Abstract
Background
Telepathology utilizing high-throughput static whole slide image scanners is proposed to address the challenge of limited pathology services in resource-restricted settings. However, the prohibitive equipment costs and sophisticated technologies coupled with large amounts of space to set up the devices make it impractical for use in resource-limited settings. Herein, we aimed to address this challenge by validating a portable whole slide imaging (WSI) device against glass slide microscopy (GSM) using lymph node biopsies from suspected lymphoma cases from Sub-Saharan Africa.
Material and methods
This was part of a multicenter prospective case–control head-to-head comparison study of liquid biopsy against conventional pathology. For the portable WSI scanner validation, the study pathologists evaluated 105 surgical lymph node specimens initially confirmed by gold-standard pathology between February and December 2021. The tissues were processed according to standard protocols for Hematoxylin and Eosin (H&E) and Immunohistochemistry (IHC) staining by well-trained histotechnicians, then digitalized the H& E and IHC slides at each center. The digital images were anonymized and uploaded to a HIPAA-compliant server by the histotechnicians. Three study pathologists independently accessed and reviewed the images after a 6-week washout. The agreement between diagnoses established on GSM and WSI across the pathologists was described and measured using Cohens’ kappa coefficient (κ).
Results
On GSM, 65.5% (n=84) of specimens were lymphoma; 25% were classified as benign, while 9.5% were metastatic. Morphological quality assessment on GSM and WSI established that 79.8% and 53.6% of cases were of high quality, respectively. When diagnoses by GSM were compared to WSI, the overall concordance for various diagnostic categories was 93%, 100%, and 86% for lymphoma, metastases, and benign conditions respectively. The sensitivity and specificity of WSI for the detection of lymphoma were 95.2% and 85.7%, respectively, with an overall inter-observer agreement (κ) of 0.86; 95% CI (0.70–0.95).
Conclusions
We demonstrate that mobile whole slide imaging (WSI) is non-inferior to conventional glass slide microscopy (GSM) for the primary diagnosis of malignant infiltration of lymph node specimens. Our results further provide proof of concept that mobile WSI can be adapted to resource-restricted settings for primary surgical pathology and would significantly improve patient outcomes.
Keywords: Validation, Portable whole slide imaging scanner, Lymphoma diagnosis, Resource-limited setting
Abbreviations: BL, Burkitt Lymphoma; CAP, College of American Pathologists; DLBCL, Diffuse Large B-cell Lymphoma; GSM, Glass slide microscopy; H&E, Hematoxylin and Eosin staining; HL, Hodgkin’s Lymphoma; IHC, Immunohistochemistry; LMICs, Low-and-middle income countries; NPV, Negative predictive value; PPV, Positive predictive value; WSI, Whole slide imaging
Highlights
-
•
The diagnosis of lymph node specimens is highly reproducible using simple scanner.
-
•
The device produces sufficiently high image quality.
-
•
The technology carries the potential for improving access to diagnostic services.
-
•
Challenges remain with the technical robustness of the device.
-
•
This will need to be addressed before this technology is considered for wider use.
Introduction
Despite the growing cancer burden, diagnostic cancer services are lacking and often of poor quality in low-middle-income countries (LMICs).1,2 High-quality pathology services providing timely and accurate cancer diagnostics are essential to improving patient outcomes in sub-Saharan Africa. However, many patients with suspected cancer currently do not have access to diagnostics; thus, they die at home or present to the hospital with advanced poor prognosis disease.3,4
A potential solution to the scarcity of pathology services in resource-limited settings in LMICs is using digital pathology technology. Snapshots of tissue biopsies taken directly from the microscope using mobile phone technology are used but require an experienced pathologist to take a representative picture. This problem has been partly addressed by using high-throughput static whole slide imaging (WSI) scanners that produce high-quality digital imagesenabling primary pathology diagnosis remotely.5,6 However, high costs and complex, sophisticated working environments are among other fundamental problems limiting the broader use of static WSI scanners in resource-limited settings.7
A potential solution would be the adoption of portable WSI scanners because they are simple to use, affordable and have minimum electricity requirements making them ideal for largely rural African settings. Moreover, mobile WSI scanners can be transported easily to peripheral healthcare centers to acquire tissue images and back to bigger centers for maintenance purposes.8
Herein, we aimed to address the issue of pathology shortage by performing validation of such a mobile WSI device (Alexapath) against glass slide microscopy (GSM) in line with the College of American Pathologists (CAP) guidelines for clinical evaluation of digital imaging tools.9 We used surgical lymph node biopsies from suspected lymphoma patients enrolled in the main study, Aggressive Infection-Related East African Lymphoma (AI-REAL).
Material and methods
Study design and study setting
This was part of a multicenter prospective case–control head-to-head comparison study of liquid biopsy against conventional pathology. The detailed design and study setting are described elsewhere.10 For the portable WSI scanner validation, study pathologists evaluated 105 initially confirmed by gold standard pathology between February 2021 and December 2021. The sites included Kilimanjaro Christian Medical Centre (KCMC), Muhimbili National Hospital (MNH) in Tanzania, and St. Mary’s Hospital Lacor, Gulu, Uganda.
Study population, tissue preparation and histopathological gold-standard assessment
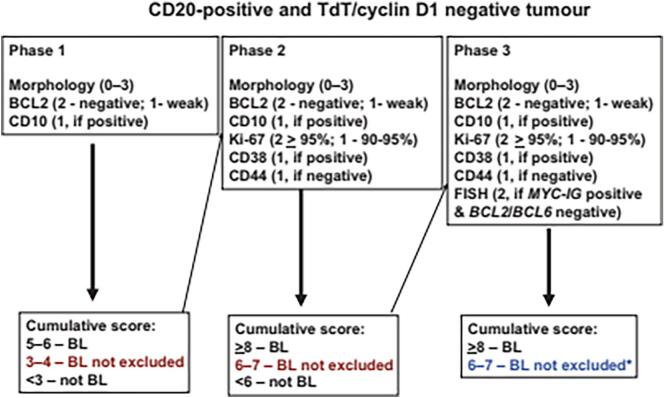
The diagnostic archives of the pathology departments of the 3 participating sites were searched to identify 105 consecutive hematopathology cases. Formalin-fixed and paraffin-embedded (FFPE) tissue blocks of the identified cases were previously processed according to standard study protocol.10 Specialist pathological assessment included a full immunohistochemistry (IHC) panel performed on the automated Roche Ventana Benchmark platform (Ventana Medical Systems Inc, USA). For suspected Burkitt’slymphoma (BL) cases, c-MYC staining was performed in addition to using the Naresh algorithm Fig. 2.11 For Hodgkin’s Lymphoma (HL), the diagnosis included the presence of Reed-Sternberg cells expressing CD30 and CD15, occasional CD20 positivity, but the absence of CD45 by IHC or as described in the study protocol.10
Fig. 2.
Diagnostic algorithm proposed by Naresh et al, (2011)11 to be used for the differential diagnosis of aggressive B-cell lymphomas in limited-resource settings with a high incidence of BL.
Training and quality control for pathology technicians and clinical pathologists
It is known that conventional telepathology often fails due to the inferior quality of tissue preparation and inadequate selection of images. All laboratory staff, therefore, followed prescribed standard operating procedures (SOPs) and kept log sheets for each study sample detailing the laboratory procedures performed on the samples in line with IsoStandard 15189 for clinical laboratory accreditation. Before the start of the study, a 5-day training pathology workshop was held to cover all technical aspects of tissue fixation, paraffin embedding, section cutting, and staining. Alexapath provided training in the practical aspects of using mobile WSI technology. Besides, the course included a refresher section focussing on the assessment of slide quality and the histopathological diagnosis and differential diagnosis of aggressive lymphoma. The 3 reporting pathologists and all laboratory scientists underwent a competency assessment according to IsoStandard 15189 at the end of the course.
Study procedure and quality assessment
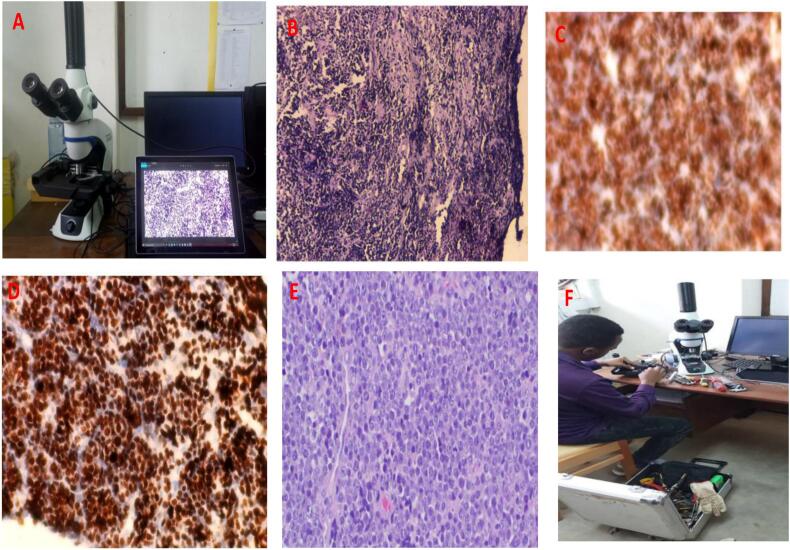
The 3 study pathologists reviewed the H&E-stained glass slides alongside IHC slides conventionally (GSM), using Olympus CX23, Japan, microscope, and independently reported their findings. The slides were then scanned by a well-trained histotechnician using Alexapath® ADA mobile scanner (200 X original magnification).12 Alexapath provided the scanners and image analysis software to each participating site (Fig. 3A). The digital images were anonymized and uploaded to a—USHealth Insurance Portability and Accountability Act (HIPAA)-compliant cloud server by the histotechnicians. Each scanned case was accompanied by relevant anonymized clinical information of the participant. After a washout period of 6 weeks of the GSM reading, each pathologist independently reviewed the scanned slides on a 24-inch monitor (Dell, Round Rock, Texas) in a blinded fashion.
Fig. 3.
A: Photograph of Alexapath mobile scanner workstation. B: Photomicroscopy of H&E stained biopsy highlighting sub-optimal quality (non-diagnostic); 100x original magnification. C: IHC stained Non-Hodgkin lymphoma demonstrating sub-optimal quality, 100x original magnification. D: IHC-stained BL demonstrating optimal quality with nearly 100% immunostaining with Ki-67, 100x original magnification. E: H&E-stained DLBCL displaying optimal quality photomicroscopy, 200x original magnification. F: A photograph showing a local biomedical engineer trying to fix a technical problem with the Alexapath scanner.
Prior to the assessment, the pathologists agreed on a 3-point quality assessment scale for GSM and digital images generated by WSI consisting of high, intermediate, and low-quality morphology based on tissue preservation and processing (i.e. fixation, embedding, staining).13, 14, 15
Cases with inadequate specimens or low-quality sections were excluded from the analysis. The 3 pathologists reviewed cases with discrepant results on GSM, and only cases for which a final consensus diagnosis could be established were included in the validation.
Data analysis
Results were categorized into broad assessments: “lymphoma (any)”, “reactive/infection condition”, or “metastatic disease”. The categories were expressed as absolute numbers and percentages. The WSI and GSM findings were described and compared to each other and summarized in a matrix with GSM as the gold-standard to establish sensitivity, specificity and PPV of mobile WSI for the diagnosis of malignancy versus reactive/benign conditions. The agreement between arbitrary pairs of observers (inter-observer agreement) was measured by kappa statistics (κ). Kappa is an index of agreement over and above that which is expected by chance alone and is scored as a number between 0 and 1. A nomenclature recommended by Landis-Koch was adopted for interpreting the strength of agreement (κ); values >0.75 are regarded as excellent agreement beyond chance, values between 0.40 and 0.75 as fair to a good agreement beyond chance and values <0.40 as poor agreement beyond chance.16 To test the quality of response rates from glass slides and scanned image diagnoses, McNemar’s test was used. An effect was considered statistically significant if the p-value of its corresponding test statistic was 5% (p <.05). We also documented our experiences and challenges encountered with the mobile scanner.
The X2 or Fisher exact test was used to compare the variables. SPSS statistical program version 20 (Chicago, Illinois, USA) was used for statistical analysis.
Results
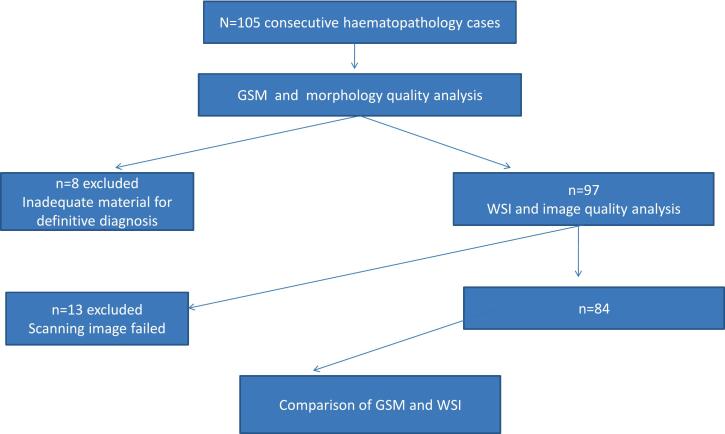
A total of 105 consecutive cases were retrieved. Of these, 21 were excluded because of inadequate or poor-quality material for definitive diagnosis (n=8) or because scanning of images had failed (n=13). Thus, 84 cases were included in this analysis. Table 1 displays the baseline and clinicopathological characteristics of the study subjects.
Table 1.
Baseline characteristics of the study subjects.
| Variable | N=84 | % |
|---|---|---|
| Age (years) | ||
| ≤15 | 31 | 36.9 |
| 16–49 | 31 | 36.9 |
| ≥50 | 22 | 26.2 |
| Sex | ||
| Male | 48 | 57.1 |
| Female | 36 | 42.9 |
| Signs/symptoms | ||
| Isolated lymphadenopathy | 38 | 45.2 |
| Generalized lymphadenopathy | 46 | 54.8 |
| Fever | 47 | 56.0 |
| Weight loss | 55 | 65.5 |
| Cough/Night sweats | 34 | 40.7 |
| Tumour site | ||
| Jaw | 13 | 15.5 |
| Abdomen | 14 | 16.7 |
| Neck | 28 | 33.3 |
| Axillar | 7 | 8.3 |
| Inguinal | 14 | 16.7 |
| Others | 8 | 9.5 |
| Duration of illness (weeks) | ||
| ≤4 | 32 | 38.1 |
| ≥5 | 52 | 61.9 |
| Documented HIV status | ||
| Positive | 6 | 7.1 |
| Negative | 38 | 45.2 |
| Missing data | 40 | 47.6 |
| Total | 84 | 100.0 |
Fig. 1 highlights the schematic flow chart of the study participants. The majority were aged 16 years or above (63.1%), were males (57.1%), presented with generalized lymphadenopathy (54.8%), fever (56%), weight loss (65.5%), cough/night sweats (40.7%); or neck mass (33.3%). Sixty-two per cent of subjects had more than 5 weeks of presenting illness. Of 44 cases with known HIV status, 7.1% were HIV-infected (Table 1).
Fig. 1.
Schematic flow chart of the study participants.
Of the 84 cases evaluated using GSM, 55, representing 65.5%, were confirmed as lymphoma. The rest were benign conditions (i.e., inflammatory, infectious, or reactive lymphadenitis, not otherwise specified; n=21; 25%), while 8 (9.5%) were established as metastatic diseases. Of the lymphomas, high-grade non-Hodgkin lymphoma, including Diffuse Large B-cell Lymphoma (DLBCL) and BL, were the most common. The remaining cases were labelled as Hodgkin’s lymphoma and other aggressive and indolent lymphomas. The results were reproduced by WSI as lymphoma (n=55; 67.9%) and benign conditions (n=21; 25.0%), while 6 (7.1%) were metastases (Table 2).
Table 2.
Histopathology evaluation of study cases by GSM or WSI.
| N (%) | % | |
|---|---|---|
| GSM | ||
| Lymphoma (any) | 55 | 65.5 |
| Inflammatory/Infectious/Reactive | 21 | 25 |
| Metastatic disease | 8 | 9.5 |
| Total | 84 | 100 |
| WSI | ||
| Lymphoma (any) | 57 | 67.9 |
| Inflammatory/Infectious/Reactive | 21 | 25 |
| Metastatic disease | 6 | 7.1 |
| Total | 84 | 100 |
Quality assessment of morphology performed by GSM showed that the majority of the cases (n=6; 79.8%) were of high quality, 13 (15.4%) were of intermediate quality, and 4 (4.8%) were of low quality (Table 3).
Table 3.
Quality assessment of the study cases by GSM or WSI.
| N | % | |
|---|---|---|
| Quality score of GSM | ||
| Low quality | 4 | 4.8 |
| Intermediate quality | 13 | 15.4 |
| High quality | 67 | 79.8 |
| Total | 84 | 100 |
| Quality score of WSI | ||
| Low quality | 12 | 14.3 |
| Intermediate quality | 27 | 32.1 |
| High quality | 45 | 53.6 |
| Total | 84 | 100 |
On the other hand, an assessment of WSI microscopy established that only about half of the cases (n=45; 53.6%) were of high quality, 27 (32.1%) were of intermediate quality, while 12 (14.3%) were of low quality (Table 3; Fig. 3).
Agreement between conventional GSM and WSI
The overall agreement of diagnoses established by the 3 study pathologists using GSM versus WSI for the principle diagnostic categories was 93%, 100%, and 86% for lymphoma, metastases, and reactive disease conditions respectively (Table 4).
Table 4.
Overall agreement of diagnoses between conventional GSM and WSI.
| GSM |
||||||
|---|---|---|---|---|---|---|
| Lymphoma | Metastatic | Reactive | Total | Concordance | ||
| WSI | Lymphoma | 53 | 1 | 3 | 57 | 93% |
| Metastatic | 0 | 6 | 0 | 6 | 100% | |
| Reactive | 2 | 1 | 18 | 21 | 86% | |
| Total | 55 | 8 | 21 | 84 | ||
ƙ = 0.83; 95 CI (0.67–0.93).
Compared to GSM, the overall sensitivity and specificity of WSI for the detection of malignancy was 60/63 (95.2%); CI (87–99) and 18/21 (85.7%); CI (64–97), respectively. Similarly, the proportion of individuals with a positive malignant result who had the disease (PPV) was 60/63 (95.2%). On the other hand, the proportion of individuals with negative malignant results who had no disease (NPV) was 18/21 (85.7%), Table 5.
Table 5.
Sensitivity, specificity, PPV, and NPV of WSI in the diagnosis of malignancy using GSM as the gold-standard.
| GSM |
|||||
|---|---|---|---|---|---|
| Malignant | Reactive | Total | Concordance | ||
| WSI | Malignant | 60 | 3 | 63 | 95% |
| Reactive | 3 | 18 | 21 | 86% | |
| Total | 63 | 21 | 84 | ||
ƙ= 0.86; 95% CI (0.7–0.95).
Discussion
We performed a clinical validation study across 2 African countries and 3 study sites to assess whether conventional GSM of H&E-stained surgical biopsies can accurately be reproduced by mobile WSI microscopy in a limited resource setting according to CAP guidelines.9 Evaluation of digital imaging systems' performance is recommended before their clinical application. Here, we established that mobile WSI (Alexapath) shows a non-inferior performance against conventional glass slide microscopy (GSM).
The severe shortage of pathology services in LMICs as one of the major bottlenecks for the successful implementation of cancer services has been well documented.2 For instance, in 2012, there were only 15 pathologists in Tanzania,3,17 and these pathologists are often placed in large urban facilities, thus depriving peripheral cancer centers of pathology services. It is not uncommon in such settings; surgical specimens are transported several hundred miles away to large urban facilities or even overseas laboratories for reporting. Consequently, diagnostic turnaround times are prolonged, and patients die before a diagnosis is established in the worst scenarios. Therefore, improving pathology services in peripheral cancer centers will improve patient outcomes and guarantee the quality of cancer registry data, including that of the WHO.18,19 Therefore, whole slide imaging with affordable portable devices and technologies has the potential to revolutionize pathology practice in resource-restricted settings without the need for specialist pathologists on site.
In our study, the overall concordance between GSM and mobile WSI for the diagnostic categories was 93%, 100%, and 86% for lymphoma, metastases, and benign conditions respectively, and is similar to validation studies for other digital pathology devices, both in resource-rich and resource-limited settings.20, 21, 22, 23 These studies reported good feasibility of using static WSI and found scanned images to be good quality. However, the scanner cost and internet speed were found to be limiting factors. Unlike the former studies, that involved a broad spectrum of specimens and organ systems, our study focused on lymph-node specimens. The low discrepancy rates observed are within the acceptable inter-observer variability range in surgical pathology practice.8,9 Our study findings imply that the Alexapath mobile WSI directly addresses the shortage of pathologists in sub-Saharan Africa by assisting local healthcare centers to confidently exclude a diagnosis of malignant lymph-node infiltration, thereby enabling local clinicians to reassure patients without the need for tissue blocks or even the patients themselves having to travel to centers of excellence.
However, many issues must be resolved before mobile WSI can be routinely implemented.
Of the initial selection of 105 cases, 21 cases were excluded from the validation study due to poor quality or quantity by GSM. Optimal morphology quality is essential for histopathological examination, irrespective of GSM or digital imaging. The quality of tissue sections can be affected by several alterations of morphological and cytological features that occur as a result of artifacts. These artifacts may occur during surgical removal, fixation, tissue processing, embedding and microtomy, staining, and mounting.24, 25, 26 Therefore, training in the best practice of tissue processing and quality control is critical for successfully adopting and implementing digital pathology.
In addition to the factors determining the quality of tissue sections that are inherent to the processing of surgically resected specimens anywhere in the world, several technical issues relating specifically to mobile WSI scanners, including that designed by Alexapath, were encountered, which may negatively affect the image quality of the scanned images. For instance, during the implementation of the study, the scanners quickly broke down (Fig. 3F). Besides, due to the absence of a z-plane, the focus of the image kept changing, especially for the thick section, while the slide was going through the scanning process. This caused uneven focus in different parts of the slide and explained the increased proportion of WSI cases with low-quality scores compared to GSM.
According to CAP, validation of the entire WSI system by trained pathologists should be performed in a manner that emulates the laboratory’s actual clinical environment. CAP recommends including at least 60 routine cases per application to assess intra-observer diagnostic concordance between digitized and glass slides viewed at least 2 weeks apart.9 The cases selected should reflect the spectrum and complexity of specimen types and diagnoses likely to be encountered during routine practice. The validation should also include all software and hardware used for imaging processing, sharing, and storage. However, these were not fully developed for the Alexapath device at the time of the study. Similarly, sustainability factors such as running costs, data security, sustainable internet connectivity, and user acceptance, and continuous training of pathologists in principles and limitations of digital pathology, are critical to the success of digital pathology implementation programs.27,28,29
Conclusions
Mobile whole slide imaging (WSI) using the Alexapath device produces sufficiently high-quality images to rapidly assess pathological sections in remote areas and differentiate between reactive changes, metastatic disease, and lymphomas. Our results imply that this simple technology can improve access to diagnostic services and patient outcomes in resource-limited settings. However, challenges remain with the technical robustness of the device and the software and hardware support for sharing and storing images. These will need to be addressed prior to the field application of this technology.
Funding
This work was supported by the NIHR/Oxford University [grant number NIHR200133]. However, the funders had no role in the study design, data collection and analysis, publication decision, or manuscript preparation.
Author contributions
All authors made substantial contributions to the paper. AM, CA, and LM conceived and designed this study. AM, CA, DM, EM, IDL, and LM were involved in collecting data. DV and AM performed data analysis. AM drafted the original manuscript version. IDL, CEM, and AS critically reviewed the manuscript. All authors read and approved the final manuscript. AM accepts to be the guarantor of this work.
Data availability statement
All data on which conclusions of this study are drawn are contained in the main manuscript.
Ethics
Ethics approval for the study was obtained from the Oxford Tropical Research Ethics Committee (OxTREC Ref: 15-19). National Institute of Medical Research (NIMR) in Tanzania (NIMR Reg No. NIMR/HQ/R.8a/Vol.IX/3408), Uganda National Council of Science and Technology (UNCST Reg No. HS529ES), and Lacor Hospital Institutional Research Ethics Committee. Initial REC approval was given on the 6th of February 2019 (protocol V3.1), and the current protocol V3.3 was approved on the 4th of April 2021 via substantial amendment.
Patient consent for publication
Not applicable.
Conflict of interests
None declared.
Acknowledgments
The authors would like to acknowledge the invaluable support from the study team members of the AI-REAL consortium (see the list), Dr Edward Wilson (Oxford University Hospital Trust), ShishirMalav and Augustine Louis (both Alexapath) for supporting this study.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. Epub 2021 Feb 4. PMID: 33538338. [DOI] [PubMed] [Google Scholar]
- 2.Kaschula R.O. The practice of pathology in Africa. Arch Pathol Lab Med. 2013 Jun;137(6):752–755. doi: 10.5858/arpa.2011-0587-ED. PMID:23721269. [DOI] [PubMed] [Google Scholar]
- 3.Rambau Peter F. Pathology Practice in a Resource-Poor Setting, Mwanza, Tanzania. Arch Pathol Lab Med. 2011;135:191–193. doi: 10.5858/135.2.191. PMID: 21284436. [DOI] [PubMed] [Google Scholar]
- 4.Wilson M.L., Fleming K.A., Kuti M.A., Looi L.M., Lago N., Ru K. Access to pathology and laboratory medicine services: a crucial gap. Lancet. 2018 May 12;391(10133):1927–1938. doi: 10.1016/S0140-6736(18)30458-6. Epub 2018 Mar 15. PMID: 29550029. [DOI] [PubMed] [Google Scholar]
- 5.Hitchcock C.L. The future of telepathology for the developing world. Arch Pathol Lab Med. 2011;135:211–214. doi: 10.5858/135.2.211. [DOI] [PubMed] [Google Scholar]
- 6.Montgomery N.D., Tomoka T., Krysiak R., Powers E., Mulenga M., Kampani C., et al. Practical Successes in Telepathology Experiences in Africa. Clin Lab Med. 2018 Mar;38(1):141–150. doi: 10.1016/j.cll.2017.10.011. Epub 2017 Dec 6. PMID: 29412878; PMCID: PMC5996143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pantanowitz L., Farahani N., Parwani A. Whole slide imaging in pathology: advantages, limitations, and emerging perspectives. Pathol Lab Med Int. 2015;7:23. [Google Scholar]
- 8.Kaushal R.K., Rajaganesan S., Rao V., Sali A., More B., Desai S.B. Validation of a portable whole-slide imaging system for frozen section diagnosis. J Pathol Inform. 2021 Sep 16;12:33. doi: 10.4103/jpi.jpi_95_20. PMID: 34760330; PMCID: PMC8529342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pantanowitz L., Sinard J.H., Henricks W.H., Fatheree L.A., Carter A.B., Contis L., et al. Validating whole slide imaging for diagnostic purposes in pathology: guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Arch Pathol Lab Med. 2013 Dec;137(12):1710–1722. doi: 10.5858/arpa.2013-0093-CP. Epub 2013 May 1. PMID: 23634907; PMCID: PMC7240346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Legason I.D., Ogwang M.D., Chamba C., Mkwizu E., El Mouden C., Mwinula H., et al. A protocol to clinically evaluate liquid biopsies as a tool to speed up diagnosis of children and young adults with aggressive infection-related lymphoma in East Africa "(AI-REAL)". BMC Cancer. 2022 May 2;22(1):484. doi: 10.1186/s12885-022-09553-w. PMID: 35501771; PMCID: PMC9059110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bancroft J.D., Gamble M. 6th ed. Churchill Livingstone, Elsevier Health Sciences; Philadelphia: 2008. Theory and Practice of Histological Technique; pp. 53–105. [Google Scholar]
- 12.Naresh K.N., Ibrahim H.A., Lazzi S., Rince P., Onorati M., Ambrosio M.R., et al. Diagnosis of Burkitt lymphoma using an algorithmic approach--applicable in both resource-poor and resource-rich countries. Br J Haematol. 2011 Sep;154(6):770–776. doi: 10.1111/j.1365-2141.2011.08771.x. Epub 2011 Jul 1. PMID: 21718280. [DOI] [PubMed] [Google Scholar]
- 13.https://beta.alexapath.com
- 14.Cross S.S. Grading and scoring in histopathology. Histopathology. 1998 Aug;33(2):99–106. doi: 10.1046/j.1365-2559.1998.00495.x. PMID: 9762541. [DOI] [PubMed] [Google Scholar]
- 15.Meyerholz D.K., Tintle N.L., Beck A.P. Common pitfalls in analysis of tissue scores. Vet Pathol. 2019 Jan;56(1):39–42. doi: 10.1177/0300985818794250. Epub 2018 Aug 21. PMID: 30131009. [DOI] [PubMed] [Google Scholar]
- 16.Thrall M.J., Wimmer J.L., Schwartz M.R. Validation of multiple whole slide imaging scanners based on the guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Arch Pathol Lab Med. 2015 May;139(5):656–664. doi: 10.5858/arpa.2014-0073-OA. PMID: 25927149. [DOI] [PubMed] [Google Scholar]
- 17.Landis J.R., Koch G.G. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 18.African Pathologists’ Summit Working Groups Proceedings of the African Pathologists Summit; March 22-23, 2013; Dakar, Senegal: a summary. Arch Pathol Lab Med. 2015 Jan;139(1):126–132. doi: 10.5858/arpa.2013-0732-CC. PMID: 24963539. [DOI] [PubMed] [Google Scholar]
- 19.Naresh K.N., Raphael M., Ayers L., Hurwitz N., Calbi V., Rogena E., et al. Lymphomas in sub-Saharan Africa--what can we learn and how can we help in improving diagnosis, managing patients and fostering translational research? Br J Haematol. 2011 Sep;154(6):696–703. doi: 10.1111/j.1365-2141.2011.08772.x. Epub 2011 Jun 28. PMID: 21707579; PMCID: PMC4207091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adesina A., Chumba D., Nelson A.M., Orem J., Roberts D.J., Wabinga H., et al. Improvement of pathology in sub-Saharan Africa. Lancet Oncol. 2013 Apr;14(4):e152–e157. doi: 10.1016/S1470-2045(12)70598-3. PMID: 23561746. [DOI] [PubMed] [Google Scholar]
- 21.Mpunga T., Hedt-Gauthier B.L., Tapela N., Nshimiyimana I., Muvugabigwi G., Pritchett N., et al. Implementation and validation of telepathology triage at cancer referral center in rural rwanda. J Glob Oncol. 2016 Jan 20;2(2):76–82. doi: 10.1200/JGO.2015.002162. PMID: 28717686; PMCID: PMC5495446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaushal R.K., Rajaganesan S., Rao V., Sali A., More B., Desai S.B. Validation of a portable whole-slide imaging system for frozen section diagnosis. J Pathol Inform. 2021 Sep 16;12:33. doi: 10.4103/jpi.jpi_95_20. PMID: 34760330; PMCID: PMC8529342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wamala D., Katamba A., Dworak O. Feasibility and diagnostic accuracy of Internet-based dynamic telepathology between Uganda and Germany. J Telemed Telecare. 2011;17(5):222–225. doi: 10.1258/jtt.2010.100609. Epub 2011 May 12. PMID: 21565844. [DOI] [PubMed] [Google Scholar]
- 24.Mremi A., Bentzer N.K., Mchome B., Mlay J., Blaakær J., Rasch V., et al. The role of telepathology in diagnosis of pre-malignant and malignant cervical lesions: Implementation at a tertiary hospital in Northern Tanzania. PLoS One. 2022 Apr 14;17(4) doi: 10.1371/journal.pone.0266649. PMID: 35421156; PMCID: PMC9009664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chatterjee S. Artefacts in histopathology. J Oral MaxillofacPathol. 2014;18:S111–S116. doi: 10.4103/0973-029X.141346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rastogi V., Puri N., Arora S., Kaur G., Yadav L., Sharma R. Artefacts: a diagnostic dilemma - a review. J Clin Diagn Res. 2013;7(10):2408–2413. doi: 10.7860/JCDR/2013/6170.3541. Epub 2013 Oct 5. PMID: 24298546; PMCID: PMC3843421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bindhu P., Krishnapillai R., Thomas P., Jayanthi P. Facts in artifacts. J Oral Maxillofac Pathol. 2013;17:397–401. doi: 10.4103/0973-029X.125206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madabhushi A., Lee G. Image analysis and machine learning in digital pathology: challenges and opportunities. Med Image Anal. 2016 Oct;33:170–175. doi: 10.1016/j.media.2016.06.037. Epub 2016 Jul 4.PMID: 27423409; PMCID: PMC5556681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lundin J., Dumont G. Medical mobile technologies - what is needed for a sustainable and scalable implementation on a global scale? Glob Health Action. 2017;10 doi: 10.1080/16549716.2017.1344046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data on which conclusions of this study are drawn are contained in the main manuscript.