Abstract
Background:
Oral squamous cell carcinoma (OSCC) is one of the most common malignancies and is a serious problem worldwide. The role of HPV in oral cavity squamous cell carcinoma has been studied in several researches. This present review and meta-analysis aimed to investigate the relation between human papillomavirus (HPV) and oral cancer.
Methods:
Relevant studies were found using online international databases including Science direct, Web of science (ISI), PubMed, Scopus, Embase, and Google scholar, to determine relevant studies published between 2000 and Jan 2020. Suitable studies were selected and assessed by two independent researchers. The quality of all papers were determined by a checklist. Heterogeneity assay among the primary studies was evaluated by Cochran’s Q test and I2 index. The statistical analyses were done using Stata SE, V.11 software. Trim and Fill method was applied to confirm the validity of the results.
Results:
This meta-analysis consists of 8 primary studies on the incidence of HPV infection in Iranian patients with oral diseases. The odds ratio between HPV infection and risk of oral cancer was 4.00 (95%CI: 2.31, 6.93).
Conclusion:
This meta-analysis showed associations between prevalence of HPV infection and oral cancer among Iranian patients. The chance of developing oral cancer among HPV positive patients was higher than that in HPV negative patients.
Keywords: Oral cancer, Iran, Human papillomavirus
Introduction
Oral squamous cell carcinoma (OSCC) is among the most common cancers and is a serious problem that causes 4.5% of all malignancies with 630000 new cases every year (1). Oral disease generally involves cancers of lip, buccal mucosa, tongue, gingiva, floor of the mouth, soft palate, and hard palate and their incidence and mortality are growing in many regions around the world (2)(3). Squamous cell cancers of head-and-neck occur in the oral cavity, nasopharynx, larynx, oropharynx, and hypopharynx (3). According to reports, the mortality and morbidity rates of head and neck squamous cell cancer vary around the world and found to be the highest in South-East Asia and Eastern Europe (4). Some behaviors such as the use of alcohol and marijuana alter antitumor immunity, and play a role in shifting HPV infection to HPV-related malignancies (5). Among young people with multiple sexual partners, oral sex (genital or anal) may help the virus reach the oral cavity and cause neoplasia in the oropharyngeal area (6).
Approximately 20% of malignancies are associated with viral or bacterial infection and today, HBV, HCV, EBV, and HPV cause 15% of the cancers. A recent study showed relationships between OSCC and HPV virus. Human papilloma-viruses (HPV) belong to papillomaviridae family (7). Papillomaviruses (Pvs) comprise a group of DNA viruses that cause warts and condylomas. The virion particles of HPV consist of a double-stranded circular DNA (dsDNA) with 8,000 bp in size, and approximately 55 nm in diameter. The genome of HPV structure is composed of early genes (E) and late genes (L) which encode E1 to E7 proteins and L1, L2 proteins, respectively (8). The L1 and L2 proteins form the viral capsid and E1 to E7 proteins are necessary for viral replication, virion synthesis, release, and also cell transformation (9).
So far, more than 200 different types of HPV have been recognized and HPV types that infect the mucosa are divided into two categories based on their potential to induce malignancy: low-risk (LR-HPV) genotypes (10), including 6 and 11 that were detected in genital warts and high-risk (HR-HPV) such as 16 and 18 leading to cervical dysplasia which can also cause cervical cancer (11, 12). In investigations on the role of HPV in particular subgroups in oral cavity cancer HPV16 was detected as the most frequent type in head and neck cancer. According to the association between HPV16 and oropharyngeal cancer, vaccination could be useful for prevention and decreasing this carcinoma (13).
There are different results on the associations between oral cancer and HPV (14). Therefore, systematic review and meta- analysis methods can evaluate the quality of studies and solve this problem. In this article, we aimed to investigate the relationship between HPV and oral cancer based on studies extracted from electronic databases.
Materials and Methods
Search strategy
Two independent researchers searched online international databases, including Science direct, Web of science (ISI), PubMed, Scopus, Embase, and Google scholar, to determine relevant studies published between 2000 and Jan 2020. Appropriate keywords were used: “Human papilloma virus”, “Oral cancer”, “Iran”, and “Oral squamous cell carcinoma” combined with and/or/not. In addition, to improve the search sensitivity, we examined the references of these studies. Two other researchers assessed the search approach at random and confirmed that all appropriate studies had been detected. Moreover, additional efforts have been made to identify unpublished studies.
Study selection
During the advanced search, full texts or abstracts of all articles were reviewed. First, duplicate papers were excluded from the study. Then, irrelevant papers were deleted after analyzing the titles, abstracts, and full texts.
Quality assessment
In order to assess the quality of the studies the STROBE statement was used (15). The statement is a checklist of 22 items covering all components of the methodology, including sample size, type of study, methods and instruments for data collection, variables, aims, study objectives, study population, results presentation, and statistical analysis. In current study, the lowest and highest scores for evaluation of studies were 0 and 44, respectively. The studies were divided into three groups, based on the quality analysis: low quality (<15.5), average quality (15.5–29.5), and high quality (30–44).
Inclusion criteria
All papers that passed the above assessment phases for high quality scores were selected if meeting the following conditions: 1) Case-control studies published in English and 2) Case-control studies on the prevalence of HPV infection in patients with oral cancer.
Exclusion criteria
The following studies have been ruled out: 1) Case reports or case series. 2) Articles with no access to the full-text. 3) Duplicated studies. 4) Conference abstracts with no full-text publication available. 5) Studies published in languages other than English. 6) Studies with low and average quality scores.
Data extraction
After selection of suitable studies, the following data were extracted: name of authors, year of publication, place where the study was conducted, study size, number of cases and controls, source of samples, total infection prevalence among oral cancer patients, and type of HPV.
Statistical analysis
In this study, the degree of heterogeneity in studies results was measured using Cochran’s Q test and I2 index. The odds ratio of the oral cancer was estimated based on a random-effect model. Moreover, sensitivity analysis was used for detection of heterogeneity. We also designed forest plots for estimation of odds ratio of oral cancer according to the results of primary studies with 95% confidence intervals (crossed lines). Each box in a forest plot indicated the weight of the study. For measuring the publication bias, the Funnel plot was used by subjective judgment in each study. In this study, Trim and Fill method was used to confirm the validity and reliability of the results. All statistical analyses were performed in Stata SE, V.11 software.
Results
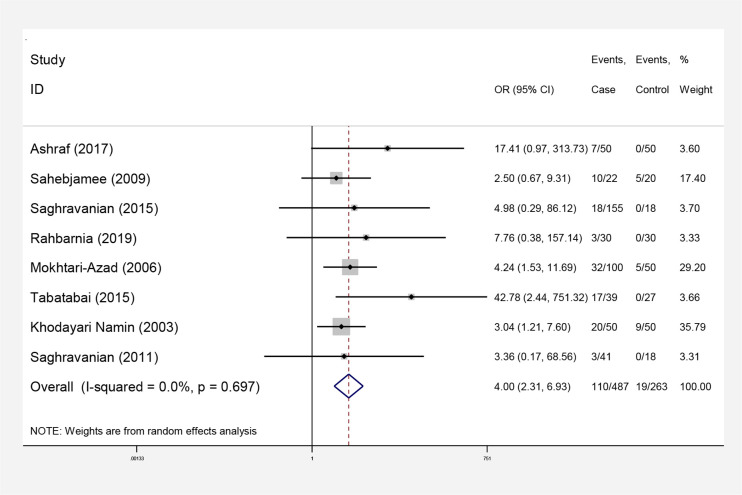
Overall, 4927 articles were detected at the starting process. The number of studies were reduced to 308 following limitation of the search strategy and exclusion of duplicates. In next step, 153 irrelevant studies were excluded after reviewing the titles and abstracts, and 147 documents were removed after reviewing the full texts. Ultimately, the meta-analysis included 8 qualifying studies (Fig. 1). Interestingly, no cohort research was established regarding the subject of the study. Accordingly, this analysis focused on case-control studies, recruited 879 people, of whom 487 had oral cancer. All case-control studies reviewed in this paper had reported higher rates of HPV in patients with oral cancer than controls (Table 1). In this case control study the prevalence of HPV infection was more common in cases than controls (Table 1). Heterogeneity was not observed in this meta-analysis (Q=4.69, P=0.697; I2=0.0%) and developing of oral cancer among people with and without HPV infection using random and fixed models were estimated 4.00 (95%CI: 2.31–6.93) (Fig. 2).
Fig. 1:
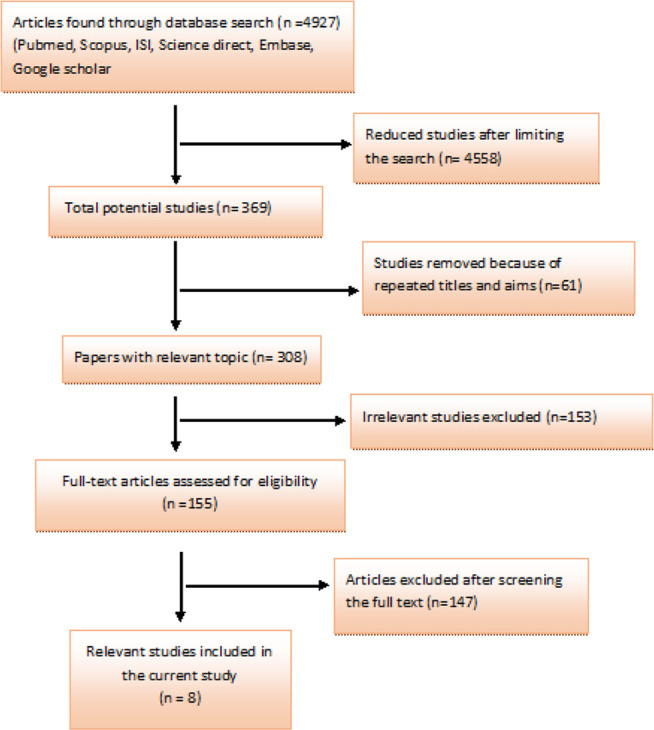
Literature search and review flowchart for selection of primary studies
Table 1:
Primary studies (case-control) included in the meta-analysis
| Reference | Publication year | Publication language | Case (N) | Control (N) | Type of HPV | Source of sample | OR (95%) | |||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Event | Total | Event | Total | Case (%) | Control (%) | |||||
| (16) | 2017 | English | 7 | 50 | 0 | 50 | Not detected | Not detected | SCC | 17.41 (0.97, 313.73) |
| (17) | 2009 | English | 10 | 22 | 5 | 20 | 6,11(30) 6 (60) 18(10) |
6,11(20) 16(80) |
SCC | 2.50 (0.67, 9.31) |
| (18) | 2015 | English | 18 | 155 | 0 | 18 | 6,11 (83.33) 16,18 (16.66) |
Not detected | SCC, VCs, LPs | 4.98 (0.29, 86.12) |
| (19) | 2019 | English | 3 | 30 | 0 | 30 | Not detected | Not detected | OSCC | 7.76 (0.38, 157.14) |
| (20) | 2006 | English | 32 | 100 | 5 | 50 | 6 (31.6) 11 (12.5) 16 (12.5) 31 (3.1) 6,16(6.3) |
Not detected | ameloblastoma | 4.24 (1.53, 11.69) |
| (21) | 2015 | English | 17 | 39 | 0 | 27 | 16,18(12.8) 16 (30.7) |
Not detected | OSCC | 42.78 (2.44, 751.32) |
| (22) | 2003 | English | 20 | 50 | 9 | 50 | 6(40) | Not detected | ameloblastoma | 3.04 (1.21, 7.60) |
| (23) | 2011 | English | 3 | 41 | 0 | 18 | 16,18(7.3) | Not detected | LPs, VCs | 3.36 (0.17, 68.56) |
| Pooled estimate (random model) | 110 | 487 | 19 | 263 | 4.00 (2.31, 6.93) | |||||
Fig. 2:
Forest plot. Odds ratio of oral cancer according to the results of primary studies and its overall estimation
In addition, sensitivity analysis was carried out to determine the reliability and stability of this meta-analysis. According to Fig. 3, there was no significant difference between these studies.
Fig. 3:
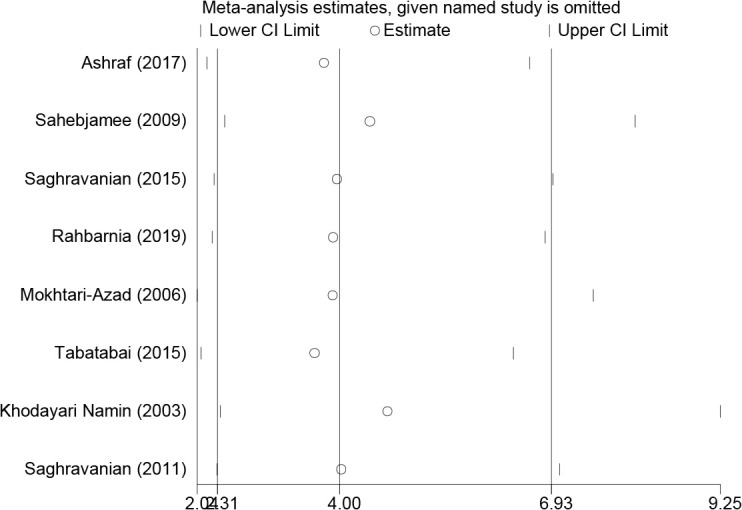
Sensitivity analysis of the studies included in this meta-analysis
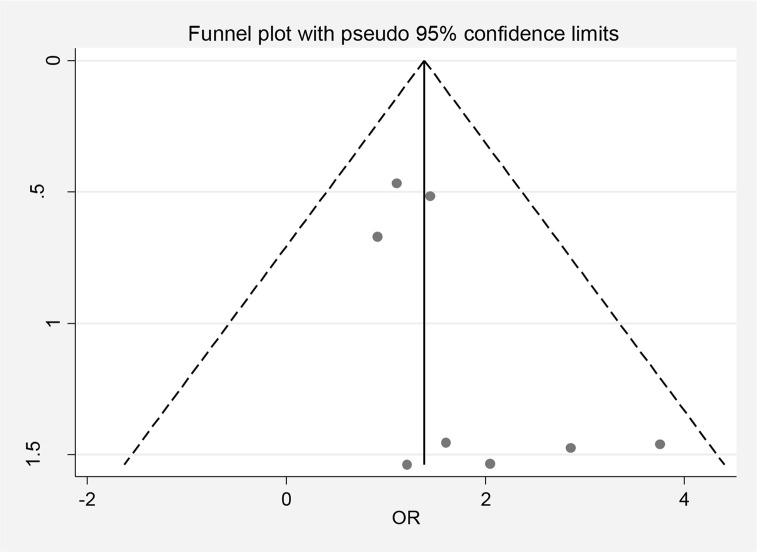
The funnel plots in Fig.4 (β=1.07, P=0.076) confirmed significant publication bias among eight studies.
Fig. 4:
Publishing bias based on Funnel plot
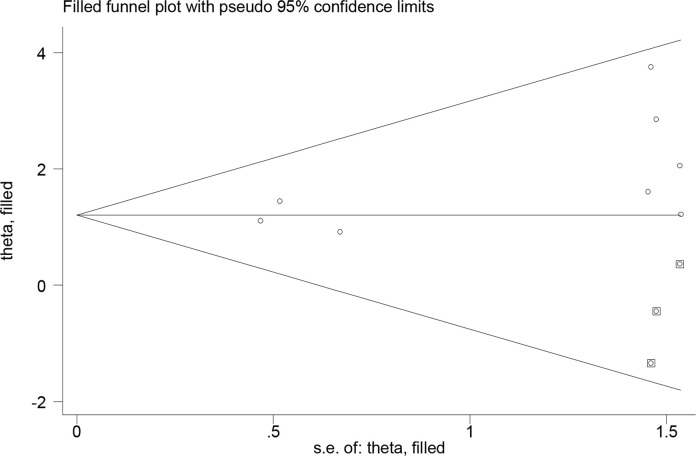
Trim and fill analysis was performed to evaluate the number of missing studies, by which three articles were found. According to the missing studies and eight primary studies, the odds ratio of oral cancer was 3.34 (95%CI: 1.98–5.63), indicating no significant difference between final studies included in the meta-analysis (Fig. 5).
Fig.5:
Trim and Fill method analysis for missing studies
Discussion
The present study showed that among 487 patients with oral cancer, 110 were positive for HPV infection. The odds ratio for developing oral cancer between people with and without HPV infection was 4.00 (95%CI: 2.31–6.93). Erythroplakia, leucoplakia, and oral submucous fibrosis (OSMF) are the most common premalignant lesions and 3%–8.1% of premalignant oral lesions are reported to transform to oral cancer (18). The incidence of HPV among oral cancer patients in Asia indicates that HPV infection combined with other cofactors is a significant risk factor for increasing rate of oral cancer (24).
The prevalence of HR-HPV among patients with OSCC is seen in various geographic regions (18). High rates of HPV positivity among patients with oral cancer were detected in Asia (33.77%) followed by America and Europe (19.65%and 16.19%, respectively and the lowest prevalence was reported in Australia (6.84%) (25). Another study reported that the prevalence of HPV following head and neck squamous cell carcinomas in Asian countries, including South East Asia, West Asia, South Asia and East Asia are 5.04%, 2.21%, 1.1–1.2%, and 3.10%, respectively (26). In Mexico and Colombia, the infection rate of HPV is reported 36% in oropharyngeal cancer (OPC) cases (27). Moreover, HPV+ oropharyngeal squamous cell carcinoma (OPSCC) was more prevalent in Sweden, Denmark, Scotland, China, South Korea, Lebanon, and the USA (28).
In a case-control study in Iran (Shiraz), the prevalence of HPV was 14% among 50 cases and no one in the control group was positive for HPV (16). Other studies suggested that the high and low prevalence of HPV infection was seen among SCC patients in Sari (North of Iran) and Ahvaz (southeast Iran) (13.25% and 3.9%, respectively) (2, 29). On the other hand, in other research study in Khorasan, HPV DNA was not detected (30).
Differences between these results may have been associated with small sample sizes and low levels of sensitivity of the assays. In almost all studies reviewed here, HPV DNA sequencing had been detected by PCR specific primers. All studies in the present study showed that the prevalence of HPV-16 infection in OSSC samples was higher than other types. Similarly, a study done by PCR among 36 Korean patients with early oral tongue SCC and 25 normal tongue mucosa showed that the prevalence of HPV was 36% and 4%, respectively. In these cases, the most prevalent genotypes were HPV-16 (85%) (31). In other study, HPV-positive head and neck squamous cell carcinoma (HPV-HNSCC) was evaluated among 207 patients and the HR-HPV was detected in 90% of the patients (32). On the other hand, a research in patients with tonsillectomy and SCC reported that HPV-16 and 18 were not associated with oral HPV (33).
HR-HPV infection plays a significant role in oral cavity carcinogenesis. It is highly linked with increased risk of oral cancer among patients and patients infected with HPV show higher stage of disease compared with negative individuals (34–35).
There are some limitations in our study, including the small number of participants in some groups. In addition, the relation between specific genotypes of HPV and oral cancer was not investigated. Further research with large sample size using more developed techniques for HPV genotyping are needed to clarify the role of HPV in the development of odontogenic lesions and tumors. Cohort studies on HPV in OSCC region could be of great benefit in determining the HPV persistence in normal cases and patients. Moreover, knowledge about the etiologic factors of odontogenic cysts could provide new approaches in preventing oral cancers and treatment of precancerous lesions.
Journalism Ethics considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
The authors would like to thank Mazandaran University of Medical Sciences for financial support (IR.MAZUMS.REC.1399.8657).
Footnotes
Conflict of interest
Author declares that no conflict of interest.
References
- 1.Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer (2009). Oral Oncol, 45(4):309–16. [DOI] [PubMed] [Google Scholar]
- 2.Akhondnezhad M, Haghshenas MR, Ghasemi M, Mousavi T. (2018). The prevalence and genotyping of human papillomavirus in patients with oral tumors in health centers and clinics of Mazandaran in Iran. Virusdisease, 29(3):297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sritippho T, Chotjumlong P, Iamaroon A. Roles of human papillomaviruses and p16 in oral cancer (2015). Asian Pac J Cancer Prev, 16(15):6193–200. [DOI] [PubMed] [Google Scholar]
- 4.Sathish N, Wang X, Yuan Y. Human papillomavirus (HPV)-associated oral cancers and treatment strategies (2014). J Dent Res, 93(7_suppl):29S–36S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baniebrahimi G, Mir F, Khanmohammadi R. Cancer stem cells and oral cancer: insights into molecular mechanisms and therapeutic approaches (2020). Cancer Cell Int, 20:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wierzbicka M, San Giorgi MR, Dikkers FG. Transmission and clearance of human papillomavirus infection in the oral cavity and its role in oropharyngeal carcinoma–A review (2023). Rev Med Virol, 33(1):e2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akram N, Imran M, Noreen M, Ahmed F, Atif M, Fatima Z, Bilal Waqar A. (2017). Oncogenic role of tumor viruses in humans. Viral Immunol, 1;30(1):20–7. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Zhao Y. (2017). Human papillomavirus infection in oral potentially malignant disorders and cancer. Arch. Oral Biol, 83:334–9. [DOI] [PubMed] [Google Scholar]
- 9.de Sanjosé S, Brotons M, Pavón MA. (2018). The natural history of human papillomavirus infection. Best Pract Res Clin Obstet Gynaecol, 47:2–13. [DOI] [PubMed] [Google Scholar]
- 10.Okunade KS. (2020). Human papillomavirus and cervical cancer. J Obstet Gynaecol, 40(5):602–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taberna M, Mena M, Pavón M, Alemany L, Gillison M, Mesía R. (2017). Human papillomavirus-related oropharyngeal cancer. Ann Oncol, 28(10):2386–98. [DOI] [PubMed] [Google Scholar]
- 12.Haghshenas MR, Mousavi T, Moosazadeh M, Afshari M. (2016). Human papillomavirus and breast cancer in Iran: a meta-analysis. Iran J Basic Med Sci, 19(3):231. [PMC free article] [PubMed] [Google Scholar]
- 13.Janecka-Widła A, Mucha-Małecka A, Majchrzyk K, Halaszka K, Przewoźnik M, Słonina D, Biesaga B. (2020). Active HPV infection and its influence on survival in head and neck squamous-cell cancer. J Cancer Res Clin Oncol, 146(7):1677–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shang Q, Peng J, Zhou Y, Chen Q, Xu H. (2020). Association of human papillomavirus with oral lichen planus and oral leukoplakia: A meta-analysis. J Evid Based Dent Pract, 1;20(4):101485. [DOI] [PubMed] [Google Scholar]
- 15.Von Elm E, Altman DG, Egger M, et al. (2007). The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med, 147(8):573–7. [DOI] [PubMed] [Google Scholar]
- 16.Ashraf MJ, Hosseini S, Monabati A, et al. (2017). The prevalence of human papilloma virus in squamous cell carcinoma of oral tongue. Iran J Pathol, 12(2):144–149. [PMC free article] [PubMed] [Google Scholar]
- 17.SahebJamee M, Boorghani M, Ghaffari SR, AtarbashiMoghadam F, Keyhani A. (2009). Human papillomavirus in saliva of patients with oral squamous cell carcinoma. Med Oral Patol Oral Cir Bucal, 14(10):e525–8. [DOI] [PubMed] [Google Scholar]
- 18.Saghravanian N, Ghazi N, Meshkat Z, Mohtasham N. (2015). Human Papillomavirus in Oral Leukoplakia, Verrucous Carcinoma, Squamous Cell Carcinoma, and Normal Mucous Membrane. Oman Med J, 30(6):455–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rahbarnia L, Farajnia S, Bayazian G, Naderpour M, Feizi H. (2019). Prevalence of Human Papillomavirus in Patients With Oral Squamous Cell Carcinoma in Tabriz, Iran. Crescent J Med Biol Sci, 6(1):105–8. [Google Scholar]
- 20.Mokhtari-Azad T, Moradi A, Khodayari-Nemin A, et al. (2006). Detection of human papillomavirus DNA in intraosseus ameloblastoma. Int J Virol, 2(1):1–6. [Google Scholar]
- 21.Tabatabai SH, Nabieyan M, Sheikhha MH, Zarmehi S, Tadbir AA, Ahmadi NA. (2015). Detection of Human papillomavirus 16 and 18 types in oral squamous cell carcinoma patients in Yazd, Iran: A Case-Control Study. J Paramed Sci, 6(1):11–17. [Google Scholar]
- 22.Namin AK, Azad TM, Eslami B, Sarkarat F, Shahrokhi M, Kashanian F. (2003). A study of the relationship between ameloblastoma and human papilloma virus. J Oral Maxillofac Surg, 61(4):467–70. [DOI] [PubMed] [Google Scholar]
- 23.Saghravanian N, Ghazvini K, Babakoohi S, Firooz A, Mohtasham N. (2011). Low prevalence of high risk genotypes of human papilloma virus in normal oral mucosa, oral leukoplakia and verrucous carcinoma. Acta Odontol Scand, 69(6):406–9. [DOI] [PubMed] [Google Scholar]
- 24.Giraldi L, Collatuzzo G, Hashim D, et al. (2021). Infection with Human Papilloma Virus (HPV) and risk of subsites within the oral cancer. Cancer Epidemiol, 1;75:102020. [DOI] [PubMed] [Google Scholar]
- 25.Yete S, D’Souza W, Saranath D. (2018). High-risk human papillomavirus in oral cancer: clinical implications. Oncology, 94(3):133–41. [DOI] [PubMed] [Google Scholar]
- 26.Bukhari N, Joseph JP, Hussain J, et al. (2019). Prevalence of human papilloma virus sub genotypes following head and neck squamous cell carcinomas in asian continent, a systematic review Article. Asian Pac J Cancer Prev, 20(11):3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinzon LM, Velazquez A, Rutkoski H, et al. (2022). Cross-cultural adaptation of a Spanish version of a previously validated HPV survey that evaluates dental students’ knowledge, perception and clinical practices in Latin America. BMC Oral Health, 22(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carlander AF, Jakobsen KK, Bendtsen SK, et al. (2021). A contemporary systematic review on repartition of HPV-positivity in oropharyngeal cancer worldwide. Viruses, 9;13(7):1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nikakhlagh S, Saki N, Shoar MH, Sartipipor A, Saki S. (2012). Incidence of etiologic factors in squamous cell carcinoma of head and neck in Ahvaz. Iran J Otorhinolaryngol, 24(67):85–90. [PMC free article] [PubMed] [Google Scholar]
- 30.Delavarian Z, Pakfetrat A, Falaki F, Pazouki M, Pazouki N. (2010). The role of viruses in oral squamous cell carcinoma in young patients in Khorasan (Northeast of Iran). J Appl Sci, 10(11):981–5. [Google Scholar]
- 31.Lee SY, Cho NH, Choi EC, et al. (2010). Relevance of human papilloma virus (HPV) infection to carcinogenesis of oral tongue cancer. Int J Oral Maxillofac Surg, 39(7):678–83. [DOI] [PubMed] [Google Scholar]
- 32.El-Salem F, Mansour M, Gitman M, et al. (2019). Real-time PCR HPV genotyping in fine needle aspirations of metastatic head and neck squamous cell carcinoma: Exposing the limitations of conventional p16 immunostaining. Oral Oncol, 1;90:74–9. [DOI] [PubMed] [Google Scholar]
- 33.Vidal Loustau AC, Dulguerov N, Curvoisier D, McKee T, Lombardi T. (2019). Low prevalence of HPV-induced oral squamous cell carcinoma in Geneva, Switzerland. Oral Dis, 25(5):1283–90. [DOI] [PubMed] [Google Scholar]
- 34.Wu W, Wang Z, Zhou Z. (2019). Role of the human papillomavirus in malignant transformation of oral leukoplakia distinct from oropharyngeal squamous cell carcinoma: A study of 76 patients with internal-control specimens. Oral Surg Oral Med Oral Pathol Oral Radiol, 1;128(3):273–9. [DOI] [PubMed] [Google Scholar]
- 35.Méndez-Martínez R, Maldonado-Frías S, Vázquez-Vega S, et al. (2020). High prevalent human papillomavirus infections of the oral cavity of asymptomatic HIV-positive men. BMC Infect Dis, 20(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]