Abstract
Nisin, a bacteriocin widely used in the food industry, and curcumin, the yellow pigment extracted from turmeric (Curcuma longa L.) stand out among the numerous natural preservatives that have antimicrobial activity. The conversion of these compounds into nanoparticles could be interesting as an alternative to improve technological aspects (such as the low water solubility of curcumin) and to evaluate how synergism could take place in the case of co-encapsulation. The main objective of the present work was to evaluate the combination of nisin (Nis) with nanoencapsulated curcumin (NCur, nanoencapsulated to promote water solubility), as well as the co-encapsulated curcumin and nisin (NCurNis), against the foodborne bacteria Staphylococcus aureus, Escherichia coli and Salmonella Typhimurium. Minimum inhibitory concentration and the minimum bactericidal concentration were evaluated for NCur and Nis, as well as their combination with the fractional inhibitory concentration assay. High effectiveness was found against S. aureus and the combination of both compounds resulted in Nis- nisin; synergism against the same microorganism. The co-encapsulation of curcumin and nisin was carried out based on the synergism tests and the characterization analyses demonstrated that a solid dispersion of the components in the PVP matrix was formed. The inhibitory effect of the curcumin and nisin co-encapsulate was improved when compared to the curcumin nanoparticles or nisin alone.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13197-022-05641-8.
Keywords: Solid dispersion, Co-encapsulation, Synergism, Natural antimicrobials, Foodborne pathogens
Introduction
Pathogenic bacteria and viruses are responsible for the largest number of outbreaks of foodborne illnesses worldwide (Souza et al. 2021). Among the most common foodborne pathogens, Staphylococcus aureus is known by its difficulty to be eliminated from the environment, which can cause food poisoning due to the ability of enterotoxigenic strains to produce staphylococcal enterotoxins in food (Zhao et al. 2017). Also, Salmonella Typhimurium is one of the main serotypes responsible for human and animal salmonellosis worldwide, and Escherichia coli can harmlessly colonize the human intestine or cause intestinal or extra-intestinal infections, including serious invasive diseases (Pakbin et al. 2021).
Foodborne diseases are caused by contamination of food and may occur at any stage of the food production, delivery, and consumption chain. They can result from several forms of environmental contamination including pollution in water, soil, or air, as well as unsafe food storage and processing (World Health Organization 2022). More and more attention has been paid to treatment with biopreservatives that may prolong shelf life and improve food safety, thus minimizing the negative effect on nutritional and flavor properties caused by contamination (Chen et al. 2020). Among such biopreservatives, nisin is a bacteriocin naturally produced by Lactococcus lactis subsp. Lactis that exhibits multiple antimicrobial actions mainly against Gram-positive bacteria: it binds to the precursor of peptidoglycan and lipid II to inhibit cell wall biosynthesis, then forms pores within the cell membrane that lead to the release of essential ions and, ultimately, to cell death (Khan and Oh 2016). Also, among the natural compounds that present antimicrobial activity, curcumin is a natural diphenolic pigment extracted from turmeric (Curcuma longa L.) with a wide range of biological activities (Almeida et al. 2018). Curcumin presents low water solubility in its native state, which reduces curcumin applicability in many food systems, and nanoencapsulation is an alternative to overcome this problem (Lemes et al. 2017).
Natural compounds that may act against foodborne bacteria are in great demand for both academia and industry and in this context, it is worth evaluating the existence of combination effects (synergism, addition, indifference, or antagonism) of nisin and curcumin. It is possible to assess the in vitro interaction of antimicrobial combinations to determine whether the effect of two antimicrobials is greater than the sum of their activities (synergism) or is simply the sum of the two antimicrobials acting together (addition) (Doern 2014). Sharma et al. (2020) evaluated the combination effect between curcumin and nisin against E. coli and S. epidermidis and found a synergic effect against both bacteria. Considering the importance of this area of research, additional studies are necessary to further explore the effect of the association of these two compounds on their antibacterial activity. For instance, co-encapsulation techniques have been used in diverse fields with promising results and could be applied to these compounds.
Co-encapsulation of bioactive compounds is an emerging field that may allow the development of food products taking into account the synergistic effect of multiple bioactives to target specific health and safety benefits (Chawda et al. 2017). Curcumin and nisin were already co-encapsulated by other authors in nanocarriers systems such as poly(lactic acid) nanoparticles produced by double emulsion (Oyeyemi et al. 2018), electrospun poly(vinyl alcohol) (Meral et al. 2019) and nisin complexation with soluble soybean polysaccharide followed by incorporation of curcumin (Luo et al. 2021). Curcumin alone was nanoencapsulated by the solid dispersion technique and evaluated for its antimicrobial activity by Almeida et al. (2018) against Gram-positive (methicillin-resistant Staphylococcus aureus (MRSA), methicillin-sensitive Staphylococcus aureus (MSSA), Enterococcus faecalis and Listeria monocytogenes), and Gram-negative (Escherichia coli, Escherichia coli ESBL, Klebsiella pneumoniae, Klebsiella pneumoniae ESBL, Morganella morganii, and Pseudomonas aeruginosa) bacteria. The authors found a MIC of 0.5 mg mL−1 against MSSA and MRSA, indicating an antibacterial activity against resistant microorganisms.
In this work, the combined effect of nisin and nanoencapsulated curcumin on the antibacterial activity, namely against S. aureus, E. coli, and S. Typhimurium were evaluated. Also, a co-encapsulated formulation of curcumin and nisin was produced, taking into account the synergism result. The in vitro antibacterial analyzes were correlated to the characterization results of the nanoparticles.
Materials and methods
Materials
Curcumin (from Curcuma longa L. powder, ≥ 65% purity, Sigma-Aldrich, St. Louis, MO, USA), polyvinylpyrrolidone (PVP, 40,000 g mol−1, Sigma-Aldrich,), Tween 80 (Dinâmica, Diadema, SP, Brazil), ethanol (99.5%, Neon, Suzano, SP, Brazil), nisin (2.5% Sigma-Aldrich, St. Louis, MO, USA), BHI broth (Sigma-Aldrich, St. Louis, MO, USA), Mueller Hinton broth and agar (Biomark Laboratories, Pune, India), Hektoen Agar (Himedia, Mumbai, India), Baird Parker Agar (Himedia, Mumbai, India) and Eosin Methylene Blue Agar (EMB, Himedia, Mumbai, India). KBr (spectroscopic grade, Sigma Aldrich, St. Louis, MO, USA) was used in the infrared spectroscopy analyses. Carbon-coated copper grids (300 mesh, Electron Microscopy Sciences, Hatfield, PA, USA) were used in the microscopy analyses.
Microorganisms
The strains of Salmonella Typhimurium (ATCC 14,028), Staphylococcus aureus (ATCC 25,923), and Escherichia coli (ATCC 25,922) were stored in Mueller Hinton agar (MHA) in a freezer at − 14 °C. For microbial reactivation, a 10 μL aliquot of each bacterium was inoculated in Brain Heart Infusion (BHI) broth and incubated for 24 h at 37 °C. After adjusting the turbidity of the medium, the inoculation was carried out by the Streak Plate method (Hektoen, Baird Parker, and EMB, for S. Typhimurium, S. aureus, and E. coli, respectively) and incubated again for 24 h at 37 °C. After this period, 3 colonies of each bacterium were inoculated in Mueller Hinton broth and incubated for 6 h at 37 °C for testing.
Production of nanoencapsulated curcumin by solid dispersion
Curcumin encapsulation and curcumin-nisin co-encapsulation were carried out using the solid dispersion technique as described by Almeida et al. (2018) with minor modifications. For the curcumin-loaded nanoparticles (NCur), PVP (100 mg) and Tween 80 (10 mg) were dissolved in ethanol (30 mL) under magnetic stirring until a translucent solution was obtained. After that, curcumin (10 mg) was added to this solution, remaining under stirring for 5 min. The mixture was then sonicated for 5 min in a pulsed condition (30 s on/10 s off) at 120 W in an ice bath and using a 1/8″ tip (Fisher Scientific, Loughborough, UK). Finally, the solvent was evaporated in an air circulation oven (Cienlab, Campinas, SP, Brazil) at 40 °C for 4 h and the resulting solid was recovered and ground with a mortar and pestle. This resulted in a 8.3%wt curcumin concentration in the solid dispersion.
The same procedure was used to co-encapsulate nisin and curcumin, with nisin (13.6 mg) being added in the same step as curcumin (10 mg). In this procedure, 100 mg of PVP and 10 mg of Tween 80, and 30 mL of absolute ethanol were used to ensure the encapsulation of both compounds. The ratio between curcumin and nisin was defined based on the results of the synergism tests. In these nanoparticles, curcumin and nisin concentration was 7.5 and 10.2%wt in the solid dispersion, respectively. It is worth pointing out that the amount of curcumin and nisin was standardized in all bacterial tests in order to allow comparison.
Nanoparticles characterization
The samples analyzed were NCur and NCurNis nanoparticles and the pure components (PVP, nisin, and curcumin). Also, physical mixtures of curcumin and PVP (MFCurPVP) and curcumin, nisin, and PVP (MFCurNisPVP) were obtained by simply mixing the components in the same proportion found in the nanoparticles to compare the results. For the Fourier Transform Infrared spectroscopy (FTIR, Shimadzu IR AFFINITY-1, Tokyo, Japan), samples were dispersed in KBr and then pelletized in a hydraulic press. Spectra were collected from 400 to 4000 cm−1 with 32 accumulations and 4 cm−1 resolution. Thermogravimetric Analysis (TGA) and Differential Scanning Calorimetry (DSC) analyzes were performed in a simultaneous thermal analyzer (STA 6000, PerkinElmer, USA). Samples (8 to 10 mg) were placed in a platinum sample holder and heated to 600 °C at 10 °C min−1 under a gaseous nitrogen atmosphere (50 mL min−1). The morphology of the nanoparticles was evaluated by Transmission Electron Microscopy at 200 kV (TEM, JEM 2100, JEOL, Peabody, MA, USA). Shortly after the production of the nanoparticles, a sample of the material was redispersed in water (0.1% wt/v) and dropped onto the copper grids and kept in a dry atmosphere until the moment of analysis. The mean size in intensity (Dz, nm) and the polydispersity index (PDI, dimensionless) of the nanoparticles were determined by Dynamic Light Scattering (DLS, Malvern Zetasizer–Nano Series instrument, Malvern, UK). The dried samples were redispersed in water (0.1% wt/v) and then analyzed in triplicate. Results were submitted to Student's t-test (p < 0.05) (Matlab, R2021a, Mathworks).
Determination of minimum inhibitory and minimum bactericidal concentrations
Initially, a stock solution of nisin was prepared in 0.02 M hydrochloric acid (HCl) and sterilized in a 0.22 μm membrane (Millipore, São Paulo, Brazil). Nanoencapsulated curcumin was solubilized in distilled water. The broth microdilution method established by the Clinical and Laboratory Standards Institute (2012) was used in the microbiological evaluation. Bacteria E. coli, S. aureus, and S. Typhimurium were used to determine the Minimum Inhibitory Concentration (MIC). The range of tested concentrations were 2000–0.94 ug/mL for Nis and 100–0.375 mg/mL for NCur, which contains 8.3%wt of pure curcumin in the encapsulate. The bacterial suspensions were standardized according to the 0.5 McFarland scale and diluted in saline solution at 0.85% to obtain 5 × 105 CFU mL−1. Each sample was added (100 μL) at an initial nanoparticles concentration of 100 mg mL−1; also, 2 mg mL−1 pure nisin was added to Mueller Hinton broth, diluted in each well of the 96 wells of the plate. A 10 μL aliquot of the bacterial suspension was then inoculated into each well and incubated at 37 °C for 24 h in a bacteriological culture incubator (FANEM, 002CB, Guarulhos, SP, Brazil). MIC was defined as the lowest concentration that visually inhibited bacterial growth. In the case of the Minimum Bactericidal Concentration (MBC), a 20 μL aliquot from the wells in which there was no microbial growth detected (in the MIC assay) was transferred to a Mueller Hinton Agar Plate. Plates were incubated at 37 °C for 24 h, and MBC was defined as the lowest concentration that did not allow bacterial growth. All tests were performed in triplicate (three independent and triplicate experiments were carried out).
Determination of the antibacterial interaction between nisin and nanoencapsulated curcumin
The assay was performed by determining the fractional inhibitory concentration (FIC) in MHB broth using the microdilution checkerboard method in a microtiter plate scheme (CLSI 2012). The FIC was calculated as follows:
Antibacterial interaction is interpreted as synergism (FIC < 0.5), addition (0.5 ≤ FIC ≤ 1), indifference (1 < FIC ≤ 4) or antagonism (FIC > 4).
Results and discussion
Nanoparticles characterization
FTIR spectra (Fig. 1) were used here to evaluate the interactions between the encapsulated compounds (curcumin or curcumin and nisin) and the encapsulant (PVP). The band related to the hydroxyl group, carbon double bonds, C–O–C bonds, alkene groups and -CH asymmetric stretch were found at 3510 cm−1 and 1512 cm−1 1026 cm−1 1628 cm−1 1429 cm−1in the spectra of curcumin, respectively. Similar results were reported in the literature (Lemes et al. 2017). For pure nisin, bands at 1647 cm−1 (amide I C=O), 1535 cm−1 (amide II N–H), and 2962 cm−1 (–CH stretching vibration) were found while PVP presented characteristic bands at 3454 cm−1 (–OH stretching), 2960 cm−1 (–CH2 asymmetric stretching), 1285 cm−1 (–CH2) and 1673 cm−1 (C=O stretching) (Kamaruddin et al. 2017).
Fig. 1.
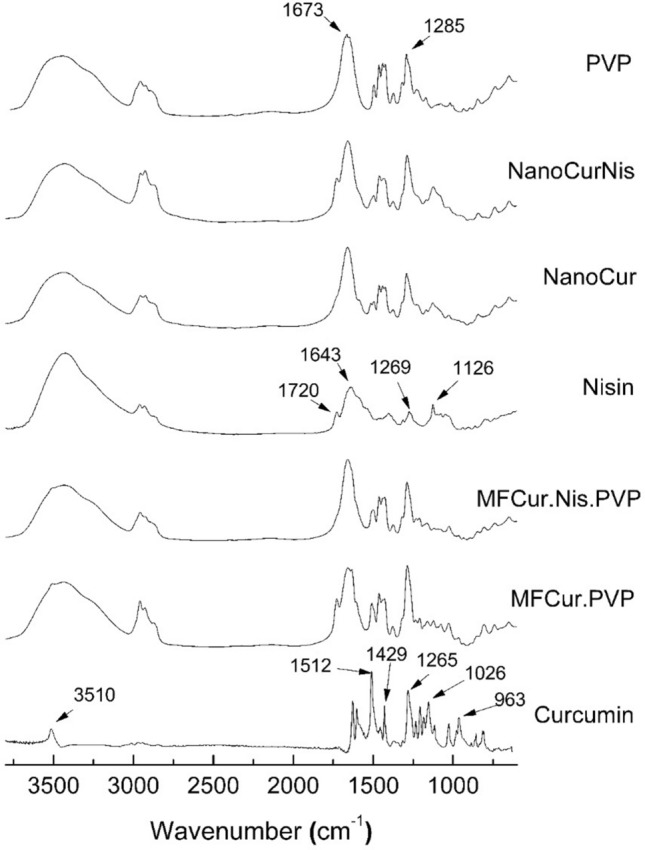
FTIR spectra: pure curcumin, nisin, and PVP; physical mixtures of nanoparticles components (MF.Cur.PVP; MF.Cur.Nis.PVP); and curcumin-loaded nanoparticles (NCur) and curcumin-nisin-loaded nanoparticles (NCurNis)
Absorption bands of the nanoparticles containing curcumin (NCur) were attenuated when compared to the physical mixture, mostly bands related to C–O–C of ethers, C=C of the aromatic ring and –CH asymmetric stretching. This effect is often attributed to the encapsulant coverage over the encapsulated substances. In addition, it may be noted that the band located in the region of 3450 cm−1 presented lower intensity for the NCur sample. This behavior is indicative of the interaction between the encapsulating agent and the encapsulated compound by hydrogen bonds (Karavas et al. 2006; Lemes et al. 2017).
In the case of co-encapsulated curcumin and nisin nanoparticles (NCurNis), the characteristic band located at 1647 cm−1 (C=O of amide I) was superimposed by the band relative to the C=O stretch (1673 cm−1) of the PVP. Even so, it is possible to notice the presence of both bands in the physical mixture (MF.Cur.Nis.PVP) and not in the nanoparticles, suggesting the interaction between the compounds after the nanoencapsulation (Lemes et al. 2017).
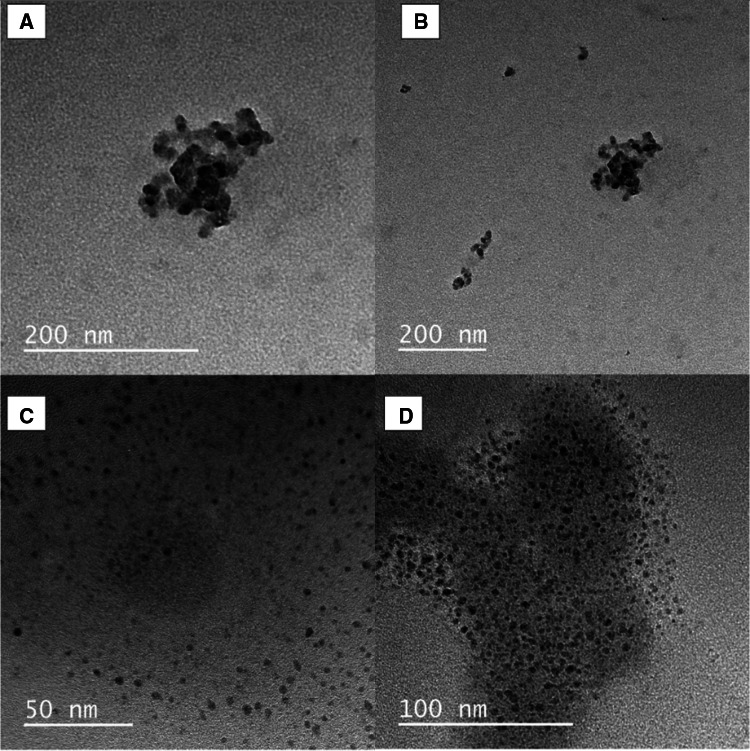
The images obtained by Transmission Electron Microscopy are presented in Fig. 2.
Fig. 2.
Transmission Electron Microscopy Images: A and B NCur; C and D NCurNis
For the NCur sample, nanoparticles with dimensions around 12 nm were clustered forming structures with approximately 160 nm. For the NCurNis, no aggregates of nanoparticles were observed, and their size was determined between 4 and 10 nm. Khan and Rathod (2014) produced curcumin nanoparticles using PVP as encapsulant, obtaining particle sizes similar to one determined for the NCur clusters. There are no reports in the literature on the nanoencapsulation of nisin in PVP by solid dispersion, however, it was possible to verify that there was the formation of nanoparticles and that these present a fairly homogeneous sizes distribution. Overall, images confirmed the formation of nanometric structures with morphology close to spherical for the two formulations (NCur and NCurNis). Also, clustering was apparently caused by the presence of nisin leading to cload-like aggregated structures.
Mean diameter values determined by DLS were 610 ± 28 nm for the NCur and 507 ± 20 nm for the NCurNis. These values are statistically different (p = 0.0067) and agreed with the TEM images that showed the larger sizes of NCur. DLS results are greater than those observed in the TEM images probably due to the agglomeration during oven drying and redispersion in water. The PDI determined for the samples was 0.55 ± 0.03 and 0.67 ± 0.02 for NCurNis and NCur, respectively, showing a statistically significant difference between them (p = 0.0061). PDI results are indicative of the width of the particle size distribution, the wider the distribution the more polydisperse the sample (Kaszuba et al. 2008).
The DSC and TGA curves are shown in Figures S1 and S2, respectively, and the temperatures and respective weight losses (%) are highlighted in Table 1.
Table 1.
Temperatures associated with weight loss (WL) determined by TGA
| Sample | Initial WL | First stage of degradation | Second stage of degradation | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ton set (°C) | Tmax (°C) | WL (%) | Ton set (°C) | Tmax (°C) | WL (%) | Ton set (°C) | Tmax (°C) | WL (%) | Total WL (%) | |
| PVP | – | 65 | 8 | 370 | 434 | 87 | – | – | – | 95 |
| Curcumin | – | – | – | 250 | 287 | 11 | 330 | 380 | 31 | 42 |
| Nisin | – | – | – | 270 | 302 | 5 | 500 | 580 | 4 | 9 |
| MFCurPVP | – | 79 | 10 | 373 | 436 | 82 | – | – | – | 92 |
| NCur | – | 70 | 6 | 209 | 303 | 59 | – | – | – | 65 |
| MFCurNisPVP | – | 116 | 12 | 360 | 430 | 76 | – | – | – | 88 |
| NCurNis | – | 61 | 8 | 276 | 287 | 4 | 367 | 435 | 72 | 84 |
In the thermogram of pure curcumin, a crystalline melting peak (Tm) at 171 °C was observed (Lemes et al. 2017), and for pure PVP the thermal degradation was detected at 427 °C (Voronova et al. 2018). For the physical mixture of curcumin and PVP (MFCurPVP), the maximum degradation peak occurred at the same temperature found for pure PVP, however in curcumin nanoparticles (NCur) the melting of curcumin was not detected. This behavior strongly indicates the formation of solid dispersion in which the two components (curcumin and PVP) are in amorphous state (Karavas et al. 2006).
The glass transition temperature (Tg) of nisin is reported in the range of 30 °C (Niaz et al. 2018a). However, it was not possible to detect the Tg in the analysis. Also, the degradation temperature of PVP in the physical mixture (MFCurNisPVP) and nisin-curcumin nanoparticles (NCurNis) was the same found on pure PVP. Possibly this result is an indicative of weak interaction between the components, or inadequate dispersion of nisin and curcumin in PVP (Karavas et al. 2006).
The thermal decomposition of pure curcumin occurred between 250 °C and 415 °C. Similar results were found by Rosa et al. (2021), who verified the ranges of thermal decomposition of curcumin between 283 °C and 363 °C and Almeida et al. (2018) with similar weight loss. In the case of pure nisin, also two stages of degradation were found starting at 270 °C and at 500 °C. Niaz et al. (2018b) found a thermal decomposition range for nisin between 200 °C and 300 °C. Also, Gruzkiene et al. (2021) determined one main degradation step with the major degradation peak at 286 °C.
PVP, physical mixtures (MF.Cur.PVP and MF.Cur.Nis.PVP), and co-encapsulated curcumin-nisin nanoparticles presented similar weight loss behavior. The initial stage of weight loss may be associated with water evaporation due to the hydrophilic nature of PVP (Almeida et al. 2018). The physical mixtures showed weight loss events near 100 °C, 400 °C, and finally 500 °C. NCur and NCurNis showed weight loss between 100 °C and 200 °C, however, only NCurNis presented a second stage close to 400 °C, similar to PVP and the physical mixtures. This behavior corroborates with DSC analysis results and indicates the formation of a solid dispersion where the two components (curcumin and PVP) are completely miscible and in amorphous form (Karavas et al. 2006).
Antimicrobial activity and combination of nanoencapsulated curcumin with nisin
Table 2 presents the MIC and MBC for curcumin nanoparticles also for nisin.
Table 2.
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) for nisin and curcumin nanoparticles for Salmonella Typhimurium, S. aureus, and E. coli
| Microorganism | NCur | Nis | ||
|---|---|---|---|---|
| MIC (mg.mL−1) | MBC (mg.mL−1) | MIC (mg.mL−1) | MBC (mg.mL−1) | |
| Salmonella Typhimurium | 6.25 | 6.25 | 1.00 | 1.00 |
| S. aureus | 0.75 | 0.75 | 0.50 | 1.00 |
| E. coli | 3.12 | 3.12 | 0.50 | 2.00 |
Both curcumin nanoparticles and nisin showed lower MICs against the S. aureus bacteria when compared to the other two bacteria tested. It was found that nisin was more effective against S. aureus, with a MIC of 0.50 mg mL−1 and MBC of 1.00 mg mL−1. Against Gram-negative bacteria, nisin has already been reported to present rupture in the bacterial outer membrane, however, bacteriocins produced by Gram-positives do not have bactericidal action against Gram-negatives (Furlanetto 2020). However, in the present work, bactericidal concentrations were identified for both Gram-negative bacteria tested (1.0 mg mL−1 for S. Typhimurium and 2.0 mg mL−1 for E. coli). Yeluri Jonnala et al. (2021) describe in their studies that the activity of nisin against Gram-negative bacteria is decreased (and not null) due to the presence of LPS in the outer membrane, which acts as a barrier and restricts the entry of the peptide nisin.Furthermore Field et al. (2012), suggested increased activity of nisin derivatives against Gram-negative bacteria due to an increased ability of nisin to cross the outer membrane, and that nisin exhibits antimicrobial activity against a variety of food-associated Gram-negative pathogens, including E. coli, Salmonella enterica serovar Typhimurium, and Cronobacter sakazakii.
Curcumin and formulations containing curcumin nanoparticles have already shown remarkable antimicrobial activity against S. aureus, as described by Taghavifar et al. (2020), who found that curcumin nanoparticles improved diabetic wounds infected with S. aureus MRSA (methicillin-resistant S. aureus) and Ma et al. (2020), who reported an excellent antibiofilm activity of curcumin nanoparticles against S. aureus.
In the present work, curcumin nanoparticles had better antibacterial activity against S. aureus, which differs from the work by Tyagi et al. (2015) who found that free curcumin was equally effective against S. aureus and E. coli. It is noteworthy that in the present work curcumin was colloidally dispersed in the water while it was fully solubilized in dimethyl sulfoxide in the work by Tyagi and coworkers, meaning that in actual use conditions the encapsulated curcumin may present better action. Leyva-Diaz et al. (2020) evaluated the impact of dietary curcumin supplementation in chickens infected with Salmonella Typhimurium, reporting a significant reduction in intestinal colonization. In the present work, both curcumin and nisin reduced antibacterial activity against Salmonella Typhimurium when compared to the other two bacteria tested.
The combined effect between nisin and the curcumin nanoparticles was evaluated against the three microorganisms and the results are shown in Table 3.
Table 3.
Synergism between antibacterial activity of Nisin (Nis–Compound A, 2 mg mL−1) and PVP nanoparticles containing curcumin (NCur–Compound B, 50 mg mL−1)
| Microorganism | MIC A | MIC B | FIC A + B | FIC B + A | FIC | Interpretation |
|---|---|---|---|---|---|---|
| Salmonella Typhimurium | 2.00 | 6.25 | 2.00 | 1.56 | 1.26 | Indifference |
| S. aureus | 0.06 | 0.75 | 0.02 | 0.01 | 0.26 | Synergism |
| E. coli | 1.00 | 6.25 | 0.06 | 6.25 | 1.06 | Indifference |
Curcumin nanoparticles showed synergism with nisin only when tested against S. aureus. Zhao et al. (2017) pointed out that nisin has an effective broad-spectrum against Gram-positive pathogens and bacteriostatic activity against bacterial spores, in addition to synergistic with a wide range of antimicrobial agents, so the combined use of curcumin and nisin nanoparticles can enhance the antibacterial action against this type of microorganism of both components tested. Takundwa et al. (2021) evaluated the antimicrobial activity of nisin and oregano essential oil combination and attributed the synergism to the acidic pH of antimicrobial solutions, reporting that acidification was facilitated by nisin and oregano essential oil, which have a pH lower when combined, resulting in better synergistic action against E. coli.
The concentration of nisin and curcumin for co-encapsulation was based on the concentrations found in the synergism test, for S. aureus being 13.6 mg mL−1 of nisin and 10 mg mL−1 of curcumin. The values found for MIC and MBC of the NCurNis were the same and, it is worth pointing out that the NCurNis had a MIC of 0.064 mg/mL showing a better inhibitory effect, when comparing the NCur and pure nisin, demonstrating that co-encapsulation of curcumin and nisin is a reliable alternative in the development of antibacterial nanocompounds.
Conclusions
The combination of nisin with nanoencapsulated curcumin against the foodborne bacteria S. aureus, E. coli, and Salmonella Typhimurium was evaluated. Images confirmed the formation of nanometric particles with spherical shapes. Components formed an amorphous structure with improved thermal stability since the degradation events were retarded after the encapsulation. In the case of the nisin-curcumin-loaded nanoparticles, FTIR analysis indicated the presence of chemical interactions between the components of the nanoparticles.
The inhibitory activity of nisin-curcumin-loaded PVP nanoparticles was satisfactory against the three bacteria tested. The antibacterial effect of both compounds had a better activity, in addition to showing synergism when tested against S. aureus. These nanoparticles showed excellent inhibitory activity when compared to nisin and curcumin nanoparticles. These results demonstrated that solid dispersions containing curcumin and nisin may act together against microorganisms, opening a wide range of possibilities of use in the food industry.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001. The authors thank the “Central Analítica Multiusuário da UTFPR Campo Mourão” (CAMulti-CM) for the analyses. Authors thank to CNPq (process number 406966/2021-4, Chamada CNPq/MCTI/FNDCT Nº 18/2021—Faixa A—Grupos Emergentes, and process number 310052/2021-1, Chamada CNPq Nº 4/2021—Bolsas de Produtividade em Pesquisa – PQ).
Abbreviations
- BHI
Brain heart infusion
- EMB
Eosin methylene blue agar
- ESBL
Extended-spectrum beta-lactamases
- DLS
Dynamic light scattering
- DSC
Differential scanning calorimetry
- Dz
Mean intensity diameter
- FIC
Fractional inhibitory concentration
- FTIR
Fourier transform infrared spectroscopy
- HCl
Hydrochloric acid
- KBr
Potassium bromide
- MBC
Minimum bactericidal concentration
- MFCurNisPVP
Physical mixture of curcumin, nisin, and polyvinylpyrrolidone
- MFCurPVP
Physical mixture of curcumin and polyvinylpyrrolidone
- MHA
Mueller hinton agar
- MHB
Mueller hinton broth
- MIC
Minimum inhibitory concentration
- MRSA
Methicillin-resistant Staphylococcus aureus
- MSSA
Methicillin-susceptible Staphylococcus aureus
- NCur
Nanoencapsulated curcumin
- NCurNis
Co-encapsulated curcumin and nisin
- Nis
Nisin
- PDI
Polydispersity index
- PVP
Polyvinylpyrrolidone
- TEM
Transmission electron microscopy
- TGA
Thermogravimetric analysis
Author contributions
MBQ: Investigation, Formal analysis, Writing—Original Draft. TFMM: Investigation, Formal analysis, Writing—Original Draft. AO: Investigation, Formal analysis, Writing—Original Draft. ASC: Investigation, Formal analysis, Writing—Original Draft. JLM: Investigation, Formal analysis, Writing—Original Draft. OHG: Conceptualization, Supervision, Writing—Review & Editing. BAAF: Conceptualization, Supervision, Writing—Review & Editing. FVL: Conceptualization, Supervision, Project administration, Funding acquisition, Writing—Review & Editing.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001 and CNPq (process number 406966/2021-4, Chamada CNPq/MCTI/FNDCT Nº 18/2021-Faixa A—Grupos Emergentes, and process number 310052/2021-1.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Benício Alves de Abreu Filho, Email: baafilho@uem.br.
Fernanda Vitória Leimann, Email: fernandaleimann@utfpr.edu.br.
References
- Gruskiene R, Kavleiskaja T, Staneviciene R, et al. (2021) Nisin-loaded ulvan particles: preparation and characterization. 10.3390/foods10051007 [DOI] [PMC free article] [PubMed]
- Almeida HHS, Barros L, Barreira JCM, et al. Bioactive evaluation and application of different formulations of the natural colorant curcumin (E100) in a hydrophilic matrix (yogurt ) Food Chem. 2018;261:224–232. doi: 10.1016/j.foodchem.2018.04.056. [DOI] [PubMed] [Google Scholar]
- Chawda PJ, Shi J, Xue S, Quek SY. Co-encapsulation of bioactives for food applications. Food Qual Saf. 2017;1(4):302–309. doi: 10.1093/fqsafe/fyx028. [DOI] [Google Scholar]
- Chen L, Song Z, Tan SY, et al. Application of bacteriocins produced from lactic acid bacteria for microbiological food safety. Curr Topic Lactic Acid Bact Probiotics. 2020;6:1–8. doi: 10.35732/ctlabp.2020.6.1.1. [DOI] [Google Scholar]
- CLSI (2012) Performance standards for antimicrobial susceptibility testing; twenty-second informational supplement
- da Rosa FC, Nogueira AL, Deretti O. Caracterização de micropartículas de plla contendo curcumina pela técnica de emulsificação/evaporação de solvente/characterization of plla microparticles containing curcumin by solvent emulsification/evaporation technique. Braz J Dev. 2021;7(3):28837–28847. doi: 10.34117/bjdv7n3-548. [DOI] [Google Scholar]
- Doern CD. When does 2 plus 2 equal 5? A review of antimicrobial synergy testing. J Clin Microbiol. 2014;52:4124–4128. doi: 10.1128/JCM.01121-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field D, Begley M, O’Connor PM, et al. Bioengineered nisin a derivatives with enhanced activity against both gram positive and gram negative pathogens. PLoS ONE. 2012;7:e46884. doi: 10.1371/journal.pone.0046884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlanetto A. Síntese de nanoemulsão e nanopartícula de ouro (AuNPs) contendo nisina e seus efeitos sobre os fatores de virulência de Staphylococcus aureus. Universidade Estadual Paulista Julio de Mesquita Filho; 2020. [Google Scholar]
- Kamaruddin ED, Sriyanti I, et al. Synthesis of polyvinylpyrrolidone (PVP)-green tea extract composite nanostructures using electrohydrodynamic spraying technique. IOP Conf Ser Mater Sci Eng. 2017;202:1–8. doi: 10.1088/1757-899X/202/1/012043. [DOI] [Google Scholar]
- Karavas E, Ktistis G, Xenakis A, Georgarakis E. Effect of hydrogen bonding interactions on the release mechanism of felodipine from nanodispersions with polyvinylpyrrolidone. Eur J Pharm Biopharm. 2006;63:103–114. doi: 10.1016/j.ejpb.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Kaszuba M, McKnight D, Connah MT, et al. Measuring sub nanometre sizes using dynamic light scattering. J Nanopart Res. 2008;10:823–829. doi: 10.1007/s11051-007-9317-4. [DOI] [Google Scholar]
- Khan I, Oh DH. Integration of nisin into nanoparticles for application in foods. Innov Food Sci Emerg Technol. 2016;34:376–384. doi: 10.1016/j.ifset.2015.12.013. [DOI] [Google Scholar]
- Khan WH, Rathod VK. Process intensification approach for preparation of curcumin nanoparticles via solvent-nonsolvent nanoprecipitation using spinning disc reactor. Chem Eng Process. 2014;80:1–10. doi: 10.1016/j.cep.2014.03.011. [DOI] [Google Scholar]
- Lemes GF, Marchiore NG, Moreira TFM, et al. Enzymatically crosslinked gelatin coating added of bioactive nanoparticles and antifungal agent: effect on the quality of Benitaka grapes. LWT Food Sci Technol. 2017;84:175–182. doi: 10.1016/j.lwt.2017.05.050. [DOI] [Google Scholar]
- Leyva-Diaz A, Hernandez-Patlan D, Solis-Cruz B, et al. Evaluation of curcumin and copper acetate against salmonella typhimurium infection, intestinal permeability, and cecal microbiota composition in broiler chickens. J Anim Sci Biotechnol. 2020 doi: 10.21203/rs.3.rs-42229/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Wu Y, Liu C, et al. Elaboration and characterization of curcumin-loaded soy soluble polysaccharide (SSPS)-based nanocarriers mediated by antimicrobial peptide nisin. Food Chem. 2021;336:127669. doi: 10.1016/J.FOODCHEM.2020.127669. [DOI] [PubMed] [Google Scholar]
- Ma S, Moser D, Han F, et al. Preparation and antibiofilm studies of curcumin loaded chitosan nanoparticles against polymicrobial biofilms of Candida albicans and Staphylococcus aureus. Carbohydr Polym. 2020 doi: 10.1016/j.carbpol.2020.116254. [DOI] [PubMed] [Google Scholar]
- Meral R, Alav A, Karakas CY, et al. Effect of electrospun nisin and curcumin loaded nanomats on the microbial quality, hardness and sensory characteristics of rainbow trout fillet. LWT. 2019;113:108292. doi: 10.1016/J.LWT.2019.108292. [DOI] [Google Scholar]
- Niaz T, Shabbir S, Noor T, et al. Polyelectrolyte multicomponent colloidosomes loaded with nisin Z for enhanced antimicrobial activity against foodborne resistant pathogens. Front Microbiol. 2018;8:1–19. doi: 10.3389/fmicb.2017.02700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niaz T, Shabbir S, Noor T, et al. Potential of polymer stabilized nano-liposomes to enhance antimicrobial activity of nisin Z against foodborne pathogens. Lwt. 2018 doi: 10.1016/j.lwt.2018.05.029. [DOI] [Google Scholar]
- Oyeyemi O, Adegbeyeni O, Oyeyemi I, et al. In vitro ovicidal activity of poly lactic acid curcumin-nisin co-entrapped nanoparticle against Fasciola spp. eggs and its reproductive toxicity. J Basic Clin Physiol Pharmacol. 2018;29:73–79. doi: 10.1515/jbcpp-2017-0045. [DOI] [PubMed] [Google Scholar]
- Pakbin B, Brück WM, Rossen JWA. Virulence factors of enteric pathogenic Escherichia coli: a review. Int J Mol Sci. 2021 doi: 10.3390/ijms22189922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma G, Dang S, Kalia M, Gabrani R. Synergistic antibacterial and anti-biofilm activity of nisin like bacteriocin with curcumin and cinnamaldehyde against ESBL and MBL producing clinical strains. Biofouling. 2020;36:710–724. doi: 10.1080/08927014.2020.1804553. [DOI] [PubMed] [Google Scholar]
- Souza JF, Souza ACF, Costa FN. Estudo retrospectivo de surtos de doenças veiculadas por alimentos, na região nordeste e Estado do Maranhão, no período de 2007 a 2019. Res Soc Dev. 2021;10(1):e36010111728. doi: 10.33448/rsd-v10i1.11728. [DOI] [Google Scholar]
- Taghavifar S, Afroughi F, SaadatiKeyvan M. Curcumin nanoparticles improved diabetic wounds infected with methicillin-resistant staphylococcus aureus sensitized with HAMLET. Int J Lower Extrem Wounds. 2020 doi: 10.1177/1534734620933079. [DOI] [PubMed] [Google Scholar]
- Takundwa BA, Bhagwat P, Pillai S, Ijabadeniyi OA. Antimicrobial efficacy of nisin, oregano and ultrasound against Escherichia coli O157:H7 and Listeria monocytogenes on lettuce. Lwt. 2021;139:110522. doi: 10.1016/j.lwt.2020.110522. [DOI] [Google Scholar]
- Tyagi P, Singh M, Kumari H, et al. Bactericidal activity of curcumin I is associated with damaging of bacterial membrane. PLoS ONE. 2015 doi: 10.1371/journal.pone.0121313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronova M, Rubleva N, Kochkina N, et al. Preparation and characterization of polyvinylpyrrolidone/cellulose nanocrystals composites. Nanomaterials. 2018 doi: 10.3390/nano8121011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2022) Foodborne diseases. https://www.who.int/health-topics/foodborne-diseases#tab=tab_1. Accessed 12 Jan 2022
- Yeluri Jonnala BR, Feehily C, O’Connor PM, et al. Assessing the ability of nisin A and derivatives thereof to inhibit gram-negative bacteria from the genus Thermus. J Dairy Sci. 2021;104:2632–2640. doi: 10.3168/jds.2020-19350. [DOI] [PubMed] [Google Scholar]
- Zhao X, Zhen Z, Wang X, Guo N. Synergy of a combination of nisin and citric acid against Staphylococcus aureus and Listeria monocytogenes. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2017;34:2058–2068. doi: 10.1080/19440049.2017.1366076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.