Abstract
Due to an increasing number of patients at risk (i.e., those with a highly compromised immune system and/or receiving aggressive chemotherapy treatment), invasive fungal infections (IFI) are increasingly being reported and associated with high mortality rates. Aspergillus spp., particularly A. fumigatus, is the major cause of IFI caused by filamentous fungi around the world followed by Fusarium spp., however, other fungi are emerging as human pathogens. The aim of this study was to explore the epidemiology and prevalence of the non-Aspergillus and non-Fusarium filamentous fungi in human clinical samples over an 11-year period in Qatar using molecular techniques. We recovered 53 filamentous fungal isolates from patients with various clinical conditions. Most patients were males (75.5%), 9.4% were immunocompromised, 20.7% had IFI, and 11.3% died within 30 days of diagnosis. The fungal isolates were recovered from a variety of clinical samples, including the nasal cavity, wounds, respiratory samples, body fluids, eye, ear, tissue, abscess, and blood specimens. Among the fungi isolated, 49% were dematiaceous fungi, followed by Mucorales (30%), with the latter group Mucorales being the major cause of IFI (5/11, 45.5%). The current study highlights the epidemiology and spectrum of filamentous fungal genera, other than Aspergillus and Fusarium, recovered from human clinical samples in Qatar, excluding superficial infections, which can aid in the surveillance of uncommon and emerging mycoses.
Keywords: filamentous fungi, invasive fungal infections, molecular epidemiology, Middle East, Qatar
Introduction
The incidence of fungal infections is increasing worldwide. About a billion people are affected with superficial (skin, hair, and nail) fungal infections worldwide.1 Life-threatening invasive fungal infections (IFI) affect primarily immunocompromised individuals with neutropenia, cancer, organ transplantation, HIV/AIDS, and those receiving immunosuppressive therapy. Other risk factors associated with serious fungal infections include asthma, chronic obstructive pulmonary disease (COPD), and tuberculosis.2 The mortality of IFI exceeds 1.6 million per year on a global scale.3–5 Recently, the World Health Organization (WHO) released the first-ever fungal pathogens priority list (WHO-FPPL) which categorizes fungal pathogens based on their public health importance and unmet research needs.6 The WHO-FPPL focuses on fungi that might cause invasive acute or subacute systemic infections as well as those which pose treatment and management difficulties. Pathogens were classified into three priority groups (critical, high, and medium). The critical group includes Cryptococcus neoformans, Candida auris, Aspergillus fumigatus, and C. albicans. Nakaseomyces glabrata (C. glabrata), Histoplasma spp., eumycetoma causative agents, Mucorales, Fusarium spp., C. tropicalis, and C. parapsilosis were assigned to the high group. Scedosporium spp., Lomentospora prolificans, Coccidioides spp., Pichia kudriavzeveii (C. krusei), Cryptococcus gattii, Talaromyces marneffei, Pneumocystis jirovecii, and Paracoccidioides spp. are pathogens in the medium category.
Filamentous fungi other than Aspergillus and Fusarium that cause human disease are emerging.7–10 These are clinically difficult to distinguish from aspergillosis and fusariosis. Moreover, many of these fungi are intrinsically resistant to the commonly used antifungal drugs, making them difficult to treat and this may lead to high mortality rates.9–12 The epidemiology of non-Aspergillus filamentous fungal infections varies geographically.13,14 For example, Fusarium is the second most common filamentous fungus causing human infections in the United States and Europe,8,15 whereas in Australia, infections caused by Scedosporium spp. were found to be more common than those caused by Fusarium spp.13
Many expatriates from high-risk regions of the world, mainly Southeast Asia, make up Qatar's population and that may explain the diverse fungal genera recovered from susceptible individuals. A few studies on the epidemiology of filamentous fungal infections have been published from the Middle East.16,17 In addition, several studies on fungal diseases in Qatar have been published, including mucormycosis,18–20Candida infections,21–25 fusariosis,26,27 and aspergillosis,28–30 however, most of these studies were case reports. Furthermore, the burden of fungal infections in Qatar was estimated by Taj-Aldeen et al. from January 2009 to December 2014.31 Except for mucormycosis,18–20,32–35 studies from the Middle East reported only a few cases of filamentous fungal infections other than aspergillosis and fusariosis.36–40
The current study aimed to investigate the epidemiology of pathogenic filamentous fungi in Qatar other than Aspergillus and Fusarium, as these genera have been addressed elsewhere,26,27,30 using internal transcribed spacer (ITS) region sequences for identification.
Materials and methods
Patients and specimens
A total of 53 clinical specimens positive for filamentous fungi belonging to 53 patients were recorded in about 11 years (September 2003–November 2014) (Table 1). These specimens were received from various facilities of the Hamad Medical Corporation (HMC) in addition to primary health centres and private hospitals in Qatar. They were isolated and identified by morphology according to the standard operating procedures of the Microbiology Laboratory at Hamad General Hospital, Qatar.
Table 1.
Patients’ demographics, clinical data, mortality, and fungi isolated.
| Specimen | Gender/ | Clinical | Mortality | Identification | Genbank | ||||
|---|---|---|---|---|---|---|---|---|---|
| S. No | number | age | Origin | data | Histopathology | (30 days) | Specimen type | (ITS) | accession# |
| 1 | Q0466 | M/63 | Pakistan | NAa | NA | Alive | Wound tissue | Aureobasidium mangrovei | ON387555 |
| 2 | Q0894 | F/23 | Qatar | Nasal polyp | Positive (Proven) | Alive | Nasal polyp | Curvularia sp. | ON387540 |
| 3 | Q6540 | F/54 | Syria | Breast cancer | NA (Proven by blood) | Died | Blood | Sarocladium kiliense | ON387561 |
| 4 | Q1292 | M/41 | Qatar | Invasive fungal sinusitis renal transplant | Positive (Proven) | Alive | Nasal swab | Rhizopus oryzae | ON387607 |
| 5 | Q0888 | M/21 | Qatar | Allergic fungal sinusitis | NA | Alive | Nasal polyp | Curvularia cf. buchloes | ON387527 |
| 6 | Q0051 | M/14 | India | Paranasal fungal sinusitis | NA | Alive | Nasal swab | Curvularia cf. buchloes | ON387526 |
| 7 | Q0141 | M/29 | Egypt | Trauma | NA | Alive | Foot tissue | Lichtheimia hongkongensis | ON387599 |
| 8 | Q0268 | M/62 | Palestine | COPDb | NA | Alive | Sputum | Alternaria alternata | ON387548 |
| 9 | Q0518 | M/34 | Sudan | Eumycetoma (Madura foot) | Positive (Proven) | Alive | Pus swab (foot) | Acremonium breve | ON387562 |
| 10 | Q0767 | M/26 | Burma | NA | NA | Alive | Plate culture | Lichtheimia hongkongensis | ON387600 |
| 11 | Q1088 | M/55 | Qatar | Liver transplant | Positive from leg ulcer (Proven) | Alive | BALd | Mucor indicus | ON387609 |
| 12 | Q0947 | M/26 | Nepal | Corneal abscess | NA | Alive | Corneal scrapings | Dothichiza pimprina | ON387551 |
| 13 | Q0286 | F/59 | Qatar | Breast cancer, on chemotherapy, fungal encephalitis | Positive (Proven) | Died | Brain abscess | Rhinocladiella mackenziei | ON387593 |
| 14 | Q1003 | M/31 | India | Infected leg fracture | Positive (Proven) | Alive | Leg tissue | Rhizopus microsporus | ON387603 |
| 15 | Q1314 | M/16 | India | Allergic fungal sinusitis | Positive (allergic) | Alive | Nasal tissue | Curvularia sp. | ON387534 |
| 16 | Q0210 | M/7 | Qatar | Obstructive jaundice | NA | Alive | Gastric aspirate | Exophiala dermatitidis | ON387592 |
| 17 | Q1325 | M/78 | Palestine | Pneumonia | NA | Died | Peritoneal fluid | Curvularia sp. | ON387533 |
| 18 | Q1293 | M/73 | Qatar | Diabetic foot | NA | Alive | Toe tissue | Rhizopus oryzae | ON387608 |
| 19 | Q0748 | M/39 | Sudan | Eye discharge | NA | Alive | Eye swab | Curvularia sp. | ON387541 |
| 20 | Q0784 | M/22 | Qatar | Fungal sinusitis | Positive (Proven) | Alive | Nasal tissue | Curvularia sp. | ON387528 |
| 21 | Q0852 | M/43 | Philippines | Dyspnea | Negative | Alive | Bronchial wash | Paecilomyces formosus | ON387591 |
| 22 | Q1036 | M/23 | Qatar | Allergic fungal sinusitis | Positive (allergic) | Alive | Nasal tissue | Curvularia sp. | ON387544 |
| 23 | Q7012 | M/53 | India | Corneal abscess | NA | Alive | Corneal scrapings | Curvularia lunata | ON387542 |
| 24 | Q1343 | M/48 | Egypt | Trauma | NA | Died | Wound | Curvularia sp. | ON387532 |
| 25 | Q1337 | M/36 | Oman | Left leg cellulitis | Negative | Alive | Leg tissue | Rhizopus oryzae | ON387606 |
| 26 | Q6551 | M/62 | Iran | Corneal abscess | NA | Alive | Corneal scrapping | Curvularia sp. | ON387529 |
| 27 | Q0167 | M/79 | Qatar | Abdominal aortic aneurysm | NA | Died | Bronchial wash | Lichtheimia sp.** | - |
| 28 | Q0786 | M/24 | Nepal | NA | NA | Alive | Ear swab | Scopulariopsis brevicaulis | ON387563 |
| 29 | Q1963 | M/20 | Sri Lanka | Leg fracture | Negative | Alive | Wound swab | Scedosporium apiospermum | ON387565 |
| 30 | Q2374 | M/36 | Nepal | Trauma | NA | Alive | J-Vac fluid | Mucor indicus | ON387610 |
| 31 | Q1066 | F/26 | Qatar | NA | NA | Alive | Nasal swab | Curvularia sp. | ON387539 |
| 32 | Q5775 | M/34 | India | Corneal abscess | NA | Alive | Eye swab | Subramaniula asteroides | ON387556 |
| 33 | Q1249 | F/58 | Sudan | Infected Sternal wound | NA | Alive | Wound tissue | Curvularia sp. | ON387531 |
| 34 | Q0445 | M/31 | Sudan | Allergic fungal sinusitis | Positive (allergic) | Alive | Nasal tissue | Curvularia sp. | ON387543 |
| 35 | Q4920 | M/29 | Nepal | Trauma | NA | Alive | Wound swab | Lichtheimia ornata | ON387602 |
| 36 | Q5822 | F/30 | India | Ear discharge | NA | Alive | Ear swab | Syncephalastrum monosporum | ON387612 |
| 37 | Q6111 | F/75 | Qatar | Upper respiratory tract infection | NA | Alive | Sputum | Mucor circinelloides | ON387611 |
| 38 | Q1114 | F/36 | India | Diabetic ketoacidosis, septic shock | NA | Alive | BAL | Curvularia sp. | ON387545 |
| 39 | Q9189 | M/26 | Nepal | NA | NA | Died | Wound swab | Lichtheimia sp.** | - |
| 40 | Q0450 | M/78 | Qatar | Diabetic foot | NA | Alive | Foot tissue | Scopulariopsis brevicaulis | ON387564 |
| 41 | Q0458 | M/49 | Eritrea | Hemoptysis, chronic cough | NA | Alive | BAL | Paecilomyces variotii | ON387590 |
| 42 | Q1162 | M/44 | Nepal | Fungal sinusitis | Positive (Proven) | Alive | Nasal tissue | Rhizopus oryzae | ON387605 |
| 43 | Q0513 | F/32 | Qatar | Renal failure | NA | Alive | Peritoneal dialysis fluid | Quambalaria cyanescens | ON387595 |
| 44 | Q0394 | M/54 | India | AMLc, cellulitis, below knee amputation | NA | Alive | Foot tissue | Lichtheimia corymbifera | ON387601 |
| 45 | Q0719 | F/42 | Qatar | NA | NA | Alive | Nose swab | Alternaria alternata | ON387549 |
| 46 | Q0870 | M/20 | Iran | NA | NA | Alive | Nasal swab | Trichoderma longibranchiatum | ON387560 |
| 47 | Q0926 | M/51 | India | Trauma | NA | Alive | Tissue (leg wound) | Rhytidhysteron rufulum | ON387552 |
| 48 | Q4037 | M/3 | Qatar | Trauma | NA | Alive | Thumb wound | Curvularia sp. | ON387536 |
| 49 | Q2296 | F/36 | Qatar | Pleural effusion | NA | Alive | Pleural fluid | Quambalaria cyanescens | ON387598 |
| 50 | Q1669 | F/51 | Qatar | NA | NA | Alive | Nasal swab | Schizophyllum commune | ON387594 |
| 51 | Q1687 | M/11 | Qatar | Allergic fungal sinusitis | NA | Alive | Nasal tissue | Curvularia sp. | ON387538 |
| 52 | Q1783 | M/28 | Sudan | Invasive fungal sinusitis | Positive (proven) | Alive | Nasal tissue | Curvularia sp. | ON387535 |
| 53 | Q1812 | F/37 | Philippines | Biliary pancreatitis, small bowel perforation, abdominal surgery, systemic mucormycosis | Positive (proven) | Alive | Abdominal wall tissue | Rhizopus microsporus | ON387604 |
aData not available.
bChronic obstructive pulmonary disease.
cAcute lymphoblastic leukemia.
dBroncho–alveolar lavage fluid; *Not identified using ITS sequencing; **Identified by morphology.
Isolation and identification of fungal pathogens from clinical specimens
Clinical samples were inoculated on Sabouraud dextrose agar (SDA; Difco Laboratories, Detroit, MI) with chloramphenicol (SDA), and SDA without antibiotics. Blood cultures were performed using Bactec FX automated Blood culture system (BD Diagnostic, Franklin Lakes, New Jersey, United States). Culture plates were incubated at 26 °C and 37 °C and were observed daily for growth up to 10 days except for dermatological specimens which were incubated up to 3 weeks. The isolates were harvested in glycerol cryo-tubes (Mast Diagnostics, UK) and stored at −70 °C until use.
Molecular identification
DNA extraction
All isolates were sub-cultured on homemade oatmeal agar (OA)41 and incubated for 5 days at 28 °C prior to DNA extraction. DNA was extracted using PrepMan Ultra sample preparation reagent (Applied Biosystems, Foster City, USA) according to the manufacturer's instructions. In short, a loop full of mycelium taken from the edge of the colonies was suspended in 100 μl PrepMan lysis solution in 2 ml sterile screw-cap microcentrifuge tubes and vortexed for 10–30 s. The mixture was heated at 100 °C in a heat block for 10 min and then centrifuged at 12 000 rpm for 2 min. A total of 50 μl supernatant containing the fungal DNA was transferred to another microcentrifuge tube. The DNA extracts were pipetted to a 96-well plate and the PCR master mix was added using a semi-automated multichannel pipetting robot (Integra Viaflo 96, INTEGRA Biosciences, Switzerland).
PCR and sequencing
The ITS region was amplified using the forward primer ITS5 (GGAAGTAAAAGTCGTAACAAGG)42 and reverse primer ITS4 (TCCTCCGCTTATTGATATGC).43 The PCR mixture per sample contained 6.6 μl of sterile water, 1.25 μl 10x Taq buffer, 1 μl dNTPs mix, 0.63 μl dimethylsulfoxide (DMSO), 0.25 μl of forward and reverse primers, and 0.06 μl Taq polymerase, resulting in a total volume of 10.04 μl. The PCR reactions were performed using the following conditions; an initial denaturation at 95 °C for 5 min, 35 cycles of denaturation at 95 °C for 30 s, 35 cycles of annealing at 55 °C for 45 s, 35 cycles extension at 72 °C for 70 s and finally a step of final extension at 72 °C for 10 min. The PCR products were kept on hold at 10 °C. The sequencing PCR reactions were performed using ABI PrismH Big Dye Terminator Reaction Kit v3.0 (Applied Biosystems, Inc., Foster City, CA, USA) and sequences were obtained with an ABI PRISM™ 3100 Genetic Analyzer (Applied Biosystems, Inc., Foster City, CA, USA) as mentioned previously.26 A consensus sequence was generated by combining the forward and the reverse read in the software packages Seqman and Editseq from the Lasergene package (DNAStar Inc., Madison, WI). A homology search with the generated consensus sequences was performed using the Basic Local Alignment Search Tool (BLAST) of the NCBI database.44
Phylogenetic analysis
To confirm the identification of isolates, a phylogenetic tree based on ITS sequences was constructed. The sequences were aligned using MAFFT v. 7.490 online version (https://mafft.cbrc.jp/alignment/server/). The aligned sequences were manually edited in Molecular Evolutionary Genetics Analysis version 7 software (MEGA7) and phylogenetic trees were inferred using the Maximum Likelihood method based on the Tamura 3-parameter model and 1000 bootstrap replications in MEGA7.45
Results
Patients characteristics
Filamentous fungi, other than Aspergillus and Fusarium, were isolated from 53 patients, 40 (75.5%) of them were males, with various clinical conditions. Their ages ranged from 3 to 79 years (median of 41 years), and five patients (9.4%) were <18 years old. Patients originated from 14 countries including the Middle East (n = 32, 60.3%), Southeast Asia (n = 20, 37.7%), and one patient from Eritrea (n = 1, 9%). The clinical presentations and the underlying conditions for 28 patients are presented in (Table 1). Patients included 5 (9.4%) immunocompromised individuals, 11 patients (20.7%) had proven IFI, and 6 (11.3%) died within 30 days after diagnosis. Risk factors were available for 25 patients and included trauma (n = 8, 15%), diabetes mellitus (n = 3, 5.7%), surgery (n = 4, 7.5%), cancer (n = 2, 3.8%), soft organ transplantations (SOT) (n = 2, 3.8%), and 1 case (1.9%) each of hematological malignancy, burn, COPD, and renal failure (Table 1).
Clinical specimens
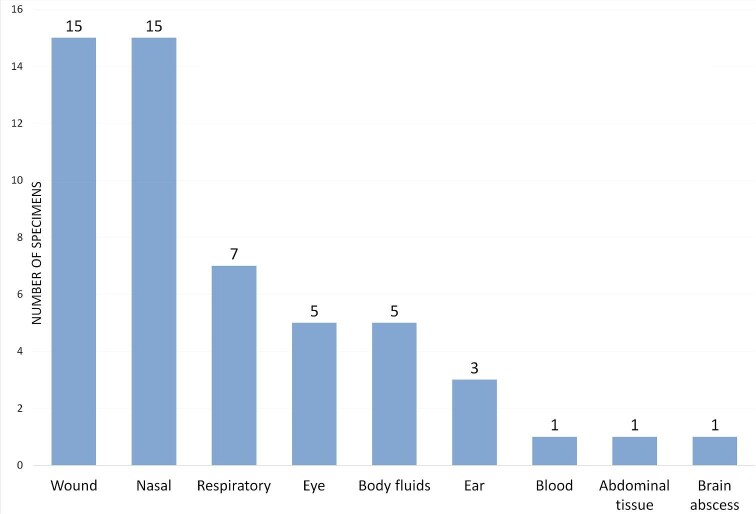
Fungi were recovered from various clinical specimens including nasal specimens (n = 15, 28.3%), wounds (n = 15, 28.3%), respiratory specimens (n = 7, 13.2%), body fluids (n = 5, 9.4%), eye (n = 5, 9.4%), ear swabs (n = 2, 3.8%), and one isolate each from an abdominal tissue, brain abscess, blood, and a clinical specimen that was received from an external facility for fungal identification with unknown specimen source (Table 3 and Fig. 1).
Table 3.
Distribution of fungal isolates and type of clinical specimen.
| Isolate (n) | Wound | Nasal | Respiratory | Eye | Body fluid | Ear | Blood | Abdominal tissue | Brain abscess | Unknown | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dematiaceous fungi (n = 26) | |||||||||||
| Alternaria alternata (2) | 1 | 1 | |||||||||
| Aureobasidium sp. (1) | 1 | ||||||||||
| Curvularia sp. (18) | 3 | 10 | 1 | 3 | 1 | ||||||
| Dothichiza pimprina (1) | 1 | ||||||||||
| Exophiala dermatitidis (1) | 1 | ||||||||||
| Rhinocladiella mackenziei (1) | 1 | ||||||||||
| Rhytidhysteron rufulum (1) | 1 | ||||||||||
| Subramaniula asteroides (1) | 1 | ||||||||||
| Mucorales (n = 16) | |||||||||||
| Rhizopus sp. (6) | 3 | 2 | 1 | ||||||||
| Lichtheimia spp. (6) | 4 | 1 | 1 | ||||||||
| Mucor sp. (3) | 2 | 1 | |||||||||
| Syncephalastrum sp. (1) | 1 | ||||||||||
| Hyaline fungi (n = 11) | |||||||||||
| Quambalaria cyanescens (2) | 2 | ||||||||||
| Sarocladium kiliense (1) | 1 | ||||||||||
| Acremonium breve (1) | 1 | ||||||||||
| Paecilomyces variotii (2) | 2 | ||||||||||
| Scopulariopsis brevicaulis (2) | 1 | 1 | |||||||||
| Scedosporium apiospermum (1) | 1 | ||||||||||
| Trichoderma sp. (1) | 1 | ||||||||||
| Schizophyllum commune (1) | 1 | ||||||||||
Figure 1.
Distribution of clinical samples.
Isolated fungi
The molecular identification of clinical fungi using ITS sequencing resulted in 51 isolates that belonged to 20 fungal genera (Table 1). The isolates were deposited to the Genbank database and their accession numbers are listed in Table 1. Two isolates were not identified due to poor sequence data, they were identified by morphological features as Lichtheimia species. Overall, dematiaceous fungi were the most isolated fungi in our study (26/53, 49%), followed by Mucorales (16/53, 30%) and other hyaline fungi (11/53, 21%) (Fig. 2). Most of the dematiaceous fungi (n = 18/26, 69%) belonged to the genus Curvularia whereas Rhizopus and Lichtheimia were the most frequently isolated genera in Mucorales, both (6/16, 37.5%).
Figure 2.
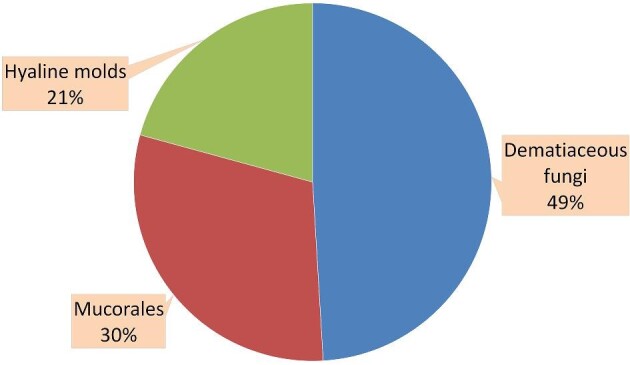
Distribution of fungal isolates.
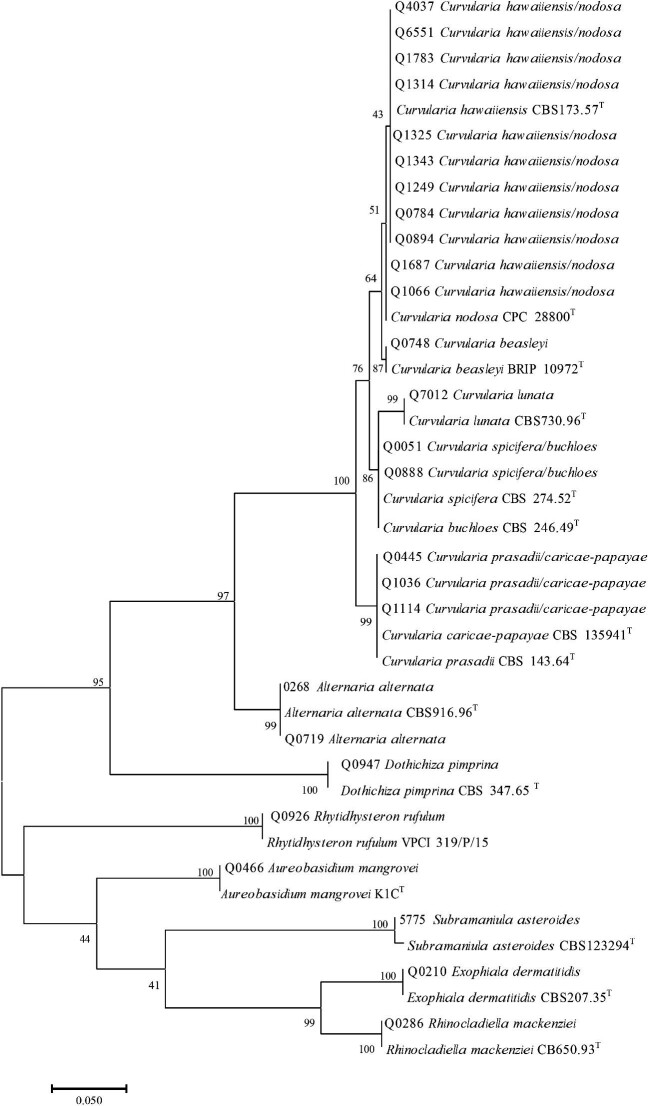
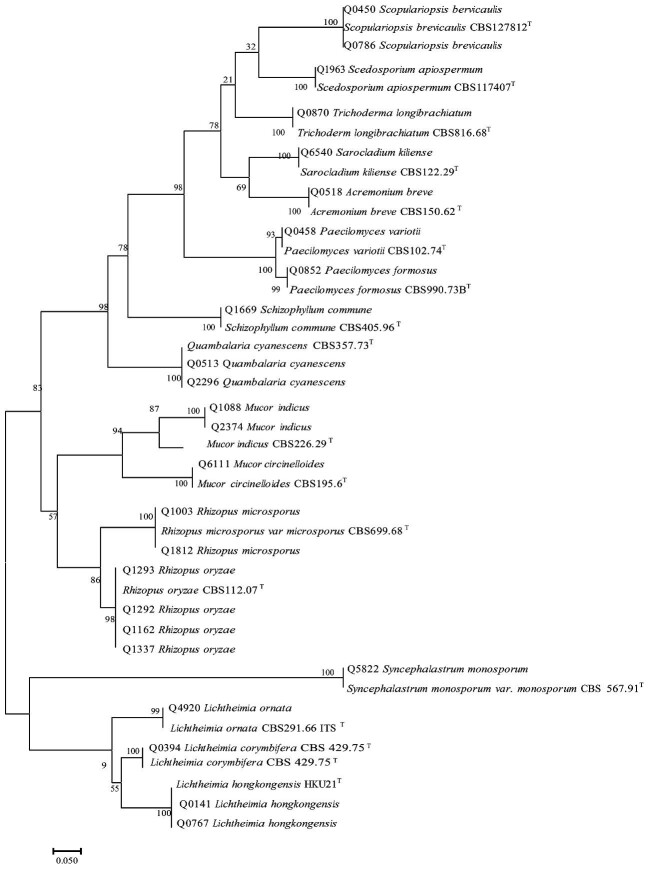
To confirm the identifications, phylogenetic trees were inferred based on the ITS sequences including type strains (Figs. 3 and 4). All isolates clustered with their corresponding type strains. However, most Curvularia species could not be sufficiently separated using ITS sequences. These included C. hawaiiensis/C. nodosa, C. spicifera/C. buchloes, and C. prasadii/C. caricae-papayae (Fig. 4). Except for two isolates, all had identical ITS sequences with more than one type strain. The isolates Q0051 and Q0888 showed 100% identity with the type strain C. buchloes CBS 246.49 and 99% identity with C. spicifera CBS 274.52 including one gap. Therefore, both isolates were identified as Curvularia cf. buchloes.
Figure 3.
Phylogenetic tree of dematiaceous fungi generated by Maximum Likelihood (ML) based on internal transcribed spacer (ITS) gene. TType strain.
Figure 4.
Phylogenetic tree of other filamentous fungi generated by Maximum Likelihood (ML) based on internal transcribed spacer (ITS) gene. T Type strain.
Invasive fungal infections
A total of 11 patients (21%) had proven IFI caused by Rhizopus spp. (4/11, 36%), Curvularia spp. (3/11, 27%), Acremonium breve (1/11, 9%), Sarocladium kiliense (1/11, 9%), Mucor indicus (1/11, 9%), and Rhinocladiella mackenziei (1/11, 9%) (Table 1). These fungi were mostly isolated from nasal specimens (5/11, 45%) and one specimen each from blood, bronchoalveolar lavage (BAL), abdominal tissue, wound tissue, foot pus, and brain abscess. Mucorales were the major cause of IFI (5/11, 45%) followed by dematiaceous fungi (4/11, 36%). Among the Mucorales, 4/5 were Rhizopus spp. and one was Mucor indicus. The dematiaceous fungi that caused IFI were Curvularia spp. (n = 3) and R. mackenziei (n = 1). We detected a rare fatal case of fungemia caused by S. kiliense in a patient with breast cancer. Acremonium spp. was recovered from a wound swab of a Sudanese patient who was diagnosed with eumycetoma (Madura foot) and this was confirmed by histopathology. The risk factors associated with IFI are shown in Table 2. They included SOT (n = 3), cancer (n = 2), abdominal surgery (n = 1), and trauma (n = 1).
Table 2.
Risk factors associated with invasive fungal infections (IFI)
| Group of fungi | Organism (n) | Risk factor | Specimen |
|---|---|---|---|
| Mucorales (5) | Mucor indicus (1) | Liver transplant | BALa |
| Rhizopus oryzae (2) | Renal transplant | Nasal | |
| NAb | Nasal | ||
| Rhizopus microsporus (2) | Abdominal surgery | Abdominal tissue | |
| Fracture | Wound tissue | ||
| Dematiaceous fungi (4) | Curvularia spp. (3) | NA | Nasal |
| NA | Nasal | ||
| NA | Nasal | ||
| Rhinocladiella mackenziei (1) | Breast cancer | Brain abscess | |
| Hyaline fungi (2) | Sarocladium kiliense (1) | Breast cancer | Blood |
| Acremonium breve (1) | NA | Foot pus |
aBroncho–alveolar lavage fluid.
bData not available.
Rare infections
We recovered clinical isolates of several fungal genera that are rarely encountered as human pathogens. However, these fungi could not be identified as infection-causing or colonizing agents. Aureobasidium spp., a black yeast-like fungus, was isolated from a wound tissue of a patient with an unknown clinical condition. Moreover, we have identified Quambalaria cyanescens isolates from two patients; one isolated from a peritoneal dialysis fluid of a patient with renal failure, and the other from a pleural fluid of a patient with unknown underlying disease. Subramaniula asteroides was isolated from an eye swab of a patient with a corneal abscess whose underlying condition was unknown. Furthermore, Exophiala dermatitidis was isolated from a gastric aspirate of a patient with obstructive jaundice. Paecilomyces spp. was isolated from BAL fluid of two patients. The underlying conditions of those patients were unknown. Another very rare species, Dothichiza pimprina, was isolated from a corneal scraping of a 26-year-old male with no clinical information mentioned. No data were available to confirm that these fungi were the etiological agents of infection.
Discussion
The epidemiology of filamentous fungal diseases in Qatar is examined in this 11-year retrospective study, excluding aspergillosis and fusariosis, which have already been covered in earlier publications.26,30,46 In a previous study, Taj-Aldeen et al. reported the burden of fungal infections in Qatar that were caused by species of Candida, Aspergillus, Fusarium, Mucorales, Cryptococcus neoformans, and Pneumocystis over a 5-year period (2009–2014).31 Their estimates were based on patients’ data retrieved from the microbiology laboratory database. The authors calculated the burden of fungal infections per 100 000 population for candidemia (15.4), Candida peritonitis (8.02), intraocular candidiasis (2.05), Candida vaginitis (3506), oral/esophageal candidiasis (6.52), cryptococcal meningitis (0.43), Pneumocystis pneumonia (0.8), mucormycosis (1.23), fusariosis (1.68), Aspergillus ear infections (23.3), onychomycosis (14.8), and rhinosinusitis (2.3).31 However, mycoses caused by other filamentous fungi were not estimated and the molecular identification of the etiological agents was not provided. Previously, we published on the molecular epidemiology and antifungal susceptibility patterns of Aspergillus30 and Fusarium26,27 species obtained from patients’ samples in Qatar. In the current study, we present the molecular epidemiology of other filamentous fungi using molecular methods for more accurate identification and to better understand the molecular diversity of fungal pathogens. In general, we were able to identify most isolates using sequencing of the ITS region, except two Curvularia isolates (C. hawaiiensis/C. nodosa, and C. prasadii/C. caricae-papayae) that could not be sufficiently separated using the ITS sequencing only, and were, therefore, identified up to genus level. Sequencing of the glyceraldehyde-3-phosphate dehydrogenase gene along with the ITS region is generally recommended for accurate identification of Curvularia species.47
Filamentous fungi were isolated from a wide range of patients from various origins, including those coming from regions where fungal diseases are common. This is reflected in the diverse genera of fungi isolated in our study. The 30-day mortality rate in the present study was 11.3%. We were, however, unable to determine whether these infections were the cause of death or whether other risk factors and underlying diseases influenced mortality. For IFI, cancer (18%) and SOT (18%) were the most common risk factors (Table 2). In a recent study from Iran, hematological malignancies and diabetes mellitus were the most prevalent underlying diseases among patients with IFI.48 Slavin et al. showed that hematological malignancies (46.7%), diabetes mellitus (23.5%), and chronic pulmonary disease were the most common comorbidities associated with IFI caused by non-Aspergillus molds in Australia.13
Mucormycosis is becoming more common worldwide,49–52 but it is especially prevalent in India and China among patients with uncontrolled diabetes mellitus.53–56 However, in a recent study where 600 articles (851 patients) of mucormycosis from January 2000 to January 2017 were analyzed using a literature search, the burden of mucormycosis was found to be slightly higher in Europe (34%) compared with Asia (31%).57 The prevalence and distribution of mucoraceous fungi varies geographically. In China, Mucor spp. was the most common pathogen causing mucormycosis (54.3%), followed by Rhizopus spp. (28.6%).58 On the other hand in a study from Europe, Rhizopus, Mucor and Lichtheimia accounted for 33.7% (58/172), 19.2% (33/172), and 18.6% (32/172) of mucormycosis cases, respectively.59Mucorales accounted for 30% (16/53) of the fungi isolated in the current study with a predominance of Rhizopus and Lichtheimia spp. (both 6/16, 37.5%), followed by Mucor spp. (3/16, 19%) and Syncephalastrum spp. (6%). Moreover, mucormycosis caused 45% (5/11) of the proven IFI in our study and 50% (3/6) of the deceased patients had mucormycosis. The burden of mucormycosis in Qatar was previously estimated to be 1.23/100 000 population.31 In neighboring countries, such as Oman, Jordan, Saudi Arabia, Iraq, and Algeria, the burden of mucormycosis was significantly lower with rates of 0.2, 0.02, 0.2, 0.034, and 0.2/100 000 individuals, respectively.60–62 In Iran, the rate of mucormycosis was relatively high (9.2/100 000 population),63 and this was attributed to the high prevalence of diabetes in the country.64
Dematiaceous fungal infections are generally caused by inhalation or inoculation of fungal spores through the skin following trauma.65,66 They usually cause superficial infections in immunocompetent patients, but they can rapidly disseminate and cause deep infections in immunocompromised patients.47,67 Superficial infections, subcutaneous nodules, and keratitis, are the most common clinical syndromes associated with dematiaceous fungi.65,66 In the current study, dematiaceous fungi were the most isolated fungi (49%), and Curvularia was the most isolated genus (69%), followed by Alternaria (7.7%). Fungal rhinosinusitis was the most common clinical presentation associated with dematiaceous fungi (11/26, 42.3%), followed by keratitis and cutaneous/subcutaneous infections (both 5/26, 19.2%). In a previous multicenter study of 23 transplant centers over 5-year period in the United States, the most common genus was Alternaria (32%), followed by Exophiala (11%).68 In contrast, Schieffelin et al. identified 27 cases of phaeohyphomycosis in SOT recipients in which Exophiala was the most recovered genus (11/27), followed by Ochroconis (3/11) and Alternaria (2/11).67 Moreover, in studies from India69 and Korea,70Exophiala was the most isolated genus causing phaeohyphomycosis (26% and 71%, respectively). However, we recovered only one case of Exophiala from a gastric aspirate specimen of a patient with obstructive jaundice admitted to the intensive care unit (ICU).
Rhinocladiella mackenziei is among the common fungi causing cerebral phaeohyphomycosis.71 The infection is almost restricted to the Middle East,72 however, few cases were reported from other regions as well.73–75 We isolated R. mackenziei from a brain abscess of a 59-year old female with breast cancer who was undergoing chemotherapy. The fungus resulted in a fatal cerebral phaeohyphomycosis that was proven by histopathology. This case was previously reported by Taj-Aldeen et al.38 and considered the second report of R. mackenziei from Qatar. The first case was reported in 1993 from brain abscess of a 55-year old male after renal transplant.72
We recovered Rhytidhysteron rufulum from a specimen of leg wound tissue of a 51 year old male following trauma. This fungus is extremely rare with only six cases reported in the literature. In all, five of them were reported from India76–79 and one case from the USA.80 Here we report the seventh case of R. rufulum from human clinical samples.
In the current study, we isolated Subramaniula asteroides from an eye swab of a 34 year old male with corneal abscess. S. asteroides is an opportunistic fungal pathogen that rarely cause fungal keratitis and skin infection.81,82 This fungus is able to grow at temperatures up to 40 °C.82 Previously reported cases of S. asteroides infections were endophthalmitis due to trauma in a noninsulin dependent diabetic patient,83 fungal rhinosinusitis in a patient without co-morbities,83 and a case of fungal keratitis after corneal trauma.84 Interestingly, S. asteroides was isolated from desert soil in Saudi Arabia.82
A corneal scraping sample obtained from a patient with a corneal abscess (Q0947) grew a fungus that had an ITS sequence which was 99.88% identical to the ITS of the isotype of Dothichiza pimprina P.N. Mathur & Thirumalachar (CBS 347.65, Genbank: MH858601.1). A review of the literature for this fungus turned up no previous reports. The isotype of D. pimprina (CBS 347.65) is the only strain available in GenBank and was isolated from India.85
From the ecological perspective, environmental studies showed that Alternaria was found to be abundant in the environment of Qatar,86–90 whereas Curvularia was less frequent.86,87 In contrast, our findings showed that Curvularia was more prevalent in clinical specimens compared with Alternaria. In general, the prevalence of pathogenic melanized fungi in the current study may be attributed to their resistance to extreme environments (such as Qatar's environment), with high temperatures, salinity, dehydration, and solar radiation.91–93
Infections caused by Quambalaria cyanescens (formerly Sporothix cyanescens94) are rare. It was previously isolated from immunocompromised95,96 and immunocompetent97–99 patients with no clinical evidence of infection in most cases. In our study, we isolated Q. cyanescens from peritoneal dialysis fluid of a patient with end stage renal disease (ESRD) and a pleural fluid from another patient with a post-surgical pleural effusion. However, there was no clinical evidence to prove infection.
Sarocladium kiliense (formerly Acremonium kiliense) was isolated in the current study from blood sample indicating a disseminated disease. Among Sarocladium, S. kiliense is associated with the majority of human infections.100–102 This fungus has been described as a cause of mycetoma,103 keratitis, endophthalmitis, endocarditis, continuous ambulatory peritoneal dialysis-associated peritonitis, and catheter-related fungemia.104 In addition, it was also linked to hospital outbreaks.105,106
We isolated Trichoderma from patient with fungal rhinosinusitis. Trichoderma was previously reported from patients with endocarditis, invasive sinusitis, keratitis, cutaneous infections, mediastinitis, peritonitis, pulmonary infections, liver infection, stomatitis, brain abscesses, infection of cardiac implantable electronic device, or disseminated infections.107
Paecilomyces variotti was obtained from BAL and bronchial wash specimens of two patients with dyspnea and chronic cough, respectively. Rosanne et al. reported that lung was the second most infected site by this fungus (27%) after the peritoneum (33%).108 Infections can affect immunocompetent109,110 and immunocompromised individuals. Patients with indwelling catheters, in particular, are at greater risk of invasive infection.108,109
In the current study, Acremonium was isolated from a patient with mycetoma. It was also previously reported to cause keratitis,111,112 osteomyelitis,113,114 disseminated infection,115–118 brain abscess,119 pulmonary infections,120–122 meningitis,123–125 endocarditis,126 subcutaneous infections,127–130 and peritonitis.131,132
Scedosporium apiospermum was isolated in our study from a wound swab of a patient following leg fracture. This fungus was reported to cause a wide range of infections in immunocompromised patients,11,133 and mostly cause local infections after traumatic inoculation in immunocompetent individuals. There have been several reports of keratitis,134–138 corioretinitis,139 vertebral osteomyelitis,140 post-traumatic brain infection,141,142 lymphocutaneous syndrome,143 lymphadenitis,144 septic arthritis,145,146 and post-tuberculosis lung infection147,148 caused by S. apiospermum.
We isolated Scopulariopsis brevicaulis from an ear swab of a patient with unknown clinical condition and a wound swab from another patient with a diabetic foot. Scopulariopsis was previously reported from cases of keratitis,149–153 otomycosis,154,155 onychomycosis,156 rhinosinusitis,157,158 and disseminated infections.159
The majority of reported Schizophyllum commune causing human infections appear to be caused by inhalation of fungal spores, resulting in sinusitis160–162 and allergic bronchopulmonary mycosis (ABPM).163 Mycoses due to S. commune is mostly prevalent in Japan compared with other parts of the world.163 Ulceration of the palate,164 brain abscess,160,165 otitis externa,166,167 meningitis,168 pneumonia,169 cutaneous granuloma,170 and onychomycosis171 caused by S. commune have also been reported. In the current study, we report the first case of S. commune from Qatar from a 55 year old female with rhinosinusitis. However, no data were available regarding tissue invasion.
Limitations of the study
Considering that only selected isolates were used, our study may not reflect the exact prevalence of these fungi. Our data, on the other hand, may provide insight into various fungal genera/species involved in human infections in the country. Furthermore, not all patients had complete data on risk factors, underlying illnesses, and clinical manifestations. Additionally, we did not sequence additional genes for species that could not be identified using ITS only. Finally, we were unable to obtain data on antifungal therapy and prophylaxis.
Conclusion
To conclude, the current study investigated the spectrum of filamentous fungi, other than Aspergillus and Fusarium that cause human diseases in Qatar. This may help clinicians and infectious diseases specialists to understand the local epidemiology and trends of these infections, particularly those caused by the emerging fungi, which may serve as a guidance for appropriate patients’ management. Identification using molecular methods can aid in accurately determining the species of fungal isolates obtained from clinical samples. However, species cannot be precisely identified using solely ITS sequencing and may require sequencing of additional genes.
Acknowledgements
We thank Bart Theelen, Dr. Anna Kolecka, Bart Kraak, and Dr. Abdullah Al-Hatmi for technical assistance.
Contributor Information
Husam Salah, Division of Microbiology, Department of Laboratory Medicine and Pathology, Hamad Medical Corporation, Doha, Qatar; Yeast Research, Westerdijk Fungal Biodiversity Institute, Utrecht, Netherlands.
Jos Houbraken, Applied and Industrial Mycology, Westerdijk Fungal Biodiversity Institute, Utrecht, Netherlands.
Teun Boekhout, Yeast Research, Westerdijk Fungal Biodiversity Institute, Utrecht, Netherlands; Institute of Biodiversity and Ecosystem Dynamics (IBED), University of Amsterdam, Amsterdam, The Netherlands.
Muna Almaslamani, Department of Medicine, Hamad Medical Corporation, Doha, Qatar.
Saad J Taj-Aldeen, Division of Microbiology, Department of Laboratory Medicine and Pathology, Hamad Medical Corporation, Doha, Qatar; Department of Biology, College of Science, University of Babylon, Hilla, Iraq.
Author contributions
Husam Salah (Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft), Jos Houbraken (Writing – review & editing), Teun Boekhout (Conceptualization, Data curation, Formal analysis, Methodology, Resources, Supervision, Writing – review & editing), Muna Almaslamani (Conceptualization, Writing – review & editing) and Saad J. Taj-Aldeen (Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – review & editing).
Funding
This study was supported by Qatar National Research Fund (QNRF), a member of Qatar Foundation [grant NPRP 5-298-3-086 (to S.T-A. and T.B.)]. Open Access funding provided by Qatar National Library.
Declaration of interest
The authors declare no conflict of interest.
References
- 1. Brown GD, Denning DW, Gow NARet al. Hidden killers: Human fungal infections. Sci Transl Med. 2012; 4. 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 2. Denning DW. The ambitious “95-95 by 2025” roadmap for the diagnosis and management of fungal diseases. Thorax. 2015; 70: 613–614. [DOI] [PubMed] [Google Scholar]
- 3. Bongomin F, Gago S, Oladele RO, Denning DW.. Global and multi-national prevalence of fungal diseases—estimate precision. J Fungi. 2017; 3: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marr KA, Carter RA, Boeckh M, Martin P, Corey L.. Invasive aspergillosis in allogeneic stem cell transplant recipients: Changes in epidemiology and risk factors. Blood. 2002; 100: 4358–4366. [DOI] [PubMed] [Google Scholar]
- 5. Guinea J, Torres-Narbona M, Gijón Pet al. Pulmonary aspergillosis in patients with chronic obstructive pulmonary disease: Incidence, risk factors, and outcome. Clin Microbiol Infect. 2010; 16: 870–877. [DOI] [PubMed] [Google Scholar]
- 6. WHO fungal priority pathogens list to guide research, development and public health action. Geneva: World Health Organization. 2022;(Licence: CC BY-NC-SA 3.0 IGO). https://www.who.int/publications/i/item/9789240060241. [Google Scholar]
- 7. Pappas PG, Alexander BD, Andes DRet al. Invasive fungal infections among organ transplant recipients: Results of the transplant-associated infection surveillance network (Transnet). Clin Infect Dis. 2010; 50: 1101–1111. [DOI] [PubMed] [Google Scholar]
- 8. Park BJ, Pappas PG, Wannemuehler KAet al. Invasive non-Aspergillus mold infections in transplant recipients, United States, 2001-2006. Emerg Infect Dis. 2011; 17: 1855–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peghin M, Monforte V, Martin-Gomez MTet al. Epidemiology of invasive respiratory disease caused by emerging non-Aspergillus molds in lung transplant recipients. Transpl Infect Dis. 2016; 18: 70–78. [DOI] [PubMed] [Google Scholar]
- 10. Jacobs SE, Wengenack NL, Walsh TJ.. Non- Aspergillus hyaline molds: Emerging causes of sino-pulmonary fungal infections and other invasive mycoses. Semin Respir Crit Care Med. 2020; 41: 115–130. [DOI] [PubMed] [Google Scholar]
- 11. Husain S, Alexander BD, Munoz Pet al. Opportunistic mycelial fungal infections in organ transplant recipients: Emerging importance of non-Aspergillus mycelial fungi. Clin Infect Dis. 2003; 37: 221–229. [DOI] [PubMed] [Google Scholar]
- 12. Pfaller MA, Diekema DJ.. Epidemiology of invasive mycoses in North America. Crit Rev Microbiol. 2010; 36: 1–53. [DOI] [PubMed] [Google Scholar]
- 13. Slavin M, van Hal S, Sorrell TCet al. Invasive infections due to filamentous fungi other than Aspergillus: Epidemiology and determinants of mortality. Clin Microbiol Infect. 2015; 21: 490.e1–490.e10. [DOI] [PubMed] [Google Scholar]
- 14. Douglas AP, Chen SCA, Slavin MA.. Emerging infections caused by non-Aspergillus filamentous fungi. Clin Microbiol Infect. 2016; 22: 670–680. [DOI] [PubMed] [Google Scholar]
- 15. Kontoyiennis DP, Marr KA, Park BJet al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: Overview of the transplant-associated infection surveillance network (TRANSNET) database. Clin Infect Dis. 2010; 50: 1091–1100. [DOI] [PubMed] [Google Scholar]
- 16. Osman M, Al Bikai A, Rafei Ret al. Update on invasive fungal infections in the Middle Eastern and North African region. Brazilian J Microbiol. 2020; 51: 1771–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kmeid J, Jabbour JF, Kanj SS.. Epidemiology and burden of invasive fungal infections in the countries of the Arab League. J Infect Public Health. 2020; 13: 2080–2086. [DOI] [PubMed] [Google Scholar]
- 18. Hilal AA, Taj-Aldeen SJ, Mirghani AH.. Rhinoorbital mucormycosis secondary to Rhizopus oryzae: A case report and literature review. Ear Nose Throat J. 2004; 83: 556–562. [PubMed] [Google Scholar]
- 19. Yassin MAD, Taj-Aldeen SJ, Khan FY, Errayes M, Aref E.. Rhino-orbital zygomycosis secondary to Rhizopus oryzae in a renal transplant recipient successfully treated with liposomal amphotericin B. Chang Gung Med J. https://pubmed.ncbi.nlm.nih.gov/18935800/. Published 2008. Accessed August 10, 2021. [PubMed] [Google Scholar]
- 20. Al Soub H, El Deeb Y, Almaslaman IM, Al Khuwaiter JY. Zygomycosis in Qatar: A retrospective review of six cases. Eur Ann Allergy Clin Immunol. 2004; 36: 387–391. https://pubmed.ncbi.nlm.nih.gov/15662967/. Accessed August 10, 2021. [PubMed] [Google Scholar]
- 21. Al Soub H, Estinoso W. Hospital-acquired candidaemia: Experience from a developing country. J Hosp Infect. 1997; 35: 141–147. [DOI] [PubMed] [Google Scholar]
- 22. Taj-Aldeen SJ, Abdulwahab A, Kolecka Aet al. Uncommon opportunistic yeast bloodstream infections from Qatar. Med Mycol. 2014; 52: 549–553. [DOI] [PubMed] [Google Scholar]
- 23. Abdul Wahab A, Salah H, Kolecka A, Boeckhout T, Taj-Aldeen S. Persistence of Candida dubliniensis in the lower airways of cystic fibrosis patients. Eur Respir J. 2015; 46 (suppl 59): PA2058. [Google Scholar]
- 24. Taj-Aldeen SJ, Salah H, Perez WBet al. Molecular analysis of resistance and detection of non-wild-type strains using etest epidemiological cutoff values for amphotericin B and echinocandins for bloodstream Candida infections from a tertiary hospital in Qatar. Antimicrob Agents Chemother. 2018; 62. 10.1128/AAC.00214-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Taj-Aldeen SJ, Kolecka A, Boesten Ret al. Epidemiology of candidemia in Qatar, the Middle East: Performance of MALDI-TOF MS for the identification of Candida species, species distribution, outcome, and susceptibility pattern. Infection. 2014; 42: 393–404. [DOI] [PubMed] [Google Scholar]
- 26. Salah H, Al-Hatmi AMS, Theelen Bet al. Phylogenetic diversity of human pathogenic Fusarium and emergence of uncommon virulent species. J Infect. 2015; 71: 658–666. [DOI] [PubMed] [Google Scholar]
- 27. Taj-Aldeen SJ, Salah H, Al-Hatmi AMSet al. In vitro resistance of clinical Fusarium species to amphotericin B and voriconazole using the EUCAST antifungal susceptibility method. Diagn Microbiol Infect Dis. 2016; 85: 438–443. [DOI] [PubMed] [Google Scholar]
- 28. Taj-Aldeen SJ, Hilal AA, Schell WA.. Allergic fungal rhinosinusitis: A report of 8 cases. Am J Otolaryngol - Head Neck Med Surg. 2004; 25: 213–218. [DOI] [PubMed] [Google Scholar]
- 29. Taj-Aldeen SJ, Hilal AA, Chong-Lopez A.. Allergic Aspergillus flavus rhinosinusitis: A case report from Qatar. Eur Arch Oto-Rhino-Laryngology. 2003; 260: 331–335. [DOI] [PubMed] [Google Scholar]
- 30. Salah H, Lackner M, Houbraken Jet al. The emergence of rare clinical Aspergillus species in Qatar: Molecular characterization and antifungal susceptibility profiles. Front Microbiol. 2019; 10. 10.3389/fmicb.2019.01677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Taj-Aldeen SJ, Chandra P, Denning DW.. Burden of fungal infections in Qatar. Mycoses. 2015; 58(Suppl 5): 51–57. [DOI] [PubMed] [Google Scholar]
- 32. El Deeb Y, Al Soub H, Almaslamani M, Al Khuwaiter J, Taj-Aldeen SJ. Post-traumatic cutaneous mucormycosis in an immunocompetent patient. Ann Saudi Med. 2005; 25: 343–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. El Zein S, El-Sheikh J, Zakhem Aet al. Mucormycosis in hospitalized patients at a tertiary care center in Lebanon: A case series. Infection. 2018; 46: 811–821. [DOI] [PubMed] [Google Scholar]
- 34. Venkatesh D, Dandagi S, Chandrappa P, Hema KN.. Mucormycosis in immunocompetent patient resulting in extensive maxillary sequestration. J Oral Maxillofac Pathol. 2018; 22: S112–S116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bellazreg F, Hattab Z, Meksi Set al. Outcome of mucormycosis after treatment: Report of five cases. New Microbes New Infect. 2015; 6: 49–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mandhan P, Hassan KO, Samaan SM, Ali MJ.. Visceral basidiobolomycosis: An overlooked infection in immunocompetent children. African J Paediatr Surg. 2015; 12: 193–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pezzani MD, Di Cristo V, Parravicini Cet al. Gastrointestinal basidiobolomycosis: An emerging mycosis difficult to diagnose but curable. Case report and review of the literature. Travel Med Infect Dis. 2019; 31: 101378. [DOI] [PubMed] [Google Scholar]
- 38. Taj-Aldeen SJ, Almaslamani M, Alkhalf Aet al. Cerebral phaeohyphomycosis due to Rhinocladiella mackenziei (formerly Ramichloridium mackenziei): A taxonomic update and review of the literature. Med Mycol. 2010; 48: 546–556. [DOI] [PubMed] [Google Scholar]
- 39. Al-Tawfiq JA, Boukhamseen A.. Cerebral phaeohyphomycosis due to Rhinocladiella mackenziei (formerly Ramichloridium mackenziei): Case presentation and literature review. J Infect Public Health. 2011; 4: 96–102. [DOI] [PubMed] [Google Scholar]
- 40. Kanj SS, Amr SS, Roberts GD.. Ramichloridium mackenziei brain abscess: Report of two cases and review of the literature. Med Mycol. 2001; 39: 97–102. [DOI] [PubMed] [Google Scholar]
- 41. Crous PW, Verkley GJM, Groenewald JZet al. , Fungal biodiversity. Westerdijk Laboratory Manual Series 1, (eds) 2019. Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands. [Google Scholar]
- 42. Ward E, Adams MJ.. Analysis of ribosomal DNA sequences of Polymyxa species and related fungi and the development of genus- and species-specific PCR primers. Mycol Res. 1998; 102: 965–974. [Google Scholar]
- 43. White TJ, Bruns T, Lee S, Taylor J.. Amplification and direct sequencing of fungal ribosomal rna genes for phylogenetics. In: PCR Protocols.; 1990: 315–322. 10.1016/b978-0-12-372180-8.50042-1. [DOI] [Google Scholar]
- 44. Sayers EW, Beck J, Bolton EEet al. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2021; 49: D10–D17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kumar S, Stecher G, Tamura K.. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016; 33: 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Taj-Aldeen SJ, Salah H, Al-Hatmi AMSet al. In vitro resistance of clinical Fusarium species to amphotericin B and voriconazole using the EUCAST antifungal susceptibility method. Diagn Microbiol Infect Dis. 2016; 85: 438–443. [DOI] [PubMed] [Google Scholar]
- 47. Chowdhary A, Meis JF, Guarro Jet al. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of systemic phaeohyphomycosis: Diseases caused by black fungi. Clin Microbiol Infect. 2014; 20: 47–75. [DOI] [PubMed] [Google Scholar]
- 48. Borjian Boroujeni Z, Shamsaei S, Yarahmadi Met al. Distribution of invasive fungal infections: Molecular epidemiology, etiology, clinical conditions, diagnosis and risk factors: A 3-year experience with 490 patients under intensive care. Microb Pathog. 2021; 152: 104616. [DOI] [PubMed] [Google Scholar]
- 49. Rees JR, Pinner RW, Hajjeh RA, Brandt ME, Reingold AL.. The epidemiological features of invasive mycotic infections in the San Francisco bay area, 1992-1993: Results of population-based laboratory active surveillance. Clin Infect Dis. 1998; 27: 1138–1150. [PubMed] [Google Scholar]
- 50. Ambrosioni J, Bouchuiguir-Wafa K, Garbino J.. Emerging invasive zygomycosis in a tertiary care center: Epidemiology and associated risk factors. Int J Infect Dis. 2010; 14 (Suppl 3): e100–e103. [DOI] [PubMed] [Google Scholar]
- 51. Saegeman V, Maertens J, Meersseman Wet al. Increasing incidence of mucormycosis in university hospital, Belgium. Emerg Infect Dis. 2010; 16: 1456–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Guinea J, Escribano P, Vena Aet al. Increasing incidence of mucormycosis in a large Spanish hospital from 2007 to 2015: Epidemiology and microbiological characterization of the isolates. PLoS One. 2017; 12: e0179136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Prakash H, Ghosh AK, Rudramurthy SMet al. A prospective multicenter study on mucormycosis in India: Epidemiology, diagnosis, and treatment. Med Mycol. 2019; 57: 395–402. [DOI] [PubMed] [Google Scholar]
- 54. Chakrabarti A, Das A, Mandal Jet al. The rising trend of invasive zygomycosis in patients with uncontrolled diabetes mellitus. Med Mycol. 2006; 44: 335–342. [DOI] [PubMed] [Google Scholar]
- 55. Chakrabarti A, Sakhuja V.. Ten years’ experience in zygomycosis at a tertiary care centre in India. J Infect. 2001; 42: 261–266. [DOI] [PubMed] [Google Scholar]
- 56. Lin E, Moua T, Limper AH.. Pulmonary mucormycosis: Clinical features and outcomes. Infection. 2017; 45: 443–448. [DOI] [PubMed] [Google Scholar]
- 57. Jeong W, Keighley C, Wolfe Ret al. The epidemiology and clinical manifestations of mucormycosis: A systematic review and meta-analysis of case reports. Clin Microbiol Infect. 2019; 25: 26–34. [DOI] [PubMed] [Google Scholar]
- 58. Chen M, Xu Y, Hong Net al. Epidemiology of fungal infections in China. Front Med. 2018; 12: 58–75. [DOI] [PubMed] [Google Scholar]
- 59. Skiada A, Pagano L, Groll Aet al. Zygomycosis in Europe: Analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin Microbiol Infect. 2011; 17: 1859–1867. [DOI] [PubMed] [Google Scholar]
- 60. Al-Hatmi AMS, Al-Shuhoumi MA, Denning DW. Estimated burden of fungal infections in Oman. J Fungi. 2021; 7: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wadi J, Denning DW.. Burden of serious fungal infections in Jordan. J Fungi. 2018; 4: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chekiri-Talbi M, Denning DW.. Burden of fungal infections in Algeria. Eur J Clin Microbiol Infect Dis. 2017; 36: 999–1004. [DOI] [PubMed] [Google Scholar]
- 63. Hedayati MT, Armaki MT, Charati JYet al. Burden of fungal infections in Iran. J Infect Dev Ctries. 2018; 12: 910–918. [DOI] [PubMed] [Google Scholar]
- 64. Esteghamati A, Etemad K, Koohpayehzadeh Jet al. Trends in the prevalence of diabetes and impaired fasting glucose in association with obesity in Iran: 2005-2011. Diabetes Res Clin Pract. 2014; 103: 319–327. [DOI] [PubMed] [Google Scholar]
- 65. Wong EH, Revankar SG.. Dematiaceous molds. Infect Dis Clin North Am. 2016; 30: 165–178. [DOI] [PubMed] [Google Scholar]
- 66. Arcobello JT, Revankar SG. Phaeohyphomycosis. Semin Respir Crit Care Med. 2020; 41: 131–140. [DOI] [PubMed] [Google Scholar]
- 67. Schieffelin JS, Garcia-Diaz JB, Loss GEet al. Phaeohyphomycosis fungal infections in solid organ transplant recipients: Clinical presentation, pathology, and treatment. Transpl Infect Dis. 2014; 16: 270–278. [DOI] [PubMed] [Google Scholar]
- 68. McCarty TP, Baddley JW, Walsh TJet al. Phaeohyphomycosis in transplant recipients: Results from the Transplant Associated Infection Surveillance Network (TRANSNET). Med Mycol. 2015; 53: 440–446. [DOI] [PubMed] [Google Scholar]
- 69. Dedwal A, Mudshingkar SS, Bhamare S, Kagal A, Karyakarte R.. Microbiological, clinical, and epidemiological profile of phaeohyphomycosis in a tertiary care hospital from Western India. Int J Infect Dis. 2020; 101: 397. [Google Scholar]
- 70. Suh MK. Phaeohyphomycosis in Korea. Japanese J Med Mycol. 2005; 46: 67–70. [DOI] [PubMed] [Google Scholar]
- 71. Revankar SG, Sutton DA, Rinaldi MG.. Primary Central nervous system phaeohyphomycosis: A review of 101 cases. Clin Infect Dis. 2004; 38: 206–216. [DOI] [PubMed] [Google Scholar]
- 72. Campbell CK, Al-Hedaithy SSA. Phaeohyphomycosis of the brain caused by Ramichloridium mackenziei sp. Nov. in middle eastern countries. Med Mycol. 1993; 31: 325–332. [Google Scholar]
- 73. Badali H, Chander J, Bansal Set al. First autochthonous case of Rhinocladiella mackenziei cerebral abscess outside the Middle East. J Clin Microbiol. 2010; 48: 646–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cristini A, Garcia-Hermoso D, Celard M, Albrand G, Lortholary O.. Cerebral phaeohyphomycosis caused by Rhinocladiella mackenziei in a woman native to Afghanistan. J Clin Microbiol. 2010; 48: 3451–3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jabeen K, Farooqi J, Zafar Aet al. Rhinocladiella mackenziei as an emerging cause of cerebral phaeohyphomycosis in Pakistan: A case series. Clin Infect Dis. 2011; 52: 213–217. [DOI] [PubMed] [Google Scholar]
- 76. Mudhigeti N, Patnayak R, Kalawat U, Yeddula SRC.. Subcutaneous Rhytidhysteron infection: A case report from South India with literature review. Cureus. 2018; 10. 10.7759/cureus.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chander J, Singla N, Kundu R, Handa U, Chowdhary A.. Phaeohyphomycosis caused by Rhytidhysteron rufulum and review of literature. Mycopathologia. 2017; 182: 403–407. [DOI] [PubMed] [Google Scholar]
- 78. Mahajan VK, Sharma V, Prabha Net al. A rare case of subcutaneous phaeohyphomycosis caused by a Rhytidhysteron species: A clinico-therapeutic experience. Int J Dermatol. 2014; 53: 1485–1489. [DOI] [PubMed] [Google Scholar]
- 79. Chowdhary A, Guarro J, Randhawa HSet al. A rare case of chromoblastomycosis in a renal transplant recipient caused by a non-sporulating species of Rhytidhysteron. Med Mycol. 2008; 46: 163–166. [DOI] [PubMed] [Google Scholar]
- 80. Braue JA, Larue RW, Boyd AS, Fine JD.. Phaeohyphomycosis caused by Rhytidhysteron rufulum. Clin Exp Dermatol. 2020; 45: 524–526. [DOI] [PubMed] [Google Scholar]
- 81. Wang XW, Houbraken J, Groenewald JZet al. Diversity and taxonomy of Chaetomium and Chaetomium-like fungi from indoor environments. Stud Mycol. 2016; 84: 145–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ahmed SA, Khan Z, Wang X weiet al. Chaetomium-like fungi causing opportunistic infections in humans: A possible role for extremotolerance. Fungal Divers. 2016; 76: 11–26. [Google Scholar]
- 83. Sun CQ, Lalitha P, Prajna NVet al. Association between in vitro susceptibility to natamycin and voriconazole and clinical outcomes in fungal keratitis. Ophthalmology. 2014; 121: 1495–1500.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Vinod Mootha V, Shahinpoor P, Sutton DAet al. Identification problems with sterile fungi, illustrated by a keratitis due to a non-sporulating Chaetomium-like species. Med Mycol. 2012; 50: 361–367. [DOI] [PubMed] [Google Scholar]
- 85. Mycobank . https://www.mycobank.org/page/Name details page/name/Dothichiza pimprina. Accessed April 11, 2022.
- 86. Moubasher AH, Turky Al-Subai AA. Soil fungi in State of Qatar. 1987. doi:10.3/JQUERY-UI.JS.
- 87. Al-Subai AAT. Air-borne fungi at Doha, Qatar. Aerobiologia (Bologna). 2002; 18: 175–183. [Google Scholar]
- 88. Fayad RK, Al-Thani RF, Al-Naemi FA, Abu-Dieyeh MH. Diversity, concentration and dynamics of culturable fungal bioaerosols at doha, qatar. Int J Environ Res Public Health. 2021; 18: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Boekhout T, Fotedar R, Kolecka Aet al. Fungal diversity in the Arabian Gulf surrounding Qatar: New species of yeasts and molds. 2019; 2016: EEPP2198. [Google Scholar]
- 90. Fotedar R, Kolecka A, Boekhout Tet al. Fungal diversity of the hypersaline Inland Sea in Qatar. Bot Mar. 2018; 61: 595–609. [Google Scholar]
- 91. Cordero RJB, Casadevall A.. Functions of fungal melanin beyond virulence. Fungal Biol Rev. 2017; 31: 99–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kejžar A, Gobec S, PlemenitaŠ A, Lenassi M. Melanin is crucial for growth of the black yeast Hortaea werneckii in its natural hypersaline environment. Fungal Biol. 2013; 117: 368–379. [DOI] [PubMed] [Google Scholar]
- 93. Fogarty R V., Tobin JM.. Fungal melanins and their interactions with metals. Enzyme Microb Technol. 1996; 19: 311–317. [DOI] [PubMed] [Google Scholar]
- 94. de Hoog GS, de Vries GA.. Two new species of Sporothrix and their relation to Blastobotrys nivea. Antonie van Leeuwenhoek Int J Gen Mol Microbiol. 1973; 39: 515–520. [DOI] [PubMed] [Google Scholar]
- 95. Sigler L, Harris JL, Dixon DMet al. Microbiology and potential virulence of Sporothrix cyanescens, a fungus rarely isolated from blood and skin. J Clin Microbiol. 1990; 28: 1009–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Tambini R, Farina C, Fiocchi Ret al. Possible pathogenic role for Sporothrix cyanescens isolated from a lung lesion in a heart transplant patient. J Med Vet Mycol. 1996; 34: 195–198. [PubMed] [Google Scholar]
- 97. Jackson L, Klotz SA, Normand RE.. A pseudoepidemic of Sporothrix cyanescens pneumonia occurring during renovation of a bronchoscopy suite. Med Mycol. 1990; 28: 455–459. [PubMed] [Google Scholar]
- 98. Fan X, Xiao M, Kong Fet al. A rare fungal species, Quambalaria cyanescens, isolated from a patient after augmentation mammoplasty - Environmental contaminant or pathogen? PLoS One. 2014; 9: e106949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kuan CS, Yew SM, Toh YFet al. Identification and characterization of a rare fungus, Quambalaria cyanescens, isolated from the peritoneal fluid of a patient after nocturnal intermittent peritoneal dialysis. PLoS One. 2015; 10: e0145932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Pérez-Cantero A, Guarro J.. Sarocladium and Acremonium infections: New faces of an old opportunistic fungus. Mycoses. 2020; 63: 1203–1214. [DOI] [PubMed] [Google Scholar]
- 101. Khan Z, Al-Obaid K, Ahmad Set al. Acremonium kiliense: Reappraisal of its clinical significance. J Clin Microbiol. 2011; 49: 2342–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Perdomo H, Sutton DA, García Det al. Spectrum of clinically relevant Acremonium species in the United States. J Clin Microbiol. 2011; 49: 243–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Dubey N, Capoor MR, Hasan ASet al. Epidemiological profile and spectrum of neglected tropical disease eumycetoma from Delhi, North India. Epidemiol Infect. 2019; 147: e294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Etienne KA, Roe CC, Smith RMet al. Whole-genome sequencing to determine origin of multinational outbreak of Sarocladium kiliense bloodstream infections. Emerg Infect Dis. 2016; 22: 476–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Bougnoux ME, Brun S, Zahar JR.. Healthcare-associated fungal outbreaks: New and uncommon species, new molecular tools for investigation and prevention. Antimicrob Resist Infect Control. 2018; 7. 10.1186/s13756-018-0338-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ioakimidou A, Vyzantiadis TA, Sakellari Iet al. An unusual cluster of Acremonium kiliense fungaemias in a haematopoietic cell transplantation unit. Diagn Microbiol Infect Dis. 2013; 75: 313–316. [DOI] [PubMed] [Google Scholar]
- 107. Sautour M, Chrétien ML, Valot Set al. First case of proven invasive pulmonary infection due to Trichoderma longibrachiatum in a neutropenic patient with acute leukemia. J Mycol Med. 2018; 28: 659–662. [DOI] [PubMed] [Google Scholar]
- 108. Sprute R, Salmanton-Garciá J, Sal Eet al. Characterization and outcome of invasive infections due to paecilomyces variotii: Analysis of patients from the FungiScope®registry and literature reports. J Antimicrob Chemother. 2021; 76: 765–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Feldman R, Cockerham L, Buchan BW, Lu Z, Huang AM.. Treatment of paecilomyces variotii pneumonia with posaconazole: Case report and literature review. Mycoses. 2016; 59: 746–750. [DOI] [PubMed] [Google Scholar]
- 110. Marques DP, Carvalho J, Rocha S, Domingos R. A case of pulmonary mycetoma caused by Paecilomyces variotii. Eur J Case Reports Intern Med. 2019; 6. 10.12890/2019_001040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Liu J, Freiberg FJ, Yeung SN, Iovieno A.. Late-onset recurrent Acremonium fungal keratitis after therapeutic penetrating keratoplasty. Can J Ophthalmol. 2021; 56: e135–e137. [DOI] [PubMed] [Google Scholar]
- 112. Wang MX, Shen DJ, Liu JCet al. Recurrent fungal keratitis and endophthalmitis. Cornea. 2000; 19: 558–560. [DOI] [PubMed] [Google Scholar]
- 113. Keynan Y, Sprecher H, Weber G.. Acremonium vertebral osteomyelitis: molecular diagnosis and response to voriconazole. Clin Infect Dis. 2007; 45: e5–e6. [DOI] [PubMed] [Google Scholar]
- 114. Khan S, Kumar A, Bhaskaran V, Chandran S, Dinesh K.. Chronic fungal osteomyelitis of the tibia due to Acremonium curvulum: A rare case. Pan Afr Med J. 2019; 34. 10.11604/pamj.2019.34.173.13737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Schell WA, Perfect JR.. Fatal, disseminated Acremonium strictum infection in a neutropenic host. J Clin Microbiol. 1996; 34: 1333–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Miyakis S, Velegraki A, Delikou Set al. Invasive Acremonium strictum infection in a bone marrow transplant recipient. Pediatr Infect Dis J. 2006; 25: 273–275. [DOI] [PubMed] [Google Scholar]
- 117. Novicki TJ, LaFe K, Bui Let al. Genetic diversity among clinical isolates of Acremonium strictum determined during an investigation of a fatal mycosis. J Clin Microbiol. 2003; 41: 2623–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Guitard J, Degulys A, Buot Get al. Acremonium sclerotigenum-Acremonium egyptiacum: A multi-resistant fungal pathogen complicating the course of aplastic anaemia. Clin Microbiol Infect. 2014; 20: O30–O32. [DOI] [PubMed] [Google Scholar]
- 119. Trupl J, Májek M, Mardiak J, Jesenská Z, Krcméry V.. Acremonium infection in two compromized patients. J Hosp Infect. 1993; 25: 299–301. [DOI] [PubMed] [Google Scholar]
- 120. Virgilio E, Mercantini P, Samra SA, Vitali M, Cavallini M.. Pleuritis caused by Acremonium strictum in a patient with metastatic testicular teratocarcinoma. Brazilian J Infect Dis. 2015; 19: 336–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Herbrecht R, Letscher-Bru V, Fohrer Cet al. Acremonium strictum pulmonary infection in a leukemic patient successfully treated with posaconazole after failure of amphotericin B. Eur J Clin Microbiol Infect Dis. 2002; 21: 814–817. [DOI] [PubMed] [Google Scholar]
- 122. Boltansky H, Kwon-Chung KJ, Macher AM, Gallin JI.. Acremonium strictum-related pulmonary infection in a patient with chronic granulomatous disease. J Infect Dis. 1984; 149: 653. [DOI] [PubMed] [Google Scholar]
- 123. Drouhet E, Martin L, Segretain G, Destombes P. Meningo-cerebral mycosis due to “Cephalosporium.” Presse Med. 1965; 73: 1809–1814. [PubMed] [Google Scholar]
- 124. Papadatos C, Pavlatou M, Alexiou D.. Cephalosporium meningitis. Pediatrics. 1969; 44: 749–751. [PubMed] [Google Scholar]
- 125. Medek S, Nemes A, Khoor Aet al. Acremonium strictum meningitis in prolonged steroid therapy. Orv Hetil. 1987; 128: 2529–2532. [PubMed] [Google Scholar]
- 126. Guarro J, del Palacio A, Gené J, Cano J, González CG.. A case of colonization of a prosthetic mitral valve by Acremonium strictum. Rev Iberoam Micol. 2009; 26: 146–148. [DOI] [PubMed] [Google Scholar]
- 127. Sharma A, Hazarika NK, Barua P, Shivaprakash MR, Chakrabarti A. Acremonium strictum: Report of a rare emerging agent of cutaneous hyalohyphomycosis with review of literatures. Mycopathologia. 2013; 176: 435–441. [DOI] [PubMed] [Google Scholar]
- 128. Hilmioglu S, Metin DY, Tasbakan Met al. Skin infection on both legs caused by Acremonium strictum (case report). Ann Saudi Med. 2015; 35: 406–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Erbagci Z, Tuncel AA, Erkilic S, Zer Y.. Successful treatment of antifungal- and cryotherapy-resistant subcutaneous hyalohyphomycosis in an immunocompetent case with topical 5% imiquimod cream. Mycopathologia. 2005; 159: 521–526. [DOI] [PubMed] [Google Scholar]
- 130. Anadolu R, Hilmioǧlu S, Oskay Tet al. Indolent Acremonium strictum infection in an immunocompetent patient. Int J Dermatol. 2001; 40: 451–453. [DOI] [PubMed] [Google Scholar]
- 131. Koc AN, Utas C, Oymak O, Sehmen E.. Peritonitis due to Acremonium strictum in a patient on continuous ambulatory peritoneal dialysis. Nephron. 1998; 79: 357–358. [DOI] [PubMed] [Google Scholar]
- 132. Gamze Sener A, Yucesoy M, Senturkun Set al. A case of Acremonium strictum peritonitis. Med Mycol. 2008; 46: 495–497. [DOI] [PubMed] [Google Scholar]
- 133. O'Bryan TA. Pseudallescheriasis in the 21st century. Expert Rev Anti Infect Ther. 2005; 3: 765–773. [DOI] [PubMed] [Google Scholar]
- 134. Wu Z, Ying H, Yiu S, Irvine J, Smith R.. Fungal keratitis caused by Scedosporium apiospermum: Report of two cases and review of treatment. Cornea. 2002; 21: 519–523. [DOI] [PubMed] [Google Scholar]
- 135. Díaz-Valle D, Del Castillo JMB, Amor Eet al. Severe keratomycosis secondary to Scedosporium apiospermum. Cornea. 2002; 21: 516–518. [DOI] [PubMed] [Google Scholar]
- 136. Sridhar MS, Garg P, Bansal AK, Sharma S.. Fungal keratitis after laser in situ keratomileusis. J Cataract Refract Surg. 2000; 26: 613–615. [DOI] [PubMed] [Google Scholar]
- 137. Tabatabaei SA, Tabatabaei M, Soleimani M, Tafti ZF.. Fungal keratitis caused by rare organisms. J Curr Ophthalmol. 2018; 30: 91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Ramakrishnan S, Mandlik K, Sathe Tet al. Ocular infections caused by Scedosporium apiospermum: A case series. Indian J Ophthalmol. 2018; 66: 137–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Kiratli H, Uzun Ö, Kiraz N, Eldem B.. Scedosporium apiospermum chorioretinitis. Acta Ophthalmol Scand. 2001; 79: 540–542. [DOI] [PubMed] [Google Scholar]
- 140. Levine NB, Kurokawa R, Fichtenbaum CJ, Howington JA, Kuntz C IV. An immunocompetent patient with primary Scedosporium apiospermum vertebral osteomyelitis. J Spinal Disord Tech. 2002; 15: 425–430. [DOI] [PubMed] [Google Scholar]
- 141. Farina C, Arosio M, Marchesi G, Amer M.. Scedosporium apiospermum post-traumatic cranial infection. Brain Inj. 2002; 16: 627–631. [DOI] [PubMed] [Google Scholar]
- 142. Sudke A, Shaikh S, Deopujari C, Sakle A. Scedosporium apiospermum: Rare cause of brain abscess in an immunocompetent patient. Neurol India. 2020; 68: 906–909. [DOI] [PubMed] [Google Scholar]
- 143. Canet JJ, Pagerols X, Sánchez C, Vives P, Garau J.. Lymphocutaneous syndrome due to Scedosporium apiospermum. Clin Microbiol Infect. 2001; 7: 648–650. [DOI] [PubMed] [Google Scholar]
- 144. Kiraz N, Guülbas Z, Akgün Y, Uzun Ö.. Lymphadenitis caused by Scedosporium apiospermum in an immunocompetent patient. Clin Infect Dis an Off Publ Infect Dis Soc Am. 2001; 32: E59–61. [DOI] [PubMed] [Google Scholar]
- 145. Tirado-Miranda R, Solera-Santos J, Brasero JCet al. Septic arthritis due to Scedosporium apiospermum: Case report and review. J Infect. 2001; 43: 210–212. [DOI] [PubMed] [Google Scholar]
- 146. Zhou N, Song Q, Zhou Let al. Suppurative arthritis induced by Scedosporium apiospermum and Mycobacterium fortuitum: A case report. Clin Lab. 2021; 67: 1303–1307. [DOI] [PubMed] [Google Scholar]
- 147. Serda Kantarcioglu A, Sybren de Hoog G, Guarro J. Clinical characteristics and epidemiology of pulmonary pseudallescheriasis. Rev Iberoam Micol. 2012; 29: 1–13. [DOI] [PubMed] [Google Scholar]
- 148. Ogata H, Harada E, Okamoto I.. Scedosporium apiospermum lung disease in a patient with nontuberculous mycobacteria. Respirol Case Reports. 2021; 9: e00691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Baptista PM, Monteiro RVS, Abreu AC, Gomes M, Snr MDCP.. Keratitis by Scopulariopsis brevicaulis fungus after lasik – a case report. Int Med Case Rep J. 2021; 14: 107–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Del Prete A, Sepe G, Ferrante Met al. Fungal keratitis due to Scopulariopsis brevicaulis in an eye previously suffering from herpetic keratitis. Ophthalmologica. 1994; 208: 333–335. [DOI] [PubMed] [Google Scholar]
- 151. Malecha MA. Fungal keratitis caused by Scopulariopsis brevicaulis treated successfully with Natamycin. Cornea. 2004; 23: 201–203. [DOI] [PubMed] [Google Scholar]
- 152. Kouyoumdjian GA, Forstot SL, Durairaj VD, Damiano RE.. Infectious keratitis after laser refractive surgery. Ophthalmology. 2001; 108: 1266–1268. [DOI] [PubMed] [Google Scholar]
- 153. Ragge NK, Dean Hart JC, Easty DL, Tyers AG. A case of fungal keratitis caused by Scopulariopsis brevicaulis: Treatment with antifungal agents and penetrating keratoplasty. Br J Ophthalmol. 1990; 74: 561–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Issakainen J, Salonen JH, Anttila VJet al. Deep, respiratory tract and ear infections caused by Pseudallescheria (Scedosporium) and Microascus (Scopulariopsis) in Finland. A 10-year retrospective multi-center study. Med Mycol. 2010; 48: 458–465. [DOI] [PubMed] [Google Scholar]
- 155. de Miguel-Martinez I, Hernandez-Cabrera PM, Armesto-Fernández MA, Martín-Sánchez AM. Necrotising otitis externa due to Scopulariopsis brevicaulis in a patient without predisposing factors. Enfermedades Infecc y Microbiol Clin (English ed). 2018; 36: 62–64. [DOI] [PubMed] [Google Scholar]
- 156. Gupta AK, Summerbell RC, Venkataraman M, Quinlan EM.. Nondermatophyte mould onychomycosis. J Eur Acad Dermatology Venereol. 2021; 35: 1628–1641. [DOI] [PubMed] [Google Scholar]
- 157. Sattler L, Sabou M, Ganeval-Stoll Aet al. Sinusitis caused by Scopulariopsis brevicaulis: Case report and review of the literature. Med Mycol Case Rep. 2014; 5: 24–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Corcino V, Beavin L, Lu S, Ross A, Sciortino C.. Coping with chronic fungal rhinosinusitis: Diagnosis to therapy. J Respir Infect. 2018; 2: 35–44. [Google Scholar]
- 159. Pérez-Cantero A, Guarro J.. Current knowledge on the etiology and epidemiology of Scopulariopsis infections. Med Mycol. 2020; 58: 145–155. [DOI] [PubMed] [Google Scholar]
- 160. Hoenigl M, Aspeck E, Valentin Tet al. Sinusitis and frontal brain abscess in a diabetic patient caused by the basidiomycete Schizophyllum commune: Case report and review of the literature. Mycoses. 2013; 56: 389–393. [DOI] [PubMed] [Google Scholar]
- 161. Ueyama M, Mizuno K, Hirose D, Kamei K, Ohta K.. A case of allergic fungal rhinosinusitis caused by Schizophyllum commune identified in both patient's nasal sputum and veranda's soil samples. J Infect Chemother. 2021; 27: 759–765. [DOI] [PubMed] [Google Scholar]
- 162. Narazaki T, Nakashima Y, Tsukamoto Yet al. Schizophyllum commune sinusitis after allogeneic bone marrow transplantation for myelodysplastic syndrome: A case report and literature review. Transpl Infect Dis. 2020; 22. 10.1111/tid.13205. [DOI] [PubMed] [Google Scholar]
- 163. Chowdhary A, Randhawa HS, Gaur SNet al. Schizophyllum commune as an emerging fungal pathogen: A review and report of two cases. Mycoses. 2013; 56: 1–10. [DOI] [PubMed] [Google Scholar]
- 164. Restrepo A, Greer DL, Robledo M, Osorio O, Mondragón H.. Ulceration of the palate caused by a basidiomycete Schizophyllum commune. Med Mycol. 1973; 11: 201–204. [PubMed] [Google Scholar]
- 165. Rihs JD, Padhye AA, Good CB.. Brain abscess caused by Schizophyllum commune: An emerging basidiomycete pathogen. J Clin Microbiol. 1996; 34: 1628–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Matos T, Tomazin R, Battelino S.. First report of otitis externa caused by Schizophyllum commune and review of the literature. Wien Klin Wochenschr. 2016; 128: 387–390. [DOI] [PubMed] [Google Scholar]
- 167. Maeda M, Maeda T, Nakamura A, Komatsu M.. A case of otitis externa caused by Schizophyllum commune: An approach to antimicrobial stewardship using gram staining of otorrhea in a medical clinic. J Infect Chemother. 2019; 25: 731–734. [DOI] [PubMed] [Google Scholar]
- 168. Chavez-Batista A, Maica J, Singer R.. Basidio-neuromycosis on man. An da Soc Biol Pernambuco. 1955; 13: 52–60. [Google Scholar]
- 169. Kim H, Yi Y, Cho SYet al. Pneumonia due to Schizophyllum commune in a patient with acute myeloid leukemia: Case report and literature review. Infect Chemother. 2021; 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Tian L, Mu Y, Zhang Het al. First report on cutaneous infectious granuloma caused by Schizophyllum commune. BMC Infect Dis. 2018; 18: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Kligman AM. A basidiomycete probably causing onychomycosis. J Invest Dermatol. 1950; 14: 67–70. [DOI] [PubMed] [Google Scholar]