Abstract
The coconut palm belongs to the Arecaceae family, which is distinct from other fruits, known for its versatility. Fresh coconut products are valuable for many food preparations owing to their nutritional and flavour properties. For example, tender coconut yields coconut water, a refreshing nutritious drink that provides good nutrients including electrolytes and other interesting compounds. The mature coconut meat which is rich in fat and protein, aids in coconut milk extraction and is a major component in the wet and dry process of oil extraction. Coconut milk has market potential owing to its increasing applications in food and beverage industries. Coconut is also known for its by-product namely coconut flour, which is rich in protein and dietary fiber, could be used in the preparation of functional foods. The different methods involved in the oil extraction process which helps in more efficient oil recovery were discussed briefly. The nutritional health-promoting functional role of coconut water and virgin coconut oil is highlighted in review paper.
Keywords: Tender coconut water, Coconut white kernel, Virgin coconut oil, Coconut flour, APCC
Introduction
Coconut is one of the important predominantly consumed nutrient-dense fruit. The nutritional and health benefits make it unique and fascinating. Every part of the tree serves one or the other benefits, provides a major contribution to human life. Coconut fruit becomes an essential component in cooking whereas other parts serve their role in fields like food industries, pharmaceuticals, cosmetics as well as unusual applications in upholstery and engineering works. Coconut (Cocos nucifera) is an important palm tree belongs to the family Arecaceae, though it is native to coastal areas but ubiquitous in all tropical and subtropical regions (Chan and Elevitch 2006). Coconut fruit generally consists of 51.7% kernel, 9.8% water, and 38.5% shell (Patil and Benjakul 2018). The fibrous covering called husk which encloses an inside hard layer called shell. The shell encloses white meat, called solid endosperm which attains a thickness of 12–15 mm upon fruit maturity. Inside the hollow shell, the large volume of space is filled with natural water called liquid endosperm or coconut water (Banzon, 1990a). Coconut milk is a white liquid obtained from grating and pressing of mature meat. Coconut milk market is also growing due to increased demand as it finds its applications in confectionery, dairy, and bakery products. All edible parts of coconut fruit are utilized by humans, contains valuable nutrients some of which have potential applications in assisting disease risk reduction (Vitrac et al. 2019). Coconut kernel extract could play a role in chemoprotection as it has been shown in a murine model to reduce oxidative stress, exhibit tumour suppression and inhibit stemness (cancer stem cell phenotype molecular programs) (Sorra et al. 2019).
Coconut being a valuable crop as it is an oil yielding tree but also known for its numerous by-products, hence found helpful for income generation by coconut growers, alleviating poverty, generating more employment opportunities. The aim of this review paper is to provide the information related to coconut nutritional facts, oil extraction methods, favorable advantage obtained from coconut by-products and their health benefits.
Nutritional composition of coconut products
Coconut is naturally enriched with a diverse amount of nutrients and known for its versatility as every part of the fruit yields nutritious benefits. It becomes an indispensable food item as it provides required and essential nutrients such as calories, protein, fat, vitamins, and minerals. According to the USDA data 2018, the protein and total fat contents (per 100 g) for coconut products are represented in Fig. 1 and Fig. 2, respectively. The nutritional facts for different coconut products are discussed below.
Fig. 1.
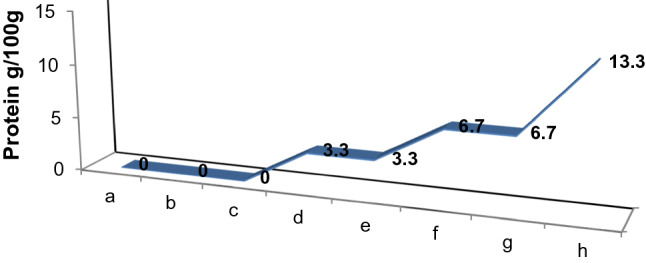
Protein content of coconut products. a Virgin coconut oil, b Refined coconut oil, c Pure coconut water, d Coconut meat, e Coconut milk, f Coconut cream, g Desiccated coconut powder, h Coconut flour. Source: USDA data, 2018
Fig. 2.
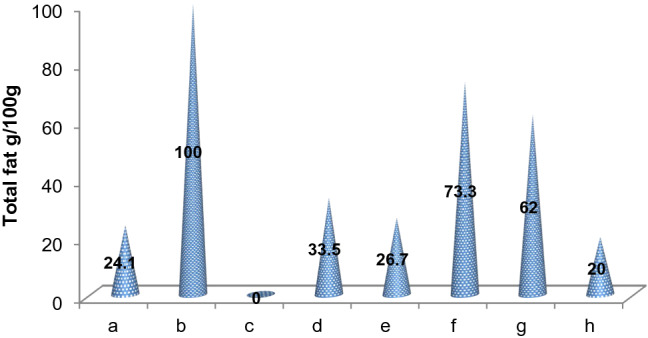
Total fat content of coconut products. a Virgin coconut oil, b Refined coconut oil, c Pure coconut water, d Coconut meat, e Coconut milk, f Coconut cream, g Desiccated coconut powder, h Coconut flour. Source: USDA data, 2018
Coconut water
This nutritious water is also referred to as a wholesome beverage. As fully mature coconut contains 250 g of water, less mature fruit considerably more the water (Banzon, 1990a). It is well known for its natural hydrating properties. The fresh Tender coconut water (TCW) is a natural sterile liquid collected from 5 to 7 months old immature coconuts. It is composed of total soluble solids 3.8–6.9°Bx, acidity of 0.072–0.4% and pH 4.5–5.5, total sugars 2.08–6.52% (BIS 2009; 2010; BSJ 2010), mineral composition of fresh coconut water: Na (1.75–31.4 mg/100 g), K (203.7–249.0 mg/100 g), Ca (3.6–27.35 mg/100 g), Mg (6.4–25.0 mg/100 g) and Fe (0.02–4.2 mg/100 g) were observed (Burns et al. 2020; Yong et al. 2009; Appaiah et al. 2015; NIIR, New Delhi). According to USDA data (2018), 100% pure coconut water contains carbohydrates (3.39 g/100 ml), sugars (1.27 g/100 ml), calcium (17 mg/100 ml), potassium (186 mg/100 ml) and sodium (28 mg/100 ml). The volume of water decreases with successive stages of maturation accompanied by its changes in chemical composition. There was a dramatic reduction in total solids, ash, and mineral contents, whereas fats and proteins, found to increase upon maturation (Jayalekshmy et al. 1988). Some studies revealed, upon maturation, as nut size increases and become larger, having greater space to hold the water inside. These changes in the volume of coconut water take place in between 7 and 10 month old mature coconut. From 7 to 9 month stage maturity, the volume of water found to be a high-fat portion and form a jelly-like structure in stage 8. As the nut gets still older and reaches the stage 10, the watery jelly endosperm becomes harder white coconut meat. The fat content increases as the coconut mature. Protein content also showed a significant increase in maturity (Jackson et al. 2004). Some findings shows that essential oils extracted from coconut water were having free radical scavenging activity and known for their antioxidant potentiality; the constituents of two different varieties were as follows: coconut green variety - esters (58.3%), ketones (33.5%), and diols (8.0%) representing 99.8% of the total extract; yellow variety- diols (74.3%), esters (16.7%), and ketones (6.2%), representing 97.2% of total extract (Fonseca et al. 2009).
The preservation of Tender coconut water (TCW) using heat treatment (85 °C for 60 s) and UV treatment (253.7 nm for 30 min) and their combinations were studied for three weeks of storage by Gunathunga et al. (2018), shows safe for consumption if stored under refrigeration (4 ± 2 °C) after treatments. Karmakar and Dev (2017) studied the preservation of TCW using hollow fiber ultra-filtration membrane technology that successfully stored TCW for 18 weeks with marginal effects on physicochemical properties.
Coconut meat and coconut milk
Coconut meat is the most useful and is a source of coconut milk. According to USDA data 2018, raw coconut meat contains 3.33 g/100 g protein, 33.49 g/100 g total fat and 9 g/100 g dietary fiber. The crude fat content and medium-chain fatty acids present in coconut meat increased at different stages of maturity, and phenolic components present in coconut meat such as gallic, caffeic, salicylic, and p-coumaric acids exhibit antioxidant properties (Mahayothee et al. 2016). Coconut protein fractions from defatted coconut meat recovered as 36% globulin, 19% albumin, 10% glutenin-1, 4% glutenin-2 and 2% prolamin fractions (Patil and Benjakul 2017). Coconut protein fractions act as an emulsifier and play a significant role in stabilizing oil droplets which becomes an important measure in assessing oil recovery.
Coconut milk is extracted from grated coconut meat of mature coconuts of usually 12–14 months. It is milky white in color, oil in water emulsion which separates into a heavy aqueous phase and a lighter cream phase. This separation is referred to as creaming (Gonzalez 1990). Coconut milk is essentially free from fiber, contains protein (3.3 g/100 g) and total fat (26.7 g/100 g) according to USDA data 2018. Freshly extracted coconut milk contains small amounts of the water-soluble B vitamins and ascorbic acid, major carbohydrate present is primarily sucrose and some starch (Seow and Gwee 1997). The global market value of coconut milk powder in 2020 was USD 59.4 million. In 2019, a share of 70% has accounted for conventional products in which the leading product was conventional milk powder. For B2B application maximum share of 66% was accounted in 2019 for coconut milk powder for the commercial production of beverages, dairy and frozen desserts, bakery and confectioneries. A fastest growth rate is expected to register in B2C with a Compound annual growth rate (CAGR) of 10.7% during forecast years 2020 to 2027. Largest market share was held with offline distribution channel of more than 77% in 2019 and online segment of 11.1% from 2020 to 2027 is expected (www.grandviewresearch.com).
Coconut flour
It is a by-product or residue obtained after extraction of coconut milk (coconut flour) and removal of fat (defatted coconut flour) from the coconut meat. After the extraction of coconut milk, coconut fiber contains about 62% of fat on a dry basis; after the extraction of fat from the coconut residue (defatting), the grinding process decreases particle size up from 1127 to 550 µm resulted in an increase in hydration properties. Higher the particle size (1127 µm), the residual oil got trapped inside the fiber matrix restricting the entry of water molecules and hence decreases in hydration property; whereas smaller the particle size (550 µm) showed better hydration properties. But, below 550 µm particle size, at 390 µm grinding damages, the fiber matrix and collapses the pores, thus reducing hydration property. Comparative studies on hydration properties revealed that the water retention capacity of coconut residue was higher than other dietary fiber sources such as sugar beet, apple, pea, wheat and carrot (Raghavendra et al. 2004, 2006). It is rich in the dietary fiber of 40 g/100 g and protein 13.3 g/100 g according to USDA data 2018. The grinding of coconut residue after extraction of fat led to the matrix rupture of honeycomb structure resulting in a flat ribbon type structure, this increases the surface area for water and fat absorption and can be utilized as dietary fiber (Rastogi 2019). Because it is a major source of dietary fiber and protein, coconut flour can be used for the preparation of functional foods.
Desiccated coconut
It is shredded dehydrated meat prepared from the fresh kernel of the coconut. It has a mild, sweet and pleasant taste and having a chewy property as it is the characteristic of coconut. As it is dehydrated coconut meat, its basic composition is similar to raw coconut meat in terms of B complex vitamins, notably niacin and pantothenic acid (Leon 1990). Asian and pacific coconut community (APCC 2009) defines its quality specification as the range of FFA should be within 0.15–0.30% as lauric and oil content should not be less than 60%. If the oil content is less than 60%, it is considered as reduced-fat desiccated coconut. For the purpose of commercialization of grated desiccated coconut, it is classified as three types according to granulometry of the product (APCC 2009) and the grated desiccated coconut weight (%) shall easily pass through a sieve of square aperture sizes as represented in Table 1.
Table 1.
Classification of grated desiccated coconut according to granulometry (
Source: APCC (2009))
| Desiccated coconut types | Sieve size (mm) |
Weight (%) |
|---|---|---|
| Extra-fine desiccated coconut | 0.85 | Not less than 90 |
| 0.50 | Max 25 | |
| Fine desiccated coconut | 1.40 | Not less than 80 |
| 0.71 | Max 20 | |
| Medium desiccated coconut | 2.80 | Not less than 90 |
| 1.40 | Max 20 |
Coconut oils
Coconut oil
It is edible oil composed of a mixture of glycerides. Upon hydrolysis, 100 g of coconut oil yields 85 g of fatty acids and 15 g of glycerol (Banzon 1990b). Some authors reported that the cold-pressed coconut oil (CO) composed of 93% medium-chain saturated fatty acid, predominantly lauric and myristic acids (Romao-Carrascoza et al. 2019). APCC (2009) provides a definition for “Crude coconut oil is the product obtained by expression and/or solvent extraction from dried kernel (copra) of the coconut, and is free from admixture with other oils and fats whereas RBD stands for refined, bleached and deodorized coconut oil obtained from crude coconut oil which has been refined by neutralization with alkali, bleached with bleaching earth or activated carbon or both and deodorized with steam; no other chemical agents being used.
Virgin coconut oil (VCO)
APCC (2009) provides definition as “It is obtained from fresh and mature kernel (12 months old from pollination) of the coconut by mechanical or natural means with or without the application of heat, which does not lead to alteration of the nature of the oil”. Virgin coconut oil is natural clear in color and has a distinct flavor and aroma and free from sediments (APCC 2009). Virgin coconut oil shows marginal differences in terms of iodine value, saponification value, refractive index, fatty acid profile, specific gravity, and moisture content when compared with copra coconut oil (Srivastava et al. 2018).
The physico-chemical stability of VCO in comparison with Refined coconut oil (RCO) was studied by Dayrit et al. (2011). The study revealed that VCO under ambient conditions, its oxidation was negligible. But, oxidation was observed in the presence of oxygen, UV radiation, ferric ions, and higher FFA content. VCO deterioration was identified by microbial decomposition when the moisture content exceeds 0.06%. The total phytosterol was higher in VCO compared to RCO, and FFA content was eight times higher than RCO. Altogether, RCO is known for its stability, VCO stability has not well established. The author opined that phenolics present in the VCO played an important role in increasing its stability. Notably, Lu and Tan (2009) studied the stability of VCO under storage, revealed that GC analysis and FTIR confirmed higher stability with no significant changes in fatty acid proportions throughout the storage period of 40 days, VCO found relatively stable to thermal treatment and found suitable for frying.
Oil extraction methods
Coconut oil extraction methods
Traditionally, the harvested coconuts were allowed to dry naturally and then coconut oil was extracted by using dried coconut meat. This is also known as the dry processing of coconut oil extraction. Banzon (1990a) detailed oil extraction by using dried coconut (copra). The dried copra is the source of coconut oil. The oil is pressed out of copra by means of ‘expeller’. The ‘oil’ is collected in the barrel, whereas the ‘deoiled cake’ is pushed out of the equipment. The oil is used extensively for the manufacture of food products, cosmetics, and other pharmaceutical purposes. The copra cake or deoiled cake is used as a component of animal feed. If the copra is hygienically pressed for coconut oil preparation, the resultant cake is further processed to obtain ‘coconut flour’, which can be used for human consumption.
Seneviratne and Jayathilaka (2016) gave a description about the dry process of coconut oil extraction using dried coconut meat (copra), which involves drying of coconut kernels by reduction of water content from 50% to about 6% from coconut kernels. The drying of copra can be done through sun drying (8 h under bright sunlight for 7–14 days), kiln drying (40–50 °C for 4–5 days) or by using hot air drier (80–90 °C for 10 h). This followed by oil extraction either by pressing (using expeller/hydraulic presser), solvent extraction (n-Hexane as solvent) or by supercritical carbon dioxide extraction method (40 °C, 280 bar pressure). The main method used for oil extraction is pressing using expeller as it is less cost and safe recovery obtained as compared to solvent extraction and supercritical carbon dioxide extraction method. The disadvantages of the solvent extraction method are; high temperatures used in the process may thermally degrade the oil, and 500–1000 mg/Kg of residual solvent may remain in the oil even after purification. The disadvantage of SC-CO2 method of pressing is much costlier to establish its operation.
Virgin coconut oil extraction methods
Coconut milk is oil in water emulsion that is stabilized by protein, predominantly by albumin and globulin fractions. The destabilization of emulsion stability by collapsing protein bonds is required in order to yield virgin coconut oil (Patil and Benjakul 2019). The different methods of VCO extraction are briefly discussed below.
Enzyme-assisted oil extraction and oil recovery
Some authors have extracted VCO using enzymatic treatment by destabilizing the coconut emulsion with good recovery of oil. The literatures of enzyme-assisted treatment for VCO extraction are represented in Table 2.
Table 2.
Yield of virgin coconut oil (VCO) by enzyme-assisted extraction method
| Enzyme / enzyme combinations | Enzyme quantity (g) |
Temperature- time combination for incubation |
Centrifugation (x g) |
VCO recovery yield (%) |
Reference |
|---|---|---|---|---|---|
| Cellulase, alpha-amylase, polygalacturonase & protease | 0.1 | Meal heated at 90 °C for 30 min & kneaded for 1 min followed by enzymatic treatment for 30 min | 12,300 × g at 12 °C for 20 min | 41.7 | Che Man et al. (1996) |
| Enzyme aspartic protease | 0.1 |
Incubated at 25 °C for 3 h Incubated at 37 °C for 3 h |
Coconut milk mixture centrifuged at 3585 × g for 10 min Coconut cream centrifuged at 4880 × g for 15 min |
76.0 83.0 |
Raghavendra & Raghavarao (2010) |
| Amylases & proteases | 1 (each) | Incubated at 40 °C & agitated for 3 h | 1792 × g for 30 min at room temperature | 65.7 | Oseni et al. (2017) |
| Crude protease extract (CPE) from over ripe pineapple | 100 | Incubated at 50 °C for 2 h | 11,200 × g for 15 min | 77.7 | Pooi-Pooi et al. (2020) |
VCO extraction by fermentation method
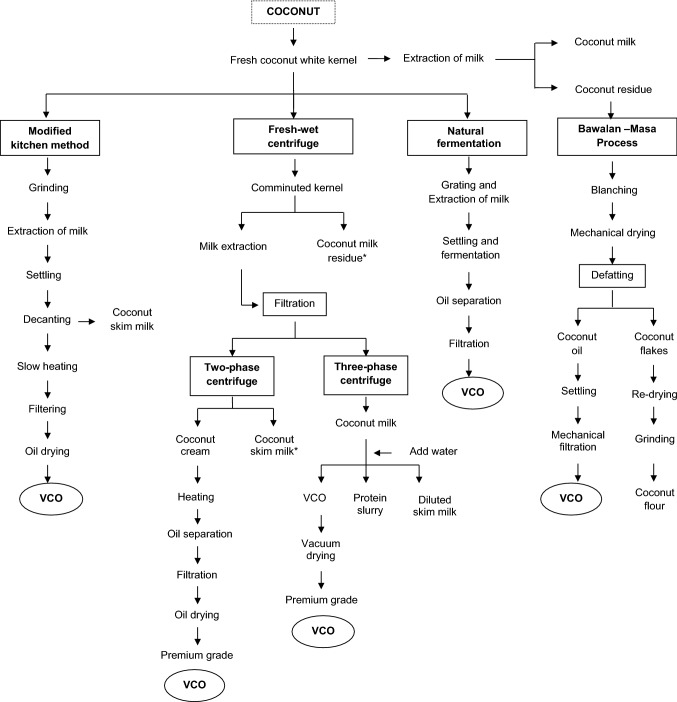
Bawalan (2011) explained the natural fermentation method which is categorized under wet processing of virgin coconut oil extraction which is represented in Fig. 3. It includes the extraction of milk followed by settling and allowed for natural fermentation leads to separation oil which is filtered out and collected as virgin coconut oil. In addition, the author Srivastava et al. (2016) extracted coconut oil by wet processing- cold extraction method, where the coconut milk was allowed to stand for 20–24 h under favorable conditions such as temperature 35–40 °C, 75% relative humidity separates oil from water and protein. The airborne lactic acid bacteria act on coconut milk mixture and ferment to break the protein bonds which helps to separate VCO. Apart from sediments, fermented skim milk (unfit for human consumption), fermented curd, the oil layer was carefully separated for recovery as VCO. Moreover, Oseni et al. 2017 studied both induced fermentation and natural fermentation method of oil extraction, where the shredded coconut meat mixed with water in the ratio 1:1 at 70 °C followed by kneading of mixture for 5 min, strained to obtain coconut milk and allowed to settle for 6 h. The resulted cream was induced to ferment by inoculation with Lactobacillus plantarum (5% w/w) for 10 h at 40 °C. Then, the mixture was centrifuged at 1792 × g for 30 min at room temperature and obtained oil recovery was 77.67%; whereas in natural fermented VCO, the mixture made at 1:2 ratio, and it was allowed to ferment naturally at 40 °C for 16 h, followed by centrifugation same as in induced fermentation, and obtained oil yield was 68.13%. Furthermore, Ghani et al. (2018) extracted coconut milk and left undisturbed for 72 h at room temperature to ferment naturally, followed by centrifugation at 4032 × g or 45 min. Later the upper layer is collected as VCO with oil recovery 9.43%.
Fig. 3.
Fresh-wet processing of Virgin coconut oil (VCO). Source: Bawalan (2011), *by-products obtained during VCO extraction process
VCO extraction by centrifugation method
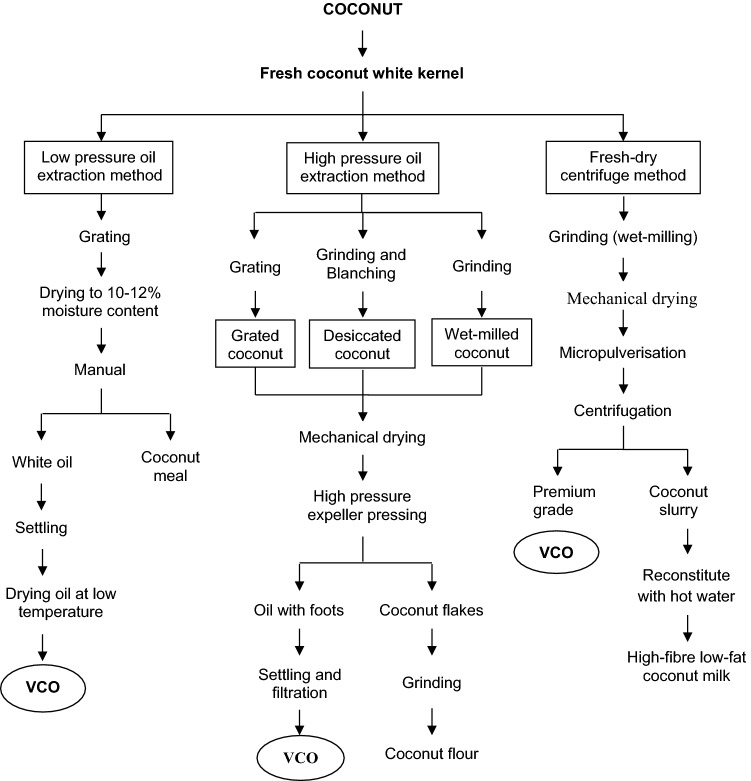
Bawalan (2011) detailed the fresh dry-centrifuge method (Fig. 4) and fresh-wet centrifuge method which includes two-phase centrifugation and three-phase centrifugation method (Fig. 3), which yield good quality virgin coconut oil. Abdurahman et al. (2011) studied VCO production by de-emulsifying the coconut milk emulsion using the centrifugation method. The optimal centrifugal processing parameters were time 60 min and 7168 × g speed; the generated heat by centrifugal rotation decreased the viscosity faster, results in increased droplet size which accelerated the settling velocity of oil droplets and separates the emulsion, and gave oil yield of 45.2% (v/v). Oseni et al. (2017) studies show that coconut meat and water in 1:1 mixture used for the extraction of coconut oil by centrifugation method using 4000 × g for 30 min at room temperature separated the cream and oil. The obtained oil recovery was 54.4%.
Fig. 4.
Fresh-dry processing of Virgin coconut oil (VCO) and its byproducts. Source: Bawalan (2011)
VCO extraction by chilling-thawing and centrifugation
Raghavendra and Raghavarao (2010) studied on oil extraction by the chilling and thawing method. Chilling at different temperatures (5, 10, 15 and 20 °C) for 6 h and thawed at ambient temperature (29 °C ± 2); followed by centrifugation at 3585 × g for 10 min to obtain a cream. Then, obtained coconut cream was further centrifuged at 4880 × g for 15 min to obtain the oil. The oil recovery varied at different chilling temperature: highest oil yield obtained at chilling temperature 5 °C was 92%; whereas at 10 °C, 15 °C and 20 °C the oil recoveries were 86%, 74%, and 65% respectively. Oseni et al. (2017) used coconut meat and water (1:1 ratio) mixture and extracted coconut milk, was centrifuged for 10 min at 3220 × g and the upper creamy layer was collected and chilled at 0 °C for 6 h. The chilled cream was then warmed slowly at room temperature to extract the oil; followed by centrifugation 1792 × g for 30 min at room temperature to obtain coconut cream, and then the cream was further centrifuged at 1792 × g for 30 min at room temperature to extract VCO. The oil recovery was 69.31%. Ghani et al. (2018) studied the extraction of VCO by chilling and centrifugation method. The coconut milk was given chilling treatment at 4 °C; the creamy layer was separated and allowed for thawing at 50 °C, followed by centrifugation at 4032 × g to separate VCO, which yielded 20.44%.
VCO extraction by pH treatment, thermal treatment, and other means
The coconut milk emulsion stability was altered with the help of pH, heat treatment and other treatments. Raghavendra and Raghavarao (2010) studied by varying the pH range, the destabilization of coconut milk emulsion were carried out. A decrease in pH from 6 to 3 destabilizes the emulsion and yielded oil to about 89% whereas an increase in the pH from 6 to 10 yielded 83% of oil recovery. The pH variation in coconut oil extraction played an important role in oil recovery. The same authors used thermal treatment to destabilize the coconut milk emulsion. Different temperatures (40, 50, 60, 70, 80 and 90 °C) were given to freshly extracted coconut milk for 20 min, followed by centrifugation at 3585 × g for 10 min to separate coconut cream. Then, the coconut cream was centrifuged to 4880 × g for 15 min to obtain clear oil. The highest yield obtained was 86% at 90 °C, due to destabilization of the emulsion by denaturation of heat liable proteins during heating which results in aggregation of oil droplets. Bawalan (2011) described VCO extraction by fresh-wet processing which includes a modified kitchen method (slow heating) (Fig. 3), and fresh-dry processing which includes a low-pressure oil extraction method (manual pressing) and high-pressure oil extraction method (high-pressure oil expeller) (Fig. 4). The author has also explained the combination of dry and wet processing (Bawalan-Masa process represented in Fig. 3. Moreover, Srivastava et al. (2016) also explained the hot extraction method of VCO by giving slow heat treatment to break protein bonds using a double-walled boiler. Then the VCO was collected from proteinaceous residue mixture by straining through a muslin cloth. Furthermore, Pooi-Pooi et al. (2020) used microwave-assisted extraction (VCO-MAE) and ultrasound-assisted extraction (VCO-UAE). VCO-MAE study includes different treatments of microwave power levels of 300 W, 450 W and 600 W with two different reaction times. The highest recovery obtained from the combination 450 W for 10 min was 58.6%. The UAE treatment includes power bath of 185 W and frequency of 40 kHz, and sonicated at 30 °C for 1 h, 2 h and 3 h. The VCO-UAE gave the highest yield of 24.1% at 2 h of treatment. The VCO from mature coconut by thermal treatment and VCO from enzyme treated aqueous extracts by authors Raghavendra & Raghavarao (2010 & 2011) shows, acid value of 0.27 and 0.6, iodine value of 4.17 and 3.92 g/100 g, saponification value of 265 and 253 min, peroxide value 0.82 and 0.81 meq O2/kg and free fatty acid of 0.14 and 0.31%, respectively.
Health benefits
Coconut water
The biologically pure and sterile water from coconut offers potential health benefits. Studies on usage of Tender coconut water (TCW) cardioprotective effect in isoproterenol (ISO) induced myocardial infarction rats. The result shows the survival rate was significantly higher in ISO treated rats administered with TCW than the control group. Serum enzymes also increased in ISO treated rats whereas the activities of such enzymes decreased, and showed significantly lower cholesterol levels in the heart and aorta, and had lower levels of triglycerides and phospholipids in the serum, heart, and aorta in ISO treated rats fed with TCW (Anurag and Rajamohan 2003a). This shows the beneficial effects of TCW in ISO induced myocardial infarction. TCW contains good amount of protein, fat, electrolytes and minerals like potassium, calcium, sodium, and magnesium (Yong et al. 2009) and could play a potential role in cardioprotection.
Administration of TCW in rats increased the activities of TCA cycle enzymes, namely succinate dehydrogenase, malate dehydrogenase and isocitrate dehydrogenase and also fatty acid oxidation enzymes, namely carnitine acetyltransferase and acyl CoA dehydrogenase in heart mitochondria. TCW fed rats also show decreased levels of malondialdehyde, an index of lipid peroxidation. The amino acid L-arginine and vitamin C present in coconut water (Prades 2012; Preetha et al. 2012; Yong et al. 2009) played an important role in radical scavenging activity and lipid peroxidation and found to be beneficial in heart mitochondrial activities (Anurag and Rajamohan 2003b). In support of this, coconut water administration in cholestero- diet fed rats resulted in increased plasma L- arginine content, urinary nitrite level, and nitric oxide synthase activity, which indicates the potential role of TCW and mature coconut water on serum and tissue lipid parameters (Sandhya and Rajamohan 2006). Another study by the authors Sandhya and Rajamohan (2008) on supplementation of coconut water in fat-cholesterol diet-fed rats, found lower levels of total cholesterol, VLDL + LDL cholesterol and serum triglycerides, and higher levels of HDL cholesterol. The administration of coconut water before its autocatalytic oxidation stage revealed protection of hemoglobin from nitrite induced oxidation to methemoglobin, mainly due to the presence of ascorbic acid, minerals and enzymes (Mantena et al. 2003). Hence, coconut water is found potent to fight against hyperlipidemic, hypercholesteremic as well as anti-oxidative conditions.
Some of the animal studies show the anti-hypertensive role of coconut water. TCW administered in fructose-induced hypertensive rats show a progressive decrease in Systolic blood pressure (SBP), as well as lowered serum triglyceride and free fatty acids, also found a significant decrease in plasma insulin, glucose, lipid peroxidation markers (malondialdehyde, hydroperoxides, and conjugated dienes) and antioxidant enzyme activity (Bhagya et al. 2012). Study by Alleyne et al. (2005) revealed that rats receiving the coconut water showed 71% decrease in mean SBP and 29% decrease in mean Diastolic blood pressure (DBP); whereas rats received coconut water-mauby mixture (two tropical drinks mixed together) showed 43% decrease in mean SBP and 57% mean DBP. This shows coconut water alone can reduce SBP, but the mixture worked better for DBP reduction. From these studies, it can be concluded that coconut water played a potential beneficial role in hypertension by upregulating the antioxidant status. A case study report by Campbell-Falck et al. (2000) in which coconut water was administered intravenously in critically ill patients of Solomon Island, due to non-availability of standard IV fluids. The patient received coconut water as a short-term intravenous hydration fluid for approximately two days, at an estimated rate of 1,200 mL/day without any adverse effects. Coconut fluid has been shown to be an effective form of intravenous hydration solution in small volumes over short periods of time, and can be considered as a temporizing alternative to standard intravenous fluids in remote areas where supplies are scarce and coconuts are abundant and inexpensive.
Virgin coconut oil
Virgin coconut oil (VCO) can be extracted from the raw coconut meat collected from fully mature coconuts. The fatty acid composition of coconut oil mainly lauric acid and myristic acid, which play a beneficial role in maintaining human health. According to the APCC (2009), VCO should contain 45–56% lauric acid (C12:0); 16–21% myristic acid (C14:0). Author Srivastava et al. (2016) remarked its benefits as it contains a high amount of polyphenol, tocopherol, phytosterol, polyphenols, monoglycerides and radical scavenging activity.
Marina et al. (2009) studied on VCO, extracted through the chilling method and fermentation method and was compared with RBD oil (Refined bleached and deodorized oil) for its antioxidant property. They found high total phenolic contents and antioxidant capacity (scavenging and reducing power), and concluded as antioxidant property could be due to the phenolics present in the VCO. In the VCO extraction process, most of the phenolics were retained, but in RBD coconut oil, due to the refining process, phenolic compounds might be lost or degraded. Hence, VCO found to have the greatest antioxidant activity than RBD coconut oil. Siddalingaswamy et al. (2011) suggested that polyphenolics and radical scavenging activity were significantly higher in hot extracted VCO (HEVCO) due to more phenolics incorporated than Cold extracted VCO (CEVCO) and Commercial coconut oil (CCO). He also studied on the anti-diabetic effect of HEVCO and CEVCO in streptozotocin-induced diabetic rats in comparison with CCO. They observed a gradual decrease in blood glucose in all HEVCO, CEVCO, and CCO fed rats. But, the complete inhibition of lipid peroxidation was observed in the HEVCO group whereas no significant reduction was seen in CEVCO and CCO group. Hepatic lipid peroxidation was also effectively inhibited by both HEVCO and CEVCO indicating better anti-diabetic effects. Studies conducted by Akinnuga et al. (2014) show a significant decrease in the concentrations of TG, TC, LDL, and VLDL whereas a significant increase in the levels of HDL in diabetic rats fed with VCO diet compared with diabetic control rats. The author suggested VCO as therapeutic as it decreases the risk of cardiovascular diseases associated with diabetes mellitus.
Studies carried out by Arunima and Rajamohan (2013) on VCO for its antioxidant potentiality against oxidative stress were compared with copra oil, olive oil and sunflower oil. Rats fed with VCO show increased activities of catalase, superoxide dismutase, glutathione peroxidase and glutathione reductase in tissues. Paraoxonase 1 activity, was known to be significantly increased in VCO administered rats and decreased the formation of lipid peroxidation and protein oxidation products like malondialdehyde, hydroperoxides, conjugated dienes and protein carbonyls in serum and tissues. The author concludes that the wet processing method followed for VCO extraction retains polyphenols and tocopherols which specify improved antioxidant status and hence preventing lipid and protein oxidation.
Nurul-Iman et al. (2013) studied the VCO effect on blood pressure in five-times-Heated palm oil (5HPO) fed rats. According to the study, the 5HPO fed rats showed overproduction of free radicals led to lower levels of plasma nitric oxide, this reduction in nitric oxide availability causes elevated blood pressure, and toxic products generated in the heated oil interrupt endothelium balance. From this study, results reveal that VCO supplementation in 5HPO fed rats significantly increased the plasma nitric oxide levels compared to 5HPO fed group, thereby decreases the elevation in blood pressure, and improving vascular reactivity. The findings revealed and author opined those antioxidants present in the VCO played a beneficial role in improving endothelial function. Alternatively, Kamisah et al. 2015 studied on the cardioprotective role of VCO in heated palm oil diet-induced hypertensive Sprague Dawley rats. Heated palm oil supplemented with VCO significantly reduced systolic blood pressure when compared with HPO fed rats.
Conclusion
As discussed, the coconut and its by-products such as coconut water, shredded meat, milk, and oil are nutrient-rich, having medicinal and antioxidant properties accommodating its top place for human consumption by showing its multitude potential in health promotion. At this stage, among different oil extraction methods, the most efficient method is chilling (5 °C)-centrifugation method yields the highest oil recovery of about 92%, whereas pH treatment (acidic pH3) yield 86% oil recovery, and thermal treatment (90 °C) yield 86% oil recovery, followed by enzymatic method used hydrolytic enzyme galactomannase also yield 86% oil recovery. From the previous studies, coconut oil revealed as it is neutral in its effects on blood cholesterol and heart-related diseases, whereas VCO found rich in phenolics serves as excellent dietary oil. It is also considered as a functional food due to its nutraceutical role in the treatment and prevention of diseases and boosts immunity. As there is a lack of information, future thrust should include work on the nutritional and therapeutic role of coconut endosperm and coconut residue/flour to enhance its suitability in food industries. However, future study needs to be focus on cost effective VCO extraction without compromising in quality of oil meeting all necessary standards, and also studies need to develop in improving the VCO storage and stability.
Acknowledgements
We thank Director, CSIR- CFTRI, Mysuru for the support.
Biological, chemical and microbiological
- APCC
Asian and pacific coconut community
- CAGR
Compound annual growth rate
- CCO
Commercial coconut oil
- CEVCO
Cold- extracted virgin coconut oil
- DBP
Diastolic blood pressure
- FFA
Free fatty acid
- HDL
High density lipoprotein
- HEVCO
Hot- extracted virgin coconut oil
- ISO
Isoproterenol
- LDL
Low density lipoprotein
- RBD
Refined bleached deodorised
- RCO
Refined coconut oil
- SBP
Systolic blood pressure
- TC
Total cholesterol
- TCW
Tender coconut water
- TG
Triglycerides
- USDA
United states department of agriculture
- VCO
Virgin coconut oil
- VCO-MAE
Virgin coconut oil- microwave assisted extraction
- VCO-UAE
Virgin coconut oil- ultrasound assisted extraction
- VLDL
Very low- density lipoprotein
- 5HPO
Five times- heated palm oil
Instrumental techniques
- FTIR
Fourier transform infrared
- GC
Gas chromatography
Authors' contributions
DPM collected literature and wrote the review; MC collected essential literature, attended reviewer comments and modified review as per new author guidelines, PB part of the literature is taken from her dissertation work, corrected and edited the article and RBS suggested for writing review, overall correction and modification.
Funding
Not applicable.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Code availability
Not applicable.
Declarations
Conflicts of interest
There is no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdurahman NH, Khoo CG, Azhari NH. Production of virgin coconut oil (VCO) by centrifugation method. ICCEIB - SOMChE: Universiti Malaysia Pahang, Kuantan; 2011. pp. 1–7. [Google Scholar]
- Akinnuga AM, Jeje SO, Bamidele O, Sunday VE (2014) Dietary consumption of virgin coconut oil ameliorates lipid profiles in diabetic rats. Physiol J, 1–5. 10.1155/2014/256236
- Alleyne T, Roache S, Thomas C, Shirley A. The control of hypertension by use of coconut water and mauby: Two tropical food drinks. West Indian Med J. 2005;54(1):3–8. doi: 10.1590/s0043-31442005000100002. [DOI] [PubMed] [Google Scholar]
- Anurag P, Rajamohan T. Cardioprotective effect of tender coconut water in experimental myocardial infarction. Plant Foods Hum Nutr. 2003;58:1–12. doi: 10.1023/B:QUAL.0000040363.64356.05. [DOI] [Google Scholar]
- Anurag P, Rajamohan T. Beneficial effects of tender coconut water against isoproterenol induced toxicity on heart mitochondrial activities in rats. Indian J Biochem Biophys. 2003;40:278–280. [PubMed] [Google Scholar]
- APCC (2009) International coconut community: APCC Quality Standards for coconut products, Indonesia
- Appaiah P, Sunil L, Prasanth Kumar PK, Gopala Krishna AG. Physico-chemical characteristics and stability aspects of coconut water and kernel at different stages of maturity. J Food Sci Technol. 2015;52(8):5196–5203. doi: 10.1007/s13197-014-1559-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arunima S, Rajamohan T. Effect of virgin coconut oil enriched diet on the antioxidant status and paraoxonase 1 activity in ameliorating the oxidative stress in rats-a comparative study. Food Funct. 2013;4:1402–1409. doi: 10.1039/c3fo60085h. [DOI] [PubMed] [Google Scholar]
- Banzon JA (1990a) The coconut palm and its fruit. In: JA Banzon, ON Gonzalez, SY de Leon, PC & Sanchez (Eds.) Coconut as food. Philippines: Philippine coconut research and development foundation Inc. pp 3–7
- Banzon JA (1990b) Coconut oil. In: JA Banzon, ON Gonzalez, SY de Leon, PC & Sanchez (Eds.) Coconut as food. Philippines: Philippine coconut research and development foundation Inc. pp. 8–12
- Bawalan DD (2011) Processing manual for virgin coconut oil, its products and by products for Pacific Island countries and territories. Secretariat of the Pacific Community, New Caledonia. pp 1–88
- Bhagya D, Prema L, Rajamohan T. Therapeutic effects of tender coconut water on oxidative stress in fructose-fed insulin resistant hypertensive rats. Asian Pac J Trop Med. 2012;5(4):270–276. doi: 10.1016/S1995-7645(12)60038-8. [DOI] [PubMed] [Google Scholar]
- Bureau of Indian Standards . Packed tender coconut water - Specification. India: New Delhi; 2009. [Google Scholar]
- Bureau of Indian Standards . Packed matured coconut water - Specification. India: New Delhi; 2010. [Google Scholar]
- Bureau of Standards Jamaica (2010) Packaged natural coconut water, Kingston, Jamaica, https://law.resource.org/pub/crs/ibr/cc.crs.3.2010.pdfs (accessed June 10, 2019)
- Burns DT, Johnston E-L, Walker MJ. Authenticity and the potability of coconut water - a critical review. J AOAC Inter. 2020;103(3):800–806. doi: 10.1093/jaocint/qsz008. [DOI] [PubMed] [Google Scholar]
- Campbell-Falck D, Thomas T, Falck TM, Tutuo N, Clem K. The intravenous use of coconut water. Ameri J Emerg Med. 2000;18(1):108–111. doi: 10.1016/S0735-6757(00)90062-7. [DOI] [PubMed] [Google Scholar]
- Chan E, Elevitch CR (2006) Cocos nucifera (Coconut). Species Profiles for Pacific Island Agroforestry, (www.traditionaltree.org), pp 1–27
- Che Man YB, Suhardiyono, Asbi AB, Azudin MN, Wei LS. Aqueous enzymatic extraction of coconut oil. J Am Oil Chem Soc. 1996;73(6):683–686. doi: 10.1007/BF02517940. [DOI] [Google Scholar]
- National Institute of Industrial Research (NIIR)-Coconut and coconut products (Cultivation and Processing) New Delhi, India. Published By Ajay, Kr. Gupta, Asia Pacific Business Press Inc., New Delhi, India
- Dayrit FM, Dimzon IKD, Valde MF, Santos JER, Garrovillas MJM, Villarino BJ. Quality characteristics of virgin coconut oil: Comparisons with refined coconut oil. Pure Appl Chem. 2011;83(9):1789–1799. doi: 10.1351/PAC-CON-11-04-01. [DOI] [Google Scholar]
- Fonseca AM, Bizerra AMC, Souza JSN, Monte FJQ, Oliveira MCF, Mattos MC, Cordell GA, Braz-Filho R, Lemos TLG. Constituents and antioxidant activity of two varieties of coconut water (Cocos nucifera L.) Rev Bras Farmacogn. 2009;19:193–198. doi: 10.1590/S0102-695X2009000200002. [DOI] [Google Scholar]
- Ghani NAA, Channip AA, Hwa PCH, Ja’afar F, Yasin HM, Usman A, Physicochemical properties, antioxidant capacities, and metal contents of virgin coconut oil produced by wet and dry processes. Food Sci Nutr. 2018;6:1298–1306. doi: 10.1002/fsn3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez ON (1990) Coconut milk. In: JA Banzon, ON Gonzalez, SY de Leon, PC & Sanchez (Eds.) Coconut as food. Philippines: Philippine coconut research and development foundation Inc. pp 15–48
- Gunathunga C, Abeywickrema S, Navaratne S. Preservation of tender coconut (Cocos nucifera L.) water by heat and UV-C treatments. Int J of Food Sci Nutr. 2018;3(3):15–19. [Google Scholar]
- https://www.grandviewresearch.com › industry-analysis
- Jackson JC, Gordon A, Wizzard G, McCook K, Rolle R. Changes in chemical composition of coconut (Cocos nucifera) water during maturation of the fruit. J Sci Food Agric. 2004;84:1049–1052. doi: 10.1002/jsfa.1783. [DOI] [Google Scholar]
- Jayalekshmy A, Arumughan C, Narayanan CS, Mathew AG. Changes in the chemical composition of coconut water during maturation. Oleagineux. 1988;43(11):409–414. [Google Scholar]
- Kamisah Y, Periyah V, Lee KT, Noor-Izwan N, Nurul-Hamizah A, Nurul-Iman BS, Subermaniam K, Jaarin K, Azman A, Faizah O, Qodriyah HMS. Cardioprotective effect of virgin coconut oil in heated palm oil diet-induced hypertensive rats. Pharm Bio. 2015;53(9):1243–1249. doi: 10.3109/13880209.2014.971383. [DOI] [PubMed] [Google Scholar]
- Karmakar S, De S. Cold sterilization and process modeling of tender coconut water by hollow fibers. J Food Eng. 2017;200:70–80. doi: 10.1016/j.jfoodeng.2016.12.021. [DOI] [Google Scholar]
- Leon SY (1990) Desiccated Coconut. In: JA Banzon, ON Gonzalez, SY de Leon, PC & Sanchez (Eds.) Coconut as food. Philippines: Philippine coconut research and development foundation Inc. pp 95–107
- Lu HFS, Tan PP. A comparative study of storage stability in virgin coconut oil and extra virgin olive oil upon thermal treatment. Int Food Res J. 2009;16:343–354. [Google Scholar]
- Mahayothee B, Koomyart I, Khuwijitjaru P, Siriwongwilaichat P, Nagle M, Müller J. Phenolic compounds, antioxidant activity, and medium chain fatty acids profiles of coconut water and meat at different maturity stages. Int J Food Prop. 2016;19:2041–2051. doi: 10.1080/10942912.2015.1099042. [DOI] [Google Scholar]
- Mantena SK, Jagadish, Badduri SR, Siripurapu KB, Unnikrishnan MK. In vitro evaluation of antioxidant properties of Cocos nucifera Linn. Water Nahrung/food. 2003;47(2):126–131. doi: 10.1002/food.200390023. [DOI] [PubMed] [Google Scholar]
- Marina AM, Che Man YB, Nazimah SAH, Amin I. Antioxidant capacity and phenolic acids of virgin coconut oil. Int J Food Sci Nutr. 2009;60(S2):114–123. doi: 10.1080/09637480802549127. [DOI] [PubMed] [Google Scholar]
- Nurul-Iman BS, Kamisah Y, Jaarin K, Qodriyah HMS. Virgin coconut oil prevents blood pressure elevation and improves endothelial functions in rats fed with repeatedly heated palm oil. Evidence-Based Complementary Alternative Med. 2013 doi: 10.1155/2013/629329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oseni NT, Fernando WMADB, Coorey R, Gold I, Jayasena V. Effect of extraction techniques on the quality of coconut oil. Afr J Food Sci. 2017;11(3):58–66. doi: 10.5897/AJFS2016.1493. [DOI] [Google Scholar]
- Patil U, Benjakul S. Characteristics of albumin and globulin from coconut meat and their role in emulsion stability without and with proteolysis. Food Hydrocoll. 2017;69:220–228. doi: 10.1016/j.foodhyd.2017.02.006. [DOI] [Google Scholar]
- Patil U, Benjakul S. Coconut Milk and Coconut Oil: Their manufacture associated with protein functionality. J Food Sci. 2018;83(8):2019–2027. doi: 10.1111/1750-3841.14223. [DOI] [PubMed] [Google Scholar]
- Patil U, Benjakul S. Use of protease from seabass pyloric caeca in combination with repeated freeze-thawing cycles increases the production efficiency of virgin coconut oil. Eur J Lipid Sci Technol. 2019;121:1–9. doi: 10.1002/ejlt.201800460. [DOI] [Google Scholar]
- Pooi-Pooi S, Ali Y, Lai OM, Kuan CH, Tang TK, Lee YY, Phuah ET. Enzymatic and mechanical extraction of virgin coconut oil. Eur J Lipid Sci Technol. 2020;122:1–13. doi: 10.1002/ejlt.201900220. [DOI] [Google Scholar]
- Prades A, Dornier M, Diop N, Pain JP. Coconut water uses, composition and properties: a review. Fruits. 2012;67:87–107. doi: 10.1051/fruits/2012002. [DOI] [Google Scholar]
- Preetha PP, Devi VG, Rajamohan T. Hypoglycemic and Antioxidant potential of coconut water in experimental diabetes. Food Funct. 2012;3:753–757. doi: 10.1039/c2fo30066d. [DOI] [PubMed] [Google Scholar]
- Raghavendra SN, Raghavarao KSMS. Effect of different treatments for the destabilization of coconut milk emulsion. J Food Engg. 2010;97:341–347. doi: 10.1016/j.jfoodeng.2009.10.027. [DOI] [Google Scholar]
- Raghavendra SN, Raghavarao KSMS. Aqueous extraction and enzymatic destabilization of coconut milk emulsions. J Am Oil Chem Soc. 2011;88:481–487. doi: 10.1007/s11746-010-1695-6. [DOI] [Google Scholar]
- Raghavendra SN, Rastogi NK, Raghavarao KSMS, Tharanathan RN. Dietary fiber from coconut residue: Effects of different treatments and particle size on the hydration properties. Eur Food Res Technol. 2004;218:563–567. doi: 10.1007/s00217-004-0889-2. [DOI] [Google Scholar]
- Raghavendra SN, Swamy SRR, Rastogi NK, Raghavarao KSMS, Kumar S, Tharanathan RN. Grinding characteristics and hydration properties of coconut residue: A source of dietary fiber. J Food Eng. 2006;72:281–286. doi: 10.1016/j.jfoodeng.2004.12.008. [DOI] [Google Scholar]
- Rastogi NK. New technologies for value added products from coconut residue. Mater Res Proceed. 2019;11:295–301. doi: 10.21741/9781644900178-25. [DOI] [Google Scholar]
- Romao-Carrascoza VS, Garcia RF, Gargaro LL, Pedrosa MMD, Brito NA, Salgueiro-Pagadigorria CL, Brito MN. Coconut oil reduces visceral adipocyte size and improves the metabolic profile of rats fed a high-carbohydrate diet. J Pharm Pharmacol. 2019;7:98–109. doi: 10.17265/2328-2150/2019.03.002. [DOI] [Google Scholar]
- Sandhya VG, Rajamohan T. Beneficial effects of coconut water feeding on lipid metabolism in cholesterol-fed rats. J Med Food. 2006;9(3):400–407. doi: 10.1089/jmf.2006.9.400. [DOI] [PubMed] [Google Scholar]
- Sandhya VG, Rajamohan T. Comparative evaluation of the hypolipidemic effects of coconut water and lovastatin in rats fed fat–cholesterol-enriched diet. Food Chem Toxicol. 2008;46:3586–3592. doi: 10.1016/jfct.2008.08.030. [DOI] [PubMed] [Google Scholar]
- Seneviratne K, Jayathilaka N (2016) Coconut oil: chemistry and nutrition. Battaramulla: Lakva Publishers, 1–142. Isbn: 978–955–1605–36–0
- Seow CC, Gwee CN. Review-Coconut milk: Chemistry and technology. Int J Food Sci Technol. 1997;32:189–201. doi: 10.1046/j.1365-2621.1997.00400.x. [DOI] [Google Scholar]
- Siddalingaswamy M, Rayaorth A, Khanum F. Anti-diabetic effects of cold and hot extracted virgin coconut oil. J Diabetes Mellitus. 2011;1(4):118–123. doi: 10.4236/jdm.2011.14016. [DOI] [Google Scholar]
- Sorra S, Talukdar J, Gogoi G, Li H, Baishya D, Das B. Coconut kernel extract as a novel chemopreventive agent that targets cancer stemness. Cancer Res. 2019;79(13):1614–1614. doi: 10.1158/1538-7445.AM2019-1614. [DOI] [Google Scholar]
- Srivastava Y, Semwal AD, Majumdar A. Quantitative and qualitative analysis of bioactive components present in virgin coconut oil. Cogent Food & Agric. 2016;2:1–13. doi: 10.1080/23311932.2016.1164929. [DOI] [Google Scholar]
- Srivastava Y, Semwal AD, Sharma GK (2018) Virgin coconut oil as functional oil. Ther Probio Unconvent Foods, 291–301. 10.1016/B978-0-12-814625-5.00015-7
- USDA data (2018) Coconut products - Food composition database: United States Department of Agriculture, Agriculture research service
- Vitrac C, Eyrans SMD, Vitrac X (2019) Coconut Shell Extracts, Compositions Containing Same and Uses. US patent- US 2019 / 0076350 A1
- Yong JWH, Ge L, Ng YF, Tan SG. The chemical composition and biological properties of coconut (Cocos nucifera L.) water. Molecules. 2009;14:5144–5164. doi: 10.3390/molecules14125144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Not applicable.