Abstract
Background
Autism spectrum disorder (ASD) is a neurodevelopmental disorder diagnosed based on social impairment, restricted interests, and repetitive behaviors. Contemporary theories posit that cerebellar pathology contributes causally to ASD by disrupting error-based learning (EBL) during infancy. The present study represents the first test of this theory in a prospective infant sample, with potential implications for ASD detection.
Methods
Data from the Infant Brain Imaging Study (n= 94, 68 male) were used to examine 6-month cerebellar functional connectivity magnetic resonance imaging in relation to later (12/24-month) ASD-associated behaviors and outcomes. Hypothesis-driven univariate analyses and machine learning–based predictive tests examined cerebellar–frontoparietal network (FPN; subserves error signaling in support of EBL) and cerebellar–default mode network (DMN; broadly implicated in ASD) connections. Cerebellar-FPN functional connectivity was used as a proxy for EBL, and cerebellar-DMN functional connectivity provided a comparative foil. Data-driven functional connectivity magnetic resonance imaging enrichment examined brain-wide behavioral associations, with post hoc tests of cerebellar connections.
Results
Cerebellar-FPN and cerebellar-DMN connections did not demonstrate associations with ASD. Functional connectivity magnetic resonance imaging enrichment identified 6-month correlates of later ASD-associated behaviors in networks of a priori interest (FPN, DMN), as well as in cingulo-opercular (also implicated in error signaling) and medial visual networks. Post hoc tests did not suggest a role for cerebellar connections.
Conclusions
We failed to identify cerebellar functional connectivity–based contributions to ASD. However, we observed prospective correlates of ASD-associated behaviors in networks that support EBL. Future studies may replicate and extend network-level positive results, and tests of the cerebellum may investigate brain-behavior associations at different developmental stages and/or using different neuroimaging modalities.
Keywords: Autism, Cerebellum, Development, Error-based learning, Functional connectivity, Infancy
Autism spectrum disorder (ASD) is a heterogeneous condition diagnosed on the basis of social impairment, restricted interests, and repetitive behaviors (1). The characteristic behavioral features of ASD generally emerge around 12 months of age (2), and ASD diagnoses have largely stabilized by 24 months of age (3). To account for postnatal developments, contemporary theories posit that cerebellar connectivity contributes to the causation of ASD by disrupting error-based learning (EBL) during infancy (4, 5, 6). EBL describes an iterative process whereby expectancy violations are interpreted against the backdrop of prior experiences to optimize prediction and inform future behaviors (7,8). During infancy, expectancy violations enhance object learning and encourage object exploration (9). As reviewed below, there is evidence implicating cerebellar connectivity in EBL (10, 11, 12), cerebellar pathology in ASD (13, 14, 15), and EBL impairment in ASD (16,17). However, cerebellar functional connectivity has not been examined in relation to ASD during infancy. Such studies are necessary to evaluate cerebellar functional connectivity as a presymptomatic risk biomarker for ASD (18).
Prospective studies of infants at high familial risk for ASD (19,20) are at the vanguard of presymptomatic risk biomarker research. These studies have identified multiple aspects of brain structure and function that correlate with behavioral variation relevant to ASD (21, 22, 23) and predict ASD diagnosis (24, 25, 26). Notably, markers of risk during infancy (e.g., increased corpus callosum volume) may be transient (27). Contextualizing presymptomatic risk biomarkers within a developmental framework that accounts for experience-dependent behavioral variation holds promise to identify modifiable pathways that may be amenable to intervention (28). To this end, the present study investigated cerebellar functional connectivity in relation to one candidate experience–dependent process: EBL.
The Cerebellum Is Important for EBL
Contextualizing and extending the cerebellum’s long-recognized role in motor control, recent data suggest that the cerebellum subserves EBL for adaptive movement, cognition, and social prediction (13,15,29,30). This capability is instantiated in polysynaptic, cerebellar-cerebral circuits (31, 32, 33, 34). Notably, cerebellar circuit-level (anatomical) disruptions are associated with cerebellar functional connectivity alterations (35), indicating that functional connectivity is sensitive to underlying neuroanatomy (34). Evidence from studies of functional connectivity suggest that the function of a given cerebellar region is determined by its network membership (34,36,37), with cerebellar regions in the somatomotor network supporting error signaling for movement (38) and cerebellar regions in the frontoparietal network (FPN) supporting error signaling following expectancy violations (36,39,40). Anatomically and functionally defined cerebellar connections are implicated in the timing and execution of EBL tasks (saccade adaption, eye-blink conditioning) (41, 42, 43, 44, 45, 46), and their protracted development represents an opportunity to leverage experience-dependent neural plasticity in service of behavioral intervention (47,48).
Cerebellar Contributions to ASD
Cerebellar contributions to ASD, in turn, are supported by basic and late developmental (child/adult) clinical research. In mice, cerebellar pathology is sufficient to produce ASD-like social and repetitive behaviors, and pharmacologic treatment targeting cerebellar Purkinje cells redresses those same behaviors (49). Among individuals with ASD, cerebellar functional connectivity differentiates cases from controls (12,50, 51, 52, 53) and scales with symptom severity (53,54), total cerebellar volume is commonly increased (55), and Purkinje cell counts are decreased (56,57). Among individuals without ASD, acquired cerebellar injury produces behaviors resembling the ASD phenotype, including difficulties with social judgment, abstract reasoning, and set shifting (58). Although there are few studies examining the infant cerebellum in relation to ASD, the available literature suggests that adult findings may generalize. The development of cerebellar white matter pathways during infancy predicts sensory responsivity and restricted, repetitive behavior among toddlers (21), and perinatal cerebellar malformations are associated with a 36-fold increase in ASD risk (5,59).
EBL May Be Disrupted in ASD
Finally, there is growing interest in EBL impairment in ASD (17,45,60). Individuals with ASD perform poorly on cerebellar-mediated tasks of EBL (45,61,62), which may alter experience-dependent learning and thereby contribute to the emergence of ASD-associated behaviors (63, 64, 65). In the social domain, EBL impairment may disrupt predictions, with downstream consequences for social learning and joint attention (66,67). In the motor domain, EBL impairment may disrupt planning following errors of precision or timing, contributing to high rates of motor discoordination in ASD (68,69). However, discerning the pathways through which these associations emerge is difficult. In the context of diminished EBL, restricted interests and repetitive behaviors may serve a compensatory function to make the immediate environment more predictable (70,71). They may also reflect alterations to neurodevelopmental processes supporting the adaptability of the motor system to environmental inputs (72,73).
Motivation for Study Design
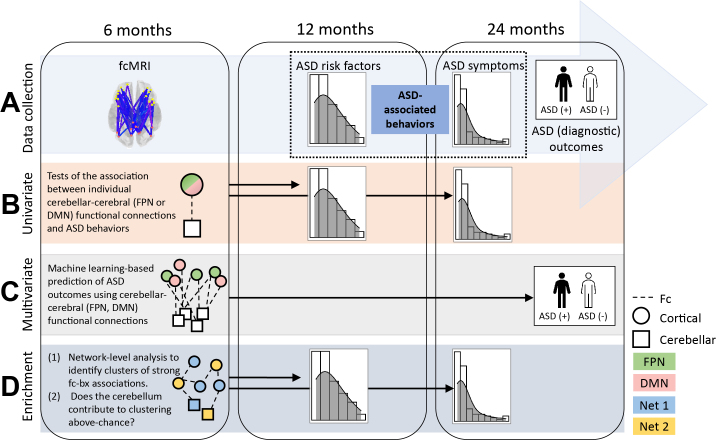
The present study examined cerebellar functional connectivity contributions to ASD in a presymptomatic infant sample. Data from the Infant Brain Imaging Study (IBIS) were used to examine 6-month cerebellar functional connectivity magnetic resonance imaging (fcMRI) in relation to 12- and 24-month ASD-associated behaviors and 24-month ASD diagnostic outcomes (Figure 1). For additional information about IBIS, refer to the Supplement (page 1). ASD-associated behaviors were selected based on hypothesized associations with EBL (Table S1), and our three-part analytic plan (univariate, multivariate machine learning, whole-brain fcMRI enrichment) was designed to inform understanding of EBL.
Figure 1.
Summary of the (A) data collection timeline and (B–D) three-part analytic plan. Functional connectivity magnetic resonance imaging (fcMRI) data were collected at 6 months, continuous measures of autism spectrum disorder (ASD)–associated behavior were collected at 12 and 24 months, and ASD diagnostic outcomes were evaluated at 24 months. Circles and squares represent regions of interest (ROIs) located in the cortex and cerebellum, respectively. Colors (green, pink, blue, yellow) denote ROI network (Net) assignments (Net 1 and Net 2 being arbitrary non–frontoparietal network [FPN] and non–default mode network [DMN], respectively). bx, behavior; Fc, functional connections/functional connectivity.
To this end, univariate and multivariate machine learning–based tests analyzed cerebellar-FPN and cerebellar–default mode network (DMN) connections. The FPN subserves error signaling in support of EBL (39,40,74) and is overrepresented in the cerebellum compared with the cortex (36). It would be extremely difficult to perform task-based neuroimaging of EBL in infants; thus, we used cerebellar-FPN functional connectivity as a proxy for EBL. Unlike the FPN, the DMN is not implicated in error processing; however, it is frequently implicated in ASD (54,75, 76, 77), making it an ideal comparative foil. Concomitant analysis of cerebellar-FPN and cerebellar-DMN functional connections afforded insight into the breadth and nature of hypothesized cerebellar disruption. Both the FPN and DMN are implicated in ASD-associated motor, social, restricted, and repetitive behaviors during early development (78, 79, 80).
To assess whether cerebellar contributions to ASD-associated behaviors are detectable in a brain-wide search space, including but not limited to the FPN and DMN, we performed data-driven fcMRI enrichment (78, 79, 80) with post hoc randomization testing. Enrichment identifies clusters of strong brain-behavior associations within and between functional brain networks, and post hoc testing evaluated whether cerebellar connections contributed to enrichment above chance. Evidence that infant cerebellar fcMRI relates to later ASD-associated behaviors and outcomes would advance current understanding of ASD pathogenesis, informing the development of targeted interventions.
Methods and Materials
Participants
This study analyzed neuroimaging and behavioral data from infants who participated in IBIS at the four original network sites: the University of North Carolina, Children’s Hospital of Philadelphia, Washington University School of Medicine, and the University of Washington. To be included, infants were required to provide usable fcMRI data at 6 months and diagnostic outcome data at 24 months. LORIS (Longitudinal Online Research and Imaging System) (81) served as the hub for data management. All families who participated in IBIS provided informed consent, approved by each site’s Human Subjects Review Board. IBIS data have been published previously [e.g., (80, 81, 82)], but IBIS studies have examined neither cerebellar fcMRI nor 6-month fcMRI in relation to later ASD-associated behaviors and outcomes.
High-risk infants were defined as having at least 1 sibling with an ASD diagnosis. High-risk-positive infants received a clinical best estimate ASD diagnosis (see below) at 24 months of age, whereas high-risk-negative infants did not. Low-risk-negative infants had at least 1 typically developing older sibling, did not have any first- or second-degree family members with ASD or intellectual disability, and did not receive an ASD diagnosis at 24 months of age. Low-risk-positive infants (n = 1) were excluded from analyses. Complete genetic and family history exclusion criteria are detailed in prior publications (79,82).
Mann-Whitney U (continuous variables) and χ2 (categorical variables) tests were used to compare participants included in analyses (n = 94) with the full IBIS sample. No differences were observed with respect to behavior, risk status, or diagnosis (ps > .05). However, the ratio of females to males was lower among participants who provided 6-month fcMRI data (p < .01). There were no effects of sex on behavior (ps > .05). Sample characteristics are reported in Table 1.
Table 1.
Sample Characteristics
| Characteristics | n | % |
|---|---|---|
| Sex | ||
| Female | 26 | 27.7% |
| Male | 68 | 72.3% |
| Outcome Group | ||
| Low-risk negative | 35 | 37.2% |
| High-risk negative | 46 | 48.9% |
| High-risk positive | 13 | 13.8% |
| Race | ||
| Biracial/multiracial | 10 | 10.6% |
| Black/African American | 2 | 2.1% |
| White | 72 | 76.6% |
| Not reported | 10 | 10.6% |
| Ethnicity | ||
| Hispanic or Latino | 6 | 6.4% |
| Not Hispanic or Latino | 78 | 83.0% |
| Not reported | 10 | 10.6% |
| Mean | SD | |
|---|---|---|
| Age at Time of Scan, mo | 6.51 | 0.59 |
| Number of BOLD Frames (After Scrubbing) | 241.13 | 57.17 |
| 12-mo Behaviors | ||
| Age at time of assessment, mo | 12.48 | 0.49 |
| CSBS-DP IJA | 1.44 | 1.38 |
| RBS-R restricteda | 0.30 | 0.85 |
| RBS-R ritualistic-samenessa | 0.63 | 1.73 |
| MSEL fine motor (T score) | 56.21 | 9.59 |
| MSEL gross motor (T score) | 48.70 | 12.75 |
| 24-mo Behaviors | ||
| Age at time of assessment, mo | 24.57 | 1.09 |
| ADOS total CSSa | 2.05 | 1.94 |
| ADOS social affect CSSa | 2.39 | 1.98 |
| ADOS RRB CSSa | 2.91 | 2.53 |
All participants included in the study sample (n = 94) provided at least 150 noncensored functional connectivity magnetic resonance imaging frames at 6 months. High scores reflect typical behaviors, unless otherwise indicated.
ADOS, Autism Diagnostic Observation Schedule; BOLD, blood oxygen level–dependent; CSBS-DP, Communication and Symbolic Behavior Scales Developmental Profile; CSS, calibrated severity score; IJA, initiation of joint attention; MSEL, Mullen Scales of Early Learning; RBS-R, Repetitive Behavior Scale–Revised; RRB, restricted interest and repetitive behavior.
Variables for which high scores reflect atypical behaviors.
Behavioral Assessment
Functional connectivity at 6 months was examined in relation to 12- and 24-month continuous behaviors (Figure 2) and 24-month diagnostic outcomes. Twelve-month behaviors indexed core and associated ASD risk factors: initiation of joint attention (79,83), fine and gross motor functioning (78,84), restricted behaviors (80), and ritualistic/sameness behaviors (80). Twenty-four-month behaviors indexed ASD symptoms: total symptom severity (85), social affect (86), and restricted interests and repetitive behaviors (RRBs) (86). We will refer to 12-month risk factors and 24-month symptoms collectively as ASD-associated behaviors (Figure 1). Groupwise descriptive statistics are provided in Table S2.
Figure 2.
Combined histograms and density plots for autism spectrum disorder–associated behaviors at 12 and 24 months. The leftmost distributions (enclosed by dashed box) were modeled using Poisson and negative binomial regression, whereas the rightmost distributions were modeled using linear regression. Blue density curves identify variables for which high scores reflect atypical behaviors; red density curves identify variables for which high scores reflect typical behaviors. CSS, calibrated severity score; IJA, initiation of joint attention; Rit/Same, ritualistic and sameness behaviors; RRB, restricted interest and repetitive behavior; V12, 12-month visit; V24, 24-month visit.
Initiation of joint attention was assessed using the Communication and Symbolic Behavior Scales Developmental Profile (87). Consistent with prior work (79), initiation of joint attention was operationalized as Communication and Symbolic Behavior Scales Developmental Profile item 7: the number of examiner-participant interactions “used to direct another’s attention to an object, event, or topic of a communicative act” (87). Fine and gross motor functioning were assessed using the Mullen Scales of Early Learning (88). The Mullen Scales of Early Learning is a standardized, clinician-administered test of developmental milestones for children 3 to 69 months of age, and it is well validated in ASD (89, 90, 91). Standardized T scores were analyzed. Restricted and ritualistic/sameness behaviors were assessed using the Repetitive Behavior Scale–Revised (RBS-R) (92). The RBS-R is a 43-item parent-report questionnaire validated for toddlers (93, 94, 95). Ritualistic and sameness subscales were combined because they load onto a common factor (93,95), and items endorsed (rather than severity scores) were examined given evidence that counts are less susceptible to rater bias (94). One outlier 7.7 SDs from the mean was excluded from analyses of ritualistic/sameness behaviors.
Twenty-four-month behaviors were indexed by the Autism Diagnostic Observation Schedule (ADOS) and Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) (96,97). Consistent with ADOS conventions, infants were administered Module 1 or 2 based on language proficiency (n = 56 ADOS Module 1, n = 5 ADOS Module 2, n = 33 ADOS-2 Module 1). Continuous measures of ASD severity were obtained for ADOS and ADOS-2 modules by computing calibrated severity scores (CSSs) across all symptoms (85), as well as within the social affect and RRB symptom domains (86). CSSs (85,86) were shifted to set minimum values to zero and eliminate discontinuities (in RRB CSSs) engendered by the scoring algorithm. As detailed in prior IBIS publications (24,25,82), clinical best estimate ASD diagnoses were made at 24 months by experienced clinicians applying the DSM-IV-TR (98) checklist to available testing and interview data.
Image Acquisition
Data were collected using cross-site calibrated 3T Siemens MAGNETOM TIM Trio scanners with 12-channel head coils. A 3-dimensional sagittal T2-weighted sequence (echo time = 497 ms, repetition time = 3200 ms, matrix 256 × 256 × 160, voxels 1 mm3) was used for coregistration with blood oxygen level–dependent scans. All sites followed identical protocols using gradient-echo echo-planar image acquisition (echo time = 27 ms, repetition time = 2500 ms, voxels 4 × 4 × 4 mm3). Infants were naturally sleeping during fMRI scanning, which involved two 6.25-minute runs (79).
Pre- and Postprocessing
We implemented the same basic functional MRI processing as previously described (25,79), with updates to improve data quality (Supplement, page 2). fcMRI processing applied global and nuisance signal regression, spatial and temporal filtering, bandpass filtering, and motion scrubbing at framewise displacement of 0.2 (99). Infants included in analyses were required to provide at least 150 noncensored frames. Neuroimaging exclusions are provided in Table S3.
Definition of Regions of Interest and fcMRI Computation
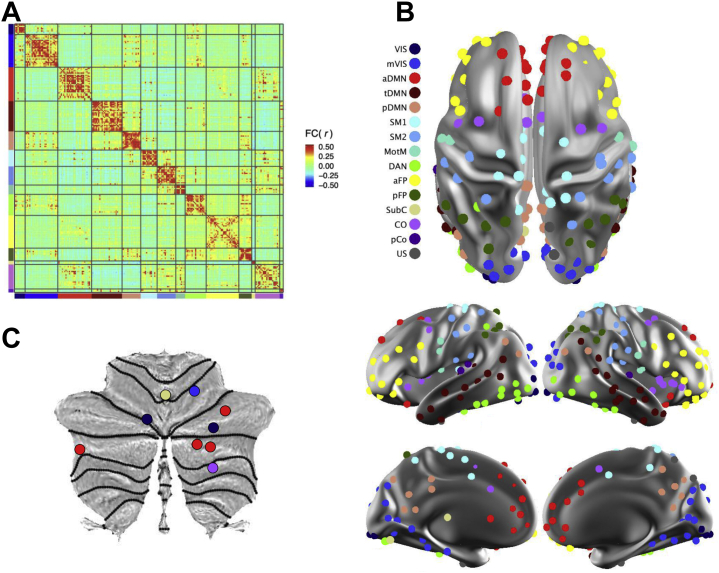
Computation of time series for the primary set of 230 regions of interest (ROIs) (10-mm diameter) were described by Pruett et al. (100), and ROI coordinates are provided in Table S4. In addition to five cerebellar ROIs in the primary 230-ROI set, we generated four new cerebellar ROIs based on their connectivity profiles with functional networks relevant to present hypotheses: the FPN and DMN. New cerebellar ROIs were centered on voxels that exhibited maximal correlations (in an independent 24-month sample) with network-average time series for the FPN or DMN, with one ROI placed for each hemisphere-network pair (left/right, FPN/DMN). Additional details regarding cerebellar ROI placement are reported in the Supplement (pages 3–4). Connectivity values were calculated as Pearson correlations between pairs of ROI time series (Figure 3A) and were Fisher r-to-z transformed for analyses.
Figure 3.
Functional network architecture in 6-month infants. (A) The sample-mean functional connectivity (FC) magnetic resonance imaging matrix depicts the correlation structure among spherical regions of interest (ROIs) (n = 234). ROIs are sorted by network assignment [see legend in panel (B)], and the color gradient illustrates the strength of correlations between ROIs. (B) Functional networks are visualized on dorsal, lateral, and medial surfaces of the brain. The color of an ROI identifies its network assignment. (C) Cerebellar ROIs, also colored by network [see legend in panel (B)], are visualized on a flattened cerebellar surface (124). aDMN, anterior default mode network; aFP, anterior frontoparietal network; CO, cingulo-opercular network; DAN, dorsal attention network; MotM, motor-mouth network; mVis, medial visual network; pCO, posterior cingulo-opercular network; pDMN, posterior default mode network; pFP, posterior frontoparietal network; SM1, somatomotor network 1; SM2, somatomotor network 2; SubC, subcortical network; tDMN, temporal default mode network; US, unspecified/unassigned; Vis, visual network.
Network Derivation
To obtain an age-appropriate network solution, we applied the Infomap community detection algorithm (101) to 6-month fcMRI data. Infomap was implemented in MATLAB release 2015b (The MathWorks, Inc.), and ROIs were sorted into networks at edge densities ranging from 2% to 10% (Figure S1). Structure-specific thresholding was applied to edges within structural components (cortical, subcortical, cerebellar), rather than across the entire brain (36). This approach integrates subcortical and cerebellar ROIs into whole-brain networks by accounting for the fact that subcortical-cortical and cerebellar-cortical correlations are relatively diminished due to acquisition factors (e.g., distance from head coil) (102). An automated procedure (102) was used to identify the consensus network structure (Figure 3B), and network names were determined by comparing our results with existing solutions, balancing neuroanatomical considerations (Figure S2). ROIs unassigned to networks (n = 4) were not analyzed.
Statistical Analysis
Univariate analyses were conducted in R (version 4.0.0; R Foundation for Statistical Computing) (103), and machine learning analyses were conducted in Python (104,105). Enrichment was implemented in Python (https://github.com/CPD-Lab/CBM_EA). In univariate and enrichment analyses, we modeled continuous outcomes because they provide more nuanced information about early behavioral development. In machine learning analyses, we predicted categorical outcomes to facilitate comparison with prior IBIS work demonstrating accurate diagnostic outcome prediction (25).
Univariate Associations
To evaluate whether 6-month cerebellar-FPN and/or cerebellar-DMN connections contribute to the development of ASD-associated behaviors, 9 (cerebellar ROIs) × 28 (12 FPN+16 DMN ROIs) correlation matrices were computed for each subject, and generalized linear models were used to examine associations between matrix elements and dimensional behaviors (Figure 2). Based on distribution shape, Poisson regression was used for the Communication and Symbolic Behavior Scales, RBS-R, and ADOS variables, whereas linear regression was used for the Mullen Scales of Early Learning fine and gross motor variables. To ascertain robustness given intermittent evidence for overdispersion (106), we compared results from Poisson and negative binomial models. Empirical p values were calculated using randomization (n = 5000). To balance statistical rigor and power (Figure S3), false discovery rate (FDR) correction was performed with respect to the number of connections (n = 252). Connections were considered significant at FDR q values <0.05.
Multivariate Machine Learning Prediction
Whereas univariate approaches are well suited for identifying strong individual functional connections, machine learning approaches examine the collective utility of many functional connections. Prior IBIS work by Emerson et al. (25) achieved a highly accurate diagnostic outcome prediction (positive and negative predictive values >95%) using 6-month whole-brain functional connectivity. To determine whether accurate diagnostic outcome prediction is attainable using exclusively cerebellar features, we replicated their approach using 6-month cerebellar-FPN and cerebellar-DMN connections (networks defined using 6-month solution).
Detailed methods are provided by Emerson et al. (25). Briefly, support vector machine learning classifiers were trained and tested in a high-risk sample (n = 59) using nested, leave-one-out cross-validation. Features were selected based on the strength of correlations with 12- and 24-month ASD-associated behaviors. To be included as a training feature in the outer loop, we required that a given functional connection exhibit at least one nominally significant behavioral correlation (p < .05) across all folds of the inner loop. Hyperparameter tuning was conducted in the inner loop over a range of regularization values (C: [0.001, 10.0]) using a linear kernel and balanced class weights (25).
fcMRI Enrichment
fcMRI enrichment identifies functional network pairs that contain clusters of strong brain-behavior associations (78, 79, 80). To assess whether cerebellar contributions to ASD-associated behaviors are detectable in a brain-wide search space, we first identified network pairs that were enriched for associations with 12- and 24-month ASD-associated behaviors, and we then performed randomization testing to quantify the extent to which cerebellar connections were overrepresented in enriched networks.
Our approach proceeded in three steps. First, the 5% strongest brain-behavior associations (hereafter referred to as hits) were identified in real and shuffled (n = 50,000) data using univariate screening (Poisson or linear regression). Second, for every network pair, enrichment p values were computed as the fraction of shuffled runs with at least as many hits as real data. Based on simulations, we determined that p values <.001 were necessary to approximate a 5% brain-wide false positive rate. To avoid overlooking potentially informative results, p values <.01 were also considered significant if they demonstrated the capacity to significantly predict behavior in secondary validation (Supplement, page 5).
Post Hoc Randomization
Between enriched networks, randomization testing (n = 10,000) examined whether cerebellar ROIs were overrepresented among hits. Cerebellar involvement was quantified as the number of hits (nC) that included at least one cerebellar ROI. Empirical p values were computed as the fraction of the randomization distribution in which nCrandom > nCreal. Aggregation of cerebellar-cerebral connections (in the top 5% of the randomization distribution) would identify important cerebellar contributions to ASD-associated behaviors, affording a whole-brain counterpart to hypothesis-driven testing.
Results
Univariate Associations
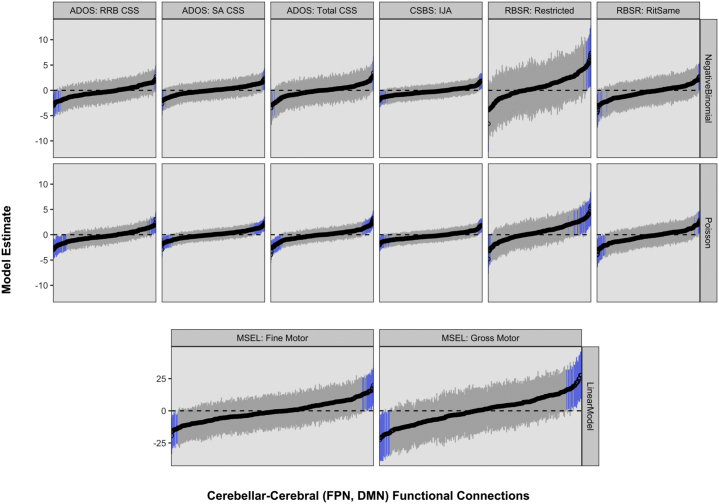
Hypothesis-driven tests of univariate associations between 6-month cerebellar-cerebral (FPN, DMN) functional connectivity and later dimensional behaviors failed to implicate the cerebellum in ASD (Figure 4) despite statistical power to detect medium-sized effects (Figure S3). Prior to FDR correction, as expected by chance, 5.6% of univariate tests were significant at p < .05. Following FDR correction, no significant results remained at q < .05. To guard against false negatives, we reanalyzed data under conditions with fewer comparisons; results remained null (Supplement, page 6).
Figure 4.
Hypothesis-driven tests of brain-behavior associations. Each subplot visualizes estimates and 95% confidence intervals (plotted on y-axis) for univariate models relating 6-month cerebellar–frontoparietal network (FPN) and cerebellar–default mode network (DMN) functional connections (n = 252 per subplot; plotted on x-axis and sorted by magnitude) to 12- and 24-month autism spectrum disorder (ASD)–associated behaviors (n = 8; indicated at top of subplots). The modeling approach (Poisson, negative binomial, or linear regression) is indicated at right, and blue confidence intervals identify significant results (empirical p < .05) prior to false discovery rate correction. Following false discovery rate correction, no significant results remained at false discovery rate–corrected q < .05. ADOS, Autism Diagnostic Observation Schedule; CSBS, Communication and Symbolic Behavior Scales; CSS, calibrated severity score; IJA, initiation of joint attention; MSEL, Mullen Scales of Early Learning; RBSR, Repetitive Behavior Scale–Revised; RitSame, ritualistic and sameness; RRB, restricted interest and repetitive behavior; SA, social affect.
Multivariate Machine Learning
In the context of familial risk (∼1/5 chance of ASD), a machine that exclusively predicts the minority class (high-risk positive) will achieve ∼20% positive predictive value, providing a baseline for classifier evaluation. Our classifier failed to meaningfully exceed 20% positive predictive value (observed positive predictive value = 23%), and performance was similarly poor with respect to other metrics (accuracy = 66%, sensitivity = 23%, specificity = 78%), indicating that cerebellar-FPN and cerebellar-DMN features are insufficient to inform diagnostic outcome prediction at 24 months.
fcMRI Enrichment
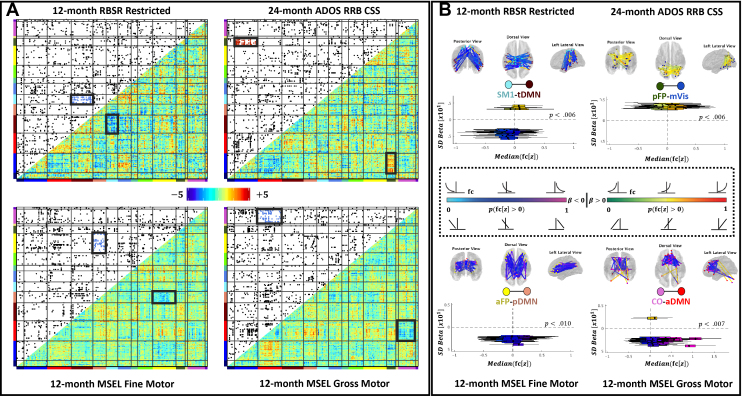
Enrichment identified four 6-month network pairs that exhibited strong associations with later ASD-associated behaviors (Figure 5), three of which passed secondary validation (Figure S4). Network pairs passing secondary validation included the posterior frontoparietal and medial visual (pFP-mVis), anterior frontoparietal and posterior default mode (aFP-pDMN), and cingulo-opercular and anterior default mode (CO-aDMN) pairings. The somatomotor-1 and temporal default mode (SM1-tDMN) network pair did not pass secondary validation (p = .15) and was not interpreted. Between the pFP and mVis, increased positive connectivity was associated with increased 24-month RRBs (p = .006). Between the anterior FPN and pDMN, increased positive connectivity was associated with decreased 12-month fine motor functioning (p = .010). Finally, between the CO and aDMN, increased positive connectivity was associated with decreased 12-month gross motor functioning (p = .007).
Figure 5.
(A) Within and between networks, clusters of brain-behavior associations were identified using enrichment. Lower triangles depict β coefficients. Upper triangles were generated by applying a 5% threshold to β coefficients. Each black dot represents a single, strong brain-behavior association. (B) Six-month functional connections between the somatomotor network 1 and temporal default mode network (SM1-tDMN), posterior frontoparietal network and medial visual network (pFP-mVis), anterior FP and posterior DMN (aFP-pDMN), and cingulo-opercular network and anterior DMN (CO-aDMN) were strongly associated with 12- and 24-month motor functioning and restricted interests and repetitive behaviors (RRBs). Within enriched network pairs, locations of strong brain-behavior associations are visualized on posterior, dorsal, and lateral views of the brain (top). Box plots (bottom) further illustrate the range of functional connectivity values in the study sample (x-axis) that underlie each brain-behavior correlation (y-axis). Blue-pink and green-red color gradients identify negative and positive brain-behavior associations, respectively. Specific colors denote the sign and strength of functional connectivity (e.g., pale blue/green = predominantly negative connectivity; pink/red = predominantly positive connectivity). All network pairs except the SM1-tDMN passed our secondary validation protocol. ADOS, Autism Diagnostic Observation Schedule; CSS, calibrated severity score; MSEL, Mullen Scales of Early Learning; RBSR, Repetitive Behavior Scale–Revised.
Post Hoc Randomization
Two of the three significant network pairs contained cerebellar ROIs: CO-aDMN and pFP-mVis. Significant aggregation of cerebellar-cerebral connections was not observed in either network pair (Figure 6).
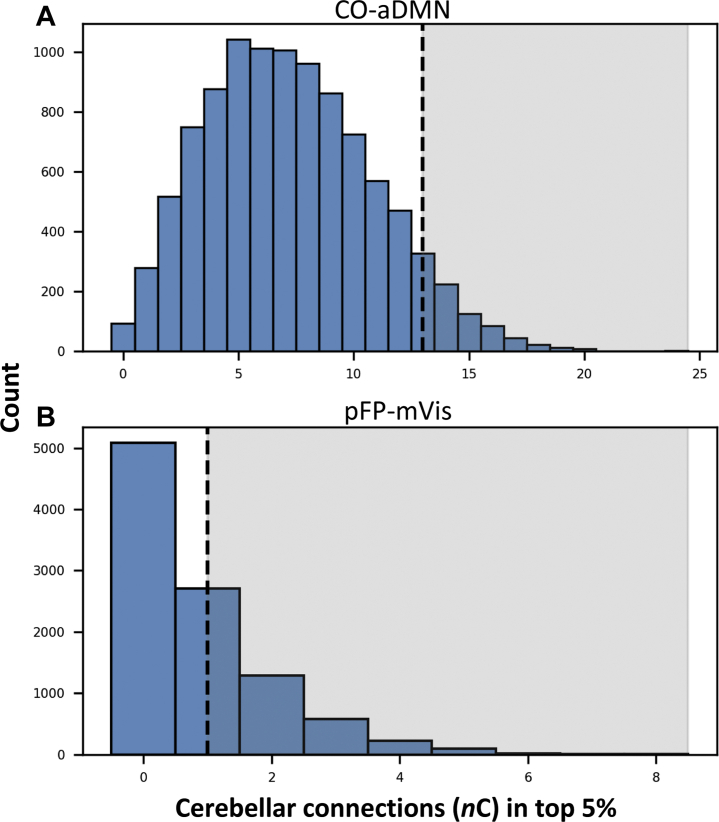
Figure 6.
Cerebellar contributions to network enrichment. Dotted lines indicate the number of cerebellar hits (nC) in real data, and shaded regions identify randomization runs in which nCrandom > nCreal. (A) Between the cingulo-opercular network and anterior default mode network (CO-aDMN), 8.41% of randomization runs included at least as many cerebellar hits as were observed in real data. (B) Between the posterior frontoparietal network and medial visual network (pFP-mVis), 49.17% of randomization runs included at least as many cerebellar hits as were observed in the real data. These results fail to support a statistically significant role for the cerebellum in the emergence of 12- and 24-month autism spectrum disorder–associated behaviors.
Discussion
The present study failed to observe a relationship between 6-month cerebellar connectivity and later ASD-associated behaviors and outcomes. Univariate tests of cerebellar-FPN and cerebellar-DMN connections did not identify associations with ASD-associated behaviors, multivariate machine learning tests of cerebellar-FPN and cerebellar-DMN connections did not achieve above-chance ASD diagnostic classification accuracy at 24 months, and fcMRI enrichment with post hoc randomization did not support a substantial role for infant cerebellar connectivity in enriched networks. Although cerebellar functional connections did not predict ASD-associated behaviors and outcomes, fcMRI enrichment identified multiple 6-month network correlates of 12- and 24-month ASD-associated behaviors. Specifically, we observed prospective correlates of motor behaviors and RRBs in functional networks implicated in error signaling (FPN) and ASD (DMN).
Cerebellar Effects May Manifest After ASD Symptoms Have Consolidated
Given strong motivation for examining the cerebellum as a presymptomatic risk biomarker for ASD, the present results should not be taken to falsify cerebellar theories of ASD pathogenesis. We examined cerebellar functional connectivity as a predictor of ASD, focusing primarily on cerebellar-FPN and cerebellar-DMN connections. Alternatively, cerebellar connectivity may differentiate individuals with ASD after symptoms have consolidated. This putative sequencing is supported by computational work, which suggests that cerebellar circuitry expedites cortical processing in situations in which established stimulus-response associations exist (8). It is also supported by recent models of neurodevelopment, which argue that disrupted sensorimotor and attentional experiences precede alterations in experience-dependent brain development (28). Densely sampling brain and behavior data at multiple time points across the first few years of life would facilitate identification of developmental epochs that may be acutely sensitive to—or predictive of—cerebellar disturbance. In addition to issues of development, research is needed to comprehensively characterize associations among cerebellar pathology (assessed using multiple neuroimaging modalities), EBL (assessed using behavioral tasks with well-described cerebellar circuitry), and ASD (assessed with respect to diagnosis and severity).
fcMRI Enrichment Identified Network Correlates of ASD-Associated Behaviors
Despite null cerebellar findings, fcMRI enrichment identified clusters of strong brain-behavior relationships in networks of a priori interest (FPN, DMN), as well as in the CO and mVis. This pattern of results is broadly consistent with the triple network model of psychopathology (107,108). Although we did not make a priori hypotheses about the CO, along with the FPN, it subserves error signaling in support of EBL (74,109,110). Together, the FPN and CO support the broader “control system” (74,111), and their involvement in enriched network pairs raises the possibility that ASD-associated behaviors reflect cortically mediated error signaling impairments. Behavioral studies are necessary to rigorously test cortical versus cerebellar learning systems (8) to determine which may account for emerging ASD-associated behaviors. DMN representation within enriched network pairs supports the developmental extension of findings obtained in older samples (54,75, 76, 77). Notably, a recent review implicated both the FPN and DMN in ASD across multiple neuroimaging modalities and heterogeneous samples (112).
To contextualize the present enrichment results between control and sensorimotor network (pFP-mVis) and control and default mode (aFPN-pDMN, CO-aDMN) network systems, we compiled results from prior fcMRI enrichment studies that also examined functional connectivity in relation to ASD-associated behaviors (Figure S5). Consistent with the present findings, more positive connectivity between control and default mode systems was reliably associated with atypical behaviors (poorer motor functioning, increased RRBs). Similar patterns have been reported in multiple psychiatric and neurodevelopmental disorders (107) and may reflect compensatory activation (i.e., recruitment of multiple brain networks for processes typically performed by a single network) (113,114) and/or network dedifferentiation (i.e., reduced segregation) (115).
Whereas brain-behavior associations between default mode and control systems exhibited consistent patterning across fcMRI enrichment studies, brain-behavior associations between sensorimotor and control systems were mixed. To account for this variation, it may be important to again consider the moderating effects of age. In early development, aspects of the control system are posited to modulate input from other regions of the brain, scaffolding age-appropriate learning and behavior (116). Whether increased connectivity between visual and control systems supports or disrupts behavioral development may depend, critically, on the age of the child (117).
Limitations
This work represents the largest analysis of cerebellar fcMRI in a prospective infant sample at high risk for ASD. There are, however, several limitations. First, although simulation-based power analyses suggested sufficient power to detect medium-sized brain-behavior effects (118), identifying small-sized, reproducible effects will require larger samples (119). Second, data were analyzed from nine 10-mm cerebellar ROIs. It is unclear whether results generalize to other regions in the cerebellum, and it is possible that ROI size and/or placement resulted in signal mixing across resting-state networks. Third, cerebellar ROI placement was optimized to test hypotheses about the FPN and DMN, and future studies might instead optimize cerebellar ROI placement in relation to other networks (e.g., CO) or anatomical structures. Fourth, at 6 months, reduced cortical-subcortical connectivity and regional subdivisions in late-maturing networks (e.g., DMN, FPN) (120,121) complicate efforts to ascribe adult-like function to infant data. Behavioral assessment of EBL is necessary to establish a more direct link to ASD phenotypes. Fifth, some measures of ASD-associated behavior (ADOS, RBS-R) were developed to characterize variation in clinical samples (85,92) and exhibited limited variability among individuals without ASD, possibly attenuating brain-behavior relationships. Finally, cerebellar ROIs placed in relation to the FPN (in an independent 24-month sample) did not exhibit preferential connectivity with the FPN in our 6-month sample (Supplement, pages 3–4). However, these cerebellar ROIs, which were placed by reverse seeding the FPN, lie in regions that map to the FPN in independent adult samples (122), indicating that ROI placement generalizes to later stages of development. We propose three explanations to reconcile these observations.
First, it is possible that patterns of cerebellar-network connectivity in infants differ markedly from patterns of cerebellar-network connectivity in toddlers. Large-scale functional brain networks exhibit rapid maturation during the first (e.g., DMN) and into the second (e.g., FPN) year of life (121), providing precedent for developmental functional network changes. Second, it is possible that components of the control system modulate sensory and motor processing to scaffold early learning [e.g., (116)], in which case relatively elevated 6-month functional connectivity between somatomotor ROIs and cerebellar ROIs placed in relation to the FPN (cf. Supplement, page 4) may support motor skill acquisition. This explanation is broadly consistent with (the extant limited number of) fcMRI enrichment studies (78, 79, 80,123) indicating that functional connectivity between control and somatomotor networks is more variable across development than functional connectivity involving the DMN (Figure S5). Third, attenuated correlations may be expected if infant cerebellar-FPN connectivity reflects presymptomatic risk for ASD. Under such conditions, interindividual variation in 6-month data may index group differences of primary interest. Univariate analyses were conducted to test, but did not find support for, this explanation. Future studies in infant and toddler samples—both enriched and not enriched for ASD—are necessary to clarify developmental network dynamics and infant ROI nomenclature.
Conclusions
We examined cerebellar connectivity as a presymptomatic risk biomarker for ASD. Contrary to hypotheses, analyses did not reveal strong associations between infant cerebellar functional connectivity and later ASD-associated behaviors and outcomes. Instead, fcMRI enrichment identified clusters of brain-behavior relationships between infant networks implicated in error signaling (FPN, CO) and ASD (DMN) (40,75). To thoroughly interrogate cerebellar theories of ASD, future studies may investigate cerebellar pathology in relation to ASD-associated behaviors and outcomes at different stages of development and/or using different neuroimaging modalities. Such efforts hold promise to identify mechanistically informed risk biomarkers for ASD, bridging scientific theory and clinical translation. Research aimed at risk biomarker discovery should also consider focusing attention on early patterns of connectivity between enriched (control, default mode, visual) networks.
Acknowledgments and Disclosures
This research was supported by grants from the Autism Science Foundation (Grant No. 19-001 [to ZWH]) and National Institutes of Health (NIH) (Grant Nos. F31 MH120918 [to ZWH], K99 MH121518 [to SM], R01 HD055741 [to KB], R01 MH093510 [to JRP], R01 MH116961 [to JJW], R01 MH118362 [to JRP and JPi], and MH118362-02S1 [to JRP]). In addition, research was supported by Washington University Institute of Clinical and Translational Sciences Grant No. UL1TR002345 from the National Center for Advancing Translational Sciences of the NIH. Computations were performed using the facilities of the Washington University Center for High Performance Computing, which were partially funded by NIH Grant Nos. 1S10RR022984-01A1 and 1S10OD018091-01. Neuroimaging processing pipelines were supported by Grant Nos. P30 NS048056 and P30 NS098577. The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.
The Infant Brain Imaging Study–Early Prediction project is a National Institute of Mental Health–funded study and consists of a consortium of 10 universities in the United States and Canada. The study includes infants at high familial risk for autism spectrum disorder, based on having an older sibling with the diagnosis. Infants are seen at 6, 12, and 24 months of age for brain magnetic resonance imaging scans and behavioral tests. Members and components of the Infant Brain Imaging Study–Early Prediction project include: Co-PIs: J.R. Pruett, Jr. & J. Piven; Clinical Sites: Children’s Hospital of Philadelphia (CHOP): R.T. Schultz, J. Pandey, J. Parish-Morris, B. Tunç, W. Guthrie; University of Minnesota (UMN): J.T. Elison, J.J. Wolff, C.A. Burrows; University of North Carolina (UNC): J. Piven, H.C. Hazlett, M.D. Shen, J.B. Girault, R. Grzadzinski; University of Washington (UW): S.R. Dager, A.M. Estes, T. St. John, D.W.W. Shaw; Washington University School of Medicine in St. Louis (WU): K.N. Botteron, R.C. McKinstry, J.N. Constantino, N. Marrus; Admin Core: WU: Alicia Rocca; UNC: Chad Chappell; Behavior Core: UW: A.M. Estes, T. St. John; University of Alberta: L. Zwaigenbaum; UMN: J.T. Elison, J.J. Wolff; University of Texas at Dallas: M.R. Swanson; MRI Core: UNC: M.A. Styner, M.D. Shen; New York University: G. Gerig; WU: J.R. Pruett, Jr., R.C. McKinstry; UMN: J.T. Elison; UW: S.R. Dager; Data Coordinating Center: Montreal Neurological Institute: A.C. Evans, L.C. MacIntyre, S. Torres-Gomez, S. Das; Statistical Analysis Core: UNC: K. Truong; Environmental Risk Core: John Hopkins University (JHU): H. Volk; Genetics Core: JHU: M.D. Fallin; UNC: M.D. Shen; EEG: University of California, Los Angeles: S.S. Jeste; Ethical, Legal, and Social Implications Core: UW: K.E. MacDuffie.
RCM serves on the advisory board of Nous Imaging, Inc., and receives funding for meals and travel from Siemens Healthineers and Philips Healthcare. JNC receives royalties from Western Psychological Services for the commercial distribution of the Social Responsiveness Scale. All other authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsgos.2021.12.004.
Supplementary Material
References
- 1.American Psychiatric Association . 5th ed. American Psychiatric Press; Washington, DC: 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 2.Ozonoff S., Iosif A.M., Baguio F., Cook I.C., Hill M.M., Hutman T., et al. A prospective study of the emergence of early behavioral signs of autism. J Am Acad Child Adolesc Psychiatry. 2010;49:256–266. [PMC free article] [PubMed] [Google Scholar]
- 3.Pierce K., Gazestani V.H., Bacon E., Barnes C.C., Cha D., Nalabolu S., et al. Evaluation of the diagnostic stability of the early autism spectrum disorder phenotype in the general population starting at 12 months. JAMA Pediatr. 2019;173:578–587. doi: 10.1001/jamapediatrics.2019.0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelly E., Escamilla C.O., Tsai P.T. Cerebellar dysfunction in autism spectrum disorders: Deriving mechanistic insights from an internal model framework. Neuroscience. 2021;462:274–287. doi: 10.1016/j.neuroscience.2020.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang S.S.H., Kloth A.D., Badura A. The cerebellum, sensitive periods, and autism. Neuron. 2014;83:518–532. doi: 10.1016/j.neuron.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fatemi S.H., Aldinger K.A., Ashwood P., Bauman M.L., Blaha C.D., Blatt G.J., et al. Consensus paper: Pathological role of the cerebellum in autism. Cerebellum. 2012;11:777–807. doi: 10.1007/s12311-012-0355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raymond J.L., Medina J.F. Computational principles of supervised learning in the cerebellum. Annu Rev Neurosci. 2018;41:233–253. doi: 10.1146/annurev-neuro-080317-061948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doya K. What are the computations of the cerebellum, the basal ganglia and the cerebral cortex? Neural Netw. 1999;12:961–974. doi: 10.1016/s0893-6080(99)00046-5. [DOI] [PubMed] [Google Scholar]
- 9.Stahl A.E., Feigenson L. Observing the unexpected enhances infants’ learning and exploration. Science. 2015;348:91–94. doi: 10.1126/science.aaa3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D'Mello A.M., Stoodley C.J. Cerebro-cerebellar circuits in autism spectrum disorder. Front Neurosci. 2015;9:408. doi: 10.3389/fnins.2015.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crippa A., Del Vecchio G., Busti Ceccarelli S., Nobile M., Arrigoni F., Brambilla P. Cortico-cerebellar connectivity in autism spectrum disorder: What do we know so far? Front Psychiatry. 2016;7:20. doi: 10.3389/fpsyt.2016.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan A.J., Nair A., Keown C.L., Datko M.C., Lincoln A.J., Müller R.A. Cerebro-cerebellar resting-state functional connectivity in children and adolescents with autism spectrum disorder. Biol Psychiatry. 2015;78:625–634. doi: 10.1016/j.biopsych.2015.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leggio M., Molinari M. Cerebellar sequencing: A trick for predicting the future. Cerebellum. 2015;14:35–38. doi: 10.1007/s12311-014-0616-x. [DOI] [PubMed] [Google Scholar]
- 14.Ito M. Control of mental activities by internal models in the cerebellum. Nat Rev Neurosci. 2008;9:304–313. doi: 10.1038/nrn2332. [DOI] [PubMed] [Google Scholar]
- 15.Sokolov A.A., Miall R.C., Ivry R.B. The cerebellum: Adaptive prediction for movement and cognition. Trends Cogn Sci. 2017;21:313–332. doi: 10.1016/j.tics.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sinha P., Kjelgaard M.M., Gandhi T.K., Tsourides K., Cardinaux A.L., Pantazis D., et al. Autism as a disorder of prediction. Proc Natl Acad Sci U S A. 2014;111:15220–15225. doi: 10.1073/pnas.1416797111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van de Cruys S., Evers K., Van der Hallen R., Van Eylen L., Boets B., de-Wit L., Wagemans J. Precise minds in uncertain worlds: Predictive coding in autism. Psychol Rev. 2014;121:649–675. doi: 10.1037/a0037665. [DOI] [PubMed] [Google Scholar]
- 18.FDA-NIH Biomarker Working Group . BEST (Biomarkers, EndpointS, and other Tools) Resource. Silver Spring, MD: U.S. Food and Drug Administration. National Institutes of Health; Bethesda, MD: 2016. Susceptibility/Risk Biomarker. [PubMed] [Google Scholar]
- 19.Ozonoff S., Young G.S., Carter A., Messinger D., Yirmiya N., Zwaigenbaum L., et al. Recurrence risk for autism spectrum disorders: A Baby Siblings Research Consortium study. Pediatrics. 2011;128:e488–e495. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolff J.J., Piven J. Predicting Autism in Infancy. J Am Acad Child Adolesc Psychiatry. 2021;60:958–967. doi: 10.1016/j.jaac.2020.07.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolff J.J., Swanson M.R., Elison J.T., Gerig G., Pruett J.R., Styner M.A., et al. Neural circuitry at age 6 months associated with later repetitive behavior and sensory responsiveness in autism. Mol Autism. 2017;8:8. doi: 10.1186/s13229-017-0126-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elison J.T., Paterson S.J., Wolff J.J., Reznick J.S., Sasson N.J., Gu H., et al. White matter microstructure and atypical visual orienting in 7-month-olds at risk for autism. Am J Psychiatry. 2013;170:899–908. doi: 10.1176/appi.ajp.2012.12091150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolff J.J., Jacob S., Elison J.T. The journey to autism: Insights from neuroimaging studies of infants and toddlers. Dev Psychopathol. 2018;30:479–495. doi: 10.1017/S0954579417000980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hazlett H.C., Gu H., Munsell B.C., Kim S.H., Styner M., Wolff J.J., et al. Early brain development in infants at high risk for autism spectrum disorder. Nature. 2017;542:348–351. doi: 10.1038/nature21369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Emerson R.W., Adams C., Nishino T., Hazlett H.C., Wolff J.J., Zwaigenbaum L., et al. Functional neuroimaging of high-risk 6-month-old infants predicts a diagnosis of autism at 24 months of age. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aag2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen M.D., Kim S.H., McKinstry R.C., Gu H., Hazlett H.C., Nordahl C.W., et al. Increased extra-axial cerebrospinal fluid in high-risk infants who later develop autism. Biol Psychiatry. 2017;82:186–193. doi: 10.1016/j.biopsych.2017.02.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolff J.J., Gerig G., Lewis J.D., Soda T., Styner M.A., Vachet C., et al. Altered corpus callosum morphology associated with autism over the first 2 years of life. Brain. 2015;138:2046–2058. doi: 10.1093/brain/awv118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piven J., Elison J.T., Zylka M.J. Toward a conceptual framework for early brain and behavior development in autism. Mol Psychiatry. 2017;22:1385–1394. doi: 10.1038/mp.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peterburs J., Desmond J.E. The role of the human cerebellum in performance monitoring. Curr Opin Neurobiol. 2016;40:38–44. doi: 10.1016/j.conb.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fiez J.A., Petersen S.E., Cheney M.K., Raichle M.E. Impaired non-motor learning and error detection associated with cerebellar damage: A single case study. Brain. 1992;115:155–178. doi: 10.1093/brain/115.1.155. [DOI] [PubMed] [Google Scholar]
- 31.Kelly R.M., Strick P.L. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci. 2003;23:8432–8444. doi: 10.1523/JNEUROSCI.23-23-08432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bostan A.C., Dum R.P., Strick P.L. Cerebellar networks with the cerebral cortex and basal ganglia. Trends Cogn Sci. 2013;17:241–254. doi: 10.1016/j.tics.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmahmann J.D., Pandya D.N. Anatomic organization of the basilar pontine projections from prefrontal cortices in rhesus monkey. J Neurosci. 1997;17:438–458. doi: 10.1523/JNEUROSCI.17-01-00438.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buckner R.L. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron. 2013;80:807–815. doi: 10.1016/j.neuron.2013.10.044. [DOI] [PubMed] [Google Scholar]
- 35.Lu J., Liu H., Zhang M., Wang D., Cao Y., Ma Q., et al. Focal pontine lesions provide evidence that intrinsic functional connectivity reflects polysynaptic anatomical pathways. J Neurosci. 2011;31:15065–15071. doi: 10.1523/JNEUROSCI.2364-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marek S., Siegel J.S., Gordon E.M., Raut R.V., Gratton C., Newbold D.J., et al. Spatial and temporal organization of the individual human cerebellum. Neuron. 2018;100:977–993. doi: 10.1016/j.neuron.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmahmann J.D., Guell X., Stoodley C.J., Halko M.A. The theory and neuroscience of cerebellar cognition. Annu Rev Neurosci. 2019;42:337–364. doi: 10.1146/annurev-neuro-070918-050258. [DOI] [PubMed] [Google Scholar]
- 38.Moberget T., Ivry R.B. Cerebellar contributions to motor control and language comprehension: Searching for common computational principles. Ann N Y Acad Sci. 2016;1369:154–171. doi: 10.1111/nyas.13094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marek S., Dosenbach N.U. The frontoparietal network: Function, electrophysiology, and importance of individual precision mapping. Dialogues Clin Neurosci. 2018;20:133–140. doi: 10.31887/DCNS.2018.20.2/smarek. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dosenbach N.U., Fair D.A., Miezin F.M., Cohen A.L., Wenger K.K., Dosenbach R.A., et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herbert J.S., Eckerman C.O., Stanton M.E. The ontogeny of human learning in delay, long-delay, and trace eyeblink conditioning. Behav Neurosci. 2003;117:1196–1210. doi: 10.1037/0735-7044.117.6.1196. [DOI] [PubMed] [Google Scholar]
- 42.Woodruff-Pak D.S., Papka M., Ivry R.B. Cerebellar involvement in eyeblink classical conditioning in humans. Neuropsychology. 1996;10:443–458. [Google Scholar]
- 43.Radell M.L., Mercado E. Modeling possible effects of atypical cerebellar processing on eyeblink conditioning in autism. Cogn Affect Behav Neurosci. 2014;14:1142–1164. doi: 10.3758/s13415-014-0263-1. [DOI] [PubMed] [Google Scholar]
- 44.Xu-Wilson M., Chen-Harris H., Zee D.S., Shadmehr R. Cerebellar contributions to adaptive control of saccades in humans. J Neurosci. 2009;29:12930–12939. doi: 10.1523/JNEUROSCI.3115-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mosconi M.W., Luna B., Kay-Stacey M., Nowinski C.V., Rubin L.H., Scudder C., et al. Saccade adaptation abnormalities implicate dysfunction of cerebellar-dependent learning mechanisms in autism spectrum disorders (ASD) PLoS One. 2013;8 doi: 10.1371/journal.pone.0063709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ivry R.B., Spencer R.M., Zelaznik H.N., Diedrichsen J. The cerebellum and event timing. Ann N Y Acad Sci. 2002;978:302–317. doi: 10.1111/j.1749-6632.2002.tb07576.x. [DOI] [PubMed] [Google Scholar]
- 47.Amemiya K., Morita T., Saito D.N., Ban M., Shimada K., Okamoto Y., et al. Local-to-distant development of the cerebrocerebellar sensorimotor network in the typically developing human brain: A functional and diffusion MRI study. Brain Struct Funct. 2019;224:1359–1375. doi: 10.1007/s00429-018-01821-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Galván A. Insights about adolescent behavior, plasticity, and policy from neuroscience research. Neuron. 2014;83:262–265. doi: 10.1016/j.neuron.2014.06.027. [DOI] [PubMed] [Google Scholar]
- 49.Tsai P.T., Rudolph S., Guo C., Ellegood J., Gibson J.M., Schaeffer S.M., et al. Sensitive periods for cerebellar-mediated autistic-like behaviors. Cell Rep. 2018;25:357–367. doi: 10.1016/j.celrep.2018.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hanaie R., Mohri I., Kagitani-Shimono K., Tachibana M., Matsuzaki J., Hirata I., et al. Aberrant cerebellar–cerebral functional connectivity in children and adolescents with autism spectrum disorder. Front Hum Neurosci. 2018;12:454. doi: 10.3389/fnhum.2018.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oldehinkel M., Mennes M., Marquand A., Charman T., Tillmann J., Ecker C., et al. Altered connectivity between cerebellum, visual, and sensory-motor networks in autism spectrum disorder: Results from the EU-AIMS longitudinal European autism project. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4:260–270. doi: 10.1016/j.bpsc.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 52.Ramos T.C., Balardin J.B., Sato J.R., Fujita A. Abnormal cortico-cerebellar functional connectivity in autism spectrum disorder. Front Syst Neurosci. 2019;12:74. doi: 10.3389/fnsys.2018.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Verly M., Verhoeven J., Zink I., Mantini D., Peeters R., Deprez S., et al. Altered functional connectivity of the language network in ASD: Role of classical language areas and cerebellum. Neuroimage Clin. 2014;4:374–382. doi: 10.1016/j.nicl.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jung M., Kosaka H., Saito D.N., Ishitobi M., Morita T., Inohara K., et al. Default mode network in young male adults with autism spectrum disorder: Relationship with autism spectrum traits. Mol Autism. 2014;5:35. doi: 10.1186/2040-2392-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Traut N., Beggiato A., Bourgeron T., Delorme R., Rondi-Reig L., Paradis A.L., Toro R. Cerebellar volume in autism: Literature meta-analysis and analysis of the autism brain imaging data exchange cohort. Biol Psychiatry. 2018;83:579–588. doi: 10.1016/j.biopsych.2017.09.029. [DOI] [PubMed] [Google Scholar]
- 56.Whitney E.R., Kemper T.L., Bauman M.L., Rosene D.L., Blatt G.J. Cerebellar Purkinje cells are reduced in a subpopulation of autistic brains: A stereological experiment using calbindin-D28k. Cerebellum. 2008;7:406–416. doi: 10.1007/s12311-008-0043-y. [DOI] [PubMed] [Google Scholar]
- 57.Bauman M., Kemper T.L. Histoanatomic observations of the brain in early infantile autism. Neurology. 1985;35 doi: 10.1212/wnl.35.6.866. 866–866. [DOI] [PubMed] [Google Scholar]
- 58.Schmahmann J.D., Sherman J.C. The cerebellar cognitive affective syndrome. Brain. 1998;121:561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- 59.Limperopoulos C., Bassan H., Gauvreau K., Robertson R.L., Sullivan N.R., Benson C.B., et al. Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors? Pediatrics. 2007;120:584–593. doi: 10.1542/peds.2007-1041. [DOI] [PubMed] [Google Scholar]
- 60.Balsters J.H., Apps M.A., Bolis D., Lehner R., Gallagher L., Wenderoth N. Disrupted prediction errors index social deficits in autism spectrum disorder. Brain. 2017;140:235–246. doi: 10.1093/brain/aww287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Freedman E.G., Foxe J.J. Eye movements, sensorimotor adaptation and cerebellar-dependent learning in autism: Toward potential biomarkers and subphenotypes. European J Neurosci. 2018;47:549–555. doi: 10.1111/ejn.13625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reeb-Sutherland B.C., Fox N.A. Eyeblink conditioning: A non-invasive biomarker for neurodevelopmental disorders. J Autism Dev Disord. 2015;45:376–394. doi: 10.1007/s10803-013-1905-9. [DOI] [PubMed] [Google Scholar]
- 63.Nebel M.B., Eloyan A., Nettles C.A., Sweeney K.L., Ament K., Ward R.E., et al. Intrinsic visual-motor synchrony correlates with social deficits in autism. Biol Psychiatry. 2016;79:633–641. doi: 10.1016/j.biopsych.2015.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nebel M.B., Joel S.E., Muschelli J., Barber A.D., Caffo B.S., Pekar J.J., Mostofsky S.H. Disruption of functional organization within the primary motor cortex in children with autism. Hum Bain Mapp. 2014;35:567–580. doi: 10.1002/hbm.22188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marko M.K., Crocetti D., Hulst T., Donchin O., Shadmehr R., Mostofsky S.H. Behavioural and neural basis of anomalous motor learning in children with autism. Brain. 2015;138:784–797. doi: 10.1093/brain/awu394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Triesch J., Teuscher C., Deák G.O., Carlson E. Gaze following: Why (not) learn it? Dev Sci. 2006;9:125–147. doi: 10.1111/j.1467-7687.2006.00470.x. [DOI] [PubMed] [Google Scholar]
- 67.Meyer M., Bekkering H., Haartsen R., Stapel J.C., Hunnius S. The role of action prediction and inhibitory control for joint action coordination in toddlers. J Exp Child Psychol. 2015;139:203–220. doi: 10.1016/j.jecp.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 68.Leonard H.C., Hill E.L. The impact of motor development on typical and atypical social cognition and language: A systematic review. Child Adolesc Ment Health. 2014;19:163–170. doi: 10.1111/camh.12055. [DOI] [PubMed] [Google Scholar]
- 69.Mous S.E., Jiang A., Agrawal A., Constantino J.N. Attention and motor deficits index non-specific background liabilities that predict autism recurrence in siblings. J Neurodev Disord. 2017;9:32. doi: 10.1186/s11689-017-9212-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pellicano E., Burr D. When the world becomes ‘too real’: A Bayesian explanation of autistic perception. Trends Cogn Sci. 2012;16:504–510. doi: 10.1016/j.tics.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 71.Lawson R.P., Rees G., Friston K.J. An aberrant precision account of autism. Front Hum Neurosci. 2014;8:302. doi: 10.3389/fnhum.2014.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.d'Souza D., d'Souza H., Karmiloff-Smith A. Precursors to language development in typically and atypically developing infants and toddlers: The importance of embracing complexity. J Child Lang. 2017;44:591–627. doi: 10.1017/S030500091700006X. [DOI] [PubMed] [Google Scholar]
- 73.Adolph K.E., Cole W.G., Vereijken B. In: Handbook of Intraindividual Variability Across the Life Span. Diehl M., Hooker K., Sliwinski M.J., editors. Routledge/Taylor & Francis Group; New York: 2015. Intraindividual variability in the development of motor skills in childhood; pp. 59–83. [Google Scholar]
- 74.Dosenbach N.U., Fair D.A., Cohen A.L., Schlaggar B.L., Petersen S.E. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nair A., Jolliffe M., Lograsso Y.S.S., Bearden C.E. A review of default mode network connectivity and its association with social cognition in adolescents with autism spectrum disorder and early-onset psychosis. Front Psychiatry. 2020;11:614. doi: 10.3389/fpsyt.2020.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Padmanabhan A., Lynch C.J., Schaer M., Menon V. The default mode network in autism. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2:476–486. doi: 10.1016/j.bpsc.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tang S., Sun N., Floris D.L., Zhang X., Di Martino A., Yeo B.T. Reconciling dimensional and categorical models of autism heterogeneity: A brain connectomics and behavioral study. Biol Psychiatry. 2020;87:1071–1082. doi: 10.1016/j.biopsych.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 78.Marrus N., Eggebrecht A.T., Todorov A., Elison J.T., Wolff J.J., Cole L., et al. Walking, gross motor development, and brain functional connectivity in infants and toddlers. Cereb Cortex. 2018;28:750–763. doi: 10.1093/cercor/bhx313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eggebrecht A.T., Elison J.T., Feczko E., Todorov A., Wolff J.J., Kandala S., et al. Joint attention and brain functional connectivity in infants and toddlers. Cereb Cortex. 2017;27:1709–1720. doi: 10.1093/cercor/bhw403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McKinnon C.J., Eggebrecht A.T., Todorov A., Wolff J.J., Elison J.T., Adams C.M., et al. Restricted and repetitive behavior and brain functional connectivity in infants at risk for developing autism spectrum disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4:50–61. doi: 10.1016/j.bpsc.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Das S., Glatard T., MacIntyre L.C., Madjar C., Rogers C., Rousseau M.E., et al. The MNI data-sharing and processing ecosystem. Neuroimage. 2016;124:1188–1195. doi: 10.1016/j.neuroimage.2015.08.076. [DOI] [PubMed] [Google Scholar]
- 82.Estes A., Zwaigenbaum L., Gu H., John T.S., Paterson S., Elison J.T., et al. Behavioral, cognitive, and adaptive development in infants with autism spectrum disorder in the first 2 years of life. J Neurodev Disord. 2015;7:24. doi: 10.1186/s11689-015-9117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dawson G., Toth K., Abbott R., Osterling J., Munson J., Estes A., Liaw J. Early social attention impairments in autism: Social orienting, joint attention, and attention to distress. Dev Psychol. 2004;40:271–283. doi: 10.1037/0012-1649.40.2.271. [DOI] [PubMed] [Google Scholar]
- 84.May T., McGinley J., Murphy A., Hinkley T., Papadopoulos N., Williams K.J., et al. A multidisciplinary perspective on motor impairment as an early behavioural marker in children with autism spectrum disorder. Aust Psychol. 2016;51:296–303. [Google Scholar]
- 85.Gotham K., Pickles A., Lord C. Standardizing ADOS scores for a measure of severity in autism spectrum disorders. J Autism Dev Disord. 2009;39:693–705. doi: 10.1007/s10803-008-0674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hus V., Gotham K., Lord C. Standardizing ADOS domain scores: Separating severity of social affect and restricted and repetitive behaviors. J Autism Dev Disord. 2014;44:2400–2412. doi: 10.1007/s10803-012-1719-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wetherby A.M., Allen L., Cleary J., Kublin K., Goldstein H. Validity and reliability of the communication and symbolic behavior scales developmental profile with very young children. J Speech Lang Hear Res. 2002;45:1202–1218. doi: 10.1044/1092-4388(2002/097). [DOI] [PubMed] [Google Scholar]
- 88.Mullen E.M. AGS; Circle Pines, MN: 1995. Mullen Scales of Early Learning. [Google Scholar]
- 89.Bishop S.L., Guthrie W., Coffing M., Lord C. Convergent validity of the Mullen Scales of Early Learning and the differential ability scales in children with autism spectrum disorders. Am J Intellect Dev Disabil. 2011;116:331–343. doi: 10.1352/1944-7558-116.5.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zwaigenbaum L., Bryson S., Rogers T., Roberts W., Brian J., Szatmari P. Behavioral manifestations of autism in the first year of life. Int J Dev Neurosci. 2005;23:143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 91.Burns T.G., King T.Z., Spencer K.S. Mullen scales of early learning: The utility in assessing children diagnosed with autism spectrum disorders, cerebral palsy, and epilepsy. Appl Neuropsychol Child. 2013;2:33–42. doi: 10.1080/21622965.2012.682852. [DOI] [PubMed] [Google Scholar]
- 92.Bodfish J.W., Symons F.J., Parker D.E., Lewis M.H. Varieties of repetitive behavior in autism: Comparisons to mental retardation. J Autism Dev Disord. 2000;30:237–243. doi: 10.1023/a:1005596502855. [DOI] [PubMed] [Google Scholar]
- 93.Mirenda P., Smith I.M., Vaillancourt T., Georgiades S., Duku E., Szatmari P., et al. Validating the repetitive behavior scale-revised in young children with autism spectrum disorder. J Autism Dev Disord. 2010;40:1521–1530. doi: 10.1007/s10803-010-1012-0. [DOI] [PubMed] [Google Scholar]
- 94.Wolff J.J., Botteron K.N., Dager S.R., Elison J.T., Estes A.M., Gu H., et al. Longitudinal patterns of repetitive behavior in toddlers with autism. J Child Psychol Psychiatry. 2014;55:945–953. doi: 10.1111/jcpp.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lam K.S., Aman M.G. The Repetitive Behavior Scale-Revised: Independent validation in individuals with autism spectrum disorders. J Autism Dev Disord. 2007;37:855–866. doi: 10.1007/s10803-006-0213-z. [DOI] [PubMed] [Google Scholar]
- 96.Lord C., Rutter M., DiLavore P.C., Risi S. Western Psychological Services; Los Angeles, CA: 1999. Autism Diagnostic Observation Schedule: Manual. [Google Scholar]
- 97.Lord C., Rutter M., DiLavore P.C., Risi S., Gotham K., Bishop S. 2nd ed. Western Psychological Services; Torrance, CA: 2012. Autism Diagnostic Observation Schedule. [Google Scholar]
- 98.American Psychiatric Association . 4th ed rev. American Psychiatric Press; Washington, DC: 2000. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 99.Nielsen A.N., Greene D.J., Gratton C., Dosenbach N.U., Petersen S.E., Schlaggar B.L. Evaluating the prediction of brain maturity from functional connectivity after motion artifact denoising. Cereb Cortex. 2019;29:2455–2469. doi: 10.1093/cercor/bhy117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pruett J.R., Jr., Kandala S., Hoertel S., Snyder A.Z., Elison J.T., Nishino T., et al. Accurate age classification of 6 and 12 month-old infants based on resting-state functional connectivity magnetic resonance imaging data. Dev Cogn Neurosci. 2015;12:123–133. doi: 10.1016/j.dcn.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rosvall M., Bergstrom C.T. Maps of random walks on complex networks reveal community structure. Proc Natl Acad Sci U S A. 2008;105:1118–1123. doi: 10.1073/pnas.0706851105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Seitzman B.A., Gratton C., Marek S., Raut R.V., Dosenbach N.U., Schlaggar B.L., et al. A set of functionally-defined brain regions with improved representation of the subcortex and cerebellum. Neuroimage. 2020;206:116290. doi: 10.1016/j.neuroimage.2019.116290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wickham H., Averick M., Bryan J., Chang W., McGowan L.D.A., François R., et al. Welcome to the Tidyverse. J Open Source Softw. 2019;4:1686. [Google Scholar]
- 104.Van Rossum G., Drake F.L., Jr. Vol. 620. Centrum voor Wiskunde en Informatica; Amsterdam: 1995. (Python Tutorial). [Google Scholar]
- 105.Pedregosa F., Varoquaux G., Gramfort A., Michel V., Thirion B., Grisel O., et al. Scikit-learn: Machine learning in Python. J Mach Learn Res. 2011;12:2825–2830. [Google Scholar]
- 106.Gardner W., Mulvey E.P., Shaw E.C. Regression analyses of counts and rates: Poisson, overdispersed Poisson, and negative binomial models. Psychol Bull. 1995;118:392–404. doi: 10.1037/0033-2909.118.3.392. [DOI] [PubMed] [Google Scholar]
- 107.Menon V. The triple network model, insight, and large-scale brain organization in autism. Biol Psychiatry. 2018;84:236–238. doi: 10.1016/j.biopsych.2018.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gratton C., Sun H., Petersen S.E. Control networks and hubs. Psychophysiology. 2018;55 doi: 10.1111/psyp.13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Power J.D., Petersen S.E. Control-related systems in the human brain. Curr Opin Neurobiol. 2013;23:223–228. doi: 10.1016/j.conb.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Neta M., Schlaggar B.L., Petersen S.E. Separable responses to error, ambiguity, and reaction time in cingulo-opercular task control regions. Neuroimage. 2014;99:59–68. doi: 10.1016/j.neuroimage.2014.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fair D.A., Dosenbach N.U., Church J.A., Cohen A.L., Brahmbhatt S., Miezin F.M., et al. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci U S A. 2007;104:13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hong S.J., Vogelstein J.T., Gozzi A., Bernhardt B.C., Yeo B.T., Milham M.P., Di Martino A. Toward neurosubtypes in autism. Biol Psychiatry. 2020;88:111–128. doi: 10.1016/j.biopsych.2020.03.022. [DOI] [PubMed] [Google Scholar]
- 113.Grady C., Sarraf S., Saverino C., Campbell K. Age differences in the functional interactions among the default, frontoparietal control, and dorsal attention networks. Neurobiol Aging. 2016;41:159–172. doi: 10.1016/j.neurobiolaging.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 114.Park D.C., Reuter-Lorenz P. The adaptive brain: Aging and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Keown C.L., Datko M.C., Chen C.P., Maximo J.O., Jahedi A., Müller R.A. Network organization is globally atypical in autism: A graph theory study of intrinsic functional connectivity. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2:66–75. doi: 10.1016/j.bpsc.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Werchan D.M., Amso D. A novel ecological account of prefrontal cortex functional development. Psychol Rev. 2017;124:720–739. doi: 10.1037/rev0000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rosen M.L., Amso D., McLaughlin K.A. The role of the visual association cortex in scaffolding prefrontal cortex development: A novel mechanism linking socioeconomic status and executive function. Dev Cogn Neurosci. 2019;39:100699. doi: 10.1016/j.dcn.2019.100699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Green P., MacLeod C.J. SIMR: An R package for power analysis of generalized linear mixed models by simulation. Methods Ecol Evol. 2016;7:493–498. [Google Scholar]
- 119.Marek S., Tervo-Clemmens B., Calabro F.J., Montez D.F., Kay B.P., Hatoum A.S., et al. Towards reproducible brain-wide association studies. bioRxiv. 2020 doi: 10.1101/2020.08.21.257758. [DOI] [Google Scholar]
- 120.Shi F., Salzwedel A.P., Lin W., Gilmore J.H., Gao W. Functional brain parcellations of the infant brain and the associated developmental trends. Cereb Cortex. 2018;28:1358–1368. doi: 10.1093/cercor/bhx062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gao W., Alcauter S., Elton A., Hernandez-Castillo C.R., Smith J.K., Ramirez J., Lin W. Functional network development during the first year: Relative sequence and socioeconomic correlations. Cereb Cortex. 2015;25:2919–2928. doi: 10.1093/cercor/bhu088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Buckner R.L., Krienen F.M., Castellanos A., Diaz J.C., Yeo B.T. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:2322–2345. doi: 10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wheelock M.D., Austin N.C., Bora S., Eggebrecht A.T., Melzer T.R., Woodward L.J., Smyser C.D. Altered functional network connectivity relates to motor development in children born very preterm. Neuroimage. 2018;183:574–583. doi: 10.1016/j.neuroimage.2018.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Diedrichsen J., Zotow E. Surface-based display of volume-averaged cerebellar imaging data. PLoS One. 2015;10 doi: 10.1371/journal.pone.0133402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.