Abstract
Background
Relative peripheral refraction (RPR) is a significant factor that participates in myopic development. Here, we evaluated the effects of atropine 0.01% eyedrops, as an antimyopia drug, on RPR.
Methods
Seventy-three children were enrolled from a randomized, double-blinded, placebo-0.01% atropine eyedrops cross-over trial. The study group had used the placebo for one year and then crossed over to atropine 0.01% eyedrops for half a year. The control group had used 0.01% atropine for one year and then crossed over to placebo eyedrops for half a year. Central and horizontal peripheral refractions (15° and 30° at the temporal and nasal retina) were measured under non-cycloplegia and cycloplegia.
Results
No significant differences in age, gender, and central refraction were identified between the two groups (P > 0.05). Under non-cycloplegia, the control group showed significant relative hyperopia in the temporal 30° retina and the nasal retina (P = 0.031; P < 0.001; P < 0.001). In the study group, the relative hyperopia in the temporal 30° retina disappeared (P = 0.983). After cycloplegia, the control group had less myopia in central refractions and less hyperopia in temporal RPR (P < 0.001; P = 0.039; P < 0.001). The study group did not present significant changes in central refractions and temporal RPR (P = 0.122; P = 0.222; P = 0.475).
Conclusions
For myopic children, atropine 0.01% eyedrops can alleviate relative hyperopia in the temporal retina and the hyperopic shift before cycloplegia. The effect might participate in myopia control.
Subject terms: Refractive errors, Paediatrics
Introduction
As a public health problem, myopia has an increasing impact on the quality of people’s lives, especially in Asia [1]. In China, over 82.3% of university students had myopia and 11.1% from high myopia [2]. In Singapore, the prevalence of myopia exceeded 70% in young teenagers [3]. With the rising incidence and related complications, like glaucoma, myopic macular degeneration, and choroidal neovascularization, more and more attention has been paid to myopia control. In 1920, atropine was first proposed to prevent myopia progression [4]. Compared with cyclopentolate 1% eyedrops, atropine 1% eyedrops had better effectiveness on retarding myopia progression after a year of observation [5]. Up to now, many researchers have found low-concentration atropine can effectively relieve myopia and reduce side effects, like poor visual feeling, photophobia, and progression rebound after stopping treatment [6, 7]. However, the mechanism of atropine control of myopia is still obscured.
Relative peripheral refraction (RPR), up to the dioptric situation and retinal shape, was the difference between central and peripheral visual refraction [8]. Since the 1970s, Hoogerheide et al. firstly found that relative peripheral hyperopia was a risk factor for myopia development and proposed its meaning in the prognosis of myopia [9]. The animal studies demonstrated that relative peripheral hyperopia and peripheral form deprivation promoted axial elongation in ocular growth [10, 11]. Soft contact lenses and orthokeratology are also considered to retard myopia progression by changing RPR [12]. However, studies about the influence of atropine on RPR are rare. Previous studies have testified that children have a difference between cycloplegic and non-cycloplegic refraction, resulting from a temporary ciliary muscle spasm and an active accommodation response in a habitual accommodative state [13–15]. Lin et al. regarded the difference spherical equivalent (DSE) between cycloplegic and non-cycloplegic autorefraction as an evaluation for excessive accommodation. They proposed that it could contribute to myopic progression [14]. Given the whole refraction state in the horizontal visual field, if there is a DSE in the central refraction, then there should also be a DSE in RPR. However, the characteristics of DSE in RPR are obscure. As a nonselective muscarinic receptor antagonist, atropine is helpful to relieve the ciliary muscle spasm and may further affect the integral refraction state. Therefore, based on a randomized, double-blinded, placebo-0.01% atropine eyedrops cross-over trial, we explore the influence of atropine 0.01% eyedrops, as an antimyopia drug, on RPR, DSE, and the two factors combined [16].
Methods
Study design
The study was conducted at the 1.5-year follow-up of the randomized, double-blinded, placebo-0.01% atropine eyedrops cross-over trial performed in Beijing Tongren Hospital [16]. All children were recruited and randomized to receive either atropine 0.01% or placebo eyedrops in both eyes once daily for one year in phase 1. The schedule generated by SAS program (SAS Institute Inc) was used to operate the randomization independently. At the end of the first year, the atropine 0.01% group will be crossed over to the placebo group, and the placebo group will be crossed over to the atropine 0.01% group for one year in phase 2. Here, the children in the study group had used the placebo for one year in Phase 1 and then crossed over to atropine 0.01% eyedrops for half a year. The children in the control group had used 0.01% atropine for one year in Phase 1 and then crossed over to placebo eyedrops for half a year. All the subjects were randomly enrolled from the cross-over trial. The study adhered to the tenets of the declaration of Helsinki and was approved by the Ethics Committee of Beijing Tongren Hospital. All participants provided written informed consent after agreeing to participate. Inclusion criteria included: aged 6–12 years, refractive error of spherical equivalent (SE) range of −1.00 D to −6.00 D in both eyes, astigmatism of −1.50 D or less in both eyes, best-corrected distance visual acuity 0.20 logMAR or better in both eyes, intraocular pressure ≤21 mm Hg. Exclusion criteria were: children with other combined ocular diseases (e.g., amblyopia, strabismus, corneal scar, cataract, glaucoma, or ocular tumor), allergy to atropine, cyclopentolate, or excipients. The trial was registered on the Chinese Clinical Trial Registry (http://www.chictr.org.cn/index.aspx). The registration number is ChiCTR-IOR-17013898 [16].
Measurement
An open-field autorefractor (WAM5500; Grand Seiko, Hiroshima, Japan) was used to measure the central and peripheral refraction of the right eyes with occluding left eyes by a patch under non-cycloplegia. Children were instructed to keep their heads stationary and fixate the central target first, and temporal targets and nasal targets followed. Five sighting targets were placed at 0, 15° temporal (−15), 30° temporal (−30), 15° nasal (+15), 30° nasal (+30) horizontal visual field angles with 5 meters radius, which correspond to the central, 15° temporal (−15), 30° temporal (−30), 15° nasal (+15), 30° nasal (+30) retina, respectively. Average values of five measurements were recorded for each angle. Cycloplegia was produced with three drops 1% cyclopentolate (Alcon) administered at 5-minute intervals. Thirty minutes after the last drop, if pupillary light reflex was still present or the pupil size was less than 6.0 mm, the fourth drop of 1% cyclopentolate was administered, and the examination was repeated 15 min later. Under cycloplegia, the refractions at five visual field angles were measured again.
SE was calculated through conventional formulas to express refractive power at different positions [17]. RPR was calculated by subtracting central SE from peripheral SEs.
where S is the sphere, C the cylinder.
Statistical analysis
Categorical data were present as counts (frequencies). Mean (standard deviation) values were used to describe continuous variables. The Kolmogorov-Smirnov test was used to examine the distributions of continuous data. Continuous data with normal distributions were analyzed with independent-samples T-tests or paired-samples T-tests. Continuous variables with abnormal distributions were analyzed with Mann–Whitney U-tests or Wilcoxon rank-sum tests. The Chi-square test was used to assess the difference in gender between the two groups. We took independent-samples T-tests and Mann–Whitney U-tests to analyze the two groups’ differences in continuous variables. Paired-samples T-tests and Wilcoxon rank-sum tests were performed to evaluate the difference between cycloplegic and non-cycloplegic SE at each position and the difference between peripheral refractions and central refractions in each group. A P value < 0.05 with two-sided was considered statistically significant. All statistical analyses were performed using commercial software (SPSS version 24.0; SPSS, Inc., Chicago, IL, United States).
Results
Seventy-three myopic children from the trial were enrolled. Thirty-nine subjects were in the study group and 34 subjects in the control group. The age of subjects was 11.61 ± 1.69 years and 11.33 ± 1.51 years in the study and the control group, respectively. The percentage of boys was 53.8% in the study group and 47.1% in the control group. No significant differences in age, gender, and central refractions no matter under non-cycloplegia or cycloplegia were identified between the two groups (P = 0.470; P = 0.563; P = 0.650; P = 0.796; Table 1).
Table 1.
The central refraction and RPRs of subjects in horizontal visual field under non-cycloplegia and cycloplegia.
| The study group | The control group | P value | P value | |||||
|---|---|---|---|---|---|---|---|---|
| Non-cycloplegia | Cycloplegia | P value | Non-cycloplegia | Cycloplegia | P value | |||
| 0° refraction, mean ± SD, (D) | −3.67 ± 1.45 | −3.49 ± 1.41 | 0.122a | −3.82 ± 1.24 | −3.57 ± 1.16 | <0.001a | 0.650c | 0.796d |
| 15° temporal RPR, mean ± SD, (D) | −0.19 ± 0.75 | −0.27 ± 0.59 | 0.222a | −0.17 ± 0.59 | −0.43 ± 0.77 | 0.039a | 0.934c | 0.336d |
| 30° temporal RPR, mean ± SD, (D) | 0.00 ± 1.39 | −0.11 ± 1.48 | 0.475a | 0.56 ± 1.43 | −0.50 ± 1.68 | <0.001b | 0.101c | 0.634e |
| 15° nasal RPR, mean ± SD, (D) | 0.58 ± 0.70 | 0.42 ± 0.82 | 0.157b | 0.68 ± 0.75 | 0.65 ± 0.74 | 0.716a | 0.581c | 0.241e |
| 30° nasal RPR, mean ± SD, (D) | 1.80 ± 1.25 | 1.76 ± 1.35 | 0.697a | 2.32 ± 1.46 | 2.14 ± 1.61 | 0.137b | 0.104c | 0.115e |
SD standard deviation, RPR relative peripheral refraction, D dioptres.
aPaired-samples T tests were used to analyze the differences between non-cycloplegic and cycloplegic refractions in each group.
bWilcoxon rank sum tests were used to analyze the differences between non-cycloplegic and cycloplegic refractions in each group.
cIndependent-sample T-tests were used to analyze the differences in non-cycloplegic refractions between the two groups.
dIndependent-sample T-tests were used to analyze the differences in cycloplegic refractions between two the groups.
eMann–Whitney U-tests were used to analyze the differences in cycloplegic refractions between two the groups.
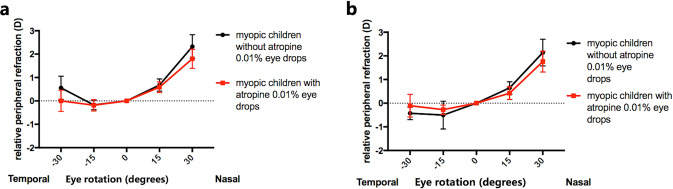
Under non-cycloplegia, the control group presented significant relative hyperopia at the temporal 30° and the nasal 15° and 30° retina (P = 0.031; P < 0.001; P < 0.001). In comparison, the significant relative hyperopia in the temporal retina disappeared in the study group. The subjects with atropine 0.01% eyedrops presented significant relative hyperopia at the nasal 15° and 30° retina (P < 0.001; P < 0.001; Table 2). Besides, all relative peripheral refractions (RPRs) in the study group were less hyperopic (or more myopic) than those in the control group (P > 0.05 for all) (Table 1; Fig. 1a).
Table 2.
Peripheral refractions of subjects in horizontal visual field under non-cycloplegia and cycloplegia.
| The study group | The control group | |||||||
|---|---|---|---|---|---|---|---|---|
| Non-cycloplegia | p value | Cycloplegia | P value | Non-cycloplegia | p value | Cycloplegia | P value | |
| 15° temporal refraction, mean ± SD, (D) | −3.86 ± 1.24 | 0.130a | −3.76 ± 1.30 | 0.006a | −3.99 ± 1.34 | 0.097a | −4.00 ± 1.04 | 0.003a |
| 30° temporal refraction, mean ± SD, (D) | −3.67 ± 1.61 | 0.983a | −3.60 ± 1.48 | 0.657a | −3.26 ± 1.39 | 0.031a | −4.07 ± 1.73 | 0.317b |
| 15° nasal refraction, mean ± SD, (D) | −3.09 ± 1.61 | <0.001b | −3.07 ± 1.79 | <0.001b | −3.14 ± 1.44 | <0.001a | −2.92 ± 1.35 | <0.001a |
| 30° nasal refraction, mean ± SD, (D) | −1.87 ± 2.07 | <0.001b | −1.73 ± 1.93 | <0.001b | −1.54 ± 1.51 | <0.001b | −1.47 ± 1.74 | <0.001b |
SD standard deviation, D dioptres.
aPaired-samples T tests were used to analyze the differences between central and peripheral refractions under non-cycloplegia and cycloplegia in each group.
bWilcoxon rank sum tests were used to analyze the differences between central and peripheral refractions under non-cycloplegia and cycloplegia in each group.
Fig. 1. The differences in relative peripheral refractions between myopic children with and without atropine 0.01% eyedrops.
a Mean relative peripheral refractions in myopic children with and without atropine 0.01% eye drops under non-cycloplegia. Error bars are 95% CIs of the means. b Mean relative peripheral refractions in myopic children with and without atropine 0.01% eyedrops under cycloplegia. Error bars are 95% CIs of the means.
Under cycloplegia, the RPRs at −15°, −30°, +15°, and +30° retina were −0.27 ± 0.59D, −0.11 ± 1.48D, 0.42 ± 0.82D, and 1.76 ± 1.35D in the study group and −0.43 ± 0.77D, −0.50 ± 1.68D, 0.65 ± 0.74D, and 2.14 ± 1.61D in the control group (Table 1). Both groups have significant relative myopia at the temporal 15° retina and significant relative hyperopia in the nasal retina (P < 0.05 for all; Table 2). Furthermore, all the absolute values of RPRs were smaller in the study group than those in the control group (P > 0.05 for all; Table 1; Fig. 1b).
No matter under non-cycloplegia or cycloplegia, both groups had more significant hyperopia in the nasal retina (P < 0.01; Table 2).
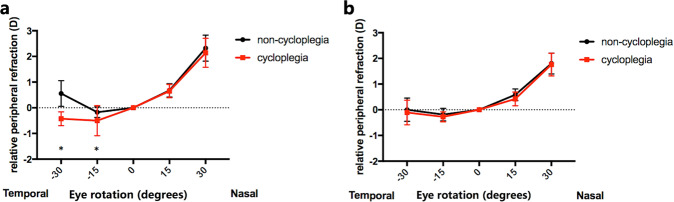
In the control group, there was significantly less myopia in central refraction after cycloplegia (P < 0.001). As a hyperopic shift in the central refraction, there were significantly myopic shifts, which meant less hyperopia in temporal RPRs (P < 0.001; P = 0.039). No significant changes in nasal RPRs occurred (Table 1; Fig. 2a). In the study group, the distributions of RPRs under non-cycloplegia and cycloplegia were close to overlap. There were no significant refraction changes presented after cycloplegia, no matter in central or peripheral visual fields (Table 1; Fig. 2b).
Fig. 2. The differences in relative peripheral refractions between the non-cycloplegic and cycloplegic refractions in myopic children.
a Mean relative peripheral refractions in myopic children without atropine 0.01% eye drops under non-cycloplegia and cycloplegia. Error bars are 95% CIs of the means. The marks show the significant differences between the non-cycloplegic and cycloplegic refractions: *P < 0.05. b Mean relative peripheral refractions in myopic children with atropine 0.01% eye drops under non-cycloplegia and cycloplegia. Error bars are 95% CIs of the means. There were no significant differences between the non-cycloplegic and cycloplegic refractions.
Discussion
As far as we know, this is the first time to evaluate the effect of atropine 0.01% eyedrops on RPR and DSE in the horizontal visual field. We performed the study based on a randomized, double-blinded, cross-over trial, which enhanced the credibility of the results. Radhakrishnan et al. demonstrated that myopes presented more peripheral hyperopia in the nasal retina than the temporal retina [18]. The study by Li et al. also showed nasal/temporal asymmetry with a higher level of relative peripheral hyperopia when the eyes rotated to the nasal visual angle [19]. In our study, myopic children presented a hook-type RPR pattern with greater relative hyperopia in the nasal retina, which was in line with previous studies. The retinal shape is asymmetric between the nasal and the temporal part along the horizontal meridian, given the optic nerve inserted into the nasal retina [8, 20–23]. The fovea also presents a temporal displacement to the optical axis [24, 25]. Moreover, the curvature of the nasal cornea and the sagittal height of the nasal sclera, which correspond to the imaging in the temporal retina, are both less than the curvature and sagittal height of the temporal part [26–29]. All these anatomic and physiological properties might work together and promote the nasal-temporal asymmetry of RPRs. On that basis, we found that atropine 0.01% eyedrops and cycloplegia had more effect on temporal RPRs. As the study by Kaufman et al. showed, during accommodation, the ciliary muscle could pull the Bruch’s membrane, the peripheral choroid, and further induce movement [30]. The optic nerve inserted into the nasal retina might reduce the impact of the accommodation on the nasal RPRs. That can explain why cycloplegia and atropine have a more significant effect on temporal RPRs, even though there is more relative hyperopia in the nasal retina. In addition, the base value of relative hyperopia in the temporal retina was lower than that in the nasal retina. Therefore, the myopic shift in peripheral fields was more liable to induce relative hyperopia disappeared or alleviated in the temporal field. Some studies also demonstrated that the temporal retina had a more significant association with myopic progression and AXL elongation than the nasal retina [22, 31]. The current result was indirectly identical to previous studies that further attracted more attention on the temporal field.
Sun et al. analyzed the effect of atropine on peripheral refraction by enrolling myopic children who had been using different concentrations of atropine for above six months. In the study, the doses of atropine were determined by ophthalmologists and could be altered during the treatment. The results showed that myopic children with atropine eye drops had significant relative peripheral myopia at the temporal 30° field. Besides, low myopes with atropine had greater relative myopia at 30° of the temporal and nasal fields when compared with subjects without atropine [32]. Our finding also suggested the atropine 0.01% relieved the relative hyperopia in the temporal retina. Under non-cycloplegia, the significantly relative hyperopia at 30° of the temporal retina presented in the subjects with placebo and disappeared in the subjects with atropine 0.01% eyedrops. In addition, compared with myopic children with placebo, the children with atropine 0.01% presented myopic shifts in all peripheral horizontal visual positions in daily life (under non-cycloplegia) and a flatter peripheral refraction pattern with smaller absolute values of RPRs under cycloplegia. Even though the differences in RPRs between the two groups were not statistically significant, the same trends were present in all visual positions. According to the previous study, a flatter RPR pattern was prone to occur in emmetropic eyes [33].
Furthermore, the once-nightly dose of atropine 0.01% eyedrops reduced the DSE in the central refraction. Previous studies regarded DSE as an over-active accommodation or excessive accommodation due to a ciliary muscle spasm, which usually occurred after sustained near work. The study by Lin et al. further proposed that a larger baseline DSE was associated with more significant myopia progression through increasing myopic retinal defocus in myopic children [14]. Near work-induced transient myopia, as accommodation tonus of the ciliary muscle after near work, has resemblance with DSE and could be a risk factor in myopia progression [34, 35]. The study conducted by Guo et al. demonstrated that atropine 0.01% eyedrops effectively reduced the magnitude of initial near work-induced transient myopia, which indirectly supported our findings [36]. For the effect of atropine 0.01% on accommodation amplitude, some studies suggested that atropine 0.01% could lower accommodation amplitude. The change was not significant [37, 38]. Here, we believed atropine 0.01% still influenced accommodation, especially on the over-active accommodation resulting from a temporary ciliary muscle spasm in distant vision.
In the meantime, the once-nightly dose of atropine 0.01% eyedrops also reduced the DSE in peripheral refractions. For temporal RPRs, myopic children with the placebo had significant myopic shifts after cycloplegia. However, these changes were not significant in myopic children with atropine 0.01% eyedrops. The RPR patterns under cycloplegia and non-cycloplegia were close to overlapping in children with atropine. As a visual stimulus, hyperopic defocus could lead to thinning choroidal thickness and scleral hypoxia [39–41]. Previous studies also showed that atropine could alleviate the choroidal thinning caused by hyperopic defocus [39, 40]. Therefore, we speculated that atropine 0.01% eyedrops could relieve relative temporal hyperopia and corresponding ocular reactions resulting from DSE, which was one of the mechanisms of atropine 0.01% eyedrops in myopia control.
At present, the meaning of RPR in myopia development and progression is debatable. Many animal studies have testified that peripheral vision plays an important role in ocular growth. Benavente-Pérez et al. proved that peripheral hyperopic defocus could induce axial myopia, and conversely, peripheral myopic defocus could induce axial hyperopia [42]. The study conducted by Smith et al. testified that peripheral form deprivation in the ocular development phase could lead to myopic refractive errors and accelerate eye growth [10]. Irving fitted chicks with myopia progression control lenses covering peripheral positive power and conventional lenses to verify whether lens-induced myopia could be reversed by peripheral myopic defocus. The results showed that the treated eyes with special peripheral designs had significantly negative axial length changes than control eyes [11]. Population-based studies also demonstrated that lens with reducing relative peripheral hyperopia could effectively lower myopia progression [43]. However, most studies showed that no matter under cycloplegia or non-cycloplegia, baseline RPR could not predict myopia development and progression in Asian and European children [18, 44, 45]. Further analyzing the previous studies, we found that most of the relevant cohort studies used the static values of RPR under cycloplegia or non-cycloplegia to predict myopia progression. Just as we can’t predict myopia development through central refraction, it’s probably hard to detect the relationship between static RPR and myopia progression. As a dynamic indicator, DSE in peripheral refraction could be a novel and useful method to study the relationship between RPR and myopia.
In the current study, myopic children in the control group has used atropine 0.01% for one year and then crossed over to placebo eyedrops for six months. According to the research by Chia et al., the effect of atropine 0.01% eyedrops on accommodation disappeared entirely after six months of stopping the drug [6]. Therefore, we considered that the control group could be used as a blank control to compare subjects using atropine 0.01% eyedrops. Some limitations of the study also should be recognized. The sample size was limited. Given that the subjects were on continuous medication and the RPR examination operation took some time, it was hard to enlarge the sample size. Besides, as a cross-sectional study, the study is hard to reflect the effect of atropine 0.01% eye drops on RPR and DSE in myopic development directly. A large, longitudinal study can provide more sound evidence for the association in myopia control with RPR and DSE.
In conclusion, we firstly assessed the impacts of atropine 0.01% eyedrops on RPR, DSE, and two factors combined in myopic children. The study shows that atropine 0.01% may alter the horizontal RPR pattern. Compared with myopic children with placebo eyedrops, atropine 0.01% eyedrops had myopic shifts (less hyperopia or more myopia) in all RPRs under non-cycloplegia and a flatter horizontal RPR pattern under cycloplegia. After cycloplegia, as a hyperopic shift in the central refraction, there were less hyperopia in temporal RPR and no significant changes in nasal RPR. Atropine 0.01% eye drops can alleviate the shifts in the central visual field and temporal RPR. According to the study, we speculated that atropine 0.01% eyedrops could control the myopia development and progression through adjusting over-active accommodation and peripheral visual signals in distant vision. The temporal RPR might have more crucial influences on myopic progression. Moreover, the difference between non-cycloplegic RPR and cycloplegic RPR could become a new, helpful indicator investigating the relationship between RPR and myopia. A study with greater sample size and longer observation could be performed to evaluate the effect of atropine 0.01% eyedrops on RPR and DSE and the related mechanism in myopic control.
Summary
What was known before
Atropine 0.01% eyedrops can effectively relieve myopia progression and reduce the side effects.
What this study adds
Atropine 0.01% eyedrops can alleviate relative peripheral hyperopia in the temporal retina and the difference between cycloplegic and non-cycloplegic autorefraction in myopic children.
Acknowledgements
The authors thank support and help from Beijing Tongren Hospital, Capital Medical University.
Author contributions
JT, SW, SL and NW: study concept and design and manuscript revision. JT, SW, WA, WB, XL and JD: performed the study. JT: drafted manuscript. JT and SW: statistical analysis. SW, SL and NW: revised the manuscript. NW: administrative, technical, material support, or study supervision. All authors participated in and provided help for the study.
Funding
The work was supported by Beihang University-CMU, Advanced Innovation Center for Big Data-Based Precision Medicine, Ophthalmic subcenter and Sanming Project of Medicine in Shenzhen (No. SZSM201512045).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Holden B, Fricke T, Wilson D, Jong M, Naidoo K, Sankaridurg P, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123:1036–42. doi: 10.1016/j.ophtha.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Wei S, Sun Y, Li S, Hu J, Yang X, Lin C, et al. Refractive errors in University Students in Central China: the Anyang University students eye study. Invest Ophthalmol Vis Sci. 2018;59:4691–700. doi: 10.1167/iovs.18-24363. [DOI] [PubMed] [Google Scholar]
- 3.Quek T, Chua C, Chong C, Chong J, Hey H, Lee J, et al. Prevalence of refractive errors in teenage high school students in Singapore. Ophthalmic Physiol Opt. 2004;24:47–55. doi: 10.1046/j.1475-1313.2003.00166.x. [DOI] [PubMed] [Google Scholar]
- 4.Gimbel H. The control of myopia with atropine. Can J Ophthalmol. 1973;8:527–32. [PubMed] [Google Scholar]
- 5.Yen M, Liu J, Kao S, Shiao C. Comparison of the effect of atropine and cyclopentolate on myopia. Ann Ophthalmol. 1989;21:180–2. [PubMed] [Google Scholar]
- 6.Chia A, Chua W, Wen L, Fong A, Goon Y, Tan D. Atropine for the treatment of childhood myopia: changes after stopping atropine 0.01%, 0.1% and 0.5% Am J Ophthalmol. 2014;157:451–7. doi: 10.1016/j.ajo.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 7.Gong Q, Janowski M, Luo M, Wei H, Chen B, Yang G, et al. Efficacy and adverse effects of atropine in childhood myopia: a meta-analysis. JAMA Ophthalmol. 2017;135:624–30. doi: 10.1001/jamaophthalmol.2017.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faria-Ribeiro M, Queirós A, Lopes-Ferreira D, Jorge J, González-Méijome J. Peripheral refraction and retinal contour in stable and progressive myopia. Optom Vis Sci. 2013;90:9–15. doi: 10.1097/OPX.0b013e318278153c. [DOI] [PubMed] [Google Scholar]
- 9.Hoogerheide J, Rempt F, Hoogenboom W. Acquired myopia in young pilots. Ophthalmologica. 1971;163:209–15. doi: 10.1159/000306646. [DOI] [PubMed] [Google Scholar]
- 10.Smith E, Kee C, Ramamirtham R, Qiao-Grider Y, Hung L. Peripheral vision can influence eye growth and refractive development in infant monkeys. Invest Ophthalmol Vis Sci. 2005;46:3965–72. doi: 10.1167/iovs.05-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Irving E, Yakobchuk-Stanger C. Myopia progression control lens reverses induced myopia in chicks. Ophthalmic Physiol Opt. 2017;37:576–584. doi: 10.1111/opo.12400. [DOI] [PubMed] [Google Scholar]
- 12.Kang P, Swarbrick H. Peripheral refraction in myopic children wearing orthokeratology and gas-permeable lenses. Optom Vis Sci. 2011;88:476–482. doi: 10.1097/OPX.0b013e31820f16fb. [DOI] [PubMed] [Google Scholar]
- 13.Sankaridurg P, He X, Naduvilath T, Lv M, Ho A, Smith E, et al. Comparison of noncycloplegic and cycloplegic autorefraction in categorizing refractive error data in children. Acta Ophthalmol. 2017;95:e633–e640. doi: 10.1111/aos.13569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin Z, Vasudevan B, Ciuffreda K, Zhou H, Mao G, Wang N, et al. The difference between cycloplegic and non-cycloplegic autorefraction and its association with progression of refractive error in Beijing urban children. Ophthalmic Physiol Opt. 2017;37:489–97.. doi: 10.1111/opo.12381. [DOI] [PubMed] [Google Scholar]
- 15.Zhao J, Mao J, Luo R, Li F, Pokharel G, Ellwein L. Accuracy of noncycloplegic autorefraction in school-age children in China. Optom Vis Sci. 2004;81:49–55. doi: 10.1097/00006324-200401000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Wei S, Li S, An W, Du J, Liang X, Sun Y, et al. Safety and efficacy of low-dose atropine eyedrops for the treatment of myopia progression in chinese children: a randomized clinical trial. JAMA Ophthalmol. 2020;138:1178–84. doi: 10.1001/jamaophthalmol.2020.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thibos L, Wheeler W, Horner D. Power vectors: an application of Fourier analysis to the description and statistical analysis of refractive error. Optom Vis Sci. 1997;74:367–75. doi: 10.1097/00006324-199706000-00019. [DOI] [PubMed] [Google Scholar]
- 18.Radhakrishnan H, Allen P, Calver R, Theagarayan B, Price H, Rae S, et al. Peripheral refractive changes associated with myopia progression. Invest Ophthalmol Vis Sci. 2013;54:1573–81. doi: 10.1167/iovs.12-10278. [DOI] [PubMed] [Google Scholar]
- 19.Li S, Li S, Liu L, Zhou Y, Yang Z, Kang M, et al. Peripheral refraction in 7- and 14-year-old children in central China: the Anyang Childhood Eye Study. Br J Ophthalmol. 2015;99:674–79. doi: 10.1136/bjophthalmol-2014-305322. [DOI] [PubMed] [Google Scholar]
- 20.Flitcroft D. The complex interactions of retinal, optical and environmental factors in myopia aetiology. Prog Retin Eye Res. 2012;31:622–60. doi: 10.1016/j.preteyeres.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Mallen E, Kashyap P. Technical note: measurement of retinal contour and supine axial length using the Zeiss IOLMaster. Ophthalmic Physiol Opt. 2007;27:404–11. doi: 10.1111/j.1475-1313.2007.00490.x. [DOI] [PubMed] [Google Scholar]
- 22.Schmid G. Association between retinal steepness and central myopic shift in children. Optom Vis Sci. 2011;88:684–90.. doi: 10.1097/OPX.0b013e3182152646. [DOI] [PubMed] [Google Scholar]
- 23.Verkicharla P, Mathur A, Mallen E, Pope J, Atchison D. Eye shape and retinal shape, and their relation to peripheral refraction. Ophthalmic Physiol Opt. 2012;32:184–99.. doi: 10.1111/j.1475-1313.2012.00906.x. [DOI] [PubMed] [Google Scholar]
- 24.Rynders M, Lidkea B, Chisholm W, Thibos LN. Statistical distribution of foveal transverse chromatic aberration, pupil centration, and angle psi in a population of young adult eyes. J Opt Soc Am A Opt Image Sci Vis. 1995;12:2348–57. doi: 10.1364/JOSAA.12.002348. [DOI] [PubMed] [Google Scholar]
- 25.Pande M, Hillman JS. Optical zone centration in keratorefractive surgery. Entrance pupil center, visual axis, coaxially sighted corneal reflex, or geometric corneal center? Ophthalmology. 1993;100:1230–7. doi: 10.1016/S0161-6420(93)31500-9. [DOI] [PubMed] [Google Scholar]
- 26.Niyazmand H, Read S, Atchison D, Collins M. Anterior eye shape in emmetropes, low to moderate myopes, and high myopes. Cont Lens Anterior Eye. 2021;44:101361. doi: 10.1016/j.clae.2020.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Fadel D. The influence of limbal and scleral shape on scleral lens design. Cont Lens Anterior Eye. 2018;41:321–28.. doi: 10.1016/j.clae.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Consejo A, Llorens-Quintana C, Bartuzel M, Iskander D. Rozema. J Rotat asymmetry Hum sclera Acta Ophthalmol. 2019;97:e266–e270. doi: 10.1111/aos.13901. [DOI] [PubMed] [Google Scholar]
- 29.Ritzmann M, Caroline P, Börret R, Korszen E. An analysis of anterior scleral shape and its role in the design and fitting of scleral contact lenses. Cont Lens Anterior Eye. 2018;41:205–13.. doi: 10.1016/j.clae.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 30.Kaufman P, Lütjen Drecoll E, Croft M. Presbyopia and glaucoma: two diseases, one pathophysiology? The 2017 Friedenwald lecture. Invest Ophthalmol Vis Sci. 2019;60:1801–12. doi: 10.1167/iovs.19-26899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stone R, Pendrak K, Sugimoto R, Lin T, Gill A, Capehart C, et al. Local patterns of image degradation differentially affect refraction and eye shape in chick. Curr Eye Res. 2006;31:91–105. doi: 10.1080/02713680500479517. [DOI] [PubMed] [Google Scholar]
- 32.Sun H, Lu W, You J, Kuo H. Peripheral refraction in myopic children with and without atropine usage. J Ophthalmol. 2020;2020:4919154. doi: 10.1155/2020/4919154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lan W, Lin Z, Yang Z, Artal P. Two-dimensional peripheral refraction and retinal image quality in emmetropic children. Sci Reps. 2019;9:16203. doi: 10.1038/s41598-019-52533-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campbell F, Westheimer G. Dynamics of accommodation responses of the human eye. J Physiol. 1960;151:285–95. doi: 10.1113/jphysiol.1960.sp006438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ciuffreda K, Vasudevan B. Nearwork-induced transient myopia (NITM) and permanent myopia–is there a link? Ophthalmic Physiol Opt. 2008;28:103–14. doi: 10.1111/j.1475-1313.2008.00550.x. [DOI] [PubMed] [Google Scholar]
- 36.Guo L, Fan L, Tao J, Hua R, Yang Q, Gu H, et al. Use of topical 0.01% atropine for controlling near work-induced transient myopia: a randomized, double-masked, placebo-controlled study. J Ocul Pharm Ther. 2020;36:97–101. doi: 10.1089/jop.2019.0062. [DOI] [PubMed] [Google Scholar]
- 37.Loughman J, Flitcroft D. The acceptability and visual impact of 0.01% atropine in a Caucasian population. Br J Ophthalmol. 2016;100:1525–9. doi: 10.1136/bjophthalmol-2015-307861. [DOI] [PubMed] [Google Scholar]
- 38.Yam J, Jiang Y, Tang S, Law A, Chan J, Wong E, et al. Low-concentration atropine for myopia progression (LAMP) study: a randomized, double-blinded, placebo-controlled trial of 0.05%, 0.025%, and 0.01% atropine eye drops in myopia control. Ophthalmology. 2019;126:113–24. doi: 10.1016/j.ophtha.2018.05.029. [DOI] [PubMed] [Google Scholar]
- 39.Chiang S, Phillips J. Effect of atropine eye drops on choroidal thinning induced by hyperopic retinal defocus. J Ophthalmol. 2018;2018:8528315. doi: 10.1155/2018/8528315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sander B, Collins M, Read S. Short-term effect of low-dose atropine and hyperopic defocus on choroidal thickness and axial length in young myopic adults. J Ophthalmol. 2019;2019:4782536. doi: 10.1155/2019/4782536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu H, Chen W, Zhao F, Zhou Q, Reinach P, Deng L, et al. Scleral hypoxia is a target for myopia control. Proc Natl Acad Sci USA. 2018;115:E7091–E7100. doi: 10.1073/pnas.1721443115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benavente-Pérez A, Nour A, Troilo D. Axial eye growth and refractive error development can be modified by exposing the peripheral retina to relative myopic or hyperopic defocus. Invest Ophthalmol Vis Sci. 2014;55:6765–73. doi: 10.1167/iovs.14-14524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berntsen D, Barr C, Mutti D, Zadnik K. Peripheral defocus and myopia progression in myopic children randomly assigned to wear single vision and progressive addition lenses. Invest Ophthalmol Vis Sci. 2013;54:5761–70. doi: 10.1167/iovs.13-11904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rotolo M, Montani G, Martin R. Myopia onset and role of peripheral refraction. Clin Optpm. 2017;9:105–11.. doi: 10.2147/OPTO.S134985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Atchison DA, Li SM, Li H, Li SY, Liu LR, Kang MT, et al. Relative peripheral hyperopia does not predict development and progression of myopia in children. Invest Ophthalmol Vis Sci. 2015;56:6162–70. doi: 10.1167/iovs.15-17200. [DOI] [PubMed] [Google Scholar]