Abstract
Rationale & Objective
There has been an increasing demand for the expertise provided by a renal genetics clinic. Such programs are limited in the United States and typically operate in a genomics research setting. Here we report a 3-year, real-world, single-center renal genetics clinic experience.
Study Design
Retrospective cohort.
Setting & Participants
Outpatient cases referred to the renal genetics clinic of the Cleveland Clinic between January 2019 and March 2022 were reviewed.
Analytical Approach
Clinical and laboratory characteristics were analyzed. All genetic testing was performed in clinical labs.
Results
309 new patients referred from 15 specialties were evaluated, including 118 males and 191 females aged 35.1 ± 20.3 years. Glomerular diseases were the leading presentation followed by cystic kidney diseases, electrolyte disorders, congenital anomalies of kidneys and urinary tract, nephrolithiasis, and tubulointerstitial kidney diseases. Dysmorphic features were noted in 27 (8.7%) patients. Genetic testing was recommended in 292 (94.5%) patients including chromosomal microarray (8.9%), single-gene tests (19.5%), multigene panels (77.3%), and exome sequencing (17.5%). 80.5% of patients received insurance coverage for genetic testing. 45% (115/256) of patients had positive results, 25% (64/256) had variants of unknown significance, and 22.3% (57/256) had negative results. 43 distinct monogenic disorders were diagnosed. Family history of kidney disease was present in 52.8% of patients and associated with positive genetic findings (OR, 2.28; 95% CI, 1.40-3.74). 69% of patients with positive results received a new diagnosis and/or a change in the diagnosis. Among these, 39.7% (31/78) of patients received a significant change in disease management.
Limitations
Retrospective and single-center study.
Conclusions
The renal genetics clinic plays important roles in the diagnosis and management of patients with genetic kidney diseases. Multigene panels are the most frequently used testing modality with a high diagnostic yield. Family history of kidney disease is a strong indication for renal genetics clinic referral.
Index Words: Genetic testing, kidney disease, renal genetics
Plain-Language Summary.
Although the demand is growing, there are still very few renal genetics clinics in the United States. This study reported a 3-year, real-world experience of the renal genetics clinic in the Cleveland Clinic. Presentations of patients referred to the renal genetics clinic were variable, and more than half of the patients had a family history of kidney disease. All genetic testing was performed in clinical labs for which the majority of patients received insurance coverage. Nearly half of the patients in the renal genetics clinic learned the genetic cause of their disease, which could also affect their family members. More than two-thirds of patients with positive testing results received a new diagnosis and or a change in the diagnosis. Among these, more than one-third of patients received a significant change in disease management. This study highlighted the importance of renal genetics clinics, which can improve the diagnosis and management of patients with genetic kidney diseases.
Kidney disease is associated with high morbidity and mortality, affecting 850 million patients worldwide.1 A positive family history is reported by 24%-34% of patients with chronic kidney disease (CKD), and familial clustering is common in patients with kidney failure,2, 3, 4 indicating that a significant fraction of kidney disease is genetic in origin. One study revealed a monogenic (single-gene disorder) cause in approximately 1 in 10 adults in a cohort of CKD patients.5 Another study showed that ∼30% of CKD with onset <25 years can be attributed to an established monogenic cause.6 To date, approximately 450 monogenic genes have been associated with kidney diseases.6 Further, copy number variation is an important cause for genetic syndromes with kidney involvement, such as congenital kidney malformations.7,8 Taken together, genetic kidney diseases may be rare individually, but they are not uncommon collectively.9 Nephrologists should be encouraged to pursue genetic evaluation for their patients.10 The expertise in renal genetic clinics could be a valuable resource, as barriers for nephrologists to adopt genetic tests have been noted.11
With advances in genetic diagnostic technology, particularly next-generation sequencing and chromosomal microarray, genetic testing is becoming more accessible and affordable for patients and families with suspected genetic kidney disease.10,12,13 A genetic assessment has important diagnostic and prognostic implications and facilitates development of personalized management strategies as well as family counseling.14,15 There is an increasing demand for renal genetics clinics, and relatively long-term studies in Australia, England, and Ireland have demonstrated multidisciplinary renal genetics clinics improve the patient diagnosis and management outcomes,16, 17, 18 but only a few medical centers have this service available in the United States.12,19,20 Further, genetic testing is often performed in a research setting.21 Data from real-world daily practice of renal genetics clinics is limited.12,19
The renal genetics clinic of the Cleveland Clinic was initiated in December 2018, and is led by a physician with dual roles as nephrologist and medical geneticist, which is uncommon. All patients undergo a thorough evaluation from nephrology and genetics, with testing performed in Clinical Laboratory Improvement Amendments-certified labs. Here we review our experience with a goal to assess the value of genetic evaluation, testing modalities, and indicators for referral to renal genetics clinics.
Methods
Study Population
This study was approved by the Cleveland Clinic Institutional Review Board (protocol number 18-705). After informed consent, patients who were evaluated between January 2019 and March 2021 at the joint Center for Personalized Genetic Healthcare-Glickman Urological and Kidney Institute Renal Genetics Clinic were enrolled. All medical records were reviewed independently by 2 researchers (X.T. and C.B.) to characterize the clinical features of patients undergoing genetic testing. Insurance information was also reviewed.
Structure of the Renal Genetics Clinic and Workflow
The renal genetics clinic of the Cleveland Clinic was started by one physician (X.W.) and rotating genetic counselors in December 2018. It has gradually developed into a group of 5 physicians including 2 additional nephrologists, 2 additional medical geneticists, 3 genetic counselors, and 1 molecular laboratory geneticist. Clinic is held 2 days weekly. Patients are first seen by a genetic counselor to collect medical history and a detailed, 3-generation pedigree. Next, they are evaluated by a physician, including physical examination and discussion of testing and management strategies. The evaluation is concluded by the genetic counselor, who performs pretest counseling, including review of the Genetic Information Nondiscrimination Act.22,23 Genetic testing is not ordered until after the confirmation of insurance pre-authorization or a self-pay decision by patients and families. Sponsored testing is supported by commercial vendors at no cost to patients and is offered to patients who are denied coverage or who are unable to pay the testing expense not covered by insurance. Testing results are interpreted collaboratively by the ordering physician and genetic counselor. Testing results are subsequently reported to the patient by the genetic counselor with post-test counseling. A follow-up visit with a physician is offered if clinically indicated or requested by the patient.
Genetic Testing
DNA samples were collected from blood or buccal swab. All tests were completed in Clinical Laboratory Improvement Amendments-certified labs including GeneDx (Gaithersburg, Maryland), PreventionGenetics (Marshfield, Wisconsin), Natera (Austin, Texas), Blueprint Genetics (Seattle, Washington), Invitae (San Francisco, California), Otolaryngology and Renal Research Laboratories in the University of Iowa (Iowa City, Iowa), Genetics and Genomics Diagnostic Laboratory at the Cincinnati Children's Hospital Medical Center, Cleveland Clinic Molecular Genetics Laboratory (Cleveland, Ohio), or the Mayo Clinic Molecular Genetics Laboratory (Rochester, Minnesota). Genetic testing methodologies included chromosomal microarray, single-gene test, multigene panel, and exome sequencing. Exome sequencing may include mitochondrial gene sequencing. Data were analyzed and interpreted according to the American College of Medical Genetics guidelines.24 Testing results were categorized into 4 groups: (1) a positive result is defined as a pathogenic or likely pathogenic variant in an autosomal dominant or X-linked disorder, a homozygous or compound heterozygous pathogenic or likely pathogenic variants in an autosomal recessive condition, an X-linked recessive condition in females, or 2 APOL1 risk alleles (G1 [rs73885319, p.S342G] and G2 [rs71785313, p.N388_Y389del]) in the homozygous or compound heterozygous state (G1/G1, G2/G2, or G1/G2); (2), carrier of autosomal recessive conditions including individuals with heterozygous pathogenic or likely pathogenic variant in an autosomal recessive disorder; (3) negative result; and (4) variant of unknown significance.24
Statistics
Patient characteristics were summarized using frequency with proportion for categorical data and mean with standard deviation for continuous data. Pie charts were used to describe genetic test decisions (suggested, suggested but declined by patients, not suggested but ordered per patient, and not suggested and not ordered), and genetic findings among those who were tested. Among those tested, a bar chart was used to summarize distribution of testing modalities used. A Venn diagram approach was applied to describe the overlap or non-overlap of genetic testing modalities. We also used bar charts to present specific genetic findings, which were stratified by testing modalities and presentations, respectively. Summary statistics were used to compare between patients with positive and negative results. Multivariable logistic regression was used to test the associations of genetic findings and patient baseline demographics (age, race), family history of kidney disease, and disease presentations. All statistical analyses were performed using R version 4.1.3.
Results
Characteristics of Patients
Between January 2019, and March 2021, 319 new patients were evaluated by the renal genetics clinic at the Cleveland Clinic main campus (Cleveland, Ohio). Of these, 309 patients from 299 pedigrees consented to participate in this study, including 118 males and 191 females aged 35.1 ± 20.3 years. Characteristics of patients at index visit are shown in Table 1. Sixty-four (20.7%) patients were under the age of 18 years. The youngest patient seen was 3 weeks old. Patient presentations included glomerular disease (33%), cystic kidney disease (25.2%), electrolyte disorders (24.9%), congenital anomalies of kidneys and urinary tract (6.8%), nephrolithiasis and or nephrocalcinosis (3.2%), tubulointerstitial kidney disease (1%), and angiomyolipoma (0.3%). Fourteen asymptomatic patients referred for a family history of kidney disease (1.6%) or for living kidney donor evaluation (2.9%) were seen. One hundred and sixty-three (52.8%) patients had a family history of kidney disease. No history of consanguinity was reported by any patient.
Table 1.
Characteristics of Patients in the Renal Genetics Clinic at Index Visit
| Factor | Total (N=309) |
|---|---|
| Age (at first visit), y, mean ± SD | 35.1 ± 20.3 |
| Male, No. (%) | 118 (38.2%) |
| White, No. (%) | 232 (75.1%) |
| African American, No. (%) | 49 (15.9%) |
| Family history of kidney disease, No. (%) | |
| Yes | 163 (52.8%) |
| No | 143 (46.3%) |
| N/A | 3 (1.0%) |
| Presentations, No. (%) | |
| Glomerular disease | 102 (33.0%) |
| Cystic kidney disease | 78 (25.2%) |
| Electrolyte disorders | 77 (24.9%) |
| Congenital anomalies of kidneys and urinary tract | 21 (6.8%) |
| Kidney stones and or nephrocalcinosis | 10 (3.2%) |
| Living donor | 9 (2.9%) |
| Tubulointerstitial disease | 3 (1.0%) |
| Renal vascular disease | 3 (1.0%) |
| Family history only | 5 (1.6%) |
| Insurance status, No. (%) | |
| Private | 184 (59.5%) |
| Medicare | 50 (16.2%) |
| Medicaid | 64 (20.7%) |
| International patient | 3 (1.0%) |
| Military | 5 (1.60%) |
| No insurance | 2 (0.60%) |
| Kidney failure, No. (%) | 33 (10.7%) |
| eGFRa, 60 mL/min/1.73 m2, mean ± SD | 88.7 ± 45.6 |
Abbreviations: eGFR, estimated glomerular filtration rate; N/A, not applicable; SD, standard deviation.
Data not available for all subjects. eGFR in pediatric patients is calculated from the Schwartz equation based on a stable serum creatinine and height.
Referring Providers
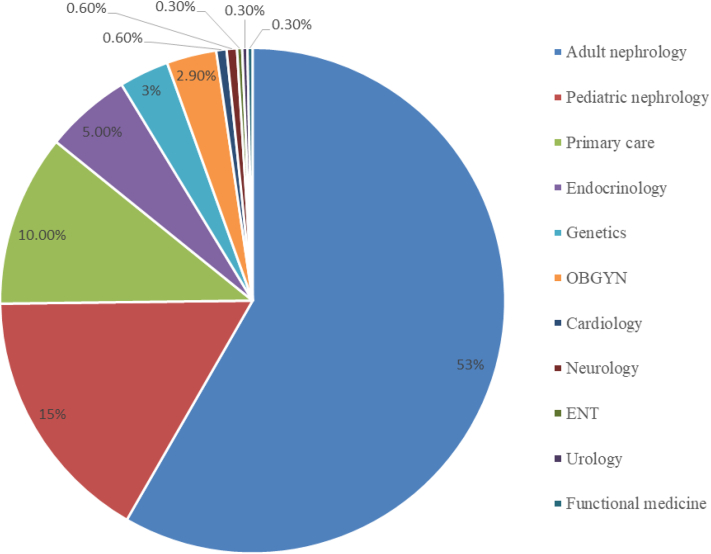
As shown in Fig 1, all patients (apart from 8 self-referred patients) were referred by medical professionals including adult nephrologists (164, 53.1%), pediatric nephrologists (46, 14.9%), geneticists (9, 2.9%), primary care providers (31, 10.0%), endocrinologists (16, 5.2 %), obstetrician-gynecologists (9, 2.9%), cardiologists (2, 0.65%), neurologists (2, 0.65%), ear, nose, and throat specialist (1, 0.3%), urologist (1, 0.3%), hepatologist (1, 0.3 %), and functional medicine physician (1, 0.3 %).
Figure 1.
Referrals from multiple specialties to the Renal Genetics Clinic. Abbreviations: OBGYN, obstetrician-gynecologist; ENT, ear, nose, and throat specialist.
Genetic Evaluation in the Renal Genetics Clinic
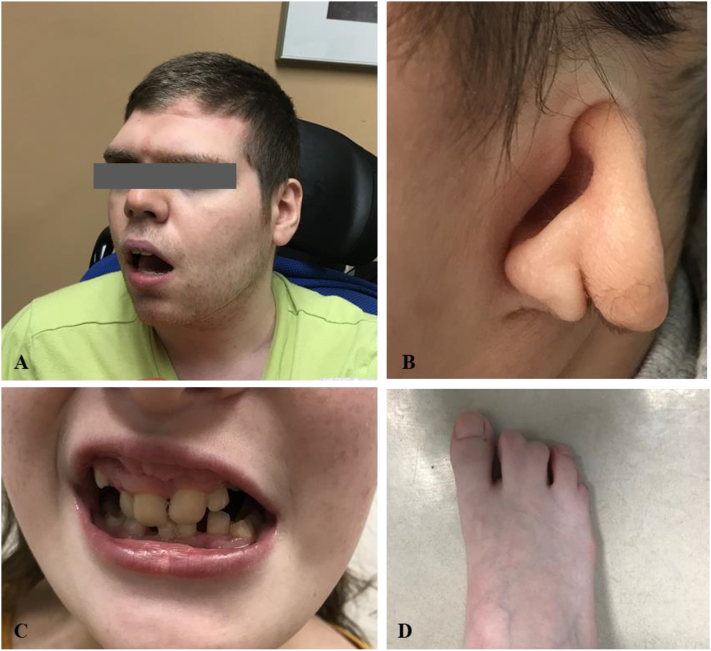
A thorough history collection and physical examination including dysmorphism assessment were completed for all patients seen at the renal genetics clinic. Variable dysmorphic features were noted in 27 (8.7%) patients (Fig 2). After clinical evaluation, 292 (95.4%) patients agreed to genetic testing, and 3 (0.97%) patients with a low likelihood for a genetic disorder were tested per patients’ request for reassurance and joint decision-making. Four (1.29%) patients declined because of concerns for life insurance discrimination. An additional 10 (3.24%) patients declined for other reasons.
Figure 2.
Examples of dysmorphic features noted in the Renal Genetics Clinic. (A) Microcephaly with backward-sloping forehead. (B) Ear malformation. (C) Dental anomaly. (D) Syndactyly.
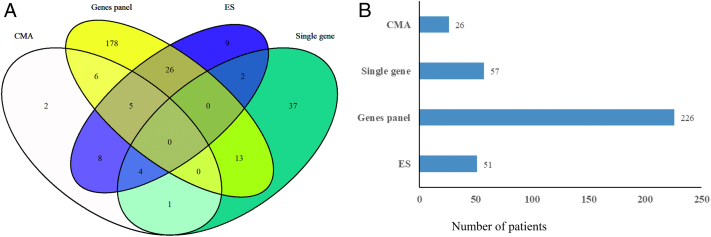
As shown in Fig 3A, multiple genetic testing modalities were utilized. For 63 patients, more than one testing modality (overlapping area) were utilized following a tiered testing approach. Exome sequencing was utilized as the last tier modality in 31 patients when other modalities, including multigene panels, yielded negative or indeterminate results. Among available testing modalities (Fig 3B), multigene panels (226, 77.4%) were most frequently utilized followed by single-gene tests (57, 19.5%), exome sequencing (51, 17.5%), and chromosomal microarray (26, 8.9%). The presence of dysmorphism was associated with increased utilization of chromosomal microarray (n=16, 59.3%) and exome sequencing (n=15, 55%).
Figure 3.
Genetics testing modalities in the Renal Genetics Clinic. (A) Venn graph showing utilization of more than one genetic testing modality (overlapping area) in patients. (B) Number of patients who were tested by each modality.
Abbreviations: CMA, chromosomal microarray; ES, exome sequencing; WES, whole exome sequencing.
Diagnostic Yield and Value in Management in the Renal Genetic Clinic
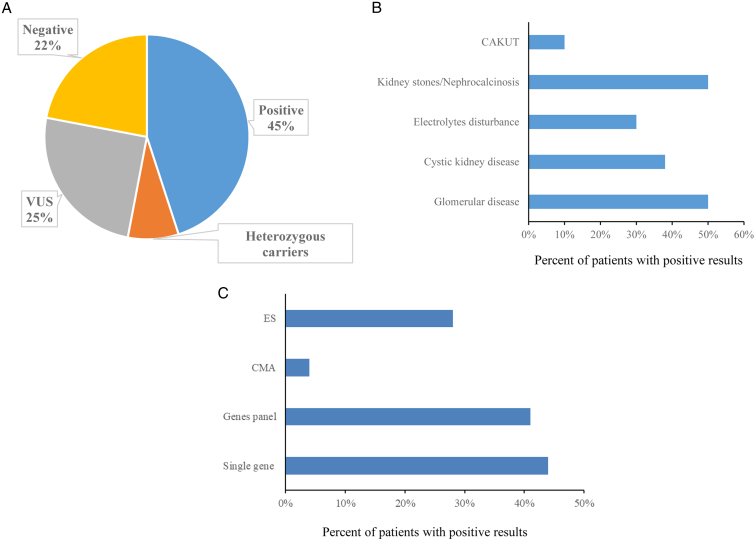
Two hundred fifty-nine patients had genetic testing results at the time of article submission. Genetic test results were negative for the 3 patients who had a low probability of a genetic disorder, and genetic testing was not physician recommended. Of the 256 patients who underwent recommended genetic testing (Fig 4A), 44.9% (115) had positive results, 7.8% (20) had only 1 pathogenic variant for a phenotype-related autosomal recessive disorder (heterozygous carrier), 25% (64) had variants of uncertain significance, and 22.3% (57) had negative results.
Figure 4.
The diagnostic yield in the Renal Genetics Clinic. (A) Test results among all patients who were suggested for genetic testing (n=256). (B) Diagnostic yield among patients with different presentations. (C) Diagnostic yield of each testing modality.
Abbreviations: CAKUT, congenital anomalies of the kidney and urinary tract; CMA, chromosomal microarray; ES, exome sequencing; VUS, variant of unknown significance.
The diagnostic yield of each testing modality is shown in Fig 4C. Forty-four percent of patients who underwent single-gene panel testing had a positive result, followed by multigene panel (40.9%), exome sequencing (28.1%), and chromosomal microarray (4%). The majority of positive results in our patients were disclosed by multigene panels (71.7%) followed by single-gene panel (18.6%), exome sequencing (8.9%), and chromosomal microarray (0.9%).
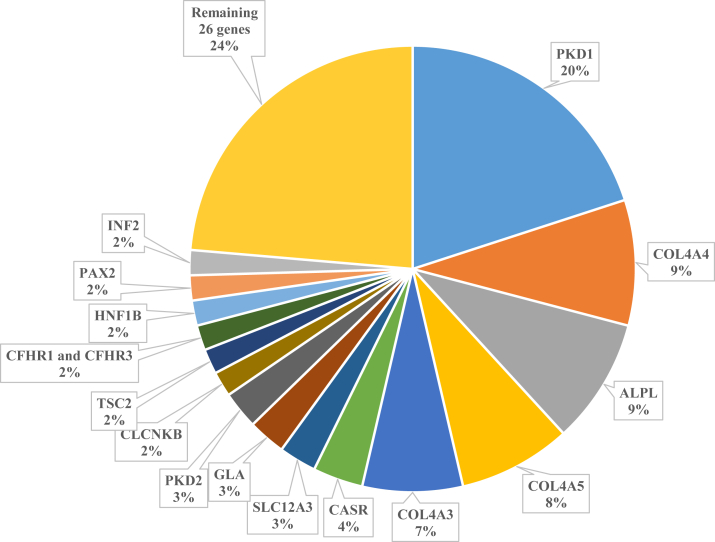
As shown in Fig 5 and Table S1, 43 distinct monogenic disorders were diagnosed among patients with positive results. Of these, 16 genes collectively accounted for 77% of the genetic diagnoses: autosomal dominant polycystic disease due to pathogenic variants in PKD1 (22 patients) or PKD2 (3); glomerulopathy due to pathogenic variants in COL4A3 (8), COL4A4 (10), COL4A5 (9), PAX2 (2), or INF2 (2); hypophosphatasia with or without nephrocalcinosis due to pathogenic variants in ALPL (10); electrolyte disorders due to pathogenic variants in CASR (4), SLC12A3 (3), or CLCNKB (2); Fabry disease due to pathogenic variants in GLA (3); thrombotic microangiopathies due to homozygous deletion of CFHR1 and CFHR3 (2); HNF1B-associated tubulointerstitial disease (2); and tuberous sclerosis due to pathogenic variants in TSC2 (2). The remaining 26 monogenic disorders identified were unique to single patients (Table S1). Two chromosomal disorders, including 22q11.2 deletion syndrome (1) and 17q12 recurrent deletion syndrome (1), were identified. Four patients were detected with 2 APOL1 risk alleles.
Figure 5.
Common genetic findings in the Renal Genetics Clinic.
As shown in Table 2, following genetic evaluation, 67.8% (78/115) of patients with positive results received a new diagnosis or a change in diagnosis. Prior diagnoses were confirmed in 32.2% (37/115) of patients. The new or changed diagnosis among patients led to a significant change in management in 39.7% (31/78) of patients, including avoidance of steroids and immunosuppression in 13 cases with a genetic form of glomerular disease, initiation of enzyme replacement therapy or chaperone therapy in 2 cases with Fabry disease, adjustment of treatment period with eculizumab in 1 case with atypical hemolytic uremic syndrome and 1 case with C3 glomerulopathy, initiation of tolvaptan in 5 cases with PKD with negative family history, avoidance of unnecessary parathyroid surgery in 1 case with familial hypocalciuric hypercalcemia, initiation of anti-FGF23 monoclonal antibody in 1 case with X-linked hypophosphatemic rickets, initiation of enzyme replacement therapy in 4 cases with hypophosphatasia, initiation of carnitine cocktail in 1 case with mitochondrial disorder, as well as personalized calcium and vitamin D management during pregnancy in 1 patient with autosomal dominant hypocalcemia25 and 1 case with 24-hydroxylase deficiency.
Table 2.
The Diagnostic Implications Among Patients Who Received Positive Results (n=115)
| No. | Percentage | |
|---|---|---|
| New diagnosis | 72 | 62.6% |
| Change of diagnosis | 6 | 5.2% |
| Confirmed a priori clinical diagnosis | 37 | 32.2% |
Characteristics Associated With a Positive Genetic Testing Result in the Renal Genetics Clinic
As shown in Table 3, positive family history of kidney disease was associated with a positive genetic testing result (P < 0.001). Compared to patients with congenital anomalies of the kidney and urinary tract and/or electrolyte disorders, patients with glomerular disease were more likely to be identified with a genetic disorder.
Table 3.
Clinical Features Associated With Positive Genetic Testing Results
| Features | Odds Ratio (95% CI) | P (χ2 test) |
|---|---|---|
| Age < 25 y | 0.81 (0.48-1.35) | 0.44 |
| Male | 1.15 (0.71-1.87) | 0.57 |
| White | 1.41 (0.81-2.51) | 0.22 |
| Family history of kidney disease | 2.28 (1.40-3.74) | 0.0007 |
| eGFR <60 mL/min/1.73 m2 | 1.2 (0.76-2.03) | 0.45 |
| Dysmorphism | 1.06 (0.45-2.42) | 0.89 |
| Presentations: CAKUT vs glomerular disease | 0.14 (0.02-0.58) | 0.004 |
| Presentations: Cystic kidney disease vs glomerular disease | 0.91 (0.47-1.75) | 0.78 |
| Presentations: Electrolytes disorders vs glomerular disease | 0.39 (0.20-0.76) | 0.005 |
| Presentations: Kidney stones or nephrocalcinosis vs glomerular disease | 0.99 (0.24-4.44) | >0.99 |
Abbreviations: CAKUT, congenital anomalies of the kidney and urinary tract; CI, confidence interval; eGFR, estimated glomerular filtration rate.
Insurance Approval and Denial for Genetic Testing
Private insurance was the predominant payer (59.5%), followed by Medicaid (20.7%) and Medicare (16.2%) (Table 1). Only 2 patients (0.6%) were without insurance. Data on insurance approval and denial was collected in 169 patients between January 2019 and June 2021. Of those, 64% received insurance pre-authorization for genetic testing. Private insurance and Medicaid had comparable approval rates (66% vs 70%, P = 0.61), whereas Medicare had a significantly lower approval rate compared to private insurance (33% vs 66%, P = 0.03) and Medicaid (33% vs 70%, P = 0.04). For patients who were denied coverage, a peer-to-peer review or appeal process was performed that resulted in overturn of the denial in 46% of patients. Insurance data for international patients and military insurance were not analyzed because of limited sample size.
Discussion
In this study, we reported the experience of a renal genetics clinic with the largest group size from a single center in a period of 3 years. The investigation revealed several important findings. First, the phenotype of patients seen at the renal genetics clinic are variable and referrals come from a broad range of specialties, which suggest the great need for kidney genetics expertise. Second, a thorough clinical evaluation including dysmorphism assessment is helpful to guide testing strategies. Multigene panels are most frequently used as a diagnostic modality in the renal genetics clinic and have a high diagnostic yield. Third, genetic evaluation plays an important role in diagnosis and management in patients with genetic kidney diseases. Family history of kidney disease is an important indicator to refer a patient to renal genetics clinic. Finally, denial by insurers for renal genetic testing is common and Medicare is most likely to decline testing coverage.
The variable presentations of patients in the renal genetics clinic represent all aspects of clinical nephrology. Compared to other renal genetics clinics16,19,26 where cystic kidney disease was cited as the most common clinical presentation, the large number of referrals for glomerular disease in our patient population reflects the diagnostic complexity in an academic referral center providing comprehensive specialty care comprised of subspecialized nephrology clinics, virtual pathology consultations, kidney transplants, and an infusion center. The increasing pursuit of a genetic diagnosis in glomerular diseases also underscores its clinical relevance in preventing invasive kidney biopsies, avoiding deleterious effects of ineffective long-term immunosuppression, and evaluating risks for related living donors. Though CKD is slightly more common in women (14%) than men (12%) in the United States,27 the female predominance (61%) in our patient population is noteworthy, likely reflecting female health consciousness and inherent maternal concern for hereditary diseases as child bearers.
Our renal genetics clinic was set up to be a 2-day clinic led by a physician with dual role as nephrologist and medical geneticist. This uncommon pairing brought focused insights and promoted the collaborative expansion of diagnostic testing in the niche field of genetic kidney diseases. A comprehensive pretest evaluation served as an initial screen for testing necessity thereby increasing overall diagnostic yield and cost-effectiveness. Compared to research genomics, clinical genomics has been suggested to be the primary medium in obtaining a genetic diagnosis as it facilitates active participation of patients in shared decision-making with short turnaround time.21 In this study, all testing was performed by Clinical Laboratory Improvement Amendments laboratories, which provided standardized interpretations of the genetic testing results. We utilized a multi-tiered approach to diagnostic testing, with multigene panels most frequently utilized. The selection of multigene panel was phenotype-driven and resulted in a high diagnostic rate in our center, which is consistent with the other studies.19,28 Exome sequencing was offered as a second or third tier test if other testing modalities were unrevealing. Together with chromosomal microarray, the multigene panel obviated the need for exome sequencing in most cases. Prior studies of exome sequencing in research settings led to identification of monogenic disorders in 29.5% of patients with steroid-resistant nephrotic syndrome29 and 29.4% with nephrolithiasis or nephrocalcinosis before the age of 25.30 In our clinical setting, exome sequencing resulted in an overall diagnostic yield of 31.2% when performed mostly as the last tier modality after a nondiagnostic multigene panel. This indicated the powerful utility of exome sequencing in ruling out genetic disorders should clinical suspicion remain.
The clinical utilities of genetic diagnosis in kidney diseases are multi-dimensional and include (1) guiding management and adoption of efficacious treatment, (2) avoiding unnecessary and potentially invasive workups, (3) promoting earlier recognition of extrarenal diseases and coordination of multidisciplinary care, (4) predicting prognosis, (5) facilitating counseling for affected patients and family members, (6) family planning, and (7) assessing risk for kidney donors.17,20,31, 32, 33, 34, 35, 36, 37 Compared to other studies that include the family counseling and or new referrals in the analysis about the impact of genetic testing in management,12,17,26 our analysis focused on significant changes by defining them as initiation or discontinuation or dosage change of medications to further highlight the importance of genetic testing in management of patients with kidney disease. Similar to experiences in other clinics,12,19 all patients in our clinic benefit from family counseling after receiving positive genetic testing results. In addition to clinical utility, the academic value of genetic testing in kidney diseases should not be understated. The identification of novel variants associated with clinical phenotype will continue to broaden our understanding of genetic kidney diseases and refine phenotype-driven testing panels.
Insurance coverage and racial disparity have been identified as barriers to the accessibility of genetic testing. Despite a potential overturn of initial denial of coverage, the appeal process inevitably deterred patients from proceeding. This generated administrative overhead and led to diagnostic delay. The scarcity of non-White patients, in stark contrast with the epidemiological profile of our patient population, may be driven by multiple factors that include late clinical presentation, missed appointments, lack of insurance coverage, access to health care, language barriers, limited health literacy, and trust in health care systems. No cost testing offered by commercial vendors served as a valuable option for expanding access to patient populations with access barriers (lack of insurance coverage or inability to pay high copay).
Consistent with finding in another cohort study utilizing exome sequencing in CKD patients,5 our study revealed that a positive family history has a strong correlation with diagnostic yield. This suggests that a family history should be incorporated by clinicians as an indispensable element in clinical encounters to prompt referrals to renal genetics clinics.
This study has several limitations. First, this is a retrospective study from a single center. Second, there is a small number of living kidney donors, which limits the analysis of the utility of genetic testing for kidney transplantation. Furthermore, the female predominance in this study may limit the findings of X-linked, particularly recessive, disorders.
In summary, this is the largest single-center study of a renal genetics clinic incorporated into clinical practice in the United States to date. Our renal genetics clinic has demonstrated its key role in the diagnosis and management of genetic kidney diseases. Multigene panels are the most frequently used testing modality with a high diagnostic yield. Further expansion of the utilization of genetic testing in kidney diseases will be primarily contingent on clinician awareness and patient access to testing modalities. Future investigation into diagnostic algorithms in different renal genetics patients such as children versus adults and Black versus non-Black patients will be much needed to guide the practice of the renal genetics clinic.
Article Information
Authors’ Full Names and Academic Degrees
Xin Yee Tan, MD, Chloe Borden, MS-2, Mary-Beth Roberts, MS, Sarah Mazzola, MS, Queenie K.-G. Tan, MD, PhD, Richard Fatica, MD, James F. Simon, MD, Juan Calle, MD, Jonathan Taliercio, DO, Katherine Dell, MD, Laura Ferreira Provenzano, MD, Diana Deitzer, DO, Hernan Rincon-Choles, MD, Ali Mehdi, MD, Michael Lioudis, MD, Emilio D. Poggio, MD, Georges Nakhoul, MD, Saul Nurko, MD, Tarek Ashour, MD,Raed N. Bou Matar, MD, Charles Kwon, MD, Brian Stephany, MD, George Thomas, MD, Yu-Wei Cheng, PhD, Deanna Leingang, MS, Adnan Alsadah, MD, Rhyan Maditz, MD, Heyka Robert, MD, Tushar Vachhrajani, MD, John R. Sedor, MD, Crystal Gadegbeku, MD, and Xiangling Wang, MD, PhD.
Authors’ Contributions
research idea and study design: XW; data acquisition: CB, XT, MR, SM, KGT RF, JFS, JC, JT, KD, LP, DD, HRC, AM, ML, EP, GN, SN, TA, RB, CK, BS, GT, YC, DL, AA, RM, HR, TV, JRS, CG;data analysis/interpretation: XT, CB, XW; statistical analysis: CB, XT, XW; supervision or mentorship: XW. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
None.
Financial Disclosure
Dr Wang serves on scientific advisory board for Natera. The remaining authors declare that they have no relevant financial interests.
Acknowledgements
We thank Pei-Chun Yu in the Cleveland Clinic for her assistance in the statistical analysis.
Peer Review
Received July 13, 2022. Evaluated by 2 external peer reviewers, with direct editorial input from the Statistical Editor, an Associate Editor, and the Editor-in-Chief. Accepted in revised form November 13, 2022.
Footnotes
Complete author and article information provided before references.
Table S1: Monogenic disorders identified at the renal genetics clinic.
Supplementary Material
Table S1.
References
- 1.Jager K.J., Kovesdy C., Langham R., Rosenberg M., Jha V., Zoccali C. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Nephrol Dial Transplant. 2019;34(11):1803–1805. doi: 10.1093/ndt/gfz174. [DOI] [PubMed] [Google Scholar]
- 2.Akrawi D.S., Li X., Sundquist J., Sundquist K., Zöller B. Familial risks of kidney failure in Sweden: a nationwide family study. PLOS ONE. 2014;9(11) doi: 10.1371/journal.pone.0113353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mallett A., Patel C., Salisbury A., Wang Z., Healy H., Hoy W. The prevalence and epidemiology of genetic renal disease amongst adults with chronic kidney disease in Australia. Orphanet J Rare Dis. 2014;9:98. doi: 10.1186/1750-1172-9-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Haan A., Eijgelsheim M., Vogt L., Knoers N.V.A.M., de Borst M.H. Diagnostic yield of next-generation sequencing in patients with chronic kidney disease of unknown etiology. Front Genet. 2019;10:1264. doi: 10.3389/fgene.2019.01264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Groopman E.E., Marasa M., Cameron-Christie S., et al. Diagnostic utility of exome sequencing for kidney disease. N Engl J Med. 2019;380(2):142–151. doi: 10.1056/NEJMoa1806891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connaughton D.M., Hildebrandt F. Personalized medicine in chronic kidney disease by detection of monogenic mutations. Nephrol Dial Transplant. 2020;35(3):390–397. doi: 10.1093/ndt/gfz028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verbitsky M., Westland R., Perez A., et al. The copy number variation landscape of congenital anomalies of the kidney and urinary tract. Nat Genet. 2019;51(1):117–127. doi: 10.1038/s41588-018-0281-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanna-Cherchi S., Kiryluk K., Burgess K.E., et al. Copy-number disorders are a common cause of congenital kidney malformations. Am J Hum Genet. 2012;91(6):987–997. doi: 10.1016/j.ajhg.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devuyst O., Knoers N.V., Remuzzi G., Schaefer F. Board of the Working Group for Inherited Kidney Diseases of the European Renal Association and European Dialysis and Transplant Association. Rare inherited kidney diseases: challenges, opportunities, and perspectives. Lancet. 2014;383(9931):1844–1859. doi: 10.1016/S0140-6736(14)60659-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knoers N., Antignac C., Bergmann C., et al. Genetic testing in the diagnosis of chronic kidney disease: recommendations for clinical practice. Nephrol Dial Transplant. 2022;37(2):239–254. doi: 10.1093/ndt/gfab218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mrug M., Bloom M.S., Seto C., et al. Genetic testing for chronic kidney diseases: clinical utility and barriers perceived by nephrologists. Kidney Med. 2021;3(6):1050–1056. doi: 10.1016/j.xkme.2021.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lundquist A.L., Pelletier R.C., Leonard C.E., et al. From theory to reality: establishing a successful kidney genetics clinic in the outpatient setting. Kidney360. 2020;1(10):1099–1106. doi: 10.34067/KID.0004262020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nestor J.G., Groopman E.E., Gharavi A.G. Towards precision nephrology: the opportunities and challenges of genomic medicine. J Nephrol. 2018;31(1):47–60. doi: 10.1007/s40620-017-0448-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cocchi E., Nestor J.G., Gharavi A.G. Clinical genetic screening in adult patients with kidney disease. Clin J Am Soc Nephrol. 2020;15(10):1497–1510. doi: 10.2215/CJN.15141219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devarajan P., Chertow G.M., Susztak K., et al. Emerging role of clinical genetics in CKD. Kidney Med. 2022;4(4) doi: 10.1016/j.xkme.2022.100435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elhassan E.A.E., Murray S.L., Connaughton D.M., et al. The utility of a genetic kidney disease clinic employing a broad range of genomic testing platforms: experience of the Irish Kidney Gene Project. J Nephrol. 2022;35(6):1655–1665. doi: 10.1007/s40620-021-01236-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alkanderi S., Yates L.M., Johnson S.A., Sayer J.A. Lessons learned from a multidisciplinary renal genetics clinic. QJM. 2017;110(7):453–457. doi: 10.1093/qjmed/hcx030. [DOI] [PubMed] [Google Scholar]
- 18.Mallett A., Fowles L.F., McGaughran J., Healy H., Patel C. A multidisciplinary renal genetics clinic improves patient diagnosis. Med J Aust. 2016;204(2):58–59. doi: 10.5694/mja15.01157. [DOI] [PubMed] [Google Scholar]
- 19.Thomas C.P., Freese M.E., Ounda A., et al. Initial experience from a renal genetics clinic demonstrates a distinct role in patient management. Genet Med. 2020;22(6):1025–1035. doi: 10.1038/s41436-020-0772-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinto E Vairo F., Kemppainen J.L., Lieske J.C., Harris P.C., Hogan M.C. Establishing a nephrology genetic clinic. Kidney Int. 2021;100(2):254–259. doi: 10.1016/j.kint.2021.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Mallett A.J., Knoers N., Sayer J., Stark Z. Clinical versus research genomics in kidney disease. Nat Rev Nephrol. 2021;17(9):570–571. doi: 10.1038/s41581-021-00436-0. [DOI] [PubMed] [Google Scholar]
- 22.Prince A.E., Roche M.I. Genetic information, non-discrimination, and privacy protections in genetic counseling practice. J Genet Couns. 2014;23(6):891–902. doi: 10.1007/s10897-014-9743-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berman J.J., Moore G.W., Hutchins G.M. U.S. Senate Bill 422: the Genetic Confidentiality and Nondiscrimination Act of 1997. Diagn Mol Pathol. 1998;7(4):192–196. doi: 10.1097/00019606-199808000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Richards S., Aziz N., Bale S., et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan X.Y., Roberts M.B., Khan L.Z., Stewart J., Wang X. The case | a pregnant female with refractory hypocalcemia. Kidney Int. 2022;102(2):453–454. doi: 10.1016/j.kint.2022.02.014. [DOI] [PubMed] [Google Scholar]
- 26.Bekheirnia N., Glinton K.E., Rossetti L., et al. Clinical utility of genetic testing in the precision diagnosis and management of pediatric patients with kidney and urinary tract diseases. Kidney360. 2021;2(1):90–104. doi: 10.34067/KID.0002272020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tomlinson L.A., Clase C.M. Sex and the incidence and prevalence of kidney disease. Clin J Am Soc Nephrol. 2019;14(11):1557–1559. doi: 10.2215/CJN.11030919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bleyer A.J., Westemeyer M., Xie J., et al. Genetic etiologies for chronic kidney disease revealed through next-generation renal gene panel. Am J Nephrol. 2022;53(4):297–306. doi: 10.1159/000522226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sadowski C.E., Lovric S., Ashraf S., et al. A single-gene cause in 29.5% of cases of steroid-resistant nephrotic syndrome. J Am Soc Nephrol. 2015;26(6):1279–1289. doi: 10.1681/ASN.2014050489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daga A., Majmundar A.J., Braun D.A., et al. Whole exome sequencing frequently detects a monogenic cause in early onset nephrolithiasis and nephrocalcinosis. Kidney Int. 2018;93(1):204–213. doi: 10.1016/j.kint.2017.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Franceschini N., Frick A., Kopp J.B. Genetic testing in clinical settings. Am J Kidney Dis. 2018;72(4):569–581. doi: 10.1053/j.ajkd.2018.02.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Groopman E.E., Rasouly H.M., Gharavi A.G. Genomic medicine for kidney disease. Nat Rev Nephrol. 2018;14(2):83–104. doi: 10.1038/nrneph.2017.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nadkarni G.N., Horowitz C.R. Genomics in CKD: is this the path forward? Adv Chronic Kidney Dis. 2016;23(2):120–124. doi: 10.1053/j.ackd.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jayasinghe K., Quinlan C., Stark Z., et al. Meeting report of the 2017 KidGen Renal Genetics Symposium. Hum Genomics. 2018;12(1):5. doi: 10.1186/s40246-018-0137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pollak M.R., Friedman D.J. The genetic architecture of kidney disease. Clin J Am Soc Nephrol. 2020;15(2):268–275. doi: 10.2215/CJN.09340819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jayasinghe K., Quinlan C., Stark Z., et al. Renal genetics in Australia: kidney medicine in the genomic age. Nephrology (Carlton) 2019;24(3):279–286. doi: 10.1111/nep.13494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mallett A., Corney C., McCarthy H., Alexander S.I., Healy H. Genomics in the renal clinic - translating nephrogenetics for clinical practice. Hum Genomics. 2015;9(1):13. doi: 10.1186/s40246-015-0035-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.