Abstract
In this study, the effect of emulsifier mixture and their concentrations on the development of nanoemulsion was studied. The impact of sonication and microfluidization processing conditions on the physicochemical properties and in vitro antimicrobial activity was also evaluated. The optimal nanoemulsion formulation was then evaluated on bread surface against B. subtilis. Results showed that a hydrophilic-lipophilic balance HLB = 12 and emulsifier: oil ratio of 1:1 allowed the formation of stable nanoemulsion. Also, both microfluidization and sonication allowed the formation of nanoscale-emulsion. Sonication treatment for 10 min allowed a maintain the total flavonoid content and a slight reduction of total phenol content. Furthermore, employing sonication resulted to the lowest polydispersity index suggesting more stable nanoemulsion. Nanoscale-emulsion showed a good in vitro antimicrobial activity against L. monocytogenes and E. coli. The application of nanoemulsion on bread surface inoculated with B. subtilis showed a delay of the decay.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13197-022-05660-5.
Keywords: Food safety, Antimicrobial activity, Nanoemulsion, Stability, Encapsulation
Introduction
Archaeological evidence and recorded history have both shown that the use of aromatic plants may be dated to 10,000 BC. The most important document containing 700 formulations of aromatic plants is so-called “Papyrus Ebers” (Baser and Buchbauer 2009). Egyptians exploited the virtues of plants to cure diseases and relieve pain and discomfort and also in food as spices, preservatives, and flavorings as well. Nevertheless, their use in food as preservatives is still limited because of their hydrophobicity which reduces their bioavailability on food surface as well as their strong aroma and toxicity at high concentrations (Ait-Ouazzou et al. 2011; Dima and Dima 2015). Nowadays, with the growing interest towards natural products a real trend of change, towards natural additives like antimicrobials and aromas of natural origin is emerging in the food industry (Aschemann-Witzel et al. 2019). To maintain their efficiency in food, natural antimicrobial agents should be incorporated in an appropriate matrix maintaining their stability and homogeneity.
Nanoemulsions may be applied as effective encapsulation systems to stabilize hydrophobic natural antimicrobials and act as carriers or delivery systems for lipophilic compounds (Choradiya and Patil 2021). Generally, nanoemulsions are highly stable against gravitational separation and droplet aggregation (de Oca-Ávalos et al. 2017). However, it is important to use the adequate emulsifiers and the emulsification methods. Emulsifiers are able to be adsorbed at the interface between the hydrophilic and hydrophobic phases decreasing the interfacial tension and preventing or slowing down the aggregation of particles of the dispersed phase by increasing repulsion forces between them (Silva et al. 2012). The preparation of nanoemulsion consists mainly on low or high energy approaches (Suyanto et al. 2019). Low energy methods are based on the selection of favorable interfacial properties and require significantly less energy input (Badruddoza et al. 2018). High energy includes several methods such as sonication and high-pressure homogenization (ie. microfluidization). These techniques have the advantages of (i) producing a smaller particle size with a narrower distribution, (ii) easy to extrapolate on a large scale and (iii) reproducible (Yukuyama et al. 2016).
The oil-in-water (o/w) emulsions have the advantage of improving the solubility of hydrophobic or oil-soluble compounds and of masking the unpleasant taste and odor of the active compounds. It can also improve the antimicrobial activity (Lohith Kumar and Sarkar 2017). Previous studies have shown that reducing the particle size of lemongrass essential oil and alginate emulsion by microfluidization improves its antimicrobial activity against E. coli (Salvia-Trujillo et al. 2014). Ghosh et al. (2014) have developed a 3% eugenol-loaded o/w emulsion composed of sesame oil, Tween 20/Tween 80 by an ultrasound cavitation method to increase the shelf-life of fresh orange juice. This improvement in the bioactivity was related to a faster penetration of the nanoemulsion to the microbial membranes due to the increased of contact surface (Salvia-Trujillo et al. 2014). This would allow reducing the concentration to reach an equivalent or even greater antibacterial effect as compared to conventional emulsions (Odriozola-Serrano et al. 2014).
Bread and other bakery products are subjected to various spoilage problems, physical, chemical, and microbial. The latter is the most serious one particularly in terms of bacterial (Bacillus sp.) growth. Bacillus subtilis is the major problem of the bread quality deterioration (Rahman et al. 2022). This microorganism contaminates raw materials (for example, flour, sugar, and yeast) and is able to survive during baking process and cooling. Bacillus spores are able to survive a heat treatment (100 °C, 10 min) which corresponds to the cooking process (Rosenkvist and Hansen 1995). It also has the capability of development under both aerobic and anaerobic conditions. Bacillus subtilis is responsible for the phenomenon of "ropiness" (Rahman et al. 2022). Bacillus spp. could contaminate both non-acidified, white and wholemeal bread (Pacher et al. 2022). However, the wholemeal breads seem to be slightly more resistant to Bacillus contamination (Rosenkvist and Hansen 1995).
The aim of this study is to evaluate the effect of the stabilization of an antimicrobial formulation based on natural antimicrobial ingredients into nanoemulsion using microfluidization and sonication method on its bioactivity. The nanoemulsion composition was firstly optimized by selecting the best hydrophilic and lipophilic balance (HLB) and oil: emulsifier ratio. Then, the evaluation of the effect of sonication and microfluidization on the physicochemical and antimicrobial activity of the nanoemulsions was carried out. Finally, the effect of nanoemulsion on the decay of white bread as a food model inoculated with B. subtilis was evaluated.
Materials and methods
Material
Cinnamon essential oil (EO) was bought from Bio Lonreco, Inc. (Dorval, QC, Canada). Water soluble citrus extracts (CE) was provided by Kerry Inc. (Beloit, WI, USA). Based on our previous study, these two compounds have a very high antibacterial activity (Ben-Fadhel et al. 2019). Their main composition is presented in the table (Online Resource 1). Sucrose monopalmitate and sunflower lecithin were kindly provided by Compass Foods Company (Singapore). Folin–Ciocalteu reagent was purchased from Sigma-Aldrich Canada Co. (Oakville, ON, Canada). Sodium carbonate was purchased by Laboratoire Mat Inc. (Quebec, QC, Canada).
Preparation of nanoemulsions
The oil in water (o/w) emulsion containing cinnamaldehyde-based EO and CE with a 1:6 (w/w) cinnamon EO:CE ratio was optimized at different ratios of emulsifier: oil phase (0.3–1.25) and various HLB values (7–18) by exploiting lecithin (HLB ~ 7) and sucrose monopalmitate (HLB ~ 18) as emulsifier. The mixture was vigorously magnetically stirred then passed through Ultra-Turrax T25 high-shear homogenizer (IKA Works Inc., Wilmington, NC, USA) at 10,000 rpm during 1 min. The resulting coarse emulsion was subjected to sonication or microfluidization treatment optimization at different amplitudes, duration, pressures and cycles to obtain the homogeneous nanoemulsions following the method of Maherani et al. (2018).
Sonication method
The coarse emulsion was subjected to sonicator processor Qsonica Q500 (Fisher Scientific Ltd, Saint-Laurent, QC, Canada) in an ice bath at 70% of full power for 10, 20 and 30 min (on-time 5 s, off-time 2 s) to obtain a homogeneous nanoemulsion (Maherani et al. 2018). Every 5 min, the ice bath was renewed to maintain constant temperature during treatment.
Microfluidization method
The coarse emulsion was treated by microfluidization, using an electric-hydraulic M-110P Microfluidizer® equipped with a diamond interaction chamber F20Y for emulsions downstream (Microfluidics International Corp., Newton, MA, USA). Optimization was performed at pressures of 69 MPa (10,000 psi), 103.4 MPa (15,000 psi) and 138.9 MPa (20,000 psi) in 1, 2, and 3 cycles (at 25 °C) (Maherani et al. 2018). Upon exiting the interaction chamber, the product flows through an external cooling coil which regulates the nanoemulsion temperature.
Size (z-average) and polydispersity index (PDI)
The mean diameter and size distribution of emulsions were determined using dynamic light scattering (DLS) technique by employing a Zetasizer Nano-ZS (ZEN3600; Malvern Instruments Inc., Westborough, MA, USA) and DTS Nano software (version 6.12). To avoid multiple scattering effects, emulsions were diluted (1:50) in Milli-Q water, and then put into a folded capillary cell DTS1060 equipped with gold electrodes (Malvern Instruments Inc.). All measurements were carried out at 20 °C by considering a medium viscosity of 1.33 and medium refractive index of 1.333. Three measurements (n = 3) were carried out for each sample.
Turbidity (τ)
Optical turbidity of the emulsions was determined by measuring their absorbance at 600 nm using a photodiode array UV–Vis spectrophotometer Scinco S-3100 (Betatek Inc., Toronto, ON, Canada) at room temperature (Maherani et al. 2018). Samples were analyzed within cells of 1 cm optical path, and deionised water was used as a blank. Triplicate measurements (n = 3) of turbidity were carried out for each sample.
Total phenol and total flavonoid content
Total phenol and total flavonoid content were determined in order to evaluate the effect of microfluidization and sonication treatment on their degradation.
Total phenol content was determined using a Folin–Ciocalteu colorimetric method according to Dewanto et al. (2002) with a standard curve ranging between 0 and 200 µg of gallic acid/mL. A quantity of 125 µL of the standard gallic acid solution or emulsions was mixed with 0.5 mL of distilled water in a test tube followed by the addition of 125 µL of Folin–Ciocalteu reagent (FCR). The vortexed samples were kept at room temperature for 6 min and then 1.25 mL of a 7% sodium carbonate aqueous solution was added to the mixture and the final volume was adjusted to 3 mL by adding water. Samples were allowed to stand for 90 min at room temperature before measurement at 760 nm versus the blank prepared similarly with water. All mean values were expressed as mg gallic acid equivalents (GAE)/g of emulsion.
Total flavonoid content was determined by using a colorimetric method (Dewanto et al. 2002). Briefly, 0.25 mL of diluted emulsions was mixed with 1.25 mL of distilled water followed by addition of 75 µL of a 5% NaNO2 solution. After 6 min, 150 µL of a 10% AlCl3⋅6 H2O solution was added and allowed to stand for 5 min at room temperature before adding 0.5 mL of 1 M NaOH. The mixture was brought to 2.5 mL with distilled water and mixed well. The absorbance was measured immediately against the blank at 510 nm in comparison with the standards prepared similarly with known ( +)-catechin concentrations. The mean results are expressed as µg catechin equivalents/g of emulsion.
Effect of the preparation method on the antimicrobial properties of emulsions
Preparation of bacterial cultures
Emulsions were evaluated for their antimicrobial activity against 4 microorganisms. Listeria monocytogenes HPB 2812 was provided by Laboratoire de Santé Publique du Québec (Sainte-Anne-de-Bellevue, QC, Canada). Escherichia coli O157:H7 CDC EDL 933 (ATCC 43,895) was provided by Prof. Charles Dozois (INRS-IAF, Canada), Bacillus subtilis 168 (ATCC 23,857) and Aspergillus flavus PDCS-4 (ATCC 26,771) were purchased from Cedarlane Laboratories (Burlington, On, Canada). For bacterial evaluation, L. monocytogenes, E. coli and B. subtilis were kept at − 80 °C in Tryptic Soy Broth (TSB; BD Difco, Fisher Scientific Ltd) containing glycerol (10%; v/v). Before each experiment, stock cultures were propagated through two consecutive 24-h growth cycles (10−1 dilution) at 35 ± 2 °C in TSB. For fungal evaluation, A. flavus were propagated through 72-h growth cycles in potato dextrose agar (PDA; BD Difco, Fisher Scientific Ltd) at 28 °C ± 2 °C. Conidia were isolated from the agar media using sterile platinum loop, suspended in sterile peptone water, and filtered through sterile cell strainer (Fisher scientific Ltd). The filtrate was adjusted using a hemocytometer to 104 conidia/mL for the MIC determination.
Minimal inhibitory concentration (MIC) determination
The MIC value of emulsions was determined in sterilized flat-bottomed 96-well microplate according to the two fold microdilution modified method of Ben-Fadhel et al. (2019). Briefly, serial two-fold dilutions of the antimicrobial compounds were made in Mueller Hinton Broth (MHB, BD Difco, Fisher Scientific Ltd) and dispensed into 96-well microplates. The concentrations ranges of emulsions were from 0.005 to 5% (w/v). Then, a volume of 100 μL of bacteria and fungi suspension (104 CFU/mL or conidia/mL) was added to 100 μL of each antimicrobial serial dilution. In the blank or negative control, 100 µL of distilled water was used instead of the working culture bacteria and fungi. The positive control (without antimicrobial agent) consisted of 100 µL of MHB and 100 µl of working culture bacteria or fungi. It should be mentioned that the sterile emulsifiers solution (0.55% w/v lecithin and 0.45% w/v sucrose monopalmitate) that used for emulsification of cinnamom EO did not have any antibacterial activity. Microplates were then incubated at 37 °C and 28 °C for 24 h and 48 h, respectively. The absorbance was measured at 595 nm in a BioTek ELx800 absorbance microplate reader (BioTek Instruments Inc., Winooski, VT, USA). The MIC is the lowest concentration of antimicrobial agent demonstrating the complete inhibition of bacterial and fungal growth as evidenced by a lack of increase in absorbance.
In situ evaluation
Bacterial and spores’ preparation
B. subtilis was selected for the evaluation of the effectiveness of the antimicrobial treatments. This microorganism was chosen as it has been determined to be the main spoilage agent of bread and it demonstrated an ability to survive to food processing conditions (frying, cooking, baking) (Rahman et al. 2022; Almada-Érix et al. 2021). To induce sporulation, nutrient agar (NA) supplemented with MnSO4 (10 mg/L) and K2HPO4 (2 g/L) was inoculated with 2 mL of 24-h grown culture of B. subtilis in TSB at 30 °C. Five days later, spores were collected by flooding the agar plate with sterile distilled water and recovered by scratching the surface with a glass spatula. After harvesting, spores were washed four times with saline water (0.85% w/v) by centrifugation at 4400 × g for 15 min at 4 °C and the pelleted cells were re-suspended in sterile double distilled water. Spore suspension was heat treated in a water bath at 80 °C for 10 min to kill remaining vegetative cells. The concentration of spore suspension was estimated by spread plating 100 µL on plates of tryptic soy agar (TSA; BD Difco, Fisher Scientific Ltd), which were incubated at 30 °C for 24 h. The spore suspension was then maintained at 4 °C until used (Ayari et al. 2012).
Challenge test of par-baked bread
Non-baked white bread balls (≈ 10 * 7 cm) were purchased from Costco (Laval, QC, Canada). Preformed dough balls were treated with 20 µL of B. subtilis at 104 CFU/mL on 4 points of bread (5 µL each). Bread was then divided into groups: untreated bread, bread treated with nanoemulsion and bread treated with coarse emulsion. Treatment was applied by spraying treating solutions on each surface of bread during 2 s. For the control samples, bread was sprayed with distilled water. Bread was then baked in convection oven for 14 min at 200 °C (10 breads per treatment). All bread balls were individually packed in Nasco Whirl–Pak™ sterile filter bags (Fisher Scientific Ltd) under atmospheric air and placed at room temperature. Bread balls were evaluated for the surface contamination (presence of rope formation) and the results were expressed as percentage of decay over time.
Statistical analysis
Each experiment was done in triplicate (n = 3). Analysis of variance (ANOVA), Duncan’s multiple range tests for equal variances and Tamhane’s test for unequal variances were performed for statistical analysis using PASW Statistics 18 software (IBM Corporation, Somers, NY, USA). Differences between means were considered significant when the confidence interval was lower than 5% (p ≤ 0.05).
Results and discussion
Preparation of nanoemulsions
Effect of the HLB on the quality of emulsions
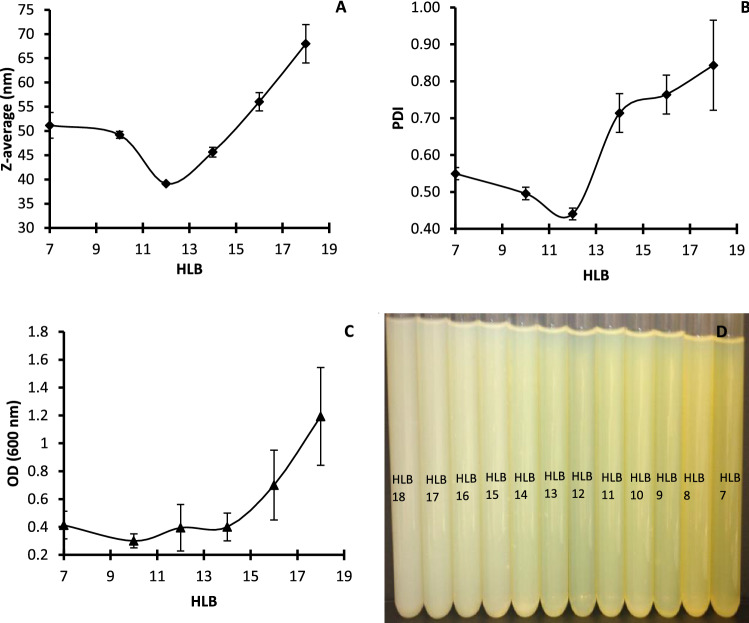
In order to prepare a stable nanoemulsion showing low PDI and mean size average, lecithin (HLB = 7) was combined with sucrose monopalmitate (HLB = 18) to prepare nanoemulsions with different HLB values. Nanoemulsions were prepared using 1 cycle of microfluidization treatment at a pressure of 15,000 psi. Results of mean size average, PDI, turbidity and appearance of emulsions were respectively detailed in Fig. 1. Results show that, z-average and PDI (Fig. 1A, B) had similar parabolic behavior showing a minimum z-average and PDI of 39.11 nm and 0.44 respectively for HLB 12 (ratio of lecithin to sucrose monopalmitate = 55:45). Afterwards, z-average and PDI increased significantly and reached 68 nm and 0.84 suggesting a non-stable nanoemulsion. Reducing the droplet size is very important factor in nanoemulsion preparation because it can result in higher retention of encapsulated components in emulsion systems (Lu et al. 2018). On the other hand, PDI value (0 < PDI < 1) indicates the uniformity and the stability of the droplet size distribution in the nanoemulsion. When the PDI ≤ 0.3, the nanoemulsion system has narrow size distribution and it is homogeneous. When the PDI ≥ 0.5, the system is heterogeneous and called broad size distribution (Pongsumpun et al. 2020). Generally, large particle size and size distribution indicate the instability of the nanoemulsion.
Fig. 1.
Effect of HLB on A the particle size, B the PDI, C the turbidity and D the appearance of nanoemulsions prepared by microfluidization at 15,000 psi/1 cycle with an emulsifier: oil ratio of 1:1 (w/w)
The turbidity and the color of nanoemulsion (Fig. 1C, D) seem to correlate with the data of droplet size and PDI. As turbidity started to increase from 0.2 (HLB = 8) to a maximum at HLB 18 with OD600 of 1.2. Similar results were also observed by Carpenter and Saharan (2017) who prepared mustard o/w nanoemulsion using span 80 and tween 80 and observed a minimum droplet size with HLB 10–11. They attributed this to the packing strength of emulsifiers at the droplet interface which had reached its saturation state at HLB 10. At higher HLB, sucrose monopalmitate molecules are present in excess and therefore will occupy maximum active sites. Lu et al. (2018) also demonstrated that emulsions appear to be opaque (white) at low HLB values and are transparent or translucent at HLB 12.
Effect of the emulsifier: oil phase ratio on the quality of emulsions
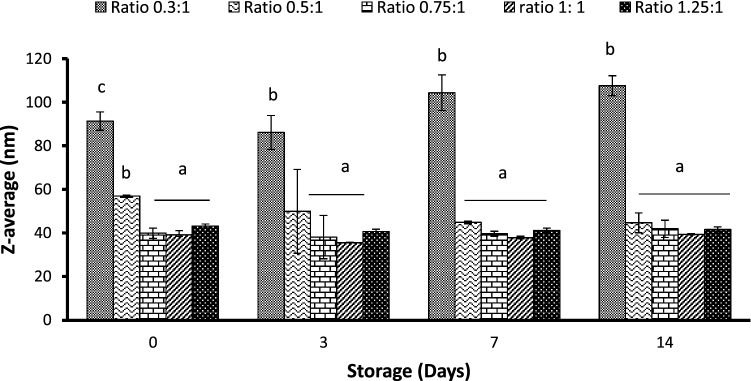
By keeping HLB 12 constant, the emulsifier: oil ratios varied successively from 0.3:1, 0.5:1, 0.75:1, 1:1 to 1.25:1. Results (Fig. 2) showed that the concentration of emulsifiers significantly affects the z-average of nanoemulsion (p ≤ 0.05). So that it adapted 91.3 nm on day 0 as compared to 56.9 nm for 0.5:1 ratio, 43.2 nm for 1:1 ratio and 39.1 nm for both ratios of 0.75:1 and 1.25:1. In the study of Lu et al. (2018), the ratio emulsifier: citral of 0.4–0.6 showed the lowest droplet size. During storage, an increase of z-average was observed for the 0.3:1 ratio and the z-average reached 107.6 nm on day 15. However, the z-average was not affected by storage time for higher emulsifier: oil ratios and the resulting emulsion was found to be stable. Bai et al. (2016) also showed that the type of emulsifier and its concentration significantly affect the mean droplet diameter and the interfacial tension. Depending on the emulsifier type, soy lecithin and gum Arabic showed the lowest droplet diameter at a ratio of 1:1 while the ratio of 0.1:1 was enough in combination with WPI to reduce droplet diameter. Qian and McClements (2011) found a minimum droplet diameter dependent on emulsifier type as SDS < Tween 20 < β-lactoglobulin < casein. They also found a decrease in mean droplet diameter with increasing emulsifier concentration explained by the presence of the emulsifier to cover any new droplet surfaces formed during homogenization. However, the reverse trend was observed for β-lactoglobulin attributed to denaturation of proteins during the high-pressure homogenization process.
Fig. 2.
Effect of the emulsifier: oil ratio on the z-average of nanoemulsions
The decrease of droplet size with increasing emulsifier: oil ratio can be explained by the presence of enough surfactant to stabilize newly formed droplets (Lu et al. 2018). However, by increasing more the surfactant ratio, droplet size would increase again due to much residual surfactant which interferes with the stability and appearance of emulsion.
Effect of the preparation method on the quality and stability of emulsions
Effect of the preparation method on the particle size, PDI and turbidity of emulsions
Results of the effect of microfluidization and sonication on stability parameters including z-average, PDI and turbidity of nanoemulsion are detailed in Table 1. Results show that both methods affect significantly (p ≤ 0.05) the z-average and the turbidity showing a z-average reduction from 189.5 nm for coarse emulsions to 45.9, 38.6 and 41.1 nm for emulsions treated by microfluidization after a pass under the pressure of 10,000, 15,000 and 20,000 psi, respectively. These values were measured as 34.1, 27.4 and 27.6 nm for emulsions treated by sonication during 10, 20 and 30 min respectively. Likewise, turbidity also diminished under the effect of the applied treatments as it decreased to ≈ 4.5 for microfluidization treatment and < 1.1 for sonication treatment as compared to ≈ 9.6 for the coarse emulsion. These results suggest that both methods were efficient to reduce droplet mean diameter and turbidity. However, sonication method seems to be the most efficient especially for reducing the turbidity and the PDI although PDI was not as sensitive as droplet size parameter to the applied treatments. The lowest PDI value of 0.283 was obtained by treatment with sonication during 10 min. Unexpectedly, a longer sonication treatment increased the PDI. This phenomenon can be referred to an “over-processing” with an increase in the Brownian motion at higher energy input leading to collision and coalescence (Mahdi Jafari et al. 2006; Lu et al. 2018). Regarding microfluidization method, increasing microfluidization pressure did not have any effect on the PDI. However, it decreased with the number of cycles applied and remained > 0.3 suggesting a non-stable nanoemulsion.
Table 1.
Z-average, PDI and turbidity of emulsions prepared by sonication versus microfluidization
| Treatments | Z-average (nm) | PDI | Turbidity |
|---|---|---|---|
| Coarse emulsion | 189.5 ± 28.3e | 0.399 ± 0.044c | 9.56 ± 0.5a |
| Microfluidization | |||
| 10,000 psi | |||
| 1 cycle | 45.9 ± 0.6d | 0.430 ± 0.008c | 4.54 ± 0.35b |
| 2 cycles | 35.3 ± 0.1c | 0.404 ± 0.002c | 3.76 ± 0.47c |
| 3 cycles | 28.3 ± 0.7a | 0.321 ± 0.006ab | 2.6 ± 0.46d |
| 15,000 psi | |||
| 1 cycle | 38.6 ± 0.2d | 0.425 ± 0.006c | 4.39 ± 0.81b |
| 2 cycles | 28.0 ± 0.4a | 0.331 ± 0.010ab | 1.84 ± 0.04e |
| 3 cycles | 28.6 ± 0.4a | 0.332 ± 0.009ab | 1.39 ± 0.22f |
| 20,000 psi | |||
| 1 cycle | 42.3 ± 0.6d | 0.488 ± 0.73d | 4.72 ± 0.01b |
| 2 cycles | 27.2 ± 0.1a | 0.340 ± 0.014b | 2.25 ± 0.10de |
| 3 cycles | 25.7 ± 0.7a | 0.313 ± 0.007ab | 2.64 ± 0.29d |
| Sonication | |||
| 10 min | 34.1 ± 0.4b | 0.283 ± 0.024a | 1.05 ± 0.11g |
| 20 min | 27.4 ± 0.4a | 0.304 ± 0.011ab | 0.43 ± 0.12h |
| 30 min | 27.6 ± 0.5a | 0.350 ± 0.017b | 0.56 ± 0.05h |
Within each column, means with the same lowercase letter are not significantly different (p > 0.05)
Effect of the preparation method on the total phenol and total flavonoid contents of emulsions
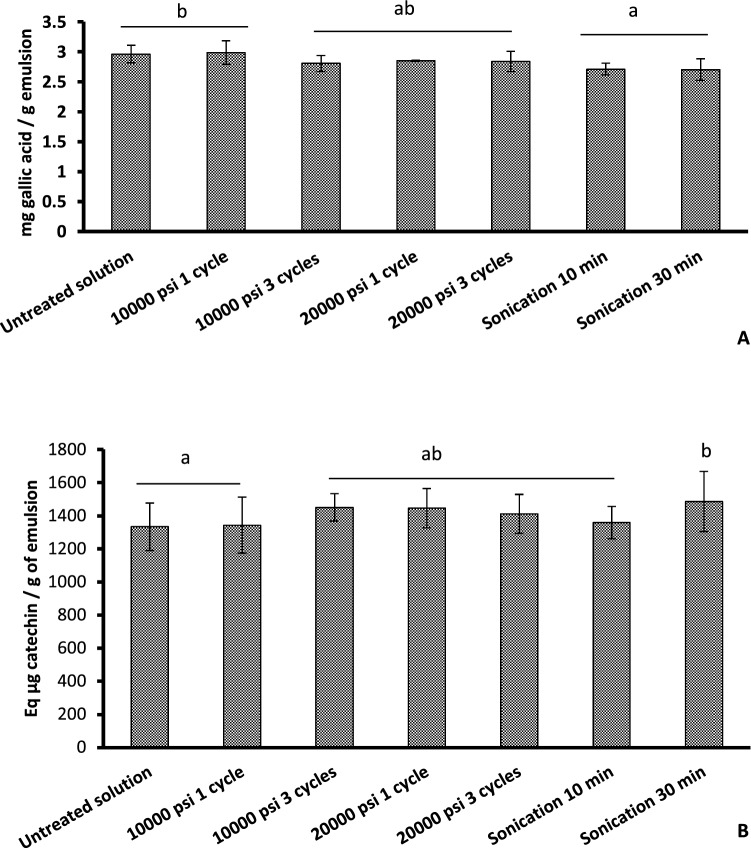
Results of total phenol content (Fig. 3A) showed that microfluidization method did not reduce significantly total phenol content of the emulsion (p > 0.05). In contrast, sonication treatment, even for 10 min, reduced significantly (p ≤ 0.05) total phenol content of nanoemulsion as compared to the coarse emulsion. These results confirmed the findings of Maherani et al. (2018) that sonication method was able to decrease total phenol content especially during storage. Such diminishment is due to polymerization and oxidation of phenolic compounds and subsequently the loss of their activity and solubility. Thus, total phenol seems to be more sensitive to sonication treatment than microfluidization.
Fig. 3.
Effect of sonication versus microfluidization on the determination of A total phenol and B total flavonoid contents
Results of total flavonoids (Fig. 3B) showed that both 10-min-sonication and microfluidization treatments did not have a significant impact on the total flavonoid contents of prepared nanoemulsion as compared to the coarse emulsion although sonication treatment for 30 min significantly increased the total flavonoids content of the nanoemulsion as compared to the coarse emulsion (p ≤ 0.05). Similar results were also observed by Maherani et al. (2018) where coarse emulsions presented lower total flavonoids content than other nanoemulsions.
Effect of the preparation method on the antimicrobial properties of emulsions
In vitro analysis
Results of the effect of sonication and microfluidization methods on the MIC of the prepared nanoemulsions are presented in Table 2. The MIC of the coarse emulsion against E. coli and L. monocytogenes were 1.25% (12,500 ppm) and 0.354% (3540 ppm) respectively, in the coarse emulsion. Sonication and microfluidization treatments reduced the MIC against L. monocytogenes to reach 0.049% (490 ppm) and E. coli to 0.078% (780 ppm) for both treatments. These results suggest that reducing droplet mean diameter affects significantly the efficiency of the emulsion against L. monocytogenes and E. coli. According to Donsì and Ferrari (2016), the improvement of the antimicrobial activity of nanoemulsion could be related to the improvement of the interaction with the cytoplasmic membranes by i) the increase of surface area and passive transport through the outer cell membrane, ii) the sustained release over time of the EO from the nanoemulsion droplets that prolongs the activity of EO iii) the fusion of the emulsifier droplets with the phospholipid bilayer of the cell membrane promotes the targeted release of the EO. Previous studies also demonstrated the effect of nanoscale droplets on the antimicrobial activity against E. coli (Salvia-Trujillo et al. 2014). However, the same authors demonstrated that sonication causes a significant loss in terms of antimicrobial activity against E. coli depending on the amplitude applied. Treatment at the concentration of 100 µm for 3 min causes a total loss of bactericidal activity of lemongrass EO, which is not the case of microfluidization. Also, a sonication treatment for 1 h can esterify more than 5% of lipids. In the current study, both microfluidization and sonication were able to improve the antimicrobial activity of Listeria and E. coli. This could be related to a better control of the temperature of the sample treated with sonication by renewing the ice bath. In the current study, treated nanoemulsions were maintained at 30 °C as compared to 47 °C for the study of Salvia-Trujillo et al. (2014).
Table 2.
MIC (%) of emulsions prepared by sonication versus microfluidization against E. coli O157:H7, L. monocytogenes, B. subtilis and A. flavus
| E. coli | L. monocytogenes | B. subtilis | A. flavus | |
|---|---|---|---|---|
| Coarse emulsion | 1.25 | 0.354 | 0.0024 | 0.07813 |
| 10 min | 0.078 | 0.029 | 0.0024 | 0.07813 |
| 20 min | 0.029 | 0.024 | 0.0024 | 0.07813 |
| 30 min | 0.039 | 0.024 | 0.0024 | 0.07813 |
| 10,000 psi/1 cycle | 0.039 | 0.029 | 0.0024 | 0.07813 |
| 10,000 psi/3 cycles | 0.078 | 0.029 | 0.0024 | 0.07813 |
| 20,000 psi/1 cycles | 0.039 | 0.049 | 0.0024 | 0.07813 |
| 20,000 psi/3 cycles | 0.039 | 0.029 | 0.0024 | 0.07813 |
Contrary to what was observed for E. coli and L. monocytogenes, results obtained with B. subtilis and A. flavus showed that the MIC was 0.0024% (24 ppm) and 0.07813% (781.3 ppm) respectively, in the coarse emulsion. No change was observed with the application of sonication and microfluidization treatments suggesting a different inhibitory mechanism of action between spores and non-spore forming microorganisms. Contrary to these observations, Pongsumpun et al. (2020) showed an improvement of the antimicrobial activity of nanoemulsion prepared with sonication treatment against A. niger, R. arrhizus, Penicillium sp and C. gloeosporioides when the disk diffusion method was employed. This suggests that the sonication method may enhance the antimicrobial activity of the volatile fraction. In fact as explained by Ben-Fadhel et al. (2019), the antimicrobial activity of EO is due to both solid and vapor-phase fractions. While the MIC method involves the liquid fraction, the antimicrobial effect of the vapor fraction is underestimated. These results suggest that the efficiency of sonication treatment and maintaining of the antimicrobial bioactivity is highly related to the control of the treatment conditions especially the temperature of the sample during treatment.
In situ study
Results of the in-situ study for B. subtilis on bread surface are detailed in Fig. 4. Results showed that for untreated samples, 100% of decay was observed on day 14 of storage at room temperature. Applying coarse emulsion on the bread surface delayed the decay by around 12 days and complete decay happened after 26 days. It is interesting to note, that applying a sonication treatment for 10 min to develop nanoemulsion improved the microbial quality of bread about 21 days as compared to the control and about 4 days as compared to coarse emulsion. As the complete decay in breads treated with nanoemulsion occurred on day 35. Similar observations were obtained in the study of Otoni et al. (2014) where an improvement in the shelf-life of sliced bread treated with clove bud and oregano EO was observed with the reduction of the droplet size. The same authors explained this efficiency to a higher bioavailability of nonpolar bioactive compounds encapsulated in smaller droplets, allowing a higher surface-to-volume ratio and easier penetration to cell membranes (Otoni et al. 2014).
Fig. 4.
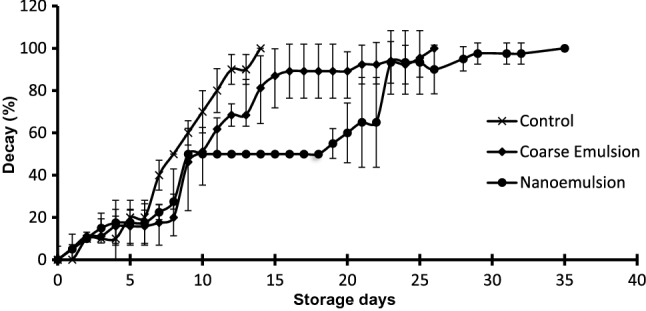
Effect of coarse emulsion and nanoemulsion on the bread decay (%) inoculated with B. subtilis and stored at room temperature
Conclusion
In this study, it was demonstrated that microfluidization and sonication treatments were able to form o/w nanoemulsions for the encapsulation of a food grade antimicrobial formulation containing cinnamon EO and CE. Based on the results of size, PDI and flavonoids content, nanoemulsion prepared with HLB12, emulsifier: oil ratio of 1:1 and with 10 min-pulsed sonication treatment gave the most stable nanoemulsion with a lower PDI and turbidity with no effect on total flavonoid content. However, 10-min-sonication treatment slightly decreased the total phenol content. Both sonication and microfluidization treatments improved the in vitro antimicrobial activity of the bioactive emulsion against E. coli and L. monocytogenes, whereas no effect was observed on the antimicrobial activity against B. subtilis and A. flavus. When applied on bread surface, the nanoemulsion allowed a better control of B. subtilis. Therefore, the stabilization of bioactive formulations based on hydrophilic and hydrophobic natural antimicrobials by using nanoemulsions is a promising solution to replace conventional synthetic preservatives used in food industry.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable
Abbreviations
- ANOVA
Analysis of variance
- A. flavus
Aspergillus flavus
- B. subtilis
Bacillus subtilis
- CE
Citrus extracts
- CFU
Colony-forming unit
- E. coli
Escherichia coli
- EO
Essential oil
- FCR
Folin–Ciocalteu reagent
- GAE
Gallic acid equivalents
- HLB
Hydrophilic and lipophilic balance
- L. monocytogenes
Listeria monocytogenes
- MHB
Mueller–Hinton broth
- MIC
Minimal inhibitory concentration
- PDI
Polydispersity index
- SD
Standard deviation
- SDS
Sodium dodecyl sulfate
- TSA
Tryptic soy agar
Author contribution
YB-F: data curation; formal analysis; investigation; methodology; software; validation; writing – original draft. MA: methodology. CM: methodology. SS: data curation; methodology; software; writing – review & editing. ZA: writing – review & editing. ML: conceptualization; data curation; funding acquisition; project administration; resources; supervision; validation; writing – review & editing.
Funding
This research work was financially supported by i) the Natural Sciences and Engineering Research Council of Canada (NSERC, Collaborative Research and Development program, project # CRDPJ 488702-15), ii) the Quebec Consortium for Industrial Bioprocess Research and Innovation (CRIBIQ, project # 2015-023-PR-C16), iii) the Ministère de l’Agriculture, des Pêcheries et de l’Alimentation du Québec (MAPAQ; Project # IA-115316), iv) their industrial partners Biosecur Lab (A Kerry Company), Skjodt-Barrett Foods Inc., Foodarom Group Inc. and Kerry Inc. and v) the MAPAQ PPIA12 Research Chair granted to Pre Monique Lacroix. Yosra Ben-Fadhel was a fellowship recipient of the Armand-Frappier Foundation. Carolina Martinez was a fellowship recipient of the MITACS.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare that they have no competing interests.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Ethical approval
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ait-Ouazzou A, Cherrat L, Espina L, Lorán S, Rota C, Pagán R. The antimicrobial activity of hydrophobic essential oil constituents acting alone or in combined processes of food preservation. Innov Food Sci Emerg Technol. 2011;12:320–329. doi: 10.1016/j.ifset.2011.04.004. [DOI] [Google Scholar]
- Almada-Érix CN, Almada CN, Pedrosa GTS, Dos Santos P, Schmiele M, Clerici MTPS, Martinez J, Lollo PC, Magnani M, Sant'ana AS. Quantifying the impact of eight unit operations on the survival of eight Bacillus strains with claimed probiotic properties. Food Res Int. 2021;142:110191. doi: 10.1016/j.foodres.2021.110191. [DOI] [PubMed] [Google Scholar]
- Aschemann-Witzel J, Varela P, Peschel AO. Consumers’ categorization of food ingredients: do consumers perceive them as ‘clean label’ producers expect? An exploration with projective mapping. Food Qual Prefer. 2019;71:117–128. doi: 10.1016/j.foodqual.2018.06.003. [DOI] [Google Scholar]
- Ayari S, Dussault D, Jerbi T, Hamdi M, Lacroix M. Radiosensitization of Bacillus cereus spores in minced meat treated with cinnamaldehyde. Radiat Phys Chem. 2012;81:1173–1176. doi: 10.1016/j.radphyschem.2012.02.022. [DOI] [Google Scholar]
- Badruddoza AZM, Gupta A, Myerson AS, Trout BL, Doyle PS. Low energy nanoemulsions as templates for the formulation of hydrophobic drugs. Adv Ther. 2018;1:1700020. doi: 10.1002/adtp.201700020. [DOI] [Google Scholar]
- Bai L, Huan S, Gu J, Mcclements DJ. Fabrication of oil-in-water nanoemulsions by dual-channel microfluidization using natural emulsifiers: Saponins, phospholipids, proteins, and polysaccharides. Food Hydrocoll. 2016;61:703–711. doi: 10.1016/j.foodhyd.2016.06.035. [DOI] [Google Scholar]
- Baser KHC, Buchbauer G (2009) Handbook of essential oils: science, technology, and applications, CRC press
- Ben-Fadhel Y, Maherani B, Aragones M, Lacroix M. Antimicrobial properties of encapsulated antimicrobial natural plant products for ready-to-eat carrots. Foods. 2019;8:535. doi: 10.3390/foods8110535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter J, Saharan VK. Ultrasonic assisted formation and stability of mustard oil in water nanoemulsion: effect of process parameters and their optimization. Ultrason Sonochem. 2017;35:422–430. doi: 10.1016/j.ultsonch.2016.10.021. [DOI] [PubMed] [Google Scholar]
- Choradiya BR, Patil SB. A comprehensive review on nanoemulsion as an ophthalmic drug delivery system. J Mol Liq. 2021;339:116751. doi: 10.1016/j.molliq.2021.116751. [DOI] [Google Scholar]
- de Oca-Ávalos JMM, Candal RJ, Herrera ML. Nanoemulsions: stability and physical properties. Curr Opin Food Sci. 2017;16:1–6. doi: 10.1016/j.cofs.2017.06.003. [DOI] [Google Scholar]
- Dewanto V, Wu X, Adom KK, Liu RH. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem. 2002;50:3010–3014. doi: 10.1021/jf0115589. [DOI] [PubMed] [Google Scholar]
- Dima C, Dima S. Essential oils in foods: extraction, stabilization, and toxicity. Curr Opin Food Sci. 2015;5:29–35. doi: 10.1016/j.cofs.2015.07.003. [DOI] [Google Scholar]
- Donsì F, Ferrari G. Essential oil nanoemulsions as antimicrobial agents in food. J Biotechnol. 2016;233:106–120. doi: 10.1016/j.jbiotec.2016.07.005. [DOI] [PubMed] [Google Scholar]
- Ghosh V, Mukherjee A, Chandrasekaran N. Eugenol-loaded antimicrobial nanoemulsion preserves fruit juice against, microbial spoilage. Colloids Surf, B. 2014;114:392–397. doi: 10.1016/j.colsurfb.2013.10.034. [DOI] [PubMed] [Google Scholar]
- Lohith Kumar DH, Sarkar P. Nanoemulsions for nutrient delivery in food. Nanosci Food Agric. 2017;5:81–121. doi: 10.1007/978-3-319-58496-6_4. [DOI] [Google Scholar]
- Lu W-C, Huang D-W, Wang C-CR, Yeh C-H, Tsai J-C, Huang Y-T, Li P-H. Preparation, characterization, and antimicrobial activity of nanoemulsions incorporating citral essential oil. J Food Drug Anal. 2018;26:82–89. doi: 10.1016/j.jfda.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdi Jafari S, He Y, Bhandari B. Nano-emulsion production by sonication and microfluidization—a comparison. Int J Food Prop. 2006;9:475–485. doi: 10.1080/10942910600596464. [DOI] [Google Scholar]
- Maherani B, Khlifi MA, Salmieri S, Lacroix M. Design of biosystems to provide healthy and safe food. Part A: effect of emulsifier and preparation technique on physicochemical, antioxidant and antimicrobial properties. Eur Food Res Technol. 2018;244:1963–1975. doi: 10.1007/s00217-018-3108-2. [DOI] [Google Scholar]
- Odriozola-Serrano I, Oms-Oliu G, Martín-Belloso O. Nanoemulsion-based delivery systems to improve functionality of lipophilic components. Front Nutr. 2014;1:24. doi: 10.3389/fnut.2014.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otoni CG, Pontes SFO, Medeiros EAA, Soares NDFF. Edible films from methylcellulose and nanoemulsions of clove bud (Syzygium aromaticum) and oregano (Origanum vulgare) essential oils as shelf life extenders for sliced bread. J Agric Food Chem. 2014;62:5214–5219. doi: 10.1021/jf501055f. [DOI] [PubMed] [Google Scholar]
- Pacher N, Burtscher J, Johler S, Etter D, Bender D, Fieseler L, Domig KJ. Ropiness in bread—a re-emerging spoilage phenomenon. Foods [online] 2022;11:3021. doi: 10.3390/foods11193021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongsumpun P, Iwamoto S, Siripatrawan U. Response surface methodology for optimization of cinnamon essential oil nanoemulsion with improved stability and antifungal activity. Ultrason Sonochem. 2020;60:104604. doi: 10.1016/j.ultsonch.2019.05.021. [DOI] [PubMed] [Google Scholar]
- Qian C, Mcclements DJ. Formation of nanoemulsions stabilized by model food-grade emulsifiers using high-pressure homogenization: factors affecting particle size. Food Hydrocolloids. 2011;25:1000–1008. doi: 10.1016/j.foodhyd.2010.09.017. [DOI] [Google Scholar]
- Rahman M, Islam R, Hasan S, Zzaman W, Rana MR, Ahmed S, Roy M, Sayem A, Matin A, Raposo A. A comprehensive review on bio-preservation of bread: an approach to adopt wholesome strategies. Foods. 2022;11:319. doi: 10.3390/foods11030319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkvist H, Hansen Å. Contamination profiles and characterisation of Bacillus species in wheat bread and raw materials for bread production. Int J Food Microbiol. 1995;26:353–363. doi: 10.1016/0168-1605(94)00147-X. [DOI] [PubMed] [Google Scholar]
- Salvia-Trujillo L, Rojas-Graü MA, Soliva-Fortuny R, Martín-Belloso O. Impact of microfluidization or ultrasound processing on the antimicrobial activity against Escherichia coli of lemongrass oil-loaded nanoemulsions. Food Control. 2014;37:292–297. doi: 10.1016/j.foodcont.2013.09.015. [DOI] [Google Scholar]
- Silva HD, Cerqueira MÂ, Vicente AA. Nanoemulsions for food applications: development and characterization. Food Bioprocess Technol. 2012;5:854–867. doi: 10.1007/s11947-011-0683-7. [DOI] [Google Scholar]
- Suyanto A, Noor E, Rusli MS, Fahma F (2019) Nano-emulsion and nano-encapsulation of fruit flavor. IOP Publishing, p 012025
- Yukuyama MN, Ghisleni DDM, Pinto TJA, Bou-Chacra NA. Nanoemulsion: process selection and application in cosmetics—a review. Int J Cosmet Sci. 2016;38:13–24. doi: 10.1111/ics.12260. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Not applicable.