Abstract
Background
Successive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants have caused severe disease in long-term care facility (LTCF) residents. Primary vaccination provides strong short-term protection, but data are limited on duration of protection following booster vaccines, particularly against the Omicron variant. We investigated the effectiveness of booster vaccination against infections, hospitalizations, and deaths among LTCF residents and staff in England.
Methods
We included residents and staff of LTCFs within the VIVALDI study (ISRCTN 14447421) who underwent routine, asymptomatic testing (December 12, 2021–March 31, 2022). Cox regression was used to estimate relative hazards of SARS-CoV-2 infection, and associated hospitalization and death at 0–13, 14–48, 49–83, 84–111, 112–139, and 140+ days after dose 3 of SARS-CoV-2 vaccination compared with 2 doses (after 84+ days), stratified by previous SARS-CoV-2 infection and adjusting for age, sex, LTCF capacity, and local SARS-CoV-2 incidence.
Results
A total of 14 175 residents and 19 793 staff were included. In residents without prior SARS-CoV-2 infection, infection risk was reduced 0–111 days after the first booster, but no protection was apparent after 112 days. Additional protection following booster vaccination waned but was still present at 140+ days for COVID-associated hospitalization (adjusted hazard ratio [aHR], 0.20; 95% CI, 0.06–0.63) and death (aHR, 0.50; 95% CI, 0.20–1.27). Most residents (64.4%) had received primary course vaccine of AstraZeneca, but this did not impact pre- or postbooster risk. Staff showed a similar pattern of waning booster effectiveness against infection, with few hospitalizations and no deaths.
Conclusions
Our findings suggest that booster vaccination provided sustained protection against severe outcomes following infection with the Omicron variant, but no protection against infection from 4 months onwards. Ongoing surveillance for SARS-CoV-2 in LTCFs is crucial.
Keywords: COVID-19, SARS-CoV-2, long-term care facilities, Omicron, vaccine effectiveness
The disproportionate impact of coronavirus disease 2019 (COVID-19) on long-term care facilities (LTCFs) has been extensively documented, both in terms of direct effects of morbidity and mortality and indirect consequences of reduced access to health care, services, and social interactions [1]. Likely reasons for this include the closed-setting environment of LTCFs, impaired immune responses due to aging, and high levels of comorbidity [2]. As such, LTCF residents and staff were prioritized for vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Vaccination programs in UK LTCFs commenced on December 8, 2020 [3, 4], with primary series delivery of homologous prime-boost with either BNT162b2 (Pfizer) or ChAdOx1 (AstraZeneca) using an 8–12-week dose interval. LTCF residents and staff were prioritized for additional booster vaccination (third dose) from September 14, 2021, with a fourth dose for adults aged 75 years and over and older residents in LTCFs from spring 2022 [5] based on evidence that protection against severe disease waned from 6 months following primary vaccination in older adults [6] and concerns about the impact of new variants.
We previously reported a high level of short-term protection against infection and severe clinical outcomes following primary course vaccination in LTCF residents and staff while Alpha and Delta variants were dominant [7]. However, we observed substantial waning of protection against infection in staff and against all outcomes (infection, hospital admission, death) in residents from 84 days (12 weeks) following the second dose, which was restored following a booster dose [7]. Substantially increased short-term protection against symptomatic disease, hospitalization, and death was also seen in adults aged >50 years following a third-dose booster vaccination during high prevalence of the Delta variant, with limited waning of protection after 70 days [8]. Data on booster vaccination effectiveness against Omicron in LTCFs are limited, particularly following a primary course of AstraZeneca. However, 3 vaccine doses have been reported to offer high levels of protection against hospitalization with the Omicron variant in community-dwelling adults aged 75 years and over, with minimal waning 2–3 months postvaccination [9].
The aim of this study was to evaluate the effectiveness of third- and fourth-dose booster vaccination against infection, hospitalizations, and death among staff and residents of LTCFs in England, from when the Omicron variant became dominant until the end of the asymptomatic testing program in LTCF residents (December 12, 2021, to March 31, 2022).
METHODS
Study Design and Setting
VIVALDI is a prospective cohort study investigating SARS-CoV-2 in residents and staff in LTCFs in England and is described in detail elsewhere [10]. In the analysis period, following national guidelines, residents were undergoing monthly routine polymerase chain reaction (PCR) testing while staff were undergoing weekly testing using a combination of PCR and lateral flow devices (LFDs). We did not require PCR confirmation of positive LFD results in our analyses. Residents with a positive PCR test were not routinely retested for 90 days unless they developed new COVID-19 symptoms [11]; however, staff resumed asymptomatic LFD testing upon return to work following infection [12].
The analysis period is defined from December 12, 2021, when the S-gene target failure (SGTF) marker for Omicron (BA.1) was first detected in the data set [13], to March 31, 2022, when asymptomatic testing in residents ended [14]. Individuals were eligible for inclusion if they had ≥1 PCR or LFD result available within the analysis period. We excluded individuals who had not received ≥2 vaccine doses at the start of the analysis period.
Data Extraction and Linkage
We retrieved all PCR results and available LFD results from routine symptomatic and asymptomatic testing in LTCFs and positive PCR results from staff and residents who underwent clinical testing in hospitals through the COVID-19 Datastore [15]. Test results, vaccination, hospitalization, and deaths data were linked to study participants using pseudo-identifiers based on individuals’ unique National Health Service (NHS) numbers [7]. COVID-19 hospitalization was defined as an admission within 14 days of a positive PCR or LFD test for SARS-CoV-2 or admission with a positive test on the same or subsequent day. COVID-19 death was defined as within 28 days of positive PCR or LFD test.
As previously [7], we linked SARS-CoV-2 serological test results for immunoglobulin G antibodies to the nucleocapsid protein (Abbott ARCHITECT system, Abbott, Maidenhead, UK) in a subset of participants who consented to blood sampling specifically for the VIVALDI study [16]. Recruitment to the blood sampling part of the study was managed within LTCFs, so this subset may not be completely representative of the population as a whole (eg, those residents less able to consent would be less likely to be recruited). We combined positive PCR and LFD results, COVID-19-related hospital admission records, and positive nucleocapsid antibody results before the analysis period into a binary variable indicating evidence of prior SARS-CoV-2 exposure.
Care Quality Commission unique location identification (CQC-ID) codes were used to link residents to LTCFs. Data on bed capacity were retrieved from Capacity Tracker [17] and requested directly from LTCF managers if unavailable. Seven-day rolling rates of SARS-CoV-2 incidence at the local authority level [18] were used to represent local infection pressure for each LTCF. A data privacy impact assessment was completed for the VIVALDI study, and a privacy notice was published [19].
Statistical Analysis
We examined individual-level vaccine effectiveness against infection, hospitalization within 14 days, and death within 28 days of a positive test. Analyses were separately conducted for residents aged ≥65 years and for staff between 18 and 75 years. Individuals were eligible for inclusion if they had complete data on sex and age, had received ≥2 vaccine doses 84 days (≥12 weeks) before the analysis start date, had ≥1 PCR or LFD test result recorded within the analysis period, and were linked to an LTCF with data on total number of staff and residents. Individuals with third vaccine dose recorded before the start of official rollout on September 14, 2021, were excluded.
We used Cox regression models to derive adjusted hazard ratios (HRs) for the risk of each outcome of interest. Vaccination status was included as a time-varying covariable. The reference category was 2 vaccine doses, with 84 days (≥12 weeks) elapsed from dose 2. The exposure categories were 0–13, 14–48, 49–83, 84–111, 112–139, and ≥140 days following dose 3 and any time following dose 4. Individuals could start in the 2-dose vaccinated state and sequentially transition through 3- and 4-dose vaccinated exposure states. Individuals entered the risk period on December 12, 2021, or the date of their first recorded PCR/LFD result within VIVALDI if later. Individuals with a positive PCR/LFD result within 30 days before December 12, 2021, entered the risk period from the 31st day post–positive test. Individuals exited the risk period at the earliest of the following: outcome of interest or end of analysis period. For hospitalization, individuals were additionally censored at 15 days post–positive SARS-CoV-2 test if there was no hospital admission by then, and similarly for mortality after 29 days. Baseline hazard was defined over calendar time. Ninety-five percent CIs were calculated using robust SEs accounting for dependence of infection events within LTCFs.
The primary analysis was stratified by evidence of SARS-CoV-2 infection before the risk period. We adjusted for sex (binary variable), age (5-knot restricted cubic spline term), LTCF size expressed as total number of beds (linear term), and local SARS-CoV-2 incidence expressed as 7-day rolling rate per 100 population. Local incidence was included as a 3-knot restricted cubic spline term with separate coefficients for each calendar month. We also evaluated the effect of AstraZeneca vs Pfizer primary vaccination course (as recorded for dose 2) on each outcome. This effect was estimated separately before and after initial booster dose (dose 3) and tested jointly using a multivariate Wald test.
We also conducted a descriptive analysis of the incidence of new and repeat SARS-CoV-2 infections in LTCF staff and residents from October 2020 onwards (when regular testing had been implemented) to evaluate whether the Omicron variant was associated with a rise in reinfections. Repeat infections were considered to be any positive PCR/LFD test recorded >30 days after the previous positive test. Participants were considered to be under follow-up from first recorded PCR/LFD test until 90 days after their last recorded test.
All statistical analyses were conducted using STATA 17.0.
Patient Consent
Patient consent was not obtained for use of data other than for the subset of patients who underwent blood sampling specifically for the VIVALDI study. The legal basis to access data from staff and residents without informed consent was provided by Regulation 3(4) of the Health Service (Control of Patient Information) Regulations 2002 (COPI) [20]. Ethical approval for the study was obtained from the South Central-Hampshire B Research Ethics Committee (20/SC/0238).
RESULTS
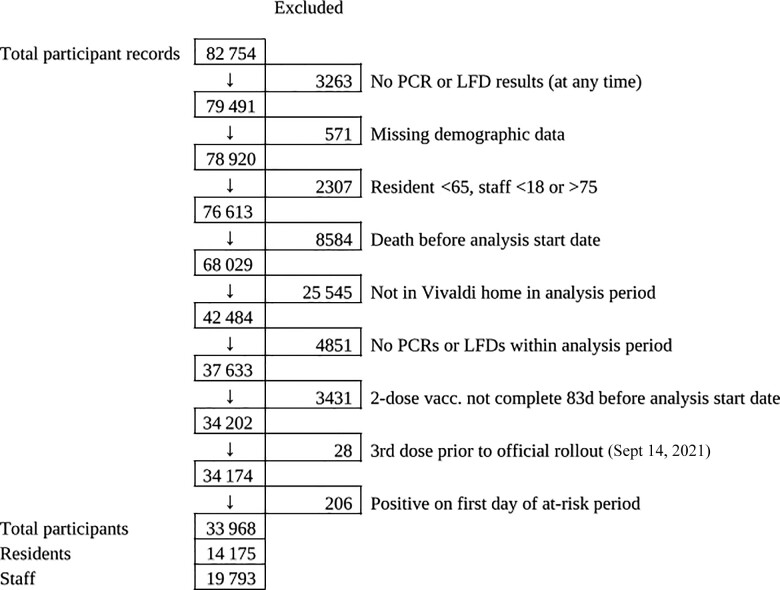
A total of 14 175 residents and 19 793 staff from 328 LTCFs (Supplementary Table 1) were included in the analysis (Figure 1). Two thousand seven hundred residents (19.0%) and 2911 staff (14.7%) had recorded evidence of SARS-CoV-2 infection before the analysis period (Table 1); 60 953 PCR (mean ± SD per month, 1.92 ± 2.34) and 27 533 LFD (0.76 ± 2.36 per month) tests for residents and 80 505 PCR (1.62 ± 2.02 per month) and 281 947 LFD (5.16 ± 6.34 per month) tests for staff were included.
Figure 1.
Flowchart for inclusion in the analysis of booster vaccine effectiveness. Abbreviations: LFD, lateral flow device; PCR, polymerase chain reaction.
Table 1.
Characteristics of Residents and Staff Included in the Analysis of Booster Vaccine Effectiveness
| Residents | Staff | All Participants | |
|---|---|---|---|
| No. | 14 175 | 19 793 | 33 968 |
| Age (IQR, range), y | 84.4 (76.4–90.4, 65.0–114.2) | 50.8 (37.0–59.0, 18.0–75.0) | 62.0 (47.0–81.8, 18.0–114.2) |
| Male sex | 4619 (32.6) | 4296 (21.7) | 8915 (26.2) |
| Female sex | 9556 (67.4) | 15 497 (78.3) | 25 053 (73.8) |
| Prior SARS-CoV-2 exposure | … | ||
| Pre-analysis PCR positive | 1958 (13.8) | 2078 (10.5) | 4036 (11.9) |
| Pre-analysis LFD positive | 77 (0.5) | 670 (3.4) | 747 (2.2) |
| Pre-analysis COVID-19 admission | 899 (6.3) | 140 (0.7) | 1039 (3.1) |
| Pre-analysis N-antibody results available | 971 (6.9) | 2147 (10.8) | 3118 (9.2) |
| Pre-analysis N-antibody positive | 304 (2.1) | 440 (2.2) | 744 (2.2) |
| Total pre-analysis infection | 2700 (19.0) | 2911 (14.7) | 5611 (16.5) |
| Vaccination | … | … | |
| First dose | … | … | |
| AstraZeneca | 8958 (63.2) | 10 496 (53.0) | 19 454 (57.3) |
| Pfizer BioNTech | 5215 (36.8) | 9091 (45.9) | 14 306 (42.1) |
| Moderna | 2 (0.0) | 197 (1.0) | 199 (0.6) |
| Second dose | … | … | |
| AstraZeneca | 9133 (64.4) | 10 484 (53.0) | 19 617 (57.8) |
| Pfizer BioNTech | 5039 (35.5) | 9104 (46.0) | 14 143 (41.6) |
| Moderna | 2 (0.0) | 196 (1.0) | 198 (0.6) |
| Second dose to analysis start (IQR, range), d | … | ||
| AstraZeneca | 248 (233–258, 84–337) | 218 (196–249, 84–305) | 241 (207–256, 84–337) |
| Pfizer BioNTech | 263 (252–271, 86–348) | 259 (216–276, 84–348) | 261 (234–275, 84–348) |
| Moderna | 129 (97–160, 97–160) | 123 (104–150, 84–289) | 123 (103–150, 84–289) |
| Booster dose (at start of period) | 12 430 (87.7) | 12 505 (63.2) | 24 935 (73.4) |
| AstraZeneca | 11 (0.1) | 9 (0.1) | 20 (0.1) |
| Pfizer BioNTech | 12 077 (97.2) | 11 160 (89.2) | 23 237 (93.2) |
| Moderna | 342 (2.8) | 1336 (10.7) | 1678 (6.7) |
| Booster dose (by end of period) | 13 487 (95.1) | 16 977 (85.8) | 30 464 (89.7) |
| AstraZeneca | 29 (0.2) | 14 (0.1) | 43 (0.1) |
| Pfizer BioNTech | 13 044 (96.7) | 14 379 (84.7) | 27 423 (90.0) |
| Moderna | 414 (3.1) | 2584 (15.2) | 2998 (9.8) |
| Booster interval from dose 2 (IQR, range), d | 203 (191–217, 25–436) | 199 (187–220, 56–436) | 201 (189–218, 25–436) |
| 2nd booster dose (by end of period) | 985 (6.9) | 173 (0.9) | 1158 (3.4) |
| AstraZeneca | 5 (0.5) | 0 (0.0) | 5 (0.4) |
| Pfizer BioNTech | 768 (78.0) | 144 (83.2) | 912 (78.8) |
| Moderna | 212 (21.5) | 29 (16.8) | 241 (20.8) |
| PCR testing (total tests) | 60 953 | 80 505 | 141 458 |
| Tests per person per month, mean (SE) | 1.92 (2.34) | 1.62 (2.02) | 1.75 (2.17) |
| LFD testing (total tests) | 27 533 | 281 995 | 309 528 |
| Tests per person per month, mean (SE) | 0.76 (2.36) | 5.16 (6.34) | 3.32 (5.52) |
Abbreviations: COVID-19, coronavirus disease 2019; IQR, interquartile range; LFD, lateral flow device; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
In the subset of 971 residents with ≥1 nucleocapsid antibody result available before the analysis period, antibodies indicating prior SARS-CoV-2 infection were found in 304 (31.3%). Of the 304, only 147 (48%) had other evidence of prior infection (ie, positive PCR or LFD test or hospital admission for COVID-19) recorded. Of the 2147 staff with ≥1 nucleocapsid antibody result available before the analysis period, antibodies indicating prior SARS-CoV-2 infection were found in 440 (20.5%). Of the 440, only 132 (30.0%) had other evidence of prior infection recorded. These findings indicate that the true proportion of residents and staff with prior infections is likely to be substantially higher than that based on the available evidence for each individual participant given that a minority of participants underwent antibody testing.
A total of 12 430 (87.7%) residents and 12 505 (63.2%) staff had received a booster vaccination dose before the analysis period, and 13 487 (95.1%) residents and 16 977 (85.8%) staff by the end. First booster doses were Pfizer in the majority (residents n = 13 044, 96.7%; staff n = 14 379, 84.7%), with Moderna used in a small number (residents n = 414, 3.1%; staff n = 2584, 15.2%). Second boosters (fourth vaccine) had been received by 6.9% of residents and 0.9% of staff by the end of the analysis period.
Infection
In residents without known prior SARS-CoV-2 infection, there was reduced risk of SARS-CoV-2 infection in the periods 0–13 days (HR, 0.56; 95% CI, 0.36–0.88), 14–48 days (HR, 0.33; 95% CI, 0.24–0.46), 49–83 days (HR, 0.37; 95% CI, 0.29–0.48), and 84–111 days (0.64; 95% CI, 0.51–0.80) after the first booster vaccine dose, relative to 2-dose vaccination (Table 2). However, no protection was apparent at 112–139 days (HR, 1.04; 95% CI, 0.71–1.53) or 140+ days (HR, 1.17; 95% CI, 0.81–1.67) following booster vaccination. Residents with known infection before the analysis period were at reduced risk of new infection relative to those without prior infection (HR, 0.50; 95% CI, 0.35–0.72), and within this group further protection following booster vaccination followed a similar pattern to that observed in infection-naïve residents. Infection rates were lower after second booster doses, but HR estimates were wide due to limited follow-up time. Additional adjustment for type of primary course before and after the booster dose did not improve model fit (P = .14).
Table 2.
Crude Event Rates and Adjusted Hazard Ratios Against PCR- or LFD-Positive SARS-CoV-2 Infections, Hospitalization Within 14 Days, and Deaths Within 28 Days of a Positive PCR or LFD Test for LTCF Residents, by Prior SARS-CoV-2 Exposure and Vaccination Status
| SARS-CoV-2 Infections | ||||||
|---|---|---|---|---|---|---|
| Prior SARS-CoV-2 Exposure | Vaccination Status | Person-Days | Infections | IR/1000pd | HR (95% CI)a | HR (95% CI)a |
| Unexposed | D2 84+ d | 50 569 | 215 | 4.25 | Ref. | … |
| … | D3 0–13 d | 10 350 | 42 | 4.06 | 0.56 (0.36–0.88) | … |
| … | D3 14–48 d | 75 307 | 142 | 1.89 | 0.33 (0.24–0.46) | … |
| … | D3 49–83 d | 245 243 | 537 | 2.19 | 0.37 (0.29–0.48) | … |
| … | D3 84–111 d | 233 204 | 737 | 3.16 | 0.64 (0.51–0.80) | … |
| … | D3 112–139 d | 193 957 | 467 | 2.41 | 1.04 (0.71–1.53) | … |
| … | D3 140+ d | 142 948 | 430 | 3.01 | 1.17 (0.81–1.67) | … |
| … | D4 0+ d | 6991 | 16 | 2.29 | 0.78 (0.37–1.65) | … |
| Exposed | D2 84+ d | 16 788 | 31 | 1.85 | 0.50 (0.35–0.72) | Ref. |
| … | D3 0–13 d | 3204 | 4 | 1.25 | … | 0.30 (0.11–0.87) |
| … | D3 14–48 d | 19 271 | 12 | 0.62 | … | 0.22 (0.12–0.42) |
| … | D3 49–83 d | 63 998 | 89 | 1.39 | … | 0.45 (0.28–0.72) |
| … | D3 84–111 d | 60 728 | 112 | 1.84 | … | 0.75 (0.47–1.18) |
| … | D3 112–139 d | 50 557 | 72 | 1.42 | … | 1.34 (0.79–2.28) |
| … | D3 140+ d | 39 885 | 70 | 1.76 | … | 1.44 (0.80–2.61) |
| … | D4 0+ d | 1718 | 1 | 0.58 | … | 0.35 (0.05–2.58) |
| SARS-CoV-2 Hospitalizations | ||||||
|---|---|---|---|---|---|---|
| Prior SARS-CoV-2 Exposure | Vaccination Status | Person-Days | Hosp. | IR/1000pd | HR (95% CI)a | HR (95% CI)a |
| Unexposed | D2 84+ d | 53 453 | 15 | 0.28 | Ref. | … |
| … | D3 0–13 d | 10 629 | 2 | 0.19 | 0.31 (0.06–1.60) | … |
| … | D3 14–48 d | 77 043 | 7 | 0.09 | 0.23 (0.08–0.64) | … |
| … | D3 49–83 d | 251 213 | 21 | 0.08 | 0.18 (0.08–0.40) | … |
| … | D3 84–111 d | 243 597 | 23 | 0.09 | 0.23 (0.11–0.47) | … |
| … | D3 112–139 d | 201 821 | 13 | 0.06 | 0.30 (0.11–0.79) | … |
| … | D3 140+ d | 147 979 | 6 | 0.04 | 0.20 (0.06–0.63) | … |
| … | D4 0+ d | 7095 | 0 | 0 | — | … |
| Exposed | D2 84+ d | 17 229 | 0 | 0 | — | Ref. |
| … | D3 0–13 d | 3259 | 0 | 0 | … | — |
| … | D3 14–48 d | 19 415 | 0 | 0 | … | — |
| … | D3 49–83 d | 64 986 | 1 | 0.02 | … | — |
| … | D3 84–111 d | 62 445 | 1 | 0.02 | … | — |
| … | D3 112–139 d | 51 754 | 1 | 0.02 | … | — |
| … | D3 140+ d | 40 806 | 0 | 0 | … | — |
| … | D4 0+ d | 1720 | 0 | 0 | … | — |
| SARS-CoV-2 Mortality | ||||||
|---|---|---|---|---|---|---|
| Prior SARS-CoV-2 Exposure | Vaccination Status | Person-Days | Deaths (w. 28 d) | IR/1000pd | HR (95% CI)a | HR (95% CI)a |
| Unexposed | D2 84+ d | 55 824 | 23 | 0.41 | Ref. | … |
| … | D3 0–13 d | 10 640 | 1 | 0.09 | 0.20 (0.03–1.55) | … |
| … | D3 14–48 d | 78 127 | 6 | 0.08 | 0.17 (0.06–0.48) | … |
| … | D3 49–83 d | 254 082 | 18 | 0.07 | 0.15 (0.07–0.31) | … |
| … | D3 84–111 d | 251 293 | 27 | 0.11 | 0.15 (0.08–0.28) | … |
| … | D3 112–139 d | 210 572 | 39 | 0.19 | 0.49 (0.23–1.01) | … |
| … | D3 140+ d | 151 975 | 17 | 0.11 | 0.50 (0.20–1.27) | … |
| … | D4 0+ d | 7222 | 0 | 0 | — | … |
| Exposed | D2 84+ d | 17 565 | 0 | 0 | — | Ref. |
| … | D3 0–13 d | 3268 | 0 | 0 | … | — |
| … | D3 14–48 d | 19 506 | 1 | 0.05 | … | — |
| … | D3 49–83 d | 65 357 | 0 | 0 | … | — |
| … | D3 84–111 d | 63 674 | 9 | 0.14 | … | — |
| … | D3 112–139 d | 53 106 | 3 | 0.06 | … | — |
| … | D3 140+ d | 41 562 | 1 | 0.02 | … | — |
| … | D4 0+ d | 1720 | 0 | 0 | … | — |
Abbreviations: HR, hazard ratio; IR, incidence rate; LCTF, long-term care facility; LFD, lateral flow device; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
HR values in the 2 columns are from mathematically identical statistical models, but HRs in right-hand column are expressed relative to “D2 84+ d” vaccine status in individuals with prior SARS-CoV-2 infection.
A protective effect of first booster vaccine dose was also seen in staff, although no protection was apparent by 112–139 days (HR, 1.21; 95% CI, 1.03–1.41) (Table 3). However, prior infection was not associated with any reduction in risk of new SARS-CoV-2 infection in this group (HR, 1.09; 95% CI, 0.94–1.25), and a similar pattern of protection from infection following the first booster dose was observed in this group. Additional adjustment for type of primary course before and after booster dose did not improve model fit (P = .13).
Table 3.
Crude Event Rates and Adjusted Hazard Ratios Against PCR- or LFD-Positive SARS-CoV-2 Infections and Crude Event Rates for Hospitalization Within 14 Days of a Positive PCR or LFD Test for LTCF Staff, by Prior SARS-CoV-2 Exposure and Vaccination Status
| SARS-CoV-2 Infections | ||||||
|---|---|---|---|---|---|---|
| Prior SARS-CoV-2 Exposure | Vaccination Status | Person-Days | Infections | IR/1000pd | HR (95% CI)a | HR (95% CI)a |
| Unexposed | D2 84+ d | 240 831 | 909 | 3.77 | Ref. | … |
| … | D3 0–13 d | 50 432 | 142 | 2.82 | 0.33 (0.27–0.41) | … |
| … | D3 14–48 d | 220 952 | 434 | 1.96 | 0.38 (0.33–0.43) | … |
| … | D3 49–83 d | 354 361 | 731 | 2.06 | 0.57 (0.51–0.64) | … |
| … | D3 84–111 d | 297 767 | 800 | 2.69 | 0.67 (0.59–0.76) | … |
| … | D3 112–139 d | 190 912 | 442 | 2.32 | 1.21 (1.03–1.42) | … |
| … | D3 140+ d | 140 821 | 461 | 3.27 | 1.72 (1.48–1.99) | … |
| … | D4 0+ d | 4818 | 7 | 1.45 | 0.77 (0.36–1.65) | … |
| Exposed | D2 84+ d | 50 290 | 200 | 3.98 | 1.09 (0.94–1.25) | Ref. |
| … | D3 0–13 d | 7822 | 16 | 2.05 | … | 0.21 (0.12–0.36) |
| … | D3 14–48 d | 30 758 | 47 | 1.53 | … | 0.28 (0.20–0.39) |
| … | D3 49–83 d | 58 004 | 141 | 2.43 | … | 0.50 (0.40–0.62) |
| … | D3 84–111 d | 52 154 | 167 | 3.2 | … | 0.59 (0.47–0.73) |
| … | D3 112–139 d | 38 584 | 94 | 2.44 | … | 1.15 (0.87–1.53) |
| … | D3 140+ d | 33 867 | 101 | 2.98 | … | 1.43 (1.13–1.81) |
| … | D4 0+ d | 479 | 0 | 0 | … | — |
| SARS-CoV-2 Hospitalizations | ||||||
|---|---|---|---|---|---|---|
| Prior SARS-CoV-2 Exposure | Vaccination Status | Person-Days | Hosp. | IR/1000pd | HR (95% CI)a | HR (95% CIa |
| Unexposed | D2 84+ d | 253 522 | 2 | 0.01 | — | … |
| … | D3 0–13 d | 51 485 | 1 | 0.02 | — | … |
| … | D3 14–48 d | 226 901 | 3 | 0.01 | — | … |
| … | D3 49–83 d | 363 558 | 7 | 0.02 | — | … |
| … | D3 84–111 d | 309 364 | 4 | 0.01 | — | … |
| … | D3 112–139 d | 198 028 | 2 | 0.01 | — | … |
| … | D3 140+ d | 146 879 | 0 | 0 | — | … |
| … | D4 0+ d | 4888 | 0 | 0 | — | … |
| Exposed | D2 84+ d | 53 279 | 2 | 0.04 | — | — |
| … | D3 0–13 d | 7925 | 0 | 0 | … | — |
| … | D3 14–48 d | 31 440 | 1 | 0.03 | … | — |
| … | D3 49–83 d | 59 781 | 1 | 0.02 | … | — |
| … | D3 84–111 d | 54 484 | 2 | 0.04 | … | — |
| … | D3 112–139 d | 40 358 | 1 | 0.02 | … | — |
| … | D3 140+ d | 35 167 | 0 | 0 | … | — |
| … | D4 0+ d | 479 | 0 | 0 | … | — |
Abbreviations: HR, hazard ratio; IR, incidence rate; LCTF, long-term care facility; LFD, lateral flow device; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
HR values in the 2 columns are from mathematically identical statistical models, but HRs in right-hand column are expressed relative to “D2 84+ d” vaccine status in individuals with prior SARS-CoV-2 infection.
Hospitalization
In residents without known prior SARS-CoV-2 infection, the first booster dose reduced risk of hospitalization within 0–13 days of SARS-CoV-2 infection (HR, 0.31; 95% CI, 0.06–1.60), which was sustained across 14–48 days (HR, 0.23; 95% CI, 0.08–0.64), 49–83 days (HR, 0.18; 95% CI, 0.08–0.40), 84–111 days (HR, 0.23; 95% CI, 0.11–0.47), 112–139 days (HR, 0.30; 95% CI, 0.11–0.79), and 140+ days (HR, 0.20; 95% CI, 0.06–0.63) from receipt of booster dose. No hospitalizations were observed after second booster doses, but follow-up time was limited. Residents with known infection before the analysis period were at reduced risk of hospitalization relative to those without prior infection, and within this group there were too few hospitalization events to reliably estimate the effect of booster vaccination. Additional adjustment for primary vaccine course type did not improve model fit (P = .60). Staff were at low risk of hospitalization following SARS-CoV-2 infection, precluding meaningful analysis of the effect of booster vaccination.
Death
In residents without known prior SARS-CoV-2 infection, the first booster reduced risk of death within 28 days of SARS-CoV-2 infection after 0–13 days (HR, 0.20; 95% CI, 0.03–1.55), 14–48 days (HR, 0.17; 95% CI, 0.06–0.48), 49–83 days (HR, 0.15; 95% CI, 0.07–0.31), and 84–111 days (HR, 0.15; 95% CI, 0.08–0.28), with apparent waning in the level of protection by 112–139 days (HR, 0.49; 95% CI, 0.23–1.01) and 140+ days (HR, 0.50; 95% CI, 0.20–1.27). Residents with known infection before the analysis period were at reduced risk of death relative to those without prior infection, and within this group there were too few deaths to evaluate the impact of booster vaccination. No deaths were observed after second booster doses, but limited follow-up time was available. Additional adjustment for primary vaccine course type did not improve model fit (P = .99). No deaths within 28 days of a positive SARS-CoV-2 test were observed among staff.
Reinfections
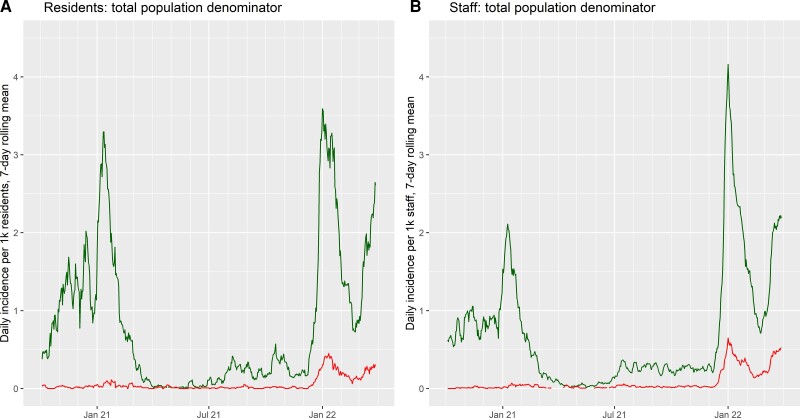
The appearance and spread of the Omicron variant in late 2021 and early 2022 were associated with a peak in incidence of new SARS-CoV-2 infections that exceeded the previous highest recorded levels in both residents and staff (Figure 2). There was also a substantial rise in the incidence of reinfections detected. In line with our Cox models, residents with prior SARS-CoV-2 infection only displayed moderately lower incidence of new infection than exposure-naïve residents during this period, and in staff the incidence of infection was unrelated to history of previous infection (Supplementary Figure 1).
Figure 2.
Rolling 7-day average incidence rate of new SARS-CoV-2 infections (green, upper lines) and repeat SARS-CoV-2 infections (red, lower lines) among residents (A) and staff (B) of long-term care facilities in the Vivaldi study. Incidence rates are calculated according to the total population under follow-up for residents and staff (and are proportional to total case counts). Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Our analysis of the risk of infection in relation to booster vaccination only includes the first observed infection in any given participant within the analysis period. However, we observed 2 infections within the analysis period, using a 30-day cutoff to define new infection episodes, in 15 residents (0.11%) and 79 staff (0.40%).
DISCUSSION
We found that SARS-CoV-2 infection and associated hospitalizations and deaths in LTCF residents who had received booster (third dose) vaccination were reduced compared with those who had only received the primary vaccine course during the period of Omicron dominance in England. However, no protection against infection was apparent in residents from 112 days after the third dose, and there was some waning of protection against hospitalization and death. While there was some evidence to suggest that infection rates were lower after second booster doses, the follow-up time was limited. Among staff, infections were reduced in those who had received booster vaccination, but the rates of hospitalization and death were too low for analysis. Overall, these findings suggest that booster vaccination provides protection in residents against infection with the Omicron variant but that this protection wanes, with more moderate waning of protection against associated severe outcomes. Those with prior infection were susceptible to reinfection with Omicron.
Few studies have explored infection and severe outcomes after booster vaccination doses specifically in LTCFs, and fewer still have explored this during the period in which the Omicron variant dominated. To the best of our knowledge, this is the only study to evaluate booster effectiveness in residents of LTCFs who have received AstraZeneca vaccine as their primary course.
A Canadian study explored the effectiveness of fourth-dose vaccination (primarily mRNA-1273), compared with third-dose, among residents aged 60 years and older in LTCFs and found evidence of improved protection against infection, symptomatic infection, and severe outcomes during the Omicron period [21]. A waning effect was observed 84 days after the third dose, but the follow-up time after the fourth dose was too limited for analysis [21]. A study conducted in the United States while Omicron was dominant estimated that VE against infection was 46.9% in LTCF residents who received a booster dose compared with those who had received 2-dose (primary course) vaccination, where booster vaccination was received ≥14 days before a positive test [22].
Our findings are also consistent with studies conducted in Israel that found that a third dose of the BNT162b2 mRNA vaccine compared with receipt of only 2 doses [23] and a fourth dose of the BN162b2 mRNA vaccine compared with 3 vaccine doses appeared to be effective in reducing risk of hospitalization, severe disease, and COVID-19-related death [24, 25]. There was evidence of waning effectiveness against infection [24], but sustained protection against severe disease [24, 25]. However, the study exploring third-dose vaccine effectiveness was conducted in the general population and excluded health care staff and LTCF residents, while the fourth-dose studies were conducted in older adults aged 60 years, only some of whom were LTCF residents. These findings may therefore not be generalizable to vulnerable residents of LTCFs.
It might be considered surprising that staff showed a similar pattern of waning protection against SARS-CoV-2 infection following receipt of a booster vaccine dose, but this is consistent with previous work in which we found similar levels and patterns of decline between residents and staff for antispike antibody levels following the second vaccination dose [26]. However, it is also relevant that booster vaccines within our analysis were based solely on the original Wuhan SARS-CoV-2 strain, and it has been shown that overall antispike antibody levels in response to such booster vaccines were not predictive of infection with the Omicron variant [27]. Despite concerns surrounding immunosenescence in older and vulnerable populations, and some uncertainty regarding the exact mechanism of protection given reduced neutralization against Omicron, it is clear that booster vaccinations did provide substantial protection over the short term in LTCF residents [21, 22].
This study has a number of strengths. Data were collected from a large cohort of LTCF residents and staff across England and linked to routinely collected and high-quality data on testing, hospitalizations, death, and vaccination. The study population underwent regular, asymptomatic testing, which enabled the systematic identification of study participants, accurate measurement of person-time at risk, and a comparatively unbiased assessment of vaccine effectiveness compared with studies that rely on symptomatic testing.
A limitation of the study is that it may have underestimated prior infection, as only a subset of participants underwent antibody testing. This will have resulted in an underestimate of the impact of past infection as people without antibody testing, along with those whose antibodies had waned, may have been misclassified as infection-naïve. For both residents and staff, a substantial proportion of those with evidence of prior infection on antibody testing did not have any other evidence of prior infection (ie, positive PCR or LFD test or hospital admission for COVID-19) recorded.
Due to the nature of the data collection for hospitalization and death records, it was not possible to distinguish between outcomes that occurred in individuals “with” and “from” COVID-19. This could potentially have led to underestimation of the protective effect of booster vaccination on severe outcomes. However, we also note that attribution of a single cause of death is not always clear-cut [28], particularly in the context of an extremely frail population.
Although our analysis focused on the period when the Omicron variant was dominant, we did not have access to sequencing data, so it was not possible to confirm the viral sublineage or to investigate whether multiple positive PCR tests from the same individual over time were genuine reinfections. It was also not possible to include data on comorbidities, which may impact estimates of vaccine effectiveness.
This study suggests that third-dose booster vaccination provided sustained protection against severe outcomes following infection with the Omicron variant in this vulnerable cohort, despite some waning, but protection against infection was not apparent from around 4 months onwards. In England, people aged over 75 years, including LTCF residents, and those who are clinically vulnerable were offered a fourth COVID-19 vaccination dose in spring 2022 [5]. Recent studies have shown fourth vaccine doses to be effective against severe illness caused by the Omicron variant when compared with a third dose administered 3–4 months previously in residents of LTCFs and older adults, respectively [21, 25], but it is not yet known whether this pattern of waning immunity will continue to be seen. It seems likely that regular vaccination will be required for residents of LTCFs to ensure continued protection against SARS-CoV-2, particularly given the potential for the rapid emergence of new variants, which may affect vaccine effectiveness. In this context, our findings underscore the critical need for continued surveillance of vaccine effectiveness against infection and severe outcomes in LTCFs to inform future decisions on the frequency and timing of vaccination in this vulnerable cohort.
Supplementary Material
Acknowledgments
We thank the staff and residents in the long-term care facilities who participated in this study and Mark Marshall at National Health Service (NHS) England, who pseudonymized the electronic health records.
Financial support. This work is independent research funded by the Department of Health and Social Care (COVID-19 surveillance studies). M.K. is funded by a Wellcome Trust Clinical PhD Fellowship (222907/Z/21/Z). L.S. is funded by a National Institute for Health Research Clinician Scientist Award (CS-2016-007). A.H. is supported by Health Data Research UK (LOND1), which is funded by the UK Medical Research Council, Engineering and Physical Sciences Research Council, Economic and Social Research Council, Department of Health and Social Care (England), Chief Scientist Office of the Scottish Government Health and Social Care Directorates, Health and Social Care Research and Development Division (Welsh Government), Public Health Agency (Northern Ireland), British Heart Foundation, and Wellcome Trust.
Disclaimer. The views expressed in this publication are those of the authors and not necessarily those of the NHS, Public Health England, or the Department of Health and Social Care.
Potential conflicts of interest. L.S. reports grants from the Department of Health and Social Care during the conduct of the study and is a member of the Social Care Working Group, which reports to the Scientific Advisory Group for Emergencies. A.I.-S. and V.B. are employed by the Department of Health and Social Care, which funded the study. A.H. reports funding from the COVID Core Studies Programme and is a member of the New and Emerging Respiratory Virus Threats Advisory Group at the Department of Health and Environmental Modelling Group of the Scientific Advisory Group for Emergencies. All other authors declare no competing interests.
Author contributions. O.S. conducted statistical analyses and drafted the Methods and Results, with M.S., T.P., and A.C. contributing to coding of data processing and analysis. N.L.A. drafted the Introduction and Discussion. M.K., H.N.-L., B.A., C.F., A.I.-S., V.B., G.T., P.M., A.H., A.C., and L.S. were involved in the planning, recruitment, and organization of the Vivaldi cohort study. G.T. and P.M. conducted lab work for the study. All authors contributed to the planning and interpretation of this analysis and contributed to the final manuscript draft.
Contributor Information
Oliver Stirrup, Institute for Global Health, University College London, London, United Kingdom.
Madhumita Shrotri, UCL Institute of Health Informatics, London, United Kingdom.
Natalie L Adams, UCL Institute of Health Informatics, London, United Kingdom.
Maria Krutikov, UCL Institute of Health Informatics, London, United Kingdom.
Hadjer Nacer-Laidi, UCL Institute of Health Informatics, London, United Kingdom.
Borscha Azmi, UCL Institute of Health Informatics, London, United Kingdom.
Tom Palmer, Institute for Global Health, University College London, London, United Kingdom.
Christopher Fuller, UCL Institute of Health Informatics, London, United Kingdom.
Aidan Irwin-Singer, Department of Health and Social Care, London, United Kingdom.
Verity Baynton, Department of Health and Social Care, London, United Kingdom.
Gokhan Tut, Institute of Immunology and Immunotherapy, University of Birmingham, Birmingham, United Kingdom.
Paul Moss, Institute of Immunology and Immunotherapy, University of Birmingham, Birmingham, United Kingdom.
Andrew Hayward, UCL Institute of Epidemiology & Healthcare, London, United Kingdom; Health Data Research UK, London, United Kingdom.
Andrew Copas, Institute for Global Health, University College London, London, United Kingdom.
Laura Shallcross, UCL Institute of Health Informatics, London, United Kingdom.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Chudasama DY, Milbourn H, Nsonwu O, et al. Penetration and impact of COVID-19 in long term care facilities in England: population surveillance study. Int J Epidemiol 2022; 50:1804–13. [DOI] [PubMed] [Google Scholar]
- 2. Castro-Herrera VM, Lown M, Fisk HL, et al. Relationships between age, frailty, length of care home residence and biomarkers of immunity and inflammation in older care home residents in the United Kingdom. Front Aging 2021; 2:599084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Department of Health and Social Care . Independent report. Optimising the COVID-19 vaccination programme for maximum short-term impact. Available at:https://www.gov.uk/government/publications/prioritising-the-first-covid-19-vaccine-dose-jcvi-statement/optimising-the-covid-19-vaccination-programme-for-maximum-short-term-impact. Accessed April 14, 2022.
- 4. Department of Health and Social Care . Independent report. Priority groups for coronavirus (COVID-19) vaccination: advice from the JCVI. December 30, 2020. Available at:https://www.gov.uk/government/publications/priority-groups-for-coronavirus-covid-19-vaccination-advice-from-the-jcvi-30-december-2020. Accessed April 14, 2022.
- 5. UK Health Security Agency . Guidance. A guide to the spring booster for those aged 75 years and older and older residents in care homes. Available at:https://www.gov.uk/government/publications/covid-19-vaccination-spring-booster-resources/a-guide-to-the-spring-booster-for-those-aged-75-years-and-older-residents-in-care-homes. Accessed June 24, 2022.
- 6. Department of Health and Social Care . Independent report. JCVI statement on the adult COVID-19 booster vaccination programme and the Omicron variant. January 7, 2022. Available at:https://www.gov.uk/government/publications/jcvi-statement-on-the-adult-covid-19-booster-vaccination-programme-and-the-omicron-variant/jcvi-statement-on-the-adult-covid-19-booster-vaccination-programme-and-the-omicron-variant-7-january-2022. Accessed June 24, 2022.
- 7. Shrotri M, Krutikov M, Nacer-Laidi H, et al. Duration of vaccine effectiveness against SARS-CoV-2 infection, hospitalisation, and death in residents and staff of long-term care facilities in England (VIVALDI): a prospective cohort study. Lancet Healthy Longev 2022; 3:e470–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Andrews N, Stowe J, Kirsebom F, et al. Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England. Nat Med 2022; 28:831–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. UK Health Security Agency . Effectiveness of COVID-19 vaccines against hospitalisation with the Omicron variant in adults aged 75 years and older. Available at:https://khub.net/documents/135939561/390853656/VE+against+hospitalisation+with+the+Omicron+variant.pdf/15c56838-2c55-5592-2653-7aeca3e24cfe. Accessed June 17, 2022.
- 10. Krutikov M, Palmer T, Donaldson A, et al. Study protocol: understanding SARS-cov-2 infection, immunity and its duration in care home residents and staff in England (VIVALDI). Wellcome Open Res 2020; 5:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. UK Health Security Agency . Changing the COVID-19 case definition. Available at:https://ukhsa.blog.gov.uk/2022/02/04/changing-the-covid-19-case-definition/. Accessed June 24, 2022.
- 12. UK Health Security Agency. Guidance [withdrawn] . COVID-19: management of staff and exposed patients or residents in health and social care settings. Available at:https://www.gov.uk/government/publications/covid-19-management-of-exposed-healthcare-workers-and-patients-in-hospital-settings/covid-19-management-of-exposed-healthcare-workers-and-patients-in-hospital-settings#repeat-testing-for-covid-19. Accessed December 12, 2022.
- 13. UK Health Security Agency . Omicron daily overview. December 17, 2021. Available at:https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1042100/20211217_OS_Daily_Omicron_Overview.pdf. Accessed April 19, 2022.
- 14. UK Health Security Agency . Guidance [withdrawn]. Coronavirus (COVID-19): testing in adult care homes. March 24, 2021. Updated April 1, 2022. Available at:https://www.gov.uk/government/publications/coronavirus-covid-19-testing-in-adult-care-homes. Accessed May 18, 2022.
- 15. NHS England . NHS COVID-19 data store. Available at:https://www.england.nhs.uk/contact-us/privacy-notice/how-we-use-your-information/covid-19-response/nhs-covid-19-data-store/. Accessed June 24, 2022.
- 16. Krutikov M, Palmer T, Tut G, et al. Prevalence and duration of detectable SARS-CoV-2 nucleocapsid antibodies in staff and residents of long-term care facilities over the first year of the pandemic (VIVALDI study): prospective cohort study in England. Lancet Healthy Longev 2022; 3:e13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. North of England Commissioning Support Unit (NECSU) . Capacity tracker. Available at:https://www.necsu.nhs.uk/capacity-tracker. Accessed June 17, 2022.
- 18. UK Health Security Agency . UK coronavirus dashboard. Available at:https://www.google.com/url?q=https://coronavirus.data.gov.uk/&sa=D&source=docs&ust=1649870894674970&usg=AOvVaw2fen5BkeN7y_PQw_fmy9cH. Accessed April 20, 2022.
- 19. University College London Institute of Health Informatics . VIVALDI privacy notice: VIVALDI (COVID-19 in care homes) study privacy notice. Available at:https://www.ucl.ac.uk/health-informatics/research/vivaldi/vivaldi-privacy-notice. Accessed April 19, 2022.
- 20. NHS Digital . Control of patient information (COPI) notice. Available at:https://digital.nhs.uk/coronavirus/coronavirus-covid-19-response-information-governance-hub/control-of-patient-information-copi-notice. Accessed April 19, 2022.
- 21. Grewal R, Kitchen SA, Nguyen L, et al. Effectiveness of a fourth dose of COVID-19 mRNA vaccine against the Omicron variant among long term care residents in Ontario, Canada: test negative design study. BMJ 2022; 378:e071502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Prasad N, Derado G, Nanduri SA, et al. Effectiveness of a COVID-19 additional primary or booster vaccine dose in preventing SARS-CoV-2 infection among nursing home residents during widespread circulation of the Omicron variant—United States, February 14–March 27, 2022. MMWR Morb Mortal Wkly Rep 2022; 71:633–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barda N, Dagan N, Cohen C, et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet 2021; 398:2093–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gazit S, Saciuk Y, Perez G, Peretz A, Pitzer VE, Patalon T. Short term, relative effectiveness of four doses versus three doses of BNT162b2 vaccine in people aged 60 years and older in Israel: retrospective, test negative, case-control study. BMJ 2022; 377:e071113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bar-On YM, Goldberg Y, Mandel M, et al. Protection by a fourth dose of BNT162b2 against Omicron in Israel. N Engl J Med 2022; 386:1712–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stirrup O, Krutikov M, Tut G, et al. Severe acute respiratory syndrome coronavirus 2 anti-spike antibody levels following second dose of ChAdOx1 nCov-19 or BNT162b2 vaccine in residents of long-term care facilities in England (VIVALDI). J Infect Dis 2022; 226:1877–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stærke NB, Reekie J, Nielsen H, et al. Levels of SARS-CoV-2 antibodies among fully vaccinated individuals with Delta or Omicron variant breakthrough infections. Nat Commun 2022; 13:4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Armstrong D. The COVID-19 pandemic and cause of death. Soc Health Illn 2021; 43:1614–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.