Abstract
Post Weaning Diarrhea (PWD) is the most important multifactorial gastroenteric disease of the weaning in pig livestock. Phytogenic (PHY) natural extracts are largely studied as alternatives to antibiotic treatments in combating the global concern of the antimicrobial resistance. The aim of this study was to evaluate the protective effect of innovative phytogenic premix with or without short and medium chain fatty acids (SCFA and MCFA) in O138 Escherichia coli challenged piglets. Twenty-seven weaned piglets were allotted into four groups fed different diets according to the following dietary treatments: CTRL (n = 13) group fed basal diet, PHY1 (n = 7) fed the basal diet supplemented with 0.2% of phytogenic premix, PHY2 (n = 7) fed the basal diet supplemented with 0.2% of phytogenic premix added with 2000 ppm of SCFA and MCFA. After 6 days of experimental diet feeding, animals were challenged (day 0) with 2 × 109 CFU of E. coli and CTRL group was divided at day 0 into positive (challenged CTRL + ; n = 6) and negative control group (unchallenged CTRL-; n = 7). Body weights were recorded at -14, -6, 0, 4 and 7 days and the feed intake was recorded daily. E. coli shedding was monitored for 4 days post-challenge by plate counting. Fecal consistency was registered daily by a four-point scale (0–3; diarrhea > 1) during the post-challenge period. Tissue samples were obtained for gene expression and histological evaluations at day 7 from four animals per group. Lower average feed intake was observed in CTRL + compared to PHY2 and CTRL during the post-challenge period. Infected groups showed higher E. coli shedding compared to CTRL- during the 4 days post-challenge (p < 0.01). PHY2 showed lower frequency of diarrhea compared to PHY1 and CTRL + from 5 to 7 days post-challenge. No significant alterations among groups were observed in histopathological evaluation. Duodenum expression of occludin tended to be lower in challenged groups compared to CTRL- at 7 days post-challenge (p = 0.066). In conclusion, dietary supplementation of PHY plus SCFA and MCFA revealed encouraging results for diarrhea prevention and growth performance in weaned piglets.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11259-022-09945-0.
Keywords: Pig, Phytochemicals, Feed additives, Alternatives to antibiotics, Fatty acids, Escherichia coli
Introduction
Post weaning diarrhea (PWD) is a gastrointestinal multifactorial disease that generally occurs during the first two weeks after the weaning phase. It is one of the most economically-relevant diseases in swine husbandry due to the costs of treatments, reduced growth, and increased of mortality (Bonetti et al. 2021). Although many factors are involved in the development of this disease, PWD is often exacerbated by many enterotoxigenic Escherichia coli pathotypes characterized by the presence of virulence factors such as toxins and adhesive fimbriae (Sun and Kim 2017). Bacterial resistance to a wide range of commonly used antibiotics is a global concern and a recent increase in prevalence and severity of PWD required alternative measures for their control (Renzhammer et al. 2020; Dell’Anno et al. 2021a, b). Reducing and replacing antimicrobials in animal farming is a crucial aim of the European policies, even if the mechanisms of cross-species transmission of resistant bacteria and their genetic elements spread from livestock to humans has not been fully understood (Rossi et al. 2014a; Cormican et al. 2017; Tang et al. 2017).
The aim of nutrition is no longer simply to satisfy the nutritional requirements, but also play a key role in the health and welfare of humans and animals (Domínguez Díaz et al. 2020; Grossi et al. 2021). Functional feed additives, which sustain the health status and reduce the risk of pathologies, have thus become fundamental in replacing or reducing antimicrobials in food-producing animals. The dietary inclusion of phytogenics (PHYs), represented by plant secondary metabolites, are largely studied as alternative growth promoters because of their biological properties which include antimicrobial, antioxidant, and nutrigenomic effects on the development of animal (Durmic and Blache 2012; Yang et al. 2015; Lillehoj et al. 2018; Reyes-Camacho et al. 2020). In particular Yan and Kim (2012) observed a significant reduction in fecal E. coli count after 1 g/kg of eugenol supplementation in pigs. A blend of oregano, anise, and citrus peel (40 mg/kg diet) supplementation to piglets’ diet has been demonstrated to evolve anti-inflammatory effect by reducing the gene expression of NF-kB and TNFα (Upadhaya et al. 2016). The dietary supplementation of thymol, cinnamaldehyde and menthol have been reported to positively affect the feed digestibility in swine (Maenner et al. 2011; Li et al. 2012).The in vivo effects, resulting from the various biological activities of the PHYs, depend on their structure, dosage, and pharmaco-kinetics, as well as the animal species, productive phase and administration period. For this reason, several combinations of natural extracts are currently studied in order to promote their possible synergistic or complementary effect on animal health. Although PHYs show antimicrobial activity in the gastrointestinal tract against specific pathogens such as Escherichia coli, Clostridium perfringens and Salmonella spp. (Thacker 2013; Mohammadi Gheisar and Kim 2018), their effectiveness can vary due to the presence and the location of functional hydroxyl and phenolic terpenoids (Dubreuil 2013). Rational combinations of PHYs have been studied in order to increase the spectrum of beneficial activities. In addition, the synergistic or complementary effect of PHYs with other compounds leads to various beneficial activities of several compounds, especially organic acid (OA). Amongst feed additives with antimicrobial activities, organic acids, in particular short-chain fatty acids (SCFAs) and medium-chain fatty acids (MCFAs), have a strong antimicrobial activity and are key to modulating intestinal health and improving animal performance (Ferronato and Prandini 2020; Jackman et al. 2020). SCFAs and MCFAs regulate the growth and virulence of enteric pathogens, such as enterohemorrhagic E. coli, Klebsiella and Salmonella (Zhang et al. 2020). They damage the bacterial structure and in some cases separate the inner and outer membranes (Hanczakowska 2017) and thus increase the concentration of IgG and IgM in piglets challenged with enterotoxigenic Escherichia coli (ETEC) strains (Han et al. 2020). A synergistic antimicrobial effect has been observed in the combination of PHYs and organic acids in vitro (Costa et al. 2013). However, the effect of their dietary supplementation on pigs' growth and the optimization of the inclusion level for diarrhea prevention against major pathogens of weaned piglets has not been fully investigated. Therefore, it was hypothesized that the dietary supplementation of phytogenic additive with or without organic acids could prevent or limit the detrimental effects of enterotoxigenic Escherichia coli infection improving animal health status.
The aim of this study was thus to evaluate the protective effect against O138 E. coli F18 + infection of an innovative phytogenic premix composed by caraway oil, lemon oil, clove, cinnamon, nutmeg, onion, pimento, orange peel, peppermint and chamomile powder with and without short and medium chain fatty acids in weaned piglets’ diet.
Materials and methods
Animal selection criteria
The trial was performed at the Experimental Animal Research and Application Centre of University of Milan and was authorized by the Italian Health Ministry (authorization n° 711/-PR) in accordance with EU regulations (Directive 2010/63/EU 2010).
Animals enrolled in the experimental trial were selected from a conventional herd free from contagious diseases (Ex A-list International Office of Epizootic, porcine reproductive and respiratory syndrome, atrophic rhinitis, Aujeszky’s disease, transmissible gastroenteritis, salmonellosis) and without a history of PWD or oedema disease. Sows were assessed for genetic susceptibility to Escherichia coli carrying F18 adhesive fimbriae (F18 E. coli) by screening the fucosyltransferase 1 (FUT1) genotypes using polymerase chain reaction (PCR) reaction according to Luise et al. (2019a, b). Briefly, genomic DNA was extracted from hair samples of sows and genotyped to identify polymorphic variants. Sows carrying the GG genotypes at FUT1 gene were considered for piglet enrolment. A further selection criterion was the absence of hemolytic E. coli in piglets feces. Microbiological analyses of selective mediums (Agar MacConkey) (Hayer et al. 2020; Li et al. 2020; Remfry et al. 2020) were thus carried out before transport and upon arrival on fecal samples collected from enrolled piglets.
Animals and experimental design
Twenty-seven weaned piglets (28 ± 2 days) balanced per weight (9.79 ± 1.25 kg) and sex, were randomly allotted in four experimental groups in randomized complete block design and, after 7 days of adaptation period, fed ad libitum for the entire experimental period according to the following dietary treatments: control group (CTRL, n = 13) fed basal diet, phytogenic additive group 1 (PHY1, n = 7) fed basal diet supplemented with 200 g/100 kg phytogenic additive, phytogenic additive group 2 (PHY2, n = 7) fed basal diet supplemented with phytogenic additive plus 2000 ppm of short and medium chain fatty acids premix.
In order to achieve the same nutrient concentrations, the control group received basal diet supplemented with the same premix carrier used for treatment groups (95% wheat meal and 5% of coconut oil) without phytogenic compounds. The iso-energetic and iso-proteic diets (Table S1) were formulated (Plurimix; Fabermatica, CR, Italy) according to animal requirements for the post weaning phase defined by the US National Research Council (NRC 2012). The phytogenic feed additive (FRESTA®F, Delacon Biotechnik GmBH), approved by EU regulation (Reg. CE 1831/2003), as zootechnical additive, was composed of essential oil from caraway oil (d-carvone 3.5–6.0 mg/g) and lemon (limonene: 2.3—9.0 mg/g), dried herbs and spices (1.5% clove powder, 10% cinnamon powder, 1.5% nutmeg powder, 5% onion powder, 2% pimento powder, 5% orange peel powder, 12.5% peppermint powder and 12.5% chamomile powder). The SCFA and MCFA premix was composed by butyric (C4), caprylic (C8), capric (C10) and lauric acid (C12). The phytogenic products (with or without SCFA and MCFA) or the premix carrier were mixed with the compound diets for 30 min in order to ensure a homogeneous distribution. Diets have been provided in meal without any technological treatments, except for mixing procedure. During the mixing process the temperature was monitored in order to do not overcome 30 °C.
Piglets were housed in two environmentally controlled rooms, in individual pens, with a plastic slatted floor and constant temperature (27° C) and humidity (60%) for the entire experimental period. The trial was divided into a pre- and post-challenge, considering the challenge as day 0 (Fig. 1).
Fig. 1.
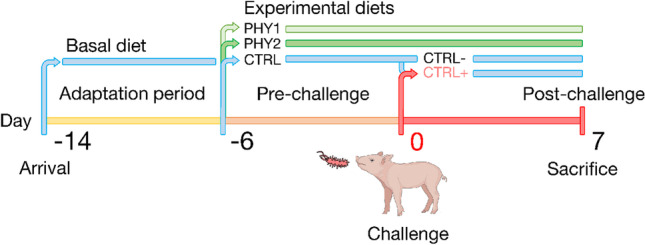
Experimental trial design from arrival (-14) to 7 days post-challenge. PHY1: treatment group fed basal diet supplemented with 200 g/100 kg of phytogenic additive; PHY2: treatment group fed basal diet supplemented with 200 g/100 kg of phytogenic additive supplemented with 2000 ppm of short and medium chain fatty acids premix; CTRL: group fed basal diet supplemented with premix carrier divided into negative control (CTRL-) and positive control (CTRL +) challenged at day 0.
Chemical analysis of experimental diets
Diets were analyzed for proximate analysis, including moisture, crude protein (CP), crude fibre (CF), ether extract (EE), and ash. The moisture determination was performed by oven-drying at 65 °C for 24 h (Regulament EC 152/2009). Crude protein content was measured according to the Kjeldahl method (Association of Official Analytical Chemists method 2001.11). Crude fiber was determined by the filter bags technique (American Oil Chemistry Society 2009). Ether extract content was determined in a Soxhlet system after hydrolysis (Association of Official Analytical Chemists method 2003.05). Ash was measured using a muffle furnace at 550 °C (Association of Official Analytical Chemists method 942.05).
Experimental challenge
E. coli challenger strain was genetically characterized by polymerase chain reaction (PCR) (Applied Biosystem 7500) in order to detect the presence of the two important virulence profile: subunit B of verocytotoxin type 2 and F18 adhesive fimbriae (Table 1).
Table 1.
PCR conditions and oligonucleotide sequences of F18 adhesive fimbriae and VTe2 (B-subunit) encoding genes
| Gene | Accession number (GenBank) | Size (pb) | Primer sequence (5’ to 3’) | PCR conditions |
|---|---|---|---|---|
| F18 adhesive fimbriae | AJ308332.1 | 519 |
5’GATCCATGAAAAGACTAGTGTTTATTTCTTTTG 3’CGAATGCGCCAATGAATGTTCATTCTCGAG |
Den. 95 °C 1’ ann. 56 °C 1′20’’ ext. 72 °C 1′30’’ 35 cycles |
| VTe2 (B-subunit) | GU459254.1 | 270 |
5’GGATCCATGAAGAAGATGTTTATAGCGG 3’AACGGGTCCACTTCAAATGATTCTCGAG |
Den. 95 °C 1’ ann. 50 °C 1′20’’ ext. 72 °C 1′30’’ 35 cycles |
Twenty piglets, except for piglets in CTRL- group (n = 7), on day 0 (challenge day) were orally infected with O138 Escherichia coli F18 + strain obtained from a permanent collection of the University of Milan and previously characterized (Rossi et al. 2014b, 2021; Dell'Anno et al. 2020).
Sixty minutes before the challenge, the piglets were sedated with azaperone (2 mL/head, Stresnil®, Janssen Cilag Spa, Milan, Italy), thereafter 30 mL of a 10% bicarbonate solution was orally administered to neutralize gastric acid and to increase the survival rate of the challenger strain in the stomach. After 10–15 min, the inoculum was given orally in a single dose of 5 mL of bacterial medium with 2 × 109 colony-forming unit (CFU) of challenger strain, using a 16G catheter (Rossi et al. 2021). Animals were fasted 3 h before and 3 h after the challenge. At the same time, piglets in CTRL- were orally inoculated with 5 mL of Luria Bertani (LB) medium to balance the level of stress associated with the oral challenge.
Zootechnical performance, clinical and fecal score
Average daily feed intake (ADFI) was recorded daily from day -6 to day 7 by measuring the refusals. Body weight (BW) was recorded on day -6 (first day of experimental diets), day 0 (challenge day), day 4 and day 7 (sacrifice day). Average daily gain (ADG) and feed efficiency were also calculated.
Piglets were individually evaluated throughout the trial by clinical examination, including observation of behavioral disturbances. In particular, oedema, epiphora, respiratory and hair scores were evaluated through three-point scales (oedema score: 0 = normal, 1 = mild, 2 = severe; epiphora score: 0 = normal, 1 = mild, 2 = severe; respiratory score: 0 = normal, 1 = slightly quick, 2 = quick; hair/bristles score: 0 = smooth, 1 = lightly brushy, 2 = highly brushy) (Rossi et al. 2021). In addition, cyanosis, a blue or red discoloration of the skin, which may or may not be localized to small areas, was considered not as a specific skin condition but as a symptom of disease. From day -6 to day 7, all piglets were evaluated for the fecal score. Clinical signs of the disease were identified according to the point scale score described by Rossi et al. (Rossi et al. 2014b). A four-point scale was adopted to score fecal consistency: 0 = normal, 1 = soft consistency, 2 = mild diarrhea, 3 = severe diarrhea; considering > 1 as an indicative of diarrhea. Fecal color was evaluated using a three-point scale: 1 = yellow, 2 = green; 3 = brown.
Microbiological evaluation of fecal samples
Individual fecal samples were collected from rectal ampulla from each piglet, on days -1, 1, 2, 3 and 4 to perform microbiological analysis and evaluate the challenger strain shedding. For each sample, 1 g of feces was homogenized with 1 ml of saline solution and incubated overnight at 37 °C on sheep blood agar plates 5% (Blood Agar Base No. 2-Oxoid) in order to examine the presence of hemolytic colonies. The total hemolytic bacteria count was performed by counting the number of colonies cultured from serial dilutions of each fecal sample in order to evaluate the presence of hemolytic E. coli in relation to the total bacteria population.
Necropsy, intestinal samples, and histopathology
At day 7 post-challenge, sixteen animals (n = 4/treatment), were randomly selected and euthanized and tissue samples were collected for histopathological and molecular analyses of intestinal tissues.
Animal care and euthanasia procedures were conducted in accordance with the European Union guidelines (86/609/EEC) and approved by the Italian Ministry of Health. Briefly, selected piglets were sedated with 2 mL/head of azaperone (Stresnil®, Janssen Cilag SpA, Milan, Italy) intramuscularly. After 20 min, animals received a bolus injection of propofol intravenously in the right and left lateral auricular vein. Anesthesia was maintained with 40 mg/kg of tiletamine/zolazepam intramuscularly (Zoletil 100, Virbac UK, Bury Saint Edmund, England). Finally, unconscious animals were euthanized by the intracardiac administration of a solution with embutramide, mebezonium iodide and tetracaine hydrochloride (0.3 mg/kg, Tanax, MSD Animal Health, Boxmeer, Netherlands). The intestine of each animal was weighed, and intestinal samples of ileum at 1 cm from ileocecal valve, mesenteric lymph nodes were harvested. For the histological evaluation, samples were diluted in 10% neutral formalin buffer and stored at 4 °C. Tissues were rinsed with sterile saline solution and transferred into 2 mL cryotubes, snap-frozen in liquid nitrogen and stored at -80 °C until further analysis.
Histological examinations of collected intestinal and lymph nodes samples for each piglet were carried out. The fixed samples were embedded in paraffin, and 5 µm thick histological sections were performed with a microtome. Cross sections were stained with hematoxylin and eosin and were blind evaluated by light microscopy. A four-point scale was adopted for inflammatory infiltrates, epithelial regeneration, fusion of villi, oedema, hyperemia, necrosis of mucosa, T atrophy, stroma, and follicular hyperplasia; considering: 0 = no evidence; 1 = slight presence; 2 = moderate; 3 = severe. Samples of duodenum were collected and frozen in liquid nitrogen for gene expression analysis.
Duodenum gene expression
Total RNA was extracted from the duodenum using FastGene Scriptase Basic (Nippon genetics) according to the manufacturer’s instructions. The integrity of total RNA was assessed by gel electrophoresis to detect the 18S and 28S rRNA bands. A combination of oligo-dT and random primers was used to reverse transcribe 100 ng of total duodenal RNA to cDNA (cDNA synthesis kit, FastGene Scriptase Basic, Nippon Genetics). Primer pairs were first tested for their specificity in qualitative PCR, using the pooled cDNA as a template. The cycling profile for the assay consisted of initial denaturation of RNA (65 °C × 5’), then the annealing of random primers (25 °C × 10’), followed by the annealing of oligo-dT and transcription (42 °C × 60’). At the end of the cycle, the enzyme deactivation (90 °C × 5’) was performed. The abundance of cytochrome c oxidase subunit I (COX1), cytochrome c oxidase subunit II (COX2), interleukin 10 (IL-10), interleukin 6 (IL-6), lysyl oxidase (LOX), glutathione peroxidase 2 (GPX2), NAD (P) H quinone dehydrogenase 1 (NQ01) claudin domain containing 1 (CLDND1) and occludin (OCLN) (Table 2) mRNA was determined using SYBR Green-based real-time quantitative PCR assays (7500 Fast Dx, Applied Biosystems). Only reaction efficiencies that were near to 100% were considered for further analysis. The mean values for the transcripts were normalized to the arithmetic mean of mRNA abundance of βactin as the reference gene within each sample. The comparative CT method was used to determine fold changes in gene expression, calculated as 2−∆∆CT. The final results were presented as the fold changes of target gene expression in a target sample relative to a reference sample, normalized to βactin rRNA (Livak and Schmittgen, 2001). The βactin rRNA was used to calculate the threshold cycles, since it previously showed constant values under all the conditions adopted.
Table 2.
Primer sequences and relative amplicon dimensions
| Gene1 | Accession number (GenBank) | Size (pb) | Primer sequence (5’ to 3’) |
|---|---|---|---|
| βactin F | DQ845171 | 76 bp | CTACGTCGCCCTGGACTTC |
| βactin R | DQ845172 | GCAGCTCGTAGCTCTTCTCC | |
| IL-6 F | JQ839263 | 112 bp | TGGGTTCAATCAGGAGACCT |
| IL-6 R | JQ839264 | CAGCCTCGACATTTCCCTTA | |
| IL-10 F | L2001 | 105 bp | TGAAGAGTGCCTTTAGCAAGCTC |
| IL-10 R | L2002 | CTCATCTTCATCGTCATGTAGGC | |
| COX1 F | EF568726 | 102 bp | GGAGCGGGTACTGGATGAAC |
| COX1 R | EF568726 | CACCTGCAAGGGTGTAGGGAGL | |
| COX2 F | AF304201 | 141 bp | AAGACGCCACTTCACCCATC |
| COX2 R | AF304201 | TCCATTGTGCTAGTGTGTGTCA | |
| GPx2 F | DQ898282 | 103 bp | GGAGATCCTGAACAGCCTCA |
| GPx2 R | DQ898282 | GCGAAGACAGGATGCTCATT | |
| LOX F | NM_001164001 | 112 bp | GTGGAGCACGAAAGCAAGACCC |
| LOX R | NM_001164001 | AAGGTGGGGTATGCATCGACAC | |
| NQ01 F | NM_001159613 | 118 bp | ATCACAGGTAAACTGAAGGACCC |
| NQ01 R | NM_001159613 | GCGGCTTCCACCTTCTTTTG | |
| CLAUDIN1 F | NM_001244539 | 90 bp | TCTTTCTTATTTCAGGTCTGGCT |
| CLAUDIN1 R | NM_001244539 | ACTGGGGTCATGGGGTCATA | |
| OCCLUDIN F | NM_001163647 | 106 bp | GTCCACCTCCTTATAGGCCTGATG |
| OCCLUDIN R | NM_001163647 | CGCTGGCTGAGAAAGCATTGG |
1CTB actin beta, IL-6 interleukin-6, IL-10 interleukin 10, COX1 cytochrome c oxidase subunit I, COX2 cytochrome c oxidase subunit II, LOX lysyl oxidase, GPX2 glutathione peroxidase 2, NQ01 NAD (P) H quinone dehydrogenase 1, CLDND1 claudin domain containing 1, OCLN occludin
Blood Samples, serum metabolite profile and serum acute phase proteins
Blood was collected from the jugular vein of each animal on day -1, day 3 and day 7 through vacutainer tubes containing ethylenediaminetetraacetic acid (EDTA) and heparin as anticoagulants. Plasma was collected after centrifugation (3000 rpm, 10 min, 4 °C), aliquoted and stored at -20 °C for further analysis. Hematocrit was evaluated on whole blood using the microhematocrit method. The concentration of total protein (g/L), albumin (g/L), globulin (g/L), albumin/globulin (A/G ratio), alanine aminotransferase (ALT-GPT; IU/L), aspartate aminotransferase (AST-GOT; IU/L), phosphatase alkaline (ALP; IU/L), glucose (mmol/L), urea (mmol/L), total bilirubin (µmol/l), total cholesterol (mmol/L), calcium (mmol/L), phosphorus (mmol/L) and magnesium (mmol/L) were analyzed in serum via standard enzymatic colorimetric analysis through a multiparametric autoanalyzer for clinical chemistry (ILab 650; Instrumentation Laboratory Company, Lexington, MA, USA) at 37 °C by the Lombardy and Emilia Romagna Experimental Zootechnic Institute (IZSLER). Porcine C-reactive protein (CRP) concentration was determined in serum with a commercial sandwich immunoassay Kit (Mybiosource, San Diego, CA, USA) following the manufacturer’s instructions. The results were read at 450 nm using a microplate reader (Model 680, Bio-Rad Laboratories, CA, USA). Haptoglobin (HP) serum concentrations were measured through a colorimetric kit (PhaseTM Range porcine Haptoglobin Assay; Tridelta Development Ltd) according to the manufacturer’s instructions. The results were read at 630 nm on a microplate reader (Model 680, Bio-Rad Laboratories, CA, USA).
Statistical analysis
Zootechnical performance and fecal microbiological analysis were analyzed using a linear model after testing the normality of data through Shapiro–Wilk test using JMP Pro 15® (SAS Inst. Inc., Cary, NC, USA). The model included the fixed effect of treatments (Trt), the effect of time (Time), and the interaction between treatment and time (Trt x Time).
Serum metabolites were evaluated performing analysis of covariance (ANCOVA) to adjust the initial variability of the pre-challenge period after testing the normality of data through Shapiro–Wilk test using JMP Pro 15® (SAS Inst. Inc., Cary, NC, USA).
Clinical score data were converted into a dichotomous variable (normal/pathological), and observed frequencies were assessed using the Chi-squared Test. Histological scores, intestinal weight and relative gene expression were analyzed using Kruskal–Wallis test (PROC NPAR1WAY of SAS 9.4 software) for non-parametric data due to the small sample size of euthanized animals at day 7. Multiple comparisons for parametric statistics were evaluated with the Tukey’s Honestly Significant Difference test (Tukey’s HSD) or Tukey–Kramer test and Steel–Dwass test was used for non-parametric multiple comparisons. The results were presented as least square means (LSMEANS) ± standard error (SE) for parametric data and as medians and range (minimum–maximum) for non-parametric results. Means or medians were considered statistically different when p ≤ 0.050 and statistical tendency was considered when p < 0.100.
Results
Chemical composition of the experimental diets
Proximate analysis of the experimental diets showed comparable contents of the principal nutrients. The inclusion of phytogenic based additives with or without MCFA and SCFA did not affect the nutrient balance of feed (Table S1).
Zootechnical performance
During the pre-challenge period, no statistically significant differences among experimental groups were observed. Considering the entire post-challenge period, ADFI of CTRL + was lower than PHY2 and CTRL- (p < 0.005; Table 3).
Table 3.
Zootechnical performance of experimental groups during the post-challenge period
| PHY1 | PHY2 | CTRL + | CTRL- | p-value | |||
|---|---|---|---|---|---|---|---|
| (n = 7) | (n = 7) | (n = 6) | (n = 7) | Trt | Time | Trt × Time | |
| BW, kg | |||||||
| d 0 | 10.39 ± 0.68 | 10.46 ± 0.68 | 10.23 ± 0.73 | 10.67 ± 0.68 | 0.342 | < 0.001 | 0.963 |
| d 4 | 11.29 ± 0.68 | 11.73 ± 0.68 | 11.25 ± 0.73 | 11.89 ± 0.68 | |||
| d 7 | 12.33 ± 0.68 | 12.96 ± 0.68 | 11.55 ± 0.73 | 13.42 ± 0.68 | |||
| ADG, kg/d | |||||||
| d 1–4 | 0.22 ± 0.09 | 0.32 ± 0.09 | 0.26 ± 0.10 | 0.35 ± 0.09 | 0.083 | 0.323 | 0.262 |
| d 5–7 | 0.35 ± 0.09 | 0.41 ± 0.09 | 0.10 ± 0.10 | 0.51 ± 0.09 | |||
| d 1–7 | 0.29 ± 0.06 | 0.36 ± 0.06 | 0.18 ± 0.07 | 0.41 ± 0.06 | |||
| ADFI, kg/d | |||||||
| d 1–4 | 0.42 ± 0.04 | 0.46 ± 0.04 | 0.35 ± 0.05 | 0.44 ± 0.04 | < 0.005 | 0.040 | 0.294 |
| d 5–7 | 0.42 ± 0.04 | 0.56 ± 0.04 | 0.37 ± 0.05 | 0.58 ± 0.04 | |||
| d 1–7 | 0.42 ± 0.03AB | 0.51 ± 0.03B | 0.36 ± 0.03A | 0.51 ± 0.03B | |||
| FCR, kg/kg | |||||||
| d 1–4 | 1.84 ± 0.41 | 1.59 ± 0.38 | 1.60 ± 0.41 | 1.41 ± 0.41 | 0.054 | 0.248 | 0.069 |
| d 5–7 | 1.57 ± 0.04 | 1.40 ± 0.38 | 3.60 ± 0.58 | 1.32 ± 0.38 | |||
| d 1–7 | 1.70 ± 0.31 | 1.49 ± 0.27 | 2.60 ± 0.36 | 1.36 ± 0.28 | |||
Data are presented as least squared means (LSMEANS) and standard errors (SE)
A−B Different uppercase letters indicate statistically significant differences between treatment groups (p < 0.01)
BW body weight, ADFI average daily feed intake, ADG average daily gain, FCR feed conversion ratio, Trt treatment effect, Time time effect, Trt × Time interaction between treatment and time
Influence of phytogenic treatments on clinical score, fecal consistency and color
During the pre-challenge period and at day 0, the piglets did not show significant differences among clinical scores, indicating a general good health status. Although statistical differences among treatments were not identified, several altered scores were registered from 1 to 4 days post-challenge. After experimental infection, considering the numerical differences of clinical score frequencies (considered as altered clinical conditions for a score of ≥ 1) revealed that the experimental procedures influenced the clinical status of piglets (Table 4). However, from 5 to 7 days post challenge, a non-normal hair score frequency tended to increase in CTRL + compared to the other experimental groups (9.52% for PHY1, 14.81% for PHY2, 33.33% for CTRL + and 14.29% for CTRL-; p = 0.071).
Table 4.
Frequencies (expressed as percentages) of clinical score ≥ 1 from 1 to 7 days post-challenge
| Treatments | p-value | ||||
| Days 1–4 |
PHY1 (n = 7) |
PHY2 (n = 7) |
CTRL + (n = 6) |
CTRL- (n = 7) |
|
| Hair | 28.57 | 10.71 | 16.67 | 14.29 | 0.325 |
| Respiratory | 3.57 | 0.00 | 4.17 | 0.00 | 0.528 |
| Oedema | 3.57 | 3.70 | 8.33 | 0.00 | 0.471 |
| Epiphora | 3.57 | 14.29 | 8.33 | 7.14 | 0.536 |
| Days 5–7 |
PHY1 (n = 7) |
PHY2 (n = 7) |
CTRL + (n = 6) |
CTRL- (n = 7) |
|
| Hair | 9.52 | 14.81 | 33.33 | 14.29 | 0.071 |
| Respiratory | 0.00 | 0.00 | 0.00 | 0.00 | - |
| Oedema | 0.00 | 0.00 | 0.00 | 0.00 | - |
| Epiphora | 0.00 | 0.00 | 0.00 | 0.00 | - |
Data are presented as a percentage of clinical score ≥ 1 registered from day 1 to day 7 post-challenge
Fecal score and color were the most informative indicators during the post-challenge period (Table 5). Significant higher frequencies of altered fecal color were recorded in challenged groups compared to CTRL- from 1 to 4 days post-challenge (p < 0.050). Significant differences in the manifestations of diarrhea (fecal consistency ≥ 2) were observed from 5 to 7 days after the challenge. In particular, PHY1 had higher number of diarrhea cases compared to PHY2, CTRL + and CTRL-, and PHY2 had a lower incidence compared to CTRL + and PHY1 (p < 0.010).
Table 5.
Frequencies (expressed as percentages) of fecal consistency ≥ 2 and fecal color = 1 registered 1 to 7 days post-challenge
| Treatments | |||||
| Days 1–4 |
PHY1 (n = 7) |
PHY2 (n = 7) |
CTRL + (n = 6) |
CTRL- (n = 7) |
p-value |
| Fecal consistency | 42.86 | 28.57 | 25.00 | 21.43 | 0.319 |
| Fecal color | 57.14A | 39.29A | 37.50A | 17.86B | 0.027 |
| Days 5–7 |
PHY1 (n = 7) |
PHY2 (n = 7) |
CTRL + (n = 6) |
CTRL- (n = 7) |
|
| Fecal consistency | 80.95A | 28.57B | 61.11C | 38.10B | 0.003 |
| Fecal color | 90.48 | 71.43 | 94.44 | 71.43 | 0.114 |
Data are presented as a percentage of fecal consistency ≥ 2 and fecal color = 1 registered from day 1 to day 7 post-challenge
A−B−CDifferent uppercase letters indicate statistically significant differences among treatment groups (p < 0.01)
Microbiological evaluation of feces and challenger strain shedding
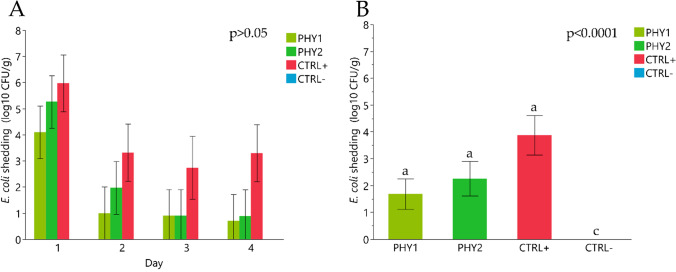
Weaned piglets did not show the presence of challenger E. coli in feces during the adaptation period and on day 0. Total bacterial count did not show statistically significant differences among groups from 1 to 4 days after the challenge (8.37 ± 0.47 log10 CFU/g for PHY1, 8.04 ± 0.47 log10 CFU/g for PHY2, 7.73 ± 0.51 log10 CFU/g for CTRL + and 7.71 ± 0.47 log10 CFU/g for CTRL-). Also after the challenge, all the experimental groups (except for negative control, CTRL-) registered fecal shedding of challenger E. coli strain (Fig. 2). Statistically significant increased fecal shedding of hemolytic E. coli was observed in challenged groups compared to CTRL- from day 1 to day 4 post-challenge (4.09 ± 0.01 log10 CFU/g for PHY1, 5.25 ± 1.10 log10 CFU/g for PHY2, 5.95 ± 1.09 log10 CFU/g for CTRL + and 0.00 ± 1.01 log10 CFU/g for CTRL-; p < 0.001).
Fig. 2.
Escherichia coli fecal shedding during the four days post-challenge where A) presents daily hemolytic E. coli fecal shedding from day 1 to day 4 post-challenge; B) presents average fecal hemolytic E. coli fecal shedding from 1 to 4 days post-challenge. Data are presented as least squared means (LSMEANS) and standard errors (SE). A−B Different uppercase letters indicate statistically significant differences among treatment groups (p < 0.001)
Histological evaluation and gene expression
Samples were examined for the presence of inflammation both in villi and in lamina propria, epithelial regeneration, fusion of villi, oedema in deep lamina propria, T atrophy, stroma (fibroconnective and histiocytes), and follicular hyperplasia.
Intestinal weight results did not reveal significant differences between treatment groups after 7 days post-challenge (Table S2). Phytogenic dietary treatments did not significantly affect ileum inflammatory infiltrates, epithelial regeneration, oedema and hyperemia after 7 days (Table 6).
Table 6.
Histological examination of ileum and lymphoid of weaned piglets fed experimental diets on day 7
| Score | Treatments | p-value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PHY1 (n = 4) | PHY2 (n = 4) | CTRL + (n = 3) | CTRL- (n = 4) | ||||||
| median | min–max | median | min–max | median | min–max | median | min–max | ||
| Ileum | |||||||||
| Inflammatory infiltrates | 1 | 0–2 | 2 | 2–3 | 2 | 1–2 | 1 | 1–2 | 0.068 |
| Epithelial regeneration | 0 | 0–0 | 0 | 0–0 | 0 | 0–0 | 0 | 0–0 | 1.000 |
| Fusion of villi | 1 | 0–3 | 3 | 2–3 | 2 | 1–3 | 1 | 0–2 | 0.223 |
| Oedema | 0 | 0–1 | 0 | 0–1 | 0 | 0–1 | 0 | 0–1 | 1.000 |
| Hyperemia | 1 | 0–1 | 1 | 1–2 | 0 | 0–2 | 1 | 0–1 | 0.382 |
| Necrosis of mucosa | 0 | 0–0 | 0 | 0–1 | 0 | 0–0 | 0 | 0–0 | 1.000 |
| Lymphoid | |||||||||
| T atrophy | 0 | 0–1 | 1 | 0–1 | 0 | 0–1 | 0 | 0–0 | 0.253 |
| Stroma | 0 | 0–1 | 1 | 1–2 | 2 | 0–3 | 0 | 0–1 | 0.073 |
| Follicular hyperplasia | 0 | 0–2 | 2 | 1–3 | 1 | 0–3 | 0 | 0–1 | 0.186 |
Data are presented as medians and minimum and maximum value (min–max)
Relative expressions of IL-10, IL-6, LOX, GPX2, NQ01 and CLDND1 were not affected by phytogenic dietary treatments (Table 7). The relative expression of occludin was downregulated at day 7 post-challenge (p < 0.012). Pairwise comparisons revealed only a tendency to increase in challenged groups compared to CTRL- (p = 0.066).
Table 7.
Duodenum expression of the main genes related to the intestinal integrity, inflammation and health of weaned piglets fed experimental diets on day 7 post-challenge
| Relative expressiona | Treatment | p-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PHY1 (n = 4) | PHY2 (n = 4) | CTRL + (n = 4) | CRTL- (n = 4) | |||||||
| median | min–max | median | min–max | median | min–max | median | min–max | |||
| IL-6 | 0.32 | 0.22–0.43 | 0.48 | 0.08–0.87 | 0.34 | 0.23–1.10 | 1.00 | 1.00–1.00 | 0.139 | |
| IL-10 | 0.26 | 0.14–0.61 | 0.94 | 0.11–1.71 | 0.24 | 0.09–0.83 | 1.00 | 1.00–1.00 | 0.110 | |
| COX1 | 0.74 | 0.58–8.15 | 4.24 | 0.51–15.31 | 3.36 | 0.56–11.36 | 1.00 | 1.00–1.00 | 0.671 | |
| COX2 | 0.46 | 0.24–1.77 | 1.57 | 0.10–4.94 | 1.34 | 0.14–2.36 | 1.00 | 1.00–1.00 | 0.734 | |
| LOX | 0.61 | 0.25–1.37 | 2.33 | 0.21–3.96 | 1.19 | 0.40–1.39 | 1.00 | 1.00–1.00 | 0.331 | |
| GPX2 | 1.04 | 0.38–1.93 | 2.98 | 0.31–3.53 | 2.11 | 0.25–6.33 | 1.00 | 1.00–1.00 | 0.426 | |
| NQ01 | 0.68 | 0.27–5.73 | 6.21 | 0.28–8.62 | 5.61 | 0.46–8.21 | 1.00 | 1.00–1.00 | 0.315 | |
| CLDND1 | 1.37 | 0.31–6.33 | 14.40 | 0.39–22.20 | 6.05 | 0.69–22.67 | 1.00 | 1.00–1.00 | 0.619 | |
| OCLN | 0.42 | 0.02–0.96 | 0.34 | 0.09–0.72 | 0.55 | 0.14–0.96 | 1.00 | 1.00–1.00 | 0.012 | |
Data are presented as medians and minimum and maximum value (min–max)
IL-6 interleukin-6, IL-10 interleukin 10, COX1 cytochrome c oxidase subunit I, COX2 cytochrome c oxidase subunit II, LOX lysyl oxidase, GPX2 glutathione peroxidase 2, NQ01 NAD (P) H quinone dehydrogenase 1, CLDND1 claudin domain containing 1, OCLN occludin
aRelative expressions of selected genes are presented as 2-.ΔΔCT
Influence of phytogenic treatments on hematological and serum metabolites
The serum metabolic profile did not show statistically significant differences between the experimental groups at day 3 after the challenge (Table S3). After 7 days post-challenge, a significantly higher level of total protein content was observed in CTRL + compared to CTRL- (p = 0.050) (Table 8). Globulin content tended to be higher in CTRL + than CTRL- at 7 days post-challenge (p = 0.055). PHY2 had a higher level of AST-GOT at day 7 compared to the other challenged groups (p < 0.050). Acute phase proteins were not affected by dietary treatments and experimental challenge and showed no statistically significant differences after 3- and 7-days post-challenge (Table S4).
Table 8.
Serum metabolites of weaned piglets fed experimental diets on day 7 post-challenge
| Treatments | |||||
|---|---|---|---|---|---|
| Blood | PHY1 (n = 7) |
PHY2 (n = 7) |
CTRL + (n = 6) |
CRTL- (n = 7) |
p-value |
| Total protein, g/L | 56.95 ± 2.67AB | 54.04 ± 2.67AB | 62.32 ± 2.89A | 50.83 ± 2.67B | 0.050 |
| Hematocrit, % | 26.03 ± 1.05 | 26.09 ± 1.05 | 25.00 ± 1.16 | 25.43 ± 1.05 | 0.880 |
| Albumin, g/L | 30.76 ± 2.85 | 32.05 ± 2.72 | 28.70 ± 3.06 | 26.11 ± 2.71 | 0.452 |
| Globulin, g/L | 28.31 ± 2.63 | 26.20 ± 2.61 | 35.31 ± 2.86 | 24.43 ± 2.61 | 0.055 |
| A/G ratio | 1.06 ± 0.09 | 1.09 ± 0.09 | 0.89 ± 0.09 | 1.14 ± 0.09 | 0.280 |
| Urea, mmol/L | 2.51 ± 0.30 | 2.09 ± 0.30 | 2.90 ± 0.33 | 1.75 ± 0.30 | 0.093 |
| ALT-GPT, IU/L | 26.51 ± 2.51 | 31.09 ± 2.54 | 24.52 ± 2.81 | 26.66 ± 2.54 | 0.375 |
| AST-GOT, IU/L | 39.11 ± 6.79A | 72.02 ± 7.42B | 37.82 ± 7.05A | 43.59 ± 6.74AB | 0.014 |
| ALP, UI/L | 170.30 ± 17.78 | 197.15 ± 17.71 | 149.83 ± 19.35 | 195.84 ± 18.32 | 0.262 |
| Total bilirubin, µmol/l | 2.25 ± 0.16 | 1.86 ± 0.16 | 1.86 ± 0.17 | 1.70 ± 0.16 | 0.123 |
| Glucose, mmol/L | 4.91 ± 0.28 | 5.52 ± 0.28 | 5.12 ± 0.31 | 4.91 ± 0.28 | 0.421 |
| Total cholesterol, mmol/L | 2.17 ± 0.11 | 2.21 ± 0.11 | 2.20 ± 0.12 | 2.19 ± 0.11 | 0.992 |
| Calcium, mmol/L | 2.55 ± 0.15 | 2.89 ± 0.15 | 2.49 ± 0.17 | 2.54 ± 0.15 | 0.243 |
| Phosphorus, mmol/L | 2.78 ± 0.11 | 3.00 ± 0.11 | 2.87 ± 0.12 | 3.04 ± 0.13 | 0.426 |
| Magnesium, mmol/L | 0.85 ± 0.04 | 0.91 ± 0.04 | 0.96 ± 0.04 | 0.87 ± 0.04 | 0.174 |
Data are presented as least squared means (LSMEANS) and standard errors (SE)
A/G albumin/globulin, ALT-GPT alanine aminotransferase, AST-GOT aspartate aminotransferase, ALP alkaline phosphatase, HDL high-density lipoprotein, LDL low density lipoprotein
A−BDifferent uppercase letters indicate statistically significant differences among treatment groups (p ≤ 0.05)
Discussion
Weaning is a critical period where piglets need to adapt to a new diet, environment and to develop their own immunity (Tretola et al. 2019). During this phase, PWD is one of the major causes of gastrointestinal disorders leading to high morbidity, antibiotic use and economic losses. Several natural extracts have been investigated for their functional proprieties to decrease diarrhea occurrence in piglets, with discordant results. The general aim of this study was to evaluate the protective effect of innovative phytogenic premix with or without MCFA and SCFA against O138 E. coli in weaned piglets. Genetic characterization of the sows led to the enrollment of piglets that were potentially susceptible to F18 fimbriae. In fact, the presence of F18 receptor (F18R) on porcine intestinal epithelium is crucial for the development of E. coli infections.
During the pre-challenge period (day -6 to day 0), the piglets showed comparable growth performance, demonstrating that the supplementation of additives did not influence their growth and feed consumption or feed palatability. Even if the effect on zootechnical performance was limited by the short experimental period (EFSA 2018), ADFI was affected by the treatment.
However, ADFI of CTRL + was significantly lower compared to PHY2 and CTRL- groups. The observed decrease in feed intake of CTRL + suggests that the challenged group without any supplementation reduced the feed consumption probably due to the detrimental effect of experimental infection. In addition, higher dietary intake is often related to a better health status (Czech et al. 2021), indicating that the treatment with PHY and organic acids could have supported animals’ health resulting in increased feed intake during the entire post-challenge period. PHY2 group showed a similar performance to CTRL- (uninfected), suggesting that dietary supplementation with the phytogenic premix, MCFA and SCFA was very effective in dealing with O138 E. coli infection, thus supporting intestinal health of animals. The addition of MCFA and SCFA may enhance animal growth by several mechanisms as previous studies described (e.g. inhibitory activity, mucosal epithelium integrity support) (Royce et al. 2013; Ferrara et al. 2017; Diao et al. 2019). In addition, phytogenic feed additives derived from spices and herbs are commonly used in animal nutrition as an alternative to in-feed antibiotics due to their antibacterial, antiviral and antioxidant properties. These effects are generally due to the presence of different bioactive compounds such as alkaloids, flavonoids, glycosides, mucilage, saponins, tannins, phenolics, polyphenols, terpenoids, and polypeptides (Upadhaya et al. 2016; Nowak et al. 2017; Caprarulo et al. 2020a; Dell’Anno et al. 2020; Reggi et al. 2020). Our results are in line with other studies demonstrating the antibacterial activity of PHYs, MCFA and SCFA on a wide range of pathogens (Dibner and Buttin 2002; Salsali et al. 2008).
In terms of clinical examination, from day 1, clinical scores were affected by experimental infection, confirming that disease development impaired the clinical status of challenged animals compared to the pre-challenge period. Moreover, significant differences in pathological hair, respiratory, oedema and epiphora scores were not detected in infected groups. This was probably due to the individual variability and the small sample size that could prevented to observe differences among groups. The O138 Escherichia coli challenger strain can impair gut health due to its capacity to adhere to the intestinal epithelium by specific fimbriae which could be followed by verocytotoxin production (Rossi et al. 2012, 2013) and in consequence may show systemic symptoms.
A slightly different situation was found during the evaluation of the fecal score and incidence of diarrhea. Experimental challenge affected transitory the fecal color and consistency during the 7 days post-challenge. Firstly, from day 1 to day 4 post-challenge, feces of yellowish color were registered more frequently in challenged group compared to CTRL- typically related to gastrointestinal disorders (Rossi et al. 2012). Considering total diarrhea cases recorded among experimental groups, from day 5 to day 7 the highest diarrhea frequency was registered, suggesting a late effect of challenge on fecal consistency compared to fecal color. These data are confirmed by a previous study by Rossi et al. (Rossi et al. 2021) showing that O138 E. coli experimental infection increased the sum of fecal score from 3 to 9 days post-challenge. Particularly, the highest diarrhea occurrence was observed in PHY1 compared to other groups, while PHY2 showed a fecal consistency comparable to CTRL- suggesting the counteracting activity of the phytogenic additives, SCFA and MCFA against experimental infection. Even if antibacterial activity of phytogenic additives was reported (Namkung et al. 2004), the observed effect on diarrhea incidence was probably related to their combined effect with SCFA and MCFA. It has been demonstrated, that SCFA and MCFA can exert an inhibitory activity (Lei et al. 2017; Swanson et al. 2018; Zhang et al. 2020) or enhance the functional properties of phytogenic additives (McKnight et al. 2019).
In addition, dietary supplementation of organic acids can modulate the intestinal environment, creating undesirable environmental conditions for pathogenic bacteria, thus also influencing the intestinal microbiota (Verstegen and Williams 2002). Even if is difficult to establish the exact mechanisms for the enhancing antimicrobial effect by the combination of PHYs with organic acids (SCFA and MCFA) in pigs, we can suppose that PHYs can act as a permeabilizing complex and modify pores of the bacterial wall, thus facilitating the entrance of organic acids with antimicrobial activity (Tugnoli et al. 2020). In addition, the reduction in undigested feed protein by organic acids reduces the negative fermentative processes, increases growth performance and repairment of damaged intestinal tissues (Jia et al. 2020). Our results suggest that the addition of MCFA and SCFA to the phytogenic premix significantly inhibited enterotoxigenic E. coli diarrhea, thus supporting intestinal health of animals.
Considering the challenger strain shedding, the proliferation started gradually from the day of challenge in line with clinical observations. Compared with the uninfected control group infected animals showed hemolytic E. coli shedding from day 1 post-challenge, thus confirming the success of the experimental infection.
Histopathological examination of the ileum, jejunum and large intestine is thus used to highlight clinical signs of E. coli infection (Luppi 2017). In our study, histological evaluation of the ileum and lymphoid of intestinal tissues did not reveal significant lesions. The animals in the experimental trial thus did not show severe signs of intestinal lesions. Although more frequent lesions were registered in the PHY2 group, these did not impair animal performance and there was a comparable growth curve to CTRL-. This was probably due to the supplementation of phytogenic with SCFA and MCFA which could have supported intestinal health.
Gene expressions showed a high individual variability in terms of inflammatory parameters and tight junctions (TJs), probably due to the limited number of animals. We thus analyzed the expression of the TJ transmembrane protein (occludin) and the observed data were in line with morphological analyses. Our findings suggested that tight junction integrity tended to be disrupted seven days after infection in challenged groups compared to the CTRL-. Intestinal permeability is regulated by the tight junctions which are a primary determinant of epithelial paracellular permeability (Zhang et al. 2021). Disruption of occludin regulation is related to many diseases. During the inflammation process, specific domains of occludin are in fact thought to mediate the transepithelial migration of neutrophils across the TJ (Feldman et al. 2005). Inflammation produces effects on epithelial barriers, increasing the leakiness of occludin, and decreasing the barrier function of this protein. Occludin responds earlier to oxidative stress than claudin, which responds later to reactive oxygen species (ROS) (Blasig et al. 2011). Intestinal bacterial infection is associated with intestinal epithelial and crypt architectural irregularity and with barrier dysfunction, leading to an increase in intestinal mucosal permeability. The observed slight downregulation of occludin after seven days in challenged groups could be due to the harmful activity of the challenger strain. Further investigations are required to better understand the effect of PHYs, SCFA and MCFA on the modulation of genes involved in inflammation and intestinal integrity.
Considering the biochemical parameters of the experimental groups (PHY1, PHY2, CTRL + and CTRL-), the values were within the reference range of weaned pigs (Klem et al. 2010; IZSLER 2017), thus confirming that phytogenic additive supplementation had no detrimental effect on serum metabolism. The metabolite profile showed an increased level of total protein and a higher globulin content in CTRL + compared to CTRL-. However, globulin together with albumin are the two major constituents of serum proteins, which play a crucial role in the inflammatory process (Balan et al. 2020; Wang et al. 2020). The increase in globulin could be associated with an inflammatory process probably due to the experimental E. coli infection leading to an increased concentration of total serum proteins. The serum AST-GOT level is a specific marker for liver tissue and represents a valuable indicator for acute hepatocyte injury or cell membranes damage (Kim 2020; Amirabagya et al. 2021). Although our results are in line with the proper range of pig physiology parameters (Klem et al. 2010; IZSLER 2017; Caprarulo et al. 2020b), AST-GOT was probably higher in the PHY2 group due to the presence of SCFA and MCFA which are immediately available for hepatic metabolism. In fact, short-chain fatty acids can activate lipid and glucose metabolism independently of the pig gut microbiota (Zhou et al. 2021).
Conclusions
Our study showed that phytogenic additive dietary supplementation limited the detrimental effect of experimental challenge. Phytogenic premix plus SCFA and MCFA revealed a positive effect on animal performance and health improving ADFI and fecal consistency during the post-challenge period compared to infected control group, suggesting that the combination of PHYs and organic acids can be considered as effective against pathogenic E. coli strains of weaned piglets. Due to the lack of studies on the argument, at this stage is too early to state that phytogenics are effective. Future studies will be necessary to confirm our results and extensively investigate how phytogenic additives and organic acids affect gene expression over time.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank the technical and veterinary staff and professors for participating in this experimental trial, and Dr. Giancarlo Selmini for his help in diets formulation.
Author contributions
Conceptualization: L. Rossi, A. Baldi; Methodology: V. Caprarulo, L. Turin, M. Hejna, S. Reggi, M. Dell’Anno, P. Riccaboni, P. Trevisi, D. Luise, A. Baldi, L. Rossi; Writing-original draft preparation: M. Dell’Anno, V. Caprarulo, M. Hejna, L. Rossi; Review and editing: M. Dell’Anno, V. Caprarulo; M. Hejna, L. Rossi, A. Baldi; Formulation and preparation of all tested diets: L. Rossi; Statistical analysis: M. Dell’Anno, V. Caprarulo. All authors drafted and approved the final version of the manuscript.
Funding
Open access funding provided by Università degli Studi di Milano within the CRUI-CARE Agreement.
Data Availability
All data generated or analyzed in this study are available from the corresponding author upon reasonable request. Data are stored on Google Drive at the following link: https://drive.google.com/drive/folders/1fpkkuaELR6a5ZIeTh3gszVoMrQJcGcMF?usp=sharing.
Declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki.
The procedures and protocols used in this study were designed in accordance with the guidelines for animal welfare and the use of animals regulated under Directive 2010/63/EU on the protection of animals used for scientific purposes. The protocol was approved by the Animal Welfare Organization of the University of Milan and by the Italian Ministry for project (authorization number: 711/2017-PR, 28/09/2017).
Consent to participate
All authors agreed to participate in this work. They also approved the content of the research and the submission of the manuscript to Veterinary Research Communication journal.
Consent to publish
All authors agree to the content of the paper for publication.
Conflicts of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- American Oil Chemistry Society (AOCS) Crude fiber analysis in feeds by filter bag technique. Champaign: Official Methods and Recommended practices; 2009. [Google Scholar]
- Amirabagya F, Hapsari RAF, Wulandari E. The Effect of Jatropha curcas L Seed Extract on AST/ALT Activity and The Central Vein Thickness in Liver. Pharmacogn J. 2021;13:66–72. doi: 10.5530/pj.2021.13.10. [DOI] [Google Scholar]
- Association of Official Analytical Chemists (AOAC) (2019) Official Methods of Analysis. Washington, DC, USA
- Balan P, Staincliffe M, Moughan PJ. Effects of spray-dried animal plasma on the growth performance of weaned piglets—A review. J Anim Physiol Anim Nutr. 2020;105:699–714. doi: 10.1111/jpn.13435. [DOI] [PubMed] [Google Scholar]
- Blasig IE, Bellmann C, Cording J, Del Vecchio G, Zwanziger D, Huber O, Haseloff RF. Occludin protein family: oxidative stress and reducing conditions. Antiox Redox Signaling. 2011;15:1195–1219. doi: 10.1089/ars.2010.3542. [DOI] [PubMed] [Google Scholar]
- Bonetti A, Tugnoli B, Piva A, Grilli E. Towards Zero Zinc Oxide: Feeding Strategies to Manage Post-Weaning Diarrhea in Piglets. Animals. 2021;11:1–24. doi: 10.3390/ani11030642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprarulo V, Giromini C, Rossi L. Chestnut and quebracho tannins in pig nutrition: the effects on performance and intestinal health. Animal. 2020;15:1–10. doi: 10.1016/j.animal.2020.100064. [DOI] [PubMed] [Google Scholar]
- Caprarulo V, Hejna M, Giromini C, Liu Y, Dell’Anno M, Sotira S, Reggi S, Sgoifo-Rossi CA, Callegari ML, Rossi L. Evaluation of Dietary Administration of Chestnut and Quebracho Tannins on Growth, Serum Metabolites and Fecal Parameters of Weaned Piglets. Animals. 2020;10:1–15. doi: 10.3390/ani10111945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commission Regulation (EC) No 152/2009 of 27 January 2009 laying down the methods of sampling and analysis for the official control of feed (Text with EEA relevance)
- Cormican M, Hopkins S, Jarlier V, Reilly J, Simonsen G, Strauss R, Vandenberg O, Zabicka D, Zarb P, Catchpole M, Heuer O, Iosifidis E, Monnet D, Plachouras D, Weist K, Ricci A, Allende A, Bolton D, Chemaly M, Davies R, Escamez P, Girones R, Herman L, Koutsoumanis K, Lindqvist R, Norrung B, Robertson L, Ru G, Sanaa M, Simmons M, Skandamis P, Snary E, Speybroeck N, Ter Kuile B, Threlfall J, Wahlstrom H, Tenhagen B, Teale C, Schuepbach G, Beloeil P, Liebana E, Stella P, Murphy D, Hauser B, Urbain B, Kozhuharov E, Bozic F, Michaelidou-Patsia A, Bures J, Vestergaard E, Baptiste K, Tiirats T, Nevalainen M, Rouby J, Hahn G, Schlumbohm W, Malemis I, Kulcsar G, Lenhardsson J, Beechinor J, Breathnach R, Pasquali P, Auce Z, Maciulskis P, Schmit M, Spiteri S, Schefferlie G, Hekman P, Bergendahl H, Swiezcicka A, Da Silva J, Taban L, Hederova J, Straus K, Madero C, Persson E, Jukes H, Weeks J, Kivilahti-Mantyla K, Moulin G, Wallmann J, Grave K, Greko C, Munoz C, Bouchard D, Catry B, Moreno M, Pomba C, Rantala M, Ruzauskas M, Sanders P, Schwarz C, van Duijkeren E, Wester A, Ignate K, Kunsagi Z, Torren-Edo J (2017) ECDC, EFSA and EMA Joint Scientific Opinion on a list of outcome indicators as regards surveillance of antimicrobial resistance and antimicrobial consumption in humans and food-producing animals. EFSA J 15. 10.2903/j.efsa.2017.5017
- Costa LB, Luciano FB, Miyada VS, Gois FD. Herbal extracts and organic acids as natural feed additives in pig diets. S Afr J Anim Sci. 2013;43:181–193. doi: 10.4314/sajas.v43i2.9. [DOI] [Google Scholar]
- Czech A, Grela ER, Kiesz M. Dietary fermented rapeseed or/and soybean meal additives on performance and intestinal health of piglets. Sci Rep. 2021;11:1–10. doi: 10.1038/s41598-021-96117-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Anno M, Sotira S, Rebucci R, Reggi S, Castiglioni B, Rossi L. In vitro evaluation of antimicrobial and antioxidant activities of algal extracts. Ital J Anim Sci. 2020;19:103–113. doi: 10.1080/1828051X.2019.1703563. [DOI] [Google Scholar]
- Dell’Anno M, Callegari ML, Reggi S, Caprarulo V, Giromini C, Spalletta A, Coranelli S, Sgoifo Rossi CA, Rossi L. Lactobacillus plantarum and Lactobacillus reuteri as Functional Feed Additives to Prevent Diarrhoea in Weaned Piglets. Animals. 2021;11:1–19. doi: 10.3390/ani11061766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell’Anno M, Hejna M, Sotira S, Caprarulo V, Reggi S, Pilu R, Miragoli F, Callegari ML, Panseri S, Rossi L. Evaluation of leonardite as a feed additive on lipid metabolism and growth of weaned piglets. Anim Feed Sci Technol. 2020;266:1–12. doi: 10.1016/j.anifeedsci.2020.114519. [DOI] [Google Scholar]
- Dell’Anno M, Reggi S, Caprarulo V, Hejna M, Sgoifo Rossi CA, Callegari ML, Baldi A, Rossi L. Evaluation of Tannin Extracts, Leonardite and Tributyrin Supplementation on Diarrhoea Incidence and Gut Microbiota of Weaned Piglets. Animals. 2021;11:1–18. doi: 10.3390/ani11061693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao H, Jiao A, Yu B, Mao X, Chen D. Gastric infusion of short-chain fatty acids can improve intestinal barrier function in weaned piglets. Genes Nutr. 2019;14:1–16. doi: 10.1186/s12263-019-0626-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibner J, Buttin P. Use of organic acids as a model to study the impact of gut microflora on nutrition and metabolism. J Appl Poult Res. 2002;11:453–463. doi: 10.1093/japr/11.4.453. [DOI] [Google Scholar]
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes Text with EEA relevance
- Domínguez Díaz L, Fernández-Ruiz V, Cámara M. The frontier between nutrition and pharma: The international regulatory framework of functional foods, food supplements and nutraceuticals. Crit Rev Food Sci Nutr. 2020;60:1738–1746. doi: 10.1080/10408398.2019.1592107. [DOI] [PubMed] [Google Scholar]
- Dubreuil JD. Antibacterial and antidiarrheal activities of plant products against enterotoxinogenic Escherichia coli. Toxins. 2013;5:2009–2041. doi: 10.3390/toxins5112009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durmic Z, Blache D. Bioactive plants and plant products: Effects on animal function, health and welfare. Anim Feed Sci Technol. 2012;176:150–162. doi: 10.1016/j.anifeedsci.2012.07.018. [DOI] [Google Scholar]
- European Food Safety Authority (EFSA), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos MdL, Bories G, Chesson A, Cocconcelli PS, Flachowsky G (2018) EFSA Panel on Additives and Products or Substances used in Animal Feed: Guidance on the assessment of the efficacy of feed additives. EFSA J 16:e05274. 10.2903/j.efsa.2018.5274 [DOI] [PMC free article] [PubMed]
- Feldman GJ, Mullin JM, Ryan MP. Occludin: structure, function and regulation. Adv Drug Delivery Rev. 2005;57:883–917. doi: 10.1016/j.addr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Ferrara F, Tedin L, Pieper R, Meyer W, Zentek J. Influence of medium-chain fatty acids and short-chain organic acids on jejunal morphology and intra-epithelial immune cells in weaned piglets. J Anim Physiol Anim Nutr. 2017;101:531–540. doi: 10.1111/jpn.12490. [DOI] [PubMed] [Google Scholar]
- Ferronato G, Prandini A. Dietary supplementation of inorganic, organic, and fatty acids in pig: A review. Animals. 2020;10:1–27. doi: 10.3390/ani10101740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossi S, Rossi L, De Marco M, Sgoifo Rossi CA. The Effect of Different Sources of Selenium Supplementation on the Meat Quality Traits of Young Charolaise Bulls during the Finishing Phase. Antioxidants. 2021;10:1–14. doi: 10.3390/antiox10040596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Zhan T, Zhao Q, Tang C, Zhang K, Han Y, Zhang J. Effects of mixed organic acids and medium chain fatty acids as antibiotic alternatives on the performance, serum immunity, and intestinal health of weaned piglets orally challenged with Escherichia coli K88. Anim Feed Sci Technol. 2020;269:1–13. doi: 10.3390/ani11051292. [DOI] [Google Scholar]
- Hanczakowska E. The use of medium-chain fatty acids in piglet feeding-a review. Ann Anim Sci. 2017;17:967–977. doi: 10.1515/aoas-2016-0099. [DOI] [Google Scholar]
- Hayer SS, Rovira A, Olsen K, Johnson TJ, Vannucci F, Rendahl A, Perez A, Alvarez J. Prevalence and trend analysis of antimicrobial resistance in clinical Escherichia coli isolates collected from diseased pigs in the USA between 2006 and 2016. Transbound Emerg Dis. 2020;67:1930–1941. doi: 10.1111/tbed.13528. [DOI] [PubMed] [Google Scholar]
- Istituto Zooprofilattico Sperimentale della Lombardia e dell'Emilia Romagna (IZSLER) (2017) Parametri di Chimica Clinica: Valori Osservati in Suini di Diversa Età. Available online: https://www.izsler.it/pls/izs_bs/v3_s2ew_consultazione.mostra_pagina?id_pagina=1494
- Jackman JA, Boyd RD, Elrod CC. Medium-chain fatty acids and monoglycerides as feed additives for pig production: towards gut health improvement and feed pathogen mitigation. J Anim Sci Biotechnol. 2020;11:1–15. doi: 10.1186/s40104-020-00446-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia M, Zhang Y, Gao Y, Ma X. Effects of Medium Chain Fatty Acids on Intestinal Health of Monogastric Animals. Curr Protein Pept Sci. 2020;21:777–784. doi: 10.2174/1389203721666191231145901. [DOI] [PubMed] [Google Scholar]
- Kim HK. The Effects of Anti-Inflammatory and Liver Function using Heat-Treated Cabbage. Int J Internet Broadcast Commun. 2020;12:131–138. doi: 10.7236/IJIBC.2020.12.3.131. [DOI] [Google Scholar]
- Klem TB, Bleken E, Morberg H, Thoresen SI, Framstad T. Hematologic and biochemical reference intervals for Norwegian crossbreed grower pigs. Vet Clin Pathol. 2010;39:221–226. doi: 10.1111/j.1939-165X.2009.00199.x. [DOI] [PubMed] [Google Scholar]
- Lei XJ, Park JW, Baek DH, Kim JK, Kim IH. Feeding the blend of organic acids and medium chain fatty acids reduces the diarrhea in piglets orally challenged with enterotoxigenic Escherichia coli K88. Anim Feed Sci Technol. 2017;224:46–51. doi: 10.1016/j.anifeedsci.2016.11.016. [DOI] [Google Scholar]
- Li P, Piao X, Ru Y, Han X, Xue L, Zhang H. Effects of adding essential oil to the diet of weaned pigs on performance, nutrient utilization, immune response and intestinal health. Asian-Australas J Anim Sci. 2012;25:1617–1626. doi: 10.5713/ajas.2012.12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Wang L, Zhou Y, Miao Z. Prevalence and characterization of virulence genes in Escherichia coli isolated from piglets suffering post-weaning diarrhoea in Shandong Province, China. Vet Med Sci. 2020;6:69–75. doi: 10.1002/vms3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillehoj H, Liu Y, Calsamiglia S, Fernandez-Miyakawa M, Chi F, Cravens R, Oh S, Gay C. Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Vet Res. 2018;49:1–18. doi: 10.1186/s13567-018-0562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Luise D, Lauridsen C, Bosi P, Trevisi P. Methodology and application of Escherichia coli F4 and F18 encoding infection models in post-weaning pigs. J Anim Sci Biotechnol. 2019;10:1–20. doi: 10.1186/s40104-019-0352-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luise D, Motta V, Bertocchi M, Salvarani C, Clavenzani P, Fanelli F, Pagotto U, Bosi P, Trevisi P. Effect of Mucine 4 and Fucosyltransferase 1 genetic variants on gut homoeostasis of growing healthy pigs. J Anim Physiol Anim Nutr. 2019;103:801–812. doi: 10.1111/jpn.13063. [DOI] [PubMed] [Google Scholar]
- Luppi A. Swine enteric colibacillosis: diagnosis, therapy and antimicrobial resistance. Porcine Health Manag. 2017;3:1–18. doi: 10.1186/s40813-017-0063-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maenner K, Vahjen W, Simon O. Studies on the effects of essential-oil-based feed additives on performance, ileal nutrient digestibility, and selected bacterial groups in the gastrointestinal tract of piglets. J Anim Sci. 2011;89:2106–2112. doi: 10.2527/jas.2010-2950. [DOI] [PubMed] [Google Scholar]
- McKnight LL, Peppler W, Wright DC, Page G, Han Y. A blend of fatty acids, organic acids, and phytochemicals induced changes in intestinal morphology and inflammatory gene expression in coccidiosis-vaccinated broiler chickens. Poult Sci. 2019;98:4901–4908. doi: 10.3382/ps/pez241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MohammadiGheisar M, Kim IH. Phytobiotics in poultry and swine nutrition–a review. Ital J Anim Sci. 2018;17:92–99. doi: 10.1080/1828051X.2017.1350120. [DOI] [Google Scholar]
- Namkung H, Li M, Gong J, Yu H, Cottrill M, De Lange C. Impact of feeding blends of organic acids and herbal extracts on growth performance, gut microbiota and digestive function in newly weaned pigs. Can J Anim Sci. 2004;84:697–704. doi: 10.4141/A04-005. [DOI] [Google Scholar]
- Nowak P, Kasprowicz-Potocka M, Zaworska A, Nowak W, Stefańska B, Sip A, Grajek W, Juzwa W, Taciak M, Barszcz M. The effect of eubiotic feed additives on the performance of growing pigs and the activity of intestinal microflora. Arch Anim Nutr. 2017;71:455–469. doi: 10.1080/1745039x.2017.1390181. [DOI] [PubMed] [Google Scholar]
- National Research Council (NRC) (2012) Nutrient Requirements of Swine. The National Academies Press, Washington, DC. 10.17226/13298
- Regulation EC 1831/2003 of the European Parliament and of the Council, of 22 September 2003 on Additives for Use in Animal Nutrition (Text with EEA Relevance); EU Commission: Brussels, Belgium, 2003
- Reggi S, Giromini C, Dell’Anno M, Baldi A, Rebucci R, Rossi L. In Vitro Digestion of Chestnut and Quebracho Tannin Extracts: Antimicrobial Effect, Antioxidant Capacity and Cytomodulatory Activity in Swine Intestinal IPEC-J2 Cells. Animals. 2020;10:1–14. doi: 10.3390/ani10020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remfry SE, Amachawadi RG, Shi X, Bai J, Woodworth JC, Tokach MD, Dritz SS, Goodband RD, DeRouchey JM, Nagaraja TG. Polymerase Chain Reaction-Based Prevalence of Serogroups of Escherichia coli Known to Carry Shiga Toxin Genes in Feces of Finisher Pigs. Foodborne Pathog Dis. 2020;17:782–791. doi: 10.1089/fpd.2020.2814. [DOI] [PubMed] [Google Scholar]
- Renzhammer R, Loncaric I, Roch F, Pinior B, Kasbohrer A, Spergser J, Ladinig A, Unterweger C. Prevalence of Virulence Genes and Antimicrobial Resistances in E. coli Associated with Neonatal Diarrhea, Postweaning Diarrhea, and Edema Disease in Pigs from Austria. Antibiotics. 2020;9:1–13. doi: 10.3390/antibiotics9040208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Camacho D, Vinyeta E, Pérez JF, Aumiller T, Criado L, Palade LM, Taranu I, Folch JM, Calvo MA, Van der Klis JD. Phytogenic actives supplemented in hyperprolific sows: effects on maternal transfer of phytogenic compounds, colostrum and milk features, performance and antioxidant status of sows and their offspring, and piglet intestinal gene expression. J Anim Sci. 2020;98:1–13. doi: 10.1093/jas/skz390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi L, Di Giancamillo A, Reggi S, Domeneghini C, Baldi A, Sala V, Dell'Orto V, Coddens A, Cox E, Fogher C. Expression of verocytotoxic Escherichia coli antigens in tobacco seeds and evaluation of gut immunity after oral administration in mouse model. J Vet Sci. 2013;14:263–270. doi: 10.4142/jvs.2013.14.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi L, Pinotti L, Agazzi A, Dell’Orto V, Baldi A. Plant bioreactors for the antigenic hook-associated flgK protein expression. Ital J Anim Sci. 2014;13:23–29. doi: 10.4081/ijas.2014.2939. [DOI] [Google Scholar]
- Rossi L, Dell'Orto V, Vagni S, Sala V, Reggi S, Baldi A. Protective effect of oral administration of transgenic tobacco seeds against verocytotoxic Escherichia coli strain in piglets. Vet Res Commun. 2014;38:39–49. doi: 10.1007/s11259-013-9583-9. [DOI] [PubMed] [Google Scholar]
- Rossi L, Turin L, Alborali GL, Demartini E, Filipe JFS, Riva F, Riccaboni P, Scanziani E, Trevisi P, Dall’Ara P, Translational Approach to Induce and Evaluate Verocytotoxic E. coli O138 Based Disease in Piglets. Animals. 2021;11:1–17. doi: 10.3390/ani11082415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi L, Vagni S, Polidori C, Alborali GL, Baldi A, Dell’Orto V. Experimental Induction of Escherichia coli Diarrhoea in Weaned Piglets. Open J Vet Med. 2012;2:1–8. doi: 10.4236/ojvm.2012.21001. [DOI] [Google Scholar]
- Royce L, Liu P, Stebbins M, Hanson B, Jarboe L. The damaging effects of short chain fatty acids on Escherichia coli membranes. Appl Microbiol Biotechnol. 2013;97:8317–8327. doi: 10.1007/s00253-013-5113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salsali H, Parker WJ, Sattar SA. The effect of volatile fatty acids on the inactivation of Clostridium perfringens in anaerobic digestion. World J Microbiol Biotechnol. 2008;24:659–665. doi: 10.1007/s11274-007-9514-4. [DOI] [Google Scholar]
- Sun Y, Kim S. Intestinal challenge with enterotoxigenic Escherichia coli in pigs, and nutritional intervention to prevent postweaning diarrhea. Anim Nutr. 2017;3:322–330. doi: 10.1016/j.aninu.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson A, Cochrane R, Amachawadi R, Remfry S, Lerner A, Nagaraja T, Pluske J, Niederwerder M, Stark C, Paulk C. 482 Determination of the Minimum Inhibitory Concentration of Various Medium Chain Fatty Acid-Based Products in E. coli, Enterotoxigenic E. coli, and Campylobacter coli. J Anim Sci. 2018;96:258–258. doi: 10.1093/jas/sky073.479. [DOI] [Google Scholar]
- Tang KL, Caffrey NP, Nóbrega DB, Cork SC, Ronksley PE, Barkema HW, Polachek AJ, Ganshorn H, Sharma N, Kellner JD. Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: a systematic review and meta-analysis. Lancet Planet Health. 2017;1:e316–e327. doi: 10.1016/s2542-5196(17)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacker PA. Alternatives to antibiotics as growth promoters for use in swine production: a review. J Anim Sci Biotechnol. 2013;4:1–12. doi: 10.1186/2049-1891-4-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretola M, Ottoboni M, Luciano A, Rossi L, Baldi A, Pinotti L. Former food products have no detrimental effects on diet digestibility, growth performance and selected plasma variables in post-weaning piglets. Ital J Anim Sci. 2019;18:987–996. doi: 10.1080/1828051X.2019.1607784. [DOI] [Google Scholar]
- Tugnoli B, Giovagnoni G, Piva A, Grilli E. From acidifiers to intestinal health enhancers: How organic acids can improve growth efficiency of pigs. Animals. 2020;10:1–18. doi: 10.3390/ani10010134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhaya SD, Kim SJ, Kim IH. Effects of gel-based phytogenic feed supplement on growth performance, nutrient digestibility, blood characteristics and intestinal morphology in weanling pigs. J Appl Anim Res. 2016;44:384–389. doi: 10.1080/09712119.2015.1091334. [DOI] [Google Scholar]
- Verstegen MW, Williams BA. Alternatives to the use of antibiotics as growth promoters for monogastric animals. Anim Biotechnol. 2002;13:113–127. doi: 10.1081/abio-120005774. [DOI] [PubMed] [Google Scholar]
- Wang D, Lindemann MD, Estienne MJ. Effect of Folic Acid Supplementation and Dietary Protein Level on Growth Performance, Serum Chemistry and Immune Response in Weanling Piglets Fed Differing Concentrations of Aflatoxin. Toxins. 2020;12:1–13. doi: 10.3390/toxins12100651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Kim I. Effect of eugenol and cinnamaldehyde on the growth performance, nutrient digestibility, blood characteristics, fecal microbial shedding and fecal noxious gas content in growing pigs. Asian-Australas J Anim Sci. 2012;25:1178–1183. doi: 10.5713/ajas.2012.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Chowdhury M, Huo Y, Gong J. Phytogenic compounds as alternatives to in-feed antibiotics: potentials and challenges in application. Pathogens. 2015;4:137–156. doi: 10.3390/pathogens4010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Dogan B, Guo C, Herlekar D, Stewart K, Scherl EJ, Simpson KW. Short Chain Fatty Acids Modulate the Growth and Virulence of Pathosymbiont Escherichia coli and Host Response. Antibiotics. 2020;9:1–20. doi: 10.3390/antibiotics9080462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Li X, Qiao S, Yang D, Li Z, Xu J, Li W, Su L, Liu W. Occludin degradation makes brain microvascular endothelial cells more vulnerable to reperfusion injury in vitro. J Neurochem. 2021;156:352–366. doi: 10.1111/jnc.15102. [DOI] [PubMed] [Google Scholar]
- Zhou H, Yu B, Sun J, Liu Z, Chen H, Ge L, Chen D. Short-chain fatty acids can improve lipid and glucose metabolism independently of the pig gut microbiota. J Anim Sci Biotechnol. 2021;12:1–14. doi: 10.1186/s40104-021-00581-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed in this study are available from the corresponding author upon reasonable request. Data are stored on Google Drive at the following link: https://drive.google.com/drive/folders/1fpkkuaELR6a5ZIeTh3gszVoMrQJcGcMF?usp=sharing.