j.ebiom.2017.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cilloniz C, Peroni HJ, Gabarrus A, et al. Lymphopenia is associated with poor outcomes of patients with community-acquired pneumonia and sepsis. OFID. 2021 doi: 10.1093/ofid/ofab169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ceccato C, Panagiotarakou M, Ranzani OT, et al. Lymphocytopenia as a predictor of mortality in patients with ICU-acquired Pneumonia. J Clin Med. 2019;8:848. doi: 10.3390/jcm8060843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Güell E, Martin-Fernandez M, de la Torre MC, et al. Impact of lymphocyte and neutrophil counts on mortality risk in severe community-acquired pneumonia with or without septic shock. J Clin Med. 2019;8:754. doi: 10.3390/jcm8050754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cilloniz C, Ewig S, Polverino E, et al. Microbial etiology of community-acquired pneumonia and its relation to severity. Thorax. 2011;66:340–346. doi: 10.1136/thx.2010.143982. [DOI] [PubMed] [Google Scholar]
- 11.Feldman C, Anderson R. The role of streptococcus pneumonia in community-acquired pneumonia. Semin Respir Crit Care Med. 2016;37:806–818. doi: 10.1055/s-0036-1592074. [DOI] [PubMed] [Google Scholar]
- 12.Molinos L, Zalacain R, Menendez R, et al. Sensitivity, specificity and positivity predictors of the pneumococcal urinary antigen test in community-acquired pneumonia. Ann Am Thorac Soc. 2015;12:1482–1489. doi: 10.1513/AnnalsATS.201505-304OC. [DOI] [PubMed] [Google Scholar]
- 13.Dominguez J, Gali N, Blanco S, et al. Detection of Streptococcus pneumoniae antigen by a rapid immunochromatographic assay in urine samples. Chest. 2001;119:243–249. doi: 10.1378/chest.119.1.243. [DOI] [PubMed] [Google Scholar]
- 14.U.S. National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. [(accessed on 1 April 2022)]; 2017 Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf.
- 15.Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 16.Menéndez R, Torres A, Aspa J, et al. Community-acquired pneumonia. New guidelines of the Spanish Society of Chest Diseases and Thoracic Surgery (SEPAR) Arch Bronconeumol. 2010;46:543–558. doi: 10.1016/j.arbres.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 17.Di Pasquale MF, Sotgiu G, Gramegna A, et al. Prevalence and etiology of community-acquired pneumonia in immunocompromised patients. Clin Infect Dis. 2019;68:1482–1493. doi: 10.1093/cid/ciy723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levy MM, Fink M, Marshall JC, et al. 2001 SCCM/ESICM/ATS/SIS international sepsis definitions conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 19.Menéndez R, Torres A, Zalacain R, et al. Risk factors to treatment failure in community acquired pneumonia: implications for disease outcome. Thorax. 2004;59:960–965. doi: 10.1136/thx.2003.017756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cilloniz C, Liapikou A, Martin-Loeches I, et al. Twenty-year trend in mortality among hospitalized patients with pneumococcal community-acquired pneumonia. PLoS ONE. 2018;13:e0200504. doi: 10.1371/journal.pone.0200504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng Z, Peng F, Xu B, Zhao J, et al. Risk factors of critical and mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020;81:e16–25. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serrano Fernández L, Ruiz Iturriaga LA, España Yandiola PP, et al. Bacteraemic pneumococcal pneumonia and SARS-CoV-2 pneumonia: differences and similarities. Int J Infect Dis. 2022;115:39–47. doi: 10.1016/j.ijid.2021.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murdoch DR. Indications for microbiological testing in pneumonia: Which patients should be tested? Clin Infect Disease. 2019;68:2034–2035. doi: 10.1093/cid/ciy829. [DOI] [PubMed] [Google Scholar]
- 24.Lim WS, Baudouin SV, George RC, et al. BTS guidelines for the management of community-acquired pneumonia in adults: update 2009. Thorax. 2009;69:iii1–iii55. doi: 10.1136/thx.2009.121434. [DOI] [PubMed] [Google Scholar]
- 25.Constantini E, Allara E, Patrucco F, et al. Adherence to guidelines for hospitalized community-acquired pneumonia over time and its impact on health outcomes and mortality. Intern Emerg Med. 2016;11:929–940. doi: 10.1007/s11739-016-1445-3. [DOI] [PubMed] [Google Scholar]
- 26.Méndez R, Menéndez R, Amara-Elori I, et al. Lymphopenic community-acquired pneumonia is associated with a dysregulated immune response and increased severity and mortality. J Infect. 2019;78:423–431. doi: 10.1016/j.jinf.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Enderman H, Meijivis SCA, Rijkers GT, et al. Systemic cytokine response in patients with community-acquired pneumonia. Eur Resp J. 2011;37:1431–1438. doi: 10.1183/09031936.00074410. [DOI] [PubMed] [Google Scholar]
- 28.Torres A, Ceccato A, Ferrer M, et al. Effect of corticosteroids on C-reactive protein in patients with severe community acquired pneumonia and high inflammatory response: the effect of lymphopenia. J Clin Med. 2019;8:1461. doi: 10.3390/jcm8091461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia-Vidal C, Fernanadez-Sabé N, Carratalá J, et al. Early mortality in patients with community-acquired pneumonia: causes and risk factors. Eur Respir J. 2008;32:733–739. doi: 10.1183/09031936.00128107. [DOI] [PubMed] [Google Scholar]
- 30.Gennè D, Kaiser L, Kinge TN, et al. Community-acquired pneumonia: causes of treatment failure in patients enrolled in clinical trials. Clin Microbiol Infect. 2003;9:949–954. doi: 10.1046/j.1469-0691.2003.00679.x. [DOI] [PubMed] [Google Scholar]
- 31.Arancibia F, Ewig S, Martinez JA. Antimicrobial treatment failures in patients with community-acquired pneumonia: causes and prognostic implications. Am J Crit Care Med. 2000;162:154–160. doi: 10.1164/ajrccm.162.1.9907023. [DOI] [PubMed] [Google Scholar]
- 32.Shoji H, Domenech A, Simonetti AF, et al. The Alere BinaxNow pneumococcal urinary antigen test: diagnostic sensitivity for adult pneumococcal pneumonia and relationship to specific serotypes. J Clin Microbiol. 2018;56:e00787–e817. doi: 10.1128/JCM.00787-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forstner C, Kolditz M, Kessemeier M, et al. Pneumococcal conjugate serotype distribution and predominating role of serotype 3 in German adults with community-acquired pneumonia. Vaccine. 2020;38:1129–1136. doi: 10.1016/j.vaccine.2019.11.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on reasonable request to the corresponding author.


