Summary
Background
In hormone-receptor positive/HER2-negative metastatic breast cancer (mBC) no randomized comparisons are available between CDK4/6 inhibitors. We undertook this systematic review and meta-analysis to assess the reliability of the likelihood of being helped or harmed (LHH).
Methods
PubMed, CENTRAL, Embase and oncological meetings websites were searched to September 13th, 2022. We included phase III randomized controlled trials (RCTs) investigating palbociclib, ribociclib and abemaciclib in addition to endocrine therapy (ET) compared to placebo in hormone-receptor positive/HER2-negative advanced or mBC. Outcomes were progression-free survival (PFS), overall survival (OS), adverse events (AEs), dose reductions and discontinuations. Hazard ratios (HRs) and risk differences were computed with a random effect model to estimate the number needed to treat/harm (NNT/NNH). LHH was computed as (1/NNT)/(1/NNH). PROSPERO registration number: CRD42022362417.
Findings
2204 records were screened and seven RCTs (4415 patients) were included. A significant PFS benefit was observed in patients treated with a CDK4/6 inhibitor compared to placebo (HR 0.549; 0.508–0.594, I2 = 0). Palbociclib, ribociclib and abemaciclib had similar NNTs (4.4, 5.0 and 4.4). Palbociclib and ribociclib showed lower LHHs for grade 3-4 neutropenia (0.33 and 0.35) and febrile neutropenia ([FN], 14.27 and 15.52), while abemaciclib the lowest LHH for any grade diarrhea (0.42). Abemaciclib had a lower LHH for grade 3-4 fatigue (9.92) and the highest LHH for all grade 3-4 AEs (0.62), while ribociclib the lowest LHH (1.75) for grade 3-4 hepatotoxicity. Palbociclib had the highest LHH for dose reductions and discontinuations (0.65 and 6.17). Considering OS, an overall benefit was observed (HR 0.788, 0.727–0.856, I2 = 0%); ribociclib and abemaciclib had lower NNTs (9.7 and 10.0). Ribociclib showed the highest LHH for diarrhea (1.29), fatigue (7.37), dose reductions (0.28) and discontinuations (2.40), while abemaciclib the highest LHHs for neutropenia (0.40), FN (12.53) and hepatotoxicity (2.23).
Interpretation
Palbociclib and ribociclib showed lower LHHs for haematological toxicities and abemaciclib for diarrhea. Palbociclib confirmed to be a manageable drug. The LHH appears to be a reliable synthesis tool for balancing risks and benefits of experimental drugs when head-to-head comparisons are missing.
Funding
None.
Keywords: Metastatic breast cancer, CDK4/6 inhibitors, LHH, NNT, NNH
Research in context.
Evidence before this study
In the last few years, CDK4/6 inhibitors deeply changed the treatment landscape in hormone-receptor-positive/HER2-negative breast cancer: palbociclib, ribociclib and abemaciclib are currently approved, due to an improvement in progression-free survival compared to placebo. However, no randomized one-to-one comparison are available. Since cross-trials comparison can be misleading, an index to compare different drugs is needed. Therefore, we conducted this analysis to evaluate the reliability of the likelihood of being helped or harmed (LHH) in this setting.
We systematically searched PubMed, the Cochrane Central Register of Controlled Trials, Embase and websites of the most relevant international meetings for phase III randomized controlled trials published up to September 13th, 2022, investigating CDK4/6 inhibitors in addition to endocrine therapy compared to placebo, in hormone-receptor positive/HER2-negative metastatic breast cancer, regardless of line of therapy. Both postmenopausal and premenopausal women were included. Keywords used for searching were “palbociclib”, “ribociclib”, “abemaciclib”, “advanced breast cancer”, “metastatic breast cancer”. No language or data restriction were applied. Recently published systematic reviews and the original papers were also screened through the reference section.
Added value of this study
This is, to the best of our knowledge, the first attempt to apply the LHH to pooled results obtained from a meta-analysis of survival outcomes to highlight differences between CDK4/6 inhibitors. Using published data available from randomized trials, this meta-analysis evaluated more than 4000 women with different characteristics, providing a quantitative comparison between CDK4/6 inhibitors.
In terms of PFS, all CDK 4/6 inhibitors showed similar efficacy, with comparable NNTs. For PFS, palbociclib and ribociclib showed a lower LHH for neutropenia compared to abemaciclib, which didn't translate into very different LHH for febrile neutropenia. On the contrary, abemaciclib showed the lowest LHH for diarrhea and fatigue, while ribociclib had the lowest LHH for hepatotoxicity. Palbociclib confirmed a favourable manageability profile, with higher LHH for dose reductions and discontinuations. When considering OS, ribociclib and abemaciclib had lower NNTs compared to placebo. For OS, ribociclib showed the highest LHH for diarrhea, fatigue, dose reduction and discontinuations, while abemaciclib presented higher LHHs for neutropenia, febrile neutropenia and transaminase increase.
Implications of all the available evidence
These findings could have several potential implications: this model is highly reproducible and can be potentially applied to any setting when making decisions about treatment strategies is required and when balancing between the magnitude of the survival advantage and toxicities is a matter. The main advantage of the LHH is its immediacy and the radar plot provides a strong visual impact to highlight differences between drugs. This analysis offers a model and a rationale for the use of the LHH in clinical practice to support clinicians in tailoring the best treatment on the single patient.
Introduction
Breast cancer (BC) is the most common neoplasm in women and the first cause of cancer-related mortality.1 Among BCs, hormone-receptor positive/HER2-negative tumours account for approximately 65–70%.2 In hormone-receptor positive/HER2-negative metastatic BC, endocrine therapy (ET) is the backbone of treatment and in the last few years cyclin dependent kinase 4 and 6 (CDK4/6) inhibitors changed clinical practice.3 In this setting palbociclib, ribociclib and abemaciclib are currently approved by the Food and Drug Administration (FDA) and European Medicines Agency (EMA) for concurrent use with aromatase inhibitors (AIs) or fulvestrant in first and subsequent lines both for pre and postmenopausal women.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 Improvements in progression-free survival (PFS) served as base for all the regulatory approvals.
Although their mechanism of action is similar, these agents have different toxicity profiles and manageability. In absence of direct comparisons, finding predictive and prognostic factors as well as clinical profiles to guide treatment decision is an ongoing challenge.16
Laupacis et colleagues proposed, among others, to use the number needed to treat (NNT) to present clinical trials results.17 The NNT is the reciprocal of the absolute risk difference (RD) and is interpreted as “how many patients need to be treated to have a benefit due to the intervention”. In the same way, we can compute the number needed to harm (NNH) which indicates “how many patients I have to treat to experience a harm caused by the treatment”.
To promote the use of these indexes, Strauss et colleagues proposed the likelihood of being helped or harmed (LHH): this index is based on the ratio between the NNT to prevent a specified outcome (the likelihood of being helped) and the NNH to experience one or more adverse events (the likelihood of being harmed).18 The LHH is calculated as the ratio of 1/NNT and 1/NNH. Because the LHH addresses how much more likely it will be for the patient to benefit from a therapy than to be harmed, the higher the LHH the greater the chances that a benefit will occur. Including statistics about survival and toxicity in a single measure, the LHH provides additional information compared to the NNT and the NNH and could be considered as a useful index. Therefore, the LHH could represent an easily understandable measure of the impact of a therapy and a patient-centred method to tailor treatments where multiple options are available.19
Herein, we undertook this analysis to provide an overview of the LHH in metastatic BC for hormone-receptor positive/HER2-negative patients.
Methods
Search strategy and selection criteria
PubMed, the Cochrane Central Register of Controlled Trials and Embase were systematically searched for phase III randomized controlled trials (RCTs) published up to September 13th, 2022. We included RCTs evaluating CDK4/6 inhibitors compared to placebo in addition to standard of care endocrine therapy (ET), in pre and postmenopausal women with hormone-receptor positive/HER2-negative advanced or metastatic BC, regardless of line of therapy. Selection was restricted to EMA/FDA currently approved agents. Endocrine therapy could include non-steroidal aromatase inhibitors (NSAI, i.e., letrozole or anastrozole), tamoxifen or fulvestrant.
Trials that assessed the efficacy of CDK 4/6 inhibitors as monotherapy or in combination with other agents (chemotherapy, immunotherapy or monoclonal antibodies) and trials conducted in (neo)adjuvant setting were excluded. Trials evaluating drugs not EMA/FDA approved were excluded. Reviews, meta-analysis, editorials, case reports and case series were also excluded. No language or data restrictions were applied.
Keywords used for searching were “palbociclib”, “ribociclib”, “abemaciclib”, “advanced breast cancer”, “metastatic breast cancer”, “clinical trial” and “randomized controlled trial”. An additional search was conducted through the websites of the American Society of Clinical Oncology (ASCO), the European Society for Medical Oncology (ESMO) and the San Antonio Breast Cancer Symposium (SABCS) to look for relevant abstracts of unpublished studies and updates of published RCTs. Further trials were searched on clinicaltrials.gov. Recently published systematic reviews and original papers were screened through the reference section to evaluate potentially eligible reports. Literature searching and eligibility assessment was conducted independently by two authors (LM and AO), disagreements were resolved through discussion or referring to a third reviewer (EB). Full search strategy is provided in Table S1.
Data analysis
Data about the following information were extracted: first author, publication year, experimental and control arm, CDK4/6 inhibitor and ET, line of therapy, median follow-up time, HRs and 95% CIs, median PFS and OS, number of patients enrolled, number of any grade and grade 3-4 adverse events (AEs) (neutropenia, febrile neutropenia [FN], diarrhea, fatigue, aspartate transaminase [AST] and/or alanine transaminase [ALT] increase, all events), number of dose reductions and discontinuations. Where multiple reports of the same study were available, the most complete and/or updated report was considered. Data extraction was independently conducted by two authors (LM and AO), disagreements were resolved through discussion or referring to a third reviewer (EB).
The primary survival outcome was investigator-assessed PFS (time from randomization to disease progression or death from any cause, whichever came first). Overall survival (time from randomization to death) was a key-secondary survival end-point, as well as in all included trials, where sample sizes were computed on PFS. Primary safety endpoints were grade 3-4 neutropenia, FN and diarrhea (any grade). Secondary outcomes were grade 3-4 fatigue, grade 3-4 hepatotoxicity (defined as both AST or ALT increase) and all grade 3-4 AEs. Dose reductions and discontinuation of treatment due to AEs were exploratory outcomes. Safety analysis was restricted to the safety population and all AEs were considered as reported by trialists.
Hazard ratios and 95% confidence intervals (CI) were extracted from investigator-assessed Kaplan–Meier curves. Pooled estimates were computed adopting a fixed-effect model according to the inverse of variance method and a random-effects model with the restricted maximum likelihood estimator. Being S(t)experimental and S(t)control the survival probabilities in the experimental and in the control arm, the NNT and respective 95% CI was computed as 1/|S(t)experimental - S(t)control|.20 Since S(t)experimental = S(t)controlHR, S(t)control was calculated by approximation of functions considering S(t)experimental = 0.5. The RDs and 95% CIs were computed from the absolute number of events for each AE with a fixed and a random effects model according to the Mantel-Haenszel method. When required, a standard continuity correction of 0.5 was applied. The NNH was calculated as 1/RD.21 Inconsistency between studies was assessed with Cochran's Q and heterogeneity was evaluated with Higgins I2 index. Results from the fixed and the random effects model are presented in supplementary materials in order to allow the evaluation of the goodness of each approach. Because they were assumed to better represent the clinical and methodological diversity of included studies, the results from the random-effects model were used to compute NNTs and NNHs. The LHH with 95% CI was determined as (1/NNT)/(1/NNH). A LHH greater than 1 means the drug is more likely to benefit than to harm, with higher values correlating to a greater benefit. Values between 0 and 1 indicate that the drug is more likely to determine an AE than an advantage. Pointed estimates with 95% CIs were presented through base ten logarithmic forest plots and radar plots. A subgroup analysis was performed to compute NNTs and NNHs for different CDK 4/6 inhibitors and a prespecified subgroup analysis was performed for ET backbone (NSAI/tamoxifen or fulvestrant). Data analysis was performed using R (version 4.1.3) packages “meta” and “ggplot2”. All tests were two-tailed and the threshold for statistical significance was set at p < 0.05.
The risk of bias was evaluated using Cochrane risk of bias tool.22 The risk of each domain was assessed and an overall low, moderate or high risk of bias was assigned to each study. Two reviewers (LM and AO) independently did the initial assessment which was confirmed by a third author (EB). Publication bias was investigated with funnel plot and tested with Egger's test. The systematic review and meta-analysis were reported following the preferred reporting items for systematic reviews and meta-analyses (PRISMA) Statement 2020.
Study protocol was registered at PROSPERO (CRD42022362417).
Role of the funding source
There was no funding source for this study. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit to publication.
Results
Studies’ characteristics
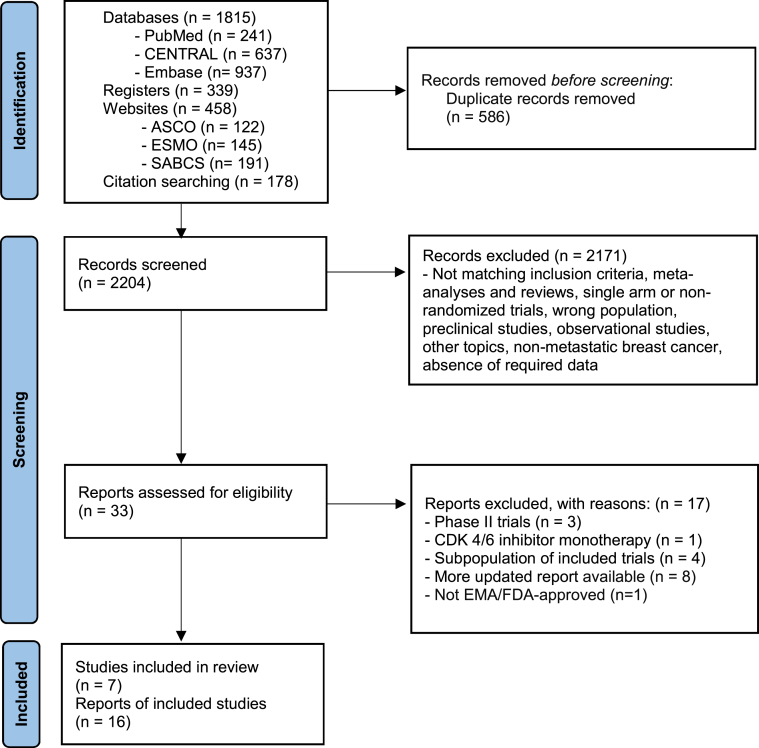
Overall, 2154 records were identified from databases and registers and 636 from websites and citation searching. 2790 records were screened with titles and abstracts and 2757 were excluded. The remaining 33 reports were assessed with full texts and 16 reports from 7 studies were considered eligible, for a total of 4415 patients. The following reports were included in the primary analysis: PALOMA-2,13,23 MONALEESA-2,6,24 MONALEESA-7,7,25 MONARCH-3,15,26,27 PALOMA-3,9,10,28 MONALEESA-3,11,29 MONARCH-2.12,30 PRISMA flow diagram is reported in Fig. 1 and full reference list in Table S2. Four studies (PALOMA-2, MONALEESA-2, MONALEESA-7 and MONARCH-3) evaluated CDK 4/6 inhibitors in association with NSAI or tamoxifen and three studies (PALOMA-3, MONALEESA-2, MONARCH-2) with fulvestrant. All trials compared the CDK4/6 inhibitor arm to placebo as an add-on to ET. The primary end-point for all included trials was PFS. Characteristics of included studies are summarized in Table 1.
Fig. 1.
Study selection. CENTRAL = Cochrane Central Register of Controlled Trials. ASCO = American Society of Clinical Oncology. ESMO = European Society for Medical Oncology. SABCS = San Antonio Breast Cancer Symposium. EMA = European Medicines Agency. n = number.
Table 1.
Characteristics of studies included in the primary analysis.
| Study | CDK4/6 inhibitor | Endocrine therapy | Line | Menopausal status | Median FUP (PFS) | HR PFS (95% CI) | Median PFS (CDK4/6i) | Median PFS (Placebo) | Median FUP (OS) | HR OS (95% CI) | Median OS (CDK4/6i) | Median OS (Placebo) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PALOMA-213,23 | Palbociclib | Letrozole | 1st line | Post- menopausal | 37.5 m | 0.563 (0.461–0.687) | 27.6 m | 14.5 m | 90.0 m | 0.956 (0.777–1.177) | 53.9 m | 51.2 m |
| MONALEESA-26,24 | Ribociclib | Letrozole | 1st line | Post-menopausal | 26.4 m | 0.568 (0.457–0.704) | 25.3 m | 16.0 m | 79.2 m | 0.760 (0.630–0.930) | 63.9 m | 51.4 m |
| MONALEESA-77,25 | Ribociclib | Tamoxifen/NSAI + Goserelin | 1st or 2nd line (after chemotherapy) | Pre-menopausal | 19.2 m | 0.553 (0.441–0.694) | 23.8 m | 13.0 m | 53.5 m | 0.763 (0.608–0.956) | 58.7 m | 48.0 m |
| MONARCH-315,26,27 | Abemaciclib | NSAI | 1st line | Post-menopausal | 39.3 m | 0.525 (0.415–0.665) | 28.2 m | 14.8 m | 70.2 m | 0.754 (0.584–0.974) | 67.1 m | 54.5 m |
| PALOMA-39,10,28 | Palbociclib | Fulvestrant | Progression after ET (adjuvant or 1st line) | Pre/post-menopausal | 14.0 m | 0.497 (0.398–0.620) | 11.2 m | 4.6 m | 73.3 m | 0.806 (0.654–0.995) | 34.8 m | 28.0 m |
| MONALEESA-311,29 | Ribociclib | Fulvestrant | 1st or 2nd line | Post-menopausal | 39.4 m | 0.590 (0.490–0.710) | 20.6 m | 12.8 m | 56.3 m | 0.726 (0.588–0.897) | 53.7 m | 41.5 m |
| MONARCH-212,30 | Abemaciclib | Fulvestrant | Progression after ET (neo/adjuvant or 1st line) | Pre/post-menopausal | 47.7 m | 0.536 (0.445–0.645) | 16.9 m | 9.3 m | 47.7 m | 0.757 (0.606–0.945) | 46.7 m | 37.3 m |
FUP = follow-up. PFS = progression-free survival. OS = overall survival. HR = hazard ratio. m = months. ET = endocrine therapy.
Forest plots for the NNT and the NNH are reported in Fig. 2, Fig. 3. Radar plots for the LHH are reported in Fig. 4, Fig. 5.
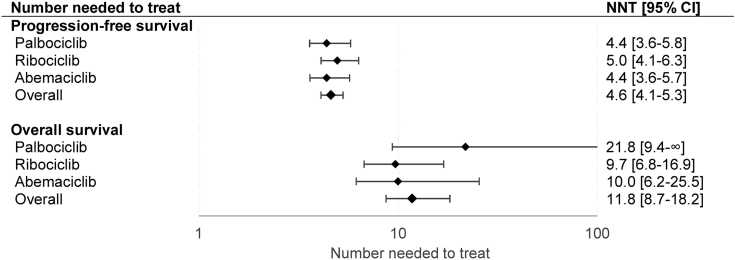
Fig. 2.
Number needed to treat. Values are displayed on a logarithmic scale. Results from the random-effects model were used to compute NNTs. Lower values correlate with greater efficacy. When the upper limit of the hazard ratio crosses 1, the confidence interval for the NNT encompasses two region values, a positive to +∞ and a negative to –∞. By convention, only positive values are reported. NNT = number needed to treat. 95% CI = 95% confidence interval.
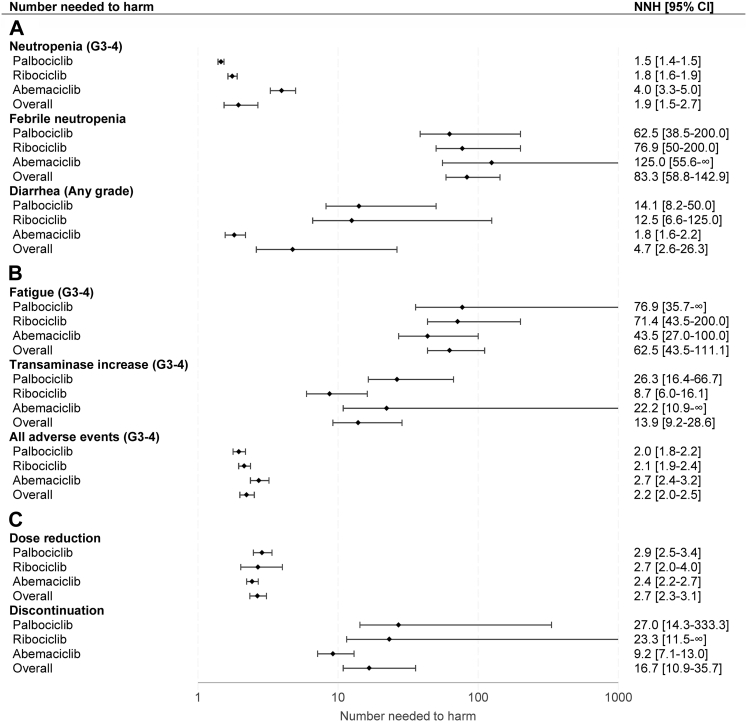
Fig. 3.
Number needed to harm for common adverse events. Values are displayed on a logarithmic scale. Results from the random-effects model were used to compute NNHs. Higher NNH correlates to less toxicity. When the lower limit of the risk difference crosses 0, the confidence interval for the NNH encompasses two region values, a positive to +∞ and a negative to ∞. By convention, only positive values are reported. NNH = number needed to harm. 95% CI = 95% confidence interval. G = grade.
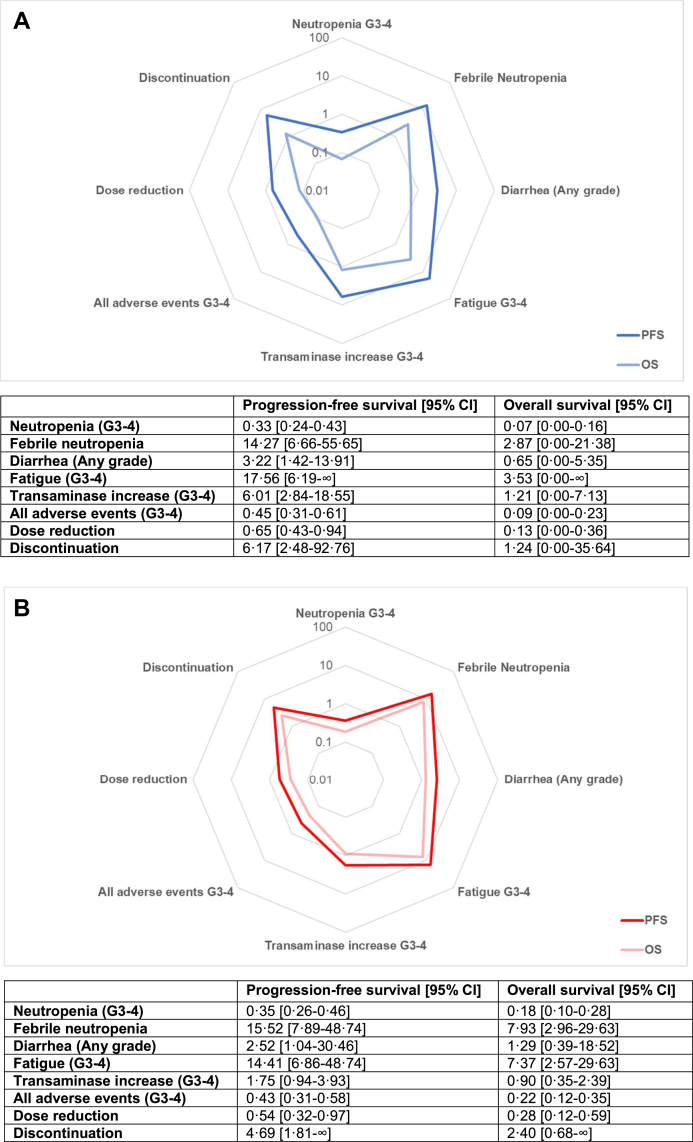
Fig. 4.
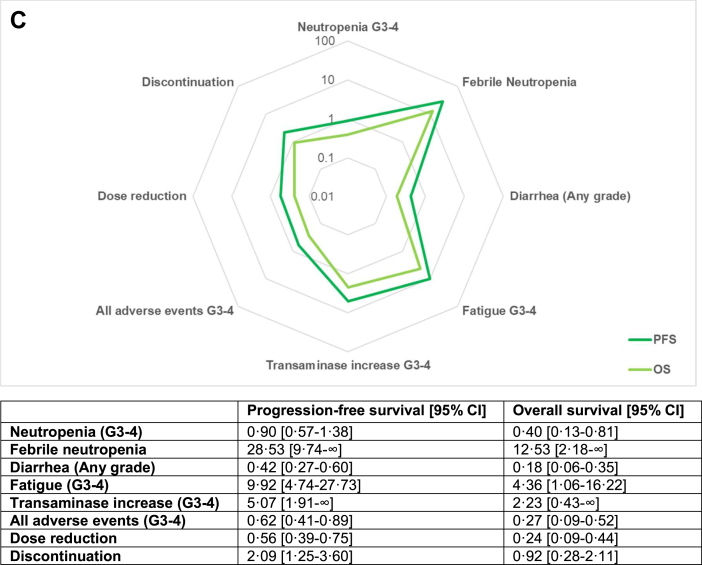
Radar plot for pointed estimates of the likelihood of being helped or harmed for progression-free survival and overall survival for palbociclib [A], ribociclib [B] and abemaciclib [C]. Values are displayed on logarithmic scale. Results from the random-effects model were used to compute the LHHs. Higher values correlate to a greater likelihood to benefit than to harm and are plotted closer to the external ring. G = grade, PFS = progression-free survival, OS = overall survival.
Fig. 5.
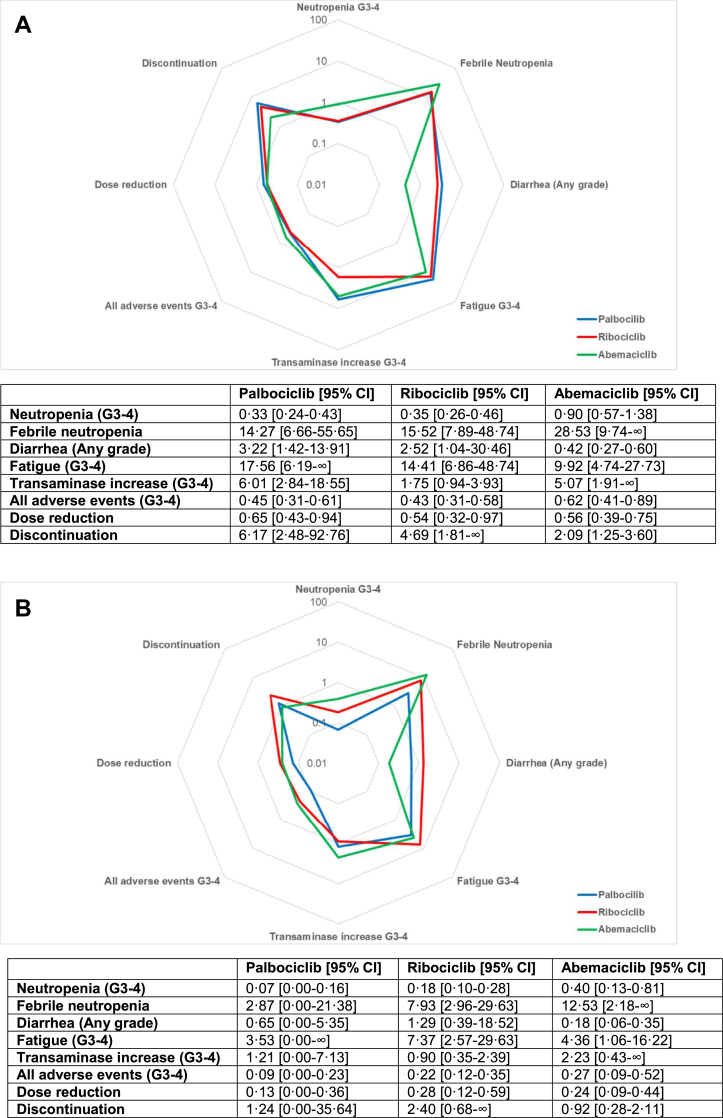
Radar plot for pointed estimates of the likelihood of being helped or harmed for palbociclib, ribociclib, and abemaciclib for progression-free survival [A] and overall survival [B]. Values are displayed on logarithmic scale. Results from the random-effects model were used to compute the LHHs. Higher values correlate to a greater likelihood to benefit than to harm and are plotted closer to the external ring. G = grade.
Number needed to treat for progression-free survival
A statistically significant advantage in PFS was observed in patients treated with a CDK4/6 inhibitor compared to placebo (HR 0.549; 0.508–0.594) and no heterogeneity was detected (I2 = 0). Pooled HRs were 0.532 (0.459–0.618) for palbociclib, 0.573 (0.508–0.646) for ribociclib and 0.532 (0.460–0.615) for abemaciclib (Fig. S3). Cumulative NNT was 4.6 (4.1–5.3), similar between palbociclib (4.4; 3.6–5.8), ribociclib (5.0; 4.1–6.3) and abemaciclib (4.4; 3.6–5.7).
Likelihood of being helped or harmed for progression-free survival
The overall RD (in percentage) for grade 3-4 neutropenia was 51.3% (37.4–65.2), with high heterogeneity (I2 = 98%) (Fig. S5). Cumulative NNH was 1.9 (1.5–2.7); 1.5 (1.4–1.5) for palbociclib, 1.8 (1.6–1.9) for ribociclib and 4.0 (3.3–5.0) for abemaciclib. Pooled LHH for PFS was 0.42 (0.29–0.65) and abemaciclib showed the lower LHH for neutropenia (0.90; 0.57–1.38) compared to palbociclib (0.33; 0.24–0.43) and ribociclib (0.35; 0.26–0.46). Cumulative RD for FN was 1.2% (0.7–1.7, I2 = 0%) (Fig. S6) and pooled NNH was 83.3 (58.8–142.9); 62.5 (38.5–200.0) for palbociclib, 76.9 (50.0–200.0) for ribociclib and 125.0 (55.6–∞ and −500.0 to −∞) for abemaciclib. Cumulative LHH for FN was 18.09 (11.12–34.87), 14.27 (6.66–55.65) for palbociclib, 15.52 (7.89–48.74) for ribociclib and 28.53 (9.74–∞ and −138.60 to −∞) for abemaciclib.
Diarrhea was the most common gastrointestinal toxicity, with a pooled RD for any grade diarrhea of 21.1% (3.8–38.4) and a high heterogeneity between different studies (I2 = 98%) (Fig. S7). Overall NNH was 4.7 (2.6–26.3), lower with abemaciclib (1.8; 1.6–2.2) compared with palbociclib (14.1; 8.2–50.0) and ribociclib (12.5; 6.6–125.0). Cumulative LHH was 1.03 (0.49–6.42), abemaciclib had the lowest LHH (0.42; 0.27–0.60) compared to palbociclib (3.22; 1.42–13.91) and ribociclib (2.52; 1.04–30.46).
Cumulative RD for grade 3-4 fatigue was 1.6% (0.9–2.3, I2 = 0%) (Fig. S8) and cumulative NNH was 62.5 (43.5–111.1); 76.9 (35.7–∞ and −33.3 to −∞) for palbociclib, 71.4 (43.5–200.0) for ribociclib and 43.5 (27.0–100.0) for abemaciclib. Pooled LHH was 13.57 (8.22–27.12); palbociclib had the highest LHH (15.56; 6.19–∞ and to −9.28 to −∞) followed by ribociclib (14.41; 6.86–48.47) and abemaciclib (9.92; 4.74–27.73). The RD for cumulative grade 3-4 ALT and AST increase was 7.2% (3.5–10.9), with significant heterogeneity (I2 = 86%) (Fig. S9). Pooled NNH was 13.9 (9.2–28.6) and ribociclib showed the lowest NNH (8.7, 6.0–16.1) compared with abemaciclib (22.2; 10.9–∞ and −5000.0 to −∞) and palbociclib (26.3; 16.4–66.7). Cumulative LHH was 3.01 (1.73–6.97), lower for ribociclib (1.75; 0.94–3.93), followed by abemaciclib (5.07; 1.91–∞ and −1386.37 to –∞) and palbociclib (6.01; 2.84–18.55). The pooled RD for all grade 3-4 toxicities was 45.0% (39.7–50.3, I2 = 67%) (Fig. S10). Cumulative NNH was 2.2 (2.0–2.5); 2.7 (2.4–3.2) for abemaciclib, 2.1 (1.9–2.4) for ribociclib and 2.0 (1.8–2.2) for palbociclib. Overall LHH was 0.48 (0.38–0.61), a higher LHH was observed with abemaciclib (0.62: 0.41–0.89) compared to ribociclib (0.43; 0.31–0.58) and palbociclib (0.45; 0.31–0.61).
Overall, the RD for dose reduction was 37.6% (32.5–42.7), with high heterogeneity between different studies (I2 = 82%) (Fig. S11). Cumulative NNH was 2.7 (2.3–3.1); 2.9 (2.5–3.4) for palbociclib, 2.7 (2.0–4.0) for ribociclib and 2.4 (2.2–2.7) for abemaciclib. Pooled LHH was 0.58 (0.44–0.75); 0.65 (0.43–0.94) for palbociclib, 0.54 (0.32–0.97) for ribociclib and 0.56 (0.39–0.75) with abemaciclib. A cumulative RD of 6.0% (2.8–9.2) was observed for discontinuation and a significant heterogeneity was detected (I2 = 83%) (Fig. S12). Pooled NNH was 16.7 (10.9–35.7); abemaciclib showed the lowest NNH (9.2; 7.1–13.0) compared to palbociclib (27.0; 14.3–333.3) and ribociclib (23.3; 11.5–∞ and −1000.0 to −∞). Cumulative LHH was 3.62 (2.06–8.72) and palbociclib had the highest LHH (6.17; 2.48–92.76) followed by ribociclib (4.69; 1.81–∞ and −243.72 to −∞) and abemaciclib (2.09; 1.25–3.60).
Number needed to treat for overall survival
An OS benefit was observed in patients treated with CDK4/6 inhibitors compared to placebo (HR 0.788, 0.727–0.856, I2 = 0%). Survival advantage was significant only for ribociclib (HR 0.750; 0.664–0.846) and abemaciclib (HR 0.756, 0.639–0.894), while a non-significant trend was observed with palbociclib (HR 0.878, 0.743–1.038) (Fig. S4). Cumulative NNT was 11.8 (8.7–18.2), lower for ribociclib (9.7; 6.8–16.8) and abemaciclib (10.0; 6.2–25.5) compared to palbociclib (21.8; 9.4–∞ and −76.4 to –∞) (Fig. 2).
Likelihood of being helped or harmed for overall survival
Overall LHH for OS for grade 3-4 neutropenia was 0.17 (0.08–0.31) and abemaciclib confirmed the highest LHH (0.40; 0.13–0.81), followed by ribociclib (0.18; 0.10–0.28) and palbociclib (0.07; 0.00–0.16 and −0.02 to −∞). Overall LHH for FN was 7.09 (3.24–16.44) and abemaciclib had the highest LHH (12.52; 2.18–∞ and −81.11 to −∞), followed by ribociclib (7.93; 2.96–29.63) and palbociclib (2.87; 0.00–21.38 and −0.50 to −∞). Cumulative LHH for any grade diarrhea was 0.40 (0.14–3.03), ribociclib showed the highest LHH (1.29; 0.39–18.52) and abemaciclib the lowest (0.18; 0.06–0.35).
Cumulative LHH for fatigue was 5.32 (2.39–12.78), with ribociclib showing the highest LHH (7.37; 2.57–29.63). Ribociclib presented the lowest LHH for transaminase increase (0.90; 0.35–2.39) and abemaciclib the highest (2.23; 0.43–∞ and −811.08 to −∞). Abemaciclib confirmed the highest LHH for all grade 3–4 AEs (0.27; 0.09–0.52), followed by ribociclib (0.22; 0.12–0.35). Similar LHH for dose reductions were observed for ribociclib (0.28; 0.12–0.59) and abemaciclib (0.24; 0.09–0.44). Ribociclib showed the highest LHH for discontinuation (2.40; 0.68–∞ and −148.14 to −∞), followed by palbociclib (1.24; 0.00–35.64 and −0.19 to −∞) and abemaciclib (0.92; 0.28–2.11).
Risk of bias
The overall risk of bias for PFS was low for all the included studies within every domain considered. In terms of OS, a high risk of bias was assigned to the PALOMA-2 study due to missing outcome data, leading to an overall moderate risk of bias. An exploratory sensitivity analysis was conducted excluding the PALOMA-2 trial and yielded consisted results with the primary analysis (Fig. S17). A detailed risk of bias assessment is reported in Fig. S1. No publication bias was detected (Fig. S2).
Discussion
According to the most recent recommendations, in hormone-receptor positive/HER2-negative metastatic BC, the combination of ET plus a CDK4/6 inhibitor is the treatment of choice, although no randomized comparisons between CDK4/6 inhibitors are currently available. In our survival analysis, no heterogeneity was observed and the addition of palbociclib, ribociclib or abemaciclib to ET significantly improved PFS with comparable NNTs among different drugs.
The most common and clinically impacting toxicities were the main focus in this analysis: since the LHH evaluates the likelihood for a patient to benefit from a therapy than to be harmed its added value in sorting out therapies is seen in the observation that drugs with the same or similar NNT may have very different NNH and LHH, because of large differences in drug-specific toxicities. When the efficacy (the NNT) is similar the LHH is weighted against side effects (the NNH); but if an efficacy difference is relevant, the LHH highlights this discrepancy trough different toxicities and efficacy outcomes.
In PALOMA-2 and PALOMA-3 trials, neutropenia was the most common grade 3-4 AE, occurring in approximately two out of three patients in the palbociclib plus endocrine therapy group and similar results were observed from the MONALEESA trials, were the incidence was about 60% in the updated analyses. On the other hand, in the MONARCH-2 and MONARCH-3 trials, grade 3-4 neutropenia was reported only in one out of three patients. As expected, our analysis highlighted statistical and clinical heterogeneity since palbociclib and ribociclib had very low NNHs (1.5 and 1.8) and LHHs (0.33 and 0.35, respectively) for this outcome while abemaciclib lesser haematological toxicity31 translated into a higher NNH (4.0) and LHH (0.90). Neutropenia is the most common AE observed with palbociclib and ribociclib32,33: myelosuppression is primarily explained due to their preferential binding to CDK6, which upregulates hematopoietic precursors proliferation.34 Neutrophils count decrease induced by CDK4/6 inhibitors is quickly reversible and generally better tolerated compared to chemotherapy-induced neutropenia, since it is characterized by a cytostatic effect and not by DNA damage.35 Some specific drug-related toxicities have a greater impact on patients' compliance and adherence to treatment and neutropenia is no exception: although very frequent, it usually does not significantly affect patients’ quality of life and can be controlled with dose delays and reductions. Despite being frequent, neutropenia does not translate into high rates of FN and infections: in the included trials FN incidence in the CDK4/6 inhibitors arms ranged approximately between 1 and 2%. In our analysis, cumulative NNT and LHH for FN were in fact respectively 83.3 and 18.09, again lower with palbociclib and ribociclib, but in absence of significant heterogeneity in the RD analysis.
Higher rates of gastrointestinal events were observed with abemaciclib: in the MONARCH-3 and MONARCH-2 pooled safety analysis, diarrhea was the most frequent AE, occurring in approximately 85% of patients, but only about 10% were grade 3-4 events.36 Diarrhea observed with abemaciclib was grade 1-2 in the majority of cases and clinically significant diarrhea (grade 2-3) occurred within a median time of 6–8 days. However, diarrhea was often limited to the first 4–6 cycles and well managed with antidiarrheal medications, taken by 69%–76% of patients.12,37 These results were confirmed in our analysis, where high heterogeneity was detected and abemaciclib showed the lowest NNH (1.8) and LHH (0.42) for any grade diarrhea compared to ribociclib and palbociclib.
Overall, grade 3-4 fatigue was reported in about 2–4% of patients in the CDK4/6 inhibitors arms, and it was more common with abemaciclib which presented the lowest NNH and LHH, followed by ribociclib and palbociclib.
As expected, significant heterogeneity was found for grade 3-4 ALT/AST increase and a higher risk was observed with ribociclib: in the MONALEESA-2 and MONALESA-3 trial the incidence of grade 3 or 4 ALT/AST increase ranged between approximately 5–10%, and it was slightly lower in the MONALEESA-7 trials (4-5%). In our analysis, NNH was 8.7 and LHH 1.75 for ribociclib: this means the likelihood of having a benefit only doubles the likelihood of being harmed, and since grade 3-4 hepatotoxicity is clinically relevant, ribociclib use should be well thought out in patients with history of or active hepatic disfunction.
Overall, all grade 3-4 AEs occurred in 60–70% of patients and the cumulative LHH was generally low. Abemaciclib showed a higher LHH, probably due to the absolute difference in terms of haematological events.
Dose reductions occur in approximately 40% of patients during the treatment with CDK 4/6 inhibitors, which are generally considered safe drugs: cumulative NNH for dose reduction was 2.7 and LHH 0.58. However, adverse events rarely lead to a definitive treatment interruption: NNH and LHH for discontinuation were respectively 16.7 and 3.62. Palbociclib confirmed to be a manageable drug, showing the highest LHH for both outcomes. In two analyses from PALOMA-2 and PALOMA-3, no PFS difference was observed in patients whose dose was reduced.38,39 This highlights how in elderly or frail patients, when the survival advantage has to be weighed on tolerability, palbociclib can be considered a reasonable choice. In absence of disease progression, when a treatment with another CDK4/6 inhibitor has to be discontinued due to scarce tolerance, a switch to another compound can be considered and if palbociclib wasn't the first choice it can contemplated. While the LHHs for dose reductions were similar, marked differences were observed for discontinuation, with ribociclib being the second best (LHH 4.69). As expected, the MONALEESA-7 trial, who enrolled only pre-menopausal women, had the highest NNH and LHH for discontinuation (166.7 and 35.74, respectively). These data confirm how, in young and fit patients, ribociclib is generally well tolerated. Despite fewer haematological toxicities, abemaciclib LHH for discontinuation was the lowest: neutropenia is often well tolerated and although anti-diarrheal drugs are effective to manage diarrhea they could negatively impact patients' compliance. Dose modifications due to diarrhea with abemaciclib are in the order of 12–19%, while discontinuation rates are around 2.3–2.9%.,40 while palbociclib and ribociclib discontinuation rates associated with neutropenia were approximately 1-2%.33 However, in both MONARCH 2 and 3 patients experienced a PFS benefit regardless of whether they had a dose reduction. Therefore, when diarrhea is not really a matter or when neutropenia is an issue, abemaciclib is absolutely a rational choice.
In our analysis, OS was a secondary end-point. So far, MONALEESA-3,41 MONALEESA-7,25 MONALEESA-2,24 and MONARCH-2,30 showed a statistically significant OS benefit in the ITT population and in the MONARCH-327 the survival difference was not significant on the basis of the pre-specified boundaries. In PALOMA-3,42 the final pre-specified survival analysis did not reach statistical significance and in the recently reported PALOMA-2 analysis OS advantage was non-significant,23 with a high percentage of missing data. Interpretation of these results requires caution, although the evidence of a clinically relevant survival difference between different CDK4/6 inhibitors should be one major criteria of choice in clinical practice. Notably, NNTs for OS tend to be higher than for PFS: this reflects the difficulty of reaching an OS advantage in cancers with long survival times. In our OS analysis, the different magnitude of the OS benefit and the trade-off between efficacy and toxicities favoured abemaciclib in terms of haematological events and hepatotoxicity, with the highest LHHs for neutropenia, FN and ALT/AST increase. On the other hand, ribociclib lowest NNT for OS resulted in the highest LHHs for diarrhea, fatigue, dose reductions and discontinuations.
Our work had some limitations. Mainly, the analysis was conducted using published hazard ratios and the cumulative number of adverse events rather than individual patient data. Since our analysis aimed to evaluate frequently reported toxicities, rarer clinically relevant AEs were not included, such as QTc prolongation, interstitial lung disease and thromboembolic events.43 From a statistical point of view, time-to event outcomes have no single NNT and reproducibility depends on the respect of the proportional hazards’ assumption, which we considered being respected in all trials. Moreover, when the treatment effect is non-significant, RDs cross 0 and the limits of the CI for the NNT and the NNH tend to infinite, encompassing two regions-value (a positive to ∞ and negative to −∞). In this case, a negative NNT indicates that the treatment could have a harmful effect and a negative NNH that the treatment could prevent an AE. This affect the interpretation of the LHH: when the survival benefit is non-significant the NNT tends to ∞ and consequently the LHH tends to 0. On the other hand, in presence of a non-significant toxicity difference the NNH and the LHH tend to ∞. In this situation is often not useful to rely too much on CIs and pointed estimates seem more trustworthy.21,44
These caveats aside, the LHH is an index of interest and presents many advantages. The lack of use of the LHH could be referred to the fact that is not widely known and, without proper explanations, it could be not immediately understandable by clinicians. Being a summary measure, even if it may be questioned about the information lost in such aggregate, its main strength is the immediacy of presented results: it could be useful not only to select treatments but also to explain what to expect in terms of toxicity and efficacy. When discussing with a patient, the LHH can be simply explained as “how many times the treatment is more likely to help you than to harm you”. In a decision-making setting, where the expected benefit of a treatment needs to be pondered against side effects, the LHH answers the question “how much I'm expecting to help my patients compared to how much I'm putting them at risk for common AEs”. The inclusion of phase III RCTs, even if patients enrolled could not reflect daily clinical practice, ensures high reproducibility in other settings. Furthermore, in absence of high heterogeneity between trials, relaying on absolute differences the LHH allows a quantitative comparison between different treatments.
In conclusion, this analysis showed how the LHH can be considered a reliable synthesis tool to help choosing the best therapy when multiple options are available: CDK4/6 inhibitors are very effective and well-tolerated drugs with a specific toxicity profile and its priority to identify clinical factors to guarantee the best treatment for every single patient.
Contributors
Study design and conceptualization: LM, AO and EB. Database and websites research, data screening and data extraction: LM, AO and EB. Data analysis and interpretation: LM, DG, AO and EB. Manuscript drafting and revision: LM, AO and EB. Figures and tables: LM. Study supervision: LM, AO and EB. Study submission: EB.
All authors red, revised and approved the final version of the manuscript. All the authors had full access to all the data in the study and the corresponding author had the final responsibility to submit the study for publication.
Data sharing statement
All the data supporting the conclusions are presented in the article or in supplemental material.
Declaration of interests
L.M., G.G., G.D. declare no conflict of interest. A.O. has declared consulting fees/advisory role for Novartis, Roche, Eli-Lilly, Amgen, Daiichi Sankyo, travel and accommodation by Daiichi Sankyo, Novartis, Roche, Pfizer. A.P. has declared consulting fees/advisory role for Amgen, MSD, Novartis, travel and accommodation by Pfizer. D.G. received educational courses fee from MSD and Amgen. A.F. has declared consulting fees/advisory role for Astra Zeneca, Daiichi Sankyo, Eisai, Eli-Lilly. Epionpharma, exact science, MSD, Novartis, Pierre Fabre, Roche, Seagen. G.T. is supported by funds of Ministero della Salute (Ricerca Corrente 2022). E.B. is currently supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC) under Investigator Grant (IG) No. IG20583. E.B. is supported by Institutional funds of Università Cattolica del Sacro Cuore (UCSC-project D1). E.B. is supported by funds of Ministero della Salute (Ricerca Corrente 2022). E.B. received speakers' and travels’ fee from MSD, Astra-Zeneca, Pfizer, Eli-Lilly, BMS, Novartis and Roche. E.B. received institutional research grants from Astra-Zeneca, Roche.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.101824.
Contributor Information
Luca Mastrantoni, Email: l.mastrantoni@hotmail.it.
Armando Orlandi, Email: armando.orlandi@policlinicogemelli.it.
Antonella Palazzo, Email: antonella.palazzo@policlinicogemelli.it.
Giovanna Garufi, Email: giovanna.garufi@unicatt.it.
Alessandra Fabi, Email: alessandra.fabi@policlinicogemelli.it.
Gennaro Daniele, Email: gennaro.daniele@policlinicogemelli.it.
Diana Giannarelli, Email: diana.giannarelli@policlinicogemelli.it.
Giampaolo Tortora, Email: giampaolo.tortora@unicatt.it.
Emilio Bria, Email: emilio.bria@unicatt.it.
Appendix A. Supplementary data
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Waks A.G., Winer E.P. Breast cancer treatment. JAMA. 2019;321(3):288. doi: 10.1001/jama.2018.20751. [DOI] [PubMed] [Google Scholar]
- 3.Burstein H.J., Somerfield M.R., Barton D.L., et al. Endocrine treatment and targeted therapy for hormone receptor–positive, human epidermal growth factor receptor 2–negative metastatic breast cancer: ASCO guideline update. J Clin Oncol. 2021;39(35):3959–3977. doi: 10.1200/JCO.21.01392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finn R.S., Crown J.P., Lang I., et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 5.Hortobagyi G.N., Stemmer S.M., Burris H.A., et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375(18):1738–1748. doi: 10.1056/NEJMoa1609709. [DOI] [PubMed] [Google Scholar]
- 6.Hortobagyi G.N., Stemmer S.M., Burris H.A., et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol. 2018;29(7):1541–1547. doi: 10.1093/annonc/mdy155. [DOI] [PubMed] [Google Scholar]
- 7.Tripathy D., Im S.A., Colleoni M., et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol. 2018;19(7):904–915. doi: 10.1016/S1470-2045(18)30292-4. [DOI] [PubMed] [Google Scholar]
- 8.Goetz M.P., Toi M., Campone M., et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35(32):3638–3646. doi: 10.1200/JCO.2017.75.6155. [DOI] [PubMed] [Google Scholar]
- 9.Turner N.C., Ro J., André F., et al. Palbociclib in hormone-receptor–positive advanced breast cancer. N Engl J Med. 2015;373(3):209–219. doi: 10.1056/NEJMoa1505270. [DOI] [PubMed] [Google Scholar]
- 10.Cristofanilli M., Turner N.C., Bondarenko I., et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425–439. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 11.Slamon D.J., Neven P., Chia S., et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor–positive, human epidermal growth factor receptor 2–negative advanced breast cancer: MONALEESA-3. J Clin Oncol. 2018;36(24):2465–2472. doi: 10.1200/JCO.2018.78.9909. [DOI] [PubMed] [Google Scholar]
- 12.Sledge G.W., Toi M., Neven P., et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2− advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35(25):2875–2884. doi: 10.1200/JCO.2017.73.7585. [DOI] [PubMed] [Google Scholar]
- 13.Rugo H.S., Finn R.S., Diéras V., et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res Treat. 2019;174(3):719–729. doi: 10.1007/s10549-018-05125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finn R.S., Martin M., Rugo H.S., et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375(20):1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 15.Johnston S., Martin M., di Leo A., et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer. 2019;5(1):5. doi: 10.1038/s41523-018-0097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daniele G., Giannarelli D., Bria E. Looking for a better measure of the benefit in clinical trials: a never-ending journey. Ann Transl Med. 2020;8(14):893. doi: 10.21037/atm.2020.03.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laupacis A., Sackett D.L., Roberts R.S. An assessment of clinically useful measures of the consequences of treatment. N Engl J Med. 1988;318(26):1728–1733. doi: 10.1056/NEJM198806303182605. [DOI] [PubMed] [Google Scholar]
- 18.Straus S.E. Individualizing treatment decisions. Eval Health Prof. 2002;25(2):210–224. doi: 10.1177/016327870202500206. [DOI] [PubMed] [Google Scholar]
- 19.Bria E., Pilotto S., Bonomi M., et al. Targeted agents (TA) for advanced solid tumors (AST): what is the likelihood of being helped or harmed (LHH)? Assessing the clinical impact of therapies with FDA/EMEA approval. J Clin Oncol. 2013;31(15_suppl):e17555. [Google Scholar]
- 20.Altman D.G., Andersen P.K. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ. 1999;319(7223):1492–1495. doi: 10.1136/bmj.319.7223.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altman D.G. Confidence intervals for the number needed to treat. BMJ. 1998;317(7168):1309. doi: 10.1136/bmj.317.7168.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins J.P.T., Sterne J.A.C., Savović J., et al. A revised tool for assessing risk of bias in randomized trials. Cochrane Database Syst Rev. 2016;10(Suppl 1) [Google Scholar]
- 23.Finn R.S., Rugo H.S., Dieras V.C., et al. Overall survival (OS) with first-line palbociclib plus letrozole (PAL+LET) versus placebo plus letrozole (PBO+LET) in women with estrogen receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer (ER+/HER2− ABC): analyses from PALOMA-2. J Clin Oncol. 2022;40(17_suppl):LBA1003. LBA1003 (abstr) [Google Scholar]
- 24.Hortobagyi G.N., Stemmer S.M., Burris H.A., et al. Overall survival with ribociclib plus letrozole in advanced breast cancer. N Engl J Med. 2022;386(10):942–950. doi: 10.1056/NEJMoa2114663. [DOI] [PubMed] [Google Scholar]
- 25.Lu Y.S., Im S.A., Colleoni M., et al. Updated overall survival of ribociclib plus endocrine therapy versus endocrine therapy alone in pre- and perimenopausal patients with HR+/HER2− advanced breast cancer in MONALEESA-7: a phase III randomized clinical trial. Clin Cancer Res. 2022;28(5):851–859. doi: 10.1158/1078-0432.CCR-21-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnston S., O'Shaughnessy J., Martin M., et al. Abemaciclib as initial therapy for advanced breast cancer: MONARCH 3 updated results in prognostic subgroups. NPJ Breast Cancer. 2021;7(1):80. doi: 10.1038/s41523-021-00289-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goetz M.P., Toi M., Huober J., et al. MONARCH 3: interim overall survival (OS) results of abemaciclib plus a nonsteroidal aromatase inhibitor (NSAI) in patients (pts) with HR+, HER2- advanced breast cancer (ABC) Ann Oncol. 2022;33(suppl_7):S808–S869. doi: 10.1016/j.annonc.2024.04.013. (abstr) [DOI] [PubMed] [Google Scholar]
- 28.Cristofanilli M., Rugo H.S., Im S.A., et al. Overall survival with palbociclib and fulvestrant in women with HR+/HER2− ABC: updated exploratory analyses of PALOMA-3, a double-blind, phase III randomized study. Clin Cancer Res. 2022;28(16):3433–3442. doi: 10.1158/1078-0432.CCR-22-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slamon D.J., Neven P., Chia S., et al. Ribociclib plus fulvestrant for postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in the phase III randomized MONALEESA-3 trial: updated overall survival. Ann Oncol. 2021;32(8):1015–1024. doi: 10.1016/j.annonc.2021.05.353. [DOI] [PubMed] [Google Scholar]
- 30.Sledge G.W., Toi M., Neven P., et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor–positive, ERBB2-negative breast cancer that progressed on endocrine therapy—MONARCH 2. JAMA Oncol. 2020;6(1):116. doi: 10.1001/jamaoncol.2019.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gelbert L.M., Cai S., Lin X., et al. Preclinical characterization of the CDK4/6 inhibitor LY2835219: in-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Invest New Drugs. 2014;32(5):825–837. doi: 10.1007/s10637-014-0120-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tripathy D., Hortobagyi G.N., Chan A., et al. Pooled safety analysis of first-line ribociclib (RIB) plus endocrine therapy (ET) in HR+/HER2– advanced breast cancer (ABC) Ann Oncol. 2019;30:iii53. [Google Scholar]
- 33.Diéras V., Rugo H.S., Schnell P., et al. Long-term pooled safety analysis of palbociclib in combination with endocrine therapy for HR+/HER2- advanced breast cancer. JNCI: J Natl Cancer Inst. 2019;111(4):419–430. doi: 10.1093/jnci/djy109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laurenti E., Frelin C., Xie S., et al. CDK6 levels regulate quiescence exit in human hematopoietic stem cells. Cell Stem Cell. 2015;16(3):302–313. doi: 10.1016/j.stem.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spring L.M., Wander S.A., Andre F., Moy B., Turner N.C., Bardia A. Cyclin-dependent kinase 4 and 6 inhibitors for hormone receptor-positive breast cancer: past, present, and future. Lancet. 2020;395(10226):817–827. doi: 10.1016/S0140-6736(20)30165-3. [DOI] [PubMed] [Google Scholar]
- 36.Rugo H.S., Huober J., García-Sáenz J.A., et al. Management of abemaciclib-associated adverse events in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: safety analysis of MONARCH 2 and MONARCH 3. Oncologist. 2021;26(1):e53–e65. doi: 10.1002/onco.13531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dickler M.N., Tolaney S.M., Rugo H.S., et al. MONARCH 1, A phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR+/HER2− metastatic breast cancer. Clin Cancer Res. 2017;23(17):5218–5224. doi: 10.1158/1078-0432.CCR-17-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diéras V., Harbeck N., Joy A.A., et al. Palbociclib with letrozole in postmenopausal women with ER+/HER2− advanced breast cancer: hematologic safety analysis of the randomized PALOMA-2 trial. Oncologist. 2019;24(12):1514–1525. doi: 10.1634/theoncologist.2019-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verma S., Bartlett C.H., Schnell P., et al. Palbociclib in combination with fulvestrant in women with hormone receptor-positive/HER2-negative advanced metastatic breast cancer: detailed safety analysis from a multicenter, randomized, placebo-controlled, phase III study (PALOMA-3) Oncologist. 2016;21(10):1165–1175. doi: 10.1634/theoncologist.2016-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.George M.A., Qureshi S., Omene C., Toppmeyer D.L., Ganesan S. Clinical and pharmacologic differences of CDK4/6 inhibitors in breast cancer. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.693104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Slamon D.J., Neven P., Chia S., et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N Engl J Med. 2020;382(6):514–524. doi: 10.1056/NEJMoa1911149. [DOI] [PubMed] [Google Scholar]
- 42.Turner N.C., Slamon D.J., Ro J., et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018;379(20):1926–1936. doi: 10.1056/NEJMoa1810527. [DOI] [PubMed] [Google Scholar]
- 43.Onesti C.E., Jerusalem G. CDK4/6 inhibitors in breast cancer: differences in toxicity profiles and impact on agent choice. A systematic review and meta-analysis. Expert Rev Anticancer Ther. 2021;21(3):283–298. doi: 10.1080/14737140.2021.1852934. [DOI] [PubMed] [Google Scholar]
- 44.McQuay H.J. Using numerical results from systematic reviews in clinical practice. Ann Intern Med. 1997;126(9):712. doi: 10.7326/0003-4819-126-9-199705010-00007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.