Abstract
Protein complexes of the mitochondrial respiratory chain assemble into respiratory supercomplexes. Here we present the high-resolution electron cryo-microscopy structure of the Arabidopsis respiratory supercomplex consisting of complex I and a complex III dimer, with a total of 68 protein subunits and numerous bound cofactors. A complex I-ferredoxin, subunit B14.7 and P9, a newly defined subunit of plant complex I, mediate supercomplex formation. The component complexes stabilize one another, enabling new detailed insights into their structure. We describe (1) an interrupted aqueous passage for proton translocation in the membrane arm of complex I; (2) a new coenzyme A within the carbonic anhydrase module of plant complex I defining a second catalytic centre; and (3) the water structure at the proton exit pathway of complex III2 with a co-purified ubiquinone in the QO site. We propose that the main role of the plant supercomplex is to stabilize its components in the membrane.
Subject terms: Plant molecular biology, Cryoelectron microscopy
A high-resolution electron cryo-microscopy structure of the mitochondrial respiratory supercomplex I + III2 from Arabidopsis thaliana shows that the component complexes stabilize each other. A complex I-ferredoxin, subunit B14.7 and the newly defined P9 subunit mediate supercomplex formation.
Main
The main electron transport pathway of the mitochondrial electron transfer chain (ETC) has three sections that each correspond to a separate membrane protein complex: (1) the NADH dehydrogenase complex, known as complex I, is the main entrance point for electrons into the ETC, where complex I catalyses electron transfer from NADH to ubiquinone; (2) cytochrome c reductase, often referred to as complex III, accepts ubiquinol from complex I and passes electrons to cytochrome c; and (3) cytochrome c oxidase, also known as complex IV, catalyses the transfer of electrons from cytochrome c to molecular oxygen. Together with complex II, which transfers electrons from succinate to ubiquinone, complexes I, III and IV were first identified in the inner membrane of mammalian mitochondria and originally assumed to exist as separate entities1. However, there was some biochemical evidence that the ETC complexes form larger assemblies in the membrane2,3. Defined ETC assemblies, referred to as respiratory supercomplexes or respirasomes, were first characterized by mild native polyacrylamide gel electrophoresis4. The main respiratory supercomplex of mammalian mitochondria consists of complex I, a complex III dimer (complex III2) and up to two monomers of complex IV. The structure of this respirasome has been determined by single-particle electron cryo-microscopy (cryo-EM)5–7 and electron cryo-tomography8. The ETC generates a proton gradient across the inner mitochondrial membrane, which is used by the ATP synthase to produce ATP9.
Apart from its main role in mitochondrial ATP production, the ETC of plants has additional functions, some of which are related to photosynthesis10. The plant ETC includes several alternative oxidoreductases, resulting in branched electron transfer pathways. Furthermore, the canonical complexes of the plant ETC comprise subunits absent in animals and fungi. For example, plant complex III2 includes the two subunits of the mitochondrial processing peptidase (MPP), which removes pre-sequences from nuclear-encoded mitochondrial proteins upon import11. In yeast and mammals, pre-protein processing is carried out by a soluble enzyme in the mitochondrial matrix12. Mitochondrial complex I of plants has a characteristic γ-type carbonic anhydrase (γCA) module13 that is thought to be involved in the re-use of mitochondrial CO2 for carbon assimilation in the chloroplasts14. The γCA module sits on the matrix side of the complex I membrane arm and has three γCAs. The structures of Arabidopsis and Polytomella complex I recently revealed a protein bridge between the γCA module and the complex I peripheral arm15 that includes an unusual ferredoxin (C1-FDX) and may stabilize the complex. Both the γCA and the bridge modules are absent in complex I from mammals and yeasts but were recently discovered in complex I from protozoa16,17, suggesting that they were complex I components of the last eukaryotic common ancestor.
The cryo-EM structures of plant complexes I, III2 and IV have recently been determined at resolutions ranging from 2.9 to 3.9 Å (refs. 15,18–20). As in mammals and fungi, the plant ETC complexes form supercomplexes21,22. However, the plant I + III2 + IV supercomplex is fragile, most likely due to the weak interaction of complexes III2 and IV (ref. 20). In contrast, the plant I + III2 supercomplex is stable and abundant21,22. Low-resolution structures of the supercomplex from Arabidopsis (18 Å) and potato (20 Å) have been obtained by negative-stain electron microscopy (EM)23,24, but high-resolution cryo-EM structures are required to understand the plant-specific features of the supercomplex, in particular the arrangement and functional interplay of the carbonic anhydrase and bridge modules of complex I and the MPP module of complex III.
In this Article, we present the high-resolution cryo-EM structure of the Arabidopsis I + III2 supercomplex. The structure offers new insights not only into the function of the individual supercomplex components in near-atomic detail, but also into supercomplex assembly. The interface between complex I and complex III2 is more extensive than in the mammalian I + III2 supercomplex25. Supercomplex formation changes the conformation of the complex I membrane arm. The change appears to be induced by the B14.7 kDa subunit, which was absent in the previously determined structures of free complex I from plants, and indirectly depends on C1-FDX.
Results
2 Å structure of the Arabidopsis I + III2 supercomplex
We determined the cryo-EM structure of the active Arabidopsis I + III2 supercomplex at 2 Å resolution (Fig. 1 and Supplementary Videos 1 and 2). The complex was isolated from digitonin-solubilized mitochondrial membranes and further purified in the synthetic digitonin analogue glyco-diosgenin (GDN) (Supplementary Fig. 1). Single-particle analysis resulted in a 2.36 Å reconstruction of the entire supercomplex (Supplementary Fig. 2). Particle subtraction and separate multibody refinement of the component complexes I and III2 improved the resolution to 2.03 Å, or 1.9 Å after density modification (Supplementary Fig. 3 and Supplementary Tables1–3). In addition, two slightly different conformations of the Arabidopsis I + III2 supercomplex were resolved by focused 3D classification with resolutions of up to 2.34 Å (Supplementary Figs. 2 and 4, and Supplementary Tables 1–3).
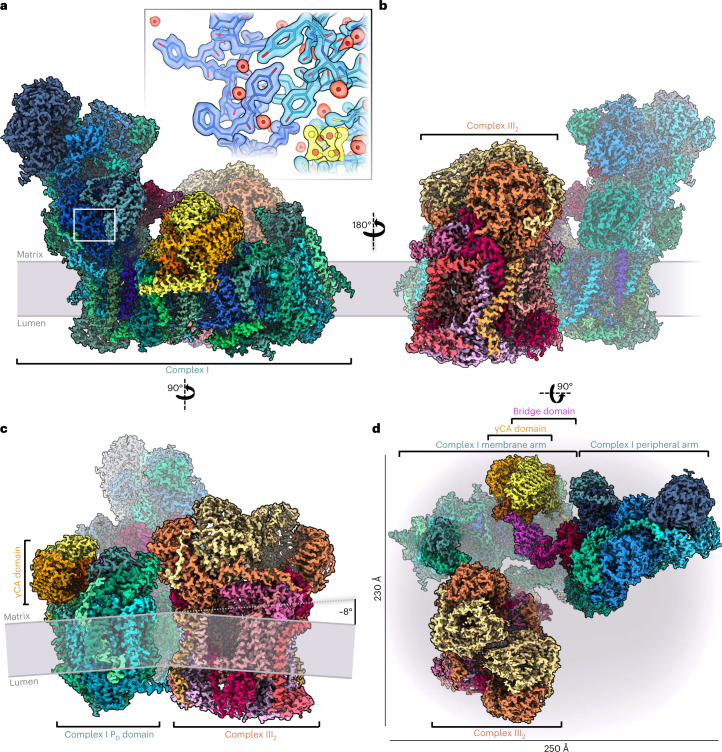
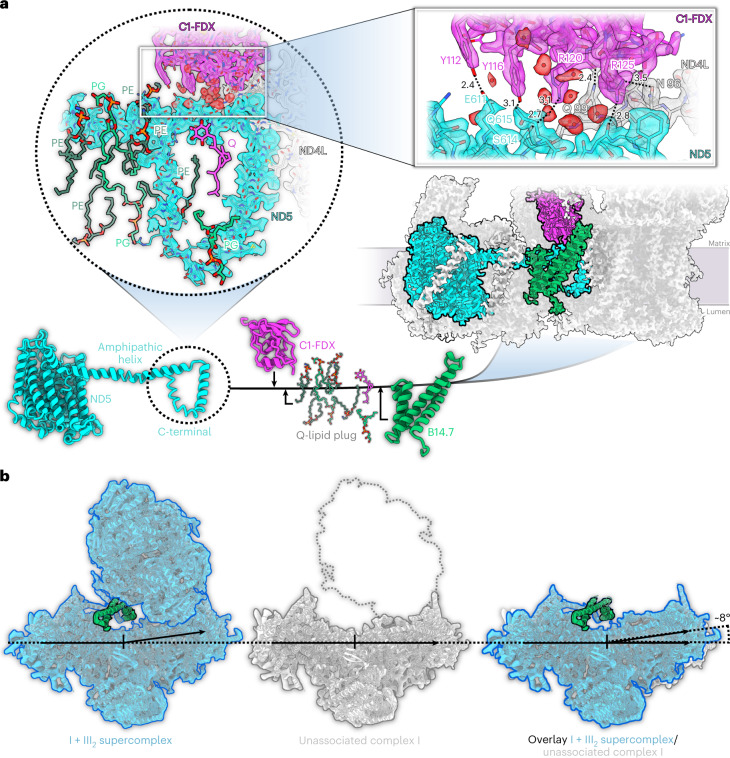
Fig. 1. 2 Å structure of the Arabidopsis I + III2 supercomplex.
a–c, Views from the plane of the inner mitochondrial membrane (grey), with complex I in front (a), with complex III2 in front (b), and from the tip of the complex I membrane arm (c). The supercomplex protrudes into the mitochondrial matrix and the cristae lumen. The 14 complex I core subunits, which are conserved in bacterial and mitochondrial complex I, are drawn in shades of blue; accessory subunits in shades of green; the three subunits of the γCA module in yellow, orange and red; the three subunits of the bridge module in pink, purple and red. In a, the inset shows a typical map region near FeS cluster N2 (yellow), and water densities are red. In c, the inner mitochondrial membrane bends by ~8° around the supercomplex. d, Matrix view of the I + III2 supercomplex. Subunits protruding from the membrane are shown in strong colours. For detailed view of the high-resolution structure, see Supplementary Videos 1 and 2. For cryo-EM data processing and activity measurements, see Supplementary Figs. 2–5. All subunits are identified in Fig. 2. For comparison with the low-resolution map of the supercomplex obtained by single-particle negative-stain EM23, see Supplementary Fig. 6.
Purified Arabidopsis I + III2 supercomplex had a NADH:cytochrome c oxidoreduction activity of 2.5 U mg−1 (Supplementary Fig. 5), similar to the mammalian I + III2 supercomplex25. Mass spectrometry (MS) identified 48 different subunits of complex I and 10 different subunits of complex III, some of which were present as pairs of isoforms (Supplementary Table 4). The cryo-EM map reveals the general architecture of the supercomplex (Fig. 1) and the arrangement of its subunits within it (Fig. 2, Table 1 and Supplementary Tables 5 and 6; for calculated molecular masses, isoelectric points and hydrophobicity of the subunits, see Supplementary Tables 7–9). The structure of complex III2 in our map agrees closely with that of mung bean complex III2 (ref. 20). Furthermore, the structure of complex I in the supercomplex generally agrees with that of the free, unassociated complex15, with the following three exceptions: (1) We identified a copy of subunit B14.7, which was not found in the previous cryo-EM maps of plant complex I15,18,19. In the supercomplex, this subunit sits at the interface of complexes I and III2 at one of their three interaction sites. (2) Subunit MNLL15 was re-assigned as NUXM because it is clearly homologous to the fungal complex I subunit NUXM rather than mammalian MNLL26. (3) The high-resolution map density allowed us to determine the sequence of a plant-specific subunit next to the ubiquinone binding site in the membrane arm15, which is encoded by the genetic locus At1g67785 (TAIR; https://www.arabidopsis.org/). We propose that this novel complex I subunit should be referred to as P9, as the other plant- and green algae-specific complex I subunits are known as P1 to P8 (refs. 15, 27, 28).
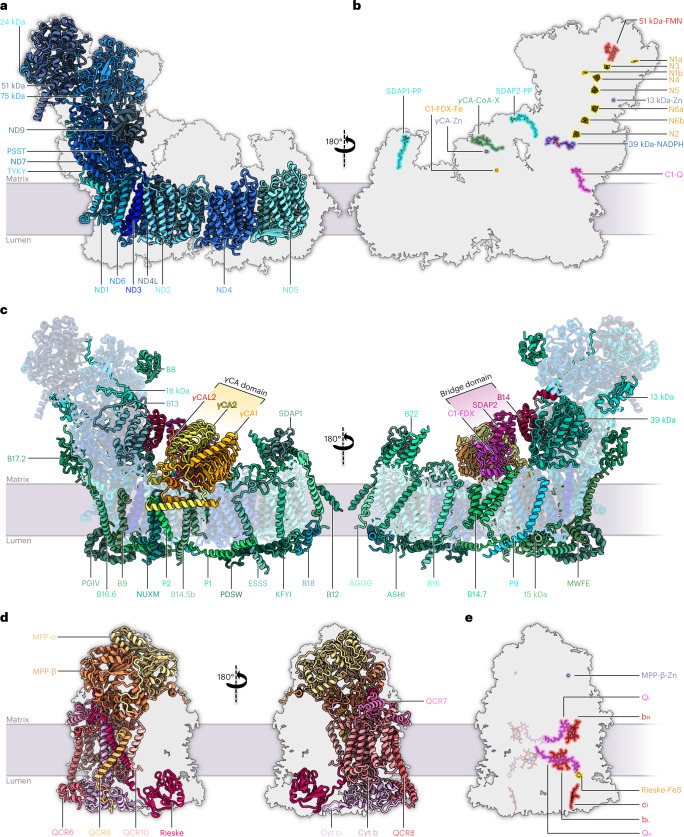
Fig. 2. Subunit composition of complex I and complex III2 within the Arabidopsis I + III2 supercomplex.
a–c, Atomic model of complex I, showing the 14 core subunits in shades of blue (a), cofactors bound to complex I (N, FeS clusters; Q, ubiquinone/ubiquinol; FMN, flavine mononucleotide) (b) and accessory subunits of mitochondrial complex I (c). Conserved accessory subunits are shown in shades of green, the subunits of the carbonic anhydrase (CA) domain in yellow and orange, and the subunits of the bridge domain in red and pink. The newly identified subunit P9 is light blue. Subunit nomenclature as for bovine complex I (ref. 80) except for non-conserved accessory subunits (for details, see Supplementary Table 5). d,e, Atomic model of complex III2, showing the structure of the ten subunits of one complex III monomer within the complex III dimer (for details, see Supplementary Table 6) from opposite directions (d) and bound cofactors of complex III2 (bH, bL, c1: haem groups attached to cytochrome b and c1; FeS: iron–sulphur cluster attached to the Rieske protein; Qi, Qo: quinone binding sites; Zn: zinc2+ bound to MPP-β) (e). For details and bound lipids, see Extended Data Fig. 2.
Table 1.
| Complex I, peripheral arm (including bridge domain), 17 subunits | |||
|---|---|---|---|
| Core subunits (7) | Conserved accessory subunits (9) | Other accessory subunit (1) | |
| 24 kDa | 13 kDa | B17.2 | C1-FDX |
| 51 kDa | 18 kDa | SDAP-2 | |
| 75 kDa | 39 kDa | ||
| TYKY-1 | B8 | ||
| PSST | B13 | ||
| ND7 | B14 | ||
| ND9 | B14.5a | ||
| Complex I, membrane arm (including carbonic anhydrase domain), 31 subunits | |||
|---|---|---|---|
| Core subunits (7) | Conserved accessory subunits (18) | Other accessory subunits (6) | |
| ND1 | 15 kDa | B18 | CA1/CA3 |
| ND2 | AGGG | B22 | CA2 |
| ND3 | ASHI | ESSS-1 | CAL2/CAL1 |
| ND4 | B9 | KFYI | P1 (SGDH) |
| ND4L | B12-2 | NUXM | P2 |
| ND5 | B14.5b | MWFE | P9 |
| ND6 | B14.7 | PDSW-1 | |
| B15 | PGIV-2 | ||
| B16.6-2 | SDAP-1 | ||
| Complex III, 10 subunits (two copies each in the dimeric complex III) | |||
|---|---|---|---|
| Core subunits (3) | Conserved accessory subunits (5) | Other accessory subunits (2) | |
| Cyt b | QCR6-1 | QCR9 | MPP-α-1 |
| Cyt c1-1 | QCR7-2 | QCR10 | MPP-β |
| Rieske-1 | QCR8-1 | ||
Core subunits are the minimal set necessary for complex I and complex III2 function and are conserved in prokaryotes and mitochondria. Accessory subunits are additional proteins forming part of the two complexes in mitochondria. They can be divided into conserved and non-conserved accessory subunits. Extensions −1/−2 indicate isoforms of Arabidopsis complex I and complex III2 subunits. The dominant isoform was fitted to the map and is shown in the structures. Subunit B14.5a was found in purified Arabidopsis I + III2 supercomplex by MS (Supplementary Table 4) but not identified in the map. For protein nomenclature and accession numbers, see Supplementary Tables 5 and 6
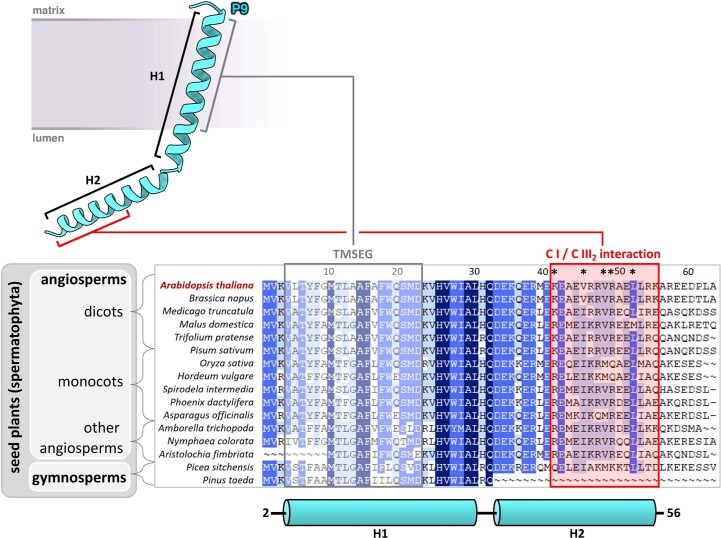
Earlier, P9 was identified in plant complex I by biochemical experiments29,30. On the basis of low sequence similarity, this subunit was suggested to possibly correspond to mammalian SGDH31, but our high-resolution cryo-EM structure now excludes this possibility. Phylogenetic analysis revealed that P9 homologues are present only in seed plants (Extended Data Fig. 1). However, the cryo-EM structure of complex I from the green alga Polytomella includes an unknown subunit exactly at the position of P9 in Arabidopsis complex I (ref. 15). As P9 is small and partially hydrophobic, homologues might have escaped detection due to limited sequence similarity. In contrast, complex I structures from animals, fungi and Tetrahymena lack a subunit at the P9 position.
Extended Data Fig. 1. Phylogenetic analysis of complex I subunit P9.
P9 is a two-helix (H1, H2) peptide that spans the inner mitochondrial membrane from the matrix to the lumenal side by its transmembrane segment (TMSEG). For phylogenetic analysis, Arabidopsis P9 (At1g67785) was used to search the non-redundant protein sequences (nr) database at the National Center for Biotechnology Information (NCBI, “https://www.ncbi.nlm.nih.gov/”) using standard settings. >100 homologous sequences were identified below an E-value of 1e-4. All sequences were from seed plants (spermatophyta). Sixteen sequences were selected for building a multiple sequence alignment using Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/); five sequences for dicots and monocots, respectively, and all identified sequences of other spermatophyta clades. Amino acid positions conserved in 16/16 sequences are highlighted in dark blue, amino acid positions conserved in ≥14/16 sequences in mid-blue and amino acid positions conserved in ≥10/16 sequences in light blue. Phylogenetic clades are indicated to the left of the alignment. The topology model of P9 is indicated above the alignment. The model was built into the cryo-EM density of the Arabidopsis I + III2 supercomplex from valine at position 2 to alanine at position 56. The positions of its two alpha-helices are shown below the alignment. The complex III2 interacting segment is shown in red. Residues K41, V45, V48, R49, L52 of Arabidopsis P9 that interact directly with complex III2 are indicated by asterisks (see Fig. 4c and d). Accession of sequences. Arabidopsis thaliana: At1g67785, Brassica napus: CAF2186799, Medicago truncatula: XP_013446710, Malus domestica: XP_028959763, Trifolium pratense: XP_045824609, Pisum sativum: XP_050898094, Oryza sativa: NP_001392381, Hordeum vulgare: XP_044978029, Spirodela intermedia: CAA2619857, Phoenix dactylifera: XP_008797106, Asparagus officinalis: XP_020253321, Amborella trichopoda: XP_020527559, Nymphaea colorata: XP_031475842, Aristolochia fimbriata: KAG9445531, Picea sitchensis: ABK24944, Pinus taeda: AFG45619.
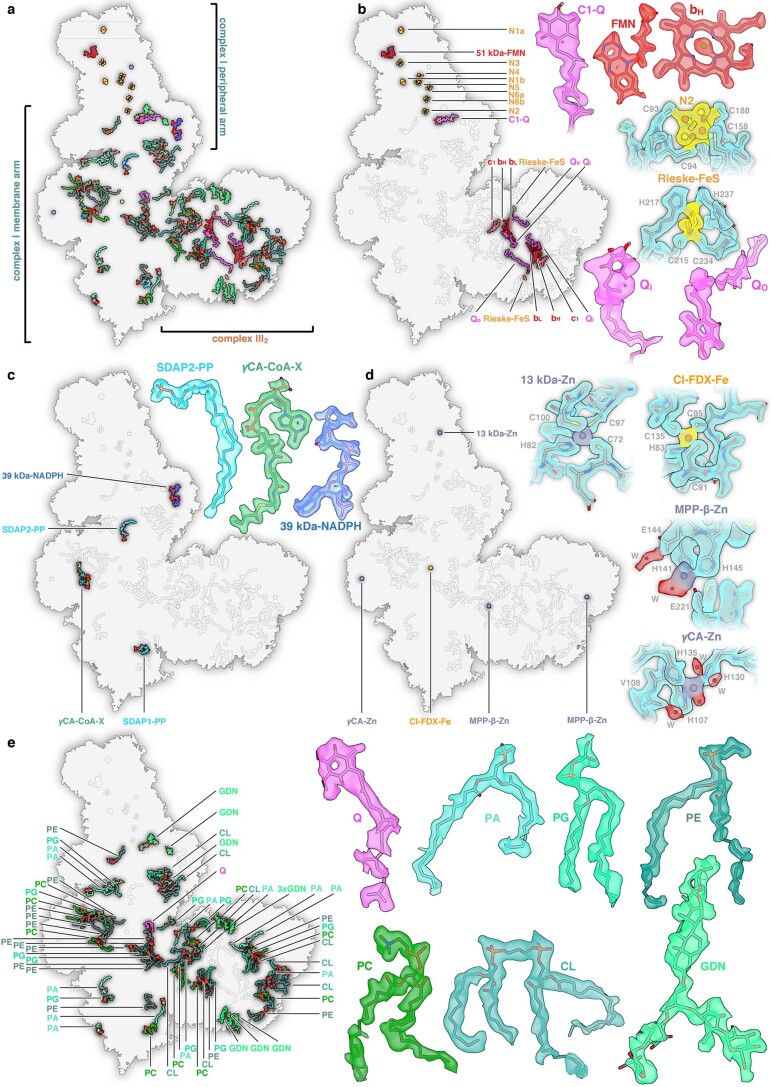
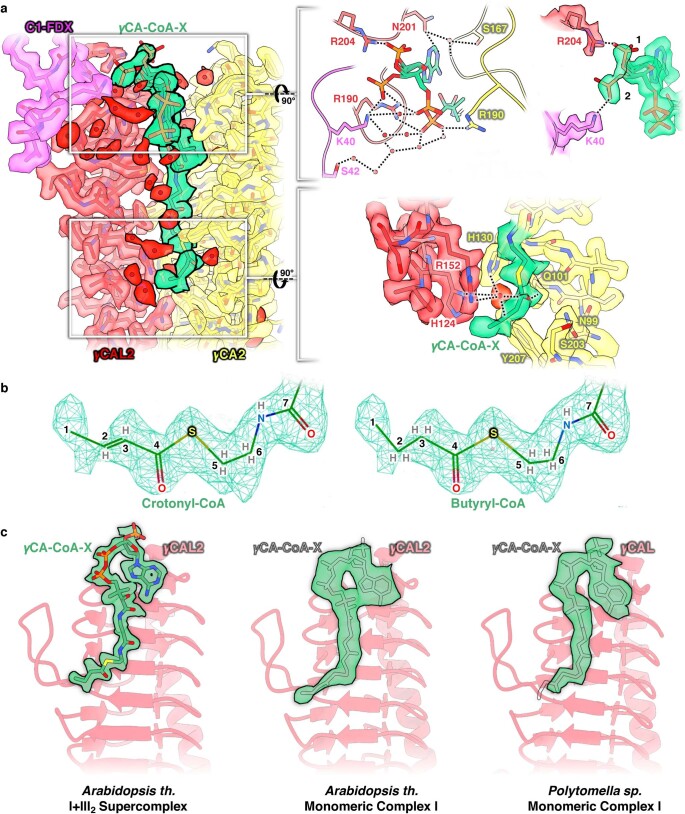
Apart from high-resolution structures of the polypeptides, our density map of the I + III2 supercomplex contains a total of 94 bound cofactors, lipids and metal ions (Fig. 2b,e and Extended Data Fig. 2), including a butyryl/crotonyl-CoA molecule in the γCA module of complex I and ubiquinone/ubiquinol (Q) in the two QO sites of the complex III dimer. Furthermore, we modelled 4,837 water molecules.
Extended Data Fig. 2. Bound cofactors, metal ions and lipids in the Arabidopsis I + III2 supercomplex.
a, Overview from the matrix side. b, Cofactors involved in respiratory electron transport. Complex I: FMN (Flavin mononucleotide), N1a, N1b, N2, N3, N4, N5, N6a, N6b (8 FeS clusters) and C1-Q (ubiquinol/ubiquinone at the Q reduction site). Complex III2: two copies each of heme bH, bL and c1, Rieske FeS, plus ubiquinone/ubiquinol at the Qo and Qi sites. c, Other cofactors: PP (S-acyl-4ʼ-phosphopantetheine bound to SDAP1 and SDAP2), CoA-X (Butyryl-or Crotonyl-Coenzym A bound to γCA), and NADPH bound to the 39 kDA subunit. d, Bound metal ions. e, Lipids and detergent molecules, with numbers of copies in round brackets. PA, phosphatidic acid (11); PC, phosphatidylcholine (9); PE, phosphatidylethanolamine (14); PG, phosphatidylglycerol (10); CL, cardiolipin (8); GDN, glyco-diosgenin (10); Q, ubiquinone/ubiquinol (not bound in the Q reduction site of complex I or in the Qo and Qi sites of complex III2). Densities and models of selected cofactors, metal ions and lipids are highlighted.
Subunit B14.7 increases the membrane arm curvature of Arabidopsis complex I
Complex III2 binds to the inner curved surface of the complex I membrane arm (Fig. 1). This overall architecture is well conserved between mammals, yeasts and plants8. In ovine mitochondria, the B14.7 subunit is located at the interface of complexes I and III2 (ref. 25). In plants, this subunit is easily lost during purification of unassociated complex I and is absent in the plant complex I structures reported so far15,18,19. In the Arabidopsis I + III2 supercomplex, B14.7 is present (Fig. 3). Together with subunit C1-FDX and a set of lipids (five phosphatidylethanolamine (PE), three phosphatidylglycerol (PG) and one Q), B14.7 binds tightly to the C-terminal loop of ND5 at the centre of the membrane arm close to the complex III2 interface (Fig. 3a). The B14.7/C1-FDX/lipid arrangement stabilizes this loop together with an amphipathic helix that runs towards the main ND5 transmembrane domain at the tip of the membrane arm. This tight interaction increases the curvature of the membrane arm, enabling close contacts to the complex III dimer (Fig. 3b), stabilizing the supercomplex.
Fig. 3. The role of subunit B14.7 and C1-FDX in I + III2 supercomplex formation.
a, Subunit B14.7 (green) and C1-FDX (magenta) interact closely with the C-terminal loop of ND5 (cyan). The loop is surrounded by a set of lipids and a Q molecule (circled inset). C1-FDX sits on top of the amphipathic helix of ND5 and stabilizes it via a tight hydrogen bond network (black dotted lines) including well-defined water molecules (square inset; all distances in Å). b, Supercomplex (left, blue) with subunit B14.7 (green) compared with the structure of unassociated complex I (centre; grey15), which does not have the B14.7 subunit. The position of the complex III dimer in the supercomplex is indicated by a grey dotted outline. An overlay (right) indicates that in the supercomplex the membrane arm of complex I rotates towards complex III2 by ~8°, resulting in a more extensive contact surface, which would stabilize the supercomplex. PE, phosphatidylethanolamine; PG, phosphatidylglycerol; Q, ubiquinone/ubiquinol.
A newly defined plant-specific subunit at the interface of complexes I and III2
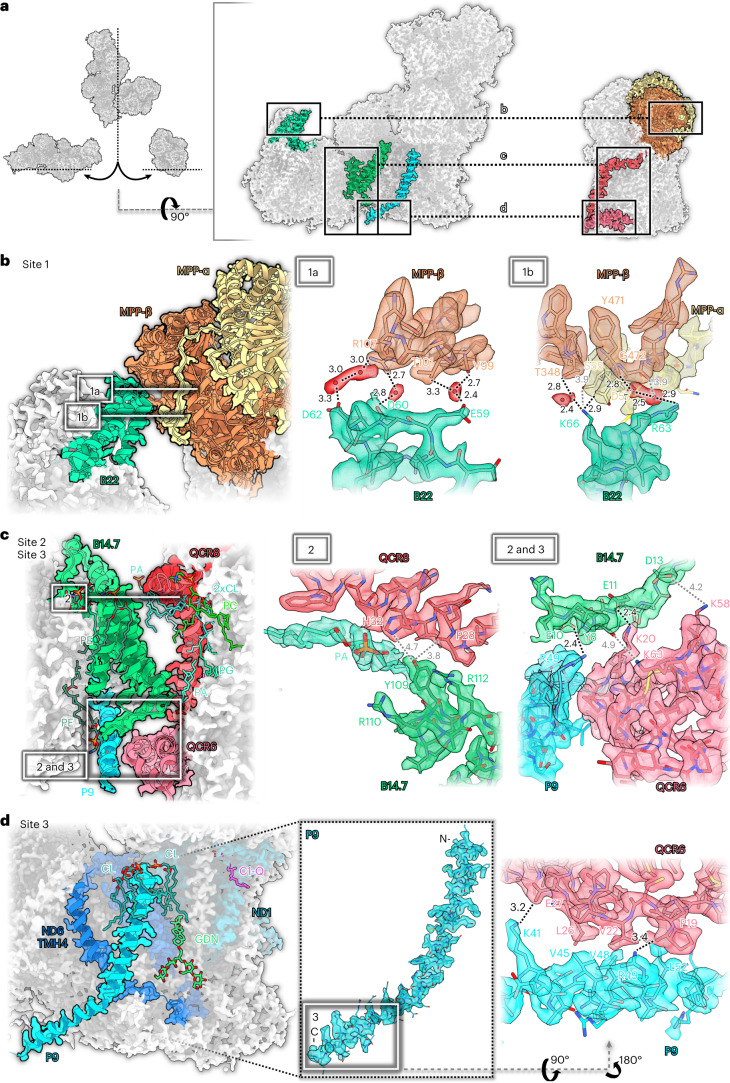
In the Arabidopsis supercomplex, complexes I and III2 interact at three distinct sites (Fig. 4). Sites 1 and 2 are partially conserved between Arabidopsis and the ovine I + III2 supercomplex. By contrast, site 3 involves the plant-specific subunit P9 and therefore appears to be unique to plants. Interactions at all three sites are based on several hydrogen bonds, salt bridges and van-der-Waals contacts and include numerous lipids and water molecules (Fig. 4). Site 1 involves subunit B22 on the complex I side, which binds to the pre-protein-processing enzyme subunits MPP-β and MPP-α of complex III2 (Fig. 4b). In the ovine I + III2 supercomplex, B22 binds only to UQCRC1, which is homologous to MPP-β of plants, but has no protein-processing activity12. In the ovine I + III2 supercomplex, subunit B15 contributes to the interaction at this site25. Site 2 marks the point where the complex I subunit B14.7 interacts with subunits QCR8 and QCR6 of complex III2 (whereas in the ovine complex, B14.7 interacts with QCR7 rather than QCR6 (ref. 25). Subunits B14.7 and QCR8 are both flanked by well-defined lipids (Fig. 4c). Finally, site 3, which is absent from the ovine complex, involves the plant-specific subunit P9 of complex I and QCR6 of complex III2. P9 is a small, 62-residue subunit that spans the inner mitochondrial membrane once near the ubiquinone binding site. Its C-terminus protrudes from the membrane into the cristae lumen, sometimes referred to as the intercristal space, where it interacts tightly with QCR6 (Fig. 4d and Extended Data Fig. 1). Notably, P9 is situated next to transmembrane helix 4 (TMH4) of ND6, which is thought to change position in the transition between the active and deactive states of mammalian complex I (ref. 32). Judging from our structures, this movement cannot take place in the Arabidopsis I + III2 supercomplex because P9 locks ND6-TMH4 in its position (Fig. 4d).
Fig. 4. Three interaction sites of complexes I and III2 in the Arabidopsis supercomplex.
a, Overview. b, Interaction site 1: B22 (green) of complex I binds to MPP-β (orange) and MPP-α (yellow) of complex III2. 1a and 1b show two roughly orthogonal views of interaction site 1. Interaction is mediated by hydrogen bonds including water molecules (red) and salt bridges. c, In site 2, B14.7 of complex I binds to QCR8 (red) and QCR6 (pink) of complex III2. In addition to polar contacts, the interaction is mediated by a set of membrane phospholipids. In site 3, binding to QCR6 involves the newly identified subunit P9 (light blue) of complex I. d, Also in site 3, the C-terminal part of P9 binds to QCR6 of complex III2 by salt bridges and hydrophobic contacts. Interacting amino acid residues are indicated by the one-letter code. Lipids: CL, cardiolipin; GDN, synthetic digitonin analogue glyco-diosgenin; PA, phosphatidic acid; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PG, phosphatidyl-glycerol; Q, ubiquinone/ubiquinol. The network of hydrogen bonds (in Å) is indicated by black (<3.5 Å) or grey (>3.5 Å) dotted lines.
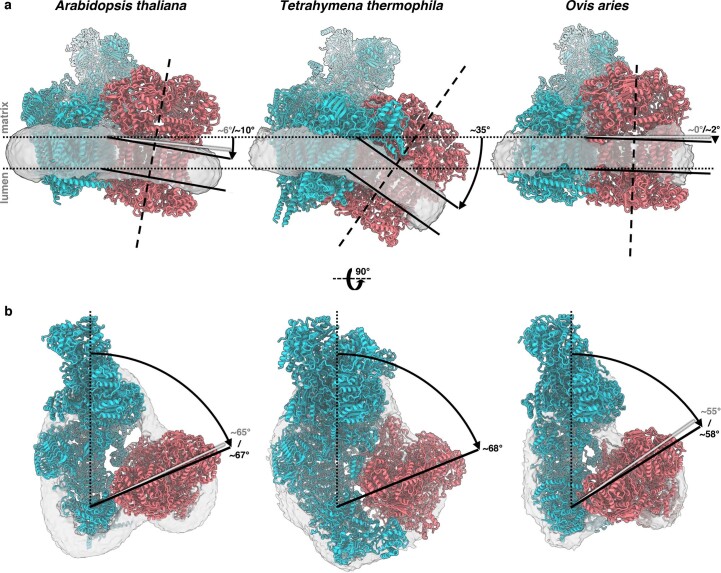
As seen from the matrix side, the consensus refinement structure as well as the two resolved I + III2 supercomplex conformations include an angle of 65–67° between the complex III dimer and the membrane arm of complex I (Extended Data Figs. 3 and 4). In the ovine supercomplex, the corresponding angle is ~55–58°. P9 increases this angle as it occupies the space between the two complexes. In Tetrahymena, even though it lacks P9, the angle is similar to Arabidopsis due to additional accessory subunits that prevent a tighter approach of complex III2 to the membrane arm of complex I.
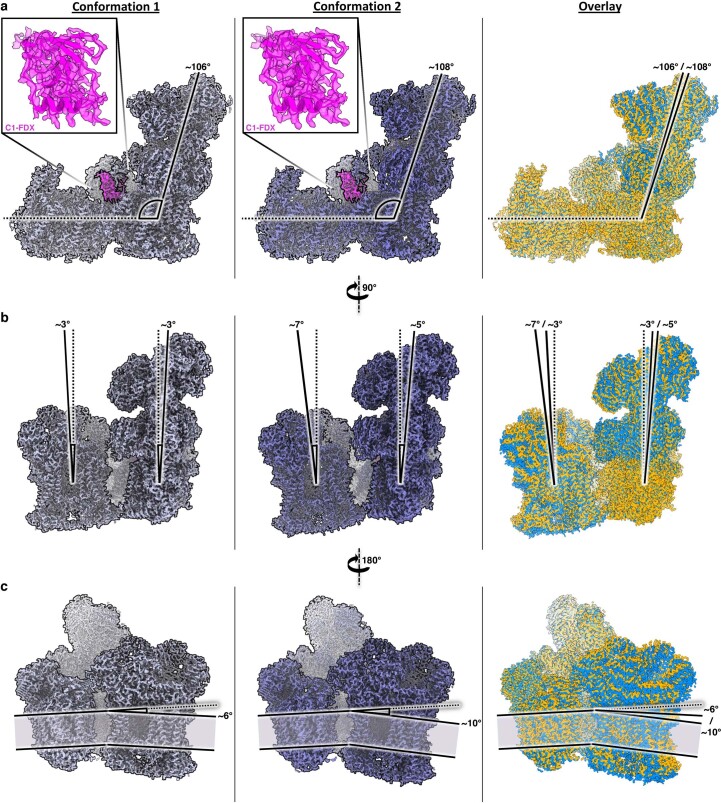
Extended Data Fig. 3. Conformations of the Arabidopsis I + III2 supercomplex.
Confromation 1 and 2 with their corresponding overlays on the right. a, View from the plane of the inner mitochondrial membrane indicating the angle between the peripheral and membrane arm of complex I in the two resolved, slightly different conformations of the I + III2 supercomplex. The angle is 106° in conformation 1 and 108° in confromation 2. The ferredoxin subunit of the bridge domain (C1-FDX) is present and well-defined in both conformations (magenta insets; complex III2 removed for clarity). b, An end-on view of the supercomplex shows that the peripheral arm tilts by 3 to 5° and C III2 by 3 to 7 ° relative to the membrane normal (dotted lines). c, A view from the opposite direction reveals that the membrane plane of complex III2 tilts by 6° or 10° relative to the membrane plane of complex I (dotted line). The panels on the right show conformation 1 in orange and conformation 2 in blue. The inner mitochondrial membrane is shown in grey. The two confromations were obtained by focused 3D classification (Supplementary Figs. 2 and 4; Supplementary Tables 1 and 2).
Extended Data Fig. 4. Conformations of the I + III2 supercomplex in different species.
Comparison of I + III2 supercomplex from Arabidopsis, Tetrahymena and ovine mitochondria. Atomic models of complex I are blue, complex III2 is red. The position of the inner mitochondrial membrane is indicated by the detergent micelles in the cryo-EM maps lowpass-filtered to 7 Å (grey). Left: conformation 2 of Arabidopsis thaliana; centre: consensus refinement of Tetrahymena thermophila; right: open conformation of Ovis aries. a, View from the tip of the complex I membrane arm, indicating the angle to which the membrane around complex III2 bends relative to complex I. The different angles in conformation 1 in Arabidopsis and the closed conformation of the ovine supercomplex are indicated by grey lines. b, Angle included between complex III2 and the complex I membrane arm as seen from the mitochondrial matrix. The dotted line indicates the approximate long axis of the complex I membrane; the straight black line is the transverse axis of complex III2. Grey lines indicate the transverse axis of Arabidopsis conformation 1 and the ovine closed conformation. The Arabidopsis structure is from this study, the structure of the open Ovis aries supercomplex is from25 (PDB: 6QC3, EMDB: 4495) and that of the Tetrahymena supercomplex from16 (PDB: 7TGH, EMDB: 25882).
In the composite structure of the Arabidopsis I + III2 supercomplex, the plane of the inner mitochondrial membrane is bent by about 8° (Fig. 1). In the two slightly different conformations of the supercomplex, the angular range is 6–10°. This indicates a slightly flexible arrangement of the two subcomplexes and raises the question whether the supercomplex actively induces membrane curvature or whether it adapts to a locally non-planar lipid bilayer. Although the lamellar cristae of plant mitochondria are known to be predominantly flat33, a minor local deviation from planarity would be difficult to detect (Extended Data Figs. 3 and 4).
Details of the γCA catalytic sites
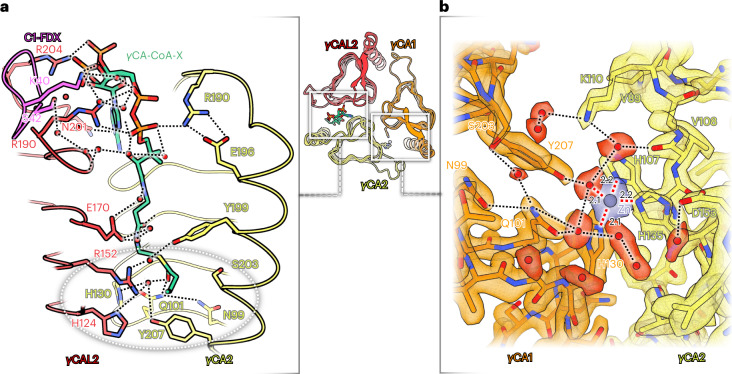
The γCA domain of Arabidopsis is a heterotrimer of two γCA subunits (γCA1 and γCA2; γCA1 can be replaced by the isoform γCA3) and one CAL2 subunit (CAL2 can be replaced by its isoform CAL1) (ref. 15). As in the homologous bacterial γCAs, which are homotrimers, three catalytic sites are located at the three subunit interfaces34. In bacteria, each of the three sites is active, as indicated by a bound metal ion coordinated by a set of three conserved histidines each. In the heterotrimeric γCA domain of Arabidopsis, only the catalytic site at the γCA1–γCA2 interface binds a metal ion15. The histidine sets at the γCA1–γCAL2 and γCA2–γCAL2 interface are incomplete and consequently cannot coordinate a metal ion. In the high-resolution structure of the Arabidopsis I + III2 supercomplex, we now find a coenzyme A at the potential catalytic site of the γCA2–γCAL2 interface (Fig. 5a and Extended Data Fig. 5). The density of this cofactor was visible in the structures of unassociated Arabidopsis and Polytomella complex I (ref. 15), but not interpreted due to insufficient resolution (Extended Data Fig. 5). Our present 2 Å map indicates that the cofactor is a butyryl- or crotonyl-CoA; the only difference between them is one single or double bond (Extended Data Fig. 5), which is not discernible. We therefore refer to this factor as γCA-CoA-X. The 3′-phosphate ADP and the diphosphate group of CoA-X interact with hydrophilic sidechains of γCA2, γCAL2 and C1-FDX either directly or via hydrogen bonds. The crotonyl or butyryl group of CoA-X is located at the catalytic site of the γCA2–γCAL2 interface, interacting via a central water molecule with two histidines and additional hydrophilic sidechains (Extended Data Fig. 5). Crotonyl-CoA is known to bind CO2 and can be carboxylated to (2S)-ethylmalonyl-CoA35. We assume that the catalytic site at the γCA2–γCAL2 interface is active and that crotonyl-CoA participates in the assimilation of CO2 for HCO3− formation. CO2 binding at crotonyl-CoA would take place exactly at the position of the CO2 molecule in the canonical active site of γCA during bicarbonate conversion. Crotonyl-CoA is formed in plant mitochondria during catabolism of lysine and possibly tryptophan36. It is further formed during mitochondrial fatty acid biosynthesis.
Fig. 5. Catalytic sites in the γCA domain.
Overview of the γCA domain (centre) with γCA1 (orange), γCA2 (yellow) and γCAL2 (red). a, Catalytic site at the γCA2–γCAL2 interface with bound butyryl- or crotonyl-CoA, here referred to as γCA-CoA-X (green). Subunits γCA2 and γCAL2 interact with the phosphate groups and the adenine ring of CoA-X either directly or across a chain (black dotted lines) of water molecules (red). The 3′ phosphate group adopts two alternative conformations, as indicated by two strong densities in the cryo-EM map (Extended Data Fig. 5). The butyryl or crotonyl group of CoA-X is located at the putative catalytic site of the γCAL2–γCA2 interface (grey dotted ellipse). b, Map density and fitted atomic model of the catalytic site at the γCA1–γCA2 interface. A zinc ion is coordinated by three histidines and one water molecule in tetrahedral geometry (red dotted lines). The active site is surrounded by a network of hydrogen bonds. For details, see Extended Data Figs. 5 and 6.
Extended Data Fig. 5. Butyryl- or Crotonyl-CoA at the γCAL2/γCA2 interface in the Arabidopsis I + III2 supercomplex.
a, Left: Overview of atomic model and cryo-EM density at the complex I/III2 interface. γCAL2, light red; γCA2, yellow; C1-FDX, pink; water molecules, red densities or spheres; Butyryl- or Crotonyl-CoA (γCA-CoA-X), green. a, Right: Details of the CoA-X 3′-phosphate ADP binding region (top). The 3′-phosphate group can adopt two conformations as indicated by two strong densities in the cryo-EM map and interacts either with K40 of C1-FDX and R190 of γCAL2, or with R204 of γCAL2. γCAL2 N201 interacts either directly with the adenine ring or via a water molecule together with γCA2 S167. γCA2 R190 and a network of water molecules connect the diphosphate group of CoA-X to the three subunits at the interface. The butyryl or crotonyl group of CoA-X is located at the catalytic site of the γCAL2/γCA2 interface (below). The CoA-X oxygen of the C4 carbonyl group interacts via a water molecule with γCAL2 R152, γCAL2 H124, γCA2 H130 and γCA2 Y207. Q101 of subunit γCA2 is in direct hydrogen bond contact to the carbonyl group. Hydrogen bonds are indicated by dotted lines. b, Butyryl- or crotonyl-CoA fit the map density equally well. The model was drawn in Coot with the cryo-EM density at a contour level of 11.5 rmsd. c, A CoA-X density is also visible in the γCA domain of free complex I from Arabidopsis and Polytomella, but was not unambiguously identified at the lower map resolution15.
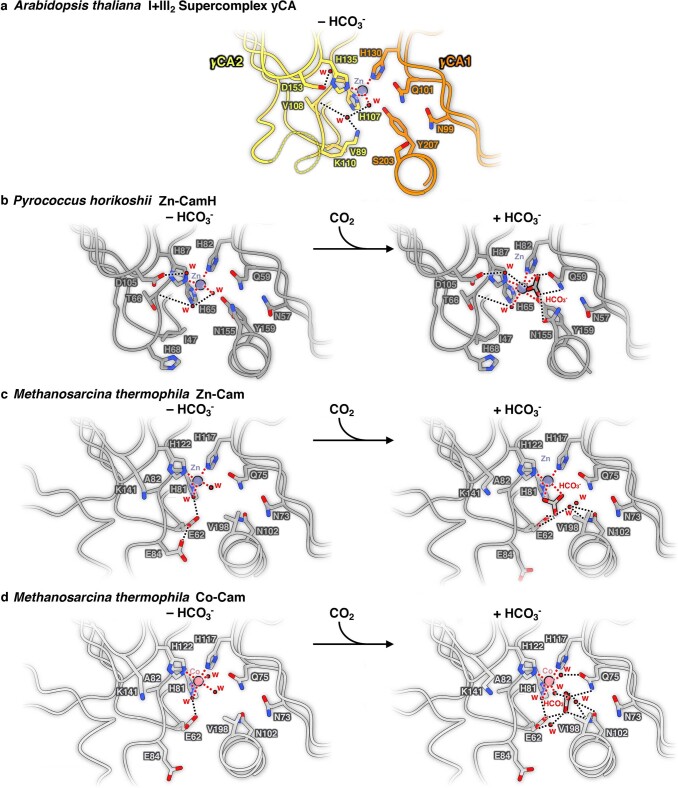
Our structure of the I + III2 supercomplex provides new insights into the architecture of the complete catalytic site at the γCA1–γCA2 interface. The higher resolution compared with the structure of the unassociated Arabidopsis complex I (ref. 15) now indicates the positions of water molecules. Apart from the three histidines, the central metal ion is coordinated by a water molecule in a tetrahedral geometry. This observation concurs with the catalytic site of the CamH γCA subclass that contains a central zinc ion and is known to be active37. Moreover, the network of hydrogen bonds and sidechains of the γCA1–γCA2 catalytic site resembles the network of CamH and differs from the one found in the Cam subclass where a zinc or cobalt ion is coordinated by two or three additional water molecules34,38. As a result, we can now clearly assign the γCA of plant complex I to the CamH subclass and the metal at the active site must be zinc (Fig. 5b and Extended Data Fig. 6). We conclude that the Arabidopsis complex I γCA1–γCA2 site is active.
Extended Data Fig. 6. Active site architecture of gamma-type carbonic anhydrases.
a, γCA-domain of complex I from Arabidopsis. The active site is located at the interface between the γCA2 (yellow) and γCA1 (orange) subunit. The three conserved histidines (H130 of γCA1, H107 and H135 of γCA2) coordinate a zinc ion (Zn) together with one water molecule (w) in a tetrahedral geometry (metal coordination shown by red dotted lines). The water molecule forms a hydrogen bond (black dotted lines) with an additional water that interacts with the polypeptide backbone of γCA2 V108 and residue γCA2 K110. A third water is coordinated by γCA2 D153. There appears to be no bound HCO3−. Coordination of Zn, the hydrogen network and conserved amino acids including Q101, N99, Y207 and S203 resemble the active Zn-CamH site of Pyrococcus horikoshii. b, Pyrococcus horikoshii Zn-CamH. Left: without HCO3− (PDB: 1V3W), right: with HCO3− (PDB: 1V67). c, Methanosarcina thermophila Zn-Cam. Left: without HCO3− (PDB: 1QRG), right: with HCO3− (PDB: 1QRL). Note that the zinc ion at the Cam site is coordinated by two further water molecules compared to the γCA2/γCA1 site in Arabidopsis and CamH. In addition, E62 and E84 that are known to be important for proton release replace V89 and K110 of γCA2 and I47 and H68 of CamH. d, Methanosarcina thermophila Co-Cam. Left: without HCO3− (PDB: 1QQ0), right: with HCO3− (PDB: 1QRE). A cobalt ion (Co) is coordinated by the three conserved histidines and three additional water molecules. In contrast to the heterotrimeric CA domain of Arabidopsis complex I, the bacterial Cam/CamH enzymes are homotrimers with three identical active sites located at the three subunit interfaces.
The aqueous passage in the membrane arm of Arabidopsis complex I
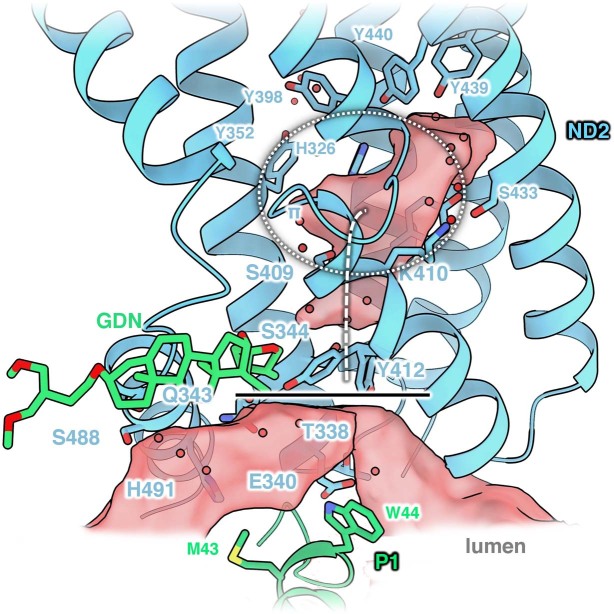
The redox reaction in the peripheral arm is energetically coupled to proton translocation in the membrane arm of complex I. Coupling is based on an aqueous passage that leads from the ubiquinone binding pocket to the ND5 subunit at the tip of the membrane arm. Ubiquinone reduction is thought to generate an electric impulse that is transferred along the aqueous passage and causes proton translocation through half-channels in the direction orthogonal to the membrane. The position, number and function of the half-channels in the membrane arm remain a matter of debate. The water structure in our high-resolution map of the Arabidopsis I + III2 supercomplex indicates three potential proton-entrance half-channels connecting the matrix to the aqueous passage in the membrane arm at core subunits ND2, ND4 and ND5 (Fig. 6a), in agreement with the structure of Yarrowia complex I (refs. 39, 40). The iron coordinated by C1-FDX is positioned close to the opening of the entrance half-channel at ND2 in a region that includes a number of ordered water molecules (Fig. 6c). The matrix channels at ND2 and ND4 seem to be closed under our experimental conditions, whereas the one of ND5 is open. On the lumenal side, two potential proton-exit half-channels from the central aqueous passage were identified. One is located at the tip of the membrane arm at ND5. This half-channel has also been seen in complex I from mammals and Yarrowia lipolytica32,39,40. In our structure, this channel is connected to the central aqueous passage while proton leakage from the open ND5 matrix half-channel is prevented by a 5–6 Å gap between ND5 H257 and T315. Our structure indicates a second lumenal half-channel at ND2 with a possible proton translocation pathway along a chain of water molecules and hydrophilic residues (Extended Data Fig. 7). This channel has been questioned. In Yarrowia, a weak water occupancy was suggested by molecular dynamics (MD) simulation at the same location39. A second half-channel on the lumenal side has been predicted on the basis of a Yarrowia mutant that lacks part of the membrane arm, including ND5, but still is partially active in proton translocation41. At the ND6 π-bulge, the hydrogen bond chain of the hydrophilic axis is interrupted (Fig. 6b). The π-bulge is identical in both our slightly different I + III2 supercomplex conformations, presumably due to the substrate-depleted conditions during sample preparation.
Fig. 6. Proton transfer path in the membrane arm of Arabidopsis complex I.
a, Conserved amino acids and water molecules (red spheres) in the membrane arm mark the central aqueous passage (white continuous line) and potential water channels (white dashed lines) to the mitochondrial matrix (transparent blue) or the cristae lumen (transparent red). The passage is interrupted at transmembrane helix 3 of subunit ND6 (red bars; see b). In subunit ND2, two potential half-channels connect the central aqueous passage to the matrix or the lumen. C1-FDX with its bound Fe ion sits next to the entrance of the ND2 matrix half-channel. The matrix and lumenal half-channel of ND5 are open. Proton leakage is prevented by a ~6 Å gap between ND5 H257 and T315 of the aqueous passage (red dotted line). b, π-Gate at the TMH3 of ND6 seen from the matrix side. Core subunits of the membrane arm are represented in shades of blue. The water chain in the central aqueous passage is interrupted by a ~9.1 Å gap. c, Density and fitted atomic model of the map region where C1-FDX (magenta) interacts with ND2 at the entrance to the potential aqueous half-channel that would connect the matrix to the central aqueous passage. The horizontal black line indicates that under our experimental conditions the channel is closed.
Extended Data Fig. 7. A potential lumenal half-channel at core subunit ND2.
View along the membrane arm. In the Arabidopsis I + III2 supercomplex, a water-filled part of the central aqueous passage around the ND2 π-bulge (transparent red, white dotted ellipse) is blocked on the lumenal side (horizontal black line) by the sidechain of ND2 Y412 and an adjacent detergent molecule (GDN, green). Waters in red; GDN, glyco-diosgenin.
A bound ubiquinone at the proton exit site of Arabidopsis complex III2
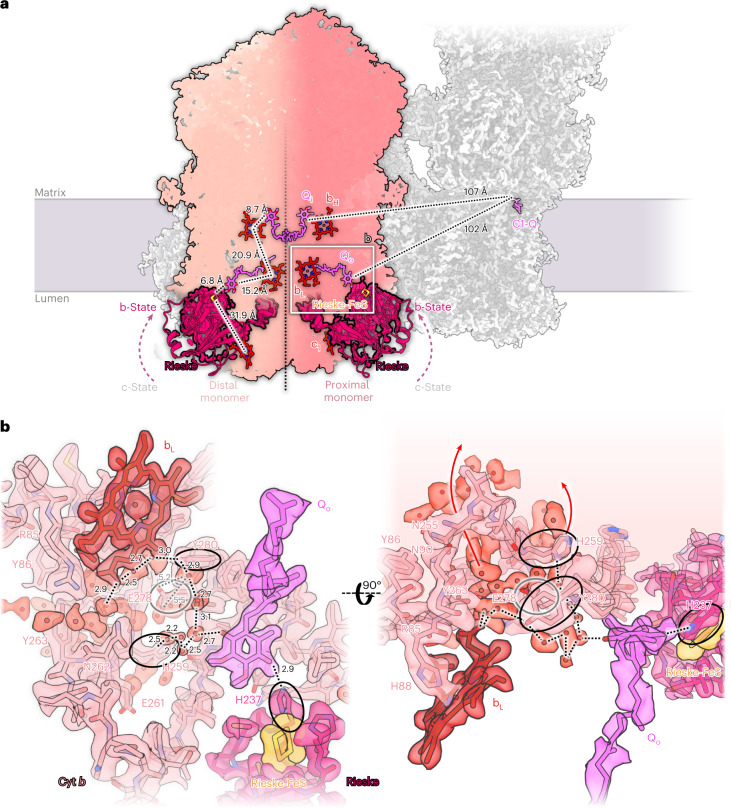
Each monomer of complex III2 has two binding sites for Q known as the Q-oxidation (Qo) and the Q-reduction site Qi42. In the structure of the I + III2 ovine supercomplex, Q densities were detected at only three of the four Q binding sites25. This was interpreted as a symmetry break in complex III2 and suggested to be relevant for supercomplex function. However, the Arabidopsis I + III2 supercomplex has Q molecules bound in all four sites, all at distances of 100–150 Å to the Q binding site of complex I (Fig. 7a).
Fig. 7. Mechanistic insights into Arabidopsis complex III2.
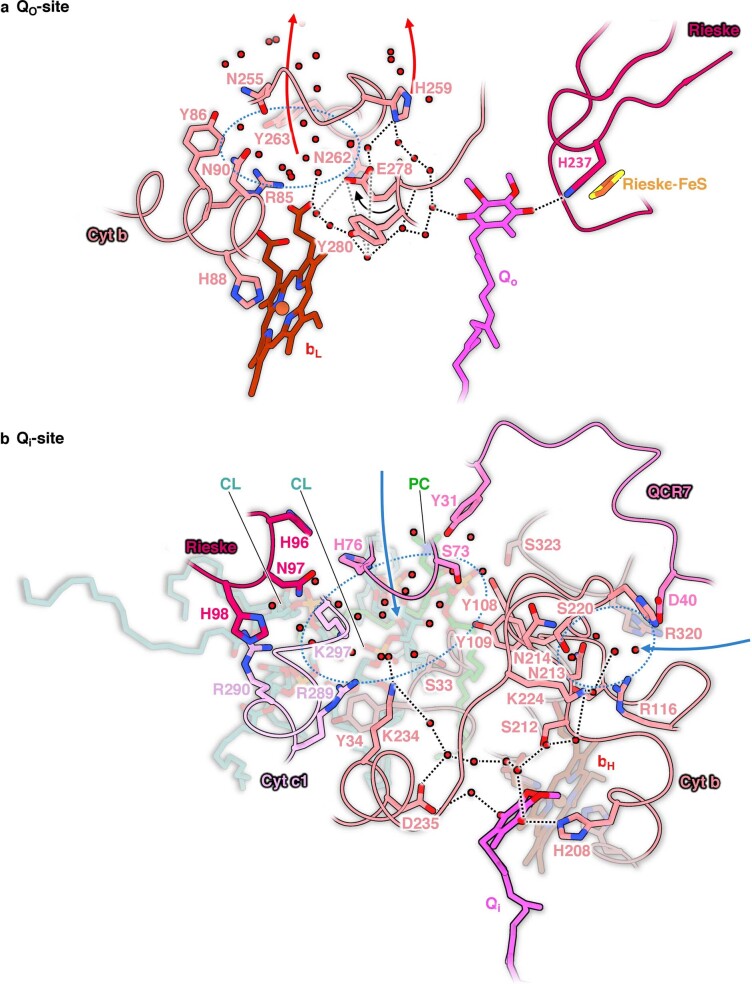
a, Overview of complex III cofactors involved in respiratory electron transport. Each monomer binds haems c1, bH, bL (red), a Rieske FeS cluster (orange) and a Q (magenta) at the reduction/oxidation site (Qi and Qo). In both monomers of complex III2, the Rieske head domain (dark red) is found in the b state. Complex III monomers proximal or distal to the ubiquinone binding site of complex I (C1-Q) are shown in different shades of pink divided by a black dotted line. Distances between the cofactors are shown for the distal complex III monomer. Distances from Qi and Qo to the quinol binding site in complex I (C1-Q) are shown for the proximal monomer. b, Detailed view of the proximal Qo site. Hydrogen network for release of the two protons during ubiquinol oxidation at the Qo site are shown by black dotted lines. One proton is transferred along a chain of water molecules (light red) via Cyt b Y280 and H259; the other can be transferred directly to H237 of the Rieske head domain. The three participating sidechains are indicated by black ellipses. Cyt b E278 (grey) may be involved in proton translocation, faces away from the bound native Q and is not part of a proton pathway (grey dotted lines). Red arrows indicate routes for proton release to the bulk solvent of the cristae lumen. For further details, see Extended Data Figs. 8 and 9.
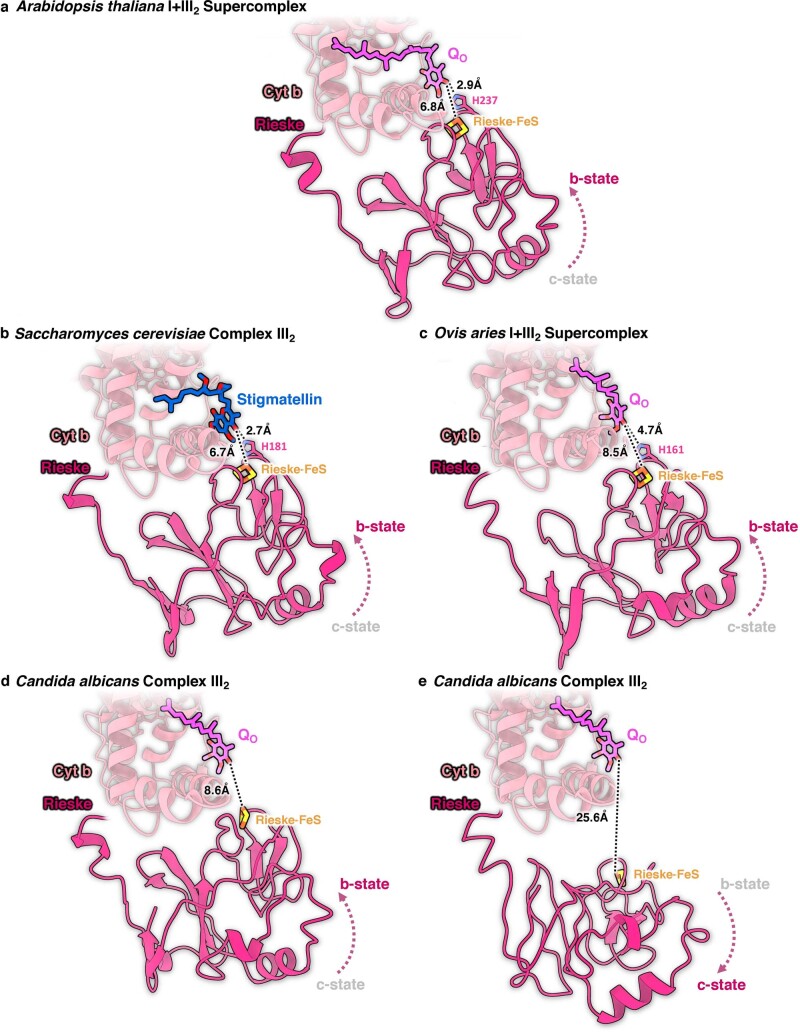
The exact details of proton release and electron transfer at Qo have long been a matter of debate, because there was no high-resolution structure of complex III with a bound Q in this site. Our 2 Å structure now shows the bound, co-purified Q substrate in each of the two Qo sites of the complex III dimer and surrounding water molecules in close-to-atomic detail. Both Rieske head domains assume the b-state, with the Rieske FeS cluster positioned 6.8 Å away from the bound Qo, and the FeS-coordinating H237 of the Rieske domain interacting with the Qo head group via a 2.9 Å hydrogen bond (Fig. 7a and Extended Data Figs. 8 and 9). These distances are in excellent agreement with the structures of yeast complex III2 with bound inhibitors stigmatellin or HHDBT43,44. In this distal binding position relative to haem bL, electrons can easily pass from Qo to the Rieske iron–sulphur cluster in the course of the Q cycle45. During the cycle, protons from the quinol are released to the cristae lumen. In our structure, as in the stigmatellin- and HHDBT-bound yeast complex, one proton can be directly transferred to H237 of the Rieske head domain. The second proton can escape via a short chain of water molecules to Cyt b H259 and then to the cristae lumen. An alternative, longer path would lead via Cyt b Y280 and the bL propionate group to a water cluster surrounded by conserved polar residues of Cyt b (R85, Y86, N90, N255, Y263 and N262). Either pathway would enable proton release to the bulk solvent without involving Cyt b E278 in the Qo motif. This residue was suggested to play an important role in proton release because in the yeast complex it interacts directly with stigmatellin. However, in the Arabidopsis supercomplex, E278 does not interact with Qo and only contributes to a water chain via its backbone nitrogen atom. This concurs with the HHDBT-inhibited yeast complex, where the inhibitor is thought to resemble the deprotonated ubisemiquinone anion state of Q. On the basis of our Qo site structure, we propose that one proton is released via H259 in subunit Cyt b and the other is taken up by H237 in the Rieske domain.
Extended Data Fig. 8. Architecture of the ubiquinol/ubiquinone (Q) binding sites in Arabidopsis complex III2.
a, The Qo site. One proton from bound ubiquinol can be transferred directly to H237 of the Rieske head domain in the b-state (black dotted line). The second proton can be released into the bulk solvent of the lumen (red arrows) along a chain of water molecules towards Cyt b H259 or towards a pool of water molecules (dotted blue ellipse) involving Cyt b Y280 and the heme bL propionate group. The sidechain of Cyt b E278 of the Qo motif faces away from the ubiquinol (black arrow) and its carboxylate group is not in hydrogen bond distance to the substrate or the water chain (grey dotted lines). b, The Qi site. Proton transfer from the bulk solvent of the matrix (blue arrows) towards a carbonyl group of bound ubiquinon can proceed via two hydrogen bond networks from clusters of water molecules (blue ellipses). One of them is surrounded by subunit QCR7, the N-terminal part of the Rieske protein, subunit Cyt c1, Cyt b and three bound lipids (two cardiolipins, CL; one phosphatidylcholine, PC). From this pool, protons can be accepted by Cyt b K234 from where they can pass to the bound ubiquinone across Cyt b D235. The other proton transfer pathway involves Cyt b K224. Our structure indicates that Cyt b H208 participates in proton transfer by forming a direct hydrogen bond to ubiquinone. Red dots, water molecules; black dotted lines, hydrogen bonds. Colors of the protein subunits (Cyt c1, Cyt b, Rieske, QCR7) as in Fig. 2. Q is drawn in pink.
Extended Data Fig. 9. Positions of the Rieske head domain in complex III structures.
In the Arabidopis I + III2 supercomplex (a), the Rieske head domain (magenta) is locked in the b-state with its FeS cluster positioned 6.8 Å and H237 2.9 Å away from the bound ubiquinone/ubiquinol that accepts the electron. These distances resemble those in the stigmatellin-bound complex III2 crystal structure of Saccharomyces cerevisiae (b) and are smaller than in the b-state in the cryo-EM structures of the Ovis aries (sheep) I + III2 supercomplex (c) and of the Candida albicans complex III2 (d) where the Rieske head group was also resolved in the c-state (e). Ubiquinone/ubiquinol is shown in pink, the inhibitor Stigmatellin in blue; distances between Qo/Rieske FeS group and Qo/FeS coordinating histidine are indicated by black dotted lines. PDB accession codes: Saccharomyces, 1EZV43; Ovis, 6Q9E25; Candida b-state, 7RJB, and Candida c-state, 7RJD81. Sidechains of the Rieske head domain histidine are not present in the Candida models.
The MPP module of complex III2
In plants, complex III2 contains an active MPP domain protruding into the mitochondrial matrix. Complex III2 thus has a dual function in respiratory electron transport and in the maturation of proteins imported into mitochondria46. In our structure of the Arabidopsis respiratory I + III2 supercomplex, subunit MPP-β of each complex III monomer contains a bound Zn ion as a cofactor for peptidase activity (Fig. 2). The Zn is coordinated by two histidines (H141 and H145) and a glutamate (E221). In addition, the map indicates a potentially coordinating water molecule (Extended Data Fig. 2). This water molecule is known to be catalytically relevant as it performs a nucleophilic attack on the carbonyl carbon of the substrate peptide bond47. However, the adjacent glycine-rich loop of subunit MPP-α is not well resolved. This loop is flexible and involved in substrate binding and product release48. The weak cryo-EM density for this region would thus indicate an active MPP domain. Due to the distance between the MPP active site and the sites involved in respiratory electron transport, it has been assumed that the two complex III2 functions are independent49. On the basis of the recently identified structure of mung bean complex III2, a potential interplay between respiratory and peptide processing functions of plant complex III2 has been conjectured, because of a perceived long-range elongation or contraction motion within or between the two monomers20. In contrast, complex III2 in the Arabidopsis supercomplex does not appear to be intrinsically flexible, but it may adopt different orientations relative to complex I (Extended Data Figs. 3 and 4).
Discussion
The 2 Å cryo-EM structure of the Arabidopsis respiratory I + III2 supercomplex provides detailed insights into how complexes I and III2 associate in the mitochondrial inner membrane (Figs. 1–4). Supercomplex formation involves protein–protein interactions at three distinct sites (Fig. 4), which explains the unusual stability of the plant supercomplex21. In Arabidopsis complex I, a defined conformation of the membrane arm promotes supercomplex formation. This conformation requires B14.7 and C1-FDX, which both interact with the C-terminal loop of ND5, thereby increasing the curvature of complex I in the membrane plane (Fig. 3). The importance of B14.7 and C1-FDX for supercomplex formation in Arabidopsis has recently been deduced from genetic experiments50. In mammalian and fungal complex I, B14.7 is likewise required for membrane arm stability51,52. In addition, the newly defined plant-specific subunit P9 contributes to the tight interaction of complexes I and III2 in Arabidopsis.
The coupling of NADH:ubiquinone oxidoreduction in the peripheral arm of complex I with proton translocation in the membrane arm may depend on long-range conformational changes32,39. Two conformations were described for the free form of Arabidopsis complex I (ref. 15), which differ in the angle between the peripheral and membrane arms. An open conformation (angle between the arms 112°) and a closed conformation (angle 106°) were reported. The C1-FDX subunit is not well defined or absent in the open conformation. On the basis of these observations, a role of C1-FDX in regulating complex I activity by adjusting the angle between the two complex I arms has been suggested15. However, in the Arabidopsis supercomplex, these two conformations are not evident. The two conformations of the supercomplex-bound form of complex I we describe in our present study are very similar and both correspond to the closed state with respect to the inter-arm angle. This angle ranges from 106° to 108° in the two conformations, and the C1-FDX subunit is present and well defined in both (Extended Data Fig. 3). In the Arabidopsis supercomplex, we do not find the open inter-arm conformation of complex I. Recently, the relevance of the inter-arm angle for complex I function has been questioned53. Rather than assigning a functional role to C1-FDX, we now conclude that the C1-FDX stabilizes complex I and that the open conformation of plant complex I (ref. 15) might be a destabilized form or assembly intermediate. However, on the basis of the supercomplex structure it seems likely that C1-FDX has an effect on proton translocation through the membrane arm, as the C1-FDX iron is located diagonally next to the proton-entry half-channel of ND2 on the matrix side of the membrane (Fig. 6a,c). It should be noted that protons are released close to this site by the activity of the carbonic anhdrase domain. Moreover, C1-FDX contributes to γCA-CoA-X binding in the γCA domain (Fig. 5a and Extended Data Fig. 5). Further investigations of C1-FDX, for example, by site-directed mutagenesis, will be necessary to clarify its role in complex I and the supercomplex.
We obtained new insights into the heterotrimeric γCA domain of complex I (Fig. 5), which is not present in mammals or fungi. The catalytic site at the γCA1–γCA2 interface perfectly resembles the active site of γCA of the bacterial CamH subclass (Extended Data Fig. 6). The two other catalytic sites lack one or two histidines for zinc binding. We now discovered a crotonyl- or butyryl-CoA at the γCA2–γCAL2 interface (Fig. 5a and Extended Data Fig. 5). Crotonyl-CoA might coordinate a CO2 molecule at this otherwise incomplete catalytic site. It is known that crotonyl-CoA can be carboxylated by crotonyl-CoA carboxylase/reductase using NADPH as a cofactor35. Further investigations, for example by site-directed mutagenesis, are needed to establish which chemical reaction is catalysed at the γCA2–γCAL2 interface of the γCA domain.
The physiological role of respiratory supercomplexes is still a matter of debate54–56. Direct channelling of ubiquinol between the Q-reduction site of complex I and the Q-oxidation site of complex III2 can be excluded, as the two substrate-binding sites are not in close proximity. Nevertheless, ubiquinol transfer from complex I to complex III2 might be facilitated by shorter diffusion distances between the substrate binding sites of the two complexes within the supercomplex. Also, supercomplex formation appears to promote respiratory chain function at other levels, for example, through the stability of individual complexes, reduction of ROS production or prevention of non-specific protein aggregation within the inner mitochondrial membrane. The I + III2 supercomplex of Arabidopsis clearly stabilizes the two component complexes.
Concluding statement
This work is published together with a study on the cryo-EM structure of supercomplex I + III2 from Vigna radiata57. No experimental data or manuscript versions were exchanged between the groups before the papers were accepted, such that the independent studies would better complement and validate one another.
Methods
Plant material
Arabidopsis thaliana was cultivated as described15. A cell suspension culture was prepared from green leaves as previously described15.
Isolation of mitochondria and purification of the I + III2 supercomplex
Mitochondria were isolated from A. thaliana cells as described15. Freshly prepared mitochondrial pellets (~100 mg, corresponding to ~10 mg mitochondrial protein) were suspended in 10 ml digitonin solubilization buffer (30 mM HEPES, pH 7.4, 150 mM potassium acetate and 5% (w/v) digitonin) and incubated for 15 min on ice. After centrifugation for 10 min at 4 °C, solubilized protein complexes were transferred onto sucrose gradients (0.3 M to 1.5 M sucrose in gradient buffer (30 mM HEPES, pH 7.8, 150 mM potassium acetate and 0.1% (w/v) digitonin)) and separated by ultracentrifugation at 146,000g and 4 °C for 20 h. Subsequently, sucrose gradients were fractionated and protein contents of relevant fractions were monitored by one-dimensional (1D) blue-native polyacrylamide gel electrophoresis58. The I + III2 supercomplex accumulates at a sucrose concentration of ~1 M (Supplementary Fig. 1a). The average protein concentration of the fractions was ~0.5 µg µl−1.
The I + III2 supercomplex was further purified by size-exclusion chromatography (Supplementary Fig. 1b). At this step, Foxglove digitonin (Merck) was replaced by synthetic digitonin (GDN, Anatrace). Supercomplex containing fractions from sucrose density ultracentrifugation were pooled and incubated for 30 min at 4 °C in cryo-EM buffer (30 mM Tris–HCl pH 7.4, 60 mM NaCl and 0,02% (w/v) GDN). They were then diluted 1:200 in the same buffer during concentration to a final volume of 50 µl using an Amicon Ultra 15 centrifugal filter unit (Merck) and a Vivaspin 500 concentrator (Merck) with 100 kDa molecular weight cut-off. The concentrated sample was loaded onto a Superose 6 Increase 3.2/300 size-exclusion chromatography column (Cytiva). Fractions containing supercomplex were eluted in cryo-EM buffer and directly used for EM grid preparation (see below).
Oxidoreductase activity of isolated I + III2 supercomplex from A. thaliana
Arabidopsis I + III2 supercomplex purified by sucrose gradient ultracentrifugation was tested for activity, using an established protocol59. The assay was carried out in 25 mM potassium phosphate (pH 7.2), 5 mM magnesium chloride, 260 µM NADH, 67 µM decylubiquinone (Sigma-Aldrich), 0.1 mM horse cytochrome c (Sigma-Aldrich) and 3,000 U ml−1 superoxide dismutase to ensure that cytochrome c remained oxidized25. The assay volume was 170 µl. Four different conditions with or without cytochrome c and decylubiquinone were tested (Supplementary Fig. 5). For each condition, measurements of two blanks (0 µg I + III2 supercomplex) as well as two measurements each of 2 µg and 4 µg I + III2 supercomplex were performed. Activity measurements were carried out with a plate reader (Multiscan Sky, Thermo Fisher Scientific) at 340 nm (NADH absorbance; extinction coefficient of NADH: 6.22 mM−1 cm−1).
Analysis of the I + III2 supercomplex by MS
Supercomplex-containing fractions from sucrose density ultracentrifugation were prepared for MS analysis via the single-pot-solid-phase-enhanced sample preparation (SP3) protocol60. Here we used a modified protocol61 adapted for the amount of available sample material. The sucrose gradient fractions were diluted in equal volumes of 2× SDT buffer (8% (w/v) sodium dodecyl sulphate, 0.2 M dithiothreitol and 0.2% Tris–HCl, pH 7.6) and incubated on a thermoshaker (TS-100, Kisker Biotech) for 1 h at 60 °C and 1,000 rpm. After centrifugation for 10 min at 20,000g, the supernatant was transferred into a new reaction tube and sonicated in a water bath for 10 min (Elmasonic S30, Elma). Proteins were alkylated via incubation in 20 mM iodoacetamide for 30 min at 600 rpm at room temperature in the dark. Alkylation was stopped by addition of 5 mM dithiothreitol.
Sera-Mag carboxylate-modified beads hydrophilic solids (GE Life Sciences) were combined 1:1 with hydrophobic solids (GE Life Sciences), and a total amount of 600 µg beads was added to each sample. Proteins were precipitated by addition of 70 µl ethanol (100%) and subsequent incubation for 10 min at 1,000 rpm at 24 °C. Beads were pelleted on a magnetic rack for 2 min, and proteins were washed three times with 140 µl of fresh 80% ethanol. After protein clean-up, beads were transferred in 80% ethanol into low-protein-binding tubes (Low Binding Micro Tubes, Sarstedt) and ethanol was removed on the magnetic racks.
Proteins were digested with 2 µg of sequencing-grade modified trypsin (V5111, Promega) in 50 mM ammonium bicarbonate at 37 °C at 1,000 rpm overnight in a total reaction volume of 60 µl. Trypsin activity was stopped the next day by addition of 1% (v/v) formic acid (FA). The pH of each sample was controlled and adjusted to <3.
Tryptic peptides were further cleaned via solid-phase extraction on SepPak Vac 1cc (50 mg) tC18 cartridges (Waters). Cartridges were wetted with 1 ml 100% acetonitrile and 1 ml 0.1% (v/v) FA in 50% (v/v) acetonitrile. Cartridge equilibration was performed by adding 2× 1 ml of 0.1% FA (v/v) in H2O. Acidified peptides (pH <3) were loaded onto the cartridges and washed two times with 0.1% FA (v/v) in H2O and eluted two times in 200 µl of 0.1% FA (v/v) in 50% (v/v) acetonitrile. Cleaned peptides were dried in a vacuum centrifuge and stored at −20 °C. Final peptide concentration was determined with the Pierce peptide quantification kit (Thermo Scientific) following the manufacturer’s instructions.
A nanoElute HPLC (Bruker Daltonics) was coupled to a timsTOF Pro ion-mobility spectrometry quadrupole time-of-flight mass spectrometer (Bruker). Peptides were reconstituted in 0.1% FA, and 400 ng peptides per sample were directly transferred onto an ‘Aurora’ reversed-phase analytical column with integrated emitter tip (25 cm × 75 μm inner diameter, IonOpticks, Fitzroy). Peptides were separated on the analytical column at 50 °C via a 70 min gradient (solvent A: 0.1% FA; solvent B: 0.1% FA in 100% acetonitrile) at a flow rate of 300 nl min−1. A linear gradient from 2% to 37% B for the first 60 min was followed by a 10 min washing step at 95% B.
The timsTOF Pro mass spectrometer was operated in DDA PASEF mode, and the pre-installed method ‘DDA PASEF-standard_1.1sec_cycletime’ was used. Automatic recalibration of ion mobility before each sample run was activated. MS and MS/MS scan range was 100–1,700 m/z, the ion mobility range (1/K0) was 0.6–1.6 V s−1 cm−2. A polygon filtering was applied in the m/z and ion mobility area to exclude the low m/z of singly charged ions for PASEF precursor selection. Ramp and accumulation time was set to 100 ms to achieve close to 100% duty cycle. The number of PASEF ramps was set to 10 with a charge maximum of 5. The quadrupole isolation width was set to 2 for m/z = 700 and 3 for m/z = 800. Collision energy was 20 eV for ion mobility (1/K0) 0.6 V s−1 cm−2 and 59 eV for ion mobility (1/K0) 1.6 V s−1 cm−2, respectively.
Evaluation of MS data
MaxQuant 2.0.3.0 (ref. 62) was used to query acquired MS/MS spectra against a modified TAIR10 database including models of mitochondrial and plastid genes after RNA editing to improve sequence coverage of affected proteins (see ref. 63). Default parameters were used with the following exception: calculation of iBAQ values64 was activated, the options ‘Log fit’ and ‘charge normalization’ were enabled. Identification transfer between individual runs via the ‘Match between runs’ feature was applied with the default parameters.
Cryo-EM grid preparation
Quantifoil R 1.2/1.3 400 Cu grids were washed for 1 h in chloroform. They were coated with a 2 nm carbon layer using a Leica EM ACE600 high vacuum sputter coater or a graphene trivial transfer single layer (ACS Material LLC). Graphene-coated grids were pre-treated as described previously65 and functionalized with 20 µM 1-polybutyric acid or just glow discharged for 15 s at 15 mA. Carbon-coated grids were just glow discharged in the same way. Supercomplex was applied at a final concentration of 0.18 mg ml−1 and the grids were plunge-frozen in liquid ethane after blotting for 4.5 s with force 20 using a Mark Vitrobot IV operating at 10 °C and 70 % humidity. Grids were stored in liquid nitrogen.
Data acquisition and image processing
Electron micrographs were collected at 300 kV with a Titan Krios G4i (Thermo Scientific) equipped with a cold field emission gun and a Falcon 4 detector operating in electron counting mode. The nominal magnification was 215,000×, corresponding to a pixel size of 0.573 Å. Electron-event representation (EER) movies consisting of 1,118 raw frames were recorded automatically with EPU software at an exposure rate of 3.4 e− pixel−1 s−1 and a total dose of 50 e− Å−2. Four datasets were collected, three from graphene grids and one from a carbon-coated grid. Data were processed separately. Movies of the single datasets were motion-corrected using MotionCor2 (ref. 66) and contrast transfer function (CTF) was estimated with CTFFind4.1.13 (ref. 67). After picking with crYOLO68, the particles were imported into Relion3.1.3 (ref. 69). Particles were binned to a pixel size of 2.558 Å, and an initial 3D classification was performed to clean the dataset. Next, a second 3D classification with a mask around the complex III dimer was performed to separate the supercomplex from unassociated complex I. Supercomplex particles were 3D-refined and re-extracted at the original pixel size of 0.573 Å. Two rounds of CTF refinement and Bayesian polishing were performed for each dataset, before they were merged. The final 3D reconstruction of the merged dataset indicated a resolution of 2.55 Å for the whole supercomplex. For an additional global non-uniform refinement, the merged particles were also imported into cryoSPARC v4.0.2 (ref. 70) and combined into a map with an overall resolution of 2.36 Å. Further processing was continued in RELION. For particle subtraction, the peripheral and membrane arm of complex I and the complex III dimer within the supercomplex were masked. Particle subtraction was followed by multibody-refinement steps for each of the separate domains. This resulted in a final resolution of 2.03 Å and 2.04 Å for the N and Q module of the CI peripheral arm, 2.13 Å and 2.29 Å for the PP and PD module of the CI membrane arm, and 2.29 Å and 2.25 Å for the MPP modules and the membrane part of complex III. Resolution estimates are based on the gold-standard Fourier shell correlation of two independent half-maps at the 0.143 cut-off71,72. The B-factor sharpened cryo-EM densities were further modified with the phenix.resolve_cryo_em tool73 using the refined maps, the two half-maps, the estimated molecular mass and a mask. Density modification improved the map quality to calculated resolutions of 1.93 Å, 1.89 Å, 2.02 Å and 2.14 Å for the N, Q, PP and PD module of complex I, and to 2.17 Å and 2.14 Å for the MPP module and membrane part of complex III. To separate different conformations of the Arabidopsis I + III2 supercomplex, focused 3D classification of the final consensus refinement was performed. Particles were first aligned by the complex I peripheral arm, using a local mask for initial 3D refinement. The particles were then 3D-classified by applying a soft mask around the membrane arm and complex III2 performing no further particle alignment with a value of T = 25. The two most dissimilar classes were further refined with a global mask, resulting in an overall resolution of 2.73 Å for conformation 1 and 2.80 Å for conformation 2. Final focused 3D refinement around the complex I peripheral arm, complex I membrane arm and complex III2 for each of the two conformations improved the resolution to 2.34 Å and 2.41 Å, respectively.
Model building
The initial model for the Arabidopsis supercomplex was built using the complete model of the A. thaliana complex I (pdb: 7ARB) and homology models for each individual subunit of complex III that were created by the SWISS-MODEL server74. Individual subunits were then assembled using the complex III2 structure of V. radiata (pdb: 7JRG) as a template. Together, the complex I and assembled complex III2 model of A. thaliana were rigid-body fitted into the cryo-EM map of the supercomplex using UCSF Chimera75. Manual building was performed in Coot76 using the post-processed maps from Relion and the density-modified maps from Phenix with additional refinement with phenix.real_space_refine77. Water molecules were built automatically into the density-modified maps using the Segger Chimera SWIM tool78, and fits were checked manually. Model quality statistics were taken from phenix.validation_cryoEM and are summarized in Supplementary Table 3. Aqueous cavities were calculated with a 1.4 Å interior and a 3.5 Å exterior probe radius using Hollow79.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Supplementary Figs. 1–6 and Tables 1–9.
Three-dimensional cryo-EM density map of the Arabidopsis I + III2 supercomplex at around 2 Å resolution with corresponding atomic model.
High-resolution map density around the N2 iron–sulphur cluster in the peripheral arm of complex I.
Acknowledgements
We thank Ö. Yildiz for help in building the atomic model and interpreting the cryo-EM structure. This work was funded by the Max Planck Society (W.K., M.D. and N.K.) and by the Deutsche Forschungsgemeinschaft (grant INST 187/791-1 FUGG to H.-P.B.; SFB 807 to W.K. and N.K.).
Extended data
Author contributions
H.-P.B. and W.K. initiated the project. J.S. purified the I + III2 supercomplex from Arabidopsis and characterized the supercomplex by activity measurements. N.R. analysed the supercomplex by MS. N.K. and M.D. optimized the sample for cryo-EM, collected cryo-EM data, performed image processing and built and analysed the atomic models. N.K. produced the figures. All authors evaluated data. H.-P.B. wrote the first draft of the manuscript with contributions from N.K. and W.K. All authors contributed in finalizing the manuscript.
Peer review
Peer review information
Nature Plants thanks Markus Schwarzlander, Ian Møller and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Funding
Open access funding provided by the Max Planck Society.
Data availability
The MS proteomics data of the mitochondrial I + III2 supercomplex from Arabidopsis thaliana are available at the PRIDE repository (https://www.ebi.ac.uk/pride/), dataset identifier PXD036482. Cryo-EM density maps and atomic models of the I + III2 supercomplex from Arabidopsis thaliana are available at the EM Data Bank (EMDB, https://www.ebi.ac.uk/emdb/, accessions EMD-15998, EMD-15999, EMD-16000, EMD-16003, EMD-16007, EMD-16008, EMD-16168, EMD-16171, EMD-16172) and Protein Data Bank (PDB, https://www.rcsb.org/, accessions 8BED, 8BEE, 8BEF, 8BEH, 8BEL, 8BEP, 8BPX, 8BQ5 and 8BQ6). Details are given in Supplementary Tables 2 and 3.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Maximilian Dreimann, Jennifer Senkler.
Contributor Information
Niklas Klusch, Email: Niklas.Klusch@biophys.mpg.de.
Hans-Peter Braun, Email: braun@genetik.uni-hannover.de.
Extended data
is available for this paper at 10.1038/s41477-022-01308-6.
Supplementary information
The online version contains supplementary material available at 10.1038/s41477-022-01308-6.
References
- 1.Hatefi Y, Haavik AG, Griffiths DE. Reconstitution of the electron transfer system I. Preparation and properties of the interacting enzyme complexes. Biochem. Biophys. Res. Commun. 1961;4:441–446. doi: 10.1016/0006-291X(61)90305-9. [DOI] [PubMed] [Google Scholar]
- 2.Fowler LR, Richardson SH. Studies on the electron transfer system. L. On the mechanism of reconstitution of the mitochondrial electron transfer system. J. Biol. Chem. 1963;238:456–463. doi: 10.1016/S0021-9258(19)84019-X. [DOI] [PubMed] [Google Scholar]
- 3.Hatefi Y, Rieske JS. The preparation and properties of DPNH-cytochrome c reductase (complex I–III of the respiratory chain) Methods Enzymol. 1967;10:225–231. doi: 10.1016/0076-6879(67)10044-X. [DOI] [Google Scholar]
- 4.Schägger H, Pfeiffer K. Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. EMBO J. 2000;19:1777–1783. doi: 10.1093/emboj/19.8.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gu J, et al. The architecture of the mammalian respirasome. Nature. 2016;537:639–643. doi: 10.1038/nature19359. [DOI] [PubMed] [Google Scholar]
- 6.Letts JA, Fiedorczuk K, Sazanov LA. The architecture of respiratory supercomplexes. Nature. 2016;537:644–648. doi: 10.1038/nature19774. [DOI] [PubMed] [Google Scholar]
- 7.Sousa JS, Mills DJ, Vonck J, Kühlbrandt W. Functional asymmetry and electron flow in the bovine respirasome. eLife. 2016;5:e21290. doi: 10.7554/eLife.21290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies KM, Blum TB, Kühlbrandt W. Conserved in situ arrangement of complex I and III2 in mitochondrial respiratory chain supercomplexes of mammals, yeast, and plants. Proc. Natl Acad. Sci. USA. 2018;115:3024–3029. doi: 10.1073/pnas.1720702115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- 10.Braun HP. The oxidative phosphorylation system of the mitochondria in plants. Mitochondrion. 2020;53:66–75. doi: 10.1016/j.mito.2020.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Braun HP, Emmermann M, Kruft V, Schmitz UK. The general mitochondrial processing peptidase from potato is an integral part of cytochrome c reductase of the respiratory chain. EMBO J. 1992;11:3219–3227. doi: 10.1002/j.1460-2075.1992.tb05399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braun HP, Schmitz UK. Are the ‘core’ proteins of the mitochondrial bc1 complex evolutionary relics of a processing protease? Trends Biochem. Sci. 1995;20:171–175. doi: 10.1016/S0968-0004(00)88999-9. [DOI] [PubMed] [Google Scholar]
- 13.Sunderhaus S, et al. Carbonic anhydrase subunits form a matrix-exposed domain attached to the membrane arm of mitochondrial complex I in plants. J. Biol. Chem. 2006;281:6482–6488. doi: 10.1074/jbc.M511542200. [DOI] [PubMed] [Google Scholar]
- 14.Zabaleta E, Martin MV, Braun HP. A basal carbon concentrating mechanism in plants? Plant Sci. 2012;187:97–104. doi: 10.1016/j.plantsci.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Klusch, N., Senkler, J., Yildiz, Ö., Kühlbrandt, W. & Braun, H. P. A ferredoxin bridge connects the two arms of plant mitochondrial complex I. Plant Cell33, 2072–2091 (2021). [DOI] [PMC free article] [PubMed]
- 16.Zhou L, Maldonado M, Padavannil A, Guo F, Letts JA. Structures of Tetrahymena’s respiratory chain reveal the diversity of eukaryotic core metabolism. Science. 2022;376:831–839. doi: 10.1126/science.abn7747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mühleip, A. et al. Structural basis of mitochondrial membrane bending by I–II–III2–IV2 supercomplex. Preprint at bioRxiv10.1101/2022.06.26.497646 (2022).
- 18.Soufari H, Parrot C, Kuhn L, Waltz F, Hashem Y. Specific features and assembly of the plant mitochondrial complex I revealed by cryo-EM. Nat. Comm. 2020;11:5195. doi: 10.1038/s41467-020-18814-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maldonado M, Padavannil A, Zhou L, Guo F, Letts JA. Atomic structure of a mitochondrial complex I intermediate from vascular plants. eLife. 2020;9:e56664. doi: 10.7554/eLife.56664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maldonado M, Guo F, Letts JA. Atomic structures of respiratory complex III2, complex IV, and supercomplex III2-IV from vascular plants. eLife. 2021;10:e62047. doi: 10.7554/eLife.62047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eubel H, Jansch L, Braun HP. New insights into the respiratory chain of plant mitochondria. Supercomplexes and a unique composition of complex II. Plant Physiol. 2003;133:274–286. doi: 10.1104/pp.103.024620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eubel H, Heinemeyer J, Braun HP. Identification and characterization of respirasomes in potato mitochondria. Plant Physiol. 2004;134:1450–1459. doi: 10.1104/pp.103.038018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dudkina NV, Eubel H, Keegstra W, Boekema EJ, Braun HP. Structure of a mitochondrial supercomplex formed by respiratory-chain complexes I and III. Proc. Natl Acad. Sci. USA. 2005;102:3225–3229. doi: 10.1073/pnas.0408870102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bultema JB, Braun HP, Boekema EJ, Kouril R. Megacomplex organization of the oxidative phosphorylation system by structural analysis of respiratory supercomplexes from potato. Biochim. Biophys. Acta. 2009;1787:60–67. doi: 10.1016/j.bbabio.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Letts JA, Fiedorczuk K, Degliesposti G, Skehel M, Sazanov LA. Structures of respiratory supercomplex I+III2 reveal functional and conformational crosstalk. Mol. Cell. 2019;75:1131–1146.e6. doi: 10.1016/j.molcel.2019.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Padavannil A, Ayala-Hernandez MG, Castellanos-Silva EA, Letts JA. The mysterious multitude: structural perspective on the accessory subunits of respiratory complex I. Front. Mol. Biosci. 2021;8:798353. doi: 10.3389/fmolb.2021.798353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyer EH. Proteomic investigations of complex I composition: how to define a subunit? Front. Plant Sci. 2012;3:106. doi: 10.3389/fpls.2012.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Subrahmanian N, Remacle C, Hamel PP. Plant mitochondrial complex I composition and assembly: a review. Biochim. Biophys. Acta. 2016;1857:1001–1014. doi: 10.1016/j.bbabio.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Meyer EH, Taylor NL, Millar AH. Resolving and identifying protein components of plant mitochondrial respiratory complexes using three dimensions of gel electrophoresis. J. Proteome Res. 2008;7:786–794. doi: 10.1021/pr700595p. [DOI] [PubMed] [Google Scholar]
- 30.Klodmann J, Sunderhaus S, Nimtz M, Jansch L, Braun HP. Internal architecture of mitochondrial complex I from Arabidopsis thaliana. Plant Cell. 2010;22:797–810. doi: 10.1105/tpc.109.073726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cardol P. Mitochondrial NADH:ubiquinone oxidoreductase (complex I) in eukaryotes: a highly conserved subunit composition highlighted by mining of protein databases. Biochim. Biophys. Acta. 2011;1807:1390–1397. doi: 10.1016/j.bbabio.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 32.Kampjut D, Sazanov LA. Structure of respiratory complex I—an emerging blueprint for the mechanism. Curr. Opin. Struct. Biol. 2022;74:102350. doi: 10.1016/j.sbi.2022.102350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Panek T, Elias M, Vancova M, Lukes J, Hashimi H. Returning to the fold for lessons in mitochondrial crista diversity and evolution. Curr. Biol. 2020;30:R575–R588. doi: 10.1016/j.cub.2020.02.053. [DOI] [PubMed] [Google Scholar]
- 34.Ferry JG. The gamma class of carbonic anhydrases. Biochim Biophys. Acta. 2010;1804:374–381. doi: 10.1016/j.bbapap.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson MC, Moore BS. Beyond ethylmalonyl-CoA: the functional role of crotonyl-CoA carboxylase/reductase homologs in expanding polyketide diversity. Nat. Prod. Rep. 2012;29:72–86. doi: 10.1039/C1NP00082A. [DOI] [PubMed] [Google Scholar]
- 36.Hildebrandt TM, Nunes Nesi A, Araujo WL, Braun HP. Amino acid catabolism in plants. Mol. Plant. 2015;8:1563–1579. doi: 10.1016/j.molp.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 37.Jeyakanthan J, et al. Observation of a calcium-binding site in the gamma-class carbonic anhydrase from Pyrococcus horikoshii. Acta Crystallogr D. 2008;64:1012–1019. doi: 10.1107/S0907444908024323. [DOI] [PubMed] [Google Scholar]
- 38.Iverson TM, Alber BE, Kisker C, Ferry JG, Rees DC. A closer look at the active site of gamma-class carbonic anhydrases: high-resolution crystallographic studies of the carbonic anhydrase from Methanosarcina thermophila. Biochemistry. 2000;39:9222–9231. doi: 10.1021/bi000204s. [DOI] [PubMed] [Google Scholar]
- 39.Parey K, et al. High-resolution structure and dynamics of mitochondrial complex I—insights into the proton pumping mechanism. Sci. Adv. 2021;7:eabj3221. doi: 10.1126/sciadv.abj3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grba DN, Hirst J. Mitochondrial complex I structure reveals ordered water molecules for catalysis and proton translocation. Nat. Struct. Mol. Biol. 2020;27:892–900. doi: 10.1038/s41594-020-0473-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dröse S, et al. Functional dissection of the proton pumping modules of mitochondrial complex I. PLoS Biol. 2011;9:e1001128. doi: 10.1371/journal.pbio.1001128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hunte C, Solmaz S, Palsdottir H, Wenz T. A structural perspective on mechanism and function of the cytochrome bc1 complex. Results Probl. Cell Differ. 2008;45:253–278. doi: 10.1007/400_2007_042. [DOI] [PubMed] [Google Scholar]
- 43.Hunte C, Koepke J, Lange C, Roßmanith T, Michel H. Structure at 2.3 Å resolution of the cytochrome bc1 complex from the yeast Saccharomyces cerevisiae co-crystallized with an antibody Fv fragment. Structure. 2000;8:669–684. doi: 10.1016/S0969-2126(00)00152-0. [DOI] [PubMed] [Google Scholar]
- 44.Palsdottir H, Lojero CG, Trumpower BL, Hunte C. Structure of the yeast cytochrome bc1 complex with a hydroxyquinone anion Qo site inhibitor bound. J. Biol. Chem. 2003;278:31303–31311. doi: 10.1074/jbc.M302195200. [DOI] [PubMed] [Google Scholar]
- 45.Kao WC, Hunte C. Quinone binding sites of cyt bc complexes analysed by X-ray crystallography and cryogenic electron microscopy. Biochem. Soc. Trans. 2022;50:877–893. doi: 10.1042/BST20190963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Braun HP. The two roles of complex III in plants. eLife. 2021;10:e65239. doi: 10.7554/eLife.65239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor AB, et al. Crystal structures of mitochondrial processing peptidase reveal the mode for specific cleavage of import signal sequences. Structure. 2001;9:615–625. doi: 10.1016/S0969-2126(01)00621-9. [DOI] [PubMed] [Google Scholar]
- 48.Nagao Y, et al. Glycine-rich region of mitochondrial processing peptidase alpha-subunit is essential for binding and cleavage of the precursor proteins. J. Biol. Chem. 2000;275:34552–34556. doi: 10.1074/jbc.M003110200. [DOI] [PubMed] [Google Scholar]
- 49.Glaser E, Dessi P. Integration of the mitochondrial-processing peptidase into the cytochrome bc1 complex in plants. J. Bioenerg. Biomembr. 1999;31:259–274. doi: 10.1023/A:1005475930477. [DOI] [PubMed] [Google Scholar]
- 50.Röhricht, H. et al. The mitochondrial ferredoxin-like is essential for the formation of complex I-containing respiratory supercomplexes in Arabidopsis thaliana. Preprint at bioRxiv (2022).
- 51.Letts JA, Sazanov LA. Clarifying the supercomplex: the higher-order organization of the mitochondrial electron transport chain. Nat. Struct. Mol. Biol. 2017;24:800–808. doi: 10.1038/nsmb.3460. [DOI] [PubMed] [Google Scholar]
- 52.Guerrero-Castillo S, et al. The assembly pathway of mitochondrial respiratory chain complex I. Cell Metab. 2017;25:128–139. doi: 10.1016/j.cmet.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 53.Laube E., Meier-Credo J., Langer J. D. & Kühlbrandt W. Conformational changes in mitochondrial complex I of the thermophilic eukaryote Chaetomium thermophilum.Sci. Adv.10.1126/sciadv.adc9952 (2022). [DOI] [PMC free article] [PubMed]
- 54.Milenkovic D, Blaza JN, Larsson NG, Hirst J. The enigma of the respiratory chain supercomplex. Cell Metab. 2017;25:765–776. doi: 10.1016/j.cmet.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 55.Hirst J. Open questions: respiratory chain supercomplexes—why are they there and what do they do? BMC Biol. 2018;16:111. doi: 10.1186/s12915-018-0577-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu M, et al. Research journey of respirasome. Protein Cell. 2020;11:318–338. doi: 10.1007/s13238-019-00681-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maldonado, M., Fan, Z., Abe, K. M. & Letts, J. A. Plant-specific features of respiratory supercomplex I + III2 from Vigna radiata. Nat. Plants10.1038/s41477-022-01306-8 (2023). [DOI] [PMC free article] [PubMed]
- 58.Wittig I, Braun HP, Schagger H. Blue native PAGE. Nat. Protoc. 2006;1:418–428. doi: 10.1038/nprot.2006.62. [DOI] [PubMed] [Google Scholar]
- 59.Birch-Machin MA, Briggs HL, Saborido AA, Bindoff LA, Turnbull DM. An evaluation of the measurement of the activities of complexes I–IV in the respiratory chain of human skeletal muscle mitochondria. Biochem. Med. Metab. Biol. 1994;51:35–42. doi: 10.1006/bmmb.1994.1004. [DOI] [PubMed] [Google Scholar]
- 60.Hughes CS, et al. Single-pot, solid-phase-enhanced sample preparation for proteomics experiments. Nat. Protoc. 2019;14:68–85. doi: 10.1038/s41596-018-0082-x. [DOI] [PubMed] [Google Scholar]
- 61.Mikulasek K, et al. SP3 protocol for proteomic plant sample preparation prior LC–MS/MS. Front. Plant Sci. 2021;12:635550. doi: 10.3389/fpls.2021.635550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tyanova S, Temu T, Cox J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protoc. 2016;11:2301–2319. doi: 10.1038/nprot.2016.136. [DOI] [PubMed] [Google Scholar]
- 63.Fuchs P, et al. Single organelle function and organization as estimated from Arabidopsis mitochondrial proteomics. Plant J. 2020;101:420–441. doi: 10.1111/tpj.14534. [DOI] [PubMed] [Google Scholar]
- 64.Schwanhäusser B, et al. Global quantification of mammalian gene expression control. Nature. 2011;473:337–342. doi: 10.1038/nature10098. [DOI] [PubMed] [Google Scholar]
- 65.D’Imprima E, et al. Protein denaturation at the air–water interface and how to prevent it. eLife. 2019;8:e42747. doi: 10.7554/eLife.42747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zheng SQ, et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods. 2017;14:331–332. doi: 10.1038/nmeth.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rohou A, Grigorieff N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 2015;192:216–221. doi: 10.1016/j.jsb.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wagner T, et al. SPHIRE-crYOLO is a fast and accurate fully automated particle picker for cryo-EM. Commun. Biol. 2019;2:218. doi: 10.1038/s42003-019-0437-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zivanov J, Nakane T, Scheres SHW. Estimation of high-order aberrations and anisotropic magnification from cryo-EM data sets in RELION-3.1. IUCrJ. 2020;7:253–267. doi: 10.1107/S2052252520000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Punjani A, Rubinstein JL, Fleet DJ, Brubaker MA. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods. 2017;14:290–296. doi: 10.1038/nmeth.4169. [DOI] [PubMed] [Google Scholar]
- 71.Scheres SH. A Bayesian view on cryo-EM structure determination. J. Mol. Biol. 2012;415:406–418. doi: 10.1016/j.jmb.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rosenthal PB, Henderson R. Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron cryomicroscopy. J. Mol. Biol. 2003;333:721–745. doi: 10.1016/j.jmb.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 73.Terwilliger TC, Ludtke SJ, Read RJ, Adams PD, Afonine PV. Improvement of cryo-EM maps by density modification. Nat. Methods. 2020;17:923–924. doi: 10.1038/s41592-020-0914-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guex N, Peitsch MC, Schwede T. Automated comparative protein structure modeling with SWISS-MODEL and Swiss-PdbViewer: a historical perspective. Electrophoresis. 2009;30:S162–S173. doi: 10.1002/elps.200900140. [DOI] [PubMed] [Google Scholar]
- 75.Pettersen EF, et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 76.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Afonine PV, et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr D. 2018;74:531–544. doi: 10.1107/S2059798318006551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang KM, Pintilie GD, Li SS, Schmid MF, Chiu W. Resolving individual atoms of protein complex by cryo-electron microscopy. Cell Res. 2020;30:1136–1139. doi: 10.1038/s41422-020-00432-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ho BK, Gruswitz F. HOLLOW: generating accurate representations of channel and interior surfaces in molecular structures. BMC Struct. Biol. 2008;8:49. doi: 10.1186/1472-6807-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Walker JE, et al. Sequences of 20 subunits of NADH-ubiquinone oxidoreductase from bovine heart-mitochondria—application of a novel strategy for sequencing proteins using the polymerase chain-reaction. J. Mol. Biol. 1992;226:1051–1072. doi: 10.1016/0022-2836(92)91052-Q. [DOI] [PubMed] [Google Scholar]
- 81.Di Trani JM, et al. Rieske head domain dynamics and indazole-derivative inhibition of Candida albicans complex III. Structure. 2022;30:129–138 e4. doi: 10.1016/j.str.2021.08.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figs. 1–6 and Tables 1–9.
Three-dimensional cryo-EM density map of the Arabidopsis I + III2 supercomplex at around 2 Å resolution with corresponding atomic model.
High-resolution map density around the N2 iron–sulphur cluster in the peripheral arm of complex I.
Data Availability Statement
The MS proteomics data of the mitochondrial I + III2 supercomplex from Arabidopsis thaliana are available at the PRIDE repository (https://www.ebi.ac.uk/pride/), dataset identifier PXD036482. Cryo-EM density maps and atomic models of the I + III2 supercomplex from Arabidopsis thaliana are available at the EM Data Bank (EMDB, https://www.ebi.ac.uk/emdb/, accessions EMD-15998, EMD-15999, EMD-16000, EMD-16003, EMD-16007, EMD-16008, EMD-16168, EMD-16171, EMD-16172) and Protein Data Bank (PDB, https://www.rcsb.org/, accessions 8BED, 8BEE, 8BEF, 8BEH, 8BEL, 8BEP, 8BPX, 8BQ5 and 8BQ6). Details are given in Supplementary Tables 2 and 3.