Abstract
While major psychiatric disorders lack signature diagnostic neuropathologies akin to dementias, classic postmortem studies have established microstructural involvement, i.e., cellular changes in neurons and glia, as a key pathophysiological finding. Advanced magnetic resonance imaging techniques allow mapping of cellular tissue architecture and microstructural abnormalities in vivo, which holds promise for advancing our understanding of the pathophysiology underlying psychiatric disorders. Here, we performed a systematic review of case-control studies using neurite orientation dispersion and density imaging (NODDI) to assess brain microstructure in psychiatric disorders and a selective review of technical considerations in NODDI. Of the 584 potentially relevant articles, 18 studies met the criteria to be included in this systematic review. We found a general theme of abnormal gray and white matter microstructure across the diagnostic spectrum. We also noted significant variability in patterns of neurite density and fiber orientation within and across diagnostic groups, as well as associations between brain microstructure and phenotypical variables. NODDI has been successfully used to detect subtle microstructure abnormalities in patients with psychiatric disorders. Given that NODDI indices may provide a more direct link to pathophysiological processes, this method may not only contribute to advancing our mechanistic understanding of disease processes, it may also be well positioned for next-generation biomarker development studies.
Keywords: Autism, Diffusion, Mood, Neurodevelopment, Psychosis, Somatic disorders
Psychiatric disorders are highly prevalent and carry a significant personal and public health burden (1,2). The negative effects of these disorders are not only apparent for a patient’s well-being and quality of life (3), they are also expressed through reduced life expectancy and increased mortality (4,5). While the development of a broad range of psychotropic pharmaceuticals has revolutionized the field, efficacy of available drugs is limited and curative treatments remain elusive (6). An important factor contributing to this reality is that our understanding of the causes and mechanisms underlying psychiatric disorders, a prerequisite for targeted drug development, remains limited (7).
Postmortem studies have established microstructural involvement, i.e., cellular changes in neurons and glia, as a key pathophysiological finding in major psychiatric disorders (8, 9, 10, 11). In parallel, diffusion tensor imaging (DTI) has been critical in confirming the presence of microstructural abnormalities in vivo, albeit without neuropathological specificity (12). For example, fractional anisotropy, a popular DTI-derived metric, can be reduced in the presence of axonal loss, reduced orientational coherence of the axons, or cerebrospinal fluid (CSF) contamination. To address this limitation, newer diffusion magnetic resonance imaging (MRI) techniques are aimed at the in vivo evaluation of cellular tissue architecture, mapping histological features noninvasively. One increasingly popular technique, neurite orientation dispersion and density imaging (NODDI) (13), enables the estimation of three key aspects of neuronal tissue for each voxel: neurite density index (NDI), which quantifies the packing density of axons or dendrites; orientation dispersion index (ODI), which assesses the orientational coherence of neurites; and the free water fraction (FWF), which estimates the extent of CSF contamination. Histological validation studies of NODDI have provided evidence that ODI matches its histological counterpart (14, 15, 16, 17, 18) and similarly for NDI (16,19). Findings from a transgenic Alzheimer’s disease model charting the evolution of diffusion-derived markers found that changes in FWF are consistent with the evolution of an inflammatory response, supporting the interpretation of FWF as a proxy of inflammation (20).
We discuss essential principles and relevant technical considerations and provide recommendations for implementation of NODDI to study brain microstructure in psychiatric disorders. To provide a synopsis of the current state of the relevant literature, we also conducted a systematic review of studies assessing NODDI abnormalities in psychiatric disorders. We hypothesized that alterations in brain microstructure would be evident in all psychiatric disorders. However, given the known heterogeneity within and across diagnostic constructs and the variability in data acquisition and analysis approaches, we expected that findings of NODDI abnormalities would differ between studies.
Methods
Selective Review of Technical Considerations in NODDI
The first part of this article describes a selective review of key principles of NODDI and technical considerations in its application to the study of brain microstructure in psychiatric disorders.
Systematic Review of Applications of NODDI in Psychiatric Disorders
Eligibility Criteria
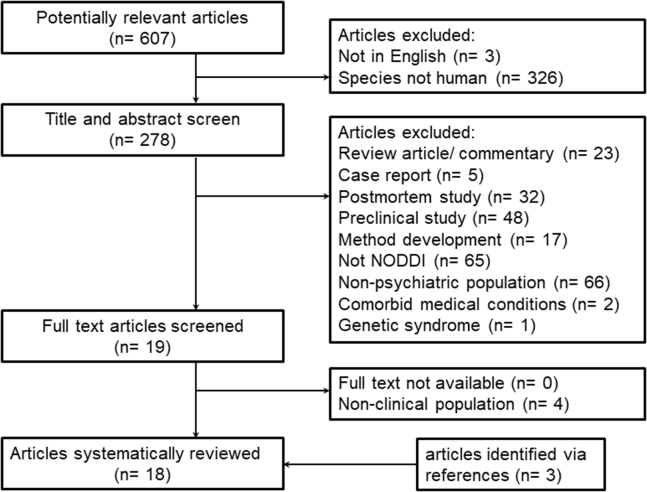
We included studies that used NODDI to assess brain microstructure in psychiatric disorders published between January 2012 and July 2021 (date of last search: September 28, 2021). We excluded review articles, studies published in languages other than English, and studies with nonhuman subjects. Studies expressly including subjects with neurologic or genetic diseases or intellectual disabilities were not considered (Figure 1).
Figure 1.
Process of study inclusion. NODDI, neurite orientation dispersion and density imaging.
Literature Search and Study Selection
Using PubMed, we used the following search term: “(NODDI OR neurite density OR orientation dispersion) AND (mental disorder OR psych∗ OR schizo∗ OR bipolar OR depress∗ OR mood OR mania OR OCD OR anxiety OR PTSD OR substance OR SUD OR addiction OR eating disorder OR anorexia OR bulimia OR attention-deficit OR autism).” We used the following filters to narrow the search: date range, January 1, 2012, to September 28, 2021; species, humans; and language, English. After removal of duplicate articles, titles and abstracts retrieved from the search were reviewed, and potentially eligible studies were selected for full text review. At this stage, we first removed review articles/commentaries and case reports, then postmortem and preclinical studies, then method development manuscripts and studies that did not use NODDI, and finally studies conducted in populations without psychiatric disorders, comorbid medical conditions, and genetic syndromes. Full texts were obtained and eligibility criteria were applied to the articles. Reference lists of those studies and major review articles of the NODDI literature were inspected for additional relevant publications.
Data Extraction
Two authors (NVK and MJS) extracted the following data for each study independently: name of first author, year of publication, number of participants per diagnostic category, diagnostic categories, demographic variables, illness duration, use of psychotropic medications, data acquisition parameters, NODDI indices used, analysis methods, regions of interest, main study outcomes, and associations between NODDI indices and clinical variables. The final summary table was developed in consensus.
Results
Selective Review of Technical Considerations in NODDI
Key Assumptions of the NODDI Model
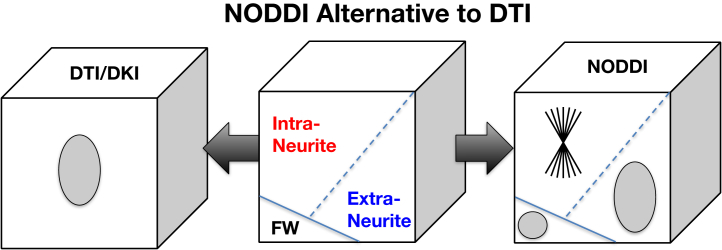
NODDI belongs to the family of diffusion MRI techniques underpinned by multicompartment models (21). Multicompartment models interpret the MRI signals in each voxel as the sum of the contributions from the individual compartments that make up the voxel. This interpretation of the voxelwise MRI signals confers the fundamental advantage over the standard DTI and the more recent diffusion kurtosis imaging (22). The metrics provided by DTI or diffusion kurtosis imaging can only provide a composite view of the multifaceted contributions in a voxel. As a result, a change to DTI-derived fractional anisotropy, as highlighted above, may be caused by a host of underlying changes to the contributing compartments. In contrast, multicompartment models aim to disentangle the contribution from each compartment, thereby enabling their individualized characterization. This distinction between DTI/diffusion kurtosis imaging and NODDI is illustrated in Figure 2.
Figure 2.
While diffusion tensor imaging (DTI)/diffusion kurtosis imaging (DKI) can only provide a composite view of the complex neuronal tissue, multicompartment models, such as neurite orientation dispersion and density imaging (NODDI), provide an alternative representation that may enable investigation of the contributing tissue components individually. FW, free water. Microstructure Imaging Group - University College London. NODDI Matlab Toolbox. Available at: http://mig.cs.ucl.ac.uk/index.php?n=Tutorial.NODDImatlab. Accessed November 20, 2021.
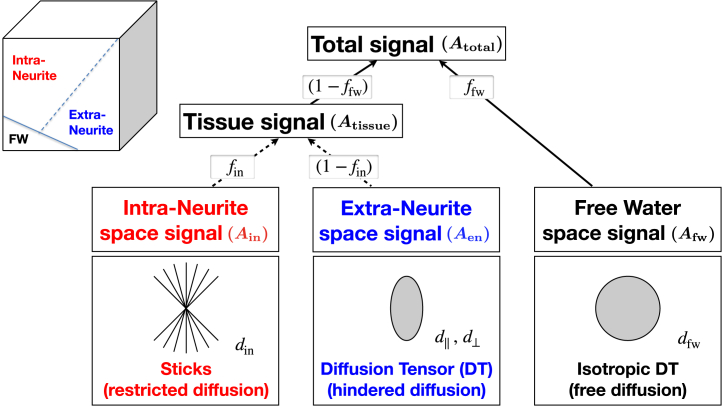
Among multicompartment models, a key distinguishing feature of the NODDI model is the number of compartments assumed to be present within a voxel. Most models contemporaneous with NODDI adopt a two-compartment representation of neural tissue, with one compartment representing the signal contribution from water confined in the intraneurite space and the other capturing the contribution from the water in the extraneurite space. However, this approach does not account for the potential contribution from CSF contamination, which is non-negligible for cortical gray matter, periventricular subcortical gray matter, and white matter. To address this, NODDI additionally includes a third compartment, enabling CSF contamination to be explicitly accounted for. The detail of the NODDI model is illustrated in Figure 3. It shows the two key metrics provided by the NODDI model: ffw, the FWF, and fin, the NDI. It also shows the assumptions made for each compartment and the parameters that the model requires known a priori. First, the intraneurite compartment models the space within individual neurites, which is approximated as a collection of sticks. One of its parameters is din, the rate of diffusion along individual neurites. This parameter is empirically estimated to be 1.7 μm2/ms and is assumed to be the same for different brain regions and for different subjects (23). Guerrero et al. (24) have investigated the influence of fixed diffusivity deviating from the true underlying value. The results show that if the true value is lower (higher) than the fixed diffusivity (1.7 μm2/ms) by 0.1 μm2/ms, NDI is estimated to be 0.025 lower (higher), FWF to be 0.01 higher (lower), and ODI with negligible changes. Given that no histological technique currently exists to estimate true diffusivity, NODDI has chosen to assume a fixed diffusivity so that it can robustly estimate parameters that we can validate histologically, such as NDI and ODI. It is conceivable that the assumption of a common fixed diffusivity may not be valid at least in some circumstances. However, any evidence that supports this will have to come from the observed NDI and ODI deviation from their histological counterparts, but such histological evidence has yet to emerge.
Figure 3.
The detailed modeling behind neurite orientation dispersion and density imaging (NODDI). The observed signal at a voxel (Atotal) is explained by the contribution from the constituting compartments, as well as how each compartment is modeled. See the text for full description. d||, parallel diffusivity; d⊥, perpendicular diffusivity; din, rate of diffusion along individual neurites; ffw, free water fraction; fin, neurite density index. Microstructure Imaging Group - University College London. NODDI Matlab Toolbox. Available at: http://mig.cs.ucl.ac.uk/index.php?n=Tutorial.NODDImatlab. Accessed November 20, 2021.
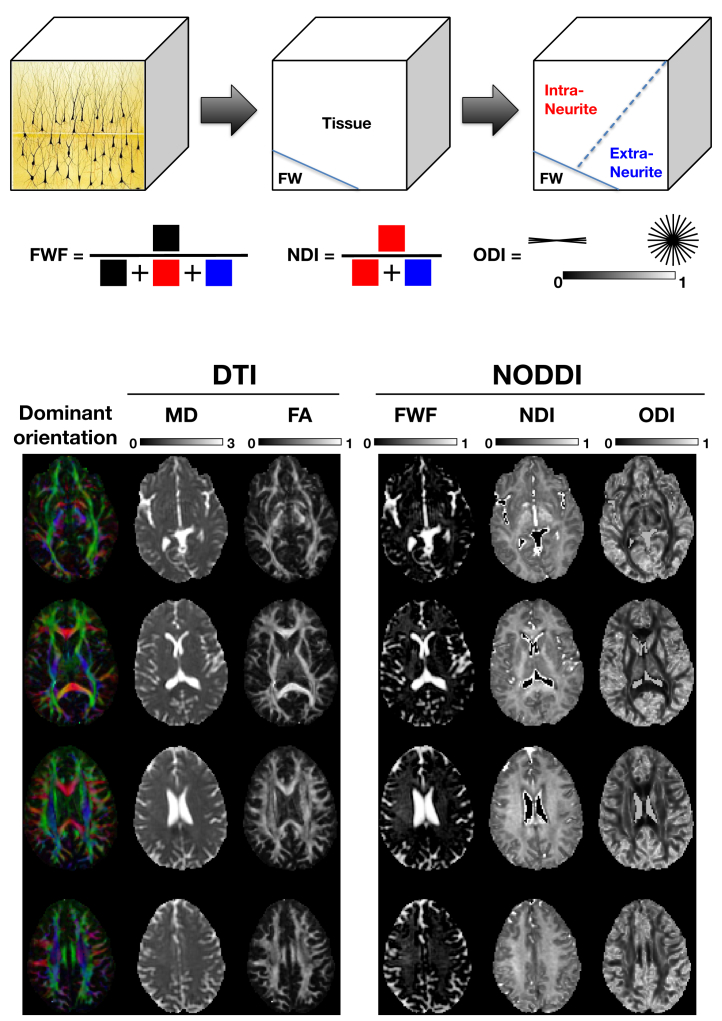
The other parameter not explicitly shown in Figure 3 is ODI, which describes how widely the orientations of the neurites spread out in space. This is the third and final key metric provided by the NODDI model. The extraneurite compartment models the space outside the neurites. This compartment, modeled as a diffusion tensor, has two parameters: d||, the parallel diffusivity, and d⊥, the perpendicular diffusivity. They are determined in terms of din, NDI, and ODI; see the original NODDI paper for details (13). Together with the intraneurite compartment, the two combine to describe the tissue space. While most contemporaneous multicompartment models stop here, as noted earlier, NODDI explicitly accounts for CSF contamination with the free water compartment. This compartment is modeled as an isotropic diffusion tensor with a diffusivity (dfw) set to 3 μm2/ms, which is the diffusivity of free water at body temperature. Figure 4 illustrates the definition of the three NODDI metrics (FWF, NDI, and ODI) and the spatial maps of these metrics of a healthy volunteer compared with standard DTI metrics (fractional anisotropy and mean diffusivity).
Figure 4.
Schematic illustration of the three neurite orientation dispersion and density imaging (NODDI) metrics. Example maps of these metrics alongside those of the standard diffusion tensor imaging (DTI) metrics are provided for comparison. FA, fractional anisotropy; FW, free water; FWF, free water fraction; MD, mean diffusivity; NDI, neurite density index; ODI, orientation dispersion index. Microstructure Imaging Group - University College London. NODDI Matlab Toolbox. Available at: http://mig.cs.ucl.ac.uk/index.php?n=Tutorial.NODDImatlab. Accessed November 20, 2021.
Acquisition Parameters
NODDI makes use of standard diffusion-weighted MRI sequences based on so-called pulsed gradient spin-echo echo-planar imaging, which images the brain, a three-dimensional object, as a set of consecutive two-dimensional axial slices. The key acquisition factors to consider are acquisition time, spatial resolution, and microstructural resolution. The challenge is to design an imaging protocol with spatial and microstructural resolutions as high as possible while keeping the acquisition time at a minimum. See the Supplement for a detailed discussion.
Optimization for Tissue Class
A recent study (24) demonstrated that the default choice of din, which was estimated from the corpus callosum (23), is suitable for white matter but not optimized for gray matter. The study has shown that for gray matter, the optimal choice is about 1.1 μm2/ms.
Limitations
Model-based approaches, such as NODDI, enable researchers to make more direct inference on microstructural features using indirect MRI measurements. Modeling assumptions are an essential part of this type of approach. Constrained by the limited information content available from typical diffusion MRI acquisitions described earlier, different multicompartment models make different trade-offs in microstructural features they can estimate. For example, by including one more compartment than most of its contemporaneous models, NODDI gains the advantage of being able to quantify free water contamination that the other models cannot. However, this choice makes it necessary to forego the ability to estimate diffusivity parameters, such as din. As a result, NODDI must estimate this quantity a priori. In the original implementation of NODDI, this quantity was estimated from the corpus callosum and assumed to be suitable for both white matter and gray matter. However, later it was shown that the appropriate value for gray matter is substantially different from the default (24). Moreover, while the appropriate din for different age groups is broadly similar across the lifespan (>10 years old), the value for infants (<1 year old) is substantially different. Another assumption that has been queried in the literature concerns d⊥, the perpendicular diffusivity of the extraneurite space. In NODDI, this value is constrained to be proportional to (1 − fin), based on the intuition that the extraneurite space is going to be reduced if fin is increased. Because this leads to a more congested extraneurite space for water molecules to diffuse, NODDI assumes that this translates into reduced d⊥. Lampinen et al. (25) have shown that NODDI is incompatible with a new class of diffusion MRI data acquired with b-tensor encoding and suggested that this constraint is the source of the incompatibility. However, Guerreri et al. (26) later demonstrated that this conclusion is unjustified and the primary source of the incompatibility is in fact din being chosen inappropriately, requiring tissue-specific optimization. Despite these limitations, NODDI is one of the few multicompartment models that have been extensively histologically validated (14, 15, 16, 17, 18).
Systematic Review of Applications of NODDI in Psychiatric Disorders
Study Identification and Characteristics
We identified a total of 18 articles that met the inclusion criteria (Figure 1). Seven studies assessed microstructure in psychosis spectrum disorders, four in mood disorders (one assessed both patients with bipolar disorder and with schizophrenia), five in neurodevelopmental and other disorders of childhood and adolescence, and one study each examined NODDI indices in binge drinking, chronic fatigue syndrome, and psychogenic nonepileptic seizures (Table 1).
Table 1.
Articles Included in Systematic Review of NODDI Studies in Psychiatry
| Author, Year | Disorder | Illness Duration | Medication Status | HC/Pt, n | Age, Years | % Male | T | Acquisition Parameters | NODDI Indices Modeled | Analysis Method | Region of Interest | Findings in Patients Compared With Control Subjects | Associations With Clinical Variables |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hanlon et al., 2021 (31) | Psychosis spectrum disorder | Chronic | Medicated | 63/150 | HC: 33.3 ± 8.3 Pt: 32.0 ± 9.4 |
HC: 65.1 Pt: 60.7 |
3 | 87 directions b = 800, 1600, 2400 |
NDI, FWF | VBM | GM, WM | ↓NDI in the lateral prefrontal cortex, superior/medial frontal gyrus, central sulci, superior temporal gyrus, and middle temporal gyrus ↓FWF in the SLF ↑FWF in the cerebral and cerebellar peduncle |
None |
| Kraguljac et al., 2021 (55) | Psychosis spectrum disorder | Firstepisode | Medication naïve | 64/78 | HC: 24.3 ± 5.9 Pt: 23.7 ± 6.0 |
HC: 64.1 Pt: 64.1 |
3 | 98 directions b = 1500, 3000 |
NDI, ODI, FWF | VBM | WM | ↓NDI in commissural and association fibers ↑NDI in projection fibers ↑ODI widespread ↑↓ FWF across regions |
Greater duration of untreated psychosis is associated greater abnormalities in all NODDI indices |
| Alkan et al., 2020 (27) | Psychosis spectrum disorder | First episode | Medicated | 18/31 | HC: 24.1 ± 4.9 Pt: 26.2 ± 5.7 |
HC: 72.2 Pt: 76.3 | 1.5 | 9, 30, 60 directions b = 300, 800, 2400 |
NDI, ODI | ROI | Hippocampus | No group differences in NDI or ODI | NDI in the hippocampus correlates with metacognitive accuracy |
| Kraguljac et al., 2019 (33) | Psychosis spectrum disorder | First episode, chronic | Unmedicated | 42/42 | HC: 27.9 ± 9.4 Pt: 26.6 ± 9.0 |
HC: 61.9 Pt: 61.9 |
3 | 30 directions b = 1000 |
ODI, FWF | VBM | WM | ↑ODI in the posterior limb of the IC | ODI is negatively correlated with subsequent antipsychotic treatment response |
| Rae et al., 2017 (29) | Psychosis spectrum disorder | First episode | Mixed | 19/35 | HC: 24.7 Pt: 26.9 |
HC: 68.4 Pt: 77.1 |
1.5 | 9, 30, 60 directions b = 300, 800, 2400 |
NDI, ODI | TBSS | WM | ↓NDI in many white matter tracts No ODI abnormalities |
None |
| Parvathaneni et al., 2017 (32) | Psychosis spectrum disorder | NR | NR | 47/67 | NR | NR | 3 | 24, 60 directions b = 1000, 2000 |
Vic, Viso, ODI | GS-BSS | GM | ↓ODI in the anterior cingulate cortex and medial frontal gyrus | NR |
| Nazeri et al., 2017 (30) | SZ, BD | Chronic | Medicated | 35/65 | HC: 33.6 ± 12.4 SZ: 35.5 ± 8.4 BD: 31.5 ± 11 |
HC: 54.3 SZ: 52.8 BD: 48.3 |
3 | 30, 30, 30 directions b = 1000, 3000, 4500 |
NDI, ODI | GS-BSS | GM | ↓NDI in the rostromedial temporal lobe in SZ ↑ODI in the PCC SMA and lateral occipital cortex in SZ No alterations in BD |
Higher NDI in frontotemporal areas was correlated with better spatial span performance |
| Sarrazin et al., 2019 (35) | BD | Chronic | Medicated | 40/41 | HC: 36.1 ± 10.1 BD/+Li: 37.1 ± 13.8 BD/−Li: 42.3 ± 12.7 |
HC: 47.5 BD/+Li: 57.7 BD/‒Li: 46.7 |
3 | 30, 60, 60 directions b = 200, 1500, 2700 |
NDI | ROI | GM | ↓NDI in patients not on Li but not in patients on Li | NDI was not correlated with Li dose or serum level in patients |
| Ota et al., 2019 (34) | BD | Chronic | Medicated | 28/31 | HC: 42.3 ± 12.6 Pt: 39.5 ± 9.3 |
HC: 39.3 Pt: 45.2 |
3 | 16 directions b = 1000, 2000 |
NDI, ODI | VBM | WM | ↓NDI in the PCC ↓ODI in the hippocampus |
None |
| Ota et al., 2018 (36) | MDD | Chronic | Medicated | 26/23 | HC: 42.9 ± 12.9 Pt: 42.3 ± 13.4 |
HC: 42.3 Pt: 39.1 |
3 | 16 directions b = 1000, 2000 |
NDI, ODI | VBM | GM, WM ROI in CC, IC, SLF, and posterior thalamic radiation | ↓NDI in the inferior frontal cortex, insula, parahippocampus, superior temporal cortex, cerebellum ↓ODI in the thalamus and occipital gyrus ↑ODI in the SLF, posterior thalamic radiation, and parietal cortex |
ODI in the inferior frontal and middle frontal gyrus was correlated with depression severity |
| Morris et al., 2018 (47) | Binge drinking | NR | NR | 38/28 | HC: 23.7 ± 3.9 Pt: 22.0 ± 4.5 |
HC: 36.8 Pt: 60.7 | 3 | 33, 65 directions b = 700, 2850 |
NDI, ODI | VBM, ROI | GM, WM, ventral striatum | ↑NDI in the superior and middle frontal gyrus, inferior parietal cortex ↓NDI in the postcentral gyrus, angular gyrus, MOG ↓ODI in the DLPFC, inferior parietal cortex, and MOG ↑ODI in the angular and supramarginal gyri, ventral striatum |
ODI in the striatum was correlated with binge drinking scores |
| Kimura et al., 2019 (46) | Chronic fatigue syndrome | Chronic | NR | 23/20 | HC: 38.2 ± 9.2 Pt: 39.8 ± 8.2 |
HC: 4.4 Pt: 5.0 |
3 | 32 directions b = 1000, 2000 |
NDI, ODI | VBM | WM | ↓NDI in the PCC and SLF ↓ODI in occipital areas, putamen, and PCC ↑ODI in occipital and temporal areas |
NR |
| Goodman et al., 2020 (44) | Psychogenic nonepileptic seizures | NR | NR | 37/34a | CGa: 39.7 ± 10.9 Pt: 36.7 ± 11.8 |
CGa: 56.8 Pt: 29.4 |
3 | 47, 46 directions b = 1500, 3000 |
ODI, NDI, FWF | VBM | WM | ↓NDI in the UF, corticospinal tract, and cingulum pathways ↓FWF in the corticospinal tract |
ICVF in the corticospinal tract and posterior cingulum were negatively correlated with depression FWF in the corticospinal tract was negatively correlated with posttraumatic stress scores |
| Andica et al., 2021 (39) | ASD | Chronic | NR | 25/26 | HC: 34.4 ± 9.0 Pt: 32.9 ± 9.2 |
HC: 68.0 Pt: 73.1 |
3 | 32 directions b = 1000, 2000 |
NDI, ODI, Viso | TBSS, ROI | WM | ↓NDI in the right hemisphere and anterior left hemisphere ↑Viso in the posterior left hemisphere |
NDI in the ATR was negatively correlated with communication scores |
| Matsuoka et al., 2020 (38) | ASD | Chronic | Mixed | 29/28 | HC: 27.3 ± 4.9 Pt: 28.9 ± 6.0 |
HC: 82.8 Pt: 85.7 |
3 | 30, 30 directions b = 1000, 2000 |
NDI, ODI | VBM | GM | ↑ODI in the occipital gyrus | ODI was positively correlated with low registration and a passive behavioral response |
| Yasuno et al., 2020 (37) | ASD | Chronic | Mixed | 27/18 | HC: 25.5 ± 2.7 Pt: 28.9 ± 8.2 |
HC: 92.6 Pt: 88.9 |
3 | 30, 30 directions b = 1000, 2000 |
NDI, ODI | VBM | GM, WM | No group differences | NDI in the fusiform and inferior parietal gyrus and CC and ODI in the ventral occipital and superior temporal regions was negatively correlated with recognition |
| Caverzasi et al., 2018 (42) | Developmental dyslexia | NR | NR | 56/39 | HC: 10.0 ± 1.8 Pt: 10.0 ± 1.9 |
HC: 57.1 Pt: 50.0 |
3 | 30, 64 directions b = 700, 2000 |
NDI, ODI | ROI | GM | Abnormal interaction between NODDI and gyrification | NR |
| Sun et al., 2020 (43) | Primary nocturnal enuresis | Chronic | NR | 34/29 | HC: 10.3 ± 1.6 Pt: 9.8 ± 1.2 |
HC: 56.0 Pt: 59.0 |
3 | 32 directions b = 700, 2000 |
NDI, ODI | TBSS | WM | ↓NDI in the IC and cingulum ↑NDI in the SLF ↓ODI in the SLF ↑ODI in the IC and ATR |
NDI in the ATR was negatively correlated with arousal from sleep score in patients |
ASD, autism spectrum disorder; ATR, anterior thalamic radiation; BD, bipolar disorder; CC, corpus callosum; CG, control group; DLPFC, dorsolateral prefrontal cortex; FWF, free water fraction; GM, gray matter; GS-BSS, gray matter surface-based spatial statistics; HC, healthy control; IC, internal capsule; ICVF, intracellular volume fraction; Li, lithium; MDD, major depressive disorder; MOG, middle occipital gyrus; NDI, neurite density index; NODDI, neurite orientation dispersion and density imaging; NR, not reported; ODI, orientation dispersion index; PCC, posterior cingulate cortex; Pt, patient; ROI, region of interest; SLF, superior longitudinal fasciculus; SMA, supplemental motor area; SZ, schizophrenia; T, tesla; TBSS, tract-based spatial statistics; UF, uncinate fasciculus; VBM, voxel-based morphometry; WM, white matter.
Clinical control group.
Findings From NODDI Studies in Psychiatric Disorders: Psychosis Spectrum Disorders
In psychosis spectrum disorders, all but one study (27) report altered brain microstructure in patients compared with control subjects. One study in antipsychotic medication–naïve patients with first-episode psychosis found that NDI is altered in many white matter tracts and differentially expressed depending on the fiber type (increased in projection fibers, decreased in commissural and association fibers), while an increase in ODI was widespread (28). Greater microstructural abnormalities were associated with a longer duration of untreated psychosis, an environmental factor that affects long-term outcomes, supporting the idea that the duration of untreated psychosis may have fundamental pathogenic effects (28). In medicated first-episode patients, a widespread decrease in white matter NDI without corresponding ODI change was reported (29). Interestingly, the authors did report a relationship between age and changes in fiber organization in patients but not in control subjects, suggestive of white matter disease progression. The two studies in first-episode patients are similar as they both detected NDI reductions in patients in association fibers, but only the first study described increased NDI in projection fibers and abnormalities in ODI. It is unclear if the discrepancies in findings are in fact due to medication exposure or to what extent differences in data acquisition (magnetic field strength and image resolution were higher in the first study) and analysis approaches (voxel-based morphometry and tract-based spatial statistics, respectively) or patient characteristics account for the variability in results. Gray matter microstructural abnormalities were assessed in three studies of patients with chronic psychosis spectrum disorders (30, 31, 32). Findings are mixed, where decreased NDI was reported in the temporal lobe (30) and frontotemporal regions (31), whereas ODI was reported to be decreased in the anterior cingulate cortex and medial frontal gyrus (32) and increased in the posterior cingulate cortex and supplemental motor area (30). While some did not report associations between brain microstructure and clinical variables (29,31), many did find relationships with clinical variables. In a cross-sectional study in medicated patients with first-episode psychosis, NDI in the hippocampus was correlated with metacognitive accuracy (27), where poorer metacognitive accuracy corresponded with lower NDI, which is consistent with reported reductions of dendritic density in the subiculum in postmortem studies and highlights the importance of microstructure in hippocampus neurocircuitry. Similarly, Nazeri et al. (30) found that gray matter NDI is relevant for cognition in patients with chronic schizophrenia. They report that higher NDI in the medial and dorsolateral prefrontal, superior temporal, and cingulate cortices, as well as the insula, striatum, and hippocampus was associated with better performance in spatial working memory (30). A longitudinal study examining effects of risperidone on white matter microstructure found that baseline ODI in unmedicated patients with psychosis spectrum disorders was predictive of subsequent treatment response, where higher whole-brain white matter ODI predicted worse clinical outcomes (33).
Findings From NODDI Studies in Psychiatric Disorders: Mood Disorders
In bipolar disorder, one study found reductions in white matter NDI and ODI in posterior cingulate regions and the hippocampus, respectively (34). A small multicenter study used a cross-sectional design and reported reduced frontal gray matter NDI in patients who were not treated with lithium but not in those who were treated with lithium (35). This provides preliminary evidence suggesting that lithium may normalize white matter microstructure, although this is in need of replication in a well-powered longitudinal study. Neither of these studies detected associations between brain microstructure and clinical variables. The only study conducted in major depressive disorder reported reduced NDI in the inferior frontal cortex, insula, parahippocampus, superior temporal cortex, and cerebellum. In contrast, ODI was reduced in the thalamus and occipital gyrus and increased in the posterior thalamic radiation and superior longitudinal fasciculi (36). In this study, only ODI in the inferior frontal and middle frontal gyri was positively correlated with depressive symptom severity.
Findings From NODDI Studies in Psychiatric Disorders: Neurodevelopmental and Other Disorders of Childhood and Adolescence
Three studies assessed NODDI indices in patients with autism spectrum disorder (37, 38, 39). One study investigated gray matter microstructure and found increased ODI in the left occipital gyrus. ODI in this region was also correlated with resting-state functional connectivity between the occipital gyrus and medial temporal gyrus and associated with atypical visual processing in patients (38). Another study in young and middle-aged adults with autism spectrum disorder examined microstructure in white matter and found widespread reductions in NDI and increases in FWF but no abnormalities in ODI (39), consistent with postmortem studies reporting reduced numbers of medium- and large-caliber axons (40,41). In patients, NDI in the anterior thalamic radiation, superior longitudinal fasciculus, and uncinate fasciculus, which are language-related and social processing–related white matter tracts, was negatively correlated with communication scores, where deficits in communication are at the core of this illness. The third study failed to detect abnormalities in NDI and ODI in either gray matter or white matter (37). When the patient group was divided into those with greater and lesser impairments in recognition of facial emotional expressions, both NDI and ODI differed between subgroups. Taken together, all studies in autism spectrum disorder have discovered microstructural features that demonstrate clinical relevance.
Two studies investigated other psychiatric disorders of childhood and adolescence. In developmental dyslexia, Caverzasi et al. (42) performed a multimodal imaging study assessing local gyrification, NDI, and ODI in regions of the language network. While they did not directly contrast microstructure indices between patients and typically developing control subjects, they examined the relationships between local gyrification and NODDI metrics and found an interaction between gyrification and ODI in the middle frontal gyrus and NDI in the medial temporal gyrus, suggesting a relationship between cortical morphology and microstructural organization. Another study examined white matter microstructure in children with primary nocturnal enuresis (43) and found a mixed picture of NDI increase (superior longitudinal fasciculus) and decrease (internal capsule and cingulum) as well as ODI increase (internal capsule and anterior thalamic radiation) and decrease (superior longitudinal fasciculus) in patients. NDI in the anterior thalamic radiation showed a negative correlation with sleep arousal scores in patients, suggesting that altered microstructure may result in the inability to wake up from sleep in response to the need to urinate.
Findings From NODDI Studies in Psychiatric Disorders: Psychiatric Disorders With Somatic Manifestations
Goodman et al. (44) examined white matter microstructure using NODDI in patients with psychogenic nonepileptic seizures. Notably, both the patient and control groups also carried a diagnosis of traumatic brain injury, a common comorbidity (45). They found decreased NDI in the fornix, uncinate fasciculus, cingulum, and corticospinal tract in patients with psychogenic nonepileptic seizures compared with those without. In addition, FWF in the corticospinal tract was lower in this group and associated with severity of posttraumatic stress symptoms. While this study suggests microstructural involvement in psychogenic nonepileptic seizures, due to the lack of inclusion of a control group, it is difficult to interpret data with respect to directionality of abnormalities.
In chronic fatigue syndrome, NDI was found to be decreased in the posterior cingulate cortex, superior longitudinal fasciculus, and frontal white matter. ODI showed a mixed picture of decrease in the posterior cingulate cortex, superior temporal gyrus, and putamen and increase in temporal and occipital areas (46). No associations with clinical or behavioral variables were tested in this study.
Findings From NODDI Studies in Psychiatric Disorders: Substance Use Disorders
In a study contrasting patients with binge drinking behaviors and healthy control subjects, Morris et al. (47) found reduced ODI in frontal cortical gray matter and increased ODI in the parietal cortex. NDI was increased in cortical white matter in regions adjacent to those with lower ODI. While alcohol use severity or binge drinking patterns were not related to overall microstructural changes, higher ODI in the ventral striatum, a key region of the reward system, was associated with binge severity in binge drinking, suggesting a role of ventral striatal neurite integrity in the pathophysiology.
Discussion
We performed a systematic review of the emerging NODDI literature in psychiatric disorders. Consistent with postmortem and neuroimaging studies that have implicated microstructural involvement in virtually all major psychiatric disorders, a general theme of abnormal gray and white matter microstructure in patients with psychiatric disorders emerged. However, findings within and across diagnostic groups were variable, both in terms of spatial patterns of microstructure abnormalities and in the directionality of alterations. In addition, we noted associations between brain microstructure and phenotypical variables, suggesting that NODDI may provide useful information in detecting clinically relevant brain signatures.
Our review highlights the increasing popularity of advanced diffusion imaging as a tool to characterize brain microstructure alterations in psychiatric disorders. This is perhaps not surprising because it holds promise to improve sensitivity to target pathologies and provide better explanations for observed abnormalities compared with traditional DTI (48). All but two studies included in our systematic review detected abnormalities in NODDI metrics in patients, and many also reported associations with clinical variables, providing proof-of-concept for detecting microstructural properties affected in psychiatric disorders (49). We also noted that findings were variable across studies within the same diagnostic group. It is an open question as to what extent this variability is a reflection of the known pathophysiological heterogeneity underlying psychiatric syndromes, the stage of the illness, or other factors such as medication effects. In addition, many of the studies did not use sequence parameters optimized for NODDI, and model parameters were typically not adjusted for tissue type, which may be a function of the relative novelty of the method. Nonetheless, NODDI is fairly robust to the choice of acquisitions; see the Supplement for details. Nevertheless, findings in psychiatric disorders to date have to be interpreted with caution, pending larger-scale replications with optimized sequence and model parameters.
To date, NODDI has been implemented in cross-sectional designs with the goal to describe microstructural abnormalities in psychiatric disorders, but there are countless opportunities for implementation of this method in studies geared at elucidating pathophysiological mechanisms, biomarker development, and clinical translation. For example, prospective studies could uncover microstructure abnormalities in at-risk patients that predispose them to developing a psychiatric condition in the future or detect abnormalities in early illness patients that prove useful in predicting the eventual clinical course. The method also lends itself well to longitudinal studies (50,51) because repeatability of NODDI is excellent, with intrasubject coefficients of variations for NDI and ODI mostly below 5% (48). This type of design is ideal for studying effects of psychotropic medications on brain microstructure and investigating the interplay between brain microstructure changes and disease progression. Finally, it could be used in multimodal studies designed to elucidate complex disease mechanisms. For example, it is not yet known if NDI or ODI abnormalities have functional consequences in psychiatric disorders, e.g., do these alterations affect connectivity or synchrony of large-scale functional brain networks, or can variance in functional network abnormalities be attributed to these microstructure deficits? Similarly, the field has only begun to study how genes, specifically those that are linked to brain development and plasticity, may be related to brain microstructure in general and NODDI indices specifically (52), and only one study has examined the impact of risk genes for psychiatric disorders on NDI and ODI (53), which could elucidate mechanistic aspects of observed microstructure alterations.
Of course, several limitations have to be discussed. While NODDI aims to provide a better characterization of microstructure tissue properties and histological evidence suggests that NDI and ODI reasonably represent histology-based neurite density and orientation dispersion, it is critical to be mindful of the model limitations when it comes to making inferences on a biological level. For example, NODDI may be sensitive to a change in neurite spine density, but we will unlikely be able to attribute any changes to NODDI metrics to a change in neurite spine density specifically. For the latter, more complex models will be required. However, estimating parameters from such models may not be possible from the data we can acquire routinely today. For example, soma and neurite density imaging is a recently developed technique that can additionally estimate soma density, but it requires data that currently can only be acquired with the Siemens Connectom scanner; there are only four such scanners in the world (54). In contrast, in the context of clinical applications, the ability to use these quantitative metrics for diagnostic and predictive processes would supersede necessity for histological accuracy. The current NODDI literature, while growing, is limited, and only approximately 60% of published papers (30,34, 35, 36, 37, 38, 39,43,46,47) included means and standard deviations of indices or a full list of clusters (cluster extent, coordinates, t statistics) with diffusion abnormalities, which prevented us from quantitatively reviewing findings with meta-analytic approaches. It will be helpful for future publications to include comprehensive summary statistics of NODDI abnormalities, which will enable coordinate-based meta-analyses. Most, but not all, studies have accounted for age (27), sex (44), or both (29,30,33, 34, 35, 36, 37, 38, 39,43,46,47,55) in their statistical models. Population and lifespan trajectory studies have demonstrated that NODDI metrics are sensitive to development and aging effects (56, 57, 58) and sex differences (59), underscoring the importance to account for these variables when studying psychiatric disorders. However, there are not sufficient data available to determine the presence or extent of sex differences in abnormal NODDI signatures in psychiatric disorders. Finally, many of the studies did not adjust (or did not report that they adjusted) model parameters for tissue type, which could bias findings, especially in studies assessing gray matter microstructure. Moving forward, it will be important to account for tissue type when assessing microstructure abnormalities and include model parameters as a standard for reporting to maximize transparency.
In summary, our review summarizes proof-of-concept that NODDI can be successfully used to detect subtle microstructure abnormalities in patients with psychiatric disorders. However, even though the number of studies is growing, the body of literature for most disorders remains small, and we have very little knowledge on the effects of medication, illness duration, clinical subtypes, or association with symptom domains and other behavioral measures relevant to psychiatric disorders. More data are needed to begin to fill these gaps in the literature. Given that NODDI indices may provide a more direct link to pathophysiological processes compared with the traditional tensor model, this method may not only contribute to advancing our mechanistic understanding of disease processes, it may also be well positioned for next-generation biomarker development studies (60).
Acknowledgments and Disclosures
This work was supported by the National Institutes of Mental Health (Grant No. R01MH118484 [to NVK]) and the Belgium FRS-FWO Excellence Of Science MEMODYN (Grant No. 30446199 [to MG and HZ]).
NVK served as consultant for Neurocrine Biosciences, Inc. All other authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsgos.2021.12.012.
Supplementary Material
References
- 1.Eaton W.W., Martins S.S., Nestadt G., Bienvenu O.J., Clarke D., Alexandre P. The burden of mental disorders. Epidemiol Rev. 2008;30:1–14. doi: 10.1093/epirev/mxn011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charlson F., van Ommeren M., Flaxman A., Cornett J., Whiteford H., Saxena S. New WHO prevalence estimates of mental disorders in conflict settings: A systematic review and meta-analysis. Lancet. 2019;394:240–248. doi: 10.1016/S0140-6736(19)30934-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Connell J., Brazier J., O’Cathain A., Lloyd-Jones M., Paisley S. Quality of life of people with mental health problems: A synthesis of qualitative research. Health Qual Life Outcomes. 2012;10:138. doi: 10.1186/1477-7525-10-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang C.K., Hayes R.D., Broadbent M., Fernandes A.C., Lee W., Hotopf M., Stewart R. All-cause mortality among people with serious mental illness (SMI), substance use disorders, and depressive disorders in southeast London: A cohort study. BMC Psychiatry. 2010;10:77. doi: 10.1186/1471-244X-10-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang C.K., Hayes R.D., Perera G., Broadbent M.T., Fernandes A.C., Lee W.E., et al. Life expectancy at birth for people with serious mental illness and other major disorders from a secondary mental health care case register in London. PLoS One. 2011;6 doi: 10.1371/journal.pone.0019590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McMahon F.J. Prediction of treatment outcomes in psychiatry—Where do we stand? Dialogues Clin Neurosci. 2014;16:455–464. doi: 10.31887/DCNS.2014.16.4/fmcmahon. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao Y., Castellanos F.X. Annual Research Review: Discovery science strategies in studies of the pathophysiology of child and adolescent psychiatric disorders—Promises and limitations. J Child Psychol Psychiatry. 2016;57:421–439. doi: 10.1111/jcpp.12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gigante A.D., Young L.T., Yatham L.N., Andreazza A.C., Nery F.G., Grinberg L.T., et al. Morphometric post-mortem studies in bipolar disorder: Possible association with oxidative stress and apoptosis. Int J Neuropsychopharmacol. 2011;14:1075–1089. doi: 10.1017/S146114571000146X. [DOI] [PubMed] [Google Scholar]
- 9.Stockmeier C.A., Rajkowska G. Cellular abnormalities in depression: Evidence from postmortem brain tissue. Dialogues Clin Neurosci. 2004;6:185–197. doi: 10.31887/DCNS.2004.6.2/cstockmeier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berdenis van Berlekom A., Muflihah C.H., Snijders G.J.L.J., MacGillavry H.D., Middeldorp J., Hol E.M., et al. Synapse pathology in schizophrenia: A meta-analysis of postsynaptic elements in postmortem brain studies. Schizophr Bull. 2020;46:374–386. doi: 10.1093/schbul/sbz060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rajkowska G. Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biol Psychiatry. 2000;48:766–777. doi: 10.1016/s0006-3223(00)00950-1. [DOI] [PubMed] [Google Scholar]
- 12.Basser P.J., Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- 13.Zhang H., Schneider T., Wheeler-Kingshott C.A., Alexander D.C. NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage. 2012;61:1000–1016. doi: 10.1016/j.neuroimage.2012.03.072. [DOI] [PubMed] [Google Scholar]
- 14.Sato K., Kerever A., Kamagata K., Tsuruta K., Irie R., Tagawa K., et al. Understanding microstructure of the brain by comparison of neurite orientation dispersion and density imaging (NODDI) with transparent mouse brain. Acta Radiol Open. 2017;6 doi: 10.1177/2058460117703816. 2058460117703816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schilling K.G., Janve V., Gao Y., Stepniewska I., Landman B.A., Anderson A.W. Histological validation of diffusion MRI fiber orientation distributions and dispersion. Neuroimage. 2018;165:200–221. doi: 10.1016/j.neuroimage.2017.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grussu F., Schneider T., Tur C., Yates R.L., Tachrount M., Ianuş A., et al. Neurite dispersion: A new marker of multiple sclerosis spinal cord pathology? Ann Clin Transl Neurol. 2017;4:663–679. doi: 10.1002/acn3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colgan N., Siow B., O’Callaghan J.M., Harrison I.F., Wells J.A., Holmes H.E., et al. Application of neurite orientation dispersion and density imaging (NODDI) to a tau pathology model of Alzheimer’s disease. Neuroimage. 2016;125:739–744. doi: 10.1016/j.neuroimage.2015.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sepehrband F., Clark K.A., Ullmann J.F., Kurniawan N.D., Leanage G., Reutens D.C., Yang Z. Brain tissue compartment density estimated using diffusion-weighted MRI yields tissue parameters consistent with histology. Hum Brain Mapp. 2015;36:3687–3702. doi: 10.1002/hbm.22872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gong N.J., Dibb R., Pletnikov M., Benner E., Liu C. Imaging microstructure with diffusion and susceptibility MR: Neuronal density correlation in disrupted-in-schizophrenia-1 mutant mice. NMR Biomed. 2020;33:e4365. doi: 10.1002/nbm.4365. [DOI] [PubMed] [Google Scholar]
- 20.Fick R.H.J., Daianu M., Pizzolato M., Wassermann D., Jacobs R.E., Thompson P.M., et al. In: Computational Diffusion MRI: MICCAI Workshop, Athens, Greece, October 2016. Fluster A., Gohosh A., Kaden E., Rathi Y., Reisert M., editors. Springer; Cham: 2017. Comparison of biomarkers in transgenic Alzheimer rats using multi-shell diffusion MRI; pp. 187–199. [Google Scholar]
- 21.Alexander D.C., Dyrby T.B., Nilsson M., Zhang H. Imaging brain microstructure with diffusion MRI: Practicality and applications. NMR Biomed. 2019;32 doi: 10.1002/nbm.3841. [DOI] [PubMed] [Google Scholar]
- 22.Jensen J.H., Helpern J.A., Ramani A., Lu H., Kaczynski K. Diffusional kurtosis imaging: The quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med. 2005;53:1432–1440. doi: 10.1002/mrm.20508. [DOI] [PubMed] [Google Scholar]
- 23.Alexander D.C., Hubbard P.L., Hall M.G., Moore E.A., Ptito M., Parker G.J., Dyrby T.B. Orientationally invariant indices of axon diameter and density from diffusion MRI. Neuroimage. 2010;52:1374–1389. doi: 10.1016/j.neuroimage.2010.05.043. [DOI] [PubMed] [Google Scholar]
- 24.Guerrero J.M., Adluru N., Bendlin B.B., Goldsmith H.H., Schaefer S.M., Davidson R.J., et al. Optimizing the intrinsic parallel diffusivity in NODDI: An extensive empirical evaluation. PLoS One. 2019;14 doi: 10.1371/journal.pone.0217118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lampinen B., Szczepankiewicz F., Mårtensson J., van Westen D., Sundgren P.C., Nilsson M. Neurite density imaging versus imaging of microscopic anisotropy in diffusion MRI: A model comparison using spherical tensor encoding. Neuroimage. 2017;147:517–531. doi: 10.1016/j.neuroimage.2016.11.053. [DOI] [PubMed] [Google Scholar]
- 26.Guerreri M, Szczepankiewicz F, Lampinen B, Palombo M, Nilsson M, Zhang H (2020): Tortuosity assumption not the cause of NODDI’s incompatibility with tensor-valued diffusion encoding. Presented at the ISMRM & SMRT Virtual Conference, August 8–14, virtual.
- 27.Alkan E., Davies G., Greenwood K., Evans S.L.H. Brain structural correlates of metacognition in first-episode psychosis. Schizophr Bull. 2020;46:552–561. doi: 10.1093/schbul/sbz116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kraguljac N.V., Anthony T., Morgan C.J., Jindal R.D., Burger M.S., Lahti A.C. White matter integrity, duration of untreated psychosis, and antipsychotic treatment response in medication-naive first-episode psychosis patients. Mol Psychiatry. 2021;26:5347–5356. doi: 10.1038/s41380-020-0765-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rae C.L., Davies G., Garfinkel S.N., Gabel M.C., Dowell N.G., Cercignani M., et al. Deficits in neurite density underlie white matter structure abnormalities in first-episode psychosis. Biol Psychiatry. 2017;82:716–725. doi: 10.1016/j.biopsych.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Nazeri A., Mulsant B.H., Rajji T.K., Levesque M.L., Pipitone J., Stefanik L., et al. Gray matter neuritic microstructure deficits in schizophrenia and bipolar disorder. Biol Psychiatry. 2017;82:726–736. doi: 10.1016/j.biopsych.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 31.Hanlon F.M., Dodd A.B., Ling J.M., Shaff N.A., Stephenson D.D., Bustillo J.R., et al. The clinical relevance of gray matter atrophy and microstructural brain changes across the psychosis continuum. Schizophr Res. 2021;229:12–21. doi: 10.1016/j.schres.2021.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parvathaneni P., Rogers B.P., Huo Y., Schilling K.G., Hainline A.E., Anderson A.W., et al. Gray matter surface based spatial statistics (GS-BSS) in diffusion microstructure. Med Image Comput Comput Assist Interv. 2017;10433:638–646. doi: 10.1007/978-3-319-66182-7_73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kraguljac N.V., Anthony T., Monroe W.S., Skidmore F.M., Morgan C.J., White D.M., et al. A longitudinal neurite and free water imaging study in patients with a schizophrenia spectrum disorder. Neuropsychopharmacology. 2019;44:1932–1939. doi: 10.1038/s41386-019-0427-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ota M., Noda T., Sato N., Hidese S., Teraishi T., Setoyama S., et al. The use of diffusional kurtosis imaging and neurite orientation dispersion and density imaging of the brain in bipolar disorder. J Affect Disord. 2019;251:231–234. doi: 10.1016/j.jad.2019.03.068. [DOI] [PubMed] [Google Scholar]
- 35.Sarrazin S., Poupon C., Teillac A., Mangin J.F., Polosan M., Favre P., et al. Higher in vivo cortical intracellular volume fraction associated with lithium therapy in bipolar disorder: A multicenter NODDI study. Psychother Psychosom. 2019;88:171–176. doi: 10.1159/000498854. [DOI] [PubMed] [Google Scholar]
- 36.Ota M., Noda T., Sato N., Hidese S., Teraishi T., Setoyama S., et al. The use of diffusional kurtosis imaging and neurite orientation dispersion and density imaging of the brain in major depressive disorder. J Psychiatr Res. 2018;98:22–29. doi: 10.1016/j.jpsychires.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 37.Yasuno F., Makinodan M., Takahashi M., Matsuoka K., Yoshikawa H., Kitamura S., et al. Microstructural anomalies evaluated by neurite orientation dispersion and density imaging are related to deficits in facial emotional recognition via perceptual-binding difficulties in autism spectrum disorder. Autism Res. 2020;13:729–740. doi: 10.1002/aur.2280. [DOI] [PubMed] [Google Scholar]
- 38.Matsuoka K., Makinodan M., Kitamura S., Takahashi M., Yoshikawa H., Yasuno F., et al. Increased dendritic orientation dispersion in the left occipital gyrus is associated with atypical visual processing in adults with autism spectrum disorder. Cereb Cortex. 2020;30:5617–5625. doi: 10.1093/cercor/bhaa121. [DOI] [PubMed] [Google Scholar]
- 39.Andica C., Kamagata K., Kirino E., Uchida W., Irie R., Murata S., Aoki S. Neurite orientation dispersion and density imaging reveals white matter microstructural alterations in adults with autism. Mol Autism. 2021;12:48. doi: 10.1186/s13229-021-00456-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wegiel J., Kaczmarski W., Flory M., Martinez-Cerdeno V., Wisniewski T., Nowicki K., et al. Deficit of corpus callosum axons, reduced axon diameter and decreased area are markers of abnormal development of interhemispheric connections in autistic subjects. Acta Neuropathol Commun. 2018;6:143. doi: 10.1186/s40478-018-0645-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zikopoulos B., Barbas H. Changes in prefrontal axons may disrupt the network in autism. J Neurosci. 2010;30:14595–14609. doi: 10.1523/JNEUROSCI.2257-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caverzasi E., Mandelli M.L., Hoeft F., Watson C., Meyer M., Allen I.E., et al. Abnormal age-related cortical folding and neurite morphology in children with developmental dyslexia. Neuroimage Clin. 2018;18:814–821. doi: 10.1016/j.nicl.2018.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun H., Xue B., Peng M., Ma H., Yu B., Hou Y., Guo Q. Abnormal neurite orientation dispersion and density imaging of white matter in children with primary nocturnal enuresis. Neuroimage Clin. 2020;28:102389. doi: 10.1016/j.nicl.2020.102389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goodman A.M., Allendorfer J.B., Blum A.S., Bolding M.S., Correia S., Ver Hoef L.W., et al. White matter and neurite morphology differ in psychogenic nonepileptic seizures. Ann Clin Transl Neurol. 2020;7:1973–1984. doi: 10.1002/acn3.51198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.LaFrance W.C., Jr., Deluca M., Machan J.T., Fava J.L. Traumatic brain injury and psychogenic nonepileptic seizures yield worse outcomes. Epilepsia. 2013;54:718–725. doi: 10.1111/epi.12053. [DOI] [PubMed] [Google Scholar]
- 46.Kimura Y., Sato N., Ota M., Shigemoto Y., Morimoto E., Enokizono M., et al. Brain abnormalities in myalgic encephalomyelitis/chronic fatigue syndrome: Evaluation by diffusional kurtosis imaging and neurite orientation dispersion and density imaging. J Magn Reson Imaging. 2019;49:818–824. doi: 10.1002/jmri.26247. [DOI] [PubMed] [Google Scholar]
- 47.Morris L.S., Dowell N.G., Cercignani M., Harrison N.A., Voon V. Binge drinking differentially affects cortical and subcortical microstructure. Addict Biol. 2018;23:403–411. doi: 10.1111/adb.12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kamiya K., Hori M., Aoki S. NODDI in clinical research. J Neurosci Methods. 2020;346:108908. doi: 10.1016/j.jneumeth.2020.108908. [DOI] [PubMed] [Google Scholar]
- 49.Nazeri A., Schifani C., Anderson J.A.E., Ameis S.H., Voineskos A.N. In vivo imaging of gray matter microstructure in major psychiatric disorders: Opportunities for clinical translation. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5:855–864. doi: 10.1016/j.bpsc.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 50.Eaton-Rosen Z., Melbourne A., Orasanu E., Cardoso M.J., Modat M., Bainbridge A., et al. Longitudinal measurement of the developing grey matter in preterm subjects using multi-modal MRI. Neuroimage. 2015;111:580–589. doi: 10.1016/j.neuroimage.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 51.Palacios E.M., Owen J.P., Yuh E.L., Wang M.B., Vassar M.J., Ferguson A.R., et al. The evolution of white matter microstructural changes after mild traumatic brain injury: A longitudinal DTI and NODDI study. Sci Adv. 2020;6 doi: 10.1126/sciadv.aaz6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elliott L.T., Sharp K., Alfaro-Almagro F., Shi S., Miller K.L., Douaud G., et al. Genome-wide association studies of brain imaging phenotypes in UK Biobank. Nature. 2018;562:210–216. doi: 10.1038/s41586-018-0571-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stauffer E.M., Bethlehem R.A.I., Warrier V., Murray G.K., Romero-Garcia R., Seidlitz J., Bullmore E.T. Grey and white matter microstructure is associated with polygenic risk for schizophrenia. Mol Psychiatry. 2021;26:7709–7718. doi: 10.1038/s41380-021-01260-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Palombo M., Ianus A., Guerreri M., Nunes D., Alexander D.C., Shemesh N., Zhang H. SANDI: A compartment-based model for non-invasive apparent soma and neurite imaging by diffusion MRI [published correction appears in Neuroimage 2021; 226:117612] Neuroimage. 2020;215:116835. doi: 10.1016/j.neuroimage.2020.116835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kraguljac N.V., Monroe W.S., Anthony T., Jindal R.D., Hill H., Lahti A.C. Neurite orientation dispersion and density imaging (NODDI) and duration of untreated psychosis in antipsychotic medication-naive first episode psychosis patients. Neuroimage Rep. 2021;1:100005. doi: 10.1016/j.ynirp.2021.100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Slater D.A., Melie-Garcia L., Preisig M., Kherif F., Lutti A., Draganski B. Evolution of white matter tract microstructure across the life span. Hum Brain Mapp. 2019;40:2252–2268. doi: 10.1002/hbm.24522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jelescu I.O., Veraart J., Adisetiyo V., Milla S.S., Novikov D.S., Fieremans E. One diffusion acquisition and different white matter models: How does microstructure change in human early development based on WMTI and NODDI? Neuroimage. 2015;107:242–256. doi: 10.1016/j.neuroimage.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chang Y.S., Owen J.P., Pojman N.J., Thieu T., Bukshpun P., Wakahiro M.L.J., et al. White matter changes of neurite density and fiber orientation dispersion during human brain maturation. PLoS One. 2015;10 doi: 10.1371/journal.pone.0123656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lawrence K.E., Nabulsi L., Santhalingam V., Abaryan Z., Villalon-Reina J.E., Nir T.M., et al. Age and sex effects on advanced white matter microstructure measures in 15,628 older adults: A UK Biobank study. Brain Imaging Behav. 2021;15:2813–2823. doi: 10.1007/s11682-021-00548-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kraguljac N.V., McDonald W.M., Widge A.S., Rodriguez C.I., Tohen M., Nemeroff C.B. Neuroimaging biomarkers in schizophrenia. Am J Psychiatry. 2021;178:509–521. doi: 10.1176/appi.ajp.2020.20030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.