Abstract
Background:
Ketamine is an intravenous anesthetic. However, whether ketamine can induce neurotoxicity and neurobehavioral deficits remains largely unknown. Delirium is a syndrome of acute brain dysfunction associated with anesthesia and surgery in patients, and tau protein may contribute to postoperative delirium. Finally, ketamine may affect the function of the endosome, the key organelle for tau release from neurons. Therefore, we set out to determine the effects of ketamine on delirium-like behavior in mice and on tau trafficking in cultured cells.
Methods:
We used the buried food test, open field test, and Y-maze test in adult mice to assess the presence of delirium-like behavior in mice. We quantified tau amounts in the serum of mice. We used cell fraction methods to determine the effects of ketamine on tau intracellular transfer, extracellular release, and endosome trafficking in cultured cells.
Results:
Ketamine induced delirium-like behavior in mice and increased tau amounts in serum of mice. The ketamine treatments also led to increased accumulation of endosomes, as evidenced by increased endosomal markers Rab5 and Rab7. Moreover, ketamine inhibited endosome maturation, demonstrated by decreased membrane-bound but increased cytoplasm amounts of Rab5 and Rab7. Consequently, ketamine increased tau in the endosomes of cultured cells and the cell culture medium.
Conclusion:
These data suggest that ketamine may interfere with intracellular tau trafficking and induce delirium-like behavior, promoting future research regarding the potential neurotoxicity of anesthetics.
Keywords: Ketamine, tau, endosome, anesthetic, delirium-like behavior, neurotoxicity
Introduction
Ketamine is a phencyclidine analog and a non-competitive antagonist of the N-methyl-D-aspartic acid (NMDA) receptor1. It is a commonly used clinical anesthetic and may also treat depression2,3. Finally, ketamine is a recreational drug owing to its ability to induce visual hallucinations in subanesthetic doses2,3. Ketamine induces cognitive impairment4 and may also be associated with delirium2. However, whether ketamine can induce delirium-like behavior in rodents and the underlying mechanism remain unknown.
Delirium is a syndrome of acute brain dysfunction potentially associated with anesthesia, surgery, pain, disease, or medication, manifesting with impaired consciousness, disorganized thinking, lack of purpose, and inability to focus5. Previous studies have demonstrated an association between tau and postoperative delirium in patients6–8.
Tau is expressed mainly in neurons and has a role in stabilizing the cytoskeleton, maintaining anchoring of membrane material, and participating in axonal transport9. Tauopathy is a hallmark of Alzheimer’s disease neuropathogenesis10 and contributes to cognitive impairment. However, it is unknown whether ketamine can induce tauopathy, especially tau trafficking. A recent study showed that anesthetic sevoflurane could induce tau trafficking from neurons to microglia via accumulation in exosomal fraction11. However, the pathways and mechanisms responsible for tau trafficking inside cells before its transition to the extracellular space remain largely unknown. We, therefore, used ketamine as a clinically relevant tool to determine intracellular tau trafficking in the present study.
Endosomes are vesicle organelles that originate from the trans-Golgi network (TGN) and have extensive bidirectional transport relationships with the TGN, cell membrane, and lysosome, which are responsible for sorting, transport, and degradation of intracellular cargo12,13. Endosomes are classified into three main categories—early endosomes (EEs), late endosomes (LEs), and recycling endosomes—based on the order of endocytic cargo transport to various endosomal vesicles14. The three types of endosomes are defined mainly based on their electron microscopic morphology, the cargo transported within the lumen, and surface markers15. Surface markers of EEs are EE antigen 1 (EEA1) and Rab5. EEs can endo-emerge to form intraluminal vesicles and gradually transform into multivesicular bodies (i.e., LEs)16. Maturation of EEs is demonstrated by increased binding of Rab5 on the endosome membrane17,18. LEs are mainly responsible for degradation, and their markers are a cluster of differentiation 63 (CD63) and Rab7. Similarly, LE maturation is demonstrated by increased binding of Rab7 on the endosome membrane13.
In this study, we investigated the effects of ketamine on delirium-like behavior and changes in tau amounts in mouse serum. Mechanistically, we assessed the effects of ketamine on the number and maturation of EEs and LEs, intracellular transport, and extracellular release of tau in the human neuroblastoma cell line (known as SH-SY5Y). The hypothesis of the present study was that ketamine causes delirium-like behavior and increases endosomal uptake and extracellular release of tau.
Methods
Ethical Approval and Consent to participate
All experimental procedures involving mice were approved by the Standing Committee on Animals at Massachusetts General Hospital, Boston, MA (protocol number: 2006N000219) and conformed to National Institutes of Health (Bethesda, MD) guidelines. This article was written according to applicable ARRIVE (Animal Research: Reporting In Vivo Experiments) guidelines. Efforts were made to minimize the number of mice used in the studies.
Antibodies and reagents
Details of the antibodies used are listed in Table 1.
Table 1.
Antibody information
| Antigen | Host species | Dilution | Manufacturer | Catalog No. |
|---|---|---|---|---|
| CD63 | Rabbit | 1:1000 | Abcam | ab216230 |
| EEA1 | Mouse | 1:1000 | Cell Signaling Technology | 48453S |
| GAPDH | Rabbit | 1:2000 | Cell Signaling Technology | 5174S |
| LAMP2 | Rabbit | 1:1000 | Abcam | ab199947 |
| Na-K ATPase | Rabbit | 1:20000 | Abcam | ab76020 |
| Rab5 | Mouse | 1:1000 | Cell Signaling Technology | 46449S |
| Rab7 | Rabbit | 1:1000 | Abcam | ab126712 |
| Total tau | Mouse | 1:1000 | Abcam | ab80579 |
| Mouse (secondary antibody) | Goat | 1:2500 | Sigma-Aldrich | SAB3701095 |
| Rabbit (secondary antibody) | Goat | 1:10000 | Invitrogen | G-21234 |
Primary antibodies were diluted with western blot blocking buffer (see western blot subsection for details), and secondary antibodies were diluted with 1×TBST.
Ketamine injection (Ketalar) was purchased from Hikma Pharmaceuticals PLC (London, United Kingdom).
Animals
A total of 48 8-week-old wild-type adult male C57BL/6J mice were purchased from Jackson Laboratory (Bar Harbor, ME) with an average weight of 23 g. No significant difference in mouse weight was found between groups at the beginning of the study (Supplemental Figure S1 A1, A2). Mice were housed with four mice per cage and maintained on a 12-hour light/dark cycle (lights out at 18:00). Mice had unlimited access to water and food in their home cages. Sample size was decided by a previous pilot experiment and power analysis (α = 0.05, β = 0.1, δ(p1-p2) = 0.51, pilot experiment p1 = 0.97). Mice were divided into 3 post-injection duration groups, 1 h group (24 mice), 2 h group (12 mice), and 6 h group (12 mice) (Fig. 1). Each post-injection duration group was divided equally into 2 treatment subgroups, a saline control group (CON) and a ketamine group (KET). Each grouping was done using the complete randomization method. Each mouse was injected with 2 μl/g via intraperitoneal injection. The dosage of the KET group was 40 mg/kg according to the previous studies19,20.
Figure 1. The diagram of experimental design.
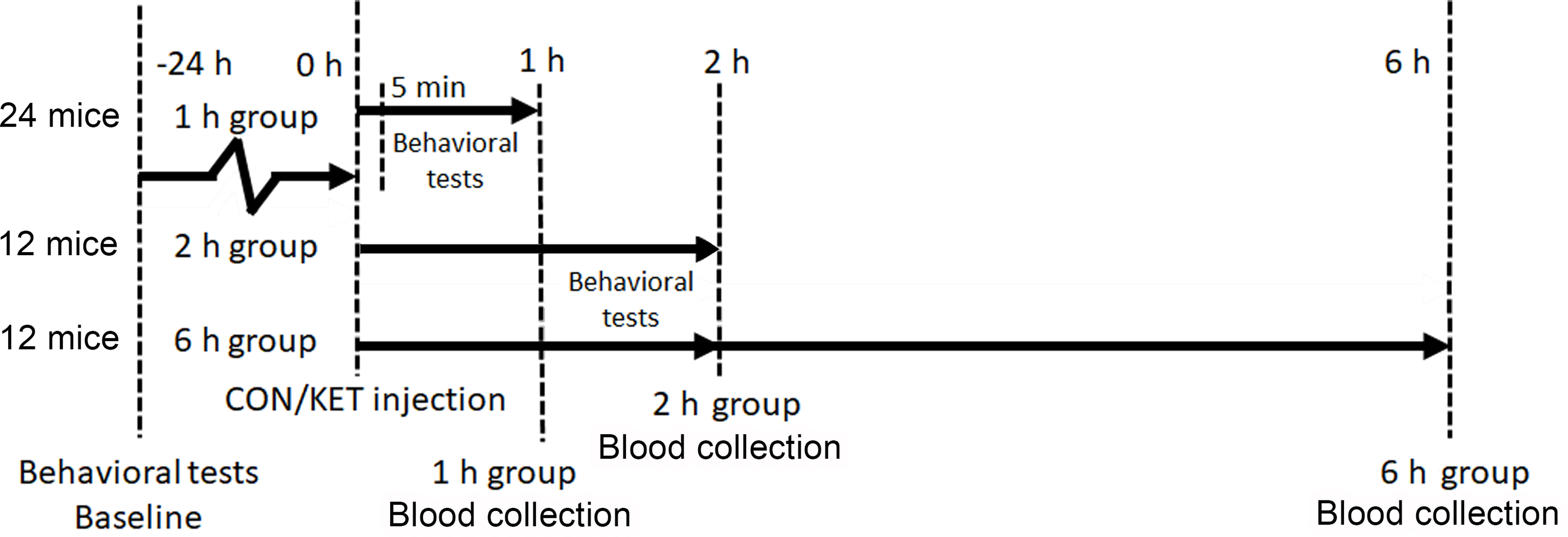
Mice had behavioral tests 24 hours before and then 1 and 2 hours after the ketamine treatment or control condition. We collected the blood of mice at 1, 2, and 6 hours after the ketamine treatment or control condition in the mice.
Behavioral tests
The animal behavioral tests battery in the present study assessed the natural behaviors with the buried food test (BFT) and open field test (OFT) to probe for attention and awareness, and the learned behaviors with the Y-maze test (YMT) to probe for cognition, which could determine the delirium-like behaviors in mice as performed in previous studies21–23. These tests focused on assessing delirium-like behaviors rather than only cognitive function in mice. All mice had multiple behavioral tests starting with the YMT training trail, followed by OFT, BFT, and finally the YMT retention trail at 24 h before (baseline) injection and 1 h or 2 h after injection (Fig. 1). Mice in the 6 h group only had the behavioral tests once at 2 h after the injection of ketamine. The equipment for behavioral tests was cleaned with 70% ethanol solution between trials. A video camera linked to AnyMaze (Stoelting Co., Wood Dale, IL) animal tracking system software was applied to monitor and analyze the activity of mice in YMT and OFT.
Y maze test.
The YMT was performed as described previously20,24,25. Precisely, the maze consists of 3 arms, including the start arm, in which the mouse starts to explore (always open); the novel arm, which is blocked at the first trial but opened at the second trial; and another familiar arm (always open). The YMT consisted of two trials separated by an inter-trial interval. The first trial (training) was 10 min in duration, which allowed the mouse to explore start arm and familiar arm of the maze, with the novel arm blocked. After a 1 h inter-trial interval, the second trial (retention) was conducted. The mouse was placed back in the maze in the same start arm with free access to all three arms for 5 min. The time spent in and entries into the novel arms were recorded and analyzed.
Open field test.
The OFT was performed as described previously20,26. Specifically, a mouse was gently placed in the center of an open field chamber under dim light. The total distance moved (meters), time (seconds) spent in the center of the open field, freezing time (seconds), and latency (time in seconds for the mouse to reach the location of the first attempt) to the center of the open field were recorded and analyzed.
Buried food test.
The BFT was performed as described in previous studies27,28. Specifically, two days before the test, we gave each mouse two pieces of sunflower seed. The test cage was prepared with clean bedding (3 cm high). We buried one sunflower seed 0.5 cm below the surface of the bedding. The location of the food pellet was changed every time randomly. We placed the mouse in the test cage and measured the latency of the mouse to eat the food. Latency was defined as the time from when the mouse was placed in the test cage until the mouse uncovered the food pellet and grasped it in its forepaws and/or teeth. Mice were allowed to consume the pellet they found and returned to their home cage. The observation time was 5 minutes. If the mouse could not find the pellet within 5 minutes, the testing session ended, and the latency was defined as 300 seconds for that mouse.
Blood collection in mice
Mice were anesthetized with 3% isoflurane for 2 minutes when retro-orbital sinus blood sampling was performed, and then mice were euthanized by cutting the head. Blood was transferred to a 4℃ refrigerator for 15 min and centrifuged at 4℃ for 15 min at 1,200 × g. We then extracted the supernatant as serum.
Cell culture
SH-SY5Y cells were cultured at 37°C with 5% CO2 in DMEM/F12 1:1 (Gibco) supplemented with 10% fetal bovine serum (Gibco), 1% L-glutamine-penicillin-streptomycin solution (Sigma-Aldrich), 1% MEM non-essential amino acids solution (100X) (Gibco), and 1% sodium pyruvate (100 mM) (Gibco). When incubating with drugs, we replaced DMEM/F12 1:1 with DMEM/F12 1:1 with HEPES without phenol red and added 10% ketamine solution or saline and 0.1% DMSO.
Cell viability assays
MTT was purchased from Invitrogen (Waltham, MA), and all MTT assays were performed following the instructions provided with the kit to obtain cell viability data. LDH assay kit (Abcam, Cambridge, MA) was used to quantitatively analyze LDH concentration in the cell culture medium. Standard curves were plotted for each assay using positive control LDH provided in the kit with gradient concentration. All operations were performed according to the kit instructions.
Whole-cell and cell fraction protein extraction
All the buffers and lysates were pre-dissolved Pierce protease and phosphatase inhibitor mini-tablets (Thermo Scientific).
Whole-cell protein extraction.
We discarded cell medium, rinsed with DPBS (Lonza), discarded as much residue as possible to obtain treated cells. We added an appropriate amount of RIPA (Thermo Scientific) lysate. After incubation, we scraped off all cells and lysate and transferred them to a clean tube. The lysate was homogenized, and the tubes were shaken with a sonic dismembrator three times at 50% amplitude for 3 seconds with an interval of 3 seconds.
Cytoplasmic protein and membrane protein extraction.
We discarded cell medium, rinsed with DPBS, discarded as much residue as possible to obtain treated cells. We added an appropriate amount of M-PER (Thermo Scientific) lysate. We sealed the culture dish with plastic wrap and transferred it to a 4℃ refrigerated room for 30 min incubation on a shaker. After incubation, we scraped off all hanging cell debris and lysate and transferred them to a clean tube. We centrifuged tubes at 16,000 × g for 30 min at 4℃ and carefully extracted the upper clarified solution into a clean tube. This solution contained cytoplasmic and nuclear proteins, and the pellet contained membrane protein. The membrane protein pellet was dissolved into a solution using RIPA.
Endosome isolation.
Minute endosome isolation and cell fractionation kit (Invent Biotechnologies, Plymouth, MN) was used to isolate endosomes as described previously29,30. Briefly, 4 × 107 cells were collected, and 1 ml buffer A was added. The cell suspension was incubated on ice for 15 minutes, loaded into a filter cartridge, and centrifuged with 16,000 × g for 30 seconds. A filter cartridge re-pass-through was performed to enrich the field. The supernatant was transferred to a fresh tube, and 500 µl buffer B was added to the tube and incubated at 4°C overnight. After incubation, vortex briefly and centrifuge with 10,000 × g for 30 minutes. We removed the supernatant and washed the pellet with a 750 µl 2:1 mixture of buffer A/buffer B. After centrifugation with 10,000 × g for 30 minutes, the pellet contains endosomes. We did not weigh the amounts of endosome pellets directly because the amounts were too small to be weighed out. According to the protocol document, the yield is typically 20 ~ 100 µg/sample. All the centrifugations were performed at 4°C. Endosome lumen proteins and endosome membrane proteins were extracted by a similar method as that used for cytoplasmic protein and membrane protein extraction.
ELISA
Concentrations of human total tau in the cell culture medium or protein lysate and mouse total tau in serum were measured by ELISA kits (Invitrogen). Experiments were performed according to the manufacturer’s instructions.
Protein concentration
Protein concentration of lysates was obtained using the Pierce BCA protein assay kit (Thermo Scientific). Standard curves were plotted for each assay using Pierce bovine serum albumin standard (Thermo Scientific) with gradient concentration.
Western blot
Proteins were separated by NuPAGE 4–12% Bis-Tris protein gel (Invitrogen) and transferred onto PVDF membranes (Bio-Rad). Membranes were blocked in 50% SuperBlock (TBS) blocking buffer (Invitrogen), 5% bovine serum albumin (Sigma-Aldrich) in 1× TBST (8 g/L NaCl, 2.42 g/L Tris-base, 1 mL/L Tween 20, adjusted pH to 7.6 with HCl). Immunoreactive bands were detected using a SuperSignal West Pico PLUS chemiluminescent substrate (Thermo Scientific). The signal intensity was analyzed using ChemiDoc XRS + with Image Lab 5.0 software (Bio-Rad, Hercules, CA).
Composite Z-score
When pooling the battery of behavioral test data, we transformed outcome data according to the composite Z-score introduced in previous studies21,22, with some modifications. Specifically, we used absolute values of Z-scores in our calculations to avoid researcher bias in data transformation. We first calculated the difference between the measured value after injection and the baseline value for each mouse. The from the CON group was then used to calculate the mean and SD . The composite Z-score of each mouse was obtained by the formula . Finally, was included in the statistical analysis.
Statistical analysis
Statistical analyses were performed using Prism 8 (GraphPad Software) to generate curves or bar graphs. All error bars represent standard deviation (SD). Data were first tested for conformity to a normal distribution using the Shapiro-Wilk test and then tested for equality of variances using the F test. Two-tailed unpaired Student’s t-test was used for statistical analysis of two groups of samples. One-way ANOVA with Turkey’s honestly significant difference test was used to evaluate the statistical significance of multiple groups of samples. Normally distributed data were described as mean ± SD. Data that did not conform to the normal distribution were combined with the experimental records to exclude outliers using the Grubbs’ test and then re-examined for normality and F tests. Data that did not conform to the normal distribution were tested using the non-parametric independent samples Mann-Whitney exact probability test. Data were then described using median (interquartile spacing). All tests were set at α = 0.05 and β = 0.1.
Results
Ketamine induces delirium-like behavior and increases serum tau amount in mice.
We used our established animal model, consisting of the YMT, BFT, and OFT 21,22, to study the effects of ketamine on delirium-like behavior in mice (Supplemental Figure S1). A single injection of ketamine in mice significantly increased the latency to eat food in the BFT and freezing time in the OFT compared to control mice at 1, but not 2, h after ketamine administration (Supplemental Figure S2). Composite Z-scores were obtained by pooling data from six measurements. The composite Z-score increased in the ketamine-treated mice at 1, but not 2, h after the ketamine treatment compared to control mice [KET: 7.899 (10.03–6.927) versus CON: 4.657 (6.371–3.365), P = 0.0005, Mann-Whitney test; Fig. 2A, B]. These data suggest that ketamine may induce delirium-like behaviors in mice. Blood was collected from the mice 1, 2, and 6 h after administration of a single injection of ketamine, and serum was extracted. We found increased amounts of tau in the serum of mice at 1, but not 2 nor 6, h after ketamine administration compared to control mice (KET: 90.36 pg/mL ± 47.41 pg/mL versus CON: 53.81 pg/mL ± 11.64 pg/mL, P = 0.0232, Student’s t-test; Fig. 2C–E).
Figure 2. Ketamine induces delirium-like behavior and increases serum total tau amounts in mice.
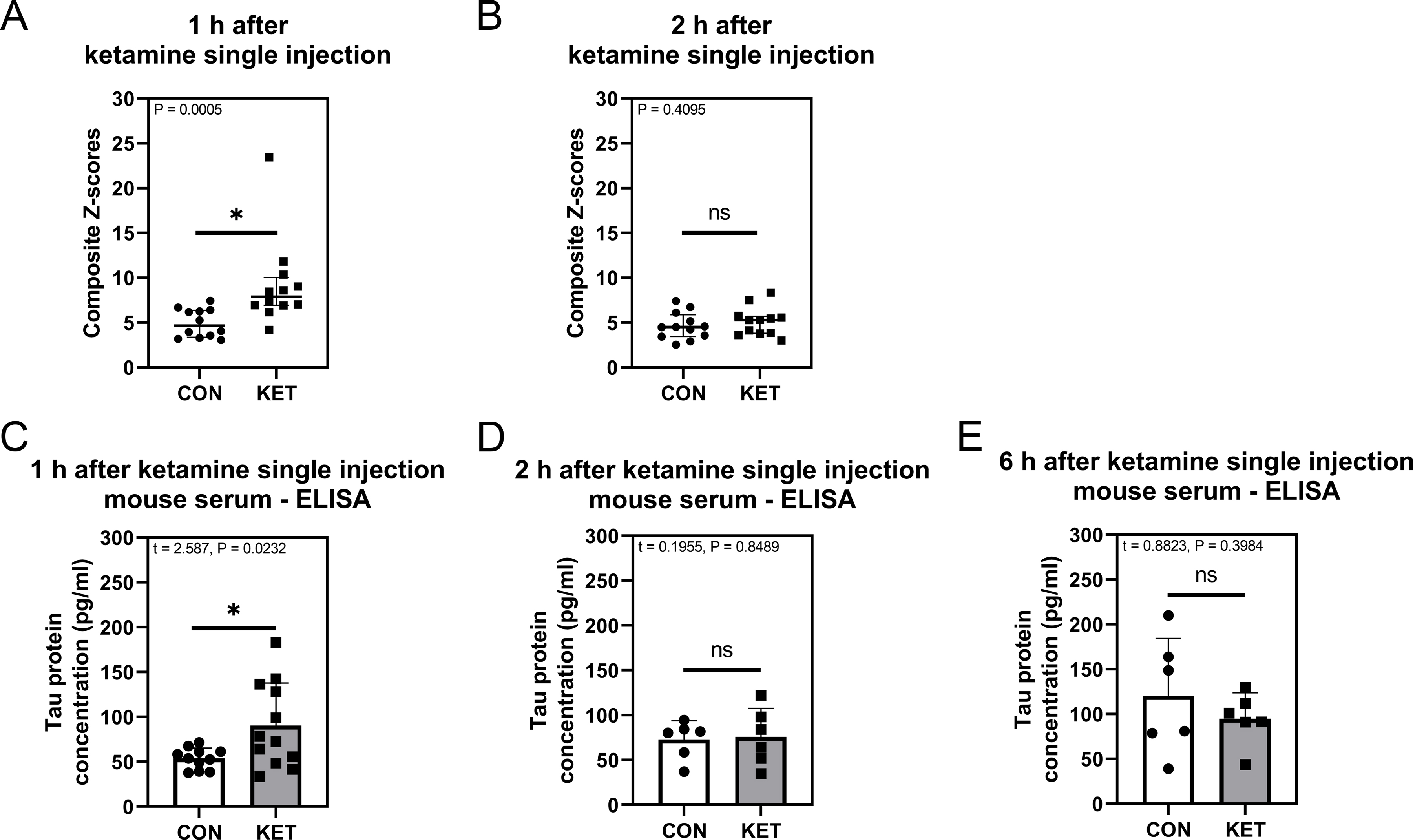
A: Composite Z-scores 1 h after ketamine administration, n = 12 in each group. B: Composite Z-scores 2 h after ketamine administration, n = 12 in each group. C: Serum total tau protein concentration 1 h after ketamine administration, n = 11 in CON group, n = 12 in KET group. D: Serum total tau protein concentration 2 h after ketamine administration, n = 6 in each group. E: Serum total tau protein concentration 6 h after ketamine administration, n = 6 in each group. Mann-Whitney test was applied to analyze data in panels A and B. Grubbs’ test was applied to identify an outlier owing to hemolysis in data in panel C. Student’s t-test was applied to analyze data in panels C, D, and E. Error bars represent 25%–75% interquartile range in panels A and B, and SD in panels C, D, and E; * indicates P < 0.05 by statistical analysis; ns indicates no significant difference between groups (P > 0.05) by statistical analysis.
Ketamine increases tau amounts in the endosome lumen and culture medium of SH-SY5Y cells.
Ketamine decreased viability of cultured SH-SY5Y cells in a dose-dependent manner (F = 155.7, P < 0.0001, one-way ANOVA; Fig 3A). Hence, we chose 156.3 μg/mL ketamine (KET1), 312.5 μg/mL ketamine (KET2), 625 μg/mL ketamine (KET3), and a control condition (CON) to treat SH-SY5Y cells. We specifically assessed the effects of ketamine on the amount of tau inside the endosome lumen and outside of the SH-SY5Y cells. Ketamine increased the amounts of tau in whole-cells (Fig. 3B, C). ELISA showed that ketamine also increased the amounts of tau in the endosomal lumen (Fig. 3D) and the cell culture medium (Fig. 3E). However, a lactate dehydrogenase (LDH) assay showed that treatment with ketamine did not increase LDH amounts in the cell culture medium (F = 1.015, P = 0.4068, one-way ANOVA; Fig. 3F), indicating that the ketamine-induced increase in tau in the cell culture medium was not due to rupture of cell membranes. These data indicate that ketamine increases endosomal uptake and extracellular release of tau.
Figure 3. Ketamine induces changes in total tau amounts in endosomes and the medium.
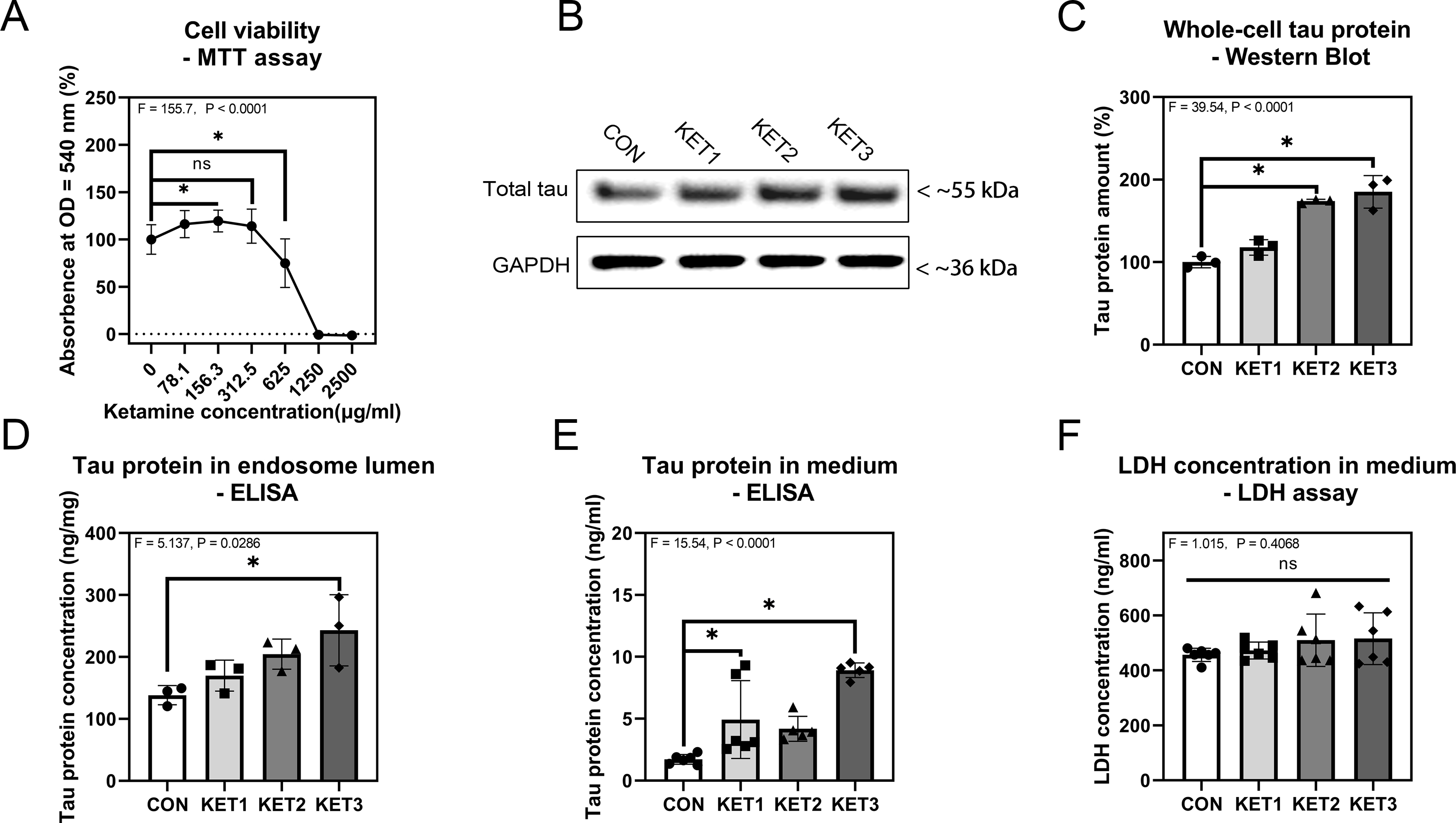
A: Cell viability via MTT assay, n = 12 biological variables in each group. B: Western blot analysis of tau in whole-cells and the internal loading control glyceraldehyde-3-phosphate dehydrogenase (GAPDH). One band is shown for each experimental condition. C: Quantitation of the western blot analysis of tau in whole-cells, n = 3 biological variables in each experimental group. D: ELISA of tau concentrations in the endosomal lumen, n = 3 biological variables in each group. E: ELISA of total tau concentrations in the cell culture medium, n = 6 biological variables in the CON and KET1 groups, n = 5 biological variables in KET2 and KET3 groups. F: LDH concentration in the cell culture medium, n = 6 biological variables in each group. One-way ANOVA was applied to analyze data in panels A and C–F. Dunnett’s test was applied for ANOVA post-hoc. SH-SY5Y cells were treated with gradient concentrations of ketamine medium. Grubbs’ test was applied to identify outliers in data in KET2 and KET3 groups in panel D. Error bars represent SD; * indicates P < 0.05 by statistical analysis.
Ketamine increases early and late endosome accumulation in SH-SY5Y cells.
Given that ketamine increased tau concentration in both the endosome and extracellular space, next, we assessed the effects of ketamine on endosome amounts and activation in SH-SY5Y cells. Quantitative western blot analysis demonstrated that after 24 h of incubation, whole-cell protein amounts of EE markers EEA1 (F = 36.48, P < 0.0001, one-way ANOVA; Fig. 4A, B) and Rab5 (F = 31.23, P < 0.0001, one-way ANOVA; Fig. 4A, C) were significantly increased, suggesting that ketamine increased amounts of EEs in the cells. Moreover, ketamine increased whole-cell protein amounts of LE marker Rab7 (F = 32.05, P < 0.0001, one-way ANOVA; Fig. 4A, E) but not CD63 (F = 2.358, P = 0.1102, one-way ANOVA; Fig. 4A, D). In addition, ketamine decreased whole-cell protein amounts of lysosome marker lysosome-associated membrane protein 2 (LAMP2) (F = 4.892, P = 0.0323, one-way ANOVA; Fig. 4A, F). These data demonstrated that ketamine increased the accumulation of EEs and LEs and decreased lysosome amounts in SH-SY5Y cells.
Figure 4. Ketamine increases endosome accumulation in SH-SY5Y cells.
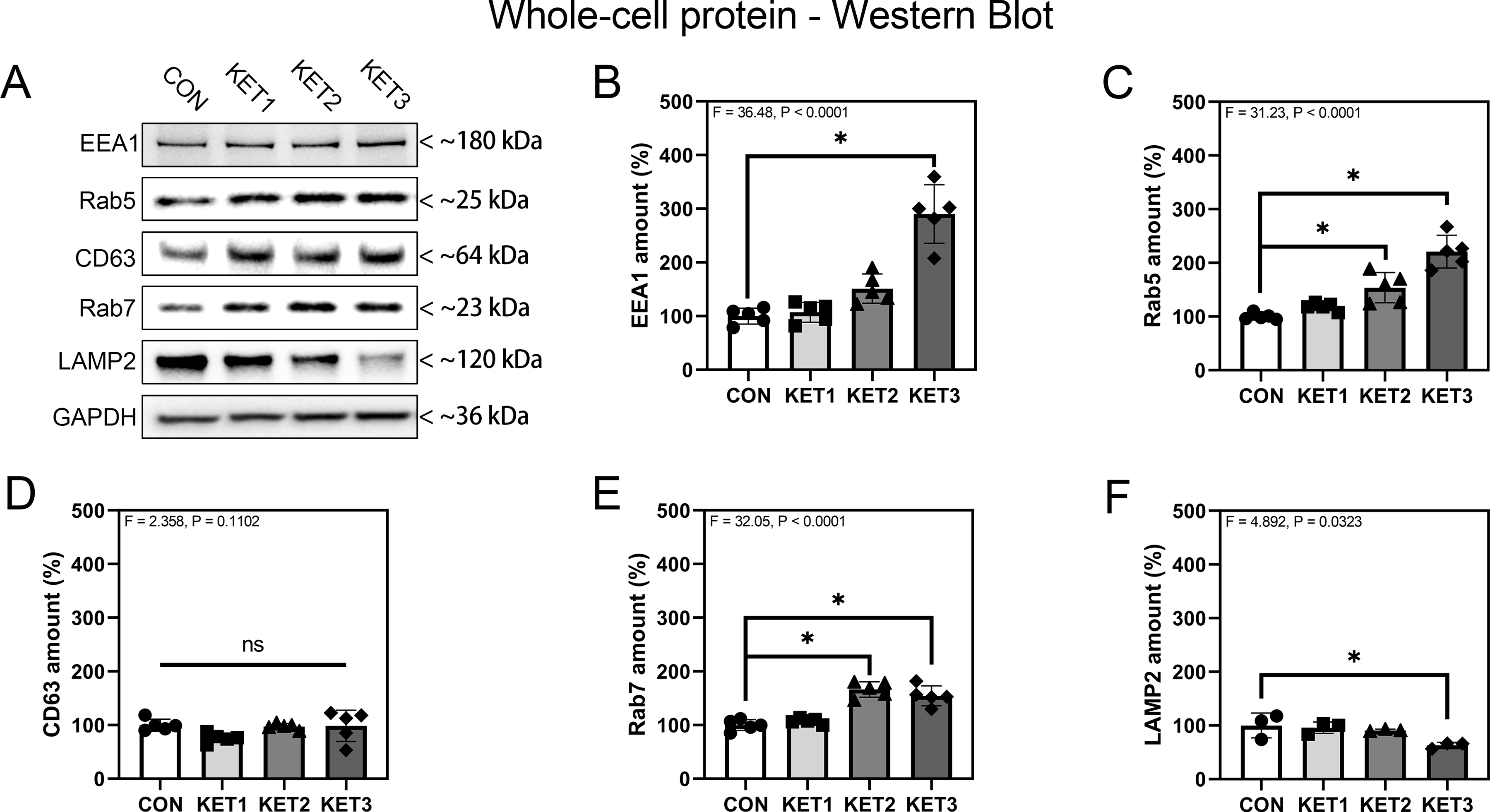
A: Effects of ketamine on whole-cell proteins (EEA1, Rab5, CD63, Rab7, LAMP2) and the internal loading control GAPDH. One band is shown for each condition. B–F: Quantitation of western blot analysis of whole-cell EEA1 (B), Rab5 (C), CD63 (D), Rab7 (E), and LAMP2 (F) proteins. n = 5 biological variables for panels B – E, n = 3 biological variables for panel F in each group. One-way ANOVA and post-hoc analysis with Dunnett’s test were applied for statistical analysis. Error bars represent SD; * indicates P < 0.05 by statistical analysis; ns indicates no significant difference between groups (P > 0.05) by statistical analysis.
Ketamine decreases endosome membrane-bound Rab5 and Rab7 but increases Rab5 and Rab7 in the cytoplasm of SH-SY5Y cells.
Given that ketamine increased the amounts of proteins associated with EEs in whole-cells, we further assessed the effects of ketamine on the amounts of these proteins bound with the endosome membrane or in the cytoplasm. First, we demonstrated that ketamine did not significantly change the amounts of sodium-potassium ATPase (Na-K ATPase) (F = 1.483, P = 0.2569, one-way ANOVA; Fig. 5A, B), indicating that Na-K ATPase could be used as an internal loading control for the amounts of the protein associated with endosome membranes. Quantitative western blot analysis demonstrated that ketamine significantly increased the amounts of cytoplasmic Rab5 (F = 11.15, P = 0.0003, KET3: 237.2% ± 84.99% versus CON: 100% ± 16.32%, one-way ANOVA; Fig. 5C, D) and Rab7 (F = 9.200, P = 0.0009, KET3: 277.6% ± 113.5% versus CON: 100% ± 26.04%, one-way ANOVA; Fig. 5C, E) 24 h after ketamine administration. Conversely, ketamine treatment significantly decreased the amounts of membrane-bound Rab5 (F = 7.410, P = 0.0025, KET1: 69.00% ± 26.86% and KET3: 55.86% ± 6.633% versus CON: 100% ± 7.792%, one-way ANOVA; Fig. 5C, F) and Rab7 (F = 8.174, P = 0.0016, KET2: 55.23% ± 16.50% and KET3: 28.56% ± 11.05% versus CON: 100% ± 41.50%, one-way ANOVA; Fig. 5C, G). These data demonstrated that ketamine could decrease endosome membrane-bound Rab5 and Rab7 but increased cytoplasmic amounts of Rab5 and Rab7, suggesting that ketamine may impair EE and LE maturation.
Figure 5. Ketamine decreases endosome membrane-bound Rab5 and Rab7 but increases cytoplasmic amounts of Rab5 and Rab7.
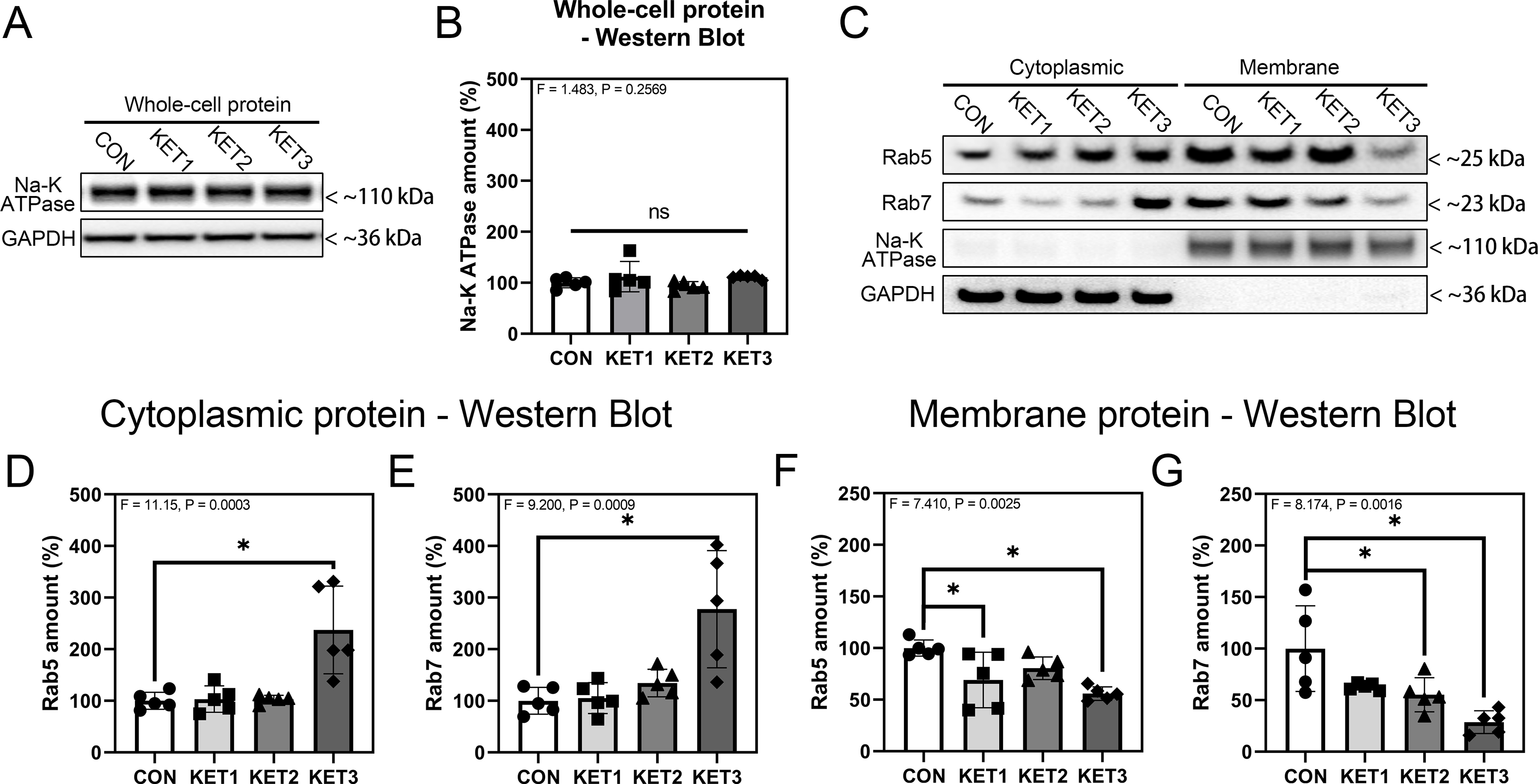
A: Effects of ketamine on whole-cell Na-K ATPase and the internal loading control GAPDH. One band is shown for each experimental condition. B: Quantitation of western blot analysis of whole-cell Na-K ATPase, n = 5 biological variables. C: Western blot analysis of cytoplasmic and membrane proteins Rab5 and Rab7 and internal loading control proteins Na-K ATPase and GAPDH. One band is shown for each experimental condition. D–G: Quantitation of western blot analysis of cytoplasmic Rab5 (D), cytoplasmic Rab7 (E), membrane Rab5 (F), and membrane Rab7 (G), n = 5 biological variables in each group. One-way ANOVA and post-hoc analysis with Dunnett’s test were applied to analyze data in panels D–G. Error bars represent SD; * indicates P < 0.05 by statistical analysis; ns indicates no significant difference among groups (P > 0.05) by statistical analysis.
Discussion
Our results show that ketamine induced delirium-like behavior and increased serum tau amounts in mice. In the mechanistic studies, ketamine impaired intracellular tau trafficking, evidenced by increasing amounts of tau inside the endosome lumen and extracellular space of SH-SY5Y cells. Moreover, ketamine might impair intracellular tau trafficking by increasing the number of endosomes but inhibiting endosome maturation. These studies, using ketamine as a clinically relevant tool, demonstrate that tau trafficking could contribute to the pathogenesis of delirium, promoting future research to study delirium pathogenesis. These data show that anesthetic ketamine could induce tauopathy and delirium-like behavior, pending confirmative studies.
We firstly found time-dependent effects of ketamine in inducing delirium-like behavior, evidenced by the findings that ketamine increased composite Z-score compared to controls. Consistently, previous studies show anesthesia/surgery induces time-dependent21 and age-dependent22 delirium-like behavior in mice. However, previous studies did not assess anesthetic effects without surgery on the delirium-like behavior in mice.
The present study showed that anesthetic ketamine without surgery could still induce delirium-like behavior in mice. Ketamine induced delirium-like behavior and increased serum tau amount 1 h after administration, suggesting an association between delirium-like behavior and increased serum tau. Consistently, a clinical observational study in 2021 concluded that postoperative plasma tau amounts could serve as a biomarker for postoperative delirium in patients7.
Endosomes are responsible for the intracellular transport of cargo, including proteins, lipids, and nucleic acids, essential for cell function31. Cell function is severely impaired when endosomes accumulate13. We found that ketamine increased endosome accumulation, which could explain the findings that ketamine increased endosomal tau amounts because more endosomes could uptake more tau. However, ketamine may also enhance the ability of single endosomes to uptake tau. We will test this hypothesis in future studies.
Previous studies showed that increased Rab5 or Rab7 in the cytoplasm and decreased amounts of membrane-bound Rab5, or Rab7 indicate inhibition of endosome maturation17,18. Endosomes become exosomes when they bind to the cell membrane32. Moreover, it has been suggested that inhibition of the endosome maturation process will increase protein release via exosomes33. In the present study, ketamine inhibited endosome maturation, evidenced by increased cytoplasm amounts of Rab5 and Rab7 and decreased membrane-bound Rab5 and Rab7. Meanwhile, ketamine also increased amounts of tau in the extracellular space. These findings indicate that ketamine inhibits endosome maturation while increasing tau uptake by endosomes, causing migration of endosomes to the cell membrane to form exosomes and releasing more tau into the extracellular space.
The mechanism of tau release into the extracellular space is unclear. However, increasing evidence shows that tau relies mainly on the non-classical protein secretion pathway and extracellular vesicles for extracellular transport34,35. Our previous study also demonstrated that the trafficking of tau to the extracellular space depended on tau phosphorylation and the generation of extracellular vesicles11. In the present study, we illustrated that ketamine increased endosome accumulation but inhibited endosome maturation, leading to more tau release into the extracellular space. Moreover, we postulate that the effect of ketamine on EEs includes blocking the conversion of EEs to LEs but does not affect the conversion of other organelles, such as TGN, to EEs.
In LEs, we found that ketamine did not significantly change CD63 protein amounts but increased Rab7 amounts. Further, ketamine decreased membrane Rab7 yet increased cytoplasmic amounts of Rab7. These data suggest that ketamine inhibits LE maturation, as demonstrated in previous studies36,37.
As an NMDA receptor antagonist, ketamine affects intracellular calcium homeostasis1. Calcium homeostasis is associated with lysosome function38, and the impaired lysosome function and calcium dysregulations contribute to the pathogenesis of Alzheimer’s disease and cognitive dysfunction39,40. Therefore, it is conceivable that ketamine may regulate endosome trafficking via impairing calcium homeostasis and lysosome function, leading to delirium-like behavior. Future studies will test this hypothesis.
There are several limitations of this study. First, we did not study whether inhibition of the ketamine-induced increase in serum tau could mitigate ketamine-induced delirium-like behavior. We could not find a specific inhibitor of tau trafficking from the intracellular to extracellular space. We will use tau knockout mice to determine the role of tau in delirium-like behavior using the established system in future studies. Second, we did not determine the effects of ketamine on the intracellular trafficking of phosphorylated tau in the present study because we wanted to focus on tau. We will use the established system to study the intracellular trafficking of both tau and phosphorylated tau in future studies. Finally, we only assessed the effects of ketamine on membrane Rab5 and Rab7 but not the specific endosome membrane Rab5 and Rab7. However, Rab5 and Rab7 are only known to bind to the endosome membrane.
In conclusion, the present study showed that ketamine induced delirium-like behavior and increased serum tau amounts in mice. In the in vitro studies, we found ketamine increased tau uptake in endosomes by increasing endosome accumulation. Ketamine then increased the release of tau to the extracellular space by inhibiting endosomal maturation. These findings will promote future studies of tau trafficking and delirium and anesthetic effects on tauopathy and delirium-like behavior.
Supplementary Material
Key Points Summary.
Question:
What are the effects and mechanism of ketamine on delirium-like behavior in mice and on tau trafficking in cultured cells?
Finding:
Ketamine induced delirium-like behavior, increased tau concentration in mouse serum, and induced accumulation of endosomes as evidenced by increased amounts of cytoplasmic Rab5 and Rab7.
Meaning:
Ketamine may induce delirium-like behavior by interfering with intracellular tau trafficking. These findings will promote future research regarding the neurotoxicity of anesthetics.
Funding:
This study was supported by a grant from the National Institutes of Health (grant No. R01AG062509 and RG041274 to Zhongcong Xie).
Glossary of Terms
- BFT
buried food test
- CD63
cluster of differentiation 63
- CON
control group
- EE
early endosome
- EEA1
early endosome antigen 1
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- KET
ketamine group
- LAMP2
lysosome-associated membrane protein 2
- LDH
lactate dehydrogenase
- LE
late endosome
- Na-K ATPase
sodium potassium ATPase
- NMDA
N-methyl-D-aspartic acid
- OFT
open field test
- TGN
trans-Golgi network
- Tau
microtubule-associated protein τ
- YMT
Y-maze test
Footnotes
Conflicts of interests
Dr. Zhongcong Xie has provided consulting services to Baxter, Shanghai 9th and 10th hospitals in the last 36 months.
References
- 1.Sinner B, Graf BM. Ketamine. Handb Exp Pharmacol 2008; (182):313–33. doi: 10.1007/978-3-540-74806-9_15 [DOI] [PubMed] [Google Scholar]
- 2.Sassano-Higgins S, Baron D, Juarez G, Esmaili N, Gold M. A Review of Ketamine Abuse and Diversion. Depress Anxiety Aug 2016;33(8):718–27. doi: 10.1002/da.22536 [DOI] [PubMed] [Google Scholar]
- 3.Jansen KL. A review of the nonmedical use of ketamine: use, users and consequences. Journal Article; Review. J Psychoactive Drugs 2000-October-01 2000;32(4):419–33. doi: 10.1080/02791072.2000.10400244 [DOI] [PubMed] [Google Scholar]
- 4.Zhang C, Xu Y, Zhang B, Hao W, Tang WK. Cognitive impairment in chronic ketamine abusers. Psychiatry Res Sep 2020;291:113206. doi: 10.1016/j.psychres.2020.113206 [DOI] [PubMed] [Google Scholar]
- 5.Setters B, Solberg LM. Delirium. Primary Care: Clinics in Office Practice Sep 2017;44(3):541–559. doi: 10.1016/j.pop.2017.04.010 [DOI] [PubMed] [Google Scholar]
- 6.Xie Z, Swain CA, Ward SA, et al. Preoperative cerebrospinal fluid beta-Amyloid/Tau ratio and postoperative delirium. Ann Clin Transl Neurol May 1 2014;1(5):319–328. doi: 10.1002/acn3.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ballweg T, White M, Parker M, et al. Association between plasma tau and postoperative delirium incidence and severity: a prospective observational study. Br J Anaesth Feb 2021;126(2):458–466. doi: 10.1016/j.bja.2020.08.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKay TB, Qu J, Liang F, et al. Tau as a serum biomarker of delirium after major cardiac surgery: a single centre case-control study. Br J Anaesth Apr 21 2022;doi: 10.1016/j.bja.2022.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin L, Latypova X, Wilson CM, et al. Tau protein kinases: involvement in Alzheimer’s disease. Ageing Res Rev Jan 2013;12(1):289–309. doi: 10.1016/j.arr.2012.06.003 [DOI] [PubMed] [Google Scholar]
- 10.Querfurth HW, LaFerla FM. Alzheimer’s disease. Journal Article; Review. N Engl J Med 2010-January-28 2010;362(4):329–44. doi: 10.1056/NEJMra0909142 [DOI] [PubMed] [Google Scholar]
- 11.Dong Y, Liang F, Huang L, et al. The anesthetic sevoflurane induces tau trafficking from neurons to microglia. Commun Biol May 12 2021;4(1):560. doi: 10.1038/s42003-021-02047-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stoorvogel W, Strous GJ, Geuze HJ, Oorschot V, Schwartz AL. Late endosomes derive from early endosomes by maturation. Cell May 3 1991;65(3):417–27. doi: 10.1016/0092-8674(91)90459-c [DOI] [PubMed] [Google Scholar]
- 13.Langemeyer L, Fröhlich F, Ungermann C. Rab GTPase Function in Endosome and Lysosome Biogenesis. Trends in cell biology Nov 2018;28(11):957–970. doi: 10.1016/j.tcb.2018.06.007 [DOI] [PubMed] [Google Scholar]
- 14.Stenmark H Rab GTPases as coordinators of vesicle traffic. Nature reviews Molecular cell biology Aug 2009;10(8):513–25. doi: 10.1038/nrm2728 [DOI] [PubMed] [Google Scholar]
- 15.Thery C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 2018;7(1):1535750. doi: 10.1080/20013078.2018.1535750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neefjes J, Jongsma MML, Berlin I. Stop or Go? Endosome Positioning in the Establishment of Compartment Architecture, Dynamics, and Function. Trends in cell biology Aug 2017;27(8):580–594. doi: 10.1016/j.tcb.2017.03.002 [DOI] [PubMed] [Google Scholar]
- 17.Delevoye C, Goud B. Rab GTPases and kinesin motors in endosomal trafficking. Methods in cell biology 2015;130:235–46. doi: 10.1016/bs.mcb.2015.05.004 [DOI] [PubMed] [Google Scholar]
- 18.Nagano M, Toshima JY, Siekhaus DE, Toshima J. Rab5-mediated endosome formation is regulated at the trans-Golgi network. Communications biology 2019;2:419. doi: 10.1038/s42003-019-0670-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Wen G, Ding R, et al. Effects of Single-Dose and Long-Term Ketamine Administration on Tau Phosphorylation-Related Enzymes GSK-3beta, CDK5, PP2A, and PP2B in the Mouse Hippocampus. J Mol Neurosci Dec 2020;70(12):2068–2076. doi: 10.1007/s12031-020-01613-9 [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Shen R, Wen G, et al. Effects of Ketamine on Levels of Inflammatory Cytokines IL-6, IL-1beta, and TNF-alpha in the Hippocampus of Mice Following Acute or Chronic Administration. Front Pharmacol 2017;8:139. doi: 10.3389/fphar.2017.00139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng M, Zhang C, Dong Y, et al. Battery of behavioral tests in mice to study postoperative delirium. Sci Rep Jul 20 2016;6:29874. doi: 10.1038/srep29874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liufu N, Liu L, Shen S, et al. Anesthesia and surgery induce age-dependent changes in behaviors and microbiota. Aging (Albany NY) Jan 24 2020;12(2):1965–1986. doi: 10.18632/aging.102736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang Z, Liang F, Zhang Y, et al. Urinary Catheterization Induces Delirium-Like Behavior Through Glucose Metabolism Impairment in Mice. Anesth Analg Apr 7 2022;doi: 10.1213/ANE.0000000000006008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rayatnia F, Javadi-Paydar M, Allami N, et al. Nitric oxide involvement in consolidation, but not retrieval phase of cognitive performance enhanced by atorvastatin in mice. Eur J Pharmacol Sep 2011;666(1–3):122–30. doi: 10.1016/j.ejphar.2011.05.017 [DOI] [PubMed] [Google Scholar]
- 25.Chen Y, Liu X, Jia X, et al. Anxiety- and depressive-like behaviors in olfactory deficient Cnga2 knockout mice. Behav Brain Res Dec 15 2014;275:219–24. doi: 10.1016/j.bbr.2014.08.042 [DOI] [PubMed] [Google Scholar]
- 26.Li XM, Su F, Ji MH, et al. Disruption of hippocampal neuregulin 1-ErbB4 signaling contributes to the hippocampus-dependent cognitive impairment induced by isoflurane in aged mice. Anesthesiology Jul 2014;121(1):79–88. doi: 10.1097/ALN.0000000000000191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nathan BP, Yost J, Litherland MT, Struble RG, Switzer PV. Olfactory function in apoE knockout mice. Behav Brain Res Apr 2 2004;150(1–2):1–7. doi: 10.1016/S0166-4328(03)00219-5 [DOI] [PubMed] [Google Scholar]
- 28.Lehmkuhl AM, Dirr ER, Fleming SM. Olfactory assays for mouse models of neurodegenerative disease. J Vis Exp Aug 25 2014;(90):e51804. doi: 10.3791/51804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thapa N, Chen M, Horn HT, Choi S, Wen T, Anderson RA. Phosphatidylinositol-3-OH kinase signalling is spatially organized at endosomal compartments by microtubule-associated protein 4. Nat Cell Biol Nov 2020;22(11):1357–1370. doi: 10.1038/s41556-020-00596-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu X, Xu J, Wu J, et al. Phosphorylation-Mediated IFN-gammaR2 Membrane Translocation Is Required to Activate Macrophage Innate Response. Cell Nov 15 2018;175(5):1336–1351 e17. doi: 10.1016/j.cell.2018.09.011 [DOI] [PubMed] [Google Scholar]
- 31.Elkin SR, Lakoduk AM, Schmid SL. Endocytic pathways and endosomal trafficking: a primer. Wiener Medizinische Wochenschrift May 2016;166(7–8):196–204. doi: 10.1007/s10354-016-0432-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bebelman MP, Crudden C, Pegtel DM, Smit MJ. The Convergence of Extracellular Vesicle and GPCR Biology. Trends Pharmacol Sci Sep 2020;41(9):627–640. doi: 10.1016/j.tips.2020.07.001 [DOI] [PubMed] [Google Scholar]
- 33.Hessvik NP, Overbye A, Brech A, et al. PIKfyve inhibition increases exosome release and induces secretory autophagy. Cell Mol Life Sci Dec 2016;73(24):4717–4737. doi: 10.1007/s00018-016-2309-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen JJ, Nathaniel DL, Raghavan P, et al. Compromised function of the ESCRT pathway promotes endolysosomal escape of tau seeds and propagation of tau aggregation. J Biol Chem Dec 13 2019;294(50):18952–18966. doi: 10.1074/jbc.RA119.009432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang L, Dong H, Cao H, Ji X, Luan S, Liu J. Exosomes in Pathogenesis, Diagnosis, and Treatment of Alzheimer’s Disease. Med Sci Monit May 6 2019;25:3329–3335. doi: 10.12659/MSM.914027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zerial M, McBride H. Rab proteins as membrane organizers. Nature Reviews Molecular Cell Biology Feb 2001;2(2):107–117. doi: 10.1038/35052055 [DOI] [PubMed] [Google Scholar]
- 37.Takeda M, Koseki J, Takahashi H, et al. Disruption of Endolysosomal RAB5/7 Efficiently Eliminates Colorectal Cancer Stem Cells. Cancer Res Apr 1 2019;79(7):1426–1437. doi: 10.1158/0008-5472.CAN-18-2192 [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, Shi Y, Wei H. Calcium Dysregulation in Alzheimer’s Disease: A Target for New Drug Development. J Alzheimers Dis Parkinsonism Aug 2017;7(5)doi: 10.4172/2161-0460.1000374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei H, Xie Z. Anesthesia, calcium homeostasis and Alzheimer’s disease. Current Alzheimer research Feb 2009;6(1):30–5. doi: 10.2174/156720509787313934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei H New Approaches to Develop Drug Treatment for Alzheimer’s Disease: Targeting Calcium Dysregulation. Current Alzheimer research 2020;17(4):311–312. doi: 10.2174/156720501704200520094610 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


