Abstract
Treatment of mice with heat-killed (HK) Mycobacterium bovis BCG or 1- to 10-μm chitin particles (nonantigenic N-acetyl-d-glucosamine polymers) is known to induce innate immune responses, including gamma interferon (IFN-γ) production, which plays a Th1 adjuvant role. However, HK BCG further induces prostaglandin E2-releasing spleen macrophages (Mφ) (PGE2-Mφ), which potentially inhibit Th1 adjuvant activities. We found that chitin particles did not induce PGE2-Mφ formation. To further assess whether chitin has Th1 adjuvant effects, interleukin-10 (IL-10)-knockout (KO) mice and their wild-type (WT, C57BL/6) controls were immunized with a 30-kDa MPB-59 mycobacterial protein mixed with chitin. Immunization with MPB-59 alone induced Th2 responses, characterized by increases in total serum immunoglobulin E (IgE) and specific serum IgG1 levels and spleen Th2 cells producing IL-4, IL-5, and IL-10. No IFN-γ-producing spleen Th1 cells, specific serum IgG2a, or delayed-type hypersentivity (DTH) footpad reactions were detected. On the other hand, chitin–MPB-59 immunization significantly increased spleen Th1 responses, DTH reaction, and serum IgG2a levels along with decreases of Th2 responses. The magnitude of these Th1 adjuvant effects was greater in IL-10-KO mice than in WT mice. In contrast, immunization with HK BCG–MPB-59 showed little or no Th1 adjuvant effect. These data indicate that chitin has a unique Th1 adjuvant effect on the development of Th1 immunity against a mycobacterial antigen. IL-10 down-regulates the adjuvant effect of chitin.
To develop protective immunity against intracellular infections such as tuberculosis, Th1 adjuvants play an important role. Live Mycobacterium bovis Calmette-Guerin bacillus (BCG) and Freund's complete adjuvant (FCA; heat-killed [HK] M. tuberculosis in mineral oil) have been used as Th1 adjuvants in experimental animals (15, 22, 52). Relatively high doses of HK BCG in saline compared with those of live BCG or FCA are required for the induction of nonspecific (innate) immune responses (26). However, HK BCG at high doses also induces prostaglandin E2 (PGE2)-releasing “suppressor” macrophages (Mφ) (13, 30, 36). PGE2 differentially modulates Th1 and Th2 immune responses. PGE2 strongly inhibits the production of Th1 cytokines, such as interleukin-2 (IL-2), IL-12, and gamma interferon (IFN-γ), and, PGE2, depending on stimulatory conditions, either has no effect or enhances production of the Th2-associated cytokines, such as IL-4, IL-5, and IL-10 (6, 16, 45, 47). Therefore, PGE2-Mφ appear to reduce Th1 adjuvant effects (14).
Recently, we have observed that Mφ phagocytose HK BCG and HK Propionibacterium parvum (Corynebacterium parvum) through mannose receptors that recognize carbohydrates of cell walls, including N-acetyl-d-glucosamine, and produce Th1 cytokines, such as IL-12, IL-18, and tumor necrosis factor α (TNF-α) (38–40). To further study this mechanism, we have designed 1- to 10-μm N-acetyl-d-glucosamine polymer (chitin) particles that induce Mφ to produce the cytokines at levels comparable to those stimulated by HK BCG or HK C. parvum (38, 39). However, unlike HK BCG or HK C. parvum, chitin particles do not induce PGE2-Mφ formation (this study). These observations suggest that chitin is a better Th1 adjuvant than HK BCG.
In this study, to determine Th1 adjuvant effects of chitin, we have examined whether soluble MPB-59 antigen mixed with chitin promotes Th1 immunity specific for MPB-59. MPB-59 is one of the 30-kDa mycobacterial antigens that are produced by proliferative BCG and M. tuberculosis and are predominant immunogens (21, 33, 35, 42, 49). When mice develop Th1 immunity against these antigens, they resist bacterial challenges (1, 20, 23, 32, 35). However, immunization with soluble MPB-59 alone resulted in typical Th2 responses including increases in specific serum immunoglobulin E (IgE) and splenic Th2 cells producing IL-4, IL-5, and IL-10. In this study, we present the results of the treatment with chitin as a Th1 adjuvant compared with those of the treatments with FCA or HK BCG suspended in saline.
Since it is established that endogenous IL-10 down-regulates various immune responses, including Th1 and Th2 responses (11, 18, 25, 28), we also employed IL-10-knockout (KO) mice, which were expected to provide a significantly higher magnitude of the chitin adjuvant effects.
MATERIALS AND METHODS
Mice.
Breeding pairs of IL-10-KO (C57BL/6-II10tm1Cgn) mice (28) were obtained from the Jackson Laboratory (Bar Harbor, Maine). Offspring were raised under pathogen-free conditions. No mice used in this study showed colitis (39). Nonpregnant females, 8 to 14 weeks old, were used for experiments. Age-matched female C57BL/6 mice were obtained from the Jackson Laboratory and used as wild-type (WT) control mice. Both IL-10-KO and WT mice were maintained in barrier-filtered cages and fed Purina laboratory chow and tap water ad libitum. Experimental protocols employed in this study were approved by IACUC of East Carolina University Brody School of Medicine.
Preparations of chitin particles and HK BCG.
As described previously (38, 40), chitin particles (1 to 10 μm) were prepared from purified chitin powders (Sigma Chemical Co., St. Louis, Mo.), suspended in saline (20 mg/ml), autoclaved, and stored at 4°C until use. The cultured bacteria of M. bovis BCG Tokyo 172 strain (the Japanese vaccine) were washed, autoclaved, and lyophilized. The powder of HK BCG was suspended in saline immediately before use. The suspensions of both chitin and HK BCG were dispersed by brief sonication (10 s) prior to injection. These chitin and HK BCG preparations contained undetectable levels of endotoxin (<0.03 endotoxin units/ml), as determined by the Limulus amebocyte lysate assay (Sigma) (39). Similarly, HK C. parvum suspensions were prepared as previously described (36).
Purified MPB-59.
MPB-59 (30 kDa) was prepared from culture filtrates of M. bovis BCG Tokyo 172 as described previously (19). The bacteria were cultured in Sauton synthetic medium at 37°C without aeration for 8 days. Sixty liters of culture filtrates was concentrated with ultrafiltration with a Pellicon Cassette system (XX42PEL60; Millipore, Bedford, Mass.) with a molecular weight 5,000 cutoff membrane (YM-3; Amicon, Beverly, Mass.). Proteins were further concentrated with 60% saturated ammonium sulfate and fractionated high-pressure liquid chromatography (i) affinity chromatography with phenyl Sepharose CL-4B, (ii) DEAE Sepharose CL-6B ion exchange, (iii) Sephacryl S200 HR gel filtration, and (iv) re-ion-exchange with DEAE Sepharose CL-6B (all from Pharmacia LKB, Uppsala, Sweden) (19). Following sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis with 10 μg of purified MPB-59 protein, a single 30-kDa band was stained by silver (data not shown). The procedure resulted in 4 mg of purified MPB-59 from 60 liters of culture filtrates.
Endotoxin removal.
Endotoxin removal from all soluble materials for cultures and administration to mice were carried out by filtration and sterilization through 0.22-μm-pore-size Zetapore membranes (AMF-Cuno). The effectiveness of endotoxin removal was monitored by the Limulus amebocyte assay (Sigma).
Mouse immunization protocol and footpad DTH.
Groups of mice (six/group) were given MPB-59 and/or chitin four times intraperitoneally at weekly intervals as follows: group I, MPB-59 (50 μg/dose) alone; group II, 1- to 10-μm chitin (200 μg/dose) alone; group III, mixtures of MPB-59 (50 μg/dose) and chitin (200 μg/dose); and group IV, saline (0.1 ml/dose) as controls. In some experiments, to determine whether HK BCG in saline at a dose that induces innate immune responses (Fig. 1B) has a Th1 adjuvant effect, we employed HK BCG (200 μg/dose) instead of chitin. Seven days after the final immunization, footpad delayed-type hypersensitivity (DTH) reactions to the locally injected MPB-59 were assessed. Mice received 50 μl of MPB-59 solution at 1,000 μg/ml in the right footpad and saline in the left footpad (control). After 48 h, mice were euthanized and MPB-59-induced footpad swelling was monitored with a spring-loaded metric caliper (Mitutoyo, Kawasaki, Japan). Spleens and blood were also harvested.
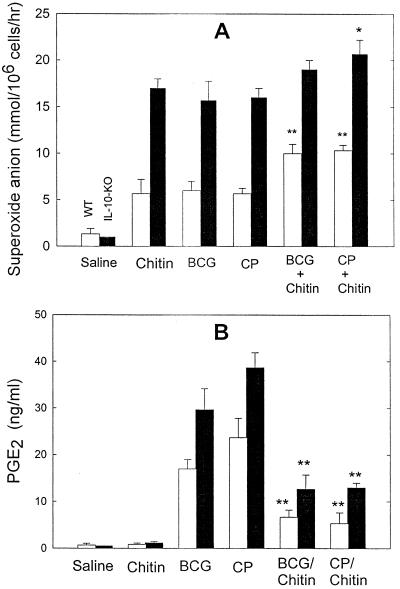
FIG. 1.
Alveolar Mφ priming and the formation of PGE2-Mφ in the spleen following HK BCG administration. WT and IL-10-KO mice intravenously received 0.5 mg of HK BCG, chitin, or HK C. parvum (CP; positive control). Mice that received 0.2 ml of saline served as negative controls. Furthermore, some groups received chitin (0.5 mg) mixed with HK BCG (0.5 mg) or HK C. parvum (0.5 mg). (A) Superoxide anion release by alveolar Mφ. On day 3, alveolar Mφ were assayed in vitro for superoxide anion release by phorbol myristate acetate (1 μM). Superoxide anion levels were measured by a cytochrome c reduction assay as described in Materials and Methods. Data are means plus standard deviation; n = 4. ∗, P < 0.05 compared with chitin alone; ∗∗, P < 0.01 compared with BCG alone or C. parvum alone. (B) PGE2 release by spleen Mφ. On day 7, splenic Mφ were isolated from the other set of experimental groups. Mφ in each group were pooled and incubated in serum-free RPMI 1640 medium containing A23187 at 10−6 M for 2 h. The levels of PGE2 were measured by ELISA. Values are means plus standard deviations; n = 3. ∗∗, P < 0.01 compared with BCG alone or C. parvum alone.
As a positive Th1 adjuvant control, 1 ml of saline with 500 μg of MPB-59 was mixed with 1 ml of FCA, and the mixture was given intraperitoneally to a group of mice (0.2 ml/dose) on days 0 and 14. Fourteen days after the final immunization, footpad DTH reactions were measured as described above.
Cytokine production in recall response—spleen cell cultures stimulated with MPB-59 antigen.
Spleens in each group of mice were isolated and pooled. Spleen cells (4 × 106 cells/ml) were suspended in RPMI 1640 plus 10% fetal bovine serum and incubated with MPB-59 at 10, 20, and 50 μg/ml for 4 days. After the incubation, the culture supernatants were collected, and the levels of selected cytokines (IL-4, IL-5, IL-10, and IFN-γ) were measured by the appropriate specific enzyme-linked immunosorbent assay (ELISA) with commercially available reagents (PharMingen [San Diego, Calif.] and Endogen).
PGE2-Mφ.
Plastic-adherent spleen Mφ were prepared as described before (36, 37) and cultured in serum-free RPMI 1640 medium with or without calcium ionophore A23187 at 10−6 M for 2 h. PGE2 levels in the culture supernatants were measured by a competitive ELISA (Cayman, Ann Arbor, Mich.).
Levels of IgE, IgG1, and IgG2a specific for MPB-59 in serum.
Total serum IgE levels were detected by ELISA using purified mouse IgE κ isotype (PharMingen) as a standard and rat anti-mouse IgE monoclonal antibody, clone R35–72 (PharMingen), as a capture antibody. Levels of MPB-59-specific IgE, IgG1, and IgG2a were measured by ELISA with 96-well plates that were coated with MPB-59 at 0.3 μg/0.1 ml/well in 0.05 M sodium carbonate buffer, pH 9.6, overnight at 4°C. Biotinylated rat monoclonal antibodies detecting IgE, IgG1, and IgG2a were clones R35-92, A85-1, and R19-15, respectively (PharMingen).
Superoxide anion release assay.
Superoxide anion levels released by alveolar Mφ were measured by a cytochrome c reduction assay as described previously (38, 39). Plastic-adherent alveolar Mφ were placed in HEPES-bicarbonate buffer containing 50 μM ferricytochrome c (Sigma) and incubated at 37°C for 1 h in the presence of phorbol myristate acetate (1 μM). The amount of reduced ferricytochrome c was measured by using a molecular extinction coefficient of 21.1 mM−1 cm−1 from the change in absorbance at 550 nm against a cell-free blank. Superoxide formation was expressed as nanomoles per 106 cells.
Statistics.
Data from this project were analyzed by one-way analysis of variance. For culture studies, tissues isolated from at least four mice were pooled; their cells were cultured in at least triplicate in each group. A P value of less than 0.05 is considered statistically significant.
RESULTS
Chitin induced alveolar Mφ priming but not splenic PGE2-Mφ formation.
Results comparable to those in Fig. 1A have been reported earlier; the present observations are included because they validate assumptions necessary for interpretation of the present findings. Previous studies (38, 39) demonstrated that intravenous injection of bacteria or chitin results in the priming of alveolar Mφ, involving the mechanisms of NK cell production of IFN-γ. To confirm whether HK BCG or chitin induces the priming of alveolar Mφ, WT and IL-10-KO mice were given 0.5 mg of HK BCG, HK C. parvum (a positive control), or chitin intravenously. We isolated alveolar Mφ from the groups and measured superoxide anion levels released by the Mφ. We found that HK BCG, HK C. parvum, and chitin induced alveolar Mφ priming at comparable levels on day 3 (Fig. 1A) but not on day 7 (data not shown). Furthermore, alveolar Mφ on day 3 from mice receiving the chitin–HK-BCG or chitin–HK-C. parvum mixture slightly increased superoxide anion release (Fig. 1A). We also confirmed that endogenous IL-10 inhibited alveolar Mφ priming levels (39).
To assess whether these treatments result in the formation of PGE2-Mφ in the spleen (36, 37), splenic Mφ were isolated on day 7 and stimulated in vitro with A23187 at 10−6 M for 2 h. As shown in Fig. 1B, PGE2 levels were unchanged in saline control and chitin-treated groups, whereas significantly higher levels of PGE2 were observed in both HK-BCG- and HK-C. parvum-treated groups. IL-10-KO mice showed more PGE2 than WT mice, suggesting that endogenous IL-10 inhibits splenic PGE2-Mφ formation. The PGE2 production in vitro was over 90% inhibited by nimesulide, a PGG/H synthase-2 inhibitor, at 1 μM (data not shown). Interestingly, the group treated with the mixture of HK BCG with chitin (0.5 mg each) showed lower levels of PGE2 than the group receiving HK BCG alone. As reported previously (36), splenic Mφ on day 3, however, showed no detectable increase in PGE2 levels in all groups (data not shown). Similar kinetics of PGE2-Mφ formation were observed when HK BCG or HK C. parvum was given intraperitoneally and subcutaneously (data not shown).
Recall responses of spleen cell cultures from mice coimmunized with MPB-59 and chitin.
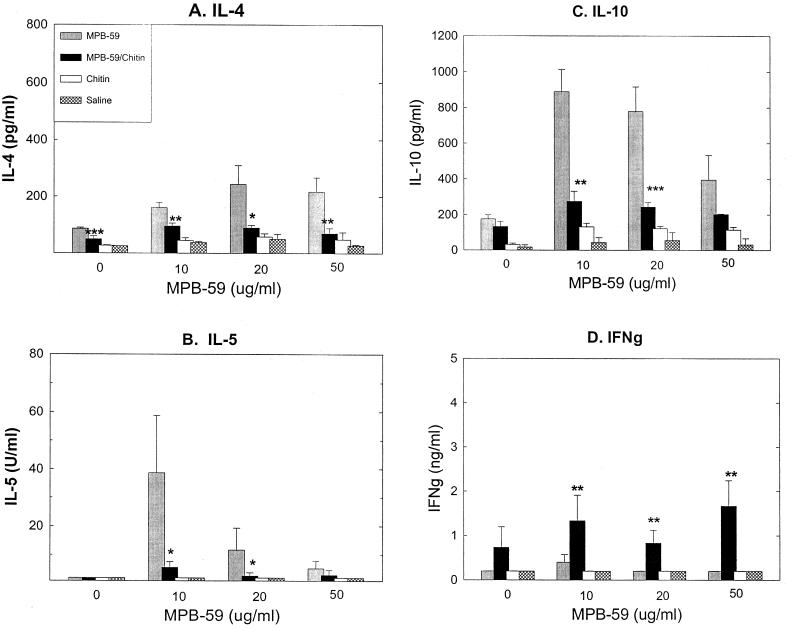
To determine whether MPB-59-induced Th2 cell development was modulated by coinjected chitin, selected cytokine levels produced by Th1 and Th2 cells were measured in recall responses of spleen cell cultures. When spleen cells were prepared from MPB-59-immunized WT mice and stimulated in vitro by MPB-59 at 10, 20, and 50 μg/ml, relatively large amounts of IL-4, IL-5, and IL-10, but not IFN-γ, were detected (Fig. 2). When mice were coimmunized with chitin and MPB-59, the levels of IL-4, IL-5, and IL-10 were significantly reduced (Fig. 2A to C). In contrast, IFN-γ production was significantly increased (Fig. 2D). However, there was little or no production of these cytokines when spleen cells were prepared from saline- or chitin-treated WT control mice and stimulated in vitro by MPB-59 antigen (Fig. 2).
FIG. 2.
Chitin-treated mouse spleen cells decreased MPB-59-stimulated IL-4, IL-5, and IL-10 production but increased MPB-59-stimulated IFN-γ production. Spleen cells were isolated from the WT mouse groups receiving the indicated treatment and stimulated in vitro with MPB-59 at 0 (medium), 10, 20, and 50 μg/ml for 4 days. The cytokine levels in the culture supernatants were measured by ELISA, as described in Materials and Methods. Values are means plus standard deviation from triplicate cultures. The data shown are representative of two independent experiments. ∗, ∗∗, and ∗∗∗, P < 0.05, P < 0.01, and P < 0.005 compared to the MPB-59-immunized group.
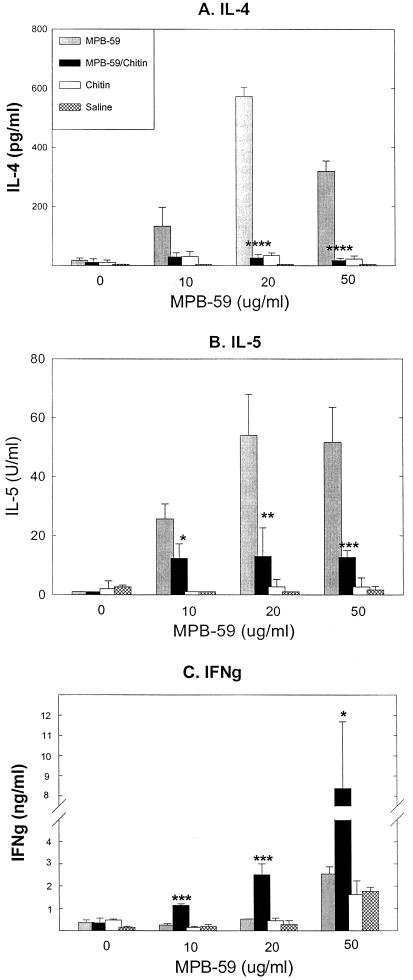
To determine whether endogenous IL-10, which is produced by diverse cell populations, including antigen-stimulated Th2 cells (28), down-regulates Th1 or Th2 responses, we immunized IL-10-KO mice with MPB-59 mixed with chitin. As shown in Fig. 3, IL-4 and IL-5 production was higher in the recall responses of MPB-59-immunized IL-10-KO mice than in those of MPB-59-immunized WT mice (Fig. 2A and B). When IL-10-KO mice were coimmunized with MPB-59–chitin, higher levels of IFN-γ were observed along with marked reduction of IL-4 and IL-5 production (Fig. 3). The results support the previous observations that IL-10 down-regulated both antigen-specific Th1 and Th2 responses (11, 18, 25, 29).
FIG. 3.
Chitin-treated mouse spleen cells decreased MPB-59-stimulated IL-4 and IL-5 production but increased MPB-59-stimulated IFN-γ production in IL-10-KO mice. IL-10-KO mice were immunized as described in Materials and Methods. Recall responses of spleen cell cultures were assayed as described in the Fig. 1 legend. Values are means plus standard deviations from triplicate cultures. The data shown are representative of two independent experiments. ∗, ∗∗, and ∗∗∗, P < 0.05, P < 0.01, and P < 0.005 compared to the MPB-59-immunized group.
Serum IgG1, IgG2a, and IgE levels in mice coimmunized with MPB-59 and chitin.
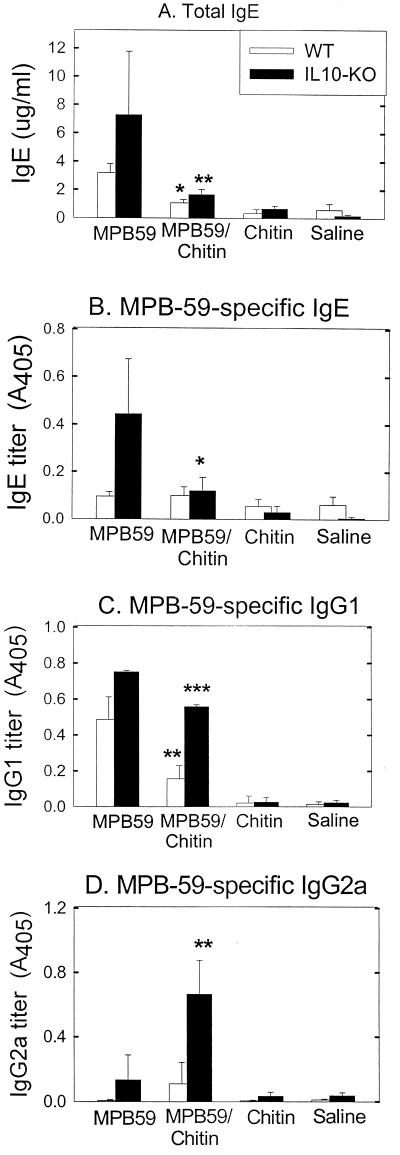
We observed that immunization of WT mice with MPB-59 resulted in increases in levels of total IgE and MPB-59-specific IgG1 in serum (Fig. 4A and C). Since endogenous IL-4 and IFN-γ isotype-switching signals antigen-specific B cells, which bias the serum IgE and IgG1 and the serum IgG2a, respectively (8, 44), we determined if these heavy-chain class switches are developed by coimmunization of MPB-59 and chitin. As shown in Fig. 4D, there was a relatively low level of serum IgG2a. In contrast, after coimmunization with MPB-59 and chitin, the levels of IgG1 and IgE were significantly reduced (Fig. 4A and C). Interestingly, MPB-59-immunized IL-10-KO mice showed a significant enhancement of total IgE, MPB-59-specific IgE, and MPB-59-specific IgG1 levels compared with those in WT mice; following immunization with MPB-59 and chitin, IgG2a levels were also significantly enhanced (Fig. 4).
FIG. 4.
Chitin treatment modulated total IgE levels and MPB-59-specific-antibody formation (IgE, IgG1, and IgG2a) in WT and IL-10-KO mice. Sera were isolated from WT and IL-10-KO mice that were immunized with MPB-59, MPB-59–chitin, chitin, and saline as described in Materials and Methods. (A) Total IgE levels in the sera were measured by a sandwich ELISA. Values are means plus standard deviations; n = 6. (B through D) MPB-59-specific IgE, IgG1, and IgG2a in sera were quantitated as described in Materials and Methods. The sera were diluted 1/5, 1/100, and 1/20 with saline before they were assayed for MPB-59-specific IgE, IgG1, and IgG2a levels, respectively. Values are mean plus standard deviations; n = 6. ∗, ∗∗, and ∗∗∗, P < 0.05, P < 0.01, and P < 0.005 compared to the MPB-59-immunized group.
Our results suggest that MPB-59 is a strong allergen which induces IL-4-dependent IgG1 and IgE production (8). Chitin-induced endogenous IFN-γ appears to regulate antibody heavy-chain class switching, resulting in higher IgG2a levels (44). Furthermore, endogenous IL-10 appears to down-regulate IL-4-dependent IgG1 and IgE production and IFN-γ-dependent IgG2a production.
Footpad DTH reaction in mice coimmunized with MPB-59 and chitin.
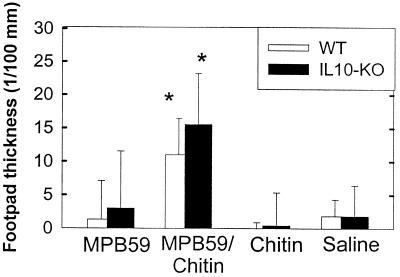
To determine if chitin has adjuvant effects to develop DTH reactions, mice were immunized with MPB-59 mixed with chitin. As shown in Fig. 5, 2 days after the challenge with MPB-59 in the footpad, the thickness of the footpads was measured. Both WT and IL-10-KO mice showed significant footpad thickness following the challenge. Although the footpad reactions seemed to be stronger in IL-10-KO than in WT mice, there was no statistically significance between MPB-59–chitin-immunized IL-10-KO and WT mice (Fig. 5).
FIG. 5.
Development of MPB-59-induced footpad DTH in WT and IL-10-KO mice coimmunized with MPB-59 and chitin. WT and IL-10-KO mice were immunized with MPB-59, MPB-59–chitin, chitin, and saline as described in Materials and Methods. Seven days after the final immunization, mice received 50 μg of MPB-59 solution in the right footpad and 50 μl of saline in the left footpad (control). After 48 h, right footpad thickness minus left footpad thickness in each group of mice was calculated. Values are means plus standard deviations; n = 6. ∗ and ∗∗, P < 0.05 and P < 0.01 compared to the MPB-59-immunized group.
Does coinjected HK BCG provide a Th1 adjuvant effect?
To determine whether HK BCG has a Th1 adjuvant effect, C57BL/6 (WT) mice (six per group) were immunized with MPB-59 mixed with HK BCG (200 μg/dose) in saline at schedules and in groups similar to those receiving coinjected chitin. As a positive Th1 adjuvant, additional mice were immunized with MPB-59 mixed with FCA.
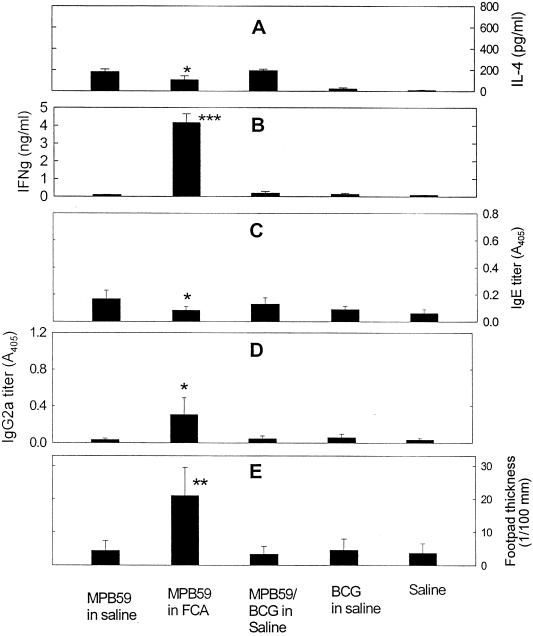
Figure 6 summarizes the IL-4 and IFN-γ levels in recall responses of spleen cell cultures, MPB-59-specific serum IgE and IgG2a, and footpad DTH reactions. MPB-59 in FCA enhanced footpad DTH reactions and antigen-specific IgG2a levels and reduced IgE levels. This Th2-to-Th1 shift was associated with relatively high IFN-γ levels and low IL-4 levels in recall responses. In contrast, mice immunized with MPB-59 mixed with HK BCG in saline showed neither up-regulation of Th1 responses nor down-regulation of Th2 responses specific for MPB-59.
FIG. 6.
Immunity against MPB-59 when mice were immunized with MPB-59 mixed with HK BCG in saline or mixed with FCA. C57BL/6 mice were immunized with MPB-59, MPB-59 in FCA, MPB-59 mixed with HK BCG in saline, HK BCG, and saline as described in Materials and Methods. (A and B) IL-4 and IFN-γ levels produced in recall responses of spleen cell cultures, respectively. Spleen cells were isolated from each group of mice stimulated in vitro with MPB-59 at 20 μg/ml for 4 days. The levels of IL-4 and IFN-γ in the culture supernatants were measured by ELISA, as described in Materials and Methods. Values are means plus standard deviations from triplicate cultures. (C and D) MPB-59-specific IgE and IgG2a titers, respectively, in serum. Immediately after footpad thickness measurements, sera were isolated from all groups of mice. MPB-59-specific IgE and IgG2a in sera were quantitated as described in Materials and Methods. Values are means plus standard deviations; n = 6. (E) DTH reaction (footpad thickness). Seven days after the final immunization (14 days after the second injection of MPB-59 in FCA), mice received 50 μg of MPB-59 solution in right footpad and 50 μl of saline in left footpad (control). After 48 h, right footpad thickness minus left footpad thickness in each group of mice was calculated. Values are means plus standard deviations; n = 6. The data are representative of two independent experiments. ∗, ∗∗, and ∗∗∗, P < 0.05; P < 0.005, and P < 0.0005 compared to the MPB-59-immunized group.
DISCUSSION
Previously, we observed that phagocytosable nonantigenic chitin, a seemingly inert molecule, as well as HK BCG and HK C. parvum, induced endogenous Th1 cytokines (IL-12, IL-18, TNF-α, and IFN-γ) (38–40). These cytokines are generally seen at early stages of infection (innate immunity) caused by mycobacteria and other intracellular bacteria (39). Innate immunity is important for protection against intracellular bacterial infections and to induce Th1 responses and cell-mediated immunity against bacteria (2). It is well established that Th1 cytokines down-regulate allergic immune (Th2) responses (34). Consistent with our previous study (41), the present study clearly demonstrated that chitin, as a Th1 adjuvant, down-regulates antigen-specific Th2 responses and up-regulates Th1 responses specific for a mycobacterial antigen.
The provocative findings are that MPB-59 induces Th2-dominant immune responses, including those of IL-4-, IL-5-, and IL-10-producing splenic Th2 cells, and increases in total serum IgE and MPB-59-specific IgG1 levels. Increases in these inflammatory parameters have been demonstrated in typical airway allergic responses (41). In this study, we found that MPB-59 immunization did not establish DTH reactions. In contrast, when mice were immunized with MPB-59 mixed with chitin, chitin down-regulated these Th2-dominant responses and up-regulated IFN-γ-producing Th1 cells. This increase in IFN-γ levels is associated with an increase in MPB-59-specific IgG2a levels that illustrates isotype switching by B cells (44). Under these Th1-dominant conditions, MPB-59 induces local DTH responses. It has been reported that DTH is IFN-γ dependent but requires additional factors such as IL-8, TNF-α, and migration inhibitory factor produced by Mφ and activated T cells (5, 9).
It is particularly important that HK BCG at a dose that induced innate immune responses including IFN-γ production did not down-regulate Th2 responses or up-regulate Th1 responses in the MPB-59 immunization model (Fig. 6). Previous studies showed that BCG immunotherapies in cancer induce suppressor T cells and suppressor Mφ (3, 13, 30) that reduce protective immunity against tuberculosis and cancer. Recent studies suggest that suppressor T cell functions can be, at least in part, explained by development of mycobacterium-specific Th2 cells (25, 46, 51, 54). Suppressor Mφ that release PGE2 would be associated with this shift of Th1-to-Th2 response (14, 16, 45, 47). It is of particular importance that effective Th1 adjuvants should not induce but inhibit the formation of PGE2-Mφ (14), although the mechanisms of chitin treatments that inhibit PGE2-Mφ formation (Fig. 1) remain to be elucidated.
It should be noted that HK BCG in light mineral oil, HK Listeria monocytogenes in Freund's incomplete adjuvant, and HK M. tuberculosis in mineral oil (FCA) have been used extensively for the enhancement of cell-mediated immunity against coinjected antigens (17, 53). The present study showed that FCA induces Th1 responses specific for coinjected MPB-59 (Fig. 6). However, cell walls isolated from BCG, M. tuberculosis, and C. parvum appear to contain essential components for the induction of splenic PGE2-Mφ formation (13). FCA at the dose used in this study (0.01 mg of HK M. tuberculosis/dose) did not induce PGE2-Mφ, while HK BCG at ≥0.1 mg/dose in either saline or mineral oil induced PGE2-Mφ (13). Therefore, the adjuvant effects of HK BCG at various concentrations suspended in mineral oil or in saline remain to be elucidated (26, 47).
Observations in our earlier (39) and present studies showed that antigen-stimulated Th2 cells, chitin-stimulated Mφ, and HK-BCG-stimulated Mφ produce IL-10. In addition, many other diverse cell populations, including bronchial epithelial cells and B cells, produce IL-10 (7, 29). Endogenous IL-10 is a powerful negative regulator for chitin- or HK-BCG-induced innate immune responses characterized by the production of IL-12, IL-18, TNF-α, and IFN-γ (39). IL-10 also inhibits protective immunity against intracellular bacterial infections due to the down-regulation of IFN-γ production (4, 11, 12). It has also been reported that IL-10 inhibits Th2 responses to allergens, most likely by inhibiting antigen-presenting cells (11, 18, 25, 39). The present study confirms that immunization of IL-10-KO mice with MPB-59 induces significantly higher levels of serum IgE- and IgG1-producing and IL-4- and IL-5-producing Th2 cells than MPB-59 immunization of WT controls. Furthermore, chitin as a Th1 adjuvant induces MPB-59-specific Th-1 cells, footpad DTH, and serum IgG2a in IL-10-KO mice. Our studies clearly support the conclusion that endogenous IL-10 down-regulates the development of antigen-specific Th1 and Th2 responses rather than inducing the shift of Th1 to Th2 responses.
It has been established that several other bacteria and their components (24, 31, 40, 43, 48, 50, 53), such as lipopolysaccharide, superantigens, and DNA with unmethylated CpG motifs, induce Th1 cytokines that up-regulate Th1 responses with down-regulation of Th2 responses. Their efficacy in regulating immune responses is limited by some toxic side effects, including splenomegaly (10, 27) as well as the formation of PGE2-Mφ in the spleen. The chitin treatments in this study accomplished significant modification without any visible adverse effects, splenomegaly (data not shown), or splenic PGE2-Mφ formation. As a result, chitin preparations of nonmicrobial origin represent a very attractive new class of Th1 adjuvant.
ACKNOWLEDGMENTS
This work was supported by grants from the East Carolina University School of Medicine and North Carolina Biotechnology Center grant 9805-ARG-0028.
REFERENCES
- 1.Andersen P. Effective vaccination of mice against Mycobacterium tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infect Immun. 1994;62:2536–2544. doi: 10.1128/iai.62.6.2536-2544.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bendelac A, Fearon D T. Innate pathways that control acquired immunity. Curr Opin Immunol. 1997;9:1–3. doi: 10.1016/s0952-7915(97)80151-3. [DOI] [PubMed] [Google Scholar]
- 3.Bennett J A, Marsh J C. Relationship of Bacillus Calmette-Guerin-induced suppressor cells to hematopoietic precursor cells. Cancer Res. 1980;40:80–85. [PubMed] [Google Scholar]
- 4.Bermudez L E, Champsi J. Infection with Mycobacterium avium induces production of interleukin-10 (IL-10), and administration of anti-IL-10 antibody is associated with enhanced resistance to infection in mice. Infect Immun. 1993;61:3093–3097. doi: 10.1128/iai.61.7.3093-3097.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernhagen J, Bacher M, Calandra T, Metz C M, Doty S B, Donnelly T, Bucala R. An essential role for macrophage migration inhibitory factor in the tuberculin delayed-type hypersensitivity reaction. J Exp Med. 1996;183:277–282. doi: 10.1084/jem.183.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Betz M, Fox B S. Prostaglandin E2 inhibits production of Th1 lymphokines but not of Th2 lymphokines. J Immunol. 1991;146:108–113. [PubMed] [Google Scholar]
- 7.Bonfield T L, Konstan M W, Burfeind P, Panuska J R, Hilliard J B, Berger M. Normal bronchial epithelial cells constitutively produce the anti-inflammatory cytokine interleukin-10, which is downregulated in cystic fibrosis. Am J Respir Cell Mol Biol. 1995;13:257–261. doi: 10.1165/ajrcmb.13.3.7544594. [DOI] [PubMed] [Google Scholar]
- 8.Coffman R L, Ohara J, Bond M W, Carty J, Zlotnik A, Paul W E. B cell stimulatory factor-1 enhances the IgE response of lipopolysaccharide-activated B cells. J Immunol. 1986;136:4538–4541. [PubMed] [Google Scholar]
- 9.Cooper A M, Dalton D K, Stewart T A, Griffin J P, Russell D G, Orme I M. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cowdery J S, Chance J H, Yi A-K, Krieg A M. Bacterial DNA induces NK cells to produce IFN-γ in vivo and increases the toxicity of lipopolysaccharides. J Immunol. 1996;156:4570–4575. [PubMed] [Google Scholar]
- 11.Dai W, Koehler G, Brombacher F. Both innate and acquired immunity to Listeria monocytogenes infection are increased in IL-10-deficient mice. J Immunol. 1997;158:2259–2267. [PubMed] [Google Scholar]
- 12.Denis M, Ghadirian E. IL-10 neutralization augments mouse resistance to systemic Mycobacterium avium infections. J Immunol. 1993;151:5425–5430. [PubMed] [Google Scholar]
- 13.Druker B J, Wepsic H T. BCG-induced macrophages as suppressor cells. Cancer Investig. 1983;1:151–161. doi: 10.3109/07357908309042417. [DOI] [PubMed] [Google Scholar]
- 14.Edwards C K, III, Hedegaard H B, Zlotnik A, Gangadharam P R, Johnston R B, Jr, Pabst M J. Chronic infection due to Mycobacterium intracellulare in mice: association with macrophage release of prostaglandin E2 and reversal by injection of indomethacin, muramyl dipeptide, or interferon-gamma. J Immunol. 1986;136:1820–1827. [PubMed] [Google Scholar]
- 15.Erb K J, Holloway J W, Sobeck A, Moll H, Le Gros G. Infection of mice with Mycobacterium bovis-Bacillus Calmette-Guerin (BCG) suppresses allergen-induced airway eosinophilia. J Exp Med. 1998;187:561–569. doi: 10.1084/jem.187.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gold K N, Weyand C M, Goronzy J J. Modulation of helper T cell function by prostaglandins. Arthritis Rheum. 1994;7:925–933. doi: 10.1002/art.1780370623. [DOI] [PubMed] [Google Scholar]
- 17.Gordon M R, Takata I, Myrvik Q N. Induction of a macrophage migration enhancement factor after desensitization of tuberculin-positive rabbits with purified protein derivative. Infect Immun. 1986;51:134–140. doi: 10.1128/iai.51.1.134-140.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grunig G, Corry D B, Leach M W, Seymour B W, Kurup V P, Rennick D M. Interleukin-10 is a natural suppressor of cytokine production and inflammation in a murine model of allergic bronchopulmonary aspergillosis. J Exp Med. 1997;17:108–1099. doi: 10.1084/jem.185.6.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haga S, Kawajiri K, Niinuma S, Honda I, Yamamoto S, Toida I, Nakamura R M, Nagai S. Effective isolation of MPB64 from a large volume of culture filtrate of Mycobacterium bovis BCG Tokyo. Jpn J Med Sci Biol. 1996;49:15–27. doi: 10.7883/yoken1952.49.15. [DOI] [PubMed] [Google Scholar]
- 20.Harris D P, Vordermeier H M, Friscia G, Roman E, Surcel H M, Pasvol G, Moreno C, Ivanyi J. Genetically permissive recognition of adjacent epitopes from the 19-kDa antigen of Mycobacterium tuberculosis by human and murine T cells. J Immunol. 1993;150:5041–5050. [PubMed] [Google Scholar]
- 21.Harth G, Lee B-Y, Wang J, Clemens D L, Horwitz M A. Novel insights into the genetics, biochemistry, and immunocytochemistry of the 30-kilodalton major extracellular protein of Mycobacterium tuberculosis. Infect Immun. 1996;64:3038–3047. doi: 10.1128/iai.64.8.3038-3047.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herz U, Gerhold K, Gruber C, Braun A, Wahn U, Renz H, Paul K. BCG infection suppresses allergic sensitization and development of increased airway reactivity in an animal model. J Allergy Clin Immunol. 1998;102:867–874. doi: 10.1016/s0091-6749(98)70030-2. [DOI] [PubMed] [Google Scholar]
- 23.Hubbard R D, Flory C M, Collins F M. Immunization of mice with mycobacterial culture filtrate proteins. Clin Exp Immunol. 1992;87:94–98. doi: 10.1111/j.1365-2249.1992.tb06419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huygen K, Abramowicz D, Vandenbussche P, Jacobs F, De Bruyn J, Kentos A, Drowart A, Van Vooren J-P, Goldman M. Spleen cell cytokine secretion in Mycobacterium bovis BCG-induced mice. Infect Immun. 1992;60:2880–2886. doi: 10.1128/iai.60.7.2880-2886.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Justice J P, Shibata Y, Sur S, Mustafa J, Fan M, Van Scott M R. IL-10 gene knockout attenuates allergen-induced airway hyperresponsiveness in C57BL/6 mice. Am J Physiol Lung Cell Mol Physiol. 2001;280:L363–L368. doi: 10.1152/ajplung.2001.280.2.L363. [DOI] [PubMed] [Google Scholar]
- 26.Kawamura I, Yang J, Takaesu Y, Fujita M, Nomoto K, Mitsuyama M. Antigen provoking gamma interferon production in response to Mycobacterium bovis BCG and functional difference in T-cell responses to this antigen between viable and killed BCG-immunized mice. Infect Immun. 1994;62:4396–4403. doi: 10.1128/iai.62.10.4396-4403.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kline J N, Waldschmidt T J, Businga T R, Lemish J E, Weinstock J V, Thorne P S, Krieg A M. Modulation of airway inflammation by CpG oligodeoxynucleotides in a murine model of asthma. J Immunol. 1998;160:2555–2559. [PubMed] [Google Scholar]
- 28.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 29.Moore K W, O'Garra A, De Waal Malefyt R, Vieira P, Mosmann T R. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura R M, Tokunaga T. Mauro Bendinelli and Herman Friedman (ed.), Mycobacterium tuberculosis—interactions with the immune system. New York, N.Y: Plenum Press; 1988. Suppressor cells in mycobacterial infections; pp. 227–241. [Google Scholar]
- 31.Okamura H, Tsutsui H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, Akita K, Namba M, Tanabe F, Konishi K, Fukuda S, Kurimoto M. Cloning of a new cytokine that induces IFNγ production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 32.Roberts A D, Sonnenberg M G, Ordway D J, Furney S K, Brennan P J, Belisle J T, Orme I M. Characteristics of protective immunity engendered by vaccination of mice with purified culture filtrate protein antigens of Mycobacterium tuberculosis. Immunology. 1995;85:502–508. [PMC free article] [PubMed] [Google Scholar]
- 33.Roche P W, Peake P W, Billman-Jacobe H, Doran T, Britton W J. T-cell determinants and antibody binding sites on the major mycobacterial secretory protein MPB59 of Mycobacterium bovis. Infect Immun. 1994;62:5319–5326. doi: 10.1128/iai.62.12.5319-5326.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scott P. IFNγ modulates the early development of Th1 and Th2 responses in a murine model of cutaneous leishmaniasis. J Immunol. 1991;147:3149–3155. [PubMed] [Google Scholar]
- 35.Sharma A K, Verma I, Tewari R, Khuller G K. Adjuvant modulation of T-cell reactivity to 30-kDa secretory protein of Mycobacterium tuberculosis H37Rv and its protective efficacy against experimental tuberculosis. J Med Microbiol. 1999;48:757–763. doi: 10.1099/00222615-48-8-757. [DOI] [PubMed] [Google Scholar]
- 36.Shibata Y. Restoration of prostaglandin-producing splenic macrophages in 89Sr-treated mice with bone marrow from Corynebacterium parvum primed donors. Reg Immunol. 1989;2:169–175. [PubMed] [Google Scholar]
- 37.Shibata Y, Bautista A P, Pennington S N, Humes J L, Volkman A. Eicosanoid production by peritoneal and splenic macrophages in mice depleted of bone marrow by 89Sr. Am J Pathol. 1987;127:75–82. [PMC free article] [PubMed] [Google Scholar]
- 38.Shibata Y, Foster L A, Metzger W J, Myrvik Q N. Alveolar macrophage priming by intravenous administration of chitin particles, polymers of N-acetyl-d-glucosamine, in mice. Infect Immun. 1997;65:1734–1741. doi: 10.1128/iai.65.5.1734-1741.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shibata Y, Foster L A, Kurimoto M, Okamura H, Nakamura R M, Kawajiri K, Justice J P, Van Scott M R, Myrvik Q N, Metzger W J. Immunoregulatory roles of IL-10 in innate immunity—IL-10 inhibits macrophage production IFN-gamma-inducing factors but enhances NK cell production of IFN-gamma. J Immunol. 1998;161:4283–4288. [PubMed] [Google Scholar]
- 40.Shibata Y, Metzger W J, Myrvik Q N. Chitin particle-induced cell mediated immunity is inhibited by mannan—mannose receptor-mediated phagocytosis initiates interleukin-12 production. J Immunol. 1997;159:2462–2467. [PubMed] [Google Scholar]
- 41.Shibata Y, Foster L A, Bradfield J F, Myrvik Q N. Oral administration of chitin down-regulates serum IgE levels and lung eosinophilia in the allergic mouse. J Immunol. 2000;164:1314–1321. doi: 10.4049/jimmunol.164.3.1314. [DOI] [PubMed] [Google Scholar]
- 42.Silver R F, Wallis R S, Ellner J J. Mapping of T cell epitopes of the 30-kDa antigen of Mycobacterium bovis strain Bacillus Calmette-Guerin in purified protein derivative (PPD)-positive individuals. J Immunol. 1995;154:4665–4674. [PubMed] [Google Scholar]
- 43.Skeen M J, Miller M A, Shinnick T M, Ziegler H K. Regulation of murine macrophage IL-12 production. Activation of macrophages in vivo, restimulation in vitro, and modulation by other cytokines. J Immunol. 1996;156:1196–1206. [PubMed] [Google Scholar]
- 44.Snapper C M, Paul W E. Interferon-γ and B cell stimulatory factor-1 reciprocally regulate lg isotype production. Science. 1987;236:944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 45.Snijdewint F G, Kalinski P, Wierenga E A, Bos J D, Kapsenberg M L. Prostaglandin E2 differentially modulates cytokine secretion profiles of human T helper lymphocytes. J Immunol. 1993;150:5321–5329. [PubMed] [Google Scholar]
- 46.Surcel H M, Troye-Blomberg M, Paulie S, Andersson G, Moreno C, Pasvol G, Ivanyi J. Th1/Th2 profiles in tuberculosis, based on the proliferation and cytokine response of blood lymphocytes to mycobacterial antigens. Immunology. 1994;81:171–176. [PMC free article] [PubMed] [Google Scholar]
- 47.van der Pouw Kraan T C, Boeije L C, Smeenk R J, Wijdenes J, Aarden L A. Prostaglandin-E2 is a potent inhibitor of human interleukin 12 production. J Exp Med. 1995;181:775–779. doi: 10.1084/jem.181.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang C C, Rook G A. Inhibition of an established allergic response to ovalbumin in BALB/c mice by killed Mycobacterium vaccae. Immunology. 1998;93:307–313. doi: 10.1046/j.1365-2567.1998.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wiker H G, Harboe M. The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis. Microbiol Rev. 1992;56:648–661. doi: 10.1128/mr.56.4.648-661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamamoto S, Kuramoto E, Shimada S, Tokunaga T. In vitro augmentation of natural killer cell activity and production of interferon-alpha/beta and -gamma with deoxyribonucleic acid fraction from Mycobacterium bovis BCG. Jpn J Cancer Res. 1988;79:866–873. doi: 10.1111/j.1349-7006.1988.tb00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamamura M, Uyemura K, Deans R J, Weinberg K, Rea T H, Bloom B R. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991;254:277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- 52.Yang J, Mitsuyama M. An essential role for endogenous interferon-gamma in the generation of protective T cells against Mycobacterium bovis BCG in mice. Immunology. 1997;91:529–935. doi: 10.1046/j.1365-2567.1997.00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yeung V P, Gieni R S, Umetsu D T, DeKruyff R H. Heat-killed Listeria monocytogenes as an adjuvant converts established murine Th2-dominated immune responses into Th1-dominated responses. J Immunol. 1998;161:4146–4152. [PubMed] [Google Scholar]
- 54.Yong A J, Grange J M, Tee R D, Beck J S, Bothamley G H, Kemeny D M, Kardjito T. Total and anti-mycobacterial lgE levels in serum from patients with tuberculosis and leprosy. Tubercle. 1989;70:273–279. doi: 10.1016/0041-3879(89)90021-4. [DOI] [PubMed] [Google Scholar]