Abstract
Objective
We aimed to compare visual and anatomical outcome in vitrectomized and non-vitrectomized eyes treated with dexamethasone (DEX) implant due to diabetic macular oedema (DMO).
Design
Multicenter, retrospective, interventional study.
Participants
236 eyes from 234 patients with DMO with or without previous vitrectomy performed with follow-up of 12 months.
Methods
Records were reviewed for cases of DMO treated with DEX implant in vitrectomized and not vitrectomized eyes. Best corrected visual acuity (BCVA), central subfoveal thickness (CST), and intraocular pressure (IOP) were recorded at baseline and 12 months after treatment with DEX implants. Correlations between vitreous status and visual and anatomical outcome, as well as safety profile were analysed.
Main outcome measures
BCVA and CST over follow-up period. Secondary outcomes: cataract rate formation, intraocular pressure increase, number of implants needed.
Results
The non-vitrectomized group included 130 eyes (55.1%), the vitrectomized group included 106 eyes (44.9%). The groups were well balanced for age and gender (p = 0.540, and p = 0.053, respectively). Both groups showed statistically significant improvement in BCVA and CST (for all groups: p < 0.001). There was no significant difference between the groups in terms of change in vision (p = 0.89) and anatomy (p = 0.65). The mean number of DEX implants given during follow-up was 3.5 in both groups, and there was no significant difference between the groups (p = 0.81).
Conclusion
We demonstrated similar anatomical and functional efficacy of DEX implant in non-vitrectomized and vitrectomized eyes. Its efficacy was not influenced by full vitrectomy for diabetic retinopathy complications. Safety profile was well balanced between groups.
Subject terms: Health care, Drug discovery
Introduction
Diabetes mellitus is a main cause of sight impairment in people of working age. Ninety-three millions people around the world are estimated to have diabetic retinopathy (DR) [1]. Diabetic macular Oedema (DMO) affects 7% of diabetic patients and it is the main cause for not only decreased vision, but also loss of sight associated with DR. Anti-vascular endothelial growth factor (VEGF) therapy has become the first line treatment for centre-involved DMO and it is effective in improving and maintaining visual acuity, and this was shown in large-scale randomised controlled trials [2–5].
Patients suffering from DMO undergoing treatment with anti-VEGF require pars plana vitrectomy (PPV) for complications such as non-clearing vitreous haemorrhage or epiretinal membrane. Frequently, these patients need ongoing intravitreal treatment for DMO even after PPV.
The vitreous gel consists of collagen and glycosaminoglycans, and is believed to act as molecular barrier to drug diffusion [6]. The fine structure of the vitreous, the flow systems operating within it, its age-related structural changes, and the presence of inflammation may have a potential effect on the movement of particles targeting the retina [7]. Drug elimination from the vitreous occurs by the aqueous outflow into the anterior chamber and permeation through the retina via retinal-choroid-sclera pathways [8]. It has been shown that after vitreous removal, the clearance of VEGF is increased. This suggests that pharmokinetics of Anti-VEGF drugs and steroids could also be affected and therefore it may be more useful to predict the final outcome of their interactions except in terms of clinical outcomes as has been done in the present study [9].
Dexamethasone (DEX) implant is a long-lasting effect system made by a bioerodible copolymer. The MEAD trial [10] revealed that DEX implant might have an anatomical and functional effect for up to 6 months in non-vitrectomized eyes. The use of DEX implant in vitrectomized eyes has not been widely studied, and only a subgroup analysis of the CHAMPLAIN pivotal trial [11] and few scarce routine clinical care reports have been reports [12, 13].
We aimed to compare visual and anatomical outcome in vitrectomized and non-vitrectomized eyes treated with DEX implant due to DMO with a long follow-up in a large cohort.
Methods
Multicenter, retrospective, interventional study. Participants: 236 eyes from 234 patients with DMO with or without previous vitrectomy performed with a follow up of at least 12 months involving multiple sites from (1) Private Retina Service, Buenos Aires, Argentina; (2) Tel Aviv Medical Center, Israel; (3) Hospital Clínic of Barcelona, Spain; (4) University of Udine, Italy; (5) University of Perugia, S.Maria della Misericordia Hospital, Perugia, Italy; (6) The Macula Foundation, Genova, Italy; (7) University of Sydney, Sydney, Australia; (8) Istanbul University Istanbul Faculty Of Medicine, Turkey; (9) Susrut Eye Foundation & Research Centre, India; (10) Azienda Ospedaliera Universitaria Sassari, Italy; (11) La Fe University Hospital of Valencia, Spain
Ethics statement Institutional review board (IRB) approval was obtained through the individual IRBs at the participating institutes for a retrospective consecutive chart review. The research adhered to the tenets of the Declaration of Helsinki. All data discussed in this study were fully anonymized before they were accessed. Informed consent was taken. Patient records from January 1, 2014 to December 1, 2020 were reviewed for cases of DMO treated with DEX implant in vitrectomized and not vitrectomized eyes.
Study participants: The following were set as study inclusion criteria: (1) age 18 years or older; (2) type 1 or 2 diabetes mellitus; (3) DMO causing visual loss (BCVA ≤ 20/32; 0.2 logMAR) in vitrectomized or not vitrectomized eyes; foveal-involving macular oedema defined by central macular thickness (CMT) of >300 μm in the central subfield; and intra- or subretinal fluid seen on SD-OCT; (4) treatment with DEX implant; (5) at least 12 months of follow-up after first DEX implant. Exclusion criteria were (1) other concomitant ocular diseases causing macular oedema (i.e. neovascular age-related macular degeneration or choroidal neovascularization due to other reasons, retinal vein occlusion, uveitis and recent intraocular surgery possibly causing), (2) uncontrolled glaucoma.
Consecutive patient charts were reviewed for demographic data; previous treatments given for DMO; stage of retinopathy (diagnosed by clinical examination, as per the International Classification) [14]; BCVA in logarithm of the minimum angle of resolution (logMAR) scale and CMT before and at last follow up after DEX implant; IOP before DEX implant and use of IOP-lowering treatment during study period; lens status and cataract grading before and at last follow up after DEX implant; number of DEX implants given trough the study period; additional treatments given for DMO during study period.
Refractory DME was defined as worsening of BCVA by 2 Early Treatment Diabetic Retinopathy Study (ETDRS) lines or reduction of less than 10% of retinal thickness on SD-OCT measured 1 month after at least 3 anti-VEGF injections that were given at monthly intervals.
All included subjects were required to have OCT scans obtained using horizontal raster pattern scans cantered on the fovea, using spectral domain-OCT (Spectralis; Heidelberg Engineering, Heidelberg, Germany, and HD-OCT Cirrus 5000, Carl Zeiss Meditec, Dublin, CA, USA). Retinal thickness was analysed using the retinal thickness map analysis protocol with nine ETDRS subfields. Central foveal subfield thickness (CST) was defined as average retinal thickness of the circular area with 1 mm diameter around the foveal centre, recorded at baseline and at last follow-up visit after DEX implant.
IOP increase was considered significant in case of (1) eyes with pre-existing glaucoma and increase in number of IOP-lowering drops or surgery during follow-up, and (2) every eye without pre-existing glaucoma that needed any IOP-lowering therapy during follow-up.
Outcome measures
Main outcome measures were BCVA and CST change before and at last follow up after DEX implant compared between vitrectomized and non-vitrectomized eyes. Secondary outcomes were number of DEX implants, additional treatments needed, the proportion of cataract surgery, and the use of IOP-lowering treatment between the two study groups during the study period.
Statistical analysis
The demographics and clinical characteristics of our study cohort were evaluated using traditional descriptive methods. To control for inter-eye correlation, we used a generalised estimating equations (GEE) procedure. Differences in baseline characteristics between the groups as well as differences in BCVA and CST from baseline to last follow-up were analysed by an univariable GEE model. Differences in outcome variables (BCVA change, CST change) between the groups were analysed by a multivariable regression model, including baseline BCVA or baseline CST respectively. All statistics were computed with SPSS Statistics 25 (IBM, Armonk, NY).
Results
The study included 236 eyes from 234 patients, with mean age of 69.5 ± 13.8 years, 120 male patients (51.3%). The non-vitrectomized group included 130 eyes, 71 (54.6%) were treatment naive, and 59 (54.4%) were previously treated for DMO. The vitrectomized group included 106 eyes, 76 (71.7%) were treatment naive, and 30 (28.3%) were previously treated for DMO. Causes for PPV were vitreous haemorrhage (95.3%, n = 225), and vitreomacular interface abnormalities in (4.7%, vitreomacular traction n = 11, epiretinal membrane n = 1). Baseline characteristics are detailed in Table 1. There was a difference between the two groups in gender (p = 0.540). However, we believe that that would not have changed the results materially.
Table 1.
Baseline characteristics.
| Non-vitrectomized eyes (n = 130) | Vitrectomized eyes (n = 106) | P* | |||
|---|---|---|---|---|---|
| Naive eyes (n = 71) | Refractory eyes (n = 59) | Naive eyes (n = 76) | Refractory eyes (n = 30) | ||
| Age, years, mean ± SD | 68.6 ± 14.3 | 69.2 ± 12.9 | 71.9 ± 14.2 | 66.7 ± 12.6 | 0.540 |
| Male, n (%) | 30/71 (42.3) | 34/59 (57.6) | 39/76 (51.3) | 18/30 (60.0) | 0.053 |
| Baseline VA, logMAR, mean ± SD | 0.59 ± 0.15 | 0.61 ± 0.19 | 0.57 ± 0.17 | 0.58 ± 0.40 | 0.810 |
| CST at baseline, µm, mean ± SD | 583 ± 94 | 565 ± 107 | 693 ± 159 | 490 ± 133 | <0.001 |
| Pseudophakia at baseline, n (%) | 55/71 (77.5) | 37/59 (62.7) | 64/76 (84.2) | 17/30 (56.7) | 0.009 |
| Glaucoma at baseline, n (%) | 1/71 (1.4) | 5/59 (8.5) | 2/76 (2.6) | 5/30 (16.7) | 0.003 |
CST central subfield thickness, VA visual acuity.
*P value for the difference among all four subgroups, tested by univariable logistic and linear GEE model.
In the non-vitrectomized group, mean baseline BCVA was 0.59 ± 0.15, and 0.61 ± 0.19 for naive and refractory eyes, respectively. In the vitrectomized group, mean baseline BCVA was 0.57 ± 0.17 logMAR, and 0.58 ± 0.40 logMAR for naive and refractory eyes, respectively (p = 0.810). In the non-vitrectomized group, mean baseline CST was 583 ± 94 µm, and 565 ± 107 µm for naive and refractory eyes, respectively. In the vitrectomized group, mean baseline CST was 693 ± 159 µm, and 490 ± 133 µm for naive and refractory eyes, respectively. CST was significantly higher in the vitrectomized eyes and it did significantly differ between the groups (p < 0.001). The proportion of pseudophakic eyes and glaucomatous eyes was significantly higher in the vitrectomized eyes and it did significantly differ between the subgroups (p = 0.009 and p = 0.003, respectively).
Functional and anatomical change after DEX implant
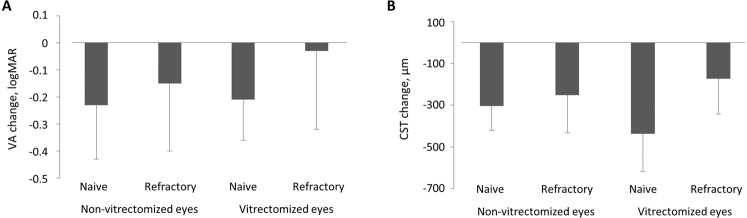
Mean baseline BCVA was 0.59 ± 0.15 logMAR, 0.61 ± 0.19 logMAR, 0.57 ± 0.17 logMAR, and 0.58 ± 0.40 logMAR and changed by −0.23 ± 0.20, −0.15 ± 0.25, −0.21 ± 0.15, and −0.03 ± 0.29 for naive and refractory non-vitrectomized and naive and refractory vitrectomized eyes, respectively (p < 0.001 for naive and refractory non-vitrectomized and naive-vitrectomized eyes; p = 0.533 for refractory vitrectomized eyes, Fig. 1A). There was no significant difference in BCVA change between vitrectomized and non-vitrectomized eyes in both groups (naive eyes: p = 0.89, refractory eyes: p = 0.10). There was a significant difference in BCVA change between naive and refractory DMO in the vitrectomized group (p = 0.001). Notably, refractory vitrectomized eyes did not gain vision after DEX-implant treatment. However, an anatomical response was noted with reduction of retinal thickness (Table 2).
Fig. 1. Funtional and anatomical changes after DEX implant.
Change in visual acuity (VA, A) and central subfield thickness (CST, B) from baseline to last follow-up. Data are mean ± SD.
Table 2.
Treatment characteristics, outcome and safety profile.
| Non-vitrectomized eyes (n = 130) | Vitrectomized eyes (n = 106) | |||
|---|---|---|---|---|
| Naive eyes (n = 71) | Refractory eyes (n = 59) | Naive eyes (n = 76) | Refractory eyes (n = 30) | |
| Follow-up period, months, mean ± SD | 24.0 ± 0.0 | 24.0 ± 0.0 | 24.3 ± 8.8 | 28.8 ± 17.0 |
| No. of DEX-I during follow-up, mean ± SD | 3.9 ± 0.5 | 3.1 ± 1.2 | 3.1 ± 1.1 | 3.9 ± 2.9 |
| Baseline VA, logMAR, mean ± SD | 0.59 ± 0.15 | 0.61 ± 0.19 | 0.57 ± 0.17 | 0.58 ± 0.40 |
| VA at last FU, logMAR, mean ± SD | 0.36 ± 0.14 | 0.47 ± 0.23 | 0.36 ± 0.16 | 0.55 ± 0.41 |
| VA change baseline-last FU, logMAR, mean ± SD | −0.23 ± 0.20 | −0.15 ± 0.25 | −0.21 ± 0.15 | −0.03 ± 0.29 |
| CST at baseline, µm, mean ± SD | 583 ± 94 | 565 ± 107 | 693 ± 159 | 490 ± 133 |
| CST at last FU, µm, mean ± SD | 279 ± 61 | 313 ± 125 | 256 ± 41 | 317 ± 85, n = 26 |
| CST change baseline-last FU, µm, mean ± SD | −304 ± 117 | −252 ± 180 | −437 ± 181 | −173 ± 170, n = 26 |
| Eyes with additional anti-VEGF injections during FU, n (%) | 1 (1.4) | 10 (16.9) | 2 (2.6) | 6 (20.0) |
| Eyes that underwent cataract surgery within FU, n (% of phakic eyes) | 15/16 (93.8) | 7/22 (31.8) | 11/12 (91.7) | 5/12 (41.7) |
| Eyes that needed IOP-lowering treatment within FU, n (%) | 5/70 (7.1) | 13/57 (22.8) | 4 (5.3) | 9 (30.0) |
CST central subfield thickness, FU follow-up, VA visual acuity.
Mean baseline CST was 583 ± 94 µm, 565 ± 107 µm, 693 ± 159 µm, and 490 ± 133 µm and changed by −304 ± 117 µm, −252 ± 180 µm, −437 ± 181 µm, and −173 ± 170 µm for naive and refractory non-vitrectomized and naive and refractory vitrectomized eyes, respectively (for all groups: p < 0.001, Fig. 1B). There was no significant difference in anatomical outcome between vitrectomized and non-vitrectomized eyes in both groups (naive eyes: p = 0.21, refractory eyes: p = 0.65,). Moreover, there were no significant differences in anatomical outcome between naive and refractory DMO in vitrectomized eyes (p = 0.059). (Table 2)
Safety profile
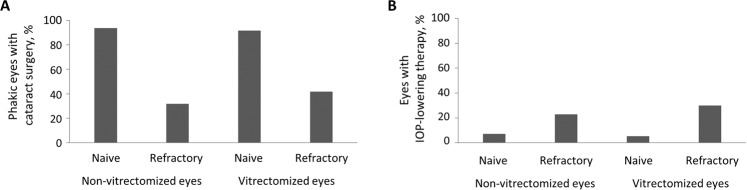
Sixteen out of 24 phakic vitrectomized eyes (66.7%) underwent cataract surgery during follow up (Fig. 2A): Within the naive eyes group 11/12 (91.7%), and within the refractory eyes group 5/12 (41.7%). In the non-vitrectomized group, 22 out of 38 eyes (57.9%) underwent cataract surgery during follow up (Fig. 2A): Within the naive eyes group 15/16 (93.8%), and within the refractory eyes group 7/22 (31.8%).
Fig. 2. Treatment characteristics, outcome and safety profile.
Eyes that underwent cataract surgery (A) and eyes that received IOP-lowering treatment (B) within the follow-up in %.
IOP increased significantly in vitrectomized eyes during follow-up in 14/106 eyes (13.2%), out of which 6/14 eyes (42.9) had controlled pre-existing glaucoma. IOP increase was treated with topical treatment in 11/14 (78.6%), with surgery in 2/14 (14.3%) and without need for treatment in 1 case (7.1%). In non-vitrectomized eyes, 18/127 (14.2%) received IOP-lowering treatment during follow-up (Fig. 2B).
The mean number of DEX implants given during follow-up was 3.5 (3.9 ± 0.5, 3.1 ± 1.2, 3.1 ± 1.1, 3.9 ± 2.9, for naive and refractory non-vitrectomized and naive and refractory vitrectomized eyes, respectively, Fig. 3A, B). There was no significant difference between vitrectomized and non-vitrectomized eyes in both groups (p = 0.81). The proportion of patients who needed additional anti-VEGF injections was 1 (1.4%), 10 (16.9%), 2 (2.6%), 6 (20%) for naive and refractory non-vitrectomized (p = 0.002) and naive and refractory vitrectomized eyes, respectively (p = 0.014).
Fig. 3. Ultra widefield fundus imaging obtained few minutes after DEX implant procedure, injection was performed in the superotemporal quadrant.
A Left eye, non-vitrectomized: The implant is maintained in the superotemporal quadrant. B Right eye of another patient, vitrectomized: The implant remained in the inferior part of the eye.
Discussion
To the best of our knowledge this is the largest study that directed to compare the functional and anatomical results with the DEX implant in vitrectomized and non-vitrectomized eyes in a multicentric cohort of DMO eyes. We showed that DEX implant caused significant functional and anatomical improvement in both groups when compared to the baseline measurements. The two groups fared the same, i.e. there was no statistical significant difference between them. Furthermore, we measured the safety profile in this multicentre international study.
Following PPV surgery, frequently repeated application of intravitreal medication may be required [15]. Previous studies have shown a shorter half-life of bevacizumab, ranibizumab and aflibercept in eyes which have undergone PPV [16, 17]. This has led to an expert opinion that fixed monthly dosing, with low threshold for increasing frequency of injection even to 2-weekly may be required, as well as close monitoring of patients to establish individual response and customise injection frequency as needed [15]. This remains controversial, as animal models have shown that intraocular pharmacokinetic properties of anti-VEGF agents in vitrectomized eyes were similar to those in non-vitrectomized eyes [18]. Therefore, it is important to know how these drugs will act on vitrectomized eyes and compare with non-vitrectomized eyes. After the vitreous is removed, the eye becomes less viscous and loses its natural role of reservoir. Therefore, a clearance of intravitreal drugs from the vitreous cavity is accelerated [9, 19].
This may be one of the reasons why in vitrectomized eyes, DEX implant has the potential to be more predictive in terms of functional and anatomical outcomes. It has been shown that DEX implant has the potential to work properly by itself, without needing in this case the vitreous [20]. DEX implant is a sustained-release preparation of DEX embedded in a bioerodible copolymer consisting of poly (lactic-co-glycolic acid). Pharmacokinetic and pharmacodynamic data suggest that when injected into the posterior segment, the DEX implant releases the active component into the vitreoretinal tissues for up to 6 months [21]. In an experimental study, the behaviour of DEX implants injected into vitrectomized eyes was modelled, and DEX implant was injected into a BSS-filled box [20]. These preclinical data are supported by clinical studies. The CHAMPLAIN study was conducted on 55 vitrectomized eyes and evaluated the safety and efficacy of DEX implant over a 26-week period [11]. The findings showed a decrease in macular thickness and an increase in BCVA after 8 weeks, and these effects continued for 26 weeks. Moreover, the combination of intravitreal DEX injection with PPV had been shown to be safe and effective for some underlying conditions that result in macular oedema, like DR, retinal vein occlusion, and uveitis [22]. The findings of our current study are in concordance with previous reports about the efficacy and safety of DEX implant in vitrectomized eyes [11–13].
Our study had certain limitations, such as its retrospective nature, however, we report comparative analysis with long-term effectiveness and safety of DEX implant in vitrectomized and non-vitrectomized eyes. Due to lack of standardised treatment or re-treatment protocol, we cannot make conclusive remarks on its efficacy and suggest treatment protocols in vitrectomized eyes using DEX implant. Moreover, CST was statistically significantly higher in the vitrectomized eyes and it did significantly differ between the groups. This might interfere with the anatomical outcome. As this was an international multicentre retrospective study, selectivity of patients may have played a part in the results, as patients with a shorter follow-up were excluded from the study.
In conclusion, this study has demonstrated similar effectiveness of DEX in non-vitrectomized and vitrectomized eyes.
Although there are postulated reasons why anti-VEGF agents may be less effective in vitrectomised eyes the clinical data is not conclusive
We strongly believe that in suitable vitrectomized patients, DEX implant might be considered as treatment option due to its efficacy and safety profile. A randomised clinical trial is needed in order to evaluate the impact of vitrectomy in the current algorithm of DMO treatment.
Summary table
What was known before
There was no certainty that Dex implant works the same in vitrectomized and non-vitrectomized eyes.
What this study adds
We demonstrated similar anatomical and functional efficacy of DEX implant in non-vitrectomized and vitrectomized eyes. Its efficacy was not influenced by full vitrectomy for Diabetic retinopathy complications. Safety profile was well balanced between groups.
Supplementary information
Author contributions
MI, CB, JC, and DZ contributed to the planning, conduction and reporting of the study. PL, VS, DV, NR, ML, ZC, SF-B, CB-M, AS-P, JZ-V, RG-P, AM, GD’AR, PU, and AL contributed to the conduction and reporting of the study.
Competing interests
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
Ethics statement
Due to the design of this study there was no need to get approval from ethical committee. IRB was obtained. Stambulian CeiSA Number ID: 6677/10.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Matias Iglicki, Catharina Busch, Jay Chhablani, Dinah Zur.
Supplementary information
The online version contains supplementary material available at 10.1038/s41433-022-01931-9.
References
- 1.Yau JWY, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–64. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L, et al. Ranibizumab for diabetic macular edema: Results from 2 phase iii randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789–801. doi: 10.1016/j.ophtha.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P, Schlingemann RO, et al. The RESTORE study: Ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118:615–25. doi: 10.1016/j.ophtha.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 4.Massin P, Bandello F, Garweg JG, Hansen LL, Harding SP, Larsen M, et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care. 2010;33:2399–405. doi: 10.2337/dc10-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heier JS, Korobelnik JF, Brown DM, Schmidt-Erfurth U, Do DV, Midena E, et al. Intravitreal Aflibercept for Diabetic Macular Edema: 148-Week Results from the VISTA and VIVID Studies. Ophthalmology. 2016;123:2376–85.. doi: 10.1016/j.ophtha.2016.07.032. [DOI] [PubMed] [Google Scholar]
- 6.Gisladottir S, Loftsson T, Stefansson E. Diffusion characteristics of vitreous humour and saline solution follow the Stokes Einstein equation. Graefe’s Arch Clin Exp Ophthalmol = Albr von Graefes Arch fur Klin und Exp Ophthalmol. 2009;247:1677–84. doi: 10.1007/s00417-009-1141-3. [DOI] [PubMed] [Google Scholar]
- 7.Mains J, Wilson CG. The vitreous humor as a barrier to nanoparticle distribution. J Ocul Pharm Ther J Assoc Ocul Pharm Ther. 2013;29:143–50. doi: 10.1089/jop.2012.0138. [DOI] [PubMed] [Google Scholar]
- 8.Awwad S, Lockwood A, Brocchini S, Khaw PT. The PK-Eye: A Novel In Vitro Ocular Flow Model for Use in Preclinical Drug Development. J Pharm Sci. 2015;104:3330–42. doi: 10.1002/jps.24480. [DOI] [PubMed] [Google Scholar]
- 9.Lee SS, Ghosn C, Yu Z, Zacharias LC, Kao H, Lanni C, et al. Vitreous VEGF clearance is increased after vitrectomy. Investig Ophthalmol Vis Sci. 2010;51:2135–8. doi: 10.1167/iovs.09-3582. [DOI] [PubMed] [Google Scholar]
- 10.Boyer DS, Yoon YH, Belfort R, Jr, Bandello F, Maturi RK, Augustin AJ, et al. Trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121:1904–14. doi: 10.1016/j.ophtha.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 11.Boyer DS, Faber D, Gupta S, Patel SS, Tabandeh H, Li XY, et al. Dexamethasone intravitreal implant for treatment of diabetic macular edema in vitrectomized patients. Retina. 2011;31:915–23. doi: 10.1097/IAE.0b013e318206d18c. [DOI] [PubMed] [Google Scholar]
- 12.Hwang S, Kang SW, Kim KT, Noh H, Kim SJ. Three-year outcomes of vitrectomy combined with intraoperative dexamethasone implantation for non-tractional refractory diabetic macular edema. Sci Rep. 2021;11:1292.. doi: 10.1038/s41598-020-80350-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bastakis GG, Dimopoulos D, Stavrakakis A, Pappas G. Long-term efficacy and duration of action of dexamethasone implant, in vitrectomised and non-vitrectomised eyes with persistent diabetic macular edema. Eye. 2019;33:411–8. doi: 10.1038/s41433-018-0219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilkinson CP, Ferris FL, 3rd, Klein RE, Lee PP, Agardh CD, Davis M, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110:1677–82. doi: 10.1016/S0161-6420(03)00475-5. [DOI] [PubMed] [Google Scholar]
- 15.Edington M, Connolly J, Chong NV. Pharmacokinetics of intravitreal anti-VEGF drugs in vitrectomized versus non-vitrectomized eyes. Expert Opin Drug Metab Toxicol. 2017;13:1217–24. doi: 10.1080/17425255.2017.1404987. [DOI] [PubMed] [Google Scholar]
- 16.Mktmostshkm O. The Clearance of Intravitreal Bevacizumab in Vitrectomized Macaque Eyes. Investig Ophthalmol Vis Sci. 2011;52:5630. [Google Scholar]
- 17.Niwa Y, Kakinoki M, Sawada T, et al. Ranibizumab and Aflibercept: Intraocular Pharmacokinetics and Their Effects on Aqueous VEGF Level in Vitrectomized and Nonvitrectomized Macaque Eyes. Investig Ophthalmol Vis Sci. 2015;56:6501–5. 10.1167/iovs.15-17279 [DOI] [PubMed]
- 18.Ahn SJ, Ahn J, Park S, Kim H, Hwang DJ, Park JH, et al. Intraocular pharmacokinetics of ranibizumab in vitrectomized versus nonvitrectomized eyes. Investig Ophthalmol Vis Sci. 2014;55:567–73. doi: 10.1167/iovs.13-13054. [DOI] [PubMed] [Google Scholar]
- 19.Patel JI, Hykin PG, Schadt M, Luong V, Fitzke F, Gregor ZJ. Pars plana vitrectomy for diabetic macular Oedema: OCT and functional correlations. Eye. 2006;20:674–80. doi: 10.1038/sj.eye.6701945. [DOI] [PubMed] [Google Scholar]
- 20.Panjaphongse R, Liu W, Pongsachareonnont P, Stewart JM. Kinematic study of ozurdex injection in balanced salt solution: modeling the behavior of an injectable drug delivery device in vitrectomized eyes. J Ocul Pharm Ther J Assoc Ocul Pharm Ther. 2015;31:174–8. doi: 10.1089/jop.2014.0134. [DOI] [PubMed] [Google Scholar]
- 21.Chang-Lin JE, Burke JA, Peng Q, Lin T, Orilla WC, Ghosn CR, et al. Pharmacokinetics of a sustained-release dexamethasone intravitreal implant in vitrectomized and nonvitrectomized eyes. Investig Ophthalmol Vis Sci. 2011;52:4605–9. doi: 10.1167/iovs.10-6387. [DOI] [PubMed] [Google Scholar]
- 22.Zheng A, Chin EK, Almeida DR, Tsang SH, Mahajan VB. Combined vitrectomy and intravitreal dexamethasone (Ozurdex) sustained-release implant. Retina. 2016;36:2087–92. doi: 10.1097/IAE.0000000000001063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.