Abstract
Toxigenic Vibrio cholerae strains are lysogens of CTXΦ, a filamentous bacteriophage which encodes cholera toxin (CT). Following infection of recipient V. cholerae cells by CTXΦ, the phage genome either integrates into the host chromosome at a specific attachment site (attRS) or exists as a replicative-form (RF) plasmid. We infected naturally occurring attRS-negative nontoxigenic V. cholerae or attenuated (CTX− attRS negative) derivatives of wild-type toxigenic strains with CTXΦ and examined the diarrheagenic potential of the strains carrying the RF of the CTXΦ genome using the adult rabbit diarrhea model. Under laboratory conditions, strains carrying the RF of CTXΦ produced more CT than corresponding lysogens as assayed by a GM1-based enzyme-linked immunosorbent assay and by fluid accumulation in ligated ileal loops of rabbits. However, when tested for diarrhea in rabbits, the attRS-negative strains (which carried the CTXΦ genome as the RF) were either negative or produced mild diarrhea, whereas the attRS-positive strains with integrated CTXΦ produced severe fatal diarrhea. Analysis of the strains after intestinal passage showed that the attRS-negative strains lost the phage genome at approximately a fivefold higher frequency than under in vitro conditions, and 75 to 90% of cells recovered from challenged rabbits after 24 h were CT negative. These results suggested that strains carrying the RF of CTXΦ are unable to cause severe disease due to rapid loss of the phage in vivo, and the gastrointestinal environment thus provides selection of toxigenic strains with an integrated CTXΦ genome. These results may have implications for the development of live V. cholerae vaccine candidates impaired in chromosomal integration of CTXΦ. These findings may also contribute to understanding of the etiology of diarrhea occasionally associated with nontoxigenic V. cholerae strains.
Cholera is a severe dehydrating diarrhea caused by toxigenic strains of the gram-negative bacterium Vibrio cholerae. The profuse watery diarrhea is mainly due to an enterotoxin, cholera toxin (CT), produced by V. cholerae (4, 21). The ctxAB operon, which encodes the A and B subunits of CT, resides in the genome of CTXΦ, a lysogenic filamentous bacteriophage (27). In the natural habitat, CTXΦ may infect nontoxigenic V. cholerae strains, leading to the origination of novel toxigenic strains (5, 6). Following entry into the recipient cells, the phage genome either integrates into the chromosome at a specific attachment site (attRS), forming stable lysogens, or exists as a plasmid referred to as the replicative form (RF) of the phage genome (27). Attenuated live vaccine strains are supposed to be protected from lysogenic conversion by CTXΦ if the attRS sequence is deleted, thus impairing the chromosomal integration of the CTXΦ genome. Recently potential vaccine strains have been developed which lack the entire CTX element, including attRS sequences (1, 14, 25, 28). A previous study has shown that V. cholerae cells carrying the RF of the CTXΦ genome can produce CT under in vitro laboratory conditions (15). However, the diarrheagenic potential of such strains in vivo was not examined.
V. cholerae strains belonging to the O1 or O139 serogroup are normally associated with epidemic cholera, whereas other serogroups of V. cholerae have been mostly associated with sporadic cases of diarrhea (11). Recent studies have recorded incidences of diarrhea outbreaks associated with non-O1 non-O139 V. cholerae, but these strains have been found to be nontoxigenic, and the pathogenic determinants for the diarrhea have not been identified (23). It is not clear, however, whether transient acquisition of the CTXΦ genome by these strains in vivo might have led to the diarrhea. In the present study CTXΦ was introduced into attRS-negative non-O1 non-O139 V. cholerae of clinical or environmental origin as well as into attenuated (Δcore ΔattRS) derivatives of clinical V. cholerae O1 and O139 strains. The resulting strains, which carried the RF of the CTXΦ genome, were tested for production of a diarrheal response in adult rabbits and for stability of the phage genome. This study was designed to examine whether potential vaccine strains carrying a deletion of attRS or naturally occurring attRS-negative V. cholerae strains can become transient diarrheal pathogens by harboring the RF of the CTXΦ genome.
MATERIALS AND METHODS
Bacterial strains, phages, and plasmids.
The V. cholerae non-O1 non-O139 strains included in this study were either isolated from surface water samples collected in Dhaka, Bangladesh, or obtained from patients with diarrhea who attended the treatment center of the International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B), located in Dhaka. A total of 37 non-O1 non-O139 strains were first tested for possession of different virulence genes and the attRS sequence, and strains which were negative for attRS were further analyzed in this study. The O1 and O139 strains were attenuated derivatives of toxigenic clinical strains described previously (1, 17, 20, 25). Strains were stored either in lyophilized form or in sealed deep nutrient agar at room temperature until used for the present study. Before use, the identities of the cultures were verified by biochemical and serological methods (29) and by using specific DNA probes as described below. The genetically marked phage MSF8.2Φ used in this study was a derivative of an El Tor CTXΦ which carried a functional ctxAB operon as well as a kanamycin resistance (Kmr) determinant. The strategy for the construction of pMSF8.2 is described below. Relevant characteristics of bacterial strains, phages, and plasmids used in this study are summarized in Table 1.
TABLE 1.
Characteristics of bacterial strains, plasmids, and phages used in this study
| Strain(s), plasmid, or phage | Relevant characteristics | Reference(s) or source |
|---|---|---|
| SM44 | Derivative of El Tor strain P-27459 in which the CTX genetic element was marked with a Kmr determinant by marker exchange disrupting the ctxAB operon | 10 |
| pRT41 | Derivative of pBR322 carrying a wild-type ctxAB operon | 26 |
| pMSF8.2 | Derivative of pCTX-Km in which the ctxAB operon was reinserted from pRT41 | This study |
| P-27459 | Toxigenic clinical V. cholerae O1 El Tor strain | Laboratory collection |
| Bang-2 | Derivative of P27459 in which the core and RS sequences including attRS was deleted (P-27459 Δcore ΔattRS) | 14 |
| Bang-2(pMSF8.2) | Strain Bang-2 carrying pMSF8.2 | This study |
| Bah-1 | Derivative of El Tor strain E-7946 in which the core region of the CTX element was deleted (E-7946 Δcore) | 14 |
| Bah-1(MSF8.2) | Bah-1 lysogenized with MSF8.2Φ | This study |
| Bah-2 | E-7946 Δcore ΔattRS | 14 |
| Bah-2(pMSF8.2) | Strain Bah-2 carrying pMSF8.2 | This study |
| MO10 | Toxigenic clinical V. cholerae O139 strain | 1, 28 |
| Bengal-2 | MO10 Δcore ΔattRS | 1, 28 |
| Bengal-2 (pMSF8.2) | Bengal-2 carrying pMSF8.2 | This study |
| 55V71, Env-81 | Environmental attRS-negative non-O1 strains | Laboratory collection |
| 55V71(pMSF8.2) | Strain 55V71 carrying pMSF8.2 | This study |
| Env-81(pMSF8.2) | Strain Env-81 carrying pMSF8.2 | This study |
| AM-15746, AM-15714 | Clinical attRS-negative non-O1 non-O139 strains | Laboratory collection |
| AM-15746(pMSF8.2) | Strain AM-15746 carrying pMSF8.2 | This study |
| AM-15714(pMSF8.2) | Strain AM-15714 carrying pMSF8.2 | This study |
Recipient strains were infected with MSF8.2Φ under in vitro conditions as described previously (6, 7). Representative infected colonies were grown in Luria broth medium containing kanamycin (50 μg/ml) and were analyzed for the presence of the phage genome. Total DNA or plasmid DNA was extracted from overnight cultures by standard methods (18) and purified using microcentrifuge filter units (Ultrafree-Probind; Sigma Chemical Company, St. Louis, Mo.). The presence of the phage genome as the RF or its integration into the chromosomes of the recipient cells was examined by comparative Southern blot analysis of total DNA and plasmid preparations as described previously (6, 7).
Recombinant DNA procedures.
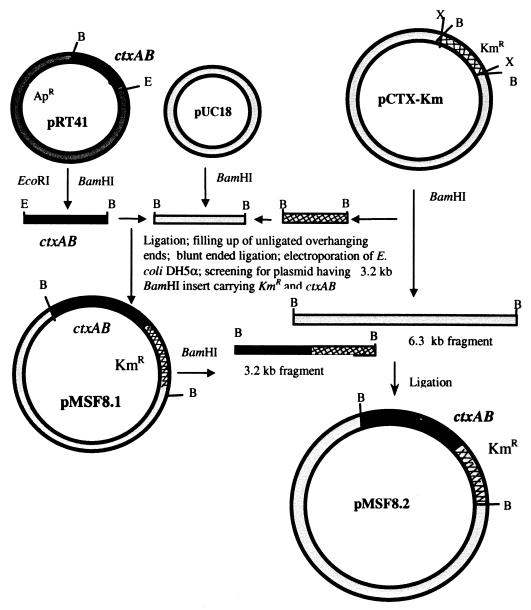
For in vitro DNA manipulations, pUC18, a chromogenic substrate (X-Gal [5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside]), and DNA restriction and DNA-modifying enzymes were purchased from Bethesda Research Laboratories (Gaithersburg, Md.) and used in accordance with the manufacturer's suggestions. The strategy for the construction of pMSF8.2, a genetically marked derivative of the RF of the CTXΦ genome carrying a functional ctxAB operon, is shown in Fig. 1. CTX-KmΦ isolated from strain SM44 (10, 27) was used to infect the classical biotype strain O395. The RF of the phage genome, pCTX-Km isolated from strain O395, carried a Kmr determinant in place of ctxAB genes. The entire ctxAB operon was obtained from another recombinant plasmid, pRT41 (26), and reinserted into pCTX-Km using a number of cloning steps to construct pMSF8.2. Briefly, pCTX-Km was digested with BamHI, and a 1.3-kb fragment encoding Kmr and the remaining 6.3-kb fragment of the phage genome were isolated. The 1.9-kb BamHI-EcoRI insert carrying the entire ctxAB operon, including the wild-type promoter, was isolated from pRT41. The DNA fragments carrying the ctxAB operon and Kmr were sequentially ligated to BamHI-cleaved dephosphorylated pUC18. The ligated DNA was isolated, and protruding ends were filled in using the Klenow fragment of Escherichia coli DNA polymerase I. The resulting blunt ends were then ligated, and the ligation mixture was used to electroporate E. coli DH5α. Colonies which were resistant to both kanamycin and ampicillin were screened for the presence of a pUC18 derivative with a 3.2-kb BamHI insert carrying the ctxAB genes and the gene encoding Kmr, and this plasmid was designated pMSF8.1. The 3.2-kb BamHI fragment of pMSF8.1 was isolated and ligated with the 6.3-kb BamHI fragment of pCTX-Km. The ligated DNA was used to electroporate V. cholerae strain O395, and colonies were selected for resistance to kanamycin. The final plasmid construct, designated pMSF8.2, thus consisted of a functional ctxAB operon, a Kmr cassette, and the RF DNA of CTXΦ and was able to support the morphogenesis of infectious phage particles.
FIG. 1.
Strategy for construction of pMSF8.2, a derivative of pCTX-Km carrying a functional ctxAB operon. Restriction sites: B, BamHI; X, XbaI; E, EcoRI. See the text for details.
Probes and PCR assays.
The gene probe used in this study to detect the CTXΦ genome was a 0.5-kb EcoRI fragment of pCVD27 carrying part of the ctxA gene (12). All strains were also tested for the presence of genes encoding the toxin-coregulated pilus (TCP) (which is the receptor for CTXΦ), the virulence regulatory gene toxR, and the CTXΦ attachment sequence attRS. The presence of the TCP pathogenicity island was determined by PCR assays specific for the tcpA, tcpI, and acfB genes as described previously (8, 13). The toxR gene probe was a 2.4-kb BamHI fragment of pVM7 (19), and the 18-bp attRS sequence was identified using a synthetic oligonucleotide corresponding to the attRS sequence (20).
Colony blots or Southern blots were prepared using nylon filters (Hybond; Amersham International plc., Ayelesbury, United Kingdom) and processed by standard methods (18). The polynucleotide probes were labeled by random priming (9) using a random-primer DNA labeling kit (Bethesda Research Laboratories) and [α-32P]dCTP (3,000 Ci/mmol; Amersham), and oligonucleotide probes were labeled by 3′ tailing using terminal deoxynucleotide transferase and [α-32P]dCTP (Amersham). Southern blots and colony blots were hybridized with the labeled probes and autoradigraphed as described previously (6–8).
Assay for CT production.
Production of CT by V. cholerae strains harboring the RF of CTXΦ as well as the CTXΦ lysogens was determined by the GM1 ganglioside-dependent enzyme-linked immunosorbent assay (ELISA) and the rabbit ileal loop assay as described previously (2, 8, 22). For each round of CT assay, 5 ml of AKI medium (1.5% Bacto Peptone, 0.4% yeast extract, 0.5% NaCl, 0.3% NaHCO3 [pH 7.4]) was inoculated with approximately 103 bacterial cells. For strains carrying the Kmr-labeled phage genome, kanamycin (50 μg/ml) was added to the culture medium to retain the phage genome. All cultures were grown for 16 h at 30°C with shaking. The culture was centrifuged at 4,000 × g for 5 min, and the supernatant was collected and filtered through 0.22-μm-pore-size Millipore filters. Aliquots of the undiluted supernatant, 10-fold and 100-fold dilutions of the supernatant, and dilutions of purified CT (Sigma) were used for the toxin assay. Quantification of CT production was done using a standard curve prepared for each batch of assay mixture. The amount of CT produced by each strain was the mean value from five different assays with the same strain and culture conditions. Toxigenic El Tor strain P-27459 and nontoxigenic El Tor strains SA-317 and SM44 were included as positive and negative control strains in each round of the assay.
Ileal loop assay.
Culture filtrates prepared for the ELISA were also tested in ileal loops of adult New Zealand White rabbits. A maximum of six ileal loops of approximately 10 cm in length were made in each rabbit (rabbits had previously been fasted for 48 h), and 1 ml of the filtrate was inoculated into each loop as described previously (2). After 18 h, rabbits were sacrificed and the loops were examined for fluid accumulation. The results were expressed as milliliters of fluid accumulated per centimeter of loop.
Assay for diarrhea in rabbits.
Diarrheal responses to V. cholerae strains were assayed in adult rabbits by using the removable intestinal tie-adult rabbit diarrhea model (24). Adult New Zealand White rabbits weighing 1.5 to 2.7 kg were used to prepare the model. Rabbits were starved for the previous 24 h, and surgery was done under a local anesthetic. The cecum of each animal was ligated to prevent it from retaining fluid secreted by the small intestine, and a temporary removable tie of the small bowl was introduced at the time of challenge. Strains were grown in Casamino Acids-yeast extract broth as described previously (24), and cells were precipitated by centrifugation and resuspended in 10 mM phosphate-buffered saline (pH 7.4) at a concentration of approximately 109 cells per ml. One millliliter of the suspension was injected into the lumen of the anterior jejunum. The removable tie in the intestine was removed after 2 h of inoculation. Each strain was inoculated in at least five different rabbits. Rabbits were observed for overt diarrhea and for death, and stools or rectal swabs were cultured on gelatin agar plates and a duplicate plate containing kanamycin (50 μg/ml) whenever appropriate to monitor shedding of the challenge organisms. Observations were made at 6-h intervals during the 7 days following inoculation; the numbers of rabbits developing moderate to severe diarrhea were arbitrarily scored, and the numbers of deaths were recorded. Rabbits that died with or without diarrhea were subjected to postmortem examinations to check for the presence of fluid in the intestine.
Stability of the CTXΦ genome.
To determine the stability of the CTXΦ genome in V. cholerae cells in vivo, the ratio of Kmr colonies to the total number of colonies recovered from stools or rectal swabs of rabbits challenged with each strain was calculated and expressed as the percentage of cells retaining the phage genome. To test the stability of the CTXΦ genome under in vitro conditions, representative colonies of the infected recipient were grown in aliquots of Luria broth either containing kanamycin (50 μg/ml) or without kanamycin. Serial dilutions of the cultures were plated on Luria agar plates containing kanamycin and on a duplicate set of Luria agar plates without the antibiotic to determine the proportion of cells retaining the phage genome.
Statistical analysis.
Statistical comparison of CT production between two groups of strains was carried out by the Mann-Whitney test. For comparison between the responses of different proportions of rabbits to different challenge strains, the χ2 statistic or Fisher's exact test was used. Differences were considered to be significant when the P value was ≤0.05. Data analyses were done by using statistical software (Sigmastat for Windows, version 2.03; Jandel Scientific, San Rafael, Calif.).
RESULTS AND DISCUSSION
We evaluated the diarrheagenic potential of strains carrying the RF of the CTXΦ genome to investigate whether strains carrying a deletion of attRS or naturally occurring attRS-negative V. cholerae strains can become transient diarrheal pathogens by temporarily harboring the CTXΦ genome. In this study, V. cholerae strains carrying the RF of the CTXΦ genome were constructed by infecting attRS-negative V. cholerae strains with a genetically marked phage, MSF8.2Φ. Infected cells were first selected by their expression of the Kmr phenotype. While all O1 and O139 strains which carried genes for the CTXΦ receptor TCP were infected by the phage, 4 of 29 TCP-negative, attRS-negative non-O1 strains were also infected. Previous studies have also reported that a small proportion of TCP-negative strains were susceptible to the phage (6). Molecular analysis of the infected strains showed the presence of RF DNA of the phage in all attRS-negative strains, although in control attRS-positive strains the phage genome integrated into the chromosome (data not shown). All O1 and O139 strains as well as the four infected non-O1 non-O139 strains (Tables 2 and 3) were further analyzed for production of CT and for causing diarrhea in the adult rabbit model. All of these strains carried the toxR gene, which is required for expression of major virulence factors in V. cholerae.
TABLE 2.
Production of CT by native V. cholerae strains and their derivatives carrying the RF DNA of a genetically marked CTXΦ
| Strain | CT productiona (GM1-ELISA) | Fluid accumulationb in rabbit ileal loops (ml/cm of ileal loop) |
|---|---|---|
| P-27459 | 2.25 ± 0.63 | 2.09 ± 0.45 |
| Bang-2 | UDc | 0 |
| Bang-2(pMSF8.2) | 2.92 ± 0.52 | 2.17 ± 0.72 |
| Bah-1 | UD | 0 |
| Bah-1(MSF8.2) | 2.19 ± 0.45 | 2.23 ± 0.63 |
| Bah-2 | UD | 0 |
| Bah-2(pMSF8.2) | 2.77 ± 0.23 | 2.39 ± 0.52 |
| MO10 | 2.83 ± 0.25 | 2.17 ± 0.32 |
| Bengal-2 | UD | 0 |
| Bengal-2(pMSF8.2) | 3.81 ± 0.73 | 2.90 ± 0.68 |
| 55V71 | UD | 0 |
| 55V71(pMSF8.2) | 2.95 ± 0.41 | 2.13 ± 0.62 |
| Env-81 | UD | 0 |
| Env-81(pMSF8.2) | 2.51 ± 0.25 | 1.92 ± 0.54 |
| AM-15746 | UD | 0 |
| AM-15746(pMSF8.2) | 2.61 ± 0.33 | 1.52 ± 0.41 |
| AM-15714 | UD | 0 |
| AM-15714(pMSF8.2) | 3.77 ± 0.47 | 2.72 ± 0.55 |
Toxin amounts are expressed in micrograms per unit of optical density of the culture at 600 nm. Values represent the averages and standard deviation from five independent observations. When assayed by GM1-ELISA, the differences in CT production by strains carrying the integrated form of the CTXΦ genome and those carrying the RF were statistically significant (P < 0.01).
Values represent the averages and standard deviations from five independent observations made in different rabbits.
UD, undetectable. The toxin amounts were less than 0.01 μg/ml, which was the lowest concentration of purified toxin used as control (see text for details).
TABLE 3.
Diarrheal response of adult rabbits to toxigenic V. cholerae strains (CTXΦ lysogens) and their derivatives carrying the RF of the phage
| Strain | Characteristics | No. of animals challenged | No. responding witha:
|
||
|---|---|---|---|---|---|
| Fatal diarrheab | Nonfatal diarrheacd | No symptomsd | |||
| Bang-2 | P-27459 Δcore ΔattRS | 4 | 0 | 0 | 4 |
| P-27459 | Toxigenic El Tor strain | 5 | 3 | 1 | 1 |
| Bang-2(pMSF8.2) | Strain Bang-2 carrying pMSF8.2 | 5 | 0 | 3 | 2 |
| Bah-1 | Strain E-7946 Δcore | 5 | 0 | 0 | 5 |
| Bah-2 | Strain E-7946 Δcore ΔattRS | 5 | 0 | 1e | 4 |
| Bah-1(MSF8.2) | Bah-1 lysogenized with MSF8.2Φ | 6 | 3 | 3 | 0 |
| Bah-2(pMSF8.2) | Strain Bah-2 carrying pMSF8.2 | 5 | 0 | 4 | 1 |
| MO10 | Toxigenic O139 strain | 6 | 4 | 2 | 0 |
| Bengal-2 | Strain MO10 Δcore ΔattRS | 5 | 0 | 0 | 5 |
| Bengal-2(pMSF8.2) | Bengal-2 carrying pMSF8.2 | 5 | 0 | 3 | 2 |
| 55V71 | Environmental attRS-negative V. cholerae non-O1 | 3 | 0 | 0 | 3 |
| 55V71(pMSF8.2) | Strain 55V71 carrying pMSF8.2 | 5 | 0 | 3 | 2 |
| Env-81 | Environmental attRS-negative V. cholerae non-O1 | 3 | 0 | 0 | 3 |
| Env-81(pMSF8.2) | Strain Env-81 carrying pMSF8.2 | 6 | 0 | 3 | 3 |
| AM-15746 | Clinical V. cholerae non-O1 | 3 | 0 | 0 | 3 |
| AM-15746(pMSF8.2) | Strain AM-15746 carrying pMSF8.2 | 5 | 0 | 4 | 1 |
| AM-15714 | Clinical V. cholerae non-O1 | 5 | 0 | 1e | 4 |
| AM-15714(pMSF8.2) | Strain AM-15714 carrying pMSF8.2 | 6 | 0 | 4 | 2 |
Differences between the proportions of rabbits responding with fatal or nonfatal diarrhea to CTXΦ lysogens and to strains carrying the RF of CTXΦ were statistically significant (P < 0.001). Differences between the proportions of rabbits responding with diarrhea to native non-O1 strains and their derivatives carrying the RF of CTXΦ were also statistically significant (P = 0.001).
Rabbits developing fatal diarrhea died within 12 h after initiation of diarrhea.
The duration of diarrhea was between 4 and 6 days for rabbits challenged with the attRS-positive toxigenic strains and between 0 and 2 days for animals challenged with attRS-negative strains carrying the RF of CTXΦ.
Animals were positive for the challenge organism in either stool or rectal swab cultures for 5 to 7 days.
These rabbits had very mild symptoms and excreted slightly mucoid, unformed stools for less than 24 h.
Expression of virulence.
Production of CT by the infected cells was initially studied in vitro by GM1-based ELISA, using an antibody against the B subunit of CT (Table 2). These assays showed that strains carrying the RF of CTXΦ produced significantly more CT than the lysogens (P < 0.01). We suspect that the higher levels of CT produced by strains carrying the RF were due to possession of multiple copies of the phage genome and hence multiple copies of the ctxAB operon. In CTXΦ lysogens, the expression of CT is regulated by the transcriptional activator ToxR, whereas in strains carrying the RF of the CTXΦ genome, expression of CT is also known to occur independently of ToxR (15). Thus, the ToxR-independent pathway of expression might also have contributed to the high levels of in vitro expression of CT by strains carrying the RF of the phage genome. To further ascertain whether the toxin was biologically active, we used the ligated ileal loop assay in rabbits and observed fluid accumulation. All culture supernatants which were positive for CT in the ELISA also caused fluid accumulation in the ileal loops of rabbits. Culture supernatants of strains carrying the RF of CTXΦ caused somewhat more fluid accumulation in the rabbit ileal loops than the lysogens did (Table 2), although the difference was not statistically significant. Considering possible variation in response to CT among individual rabbits, the number of observations was possibly less than optimum for observation of a statistically significant difference between the two groups. Nevertheless, these results confirmed a previous observation (15) that strains carrying the RF of CTXΦ produce biologically active CT in their culture supernatants.
Rabbits challenged with CTX-negative V. cholerae O1 or non-O1 strains did not show a diarrheal response (Table 3), but V. cholerae strains carrying integrated MSF8.2Φ as well as the native toxigenic strains P-27459 and MO10 produced severe diarrhea in rabbits. On the other hand, the attRS-negative strains carrying the RF of the CTXΦ genome were either negative or produced mild diarrhea (Table 3). Differences between the proportions of rabbits responding with fatal or nonfatal diarrhea to CTXΦ lysogens and to strains carrying the RF of CTXΦ were statistically significant (P < 0.001). Differences between the proportions of rabbits responding with diarrhea to native non-O1 strains and their derivatives carrying the RF of CTXΦ were also statistically significant (P = 0.001).
To examine the reason for the apparent inability of most attRS-negative strains to cause diarrhea, we monitored the stability of the CTXΦ genome in these strains (Table 4). Analysis of strains excreted by the challenged rabbits for the presence of the phage genome showed that a high proportion of cells (75 to 90%) lost the unintegrated phage genome in the first 24 h, whereas almost 99% of cells became negative for pMSF8.2 by 48 h. In the lysogens, however, ≈100% of the excreted cells retained the CTXΦ genome. These results suggested that only strains carrying the integrated CTXΦ genome can cause full-blown disease. It may be mentioned that toxigenic strains isolated from cholera patients have always been found to carry the phage genome in the prophage state. Expression of critical virulence genes in V. cholerae is known to be coordinately regulated, so that multiple genes respond in a similar fashion to environmental conditions (3). Coordinate expression of virulence genes results from the activity of a cascading system of regulatory factors. The pathogenesis of cholera involves a sequential expression of two major virulence factors (16). These include the colonization factor TCP and the enterotoxin CT, both of which are under the regulation of ToxR, a 32-kDa transmembrane protein. In the present study the time to development of diarrhea and the duration of diarrhea in rabbits challenged with CTXΦ lysogens were longer than those for strains carrying the RF (Table 3). The challenge strains were excreted by all rabbits for at least 6 days, suggesting colonization of the intestines by the strains and hence adequate expression of colonization factors. The mild and short-lived diarrhea observed in some rabbits challenged with strains carrying the RF thus appears to be a response to toxins produced early in the experiment from the RF of CTXΦ, which is known to occur independently of ToxR. After this, the CTXΦ genome was probably lost, rendering the cells nontoxigenic. The results of this study thus suggest that for a diarrheal response like that seen in cholera, a sustained expression of the ctxAB genes is required.
TABLE 4.
Loss of the CTXΦ genome from toxigenic V. cholerae strains (CTXΦ lysogens) and their derivatives carrying the RF of the CTXΦ genome in the gastrointestinal tracts of rabbits
| Strain | % of excreted cells retaining CTXΦ genome at the following day after challengea:
|
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| P-27459 | 100 | 100 | 100 | 100 | 100 |
| Bang-2(pMSF8.2) | 27.5 | 0.03 | 0.005 | 0.001 | 0 |
| Bah-1(MSF8.2) | 100 | 100 | 99.5 | 100 | 98.7 |
| Bah-2(pMSF8.2) | 18.6 | 0.72 | 0.005 | 0.001 | 0 |
| MO10 | 100 | 100 | 100 | 100 | 100 |
| Bengal-2(pMSF8.2) | 12.8 | 0.25 | 0.019 | 0.005 | 0.001 |
| 55V71(pMSF8.2) | 10.6 | 0.01 | 0.007 | 0 | 0 |
| Env-81(pMSF8.2) | 12.4 | 0.05 | 0.009 | 0 | 0.005 |
| AM-15746(pMSF8.2) | 15.2 | 0.95 | 0.007 | 0 | 0 |
| AM-15714(pMSF8.2) | 24.6 | 0.19 | 0.002 | 0 | 0 |
Results are averages of values obtained from at least three different rabbits which survived on the day of observation.
Diarrheal response to non-O1 non-O139 strains.
In the present study, although the native non-O1-non-O139 strains did not cause diarrhea in the rabbit model (Table 3), 14 of 22 rabbits (63.6%) inoculated with V. cholerae non-O1 non-O139 strains carrying pMSF8.2 developed mild to moderate diarrhea. While the O1 and O139 strains carried genes for the colonization factor TCP, the non-O1 non-O139 strains were TCP negative. Apparent colonization of rabbit intestines by these TCP-negative strains suggests that these strains probably produce some other, unknown colonization factors. Further studies are under way in our laboratory to characterize possible new colonization factors produced by the TCP-negative clinical strains. The diarrhea caused by non-O1 non-O139 strains carrying the RF of the CTXΦ derivative was of short duration (12 to 36 h), and the strains when excreted were mostly CT negative due to in vivo loss of the RF DNA. This scenario resembles clinical cases of diarrhea due to non-O1 vibrios, when strains cultured from the stool are usually CT negative (23). Besides the possibility of the diarrhea being induced by additional, unknown virulence factors, it also seems possible that in the natural habitat some non-O1 strains become transiently toxigenic by acquisition of CTXΦ. Such strains are not normally detected until they infect a host and produce diarrhea. The CTXΦ genome is probably lost in the intestinal tract following infection and production of mild diarrhea by such strains. However, it is not clear what determines the stability of the unintegrated CTXΦ genome in these cells under environmental conditions prior to infecting a mammalian host. An alternative explanation may be that such strains acquire the phage genome while inside the intestine of the host and transiently produce CT.
Selection of CTXΦ lysogens in the intestine.
Previous studies have suggested that in the gastrointestinal environment expression of CT confers a survival advantage to V. cholerae and that hypertoxigenic strains are selected in vivo by a need to upregulate the expression of CT. In the present study, strains carrying the RF of CTXΦ rapidly lost the phage genome in vivo, whereas strains carrying the integrated form of the phage retained the phage genome. We also examined the stability of the RF in V. cholerae cells under in vitro conditions. Although normally the Kmr-labeled phage genome was retained by the recipient cells when grown in the presence of kanamycin, a proportion of the cells lost the phage genome in the absence of kanamycin. However, the loss of the phage genome in vitro was more gradual than that in vivo. After 24 h of culture in the absence of kanamycin, 15 to 32% of cells lost the phage genome in vitro, whereas the frequency of loss in the rabbit intestine was between 75 and 90% for all strains tested after the first 24 h. This was unexpected, particularly since in the intestine, where TCP is adequately expressed, the CTXΦ is expected to be maintained in the cells due to continuous reinfection (15). Thus, the gastrointestinal tract seems to select CTXΦ lysogens rather than strains carrying the RF, although the latter strains produced higher levels of CT when assayed in vitro. This may explain why all naturally occurring toxigenic strains of V. cholerae carry the CTXΦ genome in the lysogenic form and particularly in serogroups which are capable of causing human disease. CTXΦ is different from the well-characterized filamentous bacteriophages derived from E. coli in that this phage has evolved to possess genes for a site-specific integration system. Integration of the CTXΦ genome into the host chromosome seems to allow it to withstand the intestinal selection, whereas the ability of the prophage to enter the replicative state maximizes horizontal propagation.
Implications for vaccine development.
The development of live oral vaccines against cholera invariably involves the deletion of the ctxA gene encoding the enzymatic subunit of CT or of the entire CTX element, thus rendering the strain nontoxigenic. The discovery of CTXΦ has shown that the possibility of reversion of attenuated strains by reacquisition of the CTXΦ genome is real. The receptor for CTXΦ for entry into a recipient V. cholerae cell is the TCP, which is also the major colonization factor. Since to elicit an adequate immune response live vaccines must colonize the intestine, TCP is retained in vaccine strains. A recent approach to protect attenuated live vaccine strains from lysogenic conversion by CTXΦ involves deleting the attRS sequence, thus impairing the chromosomal integration of the CTXΦ genome. Thus, although the attenuated strains remain susceptible to infection by CTXΦ, in strains with the attRS sequence deleted the phage genome is expected to exist as a replicative plasmid. In the present study, we found that such strains with the RF of CTXΦ lose the phage genome when introduced into the intestines of rabbits. The diarrheal response, if any, was mild, and the excreted strains were mostly nontoxigenic. These results confirmed that attenuated vaccine strains with attRS deletions are considerably protected from generating stable toxigenic revertants.
ACKNOWLEDGMENTS
This research was funded in part by the U.S. Agency for International Development under grant HRN-5986-A-00-6005-00 with the ICDDR,B and by the National Institutes of Health under grant RO1 AI39129-01A1 with the Department of International Health, Johns Hopkins University, and ICDDR,B. The ICDDR,B is supported by countries and agencies which share its concern for the health problems of developing countries.
We thank Tasnim Azim for help with the data analysis and Afjal Hossain for secretarial assistance.
REFERENCES
- 1.Coster T S, Killeen K P, Waldor M K, Beattie D T, Spriggs D R, Kenner J R, Trofa A, Sadoff J C, Mekalanos J J, Taylor D N. Safety, immunogenicity, and efficacy of live attenuated Vibrio cholerae O139 vaccine prototype. Lancet. 1995;354:949–952. doi: 10.1016/s0140-6736(95)90698-3. [DOI] [PubMed] [Google Scholar]
- 2.De S N, Chatterje D N. An experimental study of the mechanisms of action of Vibrio cholerae on the intestinal mucous membrane. J Pathol Bacteriol. 1953;46:559–562. doi: 10.1002/path.1700660228. [DOI] [PubMed] [Google Scholar]
- 3.DiRita V J. Co-ordinate expression of virulence genes by ToxR in Vibrio cholerae. Mol Microbiol. 1992;6:451–458. doi: 10.1111/j.1365-2958.1992.tb01489.x. [DOI] [PubMed] [Google Scholar]
- 4.Faruque S M, Albert M J, Mekalanos J J. Epidemiology, genetics and ecology of toxigenic Vibrio cholerae. Microbiol Mol Biol Rev. 1998;62:1301–1314. doi: 10.1128/mmbr.62.4.1301-1314.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faruque S M, Asadulghani, Alim A R M A, Albert M J, Islam K M N, Mekalanos J J. Induction of the lysogenic phage encoding cholera toxin in naturally occurring strains of toxigenic V. cholerae O1 and O139. Infect Immun. 1998;66:3752–3757. doi: 10.1128/iai.66.8.3752-3757.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faruque S M, Asadulghani, Saha M N, Alim A R M A, Albert M J, Islam K M N, Mekalanos J J. Analysis of clinical and environmental strains of nontoxigenic Vibrio cholerae for susceptibility to CTXΦ: molecular basis for the origination of new strains with epidemic potential. Infect Immun. 1998;66:5819–5825. doi: 10.1128/iai.66.12.5819-5825.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faruque S M, Rahman M M, Asadulghani, Islam K M N, Mekalanos J J. Lysogenic conversion of environmental Vibrio mimicus strains by CTXΦ. Infect Immun. 1999;67:5723–5729. doi: 10.1128/iai.67.11.5723-5729.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faruque S M, Ahmed K M, Alim A R M A, Qadri F, Siddique A K, Albert M J. Emergence of a new clone of toxigenic Vibrio cholerae biotype El Tor displacing V. cholerae O139 Bengal in Bangladesh. J Clin Microbiol. 1997;35:624–630. doi: 10.1128/jcm.35.3.624-630.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feinberg A, Volgelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1984;137:266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg I, Mekalanos J J. Effect of a recA mutation on cholera toxin gene amplification and deletion events. J Bacteriol. 1986;165:723–731. doi: 10.1128/jb.165.3.723-731.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janda J M, Powers C, Bryant R G, Abbott S L. Current perspective on the epidemiology and pathogenesis of clinically significant Vibrio spp. Clin Microbiol Rev. 1988;1:245–267. doi: 10.1128/cmr.1.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaper J B, Morris Jr J G, Nishibuchi M. DNA probes for pathogenic Vibrio species. In: Tenover F C, editor. DNA probes for infectious disease. Boca Raton, Fla: CRC Press, Inc.; 1988. pp. 65–77. [Google Scholar]
- 13.Keasler S P, Hall R H. Detection and biotyping of Vibrio cholerae O1 with multiplex polymerase chain reaction. Lancet. 1993;341:1661. doi: 10.1016/0140-6736(93)90792-f. [DOI] [PubMed] [Google Scholar]
- 14.Kenner J R, Coster T S, Taylor D N, Trofa A F, Barrera-Oro M, Hyman T, Adams J M, Beattie D T, Killeen K P, Spriggs D R, Mekalanos J J, Sadoff J C. Peru-15, an improved live attenuated oral vaccine candidate for Vibrio cholerae O1. J Infect Dis. 1995;172:1126–1129. doi: 10.1093/infdis/172.4.1126. [DOI] [PubMed] [Google Scholar]
- 15.Lazar S, Waldor M K. ToxR-independent expression of cholera toxin from the replicative form of CTXΦ. Infect Immun. 1998;66:394–397. doi: 10.1128/iai.66.1.394-397.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee S H, Hava D L, Waldor M K, Camilli A. Regulation and temporal expression patterns of Vibrio cholerae virulence genes during infection. Cell. 1999;99:625–634. doi: 10.1016/s0092-8674(00)81551-2. [DOI] [PubMed] [Google Scholar]
- 17.Lin Wei, Fullner K J, Clayton R, Sexton J A, Rogers M B, Calia K E, Calderwood S B, Fraser C, Mekalanos J J. Identification of a Vibrio cholerae RTX toxin gene cluster that is tightly linked to the cholera toxin prophage. Proc Natl Acad Sci USA. 1999;96:1071–1076. doi: 10.1073/pnas.96.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 19.Miller V L, Mekalanos J J. Synthesis of cholera toxin is positively regulated at the transcriptional level by toxR. Proc Natl Acad Sci USA. 1984;81:3471–3475. doi: 10.1073/pnas.81.11.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pearson G D N, Woods A, Chiang A, S L, Mekalanos J J. CTX genetic element encodes a site-specific recombination system and an intestinal colonization factor. Proc Natl Acad Sci USA. 1993;90:3750–3754. doi: 10.1073/pnas.90.8.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rabbani G H, Greenough W B. Cholera. In: Lebenthal E, Duffy M, editors. Text book of secretory diarrhea. New York, N.Y: Raven Press; 1990. pp. 233–253. [Google Scholar]
- 22.Sack D A, Huda S, Neogi P K B, Daniel R R, Spira W M. Microtiter ganglioside enzyme-linked immunosorbent assay for Vibrio and Escherichia coli heat labile enterotoxins and antitoxins. J Clin Microbiol. 1980;11:35–40. doi: 10.1128/jcm.11.1.35-40.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma C, Thungapathra M, Ghosh A, Mukhopadhyay A K, Basu A, Mitra R, Basu I, Bhattacharya S K, Shimada T, Ramamurthy T, Takeda T, Yamasaki S, Takeda Y, Nair G B. Molecular analysis of non-O1, non-O139 Vibrio cholerae associated with an unusual upsurge in the incidence of cholera-like disease in Calcutta, India. J Clin Microbiol. 1998;36:756–763. doi: 10.1128/jcm.36.3.756-763.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spira W M, Sack R B, Froehlich J L. Simple adult rabbit model for Vibrio cholerae and enterotoxigenic Escherichia coli diarrhea. Infect Immun. 1981;32:739–747. doi: 10.1128/iai.32.2.739-747.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor D N, Killeen K P, Hack D C, Kenner J R, Coster T S, Beattie D T, Ezzell J, Hyman T, Trofa A, Sjogren M H. Development of a live, oral, attenuated vaccine against El Tor cholera. J Infect Dis. 1994;170:1518–1523. doi: 10.1093/infdis/170.6.1518. [DOI] [PubMed] [Google Scholar]
- 26.Taylor R K, Manoil C, Mekalanos J J. Broad-host-range vectors for delivery of TnphoA: use in genetic analysis of secreted virulence determinants of Vibrio cholerae. J Bacteriol. 1989;171:1870–1878. doi: 10.1128/jb.171.4.1870-1878.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waldor M K, Mekalanos J J. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science. 1996;272:1910–1914. doi: 10.1126/science.272.5270.1910. [DOI] [PubMed] [Google Scholar]
- 28.Waldor M K, Mekalanos J J. Emergence of a new cholera pandemic: molecular analysis of virulence determinants in Vibrio cholerae O139 and development of a live vaccine prototype. J Infect Dis. 1994;170:278–283. doi: 10.1093/infdis/170.2.278. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization. Guidelines for the laboratory diagnosis of cholera. Geneva, Switzerland: Bacterial Disease Unit, World Health Organization; 1974. [Google Scholar]