Abstract
It is estimated that in the future, the number of new cancer cases worldwide will exceed the 19.3 million recorded in 2020, and the number of deaths will exceed 10 million. Cancer remains the leading cause of human mortality and lagging socioeconomic development. Intratumoral microbes have been revealed to exist in many cancer types, including pancreatic, colorectal, liver, esophageal, breast, and lung cancers. Intratumoral microorganisms affect not only the host immune system, but also the effectiveness of tumor chemotherapy. This review concentrates on the characteristics and roles of intratumoral microbes in various tumors. In addition, the potential of therapies targeting intratumoral microbes, as well as the main challenges currently delaying these therapies, are explored. Furthermore, we briefly summarize existing technical methods used to characterize intratumoral microbes. We hope to provide ideas for exploring intratumoral microbes as potential biomarkers and targets for tumor diagnosis, treatment, and prognostication.
Keywords: intratumoral bacteria, cancer, immune, 16S rRNA, therapeutic potential, challenge
Graphical abstract
Highlights
-
•
Intratumoral microbes have been revealed to exist in many cancers
-
•
Intratumoral microbes affect the host immune and tumor chemotherapy
-
•
The characteristics and roles of intratumoral microbes in tumors were summarized
-
•
Exploring biomarkers for tumor diagnosis, treatment, and prognosis
Xue et al. review the characteristics and roles of intratumoral microbes in tumors. They explore the potential and main challenges of therapies targeting these microbial communities and provide ideas about how to exploit them as biomarkers for tumor diagnosis, treatment, and prognostication.
Introduction
Cancer is a major health problem worldwide.1 According to the World Health Organization, cancer will become the leading or second-leading cause of death in patients younger than 70 in most countries.2 Among cancers, lung cancer remains the leading cause of cancer deaths, with approximately 350 people dying from lung cancer every day. The incidence of breast cancer (BC) in women is expected to surpass that of lung cancer, and BC is expected to become the most commonly diagnosed cancer in women.3 In addition, the diagnosis and treatment of cancer patients have been delayed to varying degrees because of the impact of the COVID-19 pandemic; furthermore, the incidence of cancer is expected to decrease in the short term, but the mortality rate in the later stage could increase due to untimely treatment.4 The study of cancer has continued for many years, and as a result, more means to diagnose, treat, and prevent cancer have been revealed. For example, human papillomavirus (HPV) vaccination can significantly reduce the incidence of gynecological tumors in women, such as cervical cancer (CC).5 Early screening and eradication of Helicobacter pylori have contributed to reducing the incidence of gastric cancer (GC) and improving the efficacy of early treatment and patient prognosis.6 In conclusion, the exploration of cancer and cancer therapies is an ongoing process, and a great deal of effort remains necessary.
The human microbiome is a general term that includes all microorganisms in and on the human body, e.g., those on the mucosal surfaces of the gut and skin.7 The genomes of these microorganisms are called microbial genomes. The number of genes in the microbial genomes of these organisms is more than 150 times that of the human genome.8 Therefore, the microbiome is believed to have its own genome.9 Microorganisms are able to coexist with host cells at multiple body sites, and these factors interact to influence the physiological and pathological processes of the host.10 Currently, the gut microbiome is the best known and the most studied. The normal intestinal flora is of great significance for the maintenance of intestinal mucosal integrity. The functions of gut microbes mainly include nutrient and drug metabolism, vitamin synthesis, immune regulation, and gastrointestinal structure maintenance.11,12 Microbial imbalances contribute to the development and progression of many human diseases, including cancer. For example, Ma et al. found that gut microbes regulated the level of the chemokine CXCL16 in liver sinusoidal endothelial cells (LSECs) via bile acids, thereby controlling the accumulation of CXCR6+ hepatic natural killer T (NKT) cells.13 Furthermore, these microbes exert antitumor immunity in the liver. Recently, the discovery of the intratumoral microbiome has piqued the interest of researchers. Studies have found that there are a large number of microorganisms in tumor tissue, and some of these microorganisms are involved in tumor initiation and development.
In fact, bacteria were found in tumor tissue more than 100 years ago. However, due to the lack of technical means to exclude the possibility of contamination and the very low microbial content in tumors, the presence of these bacteria was not widely recognized and explored. In 2020, Nejman et al. published a research paper on the intratumoral microbiome in Science.14 They employed more than 1,500 tumor samples and adjacent normal samples to study the relationships between seven cancers (breast, lung, ovarian, pancreatic, melanoma, bone, and brain) and bacteria. The researchers found that most tumors and their adjacent normal tissues contained different types of bacteria, mostly in cancer cells and immune cells. This research has attracted widespread attention, and intratumoral bacteria have become a popular topic in modern research. Organs and tissues, traditionally considered sterile, are increasingly being found to contain diverse microbial populations.15,16,17 Therefore, this review mainly focuses on summarizing the characteristics and roles of intratumoral microorganisms in different tumors and possible therapeutic options related to intratumoral microorganisms, and it briefly summarizes the existing technical means for detecting intratumoral microorganisms. The aim of this work is to provide guidance for the exploration of intratumoral microbes as potential biomarkers in cancer diagnosis, treatment, and prognostication.
Methods used to characterize the intratumoral microbiome
Next-generation sequencing methods achieve comprehensive and detailed analysis of all microorganisms without the need for culture. Researchers have applied high-throughput sequencing technology to analyze and generate a large number of microbiome datasets to facilitate subsequent research. Common approaches for microbiome research are based on deep sequencing of universal marker gene amplicons, such as 16S rRNA genes in prokaryotes, 18S rRNA genes, and internal transcribed spacers (ITSs) in eukaryotes, or whole metagenome-based shotgun sequencing (WMS).18,19,20 Apart from sequencing, methods such as immunohistochemistry (IHC), fluorescence in situ hybridization (FISH), D-alanine-based methods, and tissue culture can be used to explore the presence and abundance of bacteria within the tumor. A recent study also described QIIME 2 as a reproducible, interactive, and scalable microbiome data analysis system.21 It is an excellent tool for analyzing microbiome sequencing data, as are MaAsLin and Microbiome Analyst. Overall, the development of various technologies has made it possible to deeply explore the microbiomes of tumors.
16S rRNA gene sequencing
16S rRNA gene sequencing is currently one of the most commonly used amplicon sequencing methods. 16S rRNA is a conserved region with hypervariable sequences. Normally, hypervariable regions and conserved regions are alternately arranged, and a 16S rRNA fragment contains 9 hypervariable regions: V1–V9. Among them, the V4 region has good specificity and rich relevant data and is thus the best choice for bacterial diversity analysis and annotation.22 Notably, Nejman et al. performed multiplex PCR amplification and sequencing of five regions on the 16S rRNA gene.14 This method showed improved coverage and resolution of bacterial species detection compared with the traditionally used V4 or V3–V4 amplification. Generally, 16S rRNA is amplified by PCR, purified, and sequenced; finally, the sequences are clustered into operational taxonomic units (OTUs) based on similarity and are compared with existing database entries based on the representative sequences in each OTU.
WMS
Metagenomics is studied based on WMS, and many related terms are not discussed here. WMS mainly refers to the use of non-targeted sequencing methods to capture the DNA of all microorganisms present in environmental samples.23 The object of metagenomics research is to determine the total DNA content in a specific environment, not the total DNA in a specific microorganism or its cells, and WMS does not require isolation, culture, or purification of microorganisms.24 Compared with 16S rRNA gene sequencing, WMS is more precise, expensive, and complex and provides more extensive results.
IHC
The principle of IHC is the specific binding of antigen and antibody. In brief, the general procedure of this method is to first fix the sample and then coincubate it with a blocking solution. Subsequently, the sample is coincubated with primary antibody and secondary antibody followed by color development, and the sample is ultimately analyzed by microscopy. IHC is a qualitative and quantitative technique that provides location-specific data. Researchers commonly use antibodies against bacterial lipopolysaccharide (LPS) and lipoteichoic acid (LTA) to detect Gram-negative and Gram-positive bacteria in samples.25
FISH
FISH can be used to detect bacterial DNA or RNA in tissues using probes against the bacterial 16S rRNA gene labeled with fluorescent dyes. FISH takes advantage of the principle of nucleic acid complementary base pairing. A nucleic acid probe directly labeled with fluorescein is mixed with the target DNA or RNA, and the two form a hybrid. Kostic et al. explored the enrichment of Fusobacterium in colorectal cancer (CRC) by FISH.26
D-alanine-based methods
Researchers typically label live bacteria in situ with fluorescently labeled D-alanine. Almost all bacteria have alanine arginase activity, which helps to generate D-alanine, thereby producing peptidoglycan, an important cell wall component.27 The presence of living, metabolically active bacteria in tumors can be confirmed by culturing fresh tumor tissue in vitro and adding fluorescently labeled D-alanine.28
Characterization of the intratumoral microbiota in different tumors
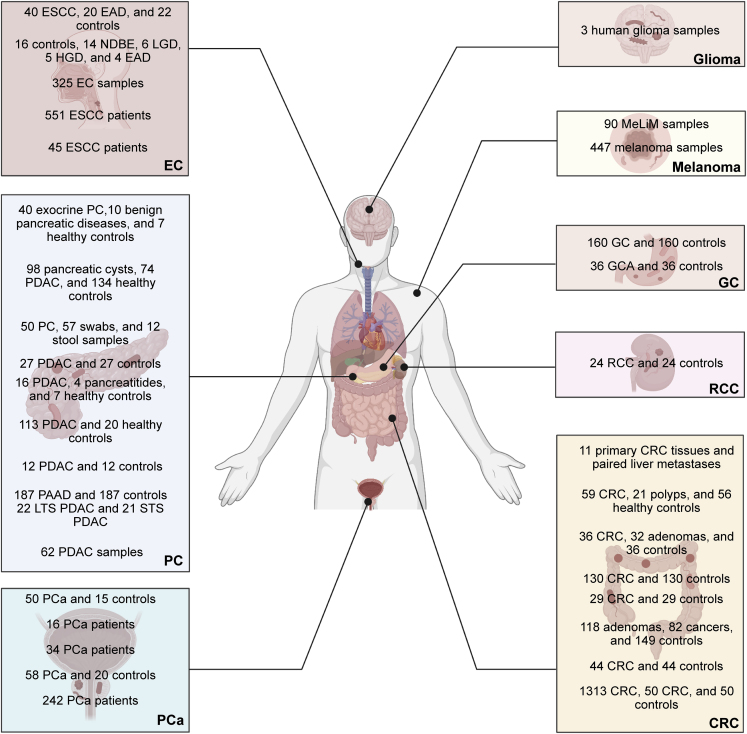
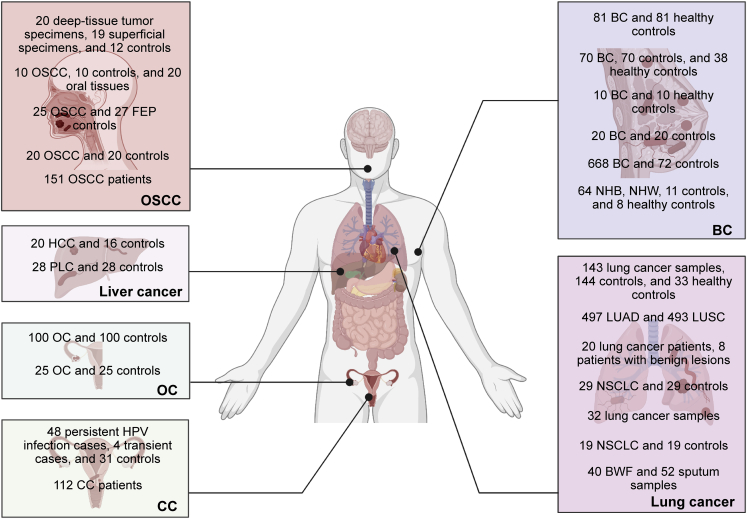
Growing evidence has confirmed the presence of microorganisms, mainly bacteria, in tumor tissues (Figures 1 and 2). Bacteria are mainly found in immune cells and tumor cells.14 The presence of bacteria within the tumor can have diagnostic value. There is also evidence that intratumoral bacteria can be associated with patient prognosis, as reported in liver and pancreatic cancer. Tables 1 and 2 provide a detailed summary of the characteristics and potential biological functions of intratumoral microbes in different tumors. Table 1 highlights the same and different intratumoral microbial types reported by different groups within the same tumor type. Table 2 mainly provides some ideas for the subsequent treatment and prevention of tumors by revealing the possible effects of intratumoral microorganisms on the occurrence and development of tumors.
Figure 1.
Intratumoral microbes are present in esophageal cancer (EC), pancreatic cancer (PC), prostate cancer (PCa), glioma, melanoma, gastric cancer (GC), renal cell cancer (RCC), and colorectal cancer (CRC)
Each box represents a cancer type. Number of clinical samples used by different study groups to study microorganisms in tumors is presented in the box.
Figure 2.
Intratumoral microbes were present in oral squamous cell carcinoma (OSCC), liver cancer, ovarian cancer (OC), cervical cancer (CC), breast cancer (BC), and lung cancer
Each box represents a cancer type. Number of clinical samples used by different study groups to study microorganisms in tumors is presented in the box.
Table 1.
The characterization of the intratumoral microbiota in various cancers
| Cancer types | Research groups | Number of clinical samples | Methods | Associated microbiota (same) | Associated microbiota (different) |
|---|---|---|---|---|---|
| Pancreatic cancer (PC) | Nilsson et al.29 | 40 exocrine PC, 14 neuroendocrine cancer, 8 multiple endocrine neoplasia, 5 chronic pancreatitis, 10 benign pancreatic disease, and 7 normal pancreas samples | PCR | – | Helicobacter pylori |
| PC | Kohi et al.30 | 134 normal pancreas control individual, 98 pancreatic cyst patient, and 74 PDAC patient samples | 16S rRNA and 18S rRNA gene sequencing | – | Enterococcaceae, Lactobacillaceae, Bifidobacteriaceae, Nakaseomyces, and Skeletocutis |
| PC | Del Castillo et al.31 | 50 PC patient samples, 189 tissue, 12 stool, and 57 swab samples | 16S rRNA gene sequencing | – | Fusobacterium, Porphyromonas and Prevotella |
| PC | Nalluri et al.32 | 27 pairs of PDAC samples and adjacent normal samples | PCR and 16S rRNA gene sequencing | Staphylococcus, Enterobacteriaceae, Pseudomonadaceae | Ruminococcaceae and Bacillaceae |
| PC | Thomas et al.33 | 7 normal, 4 pancreatitis, and 16 PDAC patient samples | 16S rRNA gene sequencing | Staphylococcus, Enterobacteriaceae | Corynebacterium, Escherichia, Propionibacterium, and Streptococcus |
| PC | Geller et al.15 | 113 PDAC samples and 20 normal samples | PCR and 16S rRNA gene sequencing | Gammaproteobacteria, Enterobacteriaceae, Pseudomonadaceae | – |
| PC | Pushalkar et al.34 | 12 pairs of PDAC samples and adjacent normal samples | PCR and 16S rRNA gene sequencing | Proteobacteria, Pseudomonadaceae | Bacteroidetes, Firmicutes, Actinobacteria, and Elizabethkingia |
| PC | Chakladar et al.35 | 187 pairs of PAAD samples and adjacent normal samples | Whole-transcriptome RNA sequencing | Proteobacteria, Gammaproteobacteria, Pseudomonadaceae | Citrobacter freundii and Shigella sonnei |
| PC | Riquelme et al.36 | 22 LTS PDAC patient and 21 STS PDAC patient samples | 16S rRNA gene sequencing | – | Saccharopolyspora, Pseudoxanthomonas, Streptomyces, and Bacillus clausii |
| PC | Guo et al.37 | 62 PDAC samples | Metagenomic sequencing and FISH | Pseudomonadaceae | Acinetobacter and Sphingopyxis |
| PC | Aykut et al.38 | – | 18S rRNA gene sequencing and FISH | – | Malassezia |
| Colorectal cancer (CRC) | Bullman et al.39 | 11 primary CRC tissues and paired liver metastases | PCR, 16S rRNA gene sequencing, and WGS | Fusobacterium, F. nucleatum | Fusobacterium necrophorum, Bacteroides fragilis, Bacteroides thetaiotaomicron, Prevotella intermedia, and Selenomonas sputigena |
| CRC | Flemer et al.40 | 59 CRC patient samples, 21 samples from individuals with polyps, and 56 samples from healthy controls | PCR and 16S rRNA gene sequencing | Firmicutes | Bacteroidetes cluster 2, Prevotella cluster, Pathogen cluster, and Firmicutes cluster 2 |
| CRC | Liu et al.41 | 36 CRC tissues, 32 adenoma tissues, and corresponding adjacent normal tissues | 16S rRNA gene sequencing | Fusobacterium, Firmicutes | Proteobacteria |
| CRC | Warren et al.42 | 130 CRC and matched normal control tissues | Metatranscriptomic analysis | Fusobacterium, Campylobacter | Leptotrichia |
| CRC | Okuda et al.43 | 29 pairs of CRC and adjacent normal tissues | 16S rRNA gene sequencing | Fusobacterium, Campylobacter | Peptostreptococcus |
| CRC | Yamamoto et al.44 | 118 adenomas, 82 cancers, and 149 matched adjacent normal tissues | PCR | F. nucleatum | – |
| CRC | Burns et al.45 | 44 pairs of CRC tissues and adjacent normal tissues | PCR and 16S rRNA gene sequencing | Fusobacterium | Providencia |
| CRC | Kosumi et al.46 | 1313 CRC patient samples, 50 pairs of CRC tissues and adjacent normal tissues | PCR and 16S rRNA gene sequencing | – | Bifidobacteria |
| Esophageal cancer (EC) | Wang et al.47 | 40 ESCC, 20 EAD, and 22 adjacent normal samples | TCMA, TCGA | Firmicutes, Proteobacteria | Firmicutes levels were significantly increased, while Proteobacteria levels were decreased in tumor samples. |
| EC | Yamamura et al.48 | 325 EC samples | PCR | F. nucleatum | – |
| EC | Liu et al.49 | 45 ESCC patient samples | 16S rRNA gene sequencing | Proteobacteria, Firmicutes | Bacteroidetes, Spirochaetes, Streptococcus, Prevotella, and Treponema |
| EC | Yamamura et al.50 | 551 ESCC patient samples | PCR | F. nucleatum | – |
| EC | Snider et al.51 | 16 control, 14 NDBE, 6 LGD, 5 HGD, and 4 EAD samples | 16S rRNA gene sequencing | Proteobacteria, Firmicutes | Proteobacteria, Enterobacteriaceae, and Akkermansia muciniphila were increased, while Firmicutes and Veillonella were reduced. |
| Oral squamous cell carcinoma (OSCC) | Hooper et al.52 | 20 deep-tissue tumor specimens, 19 corresponding superficial specimens, and 12 nontumorous control tissues | 16S rRNA gene sequencing | – | Exiguobacterium oxidotolerans, Prevotella melaninogenica, Staphylococcus aureus, Veillonella parvula |
| OSCC | Pushalkar et al.53 | 10 pairs of OSCC tissues and adjacent normal tissues, and 20 oral tissue samples | 16S rRNA gene sequencing | – | Streptococcus intermedius was present in all samples. Streptococcus sp. oral taxon 058, Peptostreptococcus stomatis, Streptococcus salivarius, Streptococcus gordonii, Gemella haemolysans, Gemella morbillorum, Johnsonella ignava, and Streptococcus parasanguinis I were highly abundant in OSCC samples. |
| OSCC | Perera et al.54 | 25 OSCC patient and 27 FEP control samples | 16S rRNA gene sequencing | P. aeruginosa | F. nucleatum subsp. polymorphum, Streptococcus dysgalactiae, Citrobacter koseri, and P. aeruginosa were significantly enriched in OSCC, while Streptococcus mitis and Staphylococcus epidermidis were abundant in FEP. |
| OSCC | Al-Hebshi et al.55 | 20 pairs of OSCC tissues and adjacent normal tissues | 16S rRNA gene sequencing | F. nucleatum, P. aeruginosa | – |
| OSCC | Neuzillet et al.56 | 151 OSCC patient samples | PCR and 16S rRNA gene sequencing | F. nucleatum | – |
| OSCC | Nakashima et al.57 | – | PCR | – | Clostridium perfringens |
| Lung cancer | Greathouse et al.58 | 33 control, 143 cancer, and 144 adjacent normal tissues | 16S rRNA gene sequencing and FISH | Firmicutes, Proteobacteria | Proteobacteria levels were increased, while Firmicutes levels were decreased. |
| Lung cancer | Wong et al.59 | 497 LUAD and 493 LUSC patients | TCGA | – | Escherichia coli str. K-12 substr. W3110, Pseudomonas putida str. KT2440 |
| Lung cancer | Lee et al.60 | 20 lung cancer patients and 8 patients with benign lesions | 16S rRNA gene sequencing | – | Veillonella, Megasphaera, Atopobium, and Selenomonas |
| Lung cancer | Dumont-Leblond et al.61 | 29 pairs of NSCLC tissues and adjacent normal tissues | 16S rRNA gene sequencing | – | Intestinal bacteria, potentially pathogenic or inflammatory bacteria |
| Lung cancer | Apostolou et al.62 | 32 lung cancer samples | PCR | – | Mycoplasma, Staphylococcus epidermidis, Streptococcus mitis, Bacillus strains, Chlamydia, Candida, Listeria, and Haemophilus influenza |
| Lung cancer | Peters et al.63 | 19 pairs of NSCLC tissues and adjacent normal tissues | 16S rRNA gene sequencing | – | In normal tissues, greater abundance of the Bacteroidaceae, Lachnospiraceae, and Ruminococcaceae families was associated with reduced RFS or DFS, whereas the Koribacteraceae family was associated with increased RFS and DFS. |
| Lung cancer | Huang et al.64 | 40 BWF samples and 52 sputum samples from lung cancer patients | 16S rRNA gene sequencing | Firmicutes, Proteobacteria | The most predominant phyla in the BWF samples were Firmicutes and Proteobacteria, and the most common genus was Prevotella. In sputum samples, Firmicutes was the predominant phylum, while Streptococcus was the most common genus. |
| Breast cancer (BC) | Urbaniak et al.65 | Breast tissues from 81 women with and without cancer | 16S rRNA gene sequencing | Firmicutes, Proteobacteria | – |
| BC | Urbaniak et al.66 | 70 pairs of BC tissues and adjacent normal tissues and 38 healthy control samples | 16S rRNA gene sequencing | – | Staphylococcus, Enterobacteriaceae, Bacillus, Staphylococcus epidermidis and Escherichia coli |
| BC | Klann et al.67 | 10 BC patients and 10 healthy controls | PCR and 16S rRNA gene sequencing | Firmicutes, Proteobacteria, Actinobacteria | Bacteroidetes |
| BC | Xuan et al.68 | 20 pairs of BC tissues and adjacent normal tissues | PCR and 16S rRNA gene sequencing | – | Methylobacterium radiotolerans was abundant in tumor tissues, while Sphingomonas yanoikuyae was abundant in paired normal tissues. |
| BC | Thompson et al.69 | 668 breast tumor tissues and 72 noncancerous adjacent tissues | TCGA and 16S rRNA gene sequencing | Firmicutes, Proteobacteria, Actinobacteria | – |
| BC | Smith et al.70 | 64 breast tumor samples from NHB and NHW women, 11 adjacent normal tissues, and 8 healthy control samples | 16S rRNA gene sequencing | Proteobacteria | – |
| BC | Fu et al.71 | Murine spontaneous breast-tumor model and human samples | PCR, 16S rRNA gene sequencing, and FISH | Firmicutes | – |
| Prostate cancer (PCa) | Banerjee et al.72 | 50 PCa samples and 15 control samples | Array-based metagenomic and capture-sequencing | – | Viruses, bacteria, fungi, and parasites |
| PCa | Cavarretta et al.73 | 16 PCa patient samples | Massive ultradeep pyrosequencing | – | Actinobacteria, Firmicutes, and Proteobacteria |
| PCa | Cohen et al.74 | 34 PCa patient samples | 16S rRNA gene sequencing | Propionibacterium acnes | – |
| PCa | Fassi Fehri et al.75 | 58 cancerous prostate tissues and 20 healthy prostate tissues | ISIF and PCR | Propionibacterium acnes | – |
| PCa | Ma et al.76 | 242 PCa patient samples | TCGA | – | Listeria monocytogenes, Methylobacterium radiotolerans JCM 2831, Xanthomonas albilineans GPE PC73, and Bradyrhizobium japonicum |
| Ovarian cancer (OC) | Al-Shabanah et al.77 | 100 pairs of OC tissues and adjacent normal tissues | PCR | – | HPV |
| OC | Zhou et al.78 | 25 OC tissues and 25 normal distal fallopian tube tissues | 16S rRNA gene sequencing | – | Proteobacteria, Firmicutes, Bacteroidetes, Actinobacteria, and Bacteria_unclassified |
| Cervical cancer (CC) | Huang et al.79 | 112 CC patient samples | PCR | – | F. nucleatum |
| CC | Liu et al.80 | 48 persistent HPV infection samples, 4 transient infection samples, and 31 negative control samples | 16S rRNA gene sequencing | – | Decreased levels of probiotics, including Shuttleworthia, Prevotella, Lactobacillus, and Sneathia, and increased levels of pathogenic bacteria, including Dispar, Streptococcus, and Faecalibacterium prausnitzii |
| Melanoma | Mrázek et al.81 | 90 samples from the MeLiM pig model | 16S rRNA gene sequencing | – | Fusobacterium and Trueperella |
| Melanoma | Zhu et al.82 | 447 melanoma patient samples | TCGA | – | Lachnoclostridium, Gelidibacter, Flammeovirga, and Acinetobacter |
| Glioma | Zhao et al.83 | 3 human glioma samples | 16S rRNA gene sequencing | – | Bacterial lipopolysaccharide |
| Gastric cancer (GC) | Yu et al.84 | 160 pairs of GC tissues and adjacent normal tissues | 16S rRNA gene sequencing | Firmicutes, Bacteroidetes, Proteobacteria | H. pylori |
| GC | Shao et al.85 | 36 pairs of GCA tissues and adjacent normal tissues | 16S rRNA gene sequencing | Firmicutes, Bacteroidetes, Proteobacteria | – |
| Renal cell carcinoma (RCC) | Wang et al.86 | 24 pairs of RCC tissues and adjacent normal tissues | 16S rRNA gene sequencing | – | Proteobacteria, Firmicutes, Bacteroidetes, and Actinobacteria, Chloroplast, Streptophyta |
| Liver cancer | Huang et al.87 | 20 HCC samples and 16 control samples | 16S rRNA gene sequencing and PCR | – | H. pylori |
| Liver cancer | Qu et al.88 | 28 pairs of PLC tissues and adjacent normal tissues | 16S rRNA gene sequencing and PCR | – | Proteobacteria, Actinobacteria, Bacteroidetes, and Firmicutes |
Abbreviations: PDAC, pancreatic ductal adenocarcinoma; PAAD, pancreatic adenocarcinoma; LTS, long-term survival; STS, short-term survival; WGS, whole-genome sequencing; ESCC, esophageal squamous cell carcinoma; EAD, esophageal adenocarcinoma; TCMA, The Cancer Microbiome Atlas; TCGA, The Cancer Genome Atlas; NDBE, Barrett’s esophagus without dysplasia; LGD, low-grade dysplasia; HGD, high-grade dysplasia; FEP, fibroepithelial polyp; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; NSCLC, non-small cell lung cancer; RFS, recurrence-free; DFS, disease-free survival; BWF, bronchial washing fluid; EMT, epithelial-to-mesenchymal transition; NHB, non-Hispanic Black; NHW, non-Hispanic White; MeLiM, melanoma-bearing Libechov minipig; GCA, gastric cardia adenocarcinoma; HCC, hepatocellular carcinoma; PLC, primary liver cancer.
Table 2.
The function and implications of the intratumoral microbiota in various cancers
| Cancer types | Associated microbiota | Main findings | Implications | Reference |
|---|---|---|---|---|
| PC | Helicobacter pylori | H. pylori was detected in PC patient tumors. | – | Nilsson et al.29 |
| PC | Enterococcaceae, Lactobacillaceae, Bifidobacteriaceae, Nakaseomyces, and Skeletocutis | The duodenal fluid microbiome profile was altered in samples from patients with PDAC compared with samples from patients with pancreatic cysts and normal pancreas samples. | PC risk and survival after PC diagnosis are affected. | Kohi et al.30 |
| PC | Fusobacterium, Porphyromonas and Prevotella | The microbial composition between PC and noncancer samples was different. | Identifying distinct bacterial taxa could shed new light on bacteria associated with etiology. | Del Castillo et al.31 |
| PC | Staphylococcus, Enterobacteriaceae, Pseudomonadaceae, Ruminococcaceae and Bacillaceae | Intratumor bacterial colonization was more likely in patients who had pancreatic head tumors, had undergone preoperative biliary drainage with stent placement, had undergone the Whipple procedure, and had undergone neoadjuvant treatment with a combination of gemcitabine and paclitaxel. | – | Nalluri et al.32 |
| PC | Staphylococcus, Enterobacteriaceae, Corynebacterium, Escherichia, Propionibacterium, and Streptococcus | The human pancreas microbiota composition was not different between normal and disease states. | – | Thomas et al.33 |
| PC | Gammaproteobacteria, Enterobacteriaceae, Pseudomonadaceae | Intratumor bacterial colonization was more likely in patients who had undergone surgical modification of the pancreatic duct. Enterobacteriaceae were found to express CDDL. | Resistance to chemotherapy is mediated. | Geller et al.15 |
| PC | Proteobacteria, Pseudomonadaceae, Bacteroidetes, Firmicutes, Actinobacteria, and Elizabethkingia | The PC microbiome promoted tumorigenesis by inducing innate and adaptive immune suppression. | PC progression is promoted. | Pushalkar et al.34 |
| PC | Proteobacteria, Gammaproteobacteria, Pseudomonadaceae, Citrobacter freundii and Shigella sonnei | The PC microbiome was linked to carcinogenesis and worse prognosis in men and smokers. | Prognosis might be affected. | Chakladar et al.35 |
| PC | Saccharopolyspora, Pseudoxanthomonas, Streptomyces, and Bacillus clausii | The tumor microbial communities were significantly different between LTS and STS. The tumor microbiome affected the immune responses that promoted T cell activation. |
Human PDAC survival could be predicted. | Riquelme et al.36 |
| PC | Pseudomonadaceae, Acinetobacter and Sphingopyxis | Distinct microbial communities in basal-like tumors played an inflammation-inducing role and promoted pancreatic carcinogenesis. | Some bacteria are highly associated with carcinogenesis. | Guo et al.37 |
| PC | Malassezia | The fungal flora promoted pancreatic tumorigenesis through MBL activation. | PC progression is promoted. | Aykut et al.38 |
| CRC | Fusobacterium, F. nucleatum, Fusobacterium necrophorum, Bacteroides fragilis, Bacteroides thetaiotaomicron, Prevotella intermedia, and Selenomonas sputigena | Fusobacterium co-occurred with other Gram-negative anaerobic bacteria in primary and matched liver metastases. Treatment of Fusobacterium-containing PDX models with metronidazole inhibited tumor growth. | Antimicrobial intervention is a potential treatment for patients with Fusobacterium-associated CRC | Bullman et al.39 |
| CRC | Bacteroidetes cluster 2, Prevotella cluster, Pathogen cluster, and Firmicutes cluster 2 | Alterations in the composition of the microbiota were not limited to cancer tissues and differed between distal and proximal cancer tissues. | – | Flemer et al.40 |
| CRC | Fusobacterium, Firmicutes, Proteobacteria | The microbiota communities of CRC and precancerous adenoma were heterogeneous. This diversity could be associated with differences in the adenoma-cancer sequence and alterations of genes correlated with CRC. | – | Liu et al.41 |
| CRC | Fusobacterium, Campylobacter, Leptotrichia | Anaerobic bacteria coexisted in CRC. Microbial characteristics were associated with the overexpression of host genes, including the gene encoding IL-8. |
– | Warren et al.42 |
| CRC | Fusobacterium, Campylobacter, Peptostreptococcus | Fusobacterium was related to ATM and PIK3CA alterations and alterations in genes encoding cell cycle proteins. Patients with high Campylobacter levels were more likely to have mutational signature 3, representing failure of double-strand DNA break repair | Substances released by bacterial infection cause DNA damage, which in turn can drive CRC progression. | Okuda et al.43 |
| CRC | F. nucleatum | The level of F. nucleatum in tumors increased with pathological stage and was highest in stage III/IV samples. | – | Yamamoto et al.44 |
| CRC | Fusobacterium, Providencia | Virulence-related bacterial genes were highly enriched in the CRC tumor microenvironment. | It is hinted that the microbiome could drive CRC progression. | Burns et al.45 |
| CRC | Bifidobacteria | There was a strong association between intratumoral Bifidobacteria and signet ring cells. | – | Kosumi et al.46 |
| EC | Firmicutes levels were significantly increased, while Proteobacteria levels were decreased in tumor samples. | The characteristics of the intratumoral microbiota were related to the subtype, stage, and prognosis of EC. | – | Wang et al.47 |
| EC | F. nucleatum |
F. nucleatum was associated with the survival of EC patients. F. nucleatum activated CCL20 to enhance the invasiveness of EC. |
Prognosis is affected. | Yamamura et al.48 |
| EC | Proteobacteria, Firmicutes, Bacteroidetes, Spirochaetes, Streptococcus, Prevotella, and Treponema | The combined abundance of Streptococcus and Prevotella was suggested to be a prognostic biomarker for ESCC patients. | Action as a prognostic biomarker is demonstrated. | Liu et al.49 |
| EC | F. nucleatum | F. nucleatum was linked to ESCC patients’ adverse reaction to neoadjuvant chemotherapy. | Treatment response to neoadjuvant chemotherapy in ESCC could be predicted. | Yamamura et al.50 |
| EC | Proteobacteria, Enterobacteriaceae, and Akkermansia muciniphila were increased, while Firmicutes and Veillonella were reduced. | The intratumoral microbiome changed dramatically during progression of Barrett’s esophagus to EAD. | – | Snider et al.51 |
| OSCC | Exiguobacterium oxidotolerans, Prevotella melaninogenica, Staphylococcus aureus, Veillonella parvula | Multiple bacterial groups were isolated from OSCC tissues.OSCC tissues differed in microbial composition from adjacent normal tissues. | – | Hooper et al.52 |
| OSCC | Streptococcus intermedius was present in all samples. Streptococcus sp. oral taxon 058, Peptostreptococcus stomatis, Streptococcus salivarius, Streptococcus gordonii, Gemella haemolysans, Gemella morbillorum, Johnsonella ignava, and Streptococcus parasanguinis I were highly abundant in OSCC samples. | Bacterial colonization was different between tumor and nontumor tissues in OSCC patients. | – | Pushalkar et al.53 |
| OSCC | F. nucleatum subsp. polymorphum, Streptococcus dysgalactiae, Citrobacter koseri, and P. aeruginosa were significantly enriched in OSCC, while Streptococcus mitis and Staphylococcus epidermidis were abundant in FEP. | Enrichment of the inflammatory bacterial population was found in OSCC. | Intratumoral microbes are pro-inflammatory. | Perera et al.54 |
| OSCC | F. nucleatum, P. aeruginosa | For the first time, F. nucleatum and P. aeruginosa were found to be related to OSCC development. | – | Al-Hebshi et al.55 |
| OSCC | F. nucleatum | F. nucleatum-related OSCC had a specific immune microenvironment and was more common in older patients without alcoholic disease. | Relationship shown to a favorable prognosis. | Neuzillet et al.56 |
| OSCC | Clostridium perfringens | Clostridium perfringens enterotoxin induced claudin-4 to activate YAP, thereby promoting progression of OSCC. | Tumor progression is promoted. | Nakashima et al.57 |
| Lung cancer | Proteobacteria levels were increased, while Firmicutes levels were decreased. | Tumors harboring TP53 mutations had distinct bacterial communities that were also relatively abundant in smoking-related tumors. | – | Greathouse et al.58 |
| Lung cancer | Escherichia coli str. K-12 substr. W3110, Pseudomonas putida str. KT2440 | Age- and sex-related differentially enriched microbes were associated with genomic alterations and immune dysregulation. | – | Wong et al.59 |
| Lung cancer | Veillonella, Megasphaera, Atopobium, and Selenomonas | Bacterial communities differed between lung cancer patients and individuals with benign lesions. | The environment for lung cancer patients might be changed. | Lee et al.60 |
| Lung cancer | intestinal bacteria, potentially pathogenic or inflammatory bacteria | Lung cancer tissues showed enrichment of intestinal and pro-inflammatory bacteria. | – | Dumont-Leblond et al.61 |
| Lung cancer | Mycoplasma, Staphylococcus epidermidis, Streptococcus mitis, Bacillus strains, Chlamydia, Candida, Listeria, and Haemophilus influenza | Multiple pathogens were identified in tissue samples surgically extracted from lung cancer patients. | – | Apostolou et al.62 |
| Lung cancer | In normal tissues, greater abundance of the Bacteroidaceae, Lachnospiraceae, and Ruminococcaceae families was associated with reduced RFS or DFS, whereas the Koribacteraceae family was associated with increased RFS and DFS. | The diversity and overall composition of the microbiota in tumor tissue were not associated with RFS or DFS. | Survival is affected. | Peters et al.63 |
| Lung cancer | The most predominant phyla in the BWF samples were Firmicutes and Proteobacteria, and the most common genus was Prevotella. In sputum samples, Firmicutes was the predominant phylum, while Streptococcus was the most common genus. | BWF samples could reflect the microbiome of lung cancer tissues better than sputum samples. | – | Huang et al.64 |
| BC | Firmicutes, Proteobacteria | Breast tissue was not sterile, and a diverse microflora was present. | – | Urbaniak et al. (2014) |
| BC | Staphylococcus, Enterobacteriaceae, Bacillus, Staphylococcus epidermidis and Escherichia coli | The bacterial profiles in adjacent normal tissues from BC patients differed from those in breast tissues of healthy women. DNA damage-causing bacteria were present in higher abundance in BC patients. |
These bacteria play a role in modulating risk of BC development. | Urbaniak et al. (2016) |
| BC | Firmicutes, Proteobacteria, Actinobacteria, and Bacteroidetes | There were differences in microbial composition and relative abundance between breast tumor tissues and normal tissues. Microbial composition varied significantly between women and between the left and right breasts of each woman. |
– | Klann et al.67 |
| BC | Methylobacterium radiotolerans was abundant in tumor tissues, while Sphingomonas yanoikuyae was abundant in paired normal tissues. | Microbial DNA was present in the breast, and microbial dysregulation was associated with BC. Bacteria and their components could affect the local immune microenvironment in BC patients. |
The local immune microenvironment was influenced. | Xuan et al.68 |
| BC | Firmicutes, Proteobacteria, Actinobacteria | Haemophilus influenza was associated with proliferation pathway-related genes. Listeria spp. were linked to gene expression profiles associated with EMT. | – | Thompson et al.69 |
| BC | Proteobacteria | The microbial community in breast tissue varied by race, stage, and breast tumor subtype. | – | Smith et al.70 |
| BC | Firmicutes | The intratumoral microbiota promoted BC cell metastasis and colonization of the lung. | BC metastasis and colonization are promoted. | Fu et al.71 |
| PCa | viruses, bacteria, fungi, and parasites | A diverse microbiome was observed in PCa samples compared with benign lesions. In some cases, viral (HPV18, KSHV) and bacterial (H. pylori) sequences led to perturbation of gene expression, in turn affecting the development of cancer. |
Tumorigenesis is influenced at the genetic level. | Banerjee et al.72 |
| PCa | Actinobacteria, Firmicutes, and Proteobacteria | The prostate contained a large number of bacteria, the distribution of which depended on the nature of the tissue. | – | Cavarretta et al.73 |
| PCa | Propionibacterium acnes | For the first time, P. acnes was discovered and isolated from PCa tissue. P. acnes was positively correlated with prostate inflammation. |
The bacterium was positively correlated with prostate inflammation. | Cohen et al.74 |
| PCa | Propionibacterium acnes | P. acnes infection promoted the occurrence and development of PCa. | PCa progress is promoted. | Fassi Fehri et al.75 |
| PCa | Listeria monocytogenes, Methylobacterium radiotolerans JCM 2831, Xanthomonas albilineans GPE PC73, and Bradyrhizobium japonicum | Microbes were more abundant in PCa samples than in normal samples. Some of these microbes were cancer promoting, and unexpectedly, most of the microbes were found to exert antitumor effects in PCa. | There is a double effect on the tumor. | Ma et al.76 |
| OC | HPV | High-risk HPV types (HPV-16, 18, 45) were closely associated with advanced stage in OC. | – | Al-Shabanah et al.77 |
| OC | Proteobacteria, Firmicutes, Bacteroidetes, Actinobacteria, and Bacteria_unclassified | The diversity and richness of bacterial communities were reduced in OC tissues compared with normal distal fallopian tube tissues. Microbiota dysbiosis was found to control OC initiation or progression by suppressing host inflammatory and immune responses. |
– | Zhou et al.78 |
| CC | F. nucleatum | The level of F. nucleatum was higher in CC tissues. High levels of F. nucleatum were associated with poorer overall survival and PFS in CC patients. F. nucleatum could be a potential diagnostic and prognostic biomarker for CC. |
The bacterium provides potential CC diagnostic and prognostic biomarkers. | Huang et al.79 |
| CC | decreased levels of probiotics including Shuttleworthia, Prevotella, Lactobacillus, and Sneathia; and increased levels of pathogenic bacteria, including Dispar, Streptococcus, and Faecalibacterium prausnitzii | The composition of the vaginal microbiota in HPV-infected cervical cancer patients varied with the duration of infection. | – | Liu et al.80 |
| Melanoma | Fusobacterium and Trueperella | There were significant differences in bacterial composition and diversity between skin and melanoma microbiomes. | – | Mrázek et al.81 |
| Melanoma | Lachnoclostridium, Gelidibacter, Flammeovirga, and Acinetobacter | The intestinal microbiota within tumors modulated chemokine (CXCL9, CXCL10 and CCL5) levels and influenced CD8+ T cell infiltration. High levels of Lachnoclostridium significantly reduced the risk of death in melanoma patients. |
The prognosis of patients receiving immunotherapy is affected. | Zhu et al.82 |
| Glioma | Bacterial lipopolysaccharide | Researchers developed a 3D quantitative in situ microbiota imaging strategy that enabled direct, contamination-free visualization of bacteria within gliomas. Bacterial components in human glioma samples were irregular and sparsely distributed, mainly located near the nuclear membrane or in the intercellular space. |
– | Zhao et al.83 |
| GC | Firmicutes, Bacteroidetes, Proteobacteria, and H. pylori | H. pylori was the most enriched member of the gastric microbiota in samples from China and Mexico. The relative abundance of H. pylori was higher in nonmalignant tissues than in malignant tissues. | – | Yu et al.84 |
| GC | Firmicutes, Bacteroidetes, Proteobacteria | Compared with the relative levels in nontumor tissues, there was decreased Helicobacter abundance and greater α diversity in GCA tumor tissues. | – | Shao et al.85 |
| RCC | Proteobacteria, Firmicutes, Bacteroidetes, and Actinobacteria Chloroplast, Streptophyta | Species diversity was reduced in RCC tissues, and the bacterial community composition was significantly different from that in normal tissues. Compared with normal tissue, 25 taxa were increased, and 47 taxa were decreased in RCC tissue. The presence of Chloroplast and Streptophyta significantly distinguished normal tissue from RCC tissue. |
– | Wang et al.86 |
| Liver cancer | H. pylori | H. pylori was present in HCC tissues | The bacterium could be associated with carcinogenic processes in the liver. | Huang et al.87 |
| Liver cancer | Proteobacteria, Actinobacteria, Bacteroidetes, and Firmicutes | Bacteria with antitumor effects, such as Pseudomonas, were reduced in PLC tissues, and their levels linearly correlated with the prognosis of PLC patients. | – | Qu et al.88 |
Abbreviations: PDAC, pancreatic ductal adenocarcinoma; PAAD, pancreatic adenocarcinoma; LTS, long-term survival; STS, short-term survival; WGS, whole-genome sequencing; ESCC, esophageal squamous cell carcinoma; EAD, esophageal adenocarcinoma; TCMA, The Cancer Microbiome Atlas; TCGA, The Cancer Genome Atlas; NDBE, Barrett’s esophagus without dysplasia; LGD, low-grade dysplasia; HGD, high-grade dysplasia; FEP, fibroepithelial polyp; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; NSCLC, non-small cell lung cancer; RFS, recurrence-free; DFS, disease-free survival; BWF, bronchial washing fluid; EMT, epithelial-to-mesenchymal transition; NHB, non-Hispanic Black; NHW, non-Hispanic White; MeLiM, melanoma-bearing Libechov minipig; GCA, gastric cardia adenocarcinoma; HCC, hepatocellular carcinoma; PLC, primary liver cancer.
Pancreatic cancer
Pancreatic cancer (PC) is a malignant tumor with a very high mortality rate.89 Pancreatic ductal adenocarcinoma (PDAC) is the most common type of PC, accounting for more than 85% of total cases.90 PDAC is inflammation-driven cancer characterized by a prominent fibroinflammatory microenvironment.91 Recently, many studies have revealed the role of intratumoral microbes in the development of PC and their effects on PC treatment. Currently, there is no clear consensus on the role of microbes in pancreatic tissue and the normal surrounding tissues of patients with PC. It is worth conducting more research to explore this issue in greater depth.
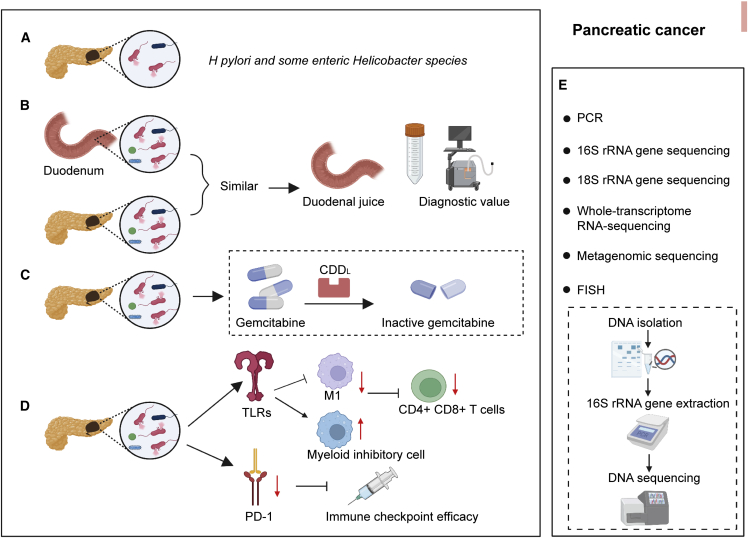
As early as 2006, Nilsson et al. found H. pylori DNA in the pancreas of 75% of PC patients.29 Since then, an increasing number of studies have revealed that the pancreas is not a sterile organ. Researchers have found that the human pancreas has its own microbiota. Interestingly, some studies have reported that the bacterial composition of PC is different from that of the normal pancreas.31,30 However, other studies have found that the composition of the bacterial community in the pancreas is not distinct between normal and disease states.32,33 This lack of difference could have been caused by the different sources, quantities, and qualities of tissue samples; the technical means; and the analysis methods used in the studies. Del Castillo et al. also found that the relative abundance of bacterial groups in the pancreas at the genus level varied significantly from person to person.31 By contrast, in the same individuals, the bacterial composition in the pancreas was very similar to that in the duodenum. In exploring the possible impacts of intratumoral microbes on tumors, many studies have revealed the potential effects of bacteria on PC treatment outcomes, prognosis, and survival.15,34,35,36,37 Gemcitabine is a common chemotherapeutic used in the treatment of PC patients. Research has indicated that bacteria in PDAC might modulate tumor sensitivity to gemcitabine. Bacteria break down gemcitabine into an inactive form via a specific isoform of cytidine deaminase.15,92 Pushalkar et al. performed 16S rRNA gene sequencing on 12 PDAC patients.34 The results showed that Proteobacteria, Bacteroidetes, and Firmicutes were the most abundant and prevalent groups of microorganisms. Further study found that ablation of the microbiome prevented the progression of pancreatic disease. Mechanistically, the microbiome generated a tolerant immune program through differential activation of selected Toll-like receptors (TLRs) in monocytes. In addition to revealing the presence of a large number of bacteria in the pancreas, researchers have recently reported the discovery of fungi within tumors. The fungal community of PDAC tumors in both mice and humans is significantly enriched for Malassezia. Ligation of mannose-binding lectin (MBL), which binds to fungal glycan to activate the complement cascade, is required for the oncogenic progression of PDAC (Figure 3).38
Figure 3.
Characterization of the intratumoral microbiota in pancreatic cancer (PC)
(A) Gastric H. pylori and low levels of intestinal H. pylori were detected in tissue from PC patients but not in tissue from controls.
(B) Researchers believe that most intratumoral bacteria in PDAC patients result from retrograde migration from the duodenum, and the microbial composition of the two is similar.
(C) PDAC contains bacteria that modulate tumor sensitivity to gemcitabine.
(D) The microbiome promotes PC tumorigenesis by inducing innate and adaptive immune suppression.
(E) Methods commonly used by researchers to study microbes in PC tumors.
Colorectal cancer
CRC is the third most common cancer worldwide and the second leading cause of cancer deaths in the United States. Intestinal flora imbalances play a significant role in the progression of CRC. In recent years, attention has been paid to the microbial communities within tumors. Currently, the mechanism of Fusobacterium nucleatum in CRC is relatively well understood.93 Researchers have revealed that the effects of F. nucleatum mainly include inhibition of immunity, modulation of virulence, regulation of micro RNA (miRNA) and long noncoding RNA (LncRNA), and modulation of metabolism.94,95
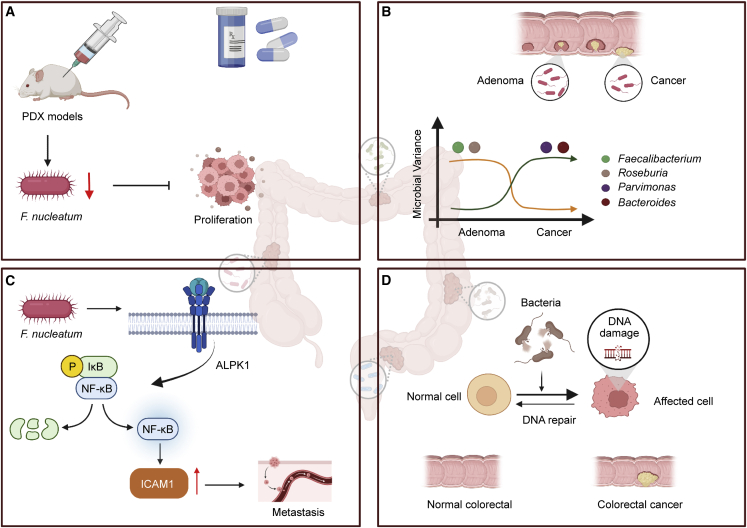
Compared with adjacent normal tissues, human CRC tissues show enrichment of Fusobacterium species.42,96,97 Fusobacterium functions as a carcinogenic factor in the progression of CRC and has emerged as an influential bacterium associated with colorectal carcinogenesis. A series of studies have confirmed these observations. F. nucleatum was associated with different immune responses in tumors with microsatellite instability.98 Its abundance was correlated with the CDKN2A methylation level and macrophage infiltration.99 Bullman et al. found co-occurrence of Fusobacterium with other Gram-negative anaerobic bacteria in primary and matched liver metastases.39 The researchers revealed consistency in the microbial community composition between paired primary and metastatic tumors. Furthermore, they reported that the treatment of Fusobacterium-containing patient-derived xenograft (PDX) models with the antibiotic metronidazole inhibited tumor proliferation and growth. Recently, Zhang et al. revealed an F. nucleatum/ALPK1/NF-κB/ICAM1 axis in CRC.100 The researchers found that the abundance of F. nucleatum in tumor tissues of CRC patients was positively correlated with ALPK1 and ICAM1 expression levels. F. nucleatum induced ALPK1 to activate the nuclear factor κB (NF-κB) pathway, leading to the upregulation of ICAM1. Ultimately, these factors promote the adhesion of CRC cells to endothelial cells and promote extravasation and metastasis. Selective targeting of Fusobacterium appears to be a potential therapeutic approach. Yamamoto et al. revealed the varied distribution of F. nucleatum in the development of CRC.44 The presence of F. nucleatum was highest in stage III/IV disease. In addition, researchers have suggested that F. nucleatum could be used as a biomarker because of its association with CRC cell proliferation. In 2015, researchers reported another genus with a pathogenic role in CRC, Providencia. In addition, researchers found that virulence-related bacterial genes were highly enriched in the CRC tumor microenvironment.45 Kosumi et al. found Bifidobacteria species in the tumors of 393 of 1,313 CRC patients.46 There was a strong association between the presence of intratumoral Bifidobacteria species and signet ring cells. Signet ring-like cells form when cells are filled with a large amount of mucus that squeezes the nucleus to one side of the cell, giving it a ring-like appearance. Other interesting findings were that changes in the microbiota composition were not limited to cancer tissues and differed between distal and proximal cancers. Prevotella and Firmicutes (found in cluster 2) were more abundant in proximal cancers, whereas Bacteroidetes (found in cluster 2) and various pathogens (found in several clusters) were more abundant only in distal cancers.40,101 Liu et al. reported microflora heterogeneity in CRC tumors.41 The composition of the microbiota in biopsies from the same site was similar but significantly different between biopsies from different sites. These findings could explain the different results revealed by different research groups. As such, 2–3 biopsy samples from each tumor should be collected whenever possible. In addition, the development of CRC might be caused in part by DNA damage induced by substances released by a bacterial infection (Figure 4).43
Figure 4.
Characterization of the intratumoral microbiota in colorectal cancer (CRC)
(A) Treatment of Fusobacterium-containing PDX models with metronidazole inhibited tumor growth.
(B) Changes in individual microbial abundance within tumors vary along the adenoma-cancer sequence. The abundance of intratumoral variants of Bacteroides and Parvimonas increases from adenoma to carcinoma. The abundance of intratumoral variants of Faecalibacterium and Roseburia decreases from adenoma to carcinoma.
(C) F. nucleatum induced ALPK1 to activate the NF-κB pathway, leading to the upregulation of ICAM1.
(D) The development of CRC could be caused in part by DNA damage caused by substances released by bacterial infection.
Esophageal cancer
Researchers have analyzed the microbial characteristics of esophageal cancer (EC) tissues based on two common databases: The Cancer Microbiome Atlas (TCMA) and The Cancer Genome Atlas (TCGA). The abundance of Firmicutes has been found to be significantly increased, while that of Proteobacteria has been found to be significantly decreased in tumor samples. In addition, studies have revealed that some microorganisms are closely related to the subtype, stage, and prognosis of EC. For example, a high abundance of Proteobacteria, Negativicutes, and Lactobacillus was linked to a favorable prognosis. The opposite was true for Clostridiales and Fusobacteriales.47 Several studies have also revealed the relationships between intratumoral microbes and the prognosis of EC. Yamamura et al. reported that F. nucleatum can be used as a prognostic biomarker due to its association with shorter survival in EC patients.48 Liu et al. suggested that the combined abundance of Streptococcus and Prevotella could predict prognosis in esophageal squamous cell carcinoma (ESCC) patients.49 The identification of similar biomarkers could be helpful for clinical prognostic assessment. In addition, researchers observed that F. nucleatum was linked to adverse reactions to neoadjuvant chemotherapy in ESCC patients.50 Interestingly, the intratumoral microbiome was found to change dramatically during the progression of Barrett’s esophagus to esophageal adenocarcinoma (EAD).51 This finding is of great significance and could guide the clinical prediction of cancer risk based on microbial changes before the development of EAD.
Oral squamous cell carcinoma
In 2006, Hooper et al. first found that there were multiple bacterial groups in oral squamous cell carcinoma (OSCC) tissues.52 The microbial composition was different between cancer and adjacent normal tissues. Subsequent studies confirmed these findings.102,53 In addition to focusing on the intratumoral microbial composition, researchers have also explored the presence of functional bacteria in OSCC. They found that inflammatory bacteria were enriched in OSCC tissue. Although the results of studies of microbial composition have varied, they suggest that microbial communities composed of different species might be functionally similar.54 Specifically, Al-Hebshi et al. were the first to discover that F. nucleatum and Pseudomonas aeruginosa, which are characteristic of inflammatory flora, were associated with OSCC development.55 However, puzzlingly, another study revealed that F. nucleatum was associated with better outcomes in OSCC patients. Specifically, the F. nucleatum burden was negatively correlated with M2-type macrophages, insensitivity to pro-inflammatory signaling, and low TLR4 signaling, and its presence ultimately led to favorable clinical outcomes.56 Both studies were limited by issues such as a small sample size, and the conclusions of the studies must be further confirmed. In addition, studies have revealed the potential utility of Clostridium perfringens enterotoxin (CPE) as a therapeutic target in OSCC.57 On the one hand, CPE enhances the proliferation, migration, and invasion ability of OSCC cells by inducing CLDN4 nuclear translocation. On the other hand, by inhibiting the phosphorylation of YAP1, CPE promotes YAP1 expression, thereby promoting tumor progression. Inhibition of CPE could be a treatment strategy for OSCC.
Lung cancer
Although the respiratory tract is exposed to the outside world, the trachea and lungs of healthy individuals are traditionally considered sterile. In 2010, Hilty et al. described the microbiota in the airways of asthmatic patients.103 Subsequently, reports of bacteria in lung cancer tissue began to appear. Researchers found that, in lung squamous cell carcinoma (LUSC), Acidovorax abundance differed between smokers and nonsmokers, with the former having a higher abundance. In addition, Acidovorax was more common in patients with TP53-mutated squamous cell carcinoma.58 Another interesting study reported age- and sex-related differences in microbiome composition in lung cancer. In addition to smoking, age and sex are important factors affecting the pathogenesis of lung cancer.104 The high incidence of lung cancer occurs in patients between the ages of 55 and 65 years old, although recently, the incidence of lung cancer in younger patients has increased. Lung cancer incidence and mortality are higher in men than in women. In LUSC, Pseudomonas putida str. KT2440 is more common in young male patients and is associated with immune infiltration. In lung adenocarcinoma (LUAD), Escherichia coli str. K-12 substr. W3110 is related to survival and genomic alterations in older female and male patients.59 Many studies have reported that bacterial communities differ between lung cancer patients and individuals with benign lesions.61,60 Relationships between the human lung tissue microbiota and epidemiological and clinical features have also been revealed.105 In addition, Lee et al. suggested that the Veillonella and Megasphaera genera could be used as biomarkers to predict lung cancer.60 The researchers found multiple species of microflora in surgically resected lung cancer tissues, with Mycoplasma found in nearly all samples. They suggested that chronic infection plays an important role in the development of lung cancer.62 Peters et al. conducted a study of the relationship between the lung microbiota and lung cancer prognosis.63 They reported that the diversity and composition of the normal lung tissue microbiome were associated with recurrence-free survival (RFS) and disease-free survival (DFS). However, the microbial composition in tumor tissue was independent of RFS and DFS.
In conclusion, the lung microbiota might alter the lung cancer environment in patients and could play a significant role in the development of lung cancer. It is worth mentioning that the samples currently used to study the intratumoral microbiome of lung cancer patients have mainly been surgically resected tumor tissue, bronchial washing fluid (BWF), bronchial brushing tissue, and sputum samples. Although studies using surgically resected tumor tissues would be ideal, researchers have often used BWF and sputum samples instead. Huang et al. explored differences between BWF and sputum samples.64 The results suggested that BWF samples might better reflect the microbiome of lung cancer tissue. Regardless of the metastatic status of lung cancer, differences between squamous cell carcinoma and adenocarcinoma have been identified. In different histological types of lung cancer, the genera related to distant metastasis were also different.
Breast cancer
As early as 2014, researchers revealed the potential of microorganisms in BC diagnosis and treatment strategies. They found that total bacterial counts were reduced in BC compared with matched normal tissue. Furthermore, bacterial DNA load was inversely associated with disease state. In addition, the expression levels of antibacterial response genes in tumors were lower than those in normal control tissues.68 Thompson et al.69 revealed a link between the microbiota and host gene expression in BC in a large-sample-size study. Some bacteria in the microbiota appeared to be closely linked to genetic profiles related to processes such as epithelial-mesenchymal transition (EMT) and proliferation. Smith et al.70 studied the microbiome of breast tissue in non-Hispanic Black and non-Hispanic White patients. They found that the microbial community in breast tissue varied by race, stage, and breast tumor subtype. Recent research has also found that the intratumoral microbiota promotes the metastasis and colonization of BC cells. Mechanistically, intratumoral bacteria enhance resistance to fluid shear stress by reorganizing the actin cytoskeleton, thereby promoting host cell survival and metastasis.71 In conclusion, intratumoral microorganisms are widely present in BC tissues, and these microorganisms might participate in the occurrence, development, and prognosis of cancer. Antibiotic therapy that precisely targets microorganisms seems to have potential, but the use of such therapy in the clinic must be evaluated.
16S rRNA gene sequencing is one of the most commonly used methods to examine the microbial composition within tumors. Costantini et al.106 reported that the V3 region of the 16S rRNA gene contains the most microbial information in breast tissue. They argued that the V4 and V6 + V7 regions were the most informative regions describing the gut microbiota, but using the same regions to describe the breast tissue microbiome might underestimate the total population analyzed. They explored the mammary tissue microbiota in various hypervariable regions (V2, V3, V4, V6+7, V8, and V9) of the 16S rRNA gene. The V3 region was ultimately found to be the most informative region in the breast tissue microbiome, accounting for 45% of all reads. They found that the results of core needle biopsy (CNB) and surgical excision biopsy (SEB) were significantly similar, while CNB is less invasive and more useful as a diagnostic modality. Interestingly, their study also revealed that the microbial composition of tumor tissue and adjacent normal tissue was similar. This finding seems to contradict some previous studies.68 Klann et al.67 suggested that this conflicting result could be due to inconsistencies in sample collection methods between studies. Normal control tissue was obtained from women who never had cancer in some studies, while in others, tissue adjacent to cancer in the same woman was used as normal control tissue.
Prostate cancer
In 2005, researchers isolated and cultured Propionibacterium acnes from prostate cancer (PCa) tissue for the first time.74 Several previous studies have revealed the presence of microbes in PCa tissue, primarily the gut microbiota.72,73 Researchers have also found that P. acnes is positively associated with prostate inflammation, which could be responsible for the subsequent development of cancer. Fehri et al.75 confirmed previous findings and further revealed a possible mechanism by which P. acnes promotes cancer. After P. acnes infection, the transcription factors NF-κB and STAT3 are activated in the body, and the COX2-prostaglandin and plasminogen-matrix metalloproteinase pathways are also activated. The host exhibits a strong inflammatory response. Prolonged exposure to P. acnes affects host cell proliferation and causes cellular transformation. Ma et al.76 conducted a large-sample-size study. They analyzed sequencing data from 242 PCa patients to gain insights into the distribution and potential roles of microbes present in PCa. The researchers found that microbes were more abundant in PCa samples than in normal samples. Some of these microbes were found to be cancer promoting, and unexpectedly, most of the microbes were found to exert antitumor effects in PCa. Much research is needed in the future to further validate these findings.
Others
Studies of intratumoral microbes in ovarian cancer (OC),77,78 CC,79,80 melanoma,81,82 glioma,83 GC,84,85 renal cell carcinoma (RCC),86 and liver cancer87,88 have also been reported (see Table 1 for details). For example, Al-Shabanah et al.77 observed a high percentage of HPV+ cells in OC (42%), while only 8% of cells in normal adjacent tissues were HPV+. HPV-16 was the predominant genotype in malignant tissues, followed by HPV-18 and HPV-45. In addition, viruses easily integrate into the host genome, resulting in gene inactivation and chromosomal instability that can promote cancer progression. A study of liver cancer and intratumoral microbes was recently reported. The researchers used 16S rRNA MiSeq to characterize the tumor microbiome of primary liver cancer (PLC) patients for the first time. They found differences in microbial populations between cancerous tissue and matched adjacent nontumorous tissue, between different histopathological subtypes, and between PLC patients with different prognoses.88 In conclusion, biomarkers reflecting the intratumoral microbiota could be noninvasive tools for cancer diagnosis and might also be useful in cancer treatment and prognostication.
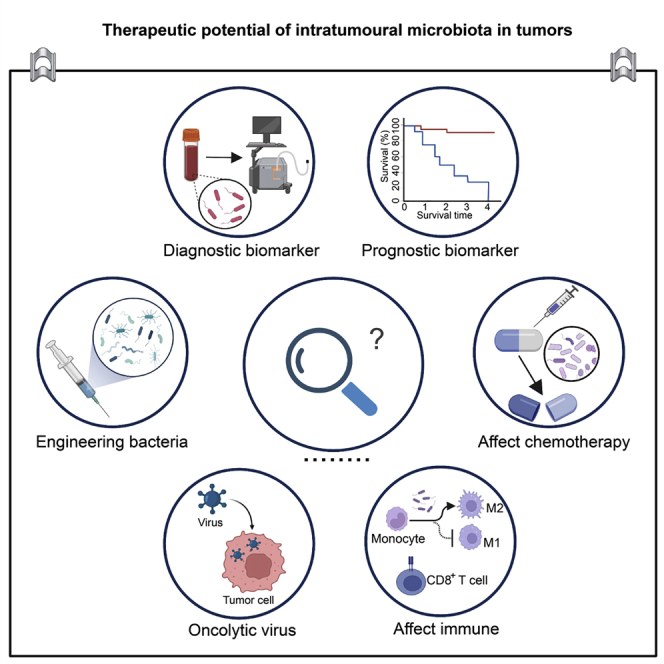
Therapeutic potential of the intratumoral microbiota in tumors
Traditional cancer treatments are mostly unable to accurately differentiate between malignant and healthy tissue, have a relatively broad scope of action, lack tumor specificity, and carry a high risk of recurrence. Furthermore, the unintended killing of healthy tissue results in side effects of varying severity. Researchers have tried for a long time to find ideal biomarkers for cancer diagnosis and treatment. Recent human microbiome studies have shown that microbes play important roles in carcinogenesis, therapeutic response, and drug resistance. Gut microbes can exert oncogenic or tumor-suppressive effects through a variety of mechanisms. Modulating the gut microbiota could be useful for cancer prevention and/or treatment. Recently, attention has shifted to the study of microbes within tumors. Although this research is mostly in the preliminary stage, many studies have begun to reveal the diagnostic value and therapeutic potential of intratumoral microbes. These microbes are closely related to immunity and can modulate the host immune system. With continuous advances in bioengineering technology, an increasing number of engineered bacteria have been designed and applied as cancer treatments. In conclusion, intratumoral microbes play a substantial role in cancer diagnosis and treatment.
Function as biomarkers in cancer
As a component of tumors, intratumoral microbes have the potential as novel diagnostic or prognostic markers. Poore et al.17 revealed unique microbial signatures by whole-genome and whole-transcriptome sequencing of 18,116 samples from 33 types of cancer. Stringent filtering criteria removed 92.3% of the total sequence data, but the researchers still identified intratumoral microbial plasma signatures that were predictive of cancer type. The study revealed the potential clinical utility of detecting markers of the intratumoral microbiome in blood and using them as a diagnostic tool. In addition, researchers have continued to report the prognostic value of intratumoral microbes in tumors.50,107 For example, Yamamura et al.50 found that in ESCC, high expression levels of C. nucleatum in tumors predicted poor patient outcomes. The intratumoral microbiome was also able to predict the prognoses of papillary thyroid carcinoma subtypes.107 In conclusion, identifying potential biomarkers and applying them in the clinic are some of the main directions of future research.
Effects on the host immune system
In recent years, immunotherapy has played a vital role in the treatment of cancer. Immune-based anticancer therapy mainly inhibits cancer by enhancing the patient’s autoimmune function or attacking cancer cells displaying foreign antigens with immune substances. Currently, monoclonal antibodies against programmed death protein 1 (PD-1)/programmed death ligand 1 (PD-L1) and cytotoxic T lymphocyte-associated protein 4 (CTLA-4) are widely used in clinical practice. Studies have found that intratumoral microbes might mediate and influence the role of immune effector cells in the tumor microenvironment. The presence of intratumoral microorganisms can increase or decrease tumor immunogenicity and promote or inhibit antitumor immunity.108 An in-depth understanding of the impact of intratumoral microbes on the cancer immune system is of great value. There is currently sufficient and extensive relevant research in PC. Pushalkar et al.34 reported that the PDAC microbiome exerts oncogenic effects by affecting the host’s immune system. The PDAC microbiome generates a tolerant immune program by activating TLRs, suppressing the activation of CD4+ T cells and differentiated CD8+ T helper 1 (Th1) cells, and inhibiting M1 macrophage differentiation. Elimination of PDAC microbes remodels the tumor microenvironment, in turn enhancing immunotherapy and potentially aiding the treatment of PDAC. The results of another study seemed to contradict the above findings. Based on 16S rRNA gene sequencing, researchers explored the microbial composition and function of the microbiota in PDAC patients. They found that the PDAC microbiota composition affects host immune responses and disease progression. They revealed that the three genera—Saccharopolyspora, Pseudoxanthomonas, and Streptomyces— in PDAC could promote antitumor immune responses by recruiting and activating CD8+ T cells.36 In head and neck squamous cell carcinoma, the pattern of immune cell infiltration in HPV+ tumor samples differs from that in non-virus-associated samples. HPV+ tumors are enriched in infiltrating CD8+ T cells, myeloid dendritic cells (DCs), and pro-inflammatory chemokines.109,110 In CRC, investigators reported that the Fap2 protein of F. nucleatum inhibits immune cell activity through TIGIT, protecting tumor cells from attack by the host immune system.111 Studies in GC have also been performed. Panda et al.112 reported benefits from treatment with the anti-PD-L1 antibody avelumab in a patient with metastatic GC. They found that the tumor tissues of Epstein-Barr virus (EBV)+ GC patients had higher lymphocyte infiltration and expression of genes related to immune checkpoint pathways, suggesting that intratumoral microbes could be involved in avelumab treatment efficacy. In conclusion, the impact of intratumoral microbes on the immune status of the host tumor microenvironment is obvious and deserves further in-depth exploration.
Effects on chemotherapy efficacy
Intratumoral microbes also influence the efficacy of cancer chemotherapy. Geller et al.15 reported the potential role of intratumoral bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. The researchers collected 15 fresh PDAC tissues and cultured them. They then found that 93% of the bacteria made CRC cells resistant to gemcitabine because the bacteria converted gemcitabine into an inactive form via the bacterial enzyme cytidine deaminase (CDD). Based on these findings, the researchers suggested that PDACs contain bacteria that could modulate tumor sensitivity to gemcitabine. In another ESCC study, the researchers found that higher levels of C. nucleatum were associated with adverse responses to neoadjuvant chemotherapy. Antibiotic therapy targeting the bacteria appeared to improve chemotherapy outcomes in patients.50 In addition, commensal bacteria have also been reported to control the therapeutic response to oxaliplatin by regulating reactive oxygen species (ROS) production.113
Application of engineered bacteria
With the continuous development of nanomaterials and bioengineering technologies, researchers have modified natural bacteria to meet special needs, producing engineered bacteria. These bacteria are microorganisms that have been modified by modern bioengineering technology to have multiple functions, high efficiency, and strong adaptability. The main effects and advantages of treatment with engineered bacteria include the following: first, such treatments can directly induce the apoptosis or autophagy of cancer cells. Second, these treatments can induce an antitumor immune response.114 Third, engineered bacteria have the ability to target and modulate tumor metabolic processes during tumor development. Fourth, engineered bacteria can be used as an excellent drug delivery system to precisely deliver drugs to tumor lesions.115 Fifth, such strategies can be employed to generate in situ synthesis of specific therapeutic drugs. For example, Canale et al.116 bioengineered a strain of E. coli Nissle 1917. The bacteria were able to colonize tumors and convert nitrogen-hydrogen compounds in tumors into L-arginine, which is a key determinant of effective antitumor T cell responses. Therefore, the engineered bacterium exerts its antitumor effect in a T cell-dependent, L-arginine-mediated manner. Similarly, Chowdhury et al.117 designed a nonpathogenic strain of E. coli. The engineered bacteria were able to synthesize CD47 nanobodies and self-destructed in vivo to spill the nanobodies, which adhered to the CD47 protein of cancer cells, decreasing their evasion of the immune response and thus rendering them more vulnerable to immune system attack. In conclusion, the development and application of engineered bacteria are important for advances in the field of cancer therapy.
Oncolytic viruses
Oncolytic viruses are a class of replication-competent tumor cell-killing viruses.118 They were discovered in a patient with CC who experienced a decrease in tumor size after being infected with the rabies virus. This phenomenon has attracted great attention from researchers. The effect of oncolytic virus therapy mainly depends on direct oncolysis, but the treatment also indirectly enhances host antitumor immunity.119 The most studied oncolytic viruses include adenovirus and type I herpes simplex virus (HSV). Liu et al.120 reported that JX-594 is a promising oncolytic virus that exerts anticancer effects in hepatocellular carcinoma (HCC). JX-594 has anti-hepatitis B virus (HBV) activity, and targeting of JX-594 can effectively inhibit tumor progression and angiogenesis in HCC patients. Intratumoral injection of JX-594 into primary or metastatic liver tumors showed therapeutic effects. In addition, oncolytic virus therapy has achieved very good results in melanoma, which responds well not only to talimogene laherparepvec (formerly OncoVEX), but also to vaccinia virus (VV) therapy.121 Oncolytic viruses have also shown synergistic effects in killing tumor cells. A study found that VV synergistically enhances the antitumor activity of vesicular stomatitis virus (VSV), which mainly depends on the active gene product VV B18R.122 Interestingly, Cronin et al.123 reported an effect of bacteria on oncolytic virus therapy for cancer. E. coli produces B18R, which greatly enhances VSVΔ51 infection in vitro and in vivo, resulting in tumor growth inhibition, in turn improving mouse survival. Oncolytic virotherapy is now routinely used to treat cancer patients who do not respond to or achieve durable responses from immune checkpoint inhibitors. In conclusion, countless oncolytic virus studies and review articles have been published, and this article provides only a brief overview.
Future perspectives and challenges
Challenges in the detection of intratumoral microbes
Characterizing the intratumoral microbiota is challenging because tumor samples are known to have low microbial biomass. Additionally, tumor samples have a very high host-to-bacterial DNA ratio, which can lead to biased amplicon-based sequencing results. In addition, because there are many steps in the process of clinical sample collection, ensuring the quality and quantity of tumor samples is also difficult. Ensuring that samples are not contaminated during the testing process and determining how to completely eliminate the influence of microbial contamination from the environment are important goals worth considering.
Challenges in determining the role of intratumoral microbes
Current research has not yet fully clarified whether specific changes in the microbes of tumors are the cause or the result of the development of cancer or whether these changes are unrelated to the cancer (for example, the result of a simple infection). The relationship between intratumoral microbes and host tumors is complex and unclear, and more research is needed to understand the mechanism.
Challenges in the therapeutic application of intratumoral microbes
As mentioned above, engineered microorganisms have been used in cancer treatment. However, their safety is an issue that cannot be ignored.124 Further testing in animal models and humans is needed.
Other potential challenges
There remain major gaps in the clinical study of the cancer microbiome, and relevant clinical interventions must still be designed. The translation of scientific research into clinical applications is difficult, and there have been several contradictory conclusions between different studies. The consistency between the results of different studies has been poor, and although some differences in microbial composition between tumors and normal tissues have been identified, some of these differences were not significant.125
Future outlook
Researchers still have a long way to go to address these challenges and to identify potential undiscovered problems. In the foreseeable future, in-depth characterization of distinct intratumoral microbiomes could enable the manipulation of these bacterial communities to achieve cancer patient therapy. In addition to addressing these potential challenges, it is also very important to dig deeper into the molecular mechanisms of the role of microorganisms in tumors and to find new therapeutic targets and biomarkers. Clearly, the contribution of microbes to cancer biology is likely to take center stage in cancer research over the next decade.
Conclusion
In conclusion, this review mainly summarized the role of intratumoral microbes in different tumor types. The therapeutic potential of intratumoral microbes in tumors was also explored. In addition, a brief summary of methods commonly used to characterize intratumoral microbes was provided. Finally, the current challenges and future research directions in this field were presented. We hope to have provided some ideas for further innovation in the field of cancer microorganisms.
Acknowledgments
This work was funded by the National Key Research and Development Program of China (2021YFC2301804).
Author contributions
L.L. and J.L. designed the work and reviewed and edited the manuscript; C.X., Q.C., and Q.Z. participated in the original draft preparation; X.Y. helped to review the manuscript; and Y.S. and Z.B. collected the references. All authors have read and approved the final manuscript.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Juan Lu, Email: lujuanzju@zju.edu.cn.
Lanjuan Li, Email: ljli@zju.edu.cn.
References
- 1.Crosby D., Bhatia S., Brindle K.M., Coussens L.M., Dive C., Emberton M., Esener S., Fitzgerald R.C., Gambhir S.S., Kuhn P., et al. Vol. 375. 2022. Early detection of cancer. Science. [DOI] [PubMed] [Google Scholar]
- 2.Organization W.H. 2020. Deaths by cause, age, sex, by Country and by region, 2000-2019 (WHO) Global Health Estimates 2020. [Google Scholar]
- 3.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN Estimates of incidence and mortality worldwide for 36 cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 4.Yabroff K.R., Wu X.C., Negoita S., Stevens J., Coyle L., Zhao J., Mumphrey B.J., Jemal A., Ward K.C. Association of the COVID-19 pandemic with patterns of Statewide cancer Services. J Natl. Cancer Inst. 2022;114:907–909. doi: 10.1093/jnci/djab122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burd E.M. Human papillomavirus and cervical cancer. Clin. Microbiol. Rev. 2003;16:1–17. doi: 10.1128/cmr.16.1.1-17.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collatuzzo G., Pelucchi C., Negri E., López-Carrillo L., Tsugane S., Hidaka A., Shigueaki Hamada G., Hernández-Ramírez R.U., López-Cervantes M., Malekzadeh R., et al. Exploring the interactions between Helicobacter pylori (Hp) infection and other risk factors of gastric cancer: a pooled analysis in the Stomach cancer Pooling (StoP) Project. Int. J. Cancer. 2021;149:1228–1238. doi: 10.1002/ijc.33678. [DOI] [PubMed] [Google Scholar]
- 7.Turnbaugh P.J., Hamady M., Yatsunenko T., Cantarel B.L., Duncan A., Ley R.E., Sogin M.L., Jones W.J., Roe B.A., Affourtit J.P., et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilbert J.A., Blaser M.J., Caporaso J.G., Jansson J.K., Lynch S.V., Knight R. Current understanding of the human microbiome. Nat. Med. 2018;24:392–400. doi: 10.1038/nm.4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turnbaugh P.J., Ley R.E., Hamady M., Fraser-Liggett C.M., Knight R., Gordon J.I. The human microbiome project. Nature. 2007;449:804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cullin N., Azevedo Antunes C., Straussman R., Stein-Thoeringer C.K., Elinav E. Microbiome and cancer. Cancer Cell. 2021;39:1317–1341. doi: 10.1016/j.ccell.2021.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Akshintala V.S., Talukdar R., Singh V.K., Goggins M. The gut microbiome in pancreatic disease. Clin. Gastroenterol. Hepatol. 2019;17:290–295. doi: 10.1016/j.cgh.2018.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jandhyala S.M., Talukdar R., Subramanyam C., Vuyyuru H., Sasikala M., Nageshwar Reddy D. Role of the normal gut microbiota. World J. Gastroenterol. 2015;21:8787–8803. doi: 10.3748/wjg.v21.i29.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma C., Han M., Heinrich B., Fu Q., Zhang Q., Sandhu M., Agdashian D., Terabe M., Berzofsky J.A., Fako V., et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science. 2018;360:eaan5931. doi: 10.1126/science.aan5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nejman D., Livyatan I., Fuks G., Gavert N., Zwang Y., Geller L.T., Rotter-Maskowitz A., Weiser R., Mallel G., Gigi E., et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science. 2020;368:973–980. doi: 10.1126/science.aay9189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geller L.T., Barzily-Rokni M., Danino T., Jonas O.H., Shental N., Nejman D., Gavert N., Zwang Y., Cooper Z.A., Shee K. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science. 2017;357:1156–1160. doi: 10.1126/science.aah5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parhi L., Alon-Maimon T., Sol A., Nejman D., Shhadeh A., Fainsod-Levi T., Yajuk O., Isaacson B., Abed J., Maalouf N., et al. Breast cancer colonization by Fusobacterium nucleatum accelerates tumor growth and metastatic progression. Nat. Commun. 2020;11:3259. doi: 10.1038/s41467-020-16967-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poore G.D., Kopylova E., Zhu Q., Carpenter C., Fraraccio S., Wandro S., Kosciolek T., Janssen S., Metcalf J., Song S.J., et al. Microbiome analyses of blood and tissues suggest cancer diagnostic approach. Nature. 2020;579:567–574. doi: 10.1038/s41586-020-2095-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Arıkan M., Mitchell A.L., Finn R.D., Gürel F. Microbial composition of Kombucha determined using amplicon sequencing and shotgun metagenomics. J. Food Sci. 2020;85:455–464. doi: 10.1111/1750-3841.14992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calgaro M., Romualdi C., Waldron L., Risso D., Vitulo N. Assessment of statistical methods from single cell, bulk RNA-seq, and metagenomics applied to microbiome data. Genome Biol. 2020;21:191. doi: 10.1186/s13059-020-02104-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y.X., Qin Y., Chen T., Lu M., Qian X., Guo X., Bai Y. A practical guide to amplicon and metagenomic analysis of microbiome data. Protein Cell. 2021;12:315–330. doi: 10.1007/s13238-020-00724-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bolyen E., Rideout J.R., Dillon M.R., Bokulich N.A., Abnet C.C., Al-Ghalith G.A., Alexander H., Alm E.J., Arumugam M., Asnicar F., et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019;37:852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lane D.J., Pace B., Olsen G.J., Stahl D.A., Sogin M.L., Pace N.R. Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses. Proc. Natl. Acad. Sci. USA. 1985;82:6955–6959. doi: 10.1073/pnas.82.20.6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharpton T.J. An introduction to the analysis of shotgun metagenomic data. Front. Plant Sci. 2014;5:209. doi: 10.3389/fpls.2014.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tringe S.G., Rubin E.M. Metagenomics: DNA sequencing of environmental samples. Nat. Rev. Genet. 2005;6:805–814. doi: 10.1038/nrg1709. [DOI] [PubMed] [Google Scholar]
- 25.Zaki S., Blau D.M., Hughes J.M., Nolte K.B., Lynfield R., Carr W., Popovic T., Centers for Disease Control and Prevention CDC CDC Grand Rounds: discovering new diseases via enhanced partnership between public health and pathology experts. MMWR Morb. Mortal. Wkly. Rep. 2014;63:121–126. [PMC free article] [PubMed] [Google Scholar]
- 26.Kostic A.D., Gevers D., Pedamallu C.S., Michaud M., Duke F., Earl A.M., Ojesina A.I., Jung J., Bass A.J., Tabernero J., et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012;22:292–298. doi: 10.1101/gr.126573.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson R.J., Bouwer H.G.A., Portnoy D.A., Frankel F.R. Pathogenicity and immunogenicity of a Listeria monocytogenes strain that requires D-alanine for growth. Infect. Immun. 1998;66:3552–3561. doi: 10.1128/iai.66.8.3552-3561.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desmarais S.M., De Pedro M.A., Cava F., Huang K.C. Peptidoglycan at its peaks: how chromatographic analyses can reveal bacterial cell wall structure and assembly. Mol. Microbiol. 2013;89:1–13. doi: 10.1111/mmi.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nilsson H.O., Stenram U., Ihse I., Wadstrom T. Helicobacter species ribosomal DNA in the pancreas, stomach and duodenum of pancreatic cancer patients. World J. Gastroenterol. 2006;12:3038–3043. doi: 10.3748/wjg.v12.i19.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kohi S., Macgregor-Das A., Dbouk M., Yoshida T., Chuidian M., Abe T., Borges M., Lennon A.M., Shin E.J., Canto M.I., Goggins M. Alterations in the duodenal fluid microbiome of patients with pancreatic cancer. Clin. Gastroenterol. Hepatol. 2022;20:e196–e227. doi: 10.1016/j.cgh.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Del Castillo E., Meier R., Chung M., Koestler D.C., Chen T., Paster B.J., Charpentier K.P., Kelsey K.T., Izard J., Michaud D.S. The microbiomes of pancreatic and duodenum tissue Overlap and are highly Subject specific but differ between pancreatic cancer and Noncancer Subjects. Cancer Epidemiol. Biomarkers Prev. 2019;28:370–383. doi: 10.1158/1055-9965.epi-18-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nalluri H., Jensen E., Staley C. Role of biliary stent and neoadjuvant chemotherapy in the pancreatic tumor microbiome. BMC Microbiol. 2021;21:280. doi: 10.1186/s12866-021-02339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas R.M., Gharaibeh R.Z., Gauthier J., Beveridge M., Pope J.L., Guijarro M.V., Yu Q., He Z., Ohland C., Newsome R., et al. Intestinal microbiota enhances pancreatic carcinogenesis in preclinical models. Carcinogenesis. 2018;39:1068–1078. doi: 10.1093/carcin/bgy073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pushalkar S., Hundeyin M., Daley D., Zambirinis C.P., Kurz E., Mishra A., Mohan N., Aykut B., Usyk M., Torres L.E., et al. The pancreatic cancer microbiome promotes oncogenesis by Induction of innate and adaptive immune suppression. Cancer Discov. 2018;8:403–416. doi: 10.1158/2159-8290.cd-17-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chakladar J., Kuo S.Z., Castaneda G., Li W.T., Gnanasekar A., Yu M.A., Chang E.Y., Wang X.Q., Ongkeko W.M. The pancreatic microbiome is associated with carcinogenesis and Worse prognosis in males and smokers. Cancers. 2020;12:2672. doi: 10.3390/cancers12092672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riquelme E., Zhang Y., Zhang L., Montiel M., Zoltan M., Dong W., Quesada P., Sahin I., Chandra V., San Lucas A., et al. Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell. 2019;178:795–806.e12. doi: 10.1016/j.cell.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo W., Zhang Y., Guo S., Mei Z., Liao H., Dong H., Wu K., Ye H., Zhang Y., Zhu Y., et al. Tumor microbiome contributes to an aggressive phenotype in the basal-like subtype of pancreatic cancer. Commun Biol. 2021;4:1019. doi: 10.1038/s42003-021-02557-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aykut B., Pushalkar S., Chen R., Li Q., Abengozar R., Kim J.I., Shadaloey S.A., Wu D., Preiss P., Verma N., et al. The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature. 2019;574:264–267. doi: 10.1038/s41586-019-1608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bullman S., Pedamallu C.S., Sicinska E., Clancy T.E., Zhang X., Cai D., Neuberg D., Huang K., Guevara F., Nelson T., et al. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science. 2017;358:1443–1448. doi: 10.1126/science.aal5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Flemer B., Lynch D.B., Brown J.M.R., Jeffery I.B., Ryan F.J., Claesson M.J., O'Riordain M., Shanahan F., O'Toole P.W. Tumour-associated and non-tumour-associated microbiota in colorectal cancer. Gut. 2017;66:633–643. doi: 10.1136/gutjnl-2015-309595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu W., Zhang X., Xu H., Li S., Lau H.C.H., Chen Q., Zhang B., Zhao L., Chen H., Sung J.J.Y., Yu J. Microbial community heterogeneity within colorectal Neoplasia and its correlation with colorectal carcinogenesis. Gastroenterology. 2021;160:2395–2408. doi: 10.1053/j.gastro.2021.02.020. [DOI] [PubMed] [Google Scholar]
- 42.Warren R.L., Freeman D.J., Pleasance S., Watson P., Moore R.A., Cochrane K., Allen-Vercoe E., Holt R.A. Co-occurrence of anaerobic bacteria in colorectal carcinomas. Microbiome. 2013;1:16. doi: 10.1186/2049-2618-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okuda S., Shimada Y., Tajima Y., Yuza K., Hirose Y., Ichikawa H., Nagahashi M., Sakata J., Ling Y., Miura N., et al. Profiling of host genetic alterations and intra-tumor microbiomes in colorectal cancer. Comput. Struct. Biotechnol. J. 2021;19:3330–3338. doi: 10.1016/j.csbj.2021.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamamoto S., Kinugasa H., Hirai M., Terasawa H., Yasutomi E., Oka S., Ohmori M., Yamasaki Y., Inokuchi T., Harada K., et al. Heterogeneous distribution of Fusobacterium nucleatum in the progression of colorectal cancer. J. Gastroenterol. Hepatol. 2021;36:1869–1876. doi: 10.1111/jgh.15361. [DOI] [PubMed] [Google Scholar]
- 45.Burns M.B., Lynch J., Starr T.K., Knights D., Blekhman R. Virulence genes are a signature of the microbiome in the colorectal tumor microenvironment. Genome Med. 2015;7:55. doi: 10.1186/s13073-015-0177-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kosumi K., Hamada T., Koh H., Borowsky J., Bullman S., Twombly T.S., Nevo D., Masugi Y., Liu L., da Silva A., et al. The amount of Bifidobacterium genus in colorectal carcinoma tissue in relation to tumor characteristics and clinical outcome. Am. J. Pathol. 2018;188:2839–2852. doi: 10.1016/j.ajpath.2018.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y., Guo H., Gao X., Wang J. The intratumor microbiota signatures associate with subtype, tumor stage, and survival status of esophageal carcinoma. Front. Oncol. 2021;11:754788. doi: 10.3389/fonc.2021.754788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamamura K., Baba Y., Nakagawa S., Mima K., Miyake K., Nakamura K., Sawayama H., Kinoshita K., Ishimoto T., Iwatsuki M., et al. Human microbiome Fusobacterium nucleatum in esophageal cancer tissue is associated with prognosis. Clin. Cancer Res. 2016;22:5574–5581. doi: 10.1158/1078-0432.ccr-16-1786. [DOI] [PubMed] [Google Scholar]
- 49.Liu Y., Lin Z., Lin Y., Chen Y., Peng X.E., He F., Liu S., Yan S., Huang L., Lu W., et al. Streptococcus and Prevotella are associated with the prognosis of oesophageal squamous cell carcinoma. J. Med. Microbiol. 2018;67:1058–1068. doi: 10.1099/jmm.0.000754. [DOI] [PubMed] [Google Scholar]
- 50.Yamamura K., Izumi D., Kandimalla R., Sonohara F., Baba Y., Yoshida N., Kodera Y., Baba H., Goel A. Intratumoral Fusobacterium nucleatum levels predict therapeutic response to neoadjuvant chemotherapy in esophageal squamous cell carcinoma. Clin. Cancer Res. 2019;25:6170–6179. doi: 10.1158/1078-0432.ccr-19-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Snider E.J., Compres G., Freedberg D.E., Khiabanian H., Nobel Y.R., Stump S., Uhlemann A.C., Lightdale C.J., Abrams J.A. Alterations to the esophageal microbiome associated with progression from Barrett's esophagus to esophageal adenocarcinoma. Cancer Epidemiol. Biomarkers Prev. 2019;28:1687–1693. doi: 10.1158/1055-9965.epi-19-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hooper S.J., Crean S.J., Lewis M.A.O., Spratt D.A., Wade W.G., Wilson M.J. Viable bacteria present within oral squamous cell carcinoma tissue. J. Clin. Microbiol. 2006;44:1719–1725. doi: 10.1128/jcm.44.5.1719-1725.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pushalkar S., Ji X., Li Y., Estilo C., Yegnanarayana R., Singh B., Li X., Saxena D. Comparison of oral microbiota in tumor and non-tumor tissues of patients with oral squamous cell carcinoma. BMC Microbiol. 2012;12:144. doi: 10.1186/1471-2180-12-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perera M., Al-Hebshi N.N., Perera I., Ipe D., Ulett G.C., Speicher D.J., Chen T., Johnson N.W. Inflammatory bacteriome and oral squamous cell carcinoma. J. Dent. Res. 2018;97:725–732. doi: 10.1177/0022034518767118. [DOI] [PubMed] [Google Scholar]
- 55.Al-Hebshi N.N., Nasher A.T., Maryoud M.Y., Homeida H.E., Chen T., Idris A.M., Johnson N.W. Inflammatory bacteriome featuring Fusobacterium nucleatum and Pseudomonas aeruginosa identified in association with oral squamous cell carcinoma. Sci. Rep. 2017;7:1834. doi: 10.1038/s41598-017-02079-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Neuzillet C., Marchais M., Vacher S., Hilmi M., Schnitzler A., Meseure D., Leclere R., Lecerf C., Dubot C., Jeannot E., et al. Prognostic value of intratumoral Fusobacterium nucleatum and association with immune-related gene expression in oral squamous cell carcinoma patients. Sci. Rep. 2021;11:7870. doi: 10.1038/s41598-021-86816-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakashima C., Yamamoto K., Kishi S., Sasaki T., Ohmori H., Fujiwara-Tani R., Mori S., Kawahara I., Nishiguchi Y., Mori T., et al. Clostridium perfringens enterotoxin induces claudin-4 to activate YAP in oral squamous cell carcinomas. Oncotarget. 2020;11:309–321. doi: 10.18632/oncotarget.27424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Greathouse K.L., White J.R., Vargas A.J., Bliskovsky V.V., Beck J.A., von Muhlinen N., Polley E.C., Bowman E.D., Khan M.A., Robles A.I., et al. Interaction between the microbiome and TP53 in human lung cancer. Genome Biol. 2018;19:123. doi: 10.1186/s13059-018-1501-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wong L.M., Shende N., Li W.T., Castaneda G., Apostol L., Chang E.Y., Ongkeko W.M. Comparative analysis of age- and gender-associated microbiome in lung adenocarcinoma and lung squamous cell carcinoma. Cancers. 2020;12:1447. doi: 10.3390/cancers12061447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee S.H., Sung J.Y., Yong D., Chun J., Kim S.Y., Song J.H., Chung K.S., Kim E.Y., Jung J.Y., Kang Y.A., et al. Characterization of microbiome in bronchoalveolar lavage fluid of patients with lung cancer comparing with benign mass like lesions. Lung Cancer. 2016;102:89–95. doi: 10.1016/j.lungcan.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 61.Dumont-Leblond N., Veillette M., Racine C., Joubert P., Duchaine C. Non-small cell lung cancer microbiota characterization: Prevalence of enteric and potentially pathogenic bacteria in cancer tissues. PLoS One. 2021;16:e0249832. doi: 10.1371/journal.pone.0249832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Apostolou P., Tsantsaridou A., Papasotiriou I., Toloudi M., Chatziioannou M., Giamouzis G. Bacterial and fungal microflora in surgically removed lung cancer samples. J. Cardiothorac. Surg. 2011;6:137. doi: 10.1186/1749-8090-6-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peters B.A., Hayes R.B., Goparaju C., Reid C., Pass H.I., Ahn J. The microbiome in lung cancer tissue and recurrence-free survival. Cancer Epidemiol. Biomarkers Prev. 2019;28:731–740. doi: 10.1158/1055-9965.epi-18-0966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang D., Su X., Yuan M., Zhang S., He J., Deng Q., Qiu W., Dong H., Cai S. The characterization of lung microbiome in lung cancer patients with different clinicopathology. Am J Cancer Res. 2019;9:2047–2063. [PMC free article] [PubMed] [Google Scholar]
- 65.Urbaniak C., Cummins J., Brackstone M., Macklaim J.M., Gloor G.B., Baban C.K., et al. Microbiota of human breast tissue. Applied and environmental microbiology. 2014;80:3007–3014. doi: 10.1128/AEM.00242-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Urbaniak C., Gloor G.B., Brackstone M., Scott L., Tangney M., Reid G. The Microbiota of Breast Tissue and Its Association with Breast Cancer. Applied and environmental microbiology. 2016;82:5039–5048. doi: 10.1128/AEM.01235-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Klann E., Williamson J.M., Tagliamonte M.S., Ukhanova M., Asirvatham J.R., Chim H., Yaghjyan L., Mai V. Microbiota composition in bilateral healthy breast tissue and breast tumors. Cancer Causes Control. 2020;31:1027–1038. doi: 10.1007/s10552-020-01338-5. [DOI] [PubMed] [Google Scholar]
- 68.Xuan C., Shamonki J.M., Chung A., Dinome M.L., Chung M., Sieling P.A., Lee D.J. Microbial dysbiosis is associated with human breast cancer. PLoS One. 2014;9:e83744. doi: 10.1371/journal.pone.0083744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thompson K.J., Ingle J.N., Tang X., Chia N., Jeraldo P.R., Walther-Antonio M.R., Kandimalla K.K., Johnson S., Yao J.Z., Harrington S.C., et al. A comprehensive analysis of breast cancer microbiota and host gene expression. PLoS One. 2017;12:e0188873. doi: 10.1371/journal.pone.0188873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Smith A., Pierre J.F., Makowski L., Tolley E., Lyn-Cook B., Lu L., Vidal G., Starlard-Davenport A. Distinct microbial communities that differ by race, stage, or breast-tumor subtype in breast tissues of non-Hispanic Black and non-Hispanic White women. Sci. Rep. 2019;9:11940. doi: 10.1038/s41598-019-48348-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fu A., Yao B., Dong T., Chen Y., Yao J., Liu Y., Li H., Bai H., Liu X., Zhang Y., et al. Tumor-resident intracellular microbiota promotes metastatic colonization in breast cancer. Cell. 2022;185:1356–1372.e26. doi: 10.1016/j.cell.2022.02.027. [DOI] [PubMed] [Google Scholar]
- 72.Banerjee S., Alwine J.C., Wei Z., Tian T., Shih N., Sperling C., Guzzo T., Feldman M.D., Robertson E.S. Microbiome signatures in prostate cancer. Carcinogenesis. 2019;40:749–764. doi: 10.1093/carcin/bgz008. [DOI] [PubMed] [Google Scholar]
- 73.Cavarretta I., Ferrarese R., Cazzaniga W., Saita D., Lucianò R., Ceresola E.R., Locatelli I., Visconti L., Lavorgna G., Briganti A., et al. The microbiome of the prostate tumor microenvironment. Eur. Urol. 2017;72:625–631. doi: 10.1016/j.eururo.2017.03.029. [DOI] [PubMed] [Google Scholar]
- 74.Cohen R.J., Shannon B.A., McNeal J.E., Shannon T., Garrett K.L. Propionibacterium acnes associated with inflammation in radical prostatectomy specimens: a possible link to cancer evolution? J. Urol. 2005;173:1969–1974. doi: 10.1097/01.ju.0000158161.15277.78. [DOI] [PubMed] [Google Scholar]
- 75.Fassi Fehri L., Mak T.N., Laube B., Brinkmann V., Ogilvie L.A., Mollenkopf H., Lein M., Schmidt T., Meyer T.F., Brüggemann H. Prevalence of Propionibacterium acnes in diseased prostates and its inflammatory and transforming activity on prostate epithelial cells. Int J Med. Microbiol. 2011;301:69–78. doi: 10.1016/j.ijmm.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 76.Ma J., Gnanasekar A., Lee A., Li W.T., Haas M., Wang-Rodriguez J., Chang E.Y., Rajasekaran M., Ongkeko W.M. Influence of intratumor microbiome on clinical outcome and immune processes in prostate cancer. Cancers. 2020;12:2524. doi: 10.3390/cancers12092524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Al-Shabanah O.A., Hafez M.M., Hassan Z.K., Sayed-Ahmed M.M., Abozeed W.N., Al-Rejaie S.S., Alsheikh A.A. Human papillomavirus genotyping and integration in ovarian cancer Saudi patients. Virol. J. 2013;10:343. doi: 10.1186/1743-422x-10-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou B., Sun C., Huang J., Xia M., Guo E., Li N., Lu H., Shan W., Wu Y., Li Y., et al. The biodiversity composition of microbiome in ovarian carcinoma patients. Sci. Rep. 2019;9:1691. doi: 10.1038/s41598-018-38031-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang S.T., Chen J., Lian L.Y., Cai H.H., Zeng H.S., Zheng M., Liu M.B. Intratumoral levels and prognostic significance of Fusobacterium nucleatum in cervical carcinoma. Aging (Albany NY) 2020;12:23337–23350. doi: 10.18632/aging.104188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu J., Luo M., Zhang Y., Cao G., Wang S. Association of high-risk human papillomavirus infection duration and cervical lesions with vaginal microbiota composition. Ann. Transl. Med. 2020;8:1161. doi: 10.21037/atm-20-5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mrázek J., Mekadim C., Kučerová P., Švejstil R., Salmonová H., Vlasáková J., Tarasová R., Čížková J., Červinková M. Melanoma-related changes in skin microbiome. Folia Microbiol (Praha) 2019;64:435–442. doi: 10.1007/s12223-018-00670-3. [DOI] [PubMed] [Google Scholar]
- 82.Zhu G., Su H., Johnson C.H., Khan S.A., Kluger H., Lu L. Intratumour microbiome associated with the infiltration of cytotoxic CD8+ T cells and patient survival in cutaneous melanoma. Eur. J. Cancer. 2021;151:25–34. doi: 10.1016/j.ejca.2021.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao J., He D., Lai H.M., Xu Y., Luo Y., Li T., Liang J., Yang X., Guo L., Ke Y., et al. Comprehensive histological imaging of native microbiota in human glioma. J Biophotonics. 2022;15:e202100351. doi: 10.1002/jbio.202100351. [DOI] [PubMed] [Google Scholar]
- 84.Yu G., Torres J., Hu N., Medrano-Guzman R., Herrera-Goepfert R., Humphrys M.S., Wang L., Wang C., Ding T., Ravel J., et al. Molecular characterization of the human stomach microbiota in gastric cancer patients. Front. Cell. Infect. Microbiol. 2017;7:302. doi: 10.3389/fcimb.2017.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shao D., Vogtmann E., Liu A., Qin J., Chen W., Abnet C.C., Wei W. Microbial characterization of esophageal squamous cell carcinoma and gastric cardia adenocarcinoma from a high-risk region of China. Cancer. 2019;125:3993–4002. doi: 10.1002/cncr.32403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang J., Li X., Wu X., Wang Z., Zhang C., Cao G., Liu K., Yan T. Uncovering the microbiota in renal cell carcinoma tissue using 16S rRNA gene sequencing. J. Cancer Res. Clin. Oncol. 2021;147:481–491. doi: 10.1007/s00432-020-03462-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huang Y., Fan X.G., Wang Z.M., Zhou J.H., Tian X.F., Li N. Identification of helicobacter species in human liver samples from patients with primary hepatocellular carcinoma. J. Clin. Pathol. 2004;57:1273–1277. doi: 10.1136/jcp.2004.018556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Qu D., Wang Y., Xia Q., Chang J., Jiang X., Zhang H. Intratumoral microbiome of human primary liver cancer. Hepatol Commun. 2022;6:1741–1752. doi: 10.1002/hep4.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 90.Cano C.E., Sandí M.J., Hamidi T., Calvo E.L., Turrini O., Bartholin L., Loncle C., Secq V., Garcia S., Lomberk G., et al. Homotypic cell cannibalism, a cell-death process regulated by the nuclear protein 1, opposes to metastasis in pancreatic cancer. EMBO Mol. Med. 2012;4:964–979. doi: 10.1002/emmm.201201255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zambirinis C.P., Levie E., Nguy S., Avanzi A., Barilla R., Xu Y., Seifert L., Daley D., Greco S.H., Deutsch M., et al. TLR9 ligation in pancreatic stellate cells promotes tumorigenesis. J. Exp. Med. 2015;212:2077–2094. doi: 10.1084/jem.20142162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McAllister F., Khan M.A.W., Helmink B., Wargo J.A. The tumor microbiome in pancreatic cancer: bacteria and beyond. Cancer Cell. 2019;36:577–579. doi: 10.1016/j.ccell.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 93.Zhou Z., Chen J., Yao H., Hu H. Fusobacterium and colorectal cancer. Front. Oncol. 2018;8:371. doi: 10.3389/fonc.2018.00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guo S., Chen J., Chen F., Zeng Q., Liu W.L., Zhang G. 2020. Exosomes derived from Fusobacterium nucleatum-infected colorectal cancer cells facilitate tumour metastasis by selectively carrying miR-1246/92b-3p/27a-3p and CXCL16. Gut. [DOI] [PubMed] [Google Scholar]
- 95.Yang Y., Weng W., Peng J., Hong L., Yang L., Toiyama Y., Gao R., Liu M., Yin M., Pan C., et al. Fusobacterium nucleatum increases proliferation of colorectal cancer cells and tumor development in mice by activating Toll-like receptor 4 signaling to nuclear factor-κB, and Up-regulating expression of MicroRNA-21. Gastroenterology. 2017;152:851–866.e24. doi: 10.1053/j.gastro.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Castellarin M., Warren R.L., Freeman J.D., Dreolini L., Krzywinski M., Strauss J., Barnes R., Watson P., Allen-Vercoe E., Moore R.A., Holt R.A. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012;22:299–306. doi: 10.1101/gr.126516.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mima K., Sukawa Y., Nishihara R., Qian Z.R., Yamauchi M., Inamura K., Kim S.A., Masuda A., Nowak J.A., Nosho K., et al. Fusobacterium nucleatum and T cells in colorectal carcinoma. JAMA Oncol. 2015;1:653–661. doi: 10.1001/jamaoncol.2015.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hamada T., Zhang X., Mima K., Bullman S., Sukawa Y., Nowak J.A., Kosumi K., Masugi Y., Twombly T.S., Cao Y., et al. Fusobacterium nucleatum in colorectal cancer Relates to immune response differentially by tumor microsatellite instability status. Cancer Immunol Res. 2018;6:1327–1336. doi: 10.1158/2326-6066.cir-18-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Park H.E., Kim J.H., Cho N.Y., Lee H.S., Kang G.H. Intratumoral Fusobacterium nucleatum abundance correlates with macrophage infiltration and CDKN2A methylation in microsatellite-unstable colorectal carcinoma. Virchows Arch. 2017;471:329–336. doi: 10.1007/s00428-017-2171-6. [DOI] [PubMed] [Google Scholar]
- 100.Zhang Y., Zhang L., Zheng S., Li M., Xu C., Jia D., Qi Y., Hou T., Wang L., Wang B., et al. Fusobacterium nucleatum promotes colorectal cancer cells adhesion to endothelial cells and facilitates extravasation and metastasis by inducing ALPK1/NF-κB/ICAM1 axis. Gut Microb. 2022;14:2038852. doi: 10.1080/19490976.2022.2038852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gao Z., Guo B., Gao R., Zhu Q., Qin H. Microbiota disbiosis is associated with colorectal cancer. Front. Microbiol. 2015;6:20. doi: 10.3389/fmicb.2015.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hooper S.J., Crean S.J., Fardy M.J., Lewis M.A.O., Spratt D.A., Wade W.G., Wilson M.J. A molecular analysis of the bacteria present within oral squamous cell carcinoma. J. Med. Microbiol. 2007;56:1651–1659. doi: 10.1099/jmm.0.46918-0. [DOI] [PubMed] [Google Scholar]
- 103.Hilty M., Burke C., Pedro H., Cardenas P., Bush A., Bossley C., Davies J., Ervine A., Poulter L., Pachter L., et al. Disordered microbial communities in asthmatic airways. PLoS One. 2010;5:e8578. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.de Groot P.M., Wu C.C., Carter B.W., Munden R.F. The epidemiology of lung cancer. Transl. Lung Cancer Res. 2018;7:220–233. doi: 10.21037/tlcr.2018.05.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yu G., Gail M.H., Consonni D., Carugno M., Humphrys M., Pesatori A.C., Caporaso N.E., Goedert J.J., Ravel J., Landi M.T. Characterizing human lung tissue microbiota and its relationship to epidemiological and clinical features. Genome Biol. 2016;17:163. doi: 10.1186/s13059-016-1021-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Costantini L., Magno S., Albanese D., Donati C., Molinari R., Filippone A., Masetti R., Merendino N. Characterization of human breast tissue microbiota from core needle biopsies through the analysis of multi hypervariable 16S-rRNA gene regions. Sci. Rep. 2018;8:16893. doi: 10.1038/s41598-018-35329-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gnanasekar A., Castaneda G., Iyangar A., Magesh S., Perez D., Chakladar J., Li W.T., Bouvet M., Chang E.Y., Ongkeko W.M. The intratumor microbiome predicts prognosis across gender and subtypes in papillary thyroid carcinoma. Comput. Struct. Biotechnol. J. 2021;19:1986–1997. doi: 10.1016/j.csbj.2021.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cogdill A.P., Gaudreau P.O., Arora R., Gopalakrishnan V., Wargo J.A. The impact of intratumoral and gastrointestinal microbiota on systemic cancer therapy. Trends Immunol. 2018;39:900–920. doi: 10.1016/j.it.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 109.An Y., Zhang W., Liu T., Wang B., Cao H. The intratumoural microbiota in cancer: new insights from inside. Biochim. Biophys. Acta Rev. Canc. 2021;1876:188626. doi: 10.1016/j.bbcan.2021.188626. [DOI] [PubMed] [Google Scholar]
- 110.Partlová S., Bouček J., Kloudová K., Lukešová E., Zábrodský M., Grega M., Fučíková J., Truxová I., Tachezy R., Špíšek R., Fialová A. Distinct patterns of intratumoral immune cell infiltrates in patients with HPV-associated compared to non-virally induced head and neck squamous cell carcinoma. OncoImmunology. 2015;4:e965570. doi: 10.4161/21624011.2014.965570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gur C., Ibrahim Y., Isaacson B., Yamin R., Abed J., Gamliel M., Enk J., Bar-On Y., Stanietsky-Kaynan N., Coppenhagen-Glazer S., et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity. 2015;42:344–355. doi: 10.1016/j.immuni.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Panda A., Mehnert J.M., Hirshfield K.M., Riedlinger G., Damare S., Saunders T., Kane M., Sokol L., Stein M.N., Poplin E., et al. Immune activation and benefit from avelumab in EBV-positive gastric cancer. J Natl Cancer Inst. 2018;110:316–320. doi: 10.1093/jnci/djx213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Iida N., Dzutsev A., Stewart C.A., Smith L., Bouladoux N., Weingarten R.A., Molina D.A., Salcedo R., Back T., Cramer S., et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342:967–970. doi: 10.1126/science.1240527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Guo Y., Chen Y., Liu X., Min J.J., Tan W., Zheng J.H. Targeted cancer immunotherapy with genetically engineered oncolytic Salmonella typhimurium. Cancer Lett. 2020;469:102–110. doi: 10.1016/j.canlet.2019.10.033. [DOI] [PubMed] [Google Scholar]
- 115.Gurbatri C.R., Lia I., Vincent R., Coker C., Castro S., Treuting P.M., Hinchliffe T.E., Arpaia N., Danino T. Engineered probiotics for local tumor delivery of checkpoint blockade nanobodies. Sci. Transl. Med. 2020;12:eaax0876. doi: 10.1126/scitranslmed.aax0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Canale F.P., Basso C., Antonini G., Perotti M., Li N., Sokolovska A., Neumann J., James M.J., Geiger S., Jin W., et al. Metabolic modulation of tumours with engineered bacteria for immunotherapy. Nature. 2021;598:662–666. doi: 10.1038/s41586-021-04003-2. [DOI] [PubMed] [Google Scholar]
- 117.Chowdhury S., Castro S., Coker C., Hinchliffe T.E., Arpaia N., Danino T. Programmable bacteria induce durable tumor regression and systemic antitumor immunity. Nat Med. 2019;25:1057–1063. doi: 10.1038/s41591-019-0498-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Russell S.J., Peng K.W., Bell J.C. Oncolytic virotherapy. Nat. Biotechnol. 2012;30:658–670. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lichty B.D., Breitbach C.J., Stojdl D.F., Bell J.C. Going viral with cancer immunotherapy. Nat. Rev. Cancer. 2014;14:559–567. doi: 10.1038/nrc3770. [DOI] [PubMed] [Google Scholar]
- 120.Liu T.C., Hwang T., Park B.H., Bell J., Kirn D.H. The targeted oncolytic poxvirus JX-594 demonstrates antitumoral, antivascular, and anti-HBV activities in patients with hepatocellular carcinoma. Mol. Ther. 2008;16:1637–1642. doi: 10.1038/mt.2008.143. [DOI] [PubMed] [Google Scholar]
- 121.Mastrangelo M.J., Maguire H.C., Jr., Eisenlohr L.C., Laughlin C.E., Monken C.E., McCue P.A., Kovatich A.J., Lattime E.C. Intratumoral recombinant GM-CSF-encoding virus as gene therapy in patients with cutaneous melanoma. Cancer Gene Ther. 1999;6:409–422. doi: 10.1038/sj.cgt.7700066. [DOI] [PubMed] [Google Scholar]
- 122.Le Boeuf F., Diallo J.S., McCart J.A., Thorne S., Falls T., Stanford M., Kanji F., Auer R., Brown C.W., Lichty B.D., et al. Synergistic interaction between oncolytic viruses augments tumor killing. Mol. Ther. 2010;18:888–895. doi: 10.1038/mt.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cronin M., Le Boeuf F., Murphy C., Roy D.G., Falls T., Bell J.C., Tangney M. Bacterial-mediated knockdown of tumor resistance to an oncolytic virus enhances therapy. Mol. Ther. 2014;22:1188–1197. doi: 10.1038/mt.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Buchta Rosean C., Feng T.Y., Azar F.N., Rutkowski M.R. Impact of the microbiome on cancer progression and response to anti-cancer therapies. Adv. Cancer Res. 2019;143:255–294. doi: 10.1016/bs.acr.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sepich-Poore G.D., Zitvogel L., Straussman R., Hasty J., Wargo J.A., Knight R. The microbiome and human cancer. Science. 2021;371:eabc4552. doi: 10.1126/science.abc4552. [DOI] [PMC free article] [PubMed] [Google Scholar]