Abstract
The effects of carboxymethyl cellulose (CMC) coating incorporated with Shirazi thyme (Zataria multiflora) oil nano emulsion (SNE), in different concentrations (10, 20, and 30 mg/ml), on the melanosis and the quality of Pacific white shrimp (Litopenaeus vannamei) was investigated during 10 days in refrigerated temperature (4 ± 0.5 °C). The results showed that incorporating SNE into CMC could significantly (P < 0.05) improve the microbial and lipid oxidation quality of the shrimp. During storage, the incremewnt of total volatile basic-nitrogen and trimethylamine in the SNEs-treated groups were lower than that of the other groups (P < 0.05). Also, the application of SNEs improved the textural, melanosis, and sensory acceptability of the coated shrimps. However, treating the shrimp with SNE in 30 mg/ml concentration caused an increase in the a* and b* values of samples and a decrease in the acceptability of this group. Hence, the SNE incorporation at lower concentrations (10, 20 mg/ml) into CMC coating could be useful in extending the shelf life of the shrimp during refrigerated storage and could be a substitute for sodium metabisulphite.
Keywords: Shirazi thyme, Zataria multiflora, Nano emulsion, Shrimp, Litopenaeus vannamei
Introduction
Shrimp is a highly nourishing, favorable sea food which is susceptible to both microbial and chemical spoilage during storage. Also, melanosis is an enzymatic harmless discoloration which is caused by melanin, insoluble black pigments, and the polymerization of phenols. These spots are an imperative element that can cause visible spoilage and reduce the shrimp’s market value. To retard the melanosis in shrimp, sulfite-based compounds have been used, but these synthetic compounds have some limitations due to their triggering of allergic reactions and potential toxicity (Nirmal and Benjakul 2011). Hence, exploring the new alternatives for these compounds is inevitably a real necessity.
Edible films and coatings, usually made from polysaccharides, proteins, and lipids, are one kind of packaging that can increase the maintenance of foods via acting as moisture, oxygen, carbon dioxide, and vapor barriers (Ojagh et al. 2010). One of the compounds used as an edible films and coatings is carboxymethyl cellulose (CMC). It is a linear, long-chain, hydrophilic, and anionic by-products of cellulose, which can make a non-toxic and viscous solutions (Tongdeesoontorn et al. 2011). According to the previous research, films and coating incorporated into preservatives can enhance the shelf life and features of foods more efficiently. However, nowadays, special attention is paid to natural preservatives; thus, Essential Oils (EO) are considered as a good candidate to be used as a natural preservative in foods.
EOs are plant-made secondary metabolites that their antioxidant and antimicrobial effects have already been confirmed (Burt 2004). A growing body of research suggests that, their performance is positively affected by its nano emulsification. The smaller size (20–200 nm), and larger specific surface, increased biological activity and facilitated dissemination in food products, the latter minimizes the impact of EOs on food flavor (Joe et al. 2012). Shirazi thyme (Zataria multiflora) is a famous medicinal plant and its EO is used in the food, cosmetic, and pharmaceutical industries. Recently, the shelf-life increment of rainbow trout fillets by Farsi gum incorporated with Shirazi thyme oil emulsion has been addressed (Dehghani et al 2018).
Despite a lot of investigations on the use of edible coatings incorporated with EO emulsions in food applications, there is limited information on the potential of nano emulsions to counteract melanosis or extend the shelf life of shrimp (Rahnama et al. 2021). Since Shirazi thyme nano emulsion (SNE) is a natural, nontoxic compound with considerable antimicrobial and antioxidant properties as well as desirable flavor, we hypothesized that its combination with CMC might prolong the shelf life of white leg shrimp (Litopenaeus vannamei) during refrigerated storage and can be used as a substitute for sulphite-based compounds in the shrimp industry.
Materials and methods
Preparation and the analysis of essential oils
Shirazi thyme essential oils were purchased from a regional market (Nourhan, Shiraz, Iran). The gas chromatography-mass spectrophotometer (GC–MS) (3420A. Beijing Beifen-Ruili Analytical Instrument Co., Ltd., Beijing, China) was used to analyze its chemical components (Dehghani et al. 2018).
Preparation of nano emulsions
To prepare a 100 ml stock of nanoemulsion, 6 ml of Shirazi thyme essential oil was added to a mixture of 4.5 ml of Tween 80 (Sigma-Aldrich, Germany) as a surfactant and 4.5 ml of ethanol (Sigma-Aldrich, Germany) as a cosurfactant. They were mixed in a closed bottle and stirred for 30 min. In order to remove ethanol, the mixture was kept for 1 h at 86 °C while the lid was open and finally made up to 100 ml with distilled water. The obtained emulsion was sonicated by ultrasound (TOMY UD-201, Japan) at a power of 200 W and a 20 kHz frequency consecutively for 15 min. The size of the droplets was measured by a dynamic light scattering (DLS) device (W3325, Microtrac, USA) after dilution with deionized water (1:10) at 25 °C and the scattering angle of 90°. Furthermore, the span (distribution width) was determined by applying the following equation:
In this equation, d10, d50, and d90 are the particle diameters related to 10, 50, and 90% intensity on a relative cumulative curve of the particle size dispersion.
Centrifugation and storage steadiness of the nano emulsions
The nano emulsions were centrifuged at 25 °C (4500 rpm for 30 min), and the stability against phase separation (creaming) and mean particle diameter of the samples were determined every 2 days during the 10 days of storage (Gahruie et al. 2017).
Sample preparation and storage conditions
White leg shrimp with the average size of 50–60 shrimp/kg were purchased immediately after harvest from a shrimp farm (Bandar-Abbas, Iran). Harvested shrimps were rinsed with the cold water and randomly divided into six different groups as follows: The control group was not treated with nano emulsion and CMC coating; the CMC control group was just treated with CMC 1% in deionized water (W/V) without essential oil. To make the CMC gel, CMC powder (Wealthy, China, MW = 262.190 KD) was gradually added to deionized water (1% W/V) while agitating the solution by magnetic stirring (Wisemix, Germany) at 80 °C for about 45 min to achieve a clear solution. Other samples were treated with CMC gel incorporated into different concentrations of SNE (10, 20, and 30 mg/ml) at a shrimp/solution proportion of 1:1 (W/V) at 4 °C (different concentrations of SNE were mixed with CMC gel (W/V) and then homogenized at 20,000 rpm for 5 min (Homogenizer, DI18B, Germany)); and the sodium metabisulphite (SMS) group was immersed in SMS (Shandong Kailong, China) (1.25 g/100 ml) at a shrimp/solution ratio of 1:2 (W/V) for 1 min at 4 °C (Kim et al. 2000). Adding the SNE to the CMC gel, dilute the tween 80 in the final shrimp batches to less than the maximum amount allowed by the FDA (Less than 1% of the weight of the finished product). Afterward, samples were drained and packaged with polyethylene bags and kept at 4 °C ± 0.5 for 10 days. All groups had a three independent replication; sampling for the evaluation of microbial, chemical, and sensorial features of the shrimp was carried out every 2 days.
Microbiological analysis
Ten grams of aseptically grounded shrimps was homogenized with 90 ml sterile normal saline, then, a tenfold serial dilution was prepared. Aerobic plate count (APC) and psychrophilic bacteria counts were done by pour plate method on plate count agar (Merck, Germany) after incubation for 2 days at 37 °C and for 10 days at 7 °C, respectively. Pour plate for counting the total lactic acid bacteria (LAB) was also performed on MRS-agar (Merck, Germany) after the incubation at 35 °C for 48 h. Most probable number (MPN) method was used for the numeration of Enterobacteriaceae using MacConkey broth (containing 1% glucose) and incubation at 37 °C for 24 h.
Chemical analysis
The pH was determined using a digital pH meter (CG824, Germany) following the homogenization of 2 g of grounded shrimp samples in 10 mL of deionized water (Basiri et al. 2015).
The assessment of total volatile base nitrogen (TVB-N) was performed as explained by AOAC (2002); briefly, minced shrimp were steam-distilled and the TVB-N scale (mg of nitrogen per 100 g of shrimp meat) was calculated based on the amount of 0.1 M HCl consumed for titration.
A mixture of minced shrimp, MgO, formaldehyde 20% (V/V), and distilled water were steam-distilled and the results were stated as TMA-N value (mg) of 100 g of shrimp meat, based on the amount of 0.05 M HCl which had been used for titration (Goulas and Kontominas’s, 2005).
Thiobarbituric acid (TBA) reactive content was established applying the method described by Benjakul and Bauer (2001), and was expressed as mg malonaldehyde per kg of samples according to the standard curve.
Texture profile analysis
To determine the hardness of shrimp’s texture, texture analyzer (Brookfield, USA) was used by imposing the force on the second segment of each shrimp with a cylindrical plunger of 0.4 cm diameter, bar probe of 0.1 P, and pace of 60 mm/min to a depth of 70% deformation.
Color properties
Surface color analysis of the shrimp, in terms of lightness (L*), redness (a*), and yellowness (b*), was determined according to the method of Yam and Papadakis (2004) with some alterations. Briefly, simple digital images were taken with a Sony color digital camera (DCR-SR65E/SR85E, Tokyo, Japan) located at a 30 cm fixed farness from the surface of the samples in a box (50 × 50 × 60 cm) with interior white color. The angle between the axis of the camera lens and the surface of the sample, and the angle between the surface of the sample and the light source (20-W fluorescent light lamp, Natural Daylight, Cixing, Zhenjiang, China) were 90° and 45°, respectively. Finally, all the surfaces of the pictures were selected and probed in the Lab mode employing Photoshop version 8.0.
Sensory and melanosis appraisement
The sensorial assessment of the raw shrimp was carried out through visual inspection by twelve proficient panelists every 2 days. Panelists gave points for quality parameters, like texture, odor, color, and general admissibility, using a 4-point expressive scale where 1 indicated the poorest quality and dislike, any higher score indicated a better quality, and 4 indicated like extremely.
Statistical analysis
All experiments were accomplished in three independent samples and analyzed using Statistical Package for Social Sciences (SPSS) software (SPSS 25 for windows, SPSS Inc, Chicago, IL, USA). The parametric data were analyzed using analysis of variance (ANOVA) and the statistical significance of differences between mean values was analyzed by Duncan’s multiple range tests. Moreover, Pearson's correlation coefficient test was applied to assess the correlation between variables. The Kruskal–Wallis, and Mann–Whitney U tests were used for determining differences in non-parametric data. Statistical Significance was considered as P < 0.05.
Result and discussion
Essential oil composition
According to GC–MS analysis, the main chemical constituent of SEO was thymol (44.52%) (Table 1). The results were consistent with those reported by Golmakani and Rezaei (2008). In contrast, Gahruie et al. (2017) reported carvacrol as the main constituent of SEO. The differences can be attributed to the extraction method and environmental factors like variations in cultivation and climate conditions (Moosavi-Nasab et al. 2016).
Table 1.
Chemical composition of Shirazi Thyme essential oil (SEO)
| Compounds | Amounts (%) |
|---|---|
| Thymol | 44.52 |
| Benzen, 1-methyl-2-(1-methyl)-(CAS gamma) | 11.64 |
| Terpinene | 6.72 |
| Alpha Pinene | 4.97 |
| Trans-Caryophyllene | 3.76 |
| Thymyl acetate | 2.5 |
| Alpha terpinene | 1.92 |
| Carvacrol methyl ether | 1.58 |
| Linalool l | 1.35 |
| Carvacryl acetate | 1.92 |
| Beta-Pinene | 1.06 |
| Caryophyllene oxide | 1.31 |
| Benzene,2-methoxy-4-methyl-1-(methylethyl) | 0.87 |
| Cyclohexanone,2-methyl-5-(1-methylethenyl)-,trans | 0.85 |
| Alpha terpineol | 0.76 |
| dl-Limonene | 0.70 |
| Spathulennol (ethyl-5) | 1.15 |
| Cyclopent-1-enyl) | 0.54 |
Droplet size and the stability of SNE
After sonication, mean droplet size was found in the range of 74.1 ± 18.24 nm, which was near to droplet size reported by Gahruie et al. (2017) (90.9 nm) for thyme nano emulsion. However, Wu et al. (2014) found higher values for mean droplet size. Various parameters including sonication conditions and oil concentration, affect the particle size of nano emulsions (Li and Chiang 2012). Initial particle size (74.1 ± 18.24 nm) of SNE increased and reached to 129.75 ± 2.05 nm after 10 days and also during this time no phase separation (creaming) was observed after centrifugation, so according to this evidence SNE had sufficient stability during 10 days storage. The distribution curve for SNE presented a monomodal shape.
Distribution width (Span) is related to the sonication time and homogeneity. Increasing the sonication time leads to greater uniformity, and lower span. Span decreased after sonication from 0.8053 to 0.5679. The initial span value was lower than the value reported by Gahruie et al. (2017) (2.586) and, higher than the value reported by Dehghani et al. (2018) (0.5). The span value was increased during storage time (0.9294) that might be attributed to cluster formation (assembly of multiple oil droplets into clusters) (Gahruie et al. 2017).
Microbiological analysis
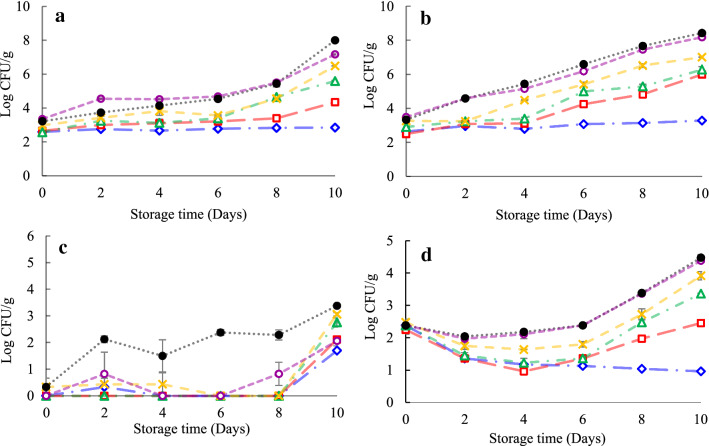
Figure 1a–d shows the changes in APC, psychrophilic, LAB, and Enterobacteriaceae counts in the treatment groups during 10 days of storage, respectively. The initial amount of APC (~ 2.5 log CFU/g) in the shrimp was similar to what had been explained in the former researches (Arancibia et al. 2015). At the 10th day of storage, an increase in APC was observed in all samples and, the APC of the control was significantly greater than that of the other groups (P < 0.05). By increasing the percentage of SNE, the APC decreased significantly (P < 0.05). On the other hand, the rise in the APC of the shrimp treated with SMS was significantly higher than that of the shrimp treated by different concentrations of SNE (P < 0.05). Additionally, the same results were observed regarding the psychrophilic count on the 10th day of the storage (Fig. 1b).
Fig. 1.
Aerobic plate count (a), psychrophilic (b), Lactic acid bacteria (c), and Enterobacteriaceae (d) count of shrimps treated with Shirazi thyme oil nanoemulsion (SNE) at three levels during refrigerated storage. ●: Control,  : CMC (Carboxy Methyl Cellulose) 1%,
: CMC (Carboxy Methyl Cellulose) 1%,  : SMS (sodium metabisulphite 1.25%),
: SMS (sodium metabisulphite 1.25%),  : SNE10 (CMC 1% with 10 mg/ml SNE),
: SNE10 (CMC 1% with 10 mg/ml SNE),  : SNE20 (CMC 1% with 20 mg/ml SNE),
: SNE20 (CMC 1% with 20 mg/ml SNE),  : SNE30 (CMC 1% with 30 mg/ml SNE) (n = 3)
: SNE30 (CMC 1% with 30 mg/ml SNE) (n = 3)
According to Fig. 1c, at the end of the storage, the SNE30 treatment was more effective in reducing the LAB counts (P < 0.05), and the LAB count of the control was considerably higher than that of the other groups (P < 0.05). Also, there were no considerable difference between the LAB count of SNE10 and SMS group (P > 0.05). Similarly, Dehghani et al. (2018) revealed that the LAB growth was slow at the refrigeration temperatures.
The Enterobacteriaceae counts of the control (4.47 ± 0.16) and CMC group (4.38 ± 0.00) from day 2 until the last day of the storage were greater than those of other groups (P < 0.05). Furthermore, a higher Enterobacteriaceae count was acquired in those exposed to SMS compared to different concentrations of SNE (P < 0.05, Fig. 1d).
Generally, SNEs showed inhibitory effect on proliferation of spoilage bacteria in shrimp through the storage. According to the previous studies, thymol is a monoterpene that can degrade microbial cells by various strategies: deteriorating outer cell membrane of Gram-negative bacteria, increasing the membrane permeability of Gram-positive bacteria, disrupting enzyme complexes, and amending the genetic contents (Burt 2004). The same results were reported by other investigators. Erkan et al. (2011) showed that bluefish (Pomatomus saltatrix) treated with thyme oil at 2 °C had lower microbial growth than the control, and shelf life of the treated samples enhanced by 3–4 days compared to the control samples. These findings are compatible to those of the Moosavy et al. (2008) who showed that carvacrol and thymol had inhibitory effects against some bacteria, such as Escherichia coli O157 H7, Listeria monocytogenes, and S. Typhimurium. According to these outcomes, it can be figured that the CMC coating incorporated into SNE can be a natural preservative for extending the shelf life of shrimp stored in refrigeration conditions.
Chemical analysis
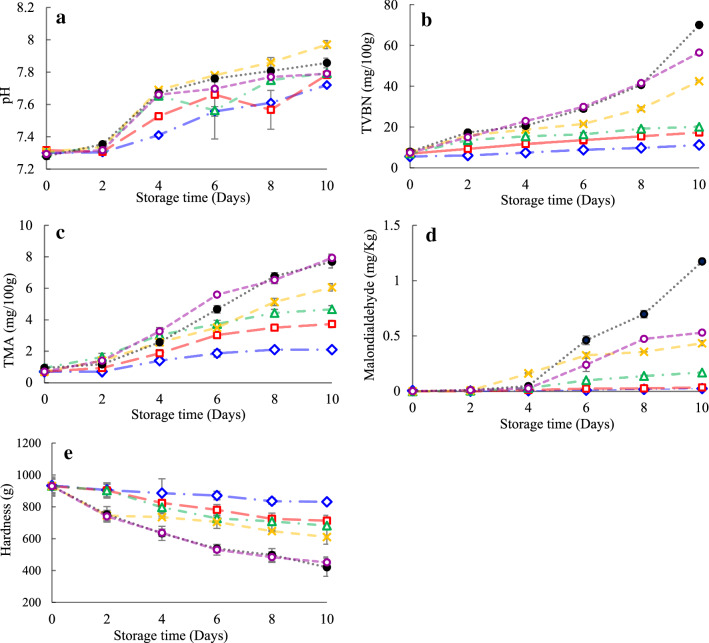
Figure 2a shows the pH changes in distinct groups of shrimps throughout the refrigerated maintenance. In the beginning, the pH of all shrimps was similar and around 7.3, which was close to the previous studies (Nirmal and Benjakul 2011; Basiri et al. 2015). On the 10th day of the storage, the minimal and the maximal pH were acquired in the SNE30 and SMS groups, respectively (P < 0.05). Also, there was no significant difference in the pH values of SNE10, SNE20, and CMC groups (P > 0.05). The aggregation of basic substances, such as TVB-N, TMA, and biogenic amines, due to microbial or enzymatic action led to a rise in pH scale of shrimp through storage (Basiri et al. 2015). Although the microbial count of SNE20 and SNE10 was noticeably lower than that of the CMC group (P < 0.05), the CMC group had lower pH value. As a result, as Huang et al. (2012) reported, it can be concluded that pH is not a suitable indicator for shrimp quality.
Fig. 2.
pH (a), TVB-N (b), TMA (c) Malondialdehyde (MDA) (d) and Hardness (e) of shrimps treated with Shirazi thyme oil nanoemulsion (SNE) at three levels during refrigerated storage. ●: Control,  : CMC (Carboxy Methyl Cellulose) 1%,
: CMC (Carboxy Methyl Cellulose) 1%,  : SMS (sodium metabisulphite 1.25%),
: SMS (sodium metabisulphite 1.25%),  : SNE10 (CMC 1% with 10 mg/ml SNE),
: SNE10 (CMC 1% with 10 mg/ml SNE),  : SNE20 (CMC 1% with 20 mg/ml SNE),
: SNE20 (CMC 1% with 20 mg/ml SNE),  : SNE30 (CMC 1% with 30 mg/ml SNE) (n = 3)
: SNE30 (CMC 1% with 30 mg/ml SNE) (n = 3)
The modifications in TVB-N and TMA for all the treatments are shown in Fig. 2b, c, respectively. The TVB-N and TMA concentration of samples rose considerably (P < 0.05) and, after 10 days of storage, the TVB-N content of the control group (70.0 mg N/100 g) and the TMA content of the control (7.70 mg N/100 g) and CMC (7.93 mg N/100 g) groups had a higher value compared to other treatments (P < 0.05). Also, the slightest TVB-N and TMA values were observed in the SNE30 group (P < 0.05). SMS group showed significantly higher TVB-N and TMA values compared to groups treated by SNEs (P < 0.05). TVB-N is an indicator that can show ammonia (primary, secondary, and tertiary amines) resulted from the spoilage bacteria’s action and autogenous enzymes (Huang et al. 2012; Arancibia et al. 2015). Furthermore, our results showed a positive significant correlation between the TVB-N content of the treated shrimps and APC (r = 0.93, P = 0.00), PSB (r = 0.90, P = 0.00), LAB (r = 0.62, P = 0.00) and Enterobacteriaceae counts (r = 0.79, P = 0.00) of them.
Trimethylamine (TMA), generated from Trimethylamine oxide via enzymatic and microbial actions, is considered to be an imperative spoilage indicator in marine products. Positive significant correlations were observed between TMA and APC (r = 0.87, P = 0.00), PBC (r = 0.92, P = 0.00), LAB (r = 0.53, P = 0.00) and Enterobacteriaceae counts (r = 0.70, P = 0.00).
Generally, the lower TVB-N and TMA values were observed in the groups treated with different concentrations of SNE; this was associated with the functionality of the phenolic compounds in SEO. These compounds are able to prevent microbial growth and decrease the microbial degradation of the products. Some other researches (Joe et al. 2012; Dehghani et al. 2018) have reported similar results. Lower TVB-N and TMA values of the SMS group could be explained by Kawai and Sakaguchi (1996), who reported that SMS could prevent enzymatic activities.
TBARS is regarded as indicators of the secondary lipid oxidation products, especially aldehydes (Cai et al. 2014). Aldehydes, like malondialdehyde (MDA), can cause red pigments to develop after reacting with thiobarbituric acid (TBA). Thus, a certain amount of these oxidation products is expressed in the TBARS value. The introductory TBARS value of the shrimp was 0.006 mg MDA/kg, that was fewer than Nirmal and Benjakul (2011). The TBARS values in all samples increased up to the 10th day (P < 0.05), but the trend was substantially slower (P < 0.05) in the SNE30 and SNE20 groups (Fig. 2d). For other groups, the TBARS followed this order: SNE10 < SMS < CMC < control. Generally, the oxygen increases the formation of TBARS (Cai et al. 2014); edible coating can reduce the rate of oxidation by reducing the entry of gases and moisture (Bravin et al. 2006). Furthermore, due to the antioxidant potential of carvacrol and thymol, their presence in SNE caused a reduction in the TBARS values in the groups treated with SNE. The SMS group showed lower oxidation than the control and the CMC group; compatible findings were reported by Basiri et al. (2015).
Texture profile analysis
Texture is considered as one of the most imperative sensory variables for consumer admissibility and among the attributes subsumed under texture, hardness seems to be the key textural attribute in meat or marine products. The hardness of shrimp treated by SNE30 was significantly higher than that of the other groups on the 6th, 8th, and 10th days of the storage (P < 0.05) (Fig. 2e). Furthermore, the trend of the decrease in hardness was dramatically higher in the CMC and control groups (P < 0.05).
Previous research has shown that protein structure and the proteolytic activity of the endogenous or microbial proteinases and collagenase of shrimp could contribute to the hardness value (Diaz-Tenorio et al. 2007). A negative correlation between the APC and the hardness of different groups (r = − 0.80, P = 0.00) could confirm these results. Our findings are in agreement with those of Alotaibi and Tahergorabi (2018), who observed that coating shrimps with sweet potato starch-based incorporated into different concentrations of thyme essential oil did not have any impact on shrimp TPA, except for hardness through the storage time.
Color properties
The alteration in L*, a*, and b* values of the shrimp are shown in Table 2. The L* value of all the samples declined significantly over the storage (P < 0.05). The result was similar to that of the previous research, which associated it to the emergence of black spots, melanosis (by oxidation of phenols), and quinines (by polyphenol oxidases) (Arancibia et al. 2015). This reduction was faster in the control group compared to that of the others from day two up to the end of the storage (P < 0.05). Also, there was no significant difference between the L* values of the coated groups at the last day of storage (10th day) (P > 0.05). In addition, the L* value of the SMS group from the 4th day of the storage was lower than that of the coated groups (P < 0.05). Generally, at the end of the storage, coating the shrimp with SNE30 caused a significant increase in the a* and b* values (P < 0.05), and no considerable difference was observed between the a* and b* values of the CMC, SNE20, and SNE10 groups (P > 0.05). Surprisingly, the lowest a* and b* values (P < 0.05) was observed in the control and the SMS groups in the last day of the storage, which can be explained by the appearing of noticeable black spots on the surface of shrimp. Similarly, Alotaibi and Tahergorabi (2018) reported that treating shrimp with thyme essential oil could significantly enhance the b* values, which could be associated with the pigmentation of thyme essential oil. On the other side, Dehghani et al. (2018) declared that the changes in the total color difference in the rainbow trout’s fillets coated by Farsi gum and enriched by clove and thyme emulsions were lower than non-coated ones.
Table 2.
Effect of Carboxy Methyl Cellulose (CMC) coating incorporated with Shirazi thyme oil nanoemulsion (SNE) at three levels, on color properties of shrimp during 10 days of refrigerated storage
| Days of storage | Treatments | ||||||
|---|---|---|---|---|---|---|---|
| Control | CMC | SMS | SNE10 | SNE20 | SNE30 | ||
| L* | 0 | 56.33 ± 1.15aC | 56.00 ± 1.73aB | 54.33 ± 3.79aD | 52.67 ± 4.62aA | 52.33 ± 2.31aB | 57.67 ± 2.08aC |
| 2 | 53.33 ± 0.58cAB | 44.33 ± 1.53aB | 49.33 ± 2.08bC | 51.67 ± 0.58cA | 51.33 ± 0.58bcB | 58.67 ± 1.53dC | |
| 4 | 42.00 ± 2.65aB | 51.67 ± 1.53cA | 46.00 ± 1.00bBC | 51.67 ± 1.15cA | 51.00 ± 1.00cAB | 58.67 ± 0.58dC | |
| 6 | 42.00 ± 1.00aB | 50.67 ± 0.58bA | 44.00 ± 2.65aABC | 50.33 ± 2.31bA | 50.67 ± 1.53bAB | 53.33 ± 2.08bB | |
| 8 | 40.67 ± 1.15aB | 50.00 ± 3.61bA | 42.67 ± 1.53aAB | 50.67 ± 5.69bA | 48.67 ± 1.53bAB | 48.67 ± 3.06bA | |
| 10 | 33.67 ± 3.51aA | 50.00 ± 1.00cA | 40.67 ± 2.31bA | 46.67 ± 2.08cA | 48.00 ± 1.00cA | 48.67 ± 1.15cA | |
| a* | 0 | 3.00 ± 0.00a | 3.33 ± 0.58a | 3.00 ± 0.00a | 3.00 ± 1.00a | 3.33 ± 0.58a | 3.33 ± 0.58a |
| 2 | 2.33 ± 0.58a | 3.00 ± 0.00a | 3.33 ± 0.58ba | 3.33 ± 1.15ba | 2.67 ± 0.58a | 4.33 ± 0.58b | |
| 4 | 3.00 ± 0.00ab | 2.33 ± 0.58a | 2.00 ± 1.00a | 3.67 ± 0.58abc | 5.33 ± 2.52c | 4.67 ± 0.58bc | |
| 6 | 2.33 ± 0.58a | 3.33 ± 0.58a | 2.33 ± 0.58a | 3.67 ± 1.53a | 6.00 ± 1.00b | 7.00 ± 1.00b | |
| 8 | 2.00 ± 1.00a | 3.33 ± 0.58ab | 2.33 ± 0.58ab | 4.00 ± 1.73ab | 4.33 ± 0.58b | 8.33 ± 1.53c | |
| 10 | 2.67 ± 0.58a | 3.67 ± 0.58ab | 2.33 ± 0.58a | 5.00 ± 1.73bc | 6.00 ± 1.00c | 18.00 ± 0.00d | |
| b* | 0 | 14.00 ± 0.00a | 14.33 ± 1.53a | 14.00 ± 1.00a | 14.00 ± 1.00a | 14.33 ± 0.58a | 14.00 ± 3.00a |
| 2 | 14.00 ± 0.58a | 14.33 ± 1.73b | 14.00 ± 1.00b | 14.00 ± 0.58b | 14.33 ± 2.52b | 14.00 ± 0.58c | |
| 4 | 10.67 ± 2.08a | 12.33 ± 1.15ab | 10.33 ± 1.15a | 14.33 ± 0.58b | 14.67 ± 1.53b | 21.67 ± 1.53c | |
| 6 | 10.33 ± 1.15a | 14.33 ± 1.15ab | 10.33 ± 1.15a | 14.67 ± 1.15ab | 17.67 ± 3.79bc | 22.00 ± 4.36c | |
| 8 | 9.33 ± 1.15a | 14.33 ± 0.58b | 8.67 ± 0.58a | 15.00 ± 1.00b | 13.33 ± 2.08b | 22.00 ± 2.00c | |
| 10 | 7.67 ± 3.06a | 14.33 ± 2.08b | 9.67 ± 0.58a | 15.00 ± 0.00b | 16.67 ± 1.15b | 34.67 ± 1.15c | |
Data are given as mean values ± standard deviation (n = 3). CMC: Carboxy Methyl Cellulose; SMS: Sodium Metabisulphite; SNE10: Shirazi thyme oil nanoemulsion 10 mg/mL; SNE20: Shirazi thyme oil nanoemulsion 20 mg/mL; SNE30: Shirazi thyme oil nanoemulsion 30 mg/mL. Minuscules indicate the significant differences within the same row and majuscule indicate significant differences within the same columns (P < 0.05)
Sensory and melanosis analysis
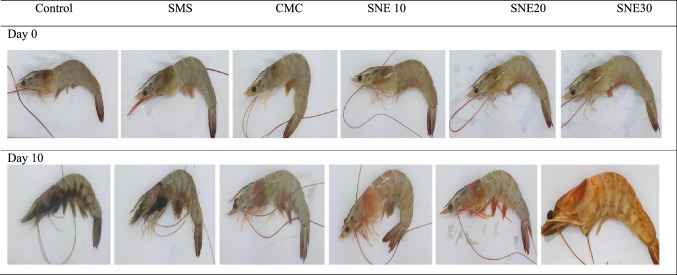
According to Table 3, significant differences in odor and texture were observed between CMC, SMS and control groups and coated shrimps containing SNEs from the 4th and the 6th day of storage respectively. But scores of colors (Fig. 3) at the end of storage showed that shrimps treated with SNE30 with color alteration to light orange (because of increase in a* and b* values), achieved lowest score and there was no significant difference between scores of SNE30, SMS and control groups. Finally, scores of acceptability at the 10th day of storage followed this order: SNE30, CMC, SMS and control < SNE20 and SNE10.
Table 3.
Effect of Carboxy Methyl Cellulose (CMC) coating incorporated with Shirazi thyme oil nanoemulsion (SNE) at three levels, on the sensory attributes of shrimp during 10 days of refrigerated storage
| Sensory attributes | Days of storage | Treatments | |||||
|---|---|---|---|---|---|---|---|
| Control | CMC | SMS | SNE10 | SNE20 | SNE30 | ||
| Odor | 0 | 4.0 ± 0.00a | 4.0 ± 0.00a | 4.0 ± 0.00a | 4.0 ± 0.00a | 4.0 ± 0.00a | 4.0 ± 0.00a |
| 2 | 3.2 ± 0.25b | 3.4 ± 0.51b | 3.8 ± 0.52a | 4.0 ± 0.00a | 4.0 ± 0.00a | 4.0 ± 0.00a | |
| 4 | 2.3 ± 0.52b | 2.5 ± 0.58b | 2.6 ± 0.58b | 3.5 ± 0.25a | 3.2 ± 0.62a | 3.5 ± 0.52a | |
| 6 | 2.1 ± 0.67b | 2.1 ± 0.45b | 2.3 ± 0.58b | 3.1 ± 0.54a | 3.2 ± 0.54a | 3.4 ± 0.51a | |
| 8 | 1.0 ± 0.00c | 1.2 ± 0.94c | 2.0 ± 0.00b | 3.1 ± 0.45a | 3.1 ± 0.52a | 3.2 ± 0.54a | |
| 10 | 1.0 ± 0.00c | 1.0 ± 0.00c | 1.6 ± 0.58b | 3.1 ± 0.52a | 3.1 ± 0.71a | 3.2 ± 0.60a | |
| Color | 0 | 4.0 ± 0.00a | 4.0 ± 0.00a | 4.0 ± 0.00a | 4.0 ± 0.00a | 4.0 ± 0.00a | 4.0 ± 0.00a |
| 2 | 3.5 ± 0.60b | 4.0 ± 0.00a | 4.0 ± 0.00a | 4.0 ± 0.00a | 4.0 ± 0.00a | 4.0 ± 0.00a | |
| 4 | 2.2 ± 0.94c | 3.8 ± 0.25a | 2.3 ± 0.58c | 3.8 ± 0.52a | 3.1 ± 0.60b | 3.1 ± 0.52b | |
| 6 | 1.3 ± 0.58c | 3.3 ± 0.53a | 1.3 ± 0.87c | 3.2 ± 0.87a | 3.3 ± 0.84a | 2.3 ± 0.58b | |
| 8 | 1.0 ± 0.00c | 2.7 ± 0.89a | 1.1 ± 0.52c | 2.8 ± 0.78a | 2.5 ± 0.58a | 2.1 ± 0.89b | |
| 10 | 1.0 ± 0.00b | 2.5 ± 0.94a | 1.0 ± 0.00b | 2.1 ± 0.25a | 2.2 ± 0.84a | 1.3 ± 0.58b | |
| Texture | 0 | 4.0 ± 0.00a | 4.0 ± 0.00a | 4.0 ± 0.00a | 4.0 ± 0.00a | 4.0 ± 0.00a | 4.0 ± 0.00a |
| 2 | 4.0 ± 0.00a | 3.8 ± 0.89a | 4.0 ± 0.00a | 4.0 ± 0.00a | 4.0 ± 0.00a | 4.0 ± 0.00a | |
| 4 | 3.1 ± 0.58b | 3.1 ± 0.52b | 3.1 ± 0.78b | 3.2 ± 0.52b | 3.2 ± 0.58b | 3.8 ± 0.52a | |
| 6 | 2.5 ± 0.52b | 2.3 ± 0.58b | 2.4 ± 0.68b | 3.1 ± 0.52a | 3.2 ± 0.84a | 3.5 ± 0.71a | |
| 8 | 2.1 ± 0.52b | 2.1 ± 0.54b | 2.2 ± 0.84b | 3.0 ± 0.00a | 2.8 ± 0.58a | 3.1 ± 0.94a | |
| 10 | 1.8 ± 0.58b | 2.1 ± 0.54b | 2.1 ± 0.60b | 3.1 ± 0.45a | 3.1 ± 0.84a | 3.0 ± 0.00a | |
| Overall acceptability | 0 | 4.0 ± 0.00a | 4.0 ± 0.00a | 4.0 ± 0.00a | 4.0 ± 0.00a | 4.0 ± 0.00a | 4.0 ± 0.00a |
| 2 | 3.2 ± 0.58b | 3.2 ± 0.68b | 3.8 ± 0.58a | 4.0 ± 0.00a | 4.0 ± 0.00a | 4.0 ± 0.00a | |
| 4 | 2.4 ± 0.94b | 2.3 ± 0.78b | 2.6 ± 0.58a | 3.2 ± 0.84a | 3.2 ± 0.54a | 3.5 ± 0.52a | |
| 6 | 2.2 ± 0.60b | 2.1 ± 0.60b | 2.3 ± 0.58b | 3.1 ± 0.60a | 3.3 ± 0.84a | 3.2 ± 0.54a | |
| 8 | 1.0 ± 0.00c | 1.0 ± 0.00c | 2.2 ± 0.94b | 3.2 ± 0.89a | 2.4 ± 0.87b | 2.3 ± 0.58b | |
| 10 | 1.0 ± 0.00b | 1.0 ± 0.00b | 1.3 ± 0.87b | 2.6 ± 0.58a | 2.3 ± 0.58a | 1.2 ± 0.52b | |
Data are given as mean values ± standard deviation (n = 3). CMC: Carboxy Methyl Cellulose; SMS: Sodium Metabisulphite; SNE10: Shirazi thyme oil nanoemulsion 10 mg/mL; SNE20: Shirazi thyme oil nanoemulsion 20 mg/mL; SNE30: Shirazi thyme oil nanoemulsion 30 mg/mL. Sensory score rating: 1: dislike; 2: neither like nor dislike; 3: like moderately; 4: like extremely. Different letters within the same row indicate significant differences (p < 0.05)
Fig. 3.
Effect of different treatments on the appearance of shrimp after 10 days of storage. CMC Carboxy methyl cellulose, SMS sodium metabisulphite, SNE10 shirazi thyme oil nanoemulsion 10 mg/mL, SNE20 Shirazi thyme oil nanoemulsion 20 mg/mL, SNE30 Shirazi thyme oil nanoemulsion 30 mg/mL
Overall, higher sensorial scores could be justified by SNE and CMC characteristics like being an antioxidant, an antimicrobial agent and oxygen barrier in comparison with the control group (Dashipour et al. 2015; Varela and Fiszman 2011) and the results of sensory assessments were paralleled with those of microbial analyses.
Conclusion
Our findings revealed that CMC coatings containing SNEs were able to extend the shelf life and maintain the quality of shrimp stored in refrigeration conditions by retarding the microbial growth, melanosis, and chemical reactions. The application of these structures could improve the texture and sensory features of the coated shrimp. Additionally, a significant inhibitory effect was seen when the SNE concentration was raised, possibly because of the great contents of thymol. It thus could be concluded that SNE could inhibit melanosis more effectively than SMS and that it can be used as a promising alternative to SMS. However, treatment of shrimp with SNE at a concentration of 30 mg/ml increased the a* and b* values of the samples, changed the shrimp’s color and decreased their acceptance. Consequently, SNE in lower concentrations (10 or 20 mg/ml) could be more beneficial in extending the shelf life of shrimp throughout the refrigerating condition.
Acknowledgements
This research was financially supported by Shiraz University, which is gratefully acknowledged. The authors would like to thank the staff of the Department of Food Hygiene and Public Health, School of Veterinary Medicine, Shiraz University.
Abbreviations
- SNE
Shirazi thyme oil nano emulsion
- CMC
Carboxymethyl cellulose
- TVB-N
Total volatile basic-nitrogen
- TMA
Trimethylamine
- TBARS
Thiobarbituric acid reactive specious
- EO
Essential oils
- DLS
Dynamic light scattering
Authors' contributions
FR Investigation, Validation, Writing the original draft. SH Conceptualization, Methodology, Review and editing the manuscript. SB Conceptualization, Investigation, Methodology, Review and editing the manuscript. MTG Conceptualization, Methodology, Review and editing the manuscript. AG Conceptualization,Methodology, Review and editing the manuscript. Seyed Shahram Shekarforoush: Conceptualization, Methodology, Project administration, Supervision, Review and editing the manuscript. (i) The work described has not been published before, (ii) it is not under consideration for publication elsewhere, (iii) its submission to JFST publication has been approved by all authors, (iv) if accepted, it will not be published elsewhere in the same form, in English or in any other language, including electronically, without the written consent of the copyright holder, and (v) JFST will not be held legally responsible should there be any claims for compensation or dispute on authorship.
Funding
This research was financially supported by Shiraz University.
Availability of data and material
The data will be provided in the editor request.
Code availability
No case.
Declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
Ethics approval
Not applicable.
Consent to participate
All participants, including sensory panelists had consented to participate in the study.
Consent for publication
All authors have consented to for publication in Journal of Food Science and Technology.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alotaibi S, Tahergorabi R. Development of a sweet potato starch-based coating and its effect on quality attributes of shrimp during refrigerated storage. LWT. 2018;88:203–209. doi: 10.1016/j.lwt.2017.10.022. [DOI] [Google Scholar]
- AOAC Official Method 999-01 (2002) Volatile bases in fish, ammonia ion selective electrode method. In: AOAC Official methods of analysis of AOAC international, vol 2, Chapter 35, pp 34–35
- Arancibia MY, Lopez-Caballero ME, Gomez-Guillen MC, Montero P. Chitosan coatings enriched with active shrimp waste for shrimp preservation. Food Control. 2015;54:259–266. doi: 10.1016/j.foodcont.2015.02.004. [DOI] [Google Scholar]
- Basiri S, Shekarforoush SS, Aminlari M, Akbari S. The effect of pomegranate peel extract (PPE) on the polyphenol oxidase (PPO) and quality of Pacific white shrimp (Litopenaeus vannamei) during refrigerated storage. LWT Food Sci Technol. 2015;60(2):1025–1033. doi: 10.1016/j.lwt.2014.10.043. [DOI] [Google Scholar]
- Benjakul S, Bauer F. Biochemical and physicochemical changes in catfish (Silurus glanis Linne) muscle as influenced by different freeze–thaw cycles. Food Chem. 2001;72(2):207–217. doi: 10.1016/S0308-8146(00)00222-3. [DOI] [Google Scholar]
- Bravin B, Peressini D, Sensidoni A. Development and application of polysaccharide–lipid edible coating to extend shelf-life of dry bakery products. J Food Eng. 2006;76(3):280–290. doi: 10.1016/j.jfoodeng.2005.05.021. [DOI] [Google Scholar]
- Burt S. Essential oils: their antibacterial properties and potential applications in foods a review. Int J Food Microbiol. 2004;94(3):223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Cai L, Wu X, Dong Z, Li X, Yi S, Li J. Physicochemical responses and quality changes of red sea bream (Pagrosomus major) to gum arabic coating enriched with ergothioneine treatment during refrigerated storage. Food Chem. 2014;160:82–89. doi: 10.1016/j.foodchem.2014.03.093. [DOI] [PubMed] [Google Scholar]
- Dashipour A, Razavilar V, Hosseini H, Shojaee-Aliabadi S, German JB, Ghanati K, Khakpour M, Khaksar R. Antioxidant and antimicrobial carboxymethyl cellulose films containing Zataria multiflora essential oil. Int J Biol Macromol. 2015;72:606–613. doi: 10.1016/j.ijbiomac.2014.09.006. [DOI] [PubMed] [Google Scholar]
- Dehghani P, Hosseini SM, Golmakani MT, Majdinasab M, Esteghlal S. Shelf-life extension of refrigerated rainbow trout fillets using total Farsi gum-based coatings containing clove and thyme essential oils emulsions. Food Hydrocoll. 2018;77:677–688. doi: 10.1016/j.foodhyd.2017.11.009. [DOI] [Google Scholar]
- Diaz-Tenorio LM, Garcia-Carreno FL, Pacheco-Aguilar RA. Comparison of freezing and thawing treatments on muscle properties of whiteleg shrimp (Litopenaeus vannamei) J Food Biochem. 2007;31(5):563–576. doi: 10.1111/j.1745-4514.2007.00130.x. [DOI] [Google Scholar]
- Erkan N, Tosun ŞY, Ulusoy Ş, Üretener G. The use of thyme and laurel essential oil treatments to extend the shelf life of bluefish (Pomatomus saltatrix) during storage in ice. J Verbrauch Lebensm. 2011;6(1):39–48. doi: 10.1007/s00003-010-0609-8. [DOI] [Google Scholar]
- Gahruie HH, Ziaee E, Eskandari MH, Hosseini SM. Characterization of basil seed gum-based edible films incorporated with Zataria multiflora essential oil nanoemulsion. Carbohydr. 2017;166:93–103. doi: 10.1016/j.carbpol.2017.02.103. [DOI] [PubMed] [Google Scholar]
- Goulas AE, Kontominas MG. Effect of salting and smoking-method on the keeping quality of chub mackerel (Scomber japonicus): biochemical and sensory attributes. Food Chem. 2005;93(3):511–520. doi: 10.1016/j.foodchem.2004.09.040. [DOI] [Google Scholar]
- Huang J, Chen Q, Qiu M, Li S. Chitosan-based edible coatings for quality preservation of postharvest whiteleg shrimp (Litopenaeus vannamei) J Food Sci. 2012;77(4):C491–C496. doi: 10.1111/j.1750-3841.2012.02651.x. [DOI] [PubMed] [Google Scholar]
- Joe MM, Chauhan PS, Bradeeba K, Shagol C, Sivakumaar PK, Sa T. Influence of sunflower oil based nanoemulsion (AUSN-4) on the shelf life and quality of Indo-Pacific king mackerel (Scomberomorus guttatus) steaks stored at 20 C. Food Control. 2012;23(2):564–570. doi: 10.1016/j.foodcont.2011.08.032. [DOI] [Google Scholar]
- Kawai T, Sakaguchi M. Fish flavor. Crit Rev Food Sci Nutr. 1996;36(3):257–298. doi: 10.1080/10408399609527725. [DOI] [PubMed] [Google Scholar]
- Kim J, Marshall MR, Wei C. Polyphenoloxidase. In: Haard NF, Simpson BK, editors. Seafood enzymes: utilization and influence on post harvest seafood quality. New York: Marcel Dekker; 2000. pp. 271–315. [Google Scholar]
- Li PH, Chiang BH. Process optimization and stability of D-limonene-in-water nanoemulsions prepared by ultrasonic emulsification using response surface methodology. Ultrason Sonochem. 2012;19(1):192–197. doi: 10.1016/j.ultsonch.2011.05.017. [DOI] [PubMed] [Google Scholar]
- Moosavi-Nasab M, Jamal Saharkhiz M, Ziaee E, Moayedi F, Koshani R, Azizi R. Chemical compositions and antibacterial activities of five selected aromatic plants essential oils against food-borne pathogens and spoilage bacteria. J Essent Oil Res. 2016;28(3):241–251. doi: 10.1080/10412905.2015.1119762. [DOI] [Google Scholar]
- Moosavy MH, Basti AA, Misaghi A, Salehi TZ, Abbasifar R, Mousavi HA, Alipour M, Razavi NE, Gandomi H, Noori N. Effect of Zataria multiflora Boiss Essential oil and nisin on Salmonella typhimurium and Staphylococcus aureus in a food model system and on the bacterial cell membranes. Food Res Int. 2008;41(10):1050–1057. doi: 10.1016/j.foodres.2008.07.018. [DOI] [Google Scholar]
- Nirmal NP, Benjakul S. Retardation of quality changes of Pacific white shrimp by green tea extract treatment and modified atmosphere packaging during refrigerated storage. Int J Food Microbiol. 2011;149(3):247–253. doi: 10.1016/j.ijfoodmicro.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Ojagh SM, Rezaei M, Razavi SH, Hosseini SM. Effect of chitosan coatings enriched with cinnamon oil on the quality of refrigerated rainbow trout. Food Chem. 2010;120(1):193–198. doi: 10.1016/j.foodchem.2009.10.006. [DOI] [Google Scholar]
- Rahnama M, Anvar SA, Ahari H, Kazempoor R. Antibacterial effects of extracted corn zein with garlic extract-based nanoemulsion on the shelf life of Vannamei prawn (Litopenaeus vannamei) at refrigerated temperature. J Food Sci. 2021;86(11):4969–4990. doi: 10.1111/1750-3841.15923. [DOI] [PubMed] [Google Scholar]
- Tongdeesoontorn W, Mauer LJ, Wongruong S, Sriburi P, Rachtanapun P. Effect of carboxymethyl cellulose concentration on physical properties of biodegradable cassava starch-based films. Chem Cent J. 2011;5(1):6. doi: 10.1186/1752-153X-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela P, Fiszman SM. Hydrocolloids in fried foods. A review. Food Hydrocoll. 2011;25(8):1801–1812. doi: 10.1016/j.foodhyd.2011.01.016. [DOI] [Google Scholar]
- Wu JE, Lin J, Zhong Q. Physical and antimicrobial characteristics of thyme oil emulsified with soluble soybean polysaccharide. Food Hydrocoll. 2014;39:144–150. doi: 10.1016/j.foodhyd.2013.12.029. [DOI] [Google Scholar]
- Yam KL, Papadakis SE. A simple digital imaging method for measuring and analyzing color of food surfaces. J Food Eng. 2004;61(1):137–142. doi: 10.1016/S0260-8774(03)00195-X. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data will be provided in the editor request.
No case.
The data that support the findings of this study are available on request from the corresponding author.