Abstract
The volatile flavor compounds are the most important indicators of the quality of cocoa beans, among which pyrazines are considered as the main and key groups affecting the cocoa flavor. In cocoa processing, roasting is an important stage in the technical treatment of cocoa and has a significant impact on chemical properties of cocoa and its flavor. The present study aimed to assess the impact of roasting (temperature and time) on alkyl pyrazines, as key flavor compounds, via gas chromatography–mass spectrometry. Additionally, other properties, including color, polyphenols, chemical properties, and sensory attributes of cocoa powder were investigated. The results indicated that with the change in roasting time and temperature, these properties changed significantly. The cocoa powder roasted at 140 °C for 40 min had the highest browning index value (OD460/OD525), tetramethylpyrazine to trimethylpyrazine (TMP/TrMP) ratio, and sensory evaluation score and the lowest polyphenol content compared to the other samples.
Keywords: Cocoa powder, Roasting, Gas chromatography–mass spectrometry (GC–MS), Color, Pyrazines, Polyphenol
Introduction
Cocoa is a product of significant economic importance in the world and a key raw material in cocoa powder and chocolate manufacturing (Kongor et al. 2016). There are several indicators for measuring the quality of cocoa beans, but the most important ones are the amount and type of flavor compounds. To date, more than 600 flavor compounds of cocoa beans and cocoa products have been identified, including aldehydes and ketones, pyrazines, pyrroles, esters, alcohols, hydrocarbons, nitriles and sulphides, ethers, furans, thiazoles, pyrones, acids, phenols, imines, amines, and oxazoles (Ziegleder 2009).
On account of the vast variety of concentration and sensory properties of these compounds, different cocoa types may exhibit various and specific flavors (Aprotosoaie et al. 2016). Flavor compounds of cocoa beans are influenced by certain factors, such as cocoa type (genotype) and cultivation characteristics, post-harvest treatments (like pulp conditioning, fermentation, and drying), industrial processes (like browning (roasting)), storage, and transportation (Kongor et al. 2016).
Roasting is one of the pivotal steps affecting the quality characteristic of cocoa beans, which determines the chemical and physical processes that occur inside the beans and the quality of the final products (Wiesława Krysiak and Motyl-Patelska 2006). During roasting, evaporation of volatile acids from the beans causes a reduction in acidity, resulting in a reduction in the sourness and bitterness of the cocoa beans. Roasting also reduces moisture and polyphenols. Owing to their antioxidant activities and beneficial effects on human health, such as cancer treatment and prevention, cardiovascular and other diseases, polyphenols have been the subject of great interest. Cocoa beans are rich in polyphenols and roasting, as a crucial step in the technical treatment of cocoa, leads to flavanol losses and modifications, particularly the epimerization of (-)-epicatechin to (-)-catechin (Arlorio et al. 2008; Kothe et al. 2013).
During roasting, polyphenols in cocoa liquor (combination of cocoa solids and cocoa butter produced after fermentation, drying, and roasting of cocoa beans) reduce viscosity and flavor of cocoa while they increase the astringency and bitterness of the produced liquor. Moreover, polyphenols play a significant role in forming the specific brown color of cocoa. Polyphenol concentration and roasting duration affect cocoa flavor (Kothe et al. 2013).
Although certain phenols are lost during the process, cocoa powder is known to be a considerably rich source of antioxidants (Gìltekin-Özgìven et al. 2016). Roasting cocoa is an essential step for creating the usual aroma of cocoa and during the roasting process, flavor compounds in cocoa beans are formed from flavor precursors generated during the fermentation and drying process (Kongor et al. 2016; Kothe et al. 2013).
During the roasting process, flavor precursors (free amino acids, short-chain peptides, and reducing sugars) produced during the fermentation and drying, produce the desired flavor compounds under Maillard reaction and Strecker degradation. The produced carbonyl derivatives react with free amino acids during Strecker decomposition, which breaks down the amino acid into aldehydes and contributes to providing the flavor. The choice of roasting parameters determines the characteristics of the chemical and physical processes that take place inside the beans, thereby determining the quality of the product. Numerous studies have confirmed that temperature and duration of roasting have considerable effects on the physical and chemical changes in cocoa beans. The optimal time for cocoa bean roasting is 15–45 min and temperatures range from 130 to 150 °C (Kongor et al. 2016).
Among the flavor compounds formed during the Maillard reaction roasting process, pyrazines are the main class of heterocyclic volatiles and key components of cocoa flavor and aroma (Aprotosoaie et al. 2016). They are recognized as the main factors determining the quality and quantity of cocoa aroma. (Alasti et al. 2020; Aprotosoaie et al. 2016; Farah et al. 2012; Jinap et al. 1998)
About 80 pyrazine compounds contribute to the overall cocoa flavor. In addition, tetramethylpyrazine and trimethylpyrazine are the most important compounds (Aprotosoaie et al. 2016). The concentration ratio of tetramethylpyrazine (TMP)/trimethylpyrazine (TrMP) has been suggested as an indicator of roasting degree. For a normal degree of roasting, the TMP/TrMP ratio is about one and for over-roasting, the value is below one. Temperature and duration of thermal processes are factors influencing the concentration of pyrazines (Serra Bonvehí and Ventura Coll 2002; Ziegleder 2009).
One other factor which affects the final quality of cocoa powder is the color. Changes in color occur due to the presence of compounds made from epicatechin that may be combined during various processes, like fermentation, drying, and roasting. These compounds increase the intensity of molecule color and make cocoa darker. The pH, humidity, time, and temperature of roasting need to be determined accurately and carefully since different types of color are likely to be formed (Beckett 2009).
Studies indicated that there appears to be a number of problems such as reduction of aroma and generation of unpleasant odor in the process of making cocoa powder. Thus, this study was implemented to solve the problem of industrial production lines and help to improve the quality of products. The preparation steps were performed on the production line and the samples were collected from the factory. The alkalization process was carried out using industrial techniques. Then, the current study was conducted in order to investigate the effect of roasting process on the alkalized cocoa and changes in their important qualitative properties to achieve the optimal conditions.
Materials and methods
Materials
In the current study, fermented and dried Cameron cocoa beans (Forastero cultivar, Cameroon) were prepared from Shirin Asal Food Industrial Group (Salimi Ind.zone-Tabriz/Iran, Po.Box: 51335-1433). Potassium carbonate, sodium hydroxide, glacial acetic acid, gallic acid, n-hexane, methanol, the Folin–Ciocalteu reagent, solvents, and the standards for GC–MS analysis, in addition to other analytical reagents, were purchased from Sigma Chemical Co. (St. Louis, Missouri). All the chemicals and reagents used in this study were of analytical grade.
Sample preparation
In Shirin Asal Food Industrial Group (Tabriz, Iran), Cameron cocoa beans (dried and fermented in Cameroon) were cleaned, dried at 100 °C, broken into cocoa nibs, and stored. At this stage, a sufficient amount of the Cameroon cocoa beans, which had undergone the cleaning step (cleaning, drying in 100 °C for 320 s), was taken from Shirin Asal Company and the alkalization process was performed on the nibs. The samples were placed in 100 mL beaker and NaOH 1.5% + K2CO3 was poured on them. Subsequently, the samples were placed on a heater at 70 °C for one hour. In next stage, the samples were divided into 75 g batches and prepared for the roasting process.
Roasting
The nibs were divided into 75-g batches and the roasting process was performed in the oven (Funke Gerber, Germany). The roasting process was performed by adjusting the oven to the desired temperatures (120, 130, and 140 °C). 75-g nibs were spread as a single layer on aluminum foil, placed in the oven, and baked for the intended time (for 20, 30, 40 min). After roasting, the samples were cooled to the room temperature (27 °C), packed in a container, and stored in refrigerator at 4 °C until the tests were performed. Prior to testing, the samples were milled and pulverized in a laboratory mill (M 20 Universal mill; IKA, Germany) at the room temperature (27 °C) for 45 s in order to obtain homogeneous samples. The liqueurs were homogenized with a mixer (CJJ-2/2A series).
Extraction of the volatile components (alkylpyrazines compounds)
Extraction of the volatile components (alkylpyrazines compounds) was performed according to the published procedure (Schultz et al. 1977). 25 g of the powdered samples were added to distilled water (200 mL) and heated for 60 min using Likens Nickerson’s simultaneous distillation extraction (SDE) apparatus. 2 mL of normal hexane was added to the round bottom flask containing the sample solution and heated using a Clevenger extraction device according to the method applied herein (Schultz et al. 1977). The volatiles were trapped in hexane.
1 µl of volatile compounds was examined on gas chromatography–mass spectrometry (GC–MS). The standard amount of five pyrazine compounds (2-methylpyrazine, 2,3-dimethylpyrazine, 2,5-dimethylpyrazine, 2,3,5-trimethylpyrazine, and 2,3,5,6-tetramethylpyrazine) had been previously injected into the GC–MS. Finally, the identification of the alkyl pyrazines compounds was carried out by comparing their retention time to the standards.
GC–MS conditions
Herein, we used an Agilent 7890A gas chromatographs equipped with mass detectors (Model 5975C, Agilent Technologies, USA), HP Chemstation software on Windows, Split/splitless injector, and HP-5 MS capillary column (30 m length, 0.25 mm i.d., 0.25 lm film thickness (Agilent, USA)).
The initial oven temperature was maintained at 80 °C for 3 min and then raised at a rate of 4–8 °C/min to 180 °C and maintained for 3 min. Helium was applied as the carrier gas at the flow speed of 1 mL min−1.
The injector was set in a split mode (split ratio of 1:500) and mass range acquisition was from 40 to 500 m/z. The mass library (Wily 2007 and NIST 2005) in the device was utilized to identify the compounds. The injection valve temperature was set at 250 °C. Data processing was carried out via Chemstation software on Windows.
Moisture measurement
Moisture content in cocoa beans and HMW fractions were measured using gravimetric analysis through drying at 105 °C (AOAC 2005).
Total polyphenol analysis
The amount of polyphenol compounds in all the treatments prepared under different roasting conditions was measured with the Folin-Ciocalteu procedure according to the published procedure (Li et al. 2014). In brief, 0.5 g of the sample was extracted with 70% acetone (v/v) under constant agitating conditions. Following the extraction, the sample was centrifuged at 80009 g for 10 min. The supernatant was transferred to a flask and the acetone was removed by rotary evaporation (N-1001S-WA, Tokyo Rikakikai Co. Ltd, Tokyo, Japan) under partial vacuum at 40 °C. The residue was transferred to a volumetric flask containing 2 mL of glacial acetic acid and diluted to 10 mL with Milli-Q Plus water. The raw extract was diluted 1/10 (v/v) with 2.5% aqueous acetic acid. The standards and the sample extracts (0.5 mL), Folin–Ciocalteu (F–C) reagent (0.5 mL), and 1.5 mL of Na2CO3 solution (20%, w/v) were blended and diluted to 10 mL with Milli-Q Plus water and kept for 60 min at the room temperature. Absorption was evaluated at 760 nm using a Model 722 spectrophotometer (Shanghai Precision and Scientific Instrument Co., Ltd., Shanghai, China). The total phenolic content was reported as Gallic Acid Equivalents (mg/g). All the samples and chemical parameters were analyzed in triplicate and the mean value was reported.
Color determination
2 g of the sample was added to 50 mL of acidified methanol (1 mL 12 N HCl:1 L methanol). The extracted pigments were filtered through Whatman 541 filter paper (GE Healthcare, Pittsburgh, PA, USA). 4 mL of the filtrated sample was transferred to a 25 mL flask and diluted to volume with the acidified methanol. The absorbance was determined at 460 and 525 nm using a Hach Model DR/4000U spectrophotometer (Hach Co., Shanghai, China).
The OD460/OD525 ratio is known as cocoa beans browning index (Emmanuel Ohene Afoakwa, Agnes Simpson Budu, Henry Mensah-Brown & Ofosu-Ansah, 2014); in terms of the color quality of roasted cocoa beans, this ratio should be at least 1.1 and if less than 1, it indicates that the cocoa is not fermented and roasted properly (Krysiak 2006; Wieslawa et al. 2013; Serra Bonvehí and Ventura Coll 2002).
Sensory evaluation
The product sensory acceptability test was performed by eight semi- trained (from Shirin Asal Company) (Bonvehí 2005). For the sensory analysis, 12 g of the powdered sample was mixed with 15 g of sucrose in a 400-mL beaker. Afterwards, 300 mL of water at 55 °C was added and the mixture was stirred to obtain a homogeneous suspension. The assessment was carried out in individual rooms under standard conditions during daytime. The samples were assessed to verify the potential acceptability of each treatment in terms of Aroma (oxidized-like oil), flavor (off-flavor), and the color using a 5-point structured hedonic scale ranging from 5 (extremely like) to 1 (extremely dislike) (Civille and Carr 2020).
Statistical method
Qualitative experiments were performed in three replications using factorial in a completely randomized design. The first factor was the roasting temperature at three levels (120, 130, 140 °C) and the second factor was the roasting time at three levels (20, 30, 40 min). The experimental data were analyzed employing Design Expert® software version 10.0.8 (Stat-Ease, Inc., USA). Duncan test was used to compare the difference between the mean values at the significance level (p ≤ 0.01).
Results and discussion
Determination of quantitative characteristics of the GC–MC method
The quantitative characteristics of the GC–MC method were investigated herein, including calibration curve equations, correlation coefficients (R), limit of detections (LODs), limit of quantification (LOQ), and linear dynamic range (LDR) (Table 1). LODs and LOQs were calculated based on Signal/Noise = 3 and Signal/Noise = 10, respectively. The recovery of alkyl pyrazines from cacao matrix were studied by adding each alkylpyrazine (at 50–100 ppb concentration range). The obtained recovery values were in the range of 96.3–98.6% for all the alkylpyrazine, which indicated that the matrix effect is negligible. The accuracy of the procedure was also determined via the mean value of the three-replicate analyses of the real samples (Table 2). The obtained data clearly showed good variability of analytical method.
Table 1.
Analytical characteristics of the GC–MC method
| Compounde name | Calibration graph equation | Ra | LODb (ppb) | LOQ (ppb) | LDRc (ppb) |
|---|---|---|---|---|---|
| 2-MP | y = 625.01x − 485,828 | 0.9979 | 150.0 | 500.0 | 250–10,000 |
| 2,3-DMP | y = 227.76x − 170,742 | 0.9959 | 52.5 | 175.0 | 100–10,000 |
| 2,5-DMP | y = 1141.8x − 862,717 | 0.9911 | 52.5 | 175.0 | 100–10,000 |
| 2,3,5-TrMP | y = 674.75x − 519,089 | 0.9905 | 22.5 | 75.0 | 50–10,000 |
| 2,3,5,6-TMP | y = 1105.9x − 828,848 | 0.9927 | 20.0 | 60.0 | 50–10,000 |
aCorrelation coefficient
bLimit of detection
cLinear dynamic range
Table 2.
Alkylpyrazines (ppb) with different roasting condition
| Temperature (°C) | Time (min) | (1) Alkylpyrazine | ||||||
|---|---|---|---|---|---|---|---|---|
| 2-MP | 2,3-DMP | 2,5-DMP | 2,3,5-TrMP | 2,3,5,6-TMP | TMP/TrMP | Total pyrazine | ||
| 120 | 20 | 801.2 ± 7.61f | 481.6 ± 16.28g | 1530.0 ± 11.60h | 8402.0 ± 15.77h | 10,230.0 ± 22.90i | 1.21 ± 0.004b | 4289 ± 0.54c |
| 30 | 793.3 ± 10.62f | 836.5 ± 23.72e | 2956.0 ± 18.60e | 10,330.0 ± 14.91g | 12,870.0 ± 28.94d | 1.24 ± 0.00a | 5556 ± 7.51b | |
| 40 | 1297.0 ± 16.98b | 960.9 ± 12.46d | 2701.0 ± 22.89f | 10,760.0 ± 16.34f | 12,480.0 ± 21.94g | 1.15 ± 0.004ef | 5640 ± 5.18b | |
| 130 | 20 | 1206.0 ± 19.52C | 687.4 ± 24.15f | 3587.0 ± 24.60c | 10,940.0 ± 18.72d | 11,890.0 ± 27.98h | 1.08 ± 0.00g | 5661 ± 18.39b |
| 30 | 1129.0 ± 27.01d | 1623.0 ± 12.82b | 6281.0 ± 15.24a | 11,030.0 ± 31.58c | 12,670.0 ± 20.13f | 1.14 ± 0.004f | 6548 ± 2.96a | |
| 40 | 1085.0 ± 19.57d | 1562.0 ± 24.78c | 4740.0 ± 22.50b | 11,770.0 ± 5.30b | 14,030.0 ± 27.32c | 1.19 ± 0.004cd | 6637 ± 6.74a | |
| 140 | 20 | 1471.0 ± 24.26a | 1573.0 ± 29.46c | 2416.0 ± 16.10g | 10,840.0 ± 10.25e | 12,790.0 ± 18.31e | 1.17 ± 0.004de | 5819 ± 5.53b |
| 30 | 1213.0 ± 26.08c | 1644.0 ± 10.57ab | 3323.0 ± 12.99d | 11,810.0 ± 16.80b | 14,930.0 ± 29.20b | 1.26 ± 0.00a | 6584 ± 7.09a | |
| 40 | 967.2 ± 22.01e | 1678. 0 ± 16.39a | 2430.0 ± 21.54g | 12,540.0 ± 24.59d | 15,070.0 ± 24.08a | 1.20 ± 0.00bc | 6537 ± 10.63a | |
Values are mean ± standard deviation of three separate determinations
MP methylpyrazine, DMP dimethylpyrazine, TrMP trimethylpyrazine, TMP tetramethylpyrazine
Mean values with different superscript letters in the same column are significantly different (p ≤ 0.01)
The effect of roasting temperature and time on the amount of alkylpyrazines and TMP/TrMP ratio in cocoa powder
GC–MS identified several volatile aromatic components in the cocoa powder. A sample chromatogram of cocoa powder is depicted in Fig. 1. According to previous studies, the main active ingredients that play role in cocoa flavor are 2-methylpyrazine, 2,5-dimethylpyrazine (DMP), 2,3-DMP, 2,3,5-trimethylpyrazine (TrMP), and 2,3,5,6-tetramethylpyrazine (TMP). Therefore, in the present study, the changes in the amounts of these five pyrazines were investigated.
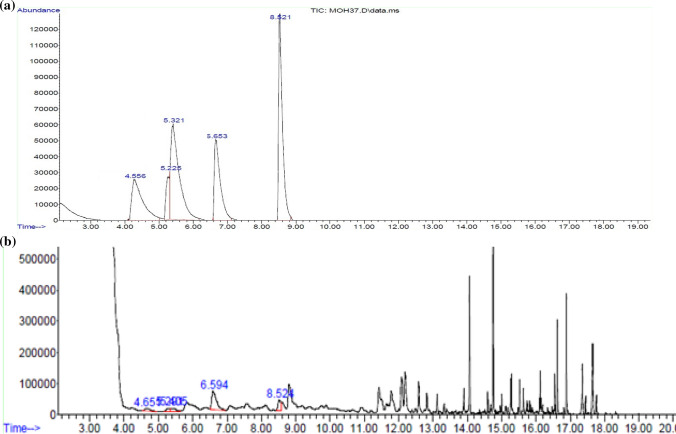
Fig. 1.
a Chromatogram of standard alkyl pyrazine compounds (concentration of each compound is 1000 ppb). b A typical chromatogram of analysis of real cocoa sample. (1) Retention time (4.55 min): 2-methylpyrazine. (2) Retention time (5.22 min): 2,5-dimethylpyrazine. (3) Retention time (5.32 min): 2,3-dimethylpyrazine. (4) Retention time (6.65 min): 2,3,5-trimethylpyrazine. (5) Retention time (8.52 min): 2,3,5,6-tetramethylpyrazine
Based on the statistical results, the amount of alkylpyrazines and TMP/TrMP ratio of the samples in different treatments had a statistically significant difference (p ≤ 0.01). As reported by other papers, the formation and concentration of pyrazines vary depending on the time and temperature of roasting (Farah et al. 2012). According to the obtained results herein, the concentration of pyrazines increased with the increase in the temperature from 120 to 140 °C. The highest amount of tetramethylpyrazine and trimethylpyrazine (15,073.2 ppb and 12,537.2 ppb, respectively) belonged to the roasted cocoa powder at 140 °C for 40 min. On the other hand, the lowest amount of these compounds (10,228.5 ppb and 8402.1 ppb, respectively) was observed in the roasted cocoa powder at 120 °C for 20 min (Table 2). This is probably because the cocoa flavor is better developed at high temperatures, yet if the roasting temperature is too high, the cocoa beans will burn and the burnt flavor conceals the flavor of cocoa (Beckett 2009). This result is in accordance with the findings obtained by other studies (Farah et al. 2012; Jinap et al. 1998). The roasting of cocoa beans at different temperatures and durations showed a linear relationship between the roasting temperature and the formation of pyrazine compounds (Ramli et al. 2006).
According to various studies, the degree of cocoa roasting has been reported to be natural when the TMP/TrMP ratio is equal to or about 1, in which case, the cocoa products show the desired aroma and flavor quality. A ratio lower than 1 indicates the overdevelopment of trimethylpyrazine, the high temperature of the cocoa roasting process, and the presence of a burnt odor. This ratio could be conducive to evaluation of the optimum temperature in cocoa processing (Serra Bonvehí and Ventura Coll 2002). A review of the sources shows that the factors influencing the TMP/TrMP ratio in roasted cocoa depend on certain parameters, such as the initial free α-amino acid content, pH solution, and roasting process (temperature, duration, the amount of alkali, and the amount of the air incorporated in brown cocoa) (Serra Bonvehí & Ventura Coll, 2002). This ratio was between 1 and 1.26 in all the samples in the present study (Table 2).
Moisture loss analysis
During roasting, the changes in the moisture content of cocoa beans are demonstrated in Table 3. A similar trend was observed at the roasting temperatures (120, 130, and 140 °C). The roasting temperatures were observed to significantly (p < 0.01) affect the moisture loss during the roasting process. Moisture loss occurred with extending time and temperatures.
Table 3.
Moisture, total polyphenols and color in different roasting condition
| Temperature (°C) | Time (min) | Moisture | Total polyphenols | Color OD460/OD525 |
|---|---|---|---|---|
| 120 | 20 | 3.80 ± 0.04a | 16.37 ± 0.05a | 1.23 ± 0.02h |
| 30 | 3.21 ± 0.02c | 16.34 ± 0.03a | 1.28 ± 0.03h | |
| 40 | 2.93 ± 0.08d | 15.92 ± 0.11b | 1.35 ± 0.04g | |
| 130 | 20 | 3.49 ± 0.04b | 15.68 ± 0.04b | 1.43 ± 0.06f |
| 30 | 2.23 ± 0.04e | 14.53 ± 0.04c | 1.52 ± 0.02e | |
| 40 | 2.00 ± 0.08f | 13.23 ± 0.08d | 1.64 ± 0.02d | |
| 140 | 20 | 2.20 ± 0.02e | 12.92 ± 0.15e | 1.71 ± 0.04c |
| 30 | 1.88 ± 0.06f | 11.80 ± 0.14f | 1.83 ± 0.02b | |
| 40 | 1.71 ± 0.10 g | 10.46 ± 0.25 g | 1.98 ± 0.02a |
Mean values with different superscript letters are significantly different (p ≤ 0.01)
Influence of roasting conditions on the total polyphenol content of the cocoa powder
Severe reduction in phenols during fermentation, drying, and roasting of cocoa beans occurs due to enzymatic and non-enzymatic oxidation (De Brito et al. 2001). Roasting is known as an important step in the cocoa powder production process, which leads to reduction in flavanols (Kothe et al. 2013). The effect of temperature, roasting time, and the interaction between temperature and time on the total polyphenols of cocoa powders (p < 0.01) was significant and as expected, the results showed that the increase in the temperature and time of roasting had negative effects on cocoa polyphenols; accordingly, the highest degradation was observed at higher temperatures and times (Table 3). Other studies have also reported similar observations (Djikeng et al. 2018; Hii et al. 2017), in which higher temperatures and longer processing times have been typically reported to reduce the amount of cocoa polyphenols (Wieslawa et al. 2013).
Kofink et al. suggested that roasting temperatures (120–160 °C for 2.5 h) could lead to catechin epimerization and degradation of procyanidin. Therefore, to maintain or reduce polyphenol damage in cocoa powder, the maximum oven temperatures of 100, 110, and 120 °C were suggested (Kofink et al. 2007). Mazor Jolić et al. reported a 16% drop at 150–140 °C for 20 min (Mazor Jolić et al. 2011). De Brito et al. reported a 16.5% drop in the total phenol at 150 °C for 30 min (De Brito et al. 2001). Arlorio measured a similar sharp drop in the total phenols, which was between 33 and 55% at 130 °C (Arlorio et al. 2008).
The reduction in polyphenol compounds in cocoa strongly depends on the oxidation of these compounds to corresponding quinones and allows the polymerization and formation of insoluble high-molecular-weight pigment compounds. Their reaction with proteins also decreases the total polyphenols content (Arlorio et al. 2008; Gìltekin-Özgìven et al. 2016).
Influence of roasting conditions on the color and colorimetric fractions (OD460/OD525 value of the cocoa powder)
Color is widely used as an indicator of the development of browning reactions in cocoa beans and temperature is the main cause of these changes) Zyzelewicz et al. 2014).
In the present study, the results revealed (Table 3) that with the increase in the temperature and roasting time, the intensity of color increased significantly (p ≤ 0.05). In the samples roasted at 140 °C and 30 and 40 min, the increase was not significant. The best color quality was obtained during roasting at 140 °C for 40 min.
Wiesława reported that this ratio in roasted cocoa beans at 135 and 150 °C was over 1.1 and apparently, its value depended on the temperature and relative air humidity. Roasted cocoa beans at these temperatures were superior in color compared to the ones roasted at lower temperatures (Krysiak 2006). This finding is in line with the results gained in this study.
The compounds responsible for proper color of beans have not been yet completely identified. It is presumed that the color of raw beans comes from polyphenolic compounds and anthocyanins, which during the fermentation process, due to various transformations, form polycondensation products, the so-called phlobaphenes.
These compounds contribute to the formation of the typical brown color of cocoa beans. During the roasting process, other changes occur, which are caused by oxidation and polymerization reactions of polyphenols and the decomposition of proteins. The formation of the characteristic color of beans is also affected by Maillard and starch dextrinization reactions (Krysiak et al. 2013).
Increased browning index with the increase in the roasting time was probably due to the increase in brown pigment formation from Maillard reactions, thermal oxidation, and formation of tannins, in addition to Strecker degradation reactions (Krysiak 2006) during the roasting process. These findings are consistent with those by other studies (Krysiak 2006; Wieslawa et al. 2013).
The results obtained from sensory evaluation of treatments
Acceptance of new products by consumers has always been a concern for manufacturers. Different roasting conditions mainly affect the flavor, color, and aroma of products, which are among the most important sensory features effective on the quality acceptance by consumers. Thus, these properties were investigated in the current work. The results of the analysis of variance of the samples indicated that the effect of temperature and roasting time on flavor and aroma of cocoa was significant (p < 0.01). Table 4 presents a summary of influence of roasting conditions on the sensory value. The scores of the aroma ranged between 1 and 2.66 and those of the flavor attribute were between 1.33 and 4. While, the interaction of temperature and time on color was not significant (p > 0.05).
Table 4.
The effect of roasting on Sensory analyze of cocoa powder
| Temperature (°C) | Time (min) | Flavor | Aroma | Color |
|---|---|---|---|---|
| 120 | 20 | 1.33 ± 0.47b | 1.00 ± 0.00c | Non-significant |
| 30 | 1.66 ± 0.4b | 1.66 ± 0.47bc | Non-significant | |
| 40 | 1.66 ± 0.4b | 1.33 ± 0.47c | Non-significant | |
| 130 | 20 | 2.00 ± 0.00b | 2.00 ± 0.00bc | Non-significant |
| 30 | 1.66 ± 0.47b | 1.66 ± 0.47bc | Non-significant | |
| 40 | 1.66 ± 0.47b | 2.00 ± 0.00bc | Non-significant | |
| 140 | 20 | 1.66 ± 0.47b | 1.66 ± 0.4bc | Non-significant |
| 30 | 2.66 ± 0.47b | 2.66 ± 0.47ab | Non-significant | |
| 40 | 4.00 ± 0.00a | 2.66 ± 0.47a | Non-significant |
Data are expressed as mean ± standard deviation (n = 3) and different letters show significant difference at the 5% level in Duncan’s test (p < 0.01)
As could be seen in Table 4, the highest flavor score (4) belonged to the sample roasted at 140 °C for 40 min. At low temperatures or shorter roasting times, cocoa samples showed a weaker flavor and aroma (120 °C–20 min). In this study, there were no significant differences between the samples roasted at a lower temperature for a longer period of time (120 °C–40 min) and the samples roasted at a higher temperature and for a shorter time (140 °C–20 min). Both of them were the least acceptable. Similar results were found by Hii et al. (2017). It seems as though the temperatures between 110 and 170 °C, which have been reported in previous studies, result in acceptable flavor and aroma up to 140 °C while higher temperatures show undesirable sensory characteristics. Similar results were found by Ramli et al. (2006); they studied the influence of roasting conditions (temperature from 120 to 170 °C and time from 20 to 50 min) on volatile flavor compound and sensory properties of roasted Malaysian cocoa beans and concluded that when the temperature increased to 160 °C and roasting time increased to 50 min, the increase in the roasting time and temperature had a considerable effect on the burnt flavor of cocoa and increased the bitter taste. The bitterness continued to increase at the higher temperature of 170 °C and with increased roasting time, alkaloids and methylpyrazines were detected in the roasted cocoa beans after exceeding the time and temperature of roasting (Ramli et al. 2006).
In this study, the linear effect of the roasting temperature and time were observed to be significant (p ≤ 0.01). It could be concluded that roasting parameters for the development of cocoa flavor could be divided into several regions: low, natural, strong, and excessive odor conditions. The optimal roasting time mainly depends on the heat transfer and temperature gradient in beans. If the temperature is high and the time is too long, there would be significant signs of over-roasting, which reduces the quality of the products.
Conclusion
Volatile chemical compounds contribute to the flavor of cocoa, among which pyrazines are considered to be one of the major groups. We found that roasting process affects the formation of color and flavor combinations during the Maillard reaction. Furthermore, increased time and temperature during the roasting process not only reduced the total polyphenols contents, but also increased the alkylpyrazine compounds. Increase in the roasting temperature to 140 °C and roasting time to 40 min resulted in the increased browning index (OD460/OD525) and a darker color was obtained. Additionally, this treatment had a high content of total alkylpyrazine and tetramethylpyrazine, but a lower content of polyphenols and humidity, which could be in general considered as a suitable treatment compared to other treatments.
Abbreviations
- GC–MS
Gas chromatography–mass spectrometry
- MP
Methylpyrazine
- DMP
Dimethylpyrazine
- TrMP
Trimethylpyrazine
- TMP
Tetramethylpyrazine
Funding
The authors have used their own funds to carry out the project.
Declaration
Competing interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Narmela Asefi, Email: n.asefi@iaut.ac.ir.
Samaneh Ebrahimzadegan, Email: eb.zadegan@gmail.com.
Ramin Maleki, Email: malekichem@gmail.com.
Seiied Sadegh Seiiedlou-Heris, Email: ss_seiedlo@yahoo.com.
References
- Alasti FM, Asefi N, Maleki R, SeiiedlouHeris SS. The influence of three different types and dosage of alkaline on the inherent properties in cocoa powder. J Food Sci Technol. 2020;57(7):2561–2571. doi: 10.1007/s13197-020-04293-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC (2005) Official methods of analysis of AOAC International. In: Association of Official Analysis Chemists International. Association of Official Analytical Chemists International, Gaithersburg
- Aprotosoaie AC, Luca SV, Miron A. Flavor chemistry of cocoa and cocoa products—an overview. Compr Rev Food Sci Food Saf. 2016;15(1):73–91. doi: 10.1111/1541-4337.12180. [DOI] [PubMed] [Google Scholar]
- Arlorio M, Locatelli M, Travaglia F, Coïsson JD, Del GE, Minassi A, Appendino G, Martelli A. Roasting impact on the contents of clovamide (N-caffeoyl-L-DOPA) and the antioxidant activity of cocoa beans (Theobroma cacao L.) Food Chem. 2008;106(3):967–975. doi: 10.1016/j.foodchem.2007.07.009. [DOI] [Google Scholar]
- Beckett ST (2009) Industrial chocolate manufacture and use, 4th edn. Wiley. 10.1002/9781444301588
- Bonvehí JS. Investigation of aromatic compounds in roasted cocoa powder. Eur Food Res Technol. 2005;221(1–2):19–29. doi: 10.1007/s00217-005-1147-y. [DOI] [Google Scholar]
- Civille GV, Carr BT. Introduction to sensory techniques. Boca Raton: CRC Press; 2020. [Google Scholar]
- De Brito ES, García NHP, Gallão MI, Cortelazzo AL, Fevereiro PS, Braga MR. Structural and chemical changes in cocoa (Theobroma cacao L.) during fermentation, drying and roasting. J Sci Food Agric. 2001;81(2):281–288. doi: 10.1002/1097-0010(20010115)81:2<281::AID-JSFA808>3.0.CO;2-B. [DOI] [Google Scholar]
- Djikeng FT, Teyomnou WT, Tenyang N, Tiencheu B, Morfor AT, Touko BAH, Houketchang SN, Boungo GT, Karuna MSL, Ngoufack FZ, Womeni HM. Effect of traditional and oven roasting on the physicochemical properties of fermented cocoa beans. Heliyon. 2018;4(2):e00533. doi: 10.1016/j.heliyon.2018.e00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah DMH, Zaibunnisa AH, Misnawi J, Zainal S. Effect of roasting process on the concentration of acrylamide and pyrizines in roasted cocoa beans from different origins. APCBEE Proc. 2012;4(4):204–208. doi: 10.1016/j.apcbee.2012.11.034. [DOI] [Google Scholar]
- Gìltekin-Özgìven M, Berktaş I, Özçelik B. Change in stability of procyanidins, antioxidant capacity and in-vitro bioaccessibility during processing of cocoa powder from cocoa beans. LWT Food Sci Technol. 2016;72:559–565. doi: 10.1016/j.lwt.2016.04.065. [DOI] [Google Scholar]
- Hii CL, Menon AS, Chiang CL, Sharif S. Kinetics of hot air roasting of cocoa nibs and product quality. J Food Process Eng. 2017;40(3):e12467. doi: 10.1111/jfpe.12467. [DOI] [Google Scholar]
- Jinap S, Wan Rosli WI, Russly AR, Nordin LM. Effect of roasting time and temperature on volatile component profiles during nib roasting of cocoa beans (Theobroma cacao) J Sci Food Agric. 1998;77(4):441–448. doi: 10.1002/(sici)1097-0010(199808)77:4<441::aid-jsfa46>3.0.co;2-#. [DOI] [Google Scholar]
- Kofink M, Papagiannopoulos M, Galensa R. Enantioseparation of catechin and epicatechin in plant food by chiral capillary electrophoresis. Eur Food Res Technol. 2007;225(3–4):569–577. doi: 10.1007/s00217-006-0455-1. [DOI] [Google Scholar]
- Kongor JE, Hinneh M, de Walle D, Van AEO, Boeckx P, Dewettinck K. Factors influencing quality variation in cocoa (Theobroma cacao) bean flavour profile: a review. Food Res Int. 2016;82:44–52. doi: 10.1016/j.foodres.2016.01.012. [DOI] [Google Scholar]
- Kothe L, Zimmermann BF, Galensa R. Temperature influences epimerization and composition of flavanol monomers, dimers and trimers during cocoa bean roasting. Food Chem. 2013;141(4):3656–3663. doi: 10.1016/j.foodchem.2013.06.049. [DOI] [PubMed] [Google Scholar]
- Krysiak W. Influence of roasting conditions on coloration of roasted cocoa beans. J Food Eng. 2006;77(3):449–453. doi: 10.1016/j.jfoodeng.2005.07.013. [DOI] [Google Scholar]
- Krysiak W, Adamski R, Żyżelewicz D. Factors affecting the colour of roasted cocoa bean. J Food Qual. 2013;36:21–31. doi: 10.1111/jfq.12009. [DOI] [Google Scholar]
- Li Y, Zhu S, Feng Y, Xu F, Ma J, Zhong F. Influence of alkalization treatment on the color quality and the total phenolic and anthocyanin contents in cocoa powder. Food Sci Biotechnol. 2014;23(1):59–63. doi: 10.1007/s10068-014-0008-5. [DOI] [Google Scholar]
- Mazor Jolić S, Radojčic Redovnikovic I, Marković K, Ivanec Šipušić D, Delonga K. Changes of phenolic compounds and antioxidant capacity in cocoa beans processing. Int J Food Sci Technol. 2011;46(9):1793–1800. doi: 10.1111/j.1365-2621.2011.02670.x. [DOI] [Google Scholar]
- Ramli N, Hassan O, Said M, Samsudin W, Idris NA. Influence of roasting conditions on volatile flavor of roasted Malaysian cocoa beans. J Food Process Preserv. 2006;30(3):280–298. doi: 10.1111/j.1745-4549.2006.00065.x. [DOI] [Google Scholar]
- Schultz TH, Flath RA, Mon TR, Eggling SB, Teranishi R. Isolation of volatile components from a model system. J Agric Food Chem. 1977;25(3):446–449. doi: 10.1021/jf60211a038. [DOI] [Google Scholar]
- Serra Bonvehí J, Ventura Coll F. Factors affecting the formation of alkylpyrazines during roasting treatment in natural and alkalinized cocoa powder. J Agric Food Chem. 2002;50(13):3743–3750. doi: 10.1021/jf011597k. [DOI] [PubMed] [Google Scholar]
- Wiesława K, Motyl-Patelska L. Effects of air parameters on changes in temperature inside roasted cocoa beans. Acta Agrophys. 2006;7(1):113–127. [Google Scholar]
- Wieslawa K, Adamski R, Zyzelewicz D. Factors affecting the color of roasted cocoa bean. J Food Qual. 2013;36(1):21–31. doi: 10.1111/jfq.12009. [DOI] [Google Scholar]
- Ziegleder G (2009) Flavour development in cocoa and chocolate. In: Industrial chocolate manufacture and use, 4th edn,pp 169–191. 10.1002/9781444301588.ch8
- Zyzelewicz D, Krysiak W, Nebesny E, Budryn G. Application of various methods for determination of the color of cocoa beans roasted under variable process parameters. Eur Food Res Technol. 2014;238(4):549–563. doi: 10.1007/s00217-013-2123-6. [DOI] [Google Scholar]