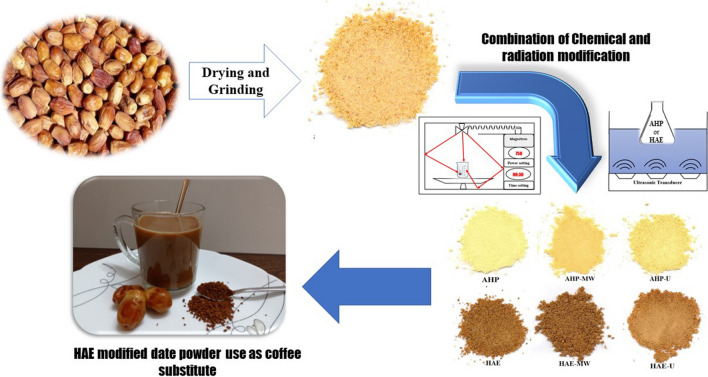
Abstract
To consider the suitability of modified date powder as a coffee substitute, the biochemical and antioxidant properties of date powder were modified by hydrochloric acid–ethanol (HAE), alkaline hydrogen peroxide (AHP), combined with ultrasound (U) microwave (MW) radiation. The results showed that the highest antioxidant activity was observed in HAE and HAE-U treated date powders. The total flavonoid content of the date powder increased by 40.8% and 100% in response to the AHP-MW and HAE-U treatments, respectively. Fourier transform infrared spectroscopy did not show any abnormal or unknown peaks in the analyzed range of the treated samples. Therefore, modification of biochemical and sensory properties of date powder by chemical and irradiation treatments did not have a detrimental effect on changing the structure of its chemical compounds or the formation of harmful compounds in it. Sensory evaluations showed that replacing coffee powder with modified date powder by up to 25% did not have significant effects on the sensory properties of the coffee drink. Finally, the results showed that modifying the biochemical and antioxidant properties of date powder by the HAE method as coffee substitute can increase the nutritional properties of coffee drinks while also reducing the expenses of the coffee industry.
Graphical abstract
Keywords: Alkaline hydrogen peroxide, Coffee substitute, Date powder, Hydrochloric acid–ethanol, Microwave, Ultrasound
Introduction
Date palm (Phoenix dactylifera) is a prehistoric and postmodern product of arid and semi-arid regions. Since ancient times, date palm has been planted not only for its high-energy profile but also for its ease of storage and transport a long distant route (Iranmanesh 2000). ‘Zahedi’ is a cultivar of dates in Iran and is also known as ‘Qasb’ in some parts of the country. The fruit is yellowish in the Khalal stage, light brown in the Rutab stage and reddish to yellowish brown in the Tamer stage. The ‘Qasb’ dates are classified as a dry and semi-dry cultivar and they have easy storage and transportation conditions. It is late-ripening and has a high rate of preharvest fruit drop.
Iran is the second largest producer of date fruits, producing almost 14% of the world’s dates (Iranmanesh 2000). Dates have an important role in the country’s agricultural economy, accounting for 30% of dried fruit exports and 10% of non-oil exports from Iran (Iranmanesh 2000). The current production of dates in producing countries exceeds the capacity of the total processing industrial complexes for date fruits in these countries. After completing the raw consumption capacity of dates, a small portion of them enter value-added industries, whereas the rest is either lost (about 30%) or sold at low prices to intermediaries and exported in bulk amounts (Iranmanesh 2000). Therefore, date fruit processing can be taken as an approach to reduce waste in the sector, while diversifying the end products of date fruits as value-added commodities.
Drying, as method for processing fruits, herbs and spice plants, is a primary action that can turn date fruits into a long shelf-life form. In general, food powders can be easily used as additives in many processed food products. Powders of dried fruits and herbs can be used as parts of instant beverage formulations, bakery raw materials, or even in drug formulations.
In the process of drying and dehydration of fruits, many functional and nutritional properties of the dried fruits are changed, thereby causing difficulties in using them in food formulations. A reliable solution to improve the functional and nutritional properties of food powders is the use of chemical and mechanical treatments. For instance, Basanta et al. (2014) reported that the alkaline hydrolysis of cherry harvesting residues fibers can increase the amount of total phenolic compound. The application of ultrasound, as a mechanical treatment, has reportedly increased the functional and antioxidant properties of sea cucumber fucoidan (Guo et al. 2014).
Phenolic compounds are considered one of the abundant classes of phytochemicals with health-promoting qualities and functions. Phenolic compounds are classified as secondary metabolites commonly distributed in the plant’s kingdom, with immense structures and functions. Phenolics are compounds that have an aromatic ring with one or more hydroxyl groups attached to it, which are derived from two pathways, acetate/malonate and shikimic acid, which the shikimic acid pathway plays a very important role in the production of this metabolites in plants. They are divided into two classes: flavonoids and non-flavonoids. flavones, flavonols, flavanones, flavan-3-ols, anthocyanidins and isoflavones are the main subclasses of flavonoids and non-flavonoid phenolic compounds include phenolic acids, hydroxy cinnamates and stilbenes. Phenolic compounds can be further grouped into water soluble compounds (phenolic acids, flavonoids, phenylpropanoids, and quinones) and water-insoluble compounds (condensed tannins, lignins, and cell-wall bound hydroxycinnamic acids). This classification is significant due to the nutritional composition or constituents since its solubility and digestibility are needed most for effective utilization within the gastrointestinal tract and some physiological operations (Ghani et al. 2021; Swallah et al. 2020).
In the process of drying dates and producing its powder, enzymatic and non-enzymatic reactions occur that make the date powder brown. These reactions usually happen in the presence of large amounts of simple carbohydrates, complex polysaccharides, protein compounds, phenolic compounds and minerals in date fruits. Specifically, the Maillard reaction and caramelization create a brown color in date powder, thereby making it potentially suitable as a coffee substitute in coffee drink formulations. Komes et al. (2015) reported that chicory root powder, along with barley flour, was roasted and then used as a partial substitute for coffee grains in coffee brews and, eventually, the roasted combination created sensory properties similar to those of coffee. Coffee is one of the most consumed beverages in the world and is the second largest traded commodity after petroleum. The high price of coffee has persuaded some researchers to come up with a suitable substitute that does not differ significantly in terms of sensory properties from coffee. With regard to the available literature on partial substitutes for coffee grains, i.e. substitutes that can replace coffee only to some limited extents so as to maintain the ultimate sensory properties of coffee, there is an absence of indication that date powder could be used relevantly. Therefore, the aim of this study was to experiment on the effects of AHP and HAE chemical treatments, as well as ultrasound and microwave treatments, to improve the phytochemical and antioxidant properties of date powder and to investigate the potential of its use as a coffee substitute in coffee drinks.
Materials and methods
Materials
Date fruits of the ‘Qasb’ cultivar are cultivated in the southern provinces of Iran, especially in the south of Fars province. In this study, dates were harvested from a commercial orchard in Jahrom city (Iran) in August 2020. Hydrochloric acid (HCl), sodium hydroxide (NaOH), ethanol, hydrogen peroxide (H2O2) and other analytical grade chemical reagents were purchased from Merck Company (Darmstadt, Germany). Double distilled water was used for preparation of solutions.
Methods
Date powder production
Unwanted residues of the date fruits of the ‘Qasb’ cultivar were removed after harvest. All dates were deseeded by hand and then cut into smaller pieces (5 mm) with a kitchen knife. Pieces of dates were poured into aluminum containers and dried in an oven using hot air convention at 80 °C for 24 h. After drying the samples, the date pieces were turned into date powder by a laboratory mill and, after being sieved with a 100-mesh screen, they were packed in moisture-impermeable polypropylene bags and stored in the refrigerator (4 °C) until the treatments were applied.
Alkaline hydrogen peroxide (AHP) treatment
According to a method by Niu et al. (2018), the AHP treatment was applied on date powder. Date powder and 2% hydrogen peroxide solution were mixed in a 1:15 ratio in a glass Erlenmeyer flask. The pH of the mixture was adjusted to 10 using a normal solution of sodium hydroxide and stirred continuously for one hour on a magnetic stirrer. The pH of the mixture was then adjusted to 7 by a normal hydrochloric acid and poured into flat polypropylene containers. They were placed in a conventional oven at 60 °C. The drying process was carried out by a hot air convention for 24 h in the said temperature. The dried date powder was completely crushed into a fine powder by a laboratory mill and was then packed in moisture-resistant polyethylene containers.
Hydrochloric acid–ethanol (HAE) treatment
Date powder and an alcoholic acidic solution [90% ethanol + 10% hydrochloric acid (37%)] were mixed in a 1: 10 ratio in an Erlenmeyer flask. The mixture was placed on a magnetic stirrer for one hour to stir well. Then, the pH of the solution was adjusted to 7 by sodium hydroxide (1 N) and poured into flat containers made of polypropylene. The drying and packaging processes were performed as mentioned.
Microwave irradiation on date powders treated with AHP and HAE
After having date powder mixed with AHP or HAE solutions, the mixtures were irradiated by 720 watts of a home microwave for 2 min, before neutralizing the pH to 7 by hydrochloric acid or sodium hydroxide. The mixtures were dried in a hot air oven for 24 h at 60 °C.
Ultrasound irradiation on date powders treated with AHP and HAE
A mixture of date powder and solutions of AHP or HAE, before neutralizing the pH, were exposed in an ultrasonic bath (6-L ultrasonic bath model H60S, Elma Germany) for 10 min at a temperature of 35 °C. The ultrasonic waves were generated by a power of 650 watts.
Extraction and biochemical measurements
Extraction of date powder were done according to the maceration method, as described by Wojdyło et al. (2007) with some minor modifications. The antioxidant activity of the extract was determined by a spectrophotometric method based on the reduction of a methanol solution from DPPH. Assays on flavones and flavonols were carried out according to Popova et al. (2004), with some minor modifications, and the results were reported as mg quercetin/g dry weigh of plant sample (Popova et al. 2004). Total flavonoid content was calculated according to the formation of a flavonoid–aluminium complex, while quercetin was selected as a standard. Total phenolic contents were analyzed using the Folin-Ciocalteu colorimetric reagent and gallic acid was used as a standard (Wojdyło et al. 2007).
Color analysis of treated date powders
Color parameters were recorded by imaging via a scanner. An amount of date powder was poured on the scanner to cover the surface. An image with a resolution of 300 dpi was prepared and saved as jpg format. The images were analyzed by Image J software (version 1.4) and were processed to render images with dimensions of 500 by 500 pixels. The colors were converted from RGB to LAB color space and the L*, a*, and b* parameters which were then calculated by the software.
Mineral composition of treated date powders
For the estimation of minerals (K, Na and Cu), 1 g of the sample was digested wet, using nitric acid. After reaching a volume of 25 ml, the extract was analyzed for these minerals by the inductively coupled plasma (ICP) technique using a Perkin Elmer atomic emission spectrophotometer (Model AAnalyst 200). All analyses were conducted in triplicate, and the results were expressed on a moisture-free basis.
Total and reducing sugar contents
The reducing sugar and total sugars were examined by Lane–Eynon General Volumetric Method (AOAC 968.28).
Fourier transform infrared (FT-IR) spectroscopic analysis
In order to investigate the effects of chemical and irradiation treatments on structural properties of date powder components, the Fourier transform infrared (FT-IR) spectra of each sample was recorded in a transmission mode on a spectrophotometer (Paragon 1000, Perkin Elmer, USA). Date powder samples were ground with potassium bromide (KBr) powder and then pressed into pellets before measurement. Spectra were obtained in a frequency range of 400–4000 cm−1 at a resolution of 1.43 cm−1. The interference of H2O and CO2 in the air was subtracted during analysis.
Sensory evaluation of coffee drink containing date powder as coffee substitute
Date powder was treated with the HAE solution and was roasted at 80 °C for 4 h to obtain a color and appearance similar to coffee powder. The resulting powder was then replaced with coffee powder in proportions of 25, 50, 75 and 100% in a 3-in-1 coffee premix formulation. The 3-in-1 coffee premix included 10% coffee powder, 34% of non-dairy creamer powder and 56% of sugar as the control sample. The sensory properties of 3-in-1 coffee premixes and coffee powder substitutes were assessed for color, odor, taste, mouthfeel, sweetness, unfavorable aftertaste, and overall acceptance. Sensory evaluations were carried out by the 5-point hedonic scale. The samples were numbered randomly using 3-digit codes and, after mixing the powders with boiling water, the samples were evaluated by 10 trained panelists who ultimately rated the samples.
Statistical analysis
In this research, two independent experiments were performed. The first experiment was related to the effect of different physical and chemical treatments on some physicochemical and biochemical properties of date powder. In this research, an experiment was conducted based on a randomized completely design (RCD) including seven treatments and three replications. In the second part, an experiment based on a randomized completely design including five treatments and three replications was conducted in relation to the evaluation of sensory and color characteristics of modified date powder as coffee substitute. Statistical analysis was performed using the JMP software version 8 and by comparison of mean values via the Tukey test (P ≤ 0.05%).
Results and discussion
Total phenolic compounds
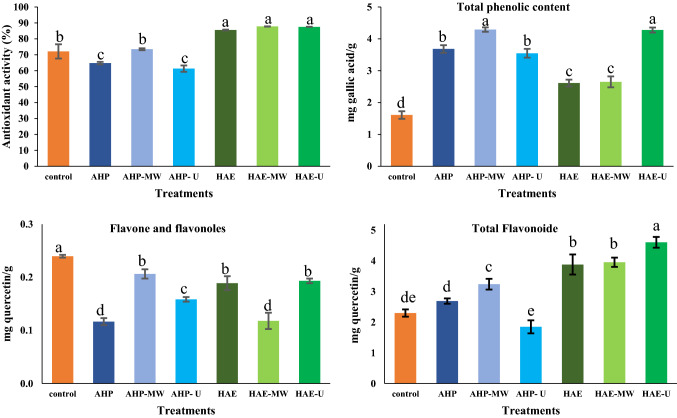
The results showed that the amount of total phenolic compounds in all samples increased significantly compared to the control sample. Treatments of AHP-MW and HAE-U made samples with the highest amount of total phenolic compounds (Fig. 1). Tareen et al. (2020) evaluated the effects of AHP treatment (3% concentration at 70 °C) on the phenolic content of oil palm trunk residue and reported that the phenolic content increased in response to the alkaline treatment. Also, the report indicated that the AHP treatment could break down lignin and release phenolic compounds therefrom. In fact, the AHP treatment can cause the release of some phenolic compounds, e.g. phenolic acid, tannins and gallic acid, from lignocellulosic biomass (Jeyavishnu et al. 2021). Furthermore, Vadivel and Brindha (2015) stated that alkaline hydrolysis brings on a higher antioxidant potential in terms of radical DPPH inhibition, removal of superoxide, and hydrogen peroxide inhibition, thereby maximizing the recovery of phenolic compounds from rice husks. The highest amount of total phenolic compounds was achieved by the AHP-MW treatment (Fig. 1). In relation to this subject, Hayat et al. (2010) reported that the amount of phenolic compounds in citrus mandarin pomace increased significantly after microwave treatment, indicating that esterified phenolic acids that are attached to glycosides can be broken down and released by microwave treatments, where appropriate strength and time are applied. This could be attributed to the heating effect caused by electromagnetic radiation during the microwave process. Ultrasound waves in both AHP and HAE treatments increased the amount of phenolic compounds compared to the control sample (Fig. 1). Ultrasound waves can destroy polysaccharide and lignin structures through cavitation energy and, ultimately, cause the release of phenolic compounds. In this regard, Golmohamadi et al. (2013) reported that ultrasound had no destructive effects on the phenolic content of red raspberry puree but could increase the transfer of phenolic compounds from the red raspberry to the extraction solvent. Although AHP treatment increased total phenolic contents, high concentrations of hydrogen peroxide can degrade and decrease total phenolic compounds. Aïder et al. (2012) reported that increasing the concentration of hydrogen peroxide significantly reduced the total phenolic compounds of whole brown flaxseed meal.
Fig. 1.
Biochemical properties of Qasb date powder under different treatments (Different lowercase letters in the top of each column denote significant differences (P < 0.05)
Antioxidant activity
The antioxidant activity of date powder increased differently in response to the various treatments, compared to the control sample (Fig. 1). In HAE samples, the rate of increase was higher than in AHP samples. While antioxidant activity usually correlates positively with total phenolic content, the results showed that HAE samples had higher antioxidant activity, with the exception of HAE-U samples which showed less phenolic compounds. This could be due to the presence of hydrophilic and hydrophobic pigments, as evidenced by the darker color of the HAE samples. Therefore, it can be concluded that a lower phenolic content, associated with large amounts of color pigments, can make the product ultimately show high levels of antioxidant activity. In this regard, Chaira et al. (2009) examined the antioxidant activity and phenolic compounds of 10 Tunisian date cultivars using the HAE treatment and reported no positive correlation between phenolic content and antioxidant activity. The report added that dates with lower phenolic compounds may have higher antioxidant activity, probably because of more pigments and various factors such as cultivar, growth conditions, geographical origin and treatments. Non-enzymatic browning reactions result in the formation of a complex set of compounds called Millard reaction products in foods under different treatments (e.g. heat, alkaline or acidic conditions). These products are often regarded as pigments which can compete with phenolic compounds in staging antioxidant activity. Date powder samples treated with HAE-U had the highest level of antioxidant activity. This could be due to the effect of ultrasound on the breakdown of hydrogen bonds, resulting in lower molecular-weight polysaccharides and the destruction of free radicals, so that short-chain polysaccharides end up with more reductive terminals that possess antioxidant properties. Guo et al. (2014) reported that the application of ultrasound treatments for 20 min increased the antioxidant activity of sea cucumber fucoidan.
Flavone, flavonols and total flavonoids
The amounts of flavones and flavonols in all treatments decreased significantly compared to the control sample (p < 0.05) (Fig. 1). These findings were similar to previous results by Kammerer et al. (2014) that flavones and flavonols are highly susceptible to damage and have a strong tendency for structural change. Putnik et al. (2016) stated that differences in flavone and flavonol contents may depend on extraction conditions such as temperature, time and method of extraction (e.g. HAE, AHP, etc.). Also, Hayat et al. (2010) reported that the amount of flavone and flavonols decreased in response to the prolonged exposure to microwave treatment. The destruction of these compounds is mostly due to microwave heating. Meng et al. (2019) investigated the effects of AHP treatment on buckwheat flavonoid levels, especially flavonols, and reported that flavonols decreased significantly as a result of the AHP treatment. This was probably due to the de-polymerization of the fiber matrix after applying the hydrogen peroxide treatment (Meng et al. 2019).
The flavonoid content of date powder samples increased under different treatments, except for the AHP-U samples which did not change significantly compared to the control (P < 0.05) (Fig. 1). The rate of increase in the flavonoid content of HAE samples was higher than AHP samples. Putnik et al. (2016) evaluated the effects of AHP and HAE treatments on the flavonoid content of grape skin pomace and reported that adding hydrochloric acid increased the total flavonoid content from 20.63 to 46.77%. They reported that the acidic solution increased the hydrolysis of phenolic structures in polymers and that the release of readily dissolved monomeric metabolites was facilitated during extraction.
The lowest amount of total flavonoids was observed in AHP-U samples, probably due to the effect of ultrasonic cavitation. This corresponded with a previous result (Gharibzahedi et al. 2019), that pectin extraction from fig skin residues by the ultrasound treatment led to a lower performance, hence a lower galacturonic acid in the samples, compared to other samples that resulted from different methods. This ultimately reduced the total flavonoids and antioxidant ability of pectin extracted by the ultrasound treatment (Gharibzahedi et al. 2019). Xiao et al. (2008) reported that increasing the microwave power led to an increase in the total flavonoid content of extraction from Chinese milkvetch. This was reportedly attributed to the direct effects of microwave energy on biomolecules via ionic conduction and bipolar rotation, thereby leading to energy dispersion in the solvent, increasing molecular motion and temperature, as well as enhancing the extraction of total flavonoids (Gfrerer and Lankmayr 2005).
Color characteristics
The results showed that the lightness (L*) of the control sample was 83.59 which increased to 94.81 in AHP but decreased to 44.15 in HAE-MW samples (Table 1). An increase in the L* index of the AHP samples can be due to the effect of high pH values on the degradation of color compounds in date powders and, thus, the increase in its lightness. Meng et al. (2019) reported that the lightness of buckwheat fiber increased after the AHP treatment. It was suggested that hydrogen peroxide, as a strong oxidizing agent, can destroy pigments that exist between layers comprising the fibers (Aïder et al. 2012). An alkaline condition can also cause hydrogen peroxide to decompose and produce pre-hydroxyl radicals (HOO). These active radicals attack pigments, oxidize and decolorize them. A decrease in the L* factor observed in HAE samples may be explained by the presence of more polyphenols, chlorophyll compounds, carotenoids, and flavonoids, which increase the likelihood of enzymatic browning reactions and lead to the appearance of dark colors (Zhang et al. 2020). In contrast, acidic conditions can facilitate the Maillard reaction and browning by first decomposing polysaccharides and long-chain fibers while also assisting in the production of oligosaccharides and simple sugars.
Table 1.
Color characteristics of modified date powders
| Treatments | L* | a* | b* |
|---|---|---|---|
| Control | 83.59c | 8.19b | 44.22cd |
| AHP | 94.81a | − 9.22e | 44.51c |
| AHP-MW | 86.93cb | 1.48c | 52.11a |
| AHP- U | 91.00ab | − 5.10d | 49.15b |
| HAE | 48.98e | 16.98a | 41.00e |
| HAE-MW | 44.15e | 17.40a | 37.14f |
| HAE-U | 61.67d | 15.77a | 42.30de |
Different lowercase letters in each column denote significant differences (P < 0.05)
The lowest and highest values of ‘a*’ factor occurred in the AHP and HAE samples in association with microwave, respectively (Table 1). In this regard, Platat et al. (2014) reported that the higher the red-green color, the higher the amount of phenolic compounds and total antioxidants, and vice versa. Here, HAE samples had the highest phenolic compounds and antioxidant activity, which corresponded with the fact that the HAE samples had the highest ‘a*’ index compared to other samples. Gharibzahedi et al. (2019) extracted pectin of fig skin using hot acidic water (citric acid) with the assistance of ultrasound and microwave. They reported that extracting pectin by the acidic method resulted in higher values of redness (a*), compared to the ultrasound-microwave method. Ultimately, it was suggested that the presence of polyphenols and pigments trapped in the pectin matrix may increase redness and reduce lightness during the reaction.
The amount of yellowness (b*) in AHP samples, however, was higher than that of the control and HAE samples (Table 1). In this regard, Yoshida and Prudencio (2020) reported that the yellowness (b*) of Okara (also known as soy pulp and doufu zha, is a fibrous residue from soymilk and tofu production) increased after AHP treatments, compared to the control sample. Also, Aïder et al. (2012) reported that the oxidation of proteins and pigments, mainly quinones resulting from phenolic oxidation, could be a reason for the increase in yellowness (b*) under the influence of the AHP treatment.
In general, by applying different chemical and radiation treatments on date powder, a different color spectrum was observed in them. According to Fig. 2, the application of HAE treatments caused a dark color in the date powder, in contrast, the AHP treatments caused a lighter color of the date powders. This color difference can increase the range of application of modified date powders in a variety of food products with different colors.
Fig. 2.
Comparison of the appearance of Qasb date powder under different chemical and radiation treatments. Comparison between HAE coffee with commercial coffee powder
Mineral compounds
The amounts of sodium and potassium increased in all treated samples, compared to the control sample. The highest amounts of sodium and potassium were observed in HAE samples (Table 2). Likewise, the amount of copper increased in all samples compared to the control, so that the highest amount was observed in AHP samples. The increase of sodium content in AHP and HAE samples could be explained by the presence of the sodium hydroxide solution which created an alkaline pH in the AHP treatments and which neutralized the acidic solution in HAE samples, respectively. It was suggested that the alkaline pH can inactivate oxidizing enzymes, thereby allowing a larger release of minerals such as potassium and iron from the structure of these enzymes.
Table 2.
Sugar and mineral contents of Qasb date powder under chemical and radiation modification
| Treatments | Reducing Sugar | Total sugar | Na | K | Cu |
|---|---|---|---|---|---|
| Control | 65.92 ± 1.87a | 73.32 ± 1.68a | 14.18 ± 0.26b | 5.41 ± 0.08f | 0.056 ± 0.00e |
| AHP | 30.08 ± 1.80d | 40.15 ± 1.92e | 12.33 ± 0.31b | 6.16 ± 0.02e | 0.095 ± 0.00b |
| AHP-MW | 48.24 ± 1.57b | 62.66 ± 1.99b | 12.97 ± 0.44b | 7.97 ± 0.15d | 0.132 ± 0.01a |
| AHP- U | 63.91 ± 1.49a | 52.66 ± 3c | 14.62 ± 0.66b | 8.93 ± 0.06c | 0.088 ± 0.01bc |
| HAE | 35.43 ± 1.35c | 41.98 ± 2.72de | 20.22 ± 1.82a | 16.59 ± 0.21a | 0.069 ± 0.00de |
| HAE-MW | 39.42 ± .79c | 49.21 ± 3.06c | 18.60 ± 0.68a | 10.98 ± 0.12b | 0.065 ± 0.01de |
| HAE-U | 35.32 ± 1.73c | 47.52 ± 0.51cd | 19.07 ± 2.56a | 8.62 ± 0.09c | 0.075 ± 0.00cd |
Different lowercase letters in each column denote significant differences (P < 0.05)
Reducing sugars and total sugars
The amount of reducing sugars and total sugars of date fruit samples decreased significantly compared to the control sample (P > 0.05) (Table 2). In general, simple sugars are unstable in alkaline and acidic environments and are converted into various compounds. Yang and Montgomery (1996) reported that alkaline environments can cause the degradation of glucose in the aqueous Ca(OH2) and thus create a complex solution with more than 50 types of compounds, including parasaccharinic acid. The products of alkaline degradation of sugars are mainly saccharinic acids (< C6), high molecular weight compounds, non-acidic compounds and unsaturated carbonyl ring compounds in small quantities. The composition of the products of the alkaline reaction is influenced by various parameters such as temperature, concentration of the alkaline solution and the nature of the monosaccharides. De Wit et al. (1979) argued that a reason for the reducing effect on alkaline-treated monosaccharides could be the increase in hydroxyl ions and the use of divalent cations which created lactic acid and changed single-carbon compounds (formic acids) to acidic products of non-lactic nature with three carbons (glyceric), while reducing the amount of four-carbon to six-carbon acids.
Strong acids, such as hydrochloric acid, release protons that break down the heterocyclic ether bonds between sugar monomers in polymer chains formed by hemicellulose, cellulose, and long-chain carbohydrates. In this regard, Bustos et al. (2003) observed that, in addition to xylose, other sugars including glucose and arabinose were produced during hydrolysis of sugar cane bagasse by hydrochloric acid. Therefore, in acidic environments, the amount of reducing sugars is expected to increase, whereas the opposite was actually observed. In fact, in acidic environments and with the presence of heat, monosaccharides are converted to furfural and hydroxymethylfurfural, meaning that the amount of reducing sugars and total sugars decrease. Aguilar et al. (2002) investigated the effect of acid treatment (sulfuric acid) on the sugar content of sugar cane bagasse, with a particular emphasis on xylose in the sugar content. It was observed that the amount of xylose increased by 2% sulfuric acid and at 122 °C. Through time and by the increase in sulfuric acid concentration to 6%, as well as with an increase in temperature to 128 °C, the amount of xylose decreased. This indicates that decomposition reactions occurred and led to the production of furfural. Bustos et al. (2003) reported that in the process of extracting sugars from sugar cane bagasse, the glucose concentration is a function of time, temperature and acid concentration. By increasing these parameters, the glucose concentration usually decreases, thereby indicating that decomposition reactions are occurring and that 5-hydroxymethylfurfural is taking form.
Fourier transform infrared (FT-IR) spectroscopic analysis
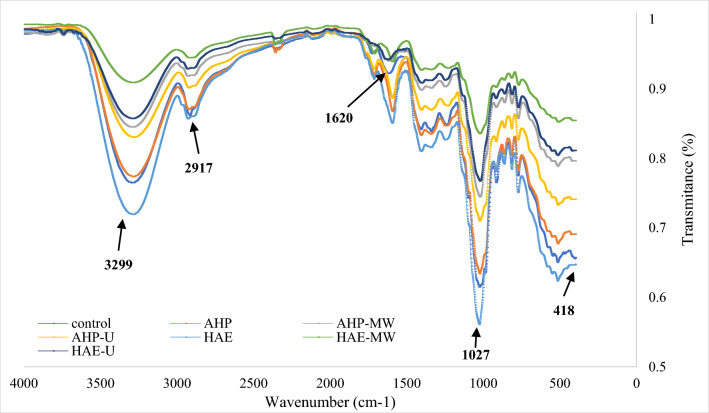
The profiles of the general spectrum of date powder (Qasb cultivar) under different treatments were similar, with the exception of some of the identified bands. According to Fig. 3, a sharp peak in the wave number 3299 cm−1 was observed for the control sample. This wave number represents the tensile O–H vibrations of hydroxyl groups as well as intramolecular hydrogen bond vibrations, mainly due to the presence of cellulose and hemicellulose, as suggested already by George et al. (2020). These researchers stated that strong absorption in the range of 3000–4000 cm−1 indicates hydrogen bond vibrations, which are mainly caused by hydroxyl polysaccharides, which is attributed to cellulose, hemicellulose and lignin in polysaccharides. The wave number in the range of 2917–2932 cm−1 in the control samples and date powder samples under the applied treatments can be related to the tensile C-H vibrations of alkyl groups, which is attributed to the presence of cellulose, hemicellulose and lignin (George et al. 2020). In general, the range 2800–3000 cm−1 could be due to the presence of methyl groups or methylene polysaccharides, and is related to the tensile C–H (Zhang et al. 2017).
Fig. 3.
FT-IR spectra of modified date powders by irradiation and chemical treatments
The wave number 1620 cm−1 in the control sample is related to the C–O stretching of conjugated or aromatic ketones and flavones in lignin (George et al. 2020). The decrease in the intensity of this peak in AHP treated samples is probably due to the degradation of lignin or hemicellulose treated with H2O2. The wave number 1027 cm−1 was related to the C–O stretching of the pyranose ring in cellulose and hemicellulose, which was previously discussed by George et al. (2020) at the wave numbers of 1022. In all samples, except those treated with the HAE, a decrease in peak intensity was observed, compared to the control sample, which could be due to cellulose or hemicellulose degradation under the applied treatments. Considering that the FT-IR spectra of different treatments are not very different from the control sample spectrum, and since no strange or unknown peaks occurred in samples of the different treatments, it can be argued that using the AHP and HAE treatments with microwave or ultrasound waves can be effective. In other words, the treatments did not damage the structure of the main components of date powder and also did not create undesirable chemicals in them.
H2O2 detection in AHP treated samples by FT-IR
One of the concerns of using hydrogen peroxide in food preprocessing is the risk of some of it remaining in the final product. The hydrogen peroxide has a low stability and after a few hours at room temperature is decomposed into two safe substances, water and oxygen, therefore the possibility of its presence after drying in treated date powder is very low. However, the possibility of the presence of hydrogen peroxide in the final product was investigated using the FT-IR technique. Şansal and Somer (1999) reported that the presence of a peak at wavelength of 418.48 cm−1 indicates the presence of hydrogen peroxide in the sample. According to Fig. 3, no absorption peak was observed in the range of 400–450 cm−1 wave numbers, so it can be inferred that all the hydrogen peroxide used in the modification of date powder by AHP method was decomposed into water and oxygen during the drying process, therefore the final modified date powder will not pose a risk to consumer health.
Selection of best treatment for producing coffee substitutes from date powder
By comparing the effects of different treatments on the functional, phytochemical and qualitative characteristics of date powder, and by using the ranking test, comparing the average rank of each treatment, the HAE method was selected as the best treatment that could properly modify the properties of the date powder and, thus, could render the date powder suitable as a coffee substitute (data not shown). As a result of the initial tests, it was observed that the HAE treatment caused the date powder to obtain optimal parameters in solubility, water holding capacity, oil holding capacity, water retention capacity, emulsifying capacity, emulsion stability (Unpublished results), antioxidant activity, total phenolic compounds, flavonoid content, flavone and flavonol content and color. The optimal values in the said parameters made the date powder a suitable option for substituting coffee.
Sensory evaluation of coffee drinks containing date powder as a coffee substitute
Coffee substitutes can be made from the seeds and roots of different plants, which, like coffee, contain large amounts of carbohydrates, proteins and other compounds, characterized by high physiological activity. After roasting, the seeds and roots usually turn brown and contain a high proportion of water-soluble substances that taste like coffee. The date powder from the HAE treatment was roasted for 4 h at 80 °C to achieve a pseudo-coffee color and appearance. The results of color evaluation showed no significant differences (according to t-test assay) between the L* of the coffee substitute powder and commercial instant coffee powder (Fig. 2 and Table 3). In fact, in terms of color darkness, which is an important feature of processed coffee, there was no significant difference between HAE-modified date powder and commercial instant coffee powder. This result is a valuable achievement in replacing HAE-modified date powder in coffee products.
Table 3.
Comparison of color parameters between modified date powder as coffee substitute vs commercial instant coffee powder
| Sample name | L* | a* | b* |
|---|---|---|---|
| HAE treated date powder | 21.63 ± 2.55a | 19.68 ± 1.72b | 17.41 ± 1.60b |
| Commercial instant coffee | 22.04 ± 2.56a | 23.45 ± 1.06a | 21.71 ± 1.68a |
Different lowercase letters in each column denote significant differences (P < 0.05)
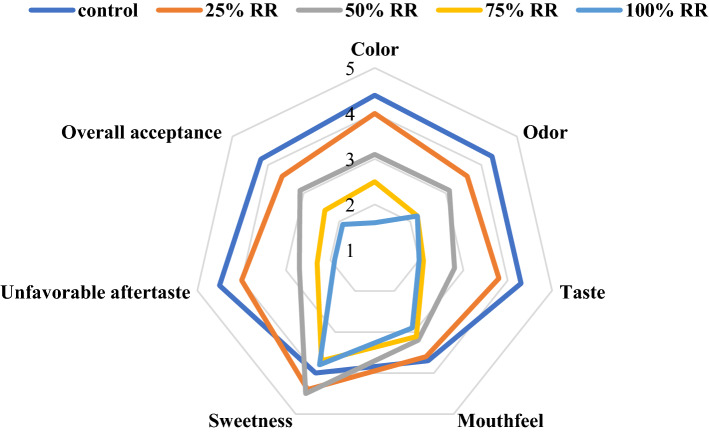
The results showed that the color of drinks did not differ significantly between the 25% replacement ratio and the commercial coffee powder (control sample) (P < 0.05) (Fig. 4), meaning that the formulation of 25% date powder + 75% coffee powder had an overall color similar to commercial coffee drinks. In contrast, the replacement ratios of 50, 75 and 100% were significantly different from the control sample (p < 0.05) and had a whitish cream color because of higher date powder ratios. Despite the similarity between the color characteristics of date powder, as a coffee substitute, and the commercial instant coffee powder, it was unfortunate that the date powder could not create a desirable color when used at high replacement ratios. Nonetheless, the 25% replacement ratio earned the highest color score from consumers and was descriptively ranked as similar to instant coffee, whereas the lowest score was attributed to the 100% replacement ratio.
Fig. 4.
The effect of replacement ratio (RR) of coffee powder by modified date powder on sensory characteristics of 3-in-1 coffee drink
In terms of aroma and odor characteristics, the sample with 25% date powder (i.e. the 25% replacement ratio) was not significantly different from the control sample (p > 0.05) (Fig. 4), meaning a promising result that could make feasible further use of date powder in a low amount (25%). To the consumer, the odor of this formulation was similar to control coffee drinks and, while the 50% replacement ratio was not significantly different from the 25% ratio in terms of odor, it was significantly different from the control sample. The 75 and 100% replacement ratios did not have the characteristic aroma of original coffee and were ranked 4th and 5th among the 5 samples, respectively. The highest aroma score was given to the control sample and the 25% ratio, together as one statistical conclusion, and the lowest score was attributed to the 100% replacement ratio. Similarly, the taste attribute earned a varied gradient of results, meaning that the 25% replacement ratio was not significantly different from the control sample in terms of taste (p > 0.05) and had a good mouthfeel in the opinion of consumers (Fig. 4). The highest taste score belonged to the control sample and the 25% replacement ratio, considered as one statistical conclusion, whereas the lowest score was given to the 100% replacement ratio. Taste scores significantly decreased in the 50, 75 and 100% replacement samples, probably due to the slightly salty taste and the presence of high sodium content in these samples, which was detected by some of the panelists. There was no significant difference among any of the five samples in terms of mouthfeel and sweetness (p > 0.05), probably because the same levels of sugar and coffee creamer were used in all formulas (Fig. 4). The results showed no significant difference in the unfavorable aftertaste index, when comparing the 25% replacement sample with the control sample (p > 0.05) (Fig. 4). An unfavorable aftertaste resulted from the 50, 75 and 100% replacement samples due to the presence of sodium, hence a slight salty taste in the samples. In the opinion of the consumers, after having these drinks, an undesirable aftertaste remained in the mouth. A comparison of overall acceptance showed that, among the samples, only the 25% replacement ratio was not significantly different from the control sample, while other replacement ratios did not achieve the desired overall acceptance score (Fig. 4). In ultimate ranking, the 25% replacement ratio was considered as the best sample from the perspective of consumers. Due to the high price of coffee, replacing 25% of coffee powder with date powder after its modification by the HAE method can be economically useful, while not affecting its sensory properties and ultimately reducing the price of the final product. Therefore, the date powder of the ‘Qasb’ date cultivar in this research can be a partial substitute for coffee powder after treating the date powder with the HAE method.
Conclusion
Many functional and physicochemical properties of food products can be modified by chemical and mechanical methods such as ultrasound and microwave irradiation. The AHP treatment alone or in combination with ultrasound or microwave increased the amount of total phenolic compounds, free radical scavenging activity of DPPH, total flavonoids and the color indices L* and b*. Furthermore, there was a decrease in flavonoids and flavonols, total sugar content and reducing sugars because of the AHP treatment, compared to the control sample of the date powder. The HAE treatment alone or in combination with ultrasound and microwave increased total phenolic compounds, DPPH free radical scavenging activity, total flavonoid content and the color index a*, while causing a decrease in flavone and flavonol, total sugars, reducing sugars, L* and b* indices. In general, the HAE treatment had better effects in improving the phytochemical and color features of date powder and was selected as the best method for modification. In testing the 3-in-1 coffee drink, it was observed that the replacement of up to 25% of coffee powder with the HAE-modified date powder had no adverse effect on sensory evaluations by the panelists. It seemed that replacing 25% of coffee powder with the modified date powder can increase the nutritional and phytochemical properties of the final product and also reduce the cost of coffee drinks. In future research, it is suggested that lower concentrations of date powder (less than 25%) be evaluated for the preparation of coffee formulations.
Abbreviations
- AHP
Alkaline hydrogen peroxide
- AHP-MW
Alkaline hydrogen peroxide-microwave
- AHP-U
Alkaline hydrogen peroxide-ultrasound
- AOAC
Association of official analytical collaboration
- CE
Catechin equivalent
- DPPH
2,2-Diphenyl-1-picrylhydrazyl
- HAE
Hydrochloric acid–ethanol
- HAE-MW
Hydrochloric acid–ethanol-microwave
- HAE-U
Hydrochloric acid–ethanol-ultrasound
- HCl
Hydrochloric acid
- H2O2
Hydrogen peroxide
- RGB
Red–green–blue
- NaOH
Sodium hydroxide
Author contributions
AHS: Conceptualization, investigation, methodology, project administration, writing—original draft preparation, writing—review and editing, supervision, RR: investigation, methodology, data curation and formal analysis, AG: investigation, writing—review and editing, supervision.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aguilar R, Ramırez J, Garrote G, Vázquez M. Kinetic study of the acid hydrolysis of sugar cane bagasse. J Food Eng. 2002;55(4):309–318. doi: 10.1016/S0260-8774(02)00106-1. [DOI] [Google Scholar]
- Aïder M, Martel A-A, Ferracci J, de Halleux D. Purification of whole brown flaxseed meal from coloring pigments by treatment in hydrogen peroxide solutions: impact on meal color. Food Bioprocess Technol. 2012;5(8):3051–3065. doi: 10.1007/s11947-011-0632-5. [DOI] [Google Scholar]
- Basanta MF, de Escalada Plá MF, Raffo MD, Stortz CA, Rojas AM. Cherry fibers isolated from harvest residues as valuable dietary fiber and functional food ingredients. J Food Eng. 2014;126:149–155. doi: 10.1016/j.jfoodeng.2013.11.010. [DOI] [Google Scholar]
- Bustos G, Ramírez JA, Garrote G, Vázquez M. Modeling of the hydrolysis of sugar cane bagasse with hydrochloric acid. Appl Biochem Biotechnol. 2003;104(1):51–68. doi: 10.1385/ABAB:104:1:51. [DOI] [PubMed] [Google Scholar]
- Chaira N, Mrabet A, Ferchichi A. Evaluation of antioxidant activity, phenolics, sugar and mineral contents in date palm fruits. J Food Biochem. 2009;33(3):390–403. doi: 10.1111/j.1745-4514.2009.00225.x. [DOI] [Google Scholar]
- De Wit G, Kieboom A, Van Bekkum H. Enolisation and isomerisation of monosaccharides in aqueous, alkaline solution. Carbohydr Res. 1979;74(1):157–175. doi: 10.1016/S0008-6215(00)84773-4. [DOI] [Google Scholar]
- George N, Andersson AA, Andersson R, Kamal-Eldin A. Lignin is the main determinant of total dietary fiber differences between date fruit (Phoenix dactylifera L.) varieties. NFS J. 2020;21:16–21. doi: 10.1016/j.nfs.2020.08.002. [DOI] [Google Scholar]
- Gfrerer M, Lankmayr E. Screening, optimization and validation of microwave-assisted extraction for the determination of persistent organochlorine pesticides. Anal Chim Acta. 2005;533(2):203–211. doi: 10.1016/j.aca.2004.11.016. [DOI] [Google Scholar]
- Ghani A, Mohtashami S, Jamalian S. Peel essential oil content and constituent variations and antioxidant activity of grapefruit (Citrus × paradisi var. red blush) during color change stages. J Food Meas Charact. 2021;15(6):4917–4928. doi: 10.1007/s11694-021-01051-0. [DOI] [Google Scholar]
- Gharibzahedi SMT, Smith B, Guo Y. Ultrasound-microwave assisted extraction of pectin from fig (Ficus carica L.) skin: optimization, characterization and bioactivity. Carbohydr Polym. 2019;222:114992. doi: 10.1016/j.carbpol.2019.114992. [DOI] [PubMed] [Google Scholar]
- Golmohamadi A, Möller G, Powers J, Nindo C. Effect of ultrasound frequency on antioxidant activity, total phenolic and anthocyanin content of red raspberry puree. Ultrason Sonochem. 2013;20(5):1316–1323. doi: 10.1016/j.ultsonch.2013.01.020. [DOI] [PubMed] [Google Scholar]
- Guo X, Ye X, Sun Y, Wu D, Wu N, Hu Y, Chen S. Ultrasound effects on the degradation kinetics, structure, and antioxidant activity of sea cucumber fucoidan. J Agric Food Chem. 2014;62(5):1088–1095. doi: 10.1021/jf404717y. [DOI] [PubMed] [Google Scholar]
- Hayat K, Zhang X, Farooq U, Abbas S, Xia S, Jia C, Zhong F, Zhang J. Effect of microwave treatment on phenolic content and antioxidant activity of citrus mandarin pomace. Food Chem. 2010;123(2):423–429. doi: 10.1016/j.foodchem.2010.04.060. [DOI] [Google Scholar]
- Iranmanesh S. The first compact book, introduction to applied technology of date production, storage, processing, packaging & export. Dayton: Aida Publishing; 2000. [Google Scholar]
- Jeyavishnu K, Thulasidharan D, Shereen MF, Arumugam A. Increased revenue with high value-added products from cashew apple (Anacardium occidentale L.)—addressing global challenges. Food Bioprocess Technol. 2021;14(6):985–1012. doi: 10.1007/s11947-021-02623-0. [DOI] [Google Scholar]
- Kammerer DR, Kammerer J, Valet R, Carle R. Recovery of polyphenols from the by-products of plant food processing and application as valuable food ingredients. Food Res Int. 2014;65:2–12. doi: 10.1016/j.foodres.2014.06.012. [DOI] [Google Scholar]
- Komes D, Bušić A, Vojvodić A, Belščak-Cvitanović A, Hruškar M. Antioxidative potential of different coffee substitute brews affected by milk addition. Eur Food Res Technol. 2015;241(1):115–125. doi: 10.1002/fsn3.2268. [DOI] [Google Scholar]
- Meng X, Liu F, Xiao Y, Cao J, Wang M, Duan X. Alterations in physicochemical and functional properties of buckwheat straw insoluble dietary fiber by alkaline hydrogen peroxide treatment. Food Chem X. 2019;3:100029. doi: 10.1016/j.fochx.2019.100029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu Y, Li N, Xia Q, Hou Y, Xu G. Comparisons of three modifications on structural, rheological and functional properties of soluble dietary fibers from tomato peels. LWT. 2018;88:56–63. doi: 10.1016/j.lwt.2017.10.003. [DOI] [Google Scholar]
- Platat C, Habib H, Al Maqbali F, Jaber N, Ibrahim W. Identification of date seeds varieties patterns to optimize nutritional benefits of date seeds. J Nutr Food Sci. 2014 doi: 10.4172/2155-9600.S8-008. [DOI] [Google Scholar]
- Popova M, Bankova V, Butovska D, Petkov V, Nikolova-Damyanova B, Sabatini AG, Marcazzan GL, Bogdanov S. Validated methods for the quantification of biologically active constituents of poplar-type propolis. Phytochem Anal Int J Plant Chem Biochem Tech. 2004;15(4):235–240. doi: 10.1002/pca.777. [DOI] [PubMed] [Google Scholar]
- Putnik P, Bursać Kovačević D, Radojčin M, Dragović-Uzelac V. Influence of acidity and extraction time on the recovery of flavonoids from grape skin pomace optimized by response surface methodology. Chem Biochem Eng Q. 2016;30(4):455–464. doi: 10.15255/CABEQ.2016.914. [DOI] [Google Scholar]
- Şansal Ü, Somer G. Detection of H2O2 in food samples by FTIR. Food Chem. 1999;65(2):259–261. doi: 10.1016/S0308-8146(98)00224-6. [DOI] [Google Scholar]
- Swallah MS, Sun H, Affoh R, Fu H, Yu H. Antioxidant potential overviews of secondary metabolites (polyphenols) in fruits. Int J Food Sci. 2020 doi: 10.1155/2020/9081686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tareen AK, Punsuvon V, Parakulsuksatid P. Investigation of alkaline hydrogen peroxide pretreatment to enhance enzymatic hydrolysis and phenolic compounds of oil palm trunk. 3 Biotech. 2020;10(4):1–12. doi: 10.1007/s13205-020-02169-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadivel V, Brindha P. Antioxidant property of solvent extract and acid/alkali hydrolysates from rice hulls. Food Biosci. 2015;11:85–91. doi: 10.1016/j.fbio.2015.06.002. [DOI] [Google Scholar]
- Wojdyło A, Oszmiański J, Czemerys R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007;105(3):940–949. doi: 10.1016/j.foodchem.2007.04.038. [DOI] [Google Scholar]
- Xiao W, Han L, Shi B. Microwave-assisted extraction of flavonoids from Radix Astragali. Sep Purif Technol. 2008;62(3):614–618. doi: 10.1016/j.seppur.2008.03.025. [DOI] [Google Scholar]
- Yang BY, Montgomery R. Alkaline degradation of glucose: effect of initial concentration of reactants. Carbohydr Res. 1996;280(1):27–45. doi: 10.1016/0008-6215(95)00294-4. [DOI] [Google Scholar]
- Yoshida BY, Prudencio SH. Alkaline hydrogen peroxide improves physical, chemical, and techno-functional properties of okara. Food Chem. 2020;323:126776. doi: 10.1016/j.foodchem.2020.126776. [DOI] [PubMed] [Google Scholar]
- Zhang W, Zeng G, Pan Y, Chen W, Huang W, Chen H, Li Y. Properties of soluble dietary fiber-polysaccharide from papaya peel obtained through alkaline or ultrasound-assisted alkaline extraction. Carbohydr Polym. 2017;172:102–112. doi: 10.1016/j.carbpol.2017.05.030. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Qi J, Zeng W, Huang Y, Yang X. Properties of dietary fiber from citrus obtained through alkaline hydrogen peroxide treatment and homogenization treatment. Food Chem. 2020;311:125873. doi: 10.1016/j.foodchem.2019.125873. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Not applicable.