Summary
Background
Vaccination reduces COVID-19-related hospitalization among older adults. However, how SARS-CoV-2 infection and vaccine regimens affect vaccine-elicited immunity remain unclear.
Methods
This is a cross-sectional study recruiting adults aged ≥70 years with comorbidities in Hong Kong. Demographic and clinical information were collected using a questionnaire. Neutralizing antibody (nAb) titers (against ancestral and Omicron strains) and SARS-CoV-2-specific T cell response were analyzed according to infection and vaccination status. Multivariable regression analysis was performed to assess the associations of BNT162b2 and booster doses with higher nAb titers, with adjustment for comorbidities.
Findings
In July 2022, 101 patients were recruited, of whom 25 (24%) had previous infection. Overall, the geometric mean titer (GMT) of BA.5 nAb was 2.8-fold lower than that against BA.2 (P < 0.0001). The ancestral strain and BA.2 titers were higher for the 3-4-dose-BNT162 group than the 2-dose-BNT162b2 group. Non-infected individuals in the 3-4-dose-CoronaVac group had a more robust T cell response than the 2-dose-CoronaVac group (P = 0.0181), but there was no significant difference between the 2-dose-BNT162b2 and 3-4-dose-BNT162b groups. Patients who had heterologous CoronaVac-BNT162b2 prime-boost regimen had 3.22-fold higher BA.5 nAb titers than those who were primed/boosted with CoronaVac (P = 0.0207). Patients with hybrid immunity had higher Omicron nAb titers than those with vaccine-only immunity. Multivariable analysis showed that BNT162b2 and booster doses were independently associated with higher ancestral strain nAb titers.
Interpretation
Our data support the use of booster doses for older adults with or without prior infection. Non-infected individuals primed with CoronaVac will benefit from heterologous mRNA vaccine booster.
Funding
Richard and Carol Yu, May Tam Mak Mei Yin, The Shaw Foundation Hong Kong, Michael Tong, Marina Lee, Government Consultancy Service (See acknowledgements for full list).
Keywords: COVID-19, Vaccination, Antibody, T cell, Booster, Hybrid immunity
Research in context.
Evidence before this study
Both mRNA and inactivated whole virion vaccine are effective in preventing severe COVID-19 complications among older adults. We searched PubMed without language restrictions on 15th August 2022 for articles using the terms “COVID-19” or “SARS-CoV-2” and the terms “vaccine”, “booster”, “hybrid immunity”, “mRNA vaccine”, or “inactivated whole virion vaccine”. Booster doses could enhance neutralizing antibody (nAb) titer and showed improve protection against hospitalization. However, most of these studies did not include patients receiving inactivated whole virion vaccine, which is used widely worldwide. Furthermore, most of these studies did not stratify their analysis based on infection status. Another limitation of previous studies was that BA.5 strain was not included in their nAb assay.
Added value of this study
We have systematically compared the effect of vaccine doses and vaccine types on humoral and cellular immunity, and stratified the analysis based on infection status. Furthermore, we compared the effect of hybrid immunity with vaccine-only immunity with the same number of exposures to SARS-CoV-2 antigens (S protein). We found that among non-infected patients, booster doses of vaccine was superior to 2 doses for nAb for Omicron variant, including BA.5 sublineage. Patients primed with CoronaVac and boosted with BNT162b2 had similar nAb titer as those with 3 doses of BNT162b2. Hybrid immunity induced higher nAb titers than vaccine only immunity for those with same number of exposures.
Implications of all the available evidence
Our data support the use of booster doses of vaccine for older adults. Patients who have completed the primary series with inactivated whole virion vaccine can have high nAb titers and T cell response after boosting with mRNA vaccine.
Introduction
Coronavirus disease 2019 (COVID-19) was associated with an estimated 18.2 million deaths between 2020 and 2021.1 Older adults, especially those with comorbidities, are at particularly high risk of severe disease.2 In Hong Kong, the case-fatality rate was 15.3-fold higher for the age group 80 years or above, and 2.4-fold higher for the 70–79 year-old age group, than the overall rate during the Omicron BA.2-dominant wave in early 2022.3
Vaccination, including mRNA and inactivated whole virion vaccines, is highly effective in preventing severe disease among older adults.4,5 However, several problems affect vaccine effectiveness among older adults. First, the magnitude of nAb and T cell response elicited by vaccination or infection are poorer among older adults when compared with younger adults.6,7 Second, waning of humoral immunity occurs after vaccination or infection. The vaccine effectiveness of BNT162b2 (Pfizer) vaccine for individuals aged ≥65 years was reduced from 80% at 1 month to 43% at 5 months after full vaccination.8 Third, comorbidities are associated with hyporesponsiveness and nondurable response to COVID-19 vaccination.9 Furthermore, novel variants are less susceptible to nAb induced by vaccination.3,10 The Omicron sublineages BA.1 or BA.2 are >10-fold less susceptible than ancestral strain to nAb elicited by COVID-19 vaccines,10, 11, 12, 13 while Omicron sublineage BA.5 is less susceptible than BA.2.13, 14, 15, 16
Vaccine booster doses have been recommended to compensate for poorer immune response among the older adults.17 Patients with a single booster had a lower risk of severe infection than those without any boosters.18 Several studies focusing on older adults found that a second booster dose reduce the risk of severe disease.19, 20, 21 In a study by Munro et al., the anti-spike IgG was significantly higher among patients with two boosters than those with one booster, while there was only a slight increase in T cell response.22
Apart from homologous booster in which patients received the same vaccine for both priming and boosting, studies have also assessed the effectiveness of heterologous booster, especially for patients who have been primed with inactivated whole virion vaccine, such as CoronaVac, which are known to induce lower levels of nAb. Patients with heterologous 3-dose regimen (2 doses of CoronaVac plus 1 dose BNT162b2) had better response than 3 doses of CoronaVac against ancestral virus and Omicron variant,23 and has similar protective effectiveness against symptomatic infection as the homologous 3 dose BNT162b2 regimen among adults aged over 60 years.24
Although studies have found that patients with hybrid immunity (vaccination plus infection) can elicit more potent and broader nAb than those with vaccine-only immunity,14 most vaccine booster studies did not differentiate patients with previous infection from those who are infection naïve, and some excluded patients with previous SARS-CoV-2 infection.18,22 To address these limitations, this study determined the nAb titers against ancestral virus and Omicron sublineages BA.2 and BA.5, and stratified our analysis to determine the effect of booster doses, vaccine type, and hybrid immunity.
Methods
Study design and participants
We did a cross-sectional study to investigate the humoral and cellular immunity against SARS-CoV-2 in older adults with comorbidities in Hong Kong. We recruited patients who attended the medical out-patient clinic of Queen Mary Hospital in Hong Kong for follow-up of their chronic medical conditions from 12 to 27 July 2022. The research nurses randomly approached patients at the clinic for recruitment. Patients were eligible for inclusion if they were aged 70 years or above, and had at least one chronic comorbid condition. Exclusion criteria included refusal to provide written informed consent, mental incapacity to provide written informed consent, or unable to collect sufficient volume of blood. We did not apply any selection criteria based on the gender of the patient. The gender of the patient was self-reported by the patient. On the day of recruitment, we collected blood specimens for antibody and T cell assays. We collected data on demographic, underlying comorbidities, vaccination history and COVID-19 infection history using a questionnaire or from the electronic medical records. Data on body mass index were not available. The primary endpoint was the proportion of patients with detectable neutralizing antibodies, while the secondary endpoints were neutralizing antibody titer, and the effect of vaccine doses, vaccine types and hybrid immunity on the neutralising antibody titers.
Ethics
This study was approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority of Hong Kong West Cluster (HKU/HA HKW IRB) (Reference number: UW 22–328). Written informed consent was obtained from all study participants.
Definitions
Based on the definition from the World Health Organization, a patient was considered to have completed the primary series of COVID-19 vaccine if they had received at least 2 doses of BNT162b2 or CoronaVac.17 Patients were classified as being previously infected with SARS-CoV-2 if they had known positive RT-PCR or rapid antigen test results; or tested positive for IgG against SARS-CoV-2 nucleocapsid (N) protein in this study if they had not received inactivated whole virion COVID-19 vaccine previously. A patient is considered to have received a dose if the blood specimen was collected at least 7 days after the dose.
SARS-CoV-2-specific antibody and T cell assays
Conventional live virus neutralization test (cVNT) was performed as described previously.3,11 The virus strains tested included an ancestral virus (GISAID accession number: EPI_ISL_434,571), an Omicron sublineage BA.2.2 virus (GISAID accession number: EPI_ISL_11,413,802) and sublineage BA.5.2 virus (GISAID accession number: EPI_ISL_13,777,658). For statistical analysis, a value of 5 was assigned if the cVNT is < 10. A serum specimen was considered to be seronegative if cytopathic effect was seen at a dilution of 1:10.
IgG against SARS-CoV-2 spike protein receptor binding domain (anti-RBD IgG) was performed using the SARS-CoV-2 IgG II Quant assay (Abbott Diagnostics, Chicago, USA). Anti-N IgG was performed using the iFlash-SARS-CoV-2 IgG assay (Shenzhen YHLO Biotech Co. Ltd., Shenzhen, China). The manufacturer's cutoff values for anti-RBD IgG and anti-N IgG were 50.0 AU/ml and 10 AU/ml, respectively.
SARS-CoV-2-specific T cell response was measured using a SARS-CoV-2 specific quantitative IFN-γ release assay (IGRA) with whole blood following the manufacturer's instructions (Wantai SARS-CoV-2 IGRA, Wantai Biopharm, Beijing, China). The results were interpreted according to the manufacturer's recommendation.
Details of the antibody and T cell assays are provided in the supplementary appendix.
Statistical analysis
Statistical analysis was performed using SPSS version 28.01.0 or GraphPad Prism 9.4.0. Fisher's exact test and Mann Whitney U test were used to compare categorical and continuous variables, respectively, between infected and non-infected groups. Chi-square for trend test was used in determining the relationship between vaccine doses and infection status. Multivariate analysis was performed to control for confounding factors in the comparison between infected and non-infected groups, and included all variables with a p value of <0.1 in the univariate analysis. Sample size was based on feasibility.
Statistical analysis involving nAb titers were performed using log-transformed nAb titers. One-way ANOVA with Dunn's multiple comparisons test was used in assessing the difference in neutralizing titers among ancestral strain and Omicron variants. We assessed the distribution of log-transformed nAb titers using the Shapiro–Wilk test, of which most were not normally distributed, and thus applied the non-parametric statistics for consistency of statistical tests conducted and reporting. Multivariable regression analysis was performed to assess the association of BNT162b2 and booster doses with higher nAb titers against ancestral strain, omicron variants, adjusting for age, sex, hypertension, diabetes mellitus, heart disease, malignancy, other endocrine disease, lung, liver disease, neurological disease, kidney disease, autoimmune disease, solid organ transplant, and haematological disease. Backward selection was used as the covariant elimination strategy. A P value of <0.05 was considered as statistically significant.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Patient characteristics
Between 12 and 27 July, 2022, 130 patients were screened for inclusion, of whom 101 were recruited (Table 1 and Supplementary Table S1). The median age was 74 years, and 26.7% (27/101) were 80 years old or above. 49.5% (50/101) were female. All patients were ethnic Chinese. The most common comorbidities were hypertension (69.3% [70/101]), diabetes mellitus (38.6% [39/101]), and chronic heart disease (35.6% [36/101]). 97% (98/101) have received at least one dose of COVID-19 vaccine, 94.1% (95/101) completed the primary vaccine series, and 69.3% (70/101) received at least 1 booster dose (≥3 doses). Among the 70 patients who have received booster doses, 8 have received heterologous prime-boost regimen (7 primed with CoronaVac and boosted with BNT162b2 [CoronaVac/BNT16b2]; 1 primed with BNT162b2 and boosted with CoronaVac [BNT162b2/CoronaVac]).
Table 1.
Characteristics of patients in this study.
| Total (n = 101) | Non-infected (n = 76) | Infecteda (n = 25) | P value (infected vs non-infected) | |
|---|---|---|---|---|
| Demographics | ||||
| Median age in years (interquartile range) | 74 (72–80) | 75 (72–81) | 74 (72–77) | 0.272 |
| Aged 80 years or above | 27 (26.7) | 25 (32.9) | 2 (8) | 0.018 |
| Sex | 0.064 | |||
| Male | 51 (50.5) | 34 (44.7) | 17 (68) | |
| Female | 50 (49.5) | 42 (55.3) | 8 (32) | |
| Chronic comorbidities | ||||
| Hypertension | 70 (69.3) | 52 (68.4) | 18 (72) | 0.807 |
| Diabetes mellitus | 39 (38.6) | 26 (34.2) | 13 (52) | 0.155 |
| Cardiovascular disease | 36 (35.6) | 27 (35.5) | 9 (36) | 1.000 |
| Malignancy | 32 (31.7) | 26 (34.2) | 6 (24) | 0.459 |
| Other endocrine disease | 29 (28.7) | 21 (27.6) | 8 (32) | 0.799 |
| Lung disease | 24 (23.8) | 16 (21.1) | 8 (32) | 0.286 |
| Liver disease | 21 (20.8) | 15 (19.7) | 6 (24) | 0.777 |
| Neurological disease | 13 (12.9) | 9 (11.8) | 4 (16) | 0.731 |
| Kidney disease | 12 (11.9) | 10 (13.2) | 2 (8) | 0.725 |
| Autoimmune disease | 7 (6.9) | 5 (6.6) | 2 (8) | 1.000 |
| Solid organ transplant | 4 (4.0) | 2 (2.6) | 2 (8) | 0.255 |
| Haematological disease | 3 (3.0) | 2 (2.6) | 1 (4) | 1.000 |
| On immunosuppressive therapyb | 8 (7.9) | 5 (6.6)c | 3 (12)d | 0.405 |
| COVID-19 vaccine history | ||||
| Received at least 1 dose of COVID-19 vaccine | 98 (97.0) | 76 (100) | 22 (88) | 0.0138 |
| Completed primary seriese | 93 (92.1) | 74 (97.4) | 19 (76) | 0.003 |
| Received at least 1 booster dose | 70 (69.3) | 60 (78.9) | 10 (40) | 0.001 |
| Number of vaccine doses | ||||
| 0 | 3 (3) | 0 (0) | 3 (12) | <0.001i |
| 1 | 5 (5) | 2 (2.6) | 3 (12)f | |
| 2 | 23 (22.8) | 14 (18.4) | 9 (36)g | |
| 3 | 62 (61.4) | 53 (69.7) | 9 (36)h | |
| 4 | 8 (7.9) | 7 (9.2) | 1 (4) | |
| Type of vaccine | ||||
| CoronaVac only | 30 (29.7) | 23 (30.3) | 7 (28) | 1.000 |
| BNT162b2 only | 60 (59.4) | 47 (61.8) | 13 (52) | 0.618 |
| At least one dose of BNT162b2 | 68 (67.3) | 53 (69.7) | 15 (60) | 0.462 |
| Both CoronaVac and BNT162b2j,k | 8 (7.9) | 6 (7.9) | 2 (8) | 1.000 |
19 patients previously tested positive for SARS-CoV-2 with RT-PCR or rapid antigen test between February and May 2022; 6 patients did not have SARS-CoV-2 positive test and did not receive CoronaVac but tested positive for anti-N IgG.
Included patients who were receiving the immunosuppressive therapy within 1 month before blood collection. The immunosuppressive therapy included chemotherapy, tyrosine kinase inhibitor, janus kinase inhibitor, cyclosporine, mycophenolate mofetil, myophenolic acid, tacrolimus.
1 transplant patient on immunosuppressants; 3 patients on chemotherapy or tyrosine kinase inhibitor for malignancy; 1 patient on Janus kinase inhibitor for haematological disorder.
2 transplant patients on immunosuppressants; 1 patient is on chemotherapy for malignancy.
Received at least 2 doses of BNT162b2 or CoronaVac.
All patients received COVID-19 vaccine before infection.
Among 7 patients with known date of diagnosis, 6 received 2nd dose before infection.
Among 8 patients with known date of diagnosis, all received 3rd dose after infection.
Chi-square for trend test.
Among seven patients who received CoronaVac for the first two doses, six received one booster dose of BNT162b2, and one received two booster doses of BNT162b2.
One patient received BNT162b2 for the first two doses, and then one booster dose of CoronaVac.
Nineteen patients (18.8%) had a history of SARS-CoV-2 infection that was confirmed with either RT-PCR or rapid antigen test, and all were diagnosed between February and May 2022. In addition, 6 of 82 patients (7.3%) who did not have a history of SARS-CoV-2 infection or CoronaVac vaccination tested positive for anti-N IgG. As anti-N IgG wanes rapidly within 3 months,25 these patients with anti-N IgG likely had recent SARS-CoV-2 infection in 2022. In this study, we classified all 25 of these patients as having previous SARS-CoV-2 infection. These 25 patients were likely infected with the Omicron variant because Omicron variant, especially the BA.2, was the dominant SARS-CoV-2 strain circulating in Hong Kong between January and June 2022.3
When compared with previously infected patients, non-infected patients were more likely to be younger than 80 years old (67.1% [51/76] vs 92% [23/25]; P = 0.018; Fisher's exact test), vaccinated (100% [76/76] vs 88% [22/25], P = 0.0138; Fisher's exact test), completed the primary series of COVID-19 vaccine (97.4% [74/76] vs 76% [19/25]; P=<0.003; Fisher's exact test) and more likely to have received more doses of COVID-19 vaccines (P < 0.001; Chi square for trend test) (Table 1). The proportion of female was lower in the infected than the non-infected group, but did not reach statistical significance (32% [8/25] vs 55.3% [42/76], P = 0.064; Fisher's exact test]. Multivariate analysis showed that aged <80 years old (P = 0.016) and higher number of vaccine doses (P < 0.001) were independently associated with non-infection.
Overall humoral and cellular immune response
NAb against BA.5 was detectable in 50.5% (51/101) of the patients, which was significantly lower than that against ancestral strain (85.1% [86/101]; P < 0.0001; Fisher's exact test) or BA.2 (81.2% [82/101]; P < 0.0001; Fisher's exact test) (Fig. 1A). Anti-RBD IgG was positive in 94.1% (95/101) of patients (Supplementary Fig. S1). SARS-CoV-2-specific T cell response, as determined by IGRA, was positive in 84.2% (85/101) of patients (Supplementary Fig. S1). Overall, 95.0% (96/101) had detectable nAb against at least one of SARS-CoV-2 strains or a positive SARS-CoV-2 IGRA result.
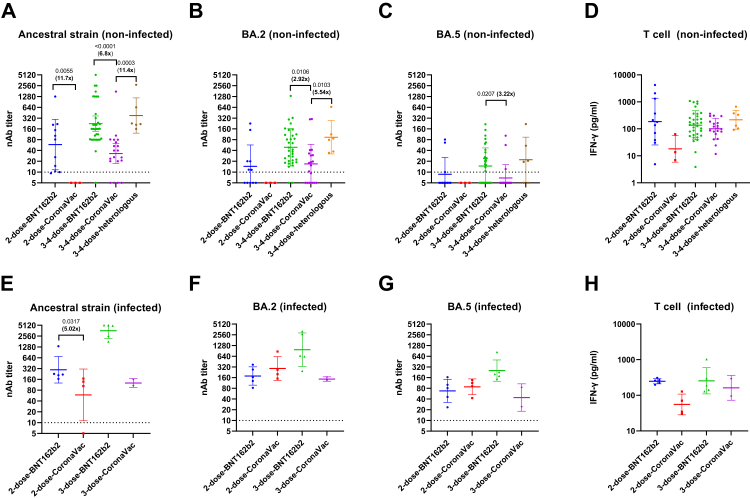
Fig. 1.
Comparison of neutralizing antibody seropositive rates (A) and titers (B–D) among different SARS-CoV-2 ancestral strain and Omicron sublineages; stratified by infection status. All 101 study participants in this study were included in this figure. (A) Error bar indicates 95% confidence interval. P values were calculated using Fisher's exact test. (B–D) Horizontal bars represent the geometric mean neutralizing antibody titer, and the error bars indicate the geometric mean standard deviation. Horizontal dotted line indicates the limit of detection. P values were calculated using Wilcoxon matched-pairs signed rank test. Fold-difference between the neutralization titer against different virus strains are shown in bold.
The nAb titer against BA.5 was also the lowest among the 3 SARS-CoV-2 strains tested. When all 101 patients were analyzed together, the nAb geometric mean titer (GMT) against BA.5 was 6.23-fold and 2.80-fold lower than those against ancestral strain and BA.2, respectively (P < 0.0001; one-way ANOVA with Dunn's multiple comparisons test) (Fig. 1B). The nAb GMT against BA.2 was 2.22-fold lower than that against the ancestral strain (P = 0.0005; one-way ANOVA with Dunn's multiple comparisons test).
Effect of infection status on humoral and cellular immune response
When stratified according to infection status, the nAb GMT against BA.5 was also significantly lower than those of BA.2 and ancestral strain for both non-infected and previously infected patients (Fig. 1C and 1D). However, there was a difference between non-infected and previously infected patients for the relative neutralization activity against BA.2 and ancestral strain. While the nAb GMT against BA.2 was statistically significantly lower than that against ancestral strain for non-infected patients (3.22-fold reduction; P < 0.001; one-way ANOVA with Dunn's multiple comparisons test) (Fig. 1C), there was no statistically significant difference between nAb titer against BA.2 and ancestral strain for previously infected patients (Fig. 1D).
Effect of booster vaccine on nAb and T cell response
Next, we assessed the effect of the number of vaccine doses on antibody and T cell response (Fig. 2). Since BNT162b2 is known to elicit higher titers of nAb than CoronaVac and infection status can affect the nAb and T cell response, we separated the analysis for the different vaccine types. As there were very few patients who have received only 1 dose of vaccine, we have excluded this group from further analysis.
Fig. 2.
Effect of increasing number of vaccine doses on neutralizing antibody and T cell response; stratified by infection status and vaccine type. A–D) Non-infected patients; E–H) infected patients. Horizontal bars represent geometric mean titer, and the error bars indicate the geometric mean standard deviation. Horizontal dotted line indicates the limit of detection for neutralizing antibody. P values were calculated using Mann Whitney U test. Fold-difference between the neutralization titer against different virus strains are shown in bold. The number of patients in each group is shown in Supplementary Table S2.
For non-infected individuals, the 3–4 dose-BNT16b2 group had significantly higher nAb titers against ancestral strain (P = 0.0323; Mann–Whitney U test) and BA.2 (P = 0.005; Mann–Whitney U test) than the 2-dose-BNT162b2 group. Furthermore, the 3-4-dose CoronaVac group had significantly higher nAb titers against ancestral strain (P = 0.0305; Mann–Whitney U test) and more robust T cell activity (P = 0.0181; Mann–Whitney U test) than 2-dose-CoronaVac group.
For previously infected individuals, the 3-4-dose-BNT162b2 group had significantly higher nAb titers against ancestral strain (P = 0.0079; Mann–Whitney U test), BA.2 (P = 0.00317; Mann–Whitney U test), and BA.5 (P = 0.00159; Mann–Whitney U test) than the 2-dose-BNT162b group. Since there were only 2 previously-individuals with 3 doses of CoronaVac, we did not perform a statistical analysis comparing 2 or 3 doses of CoronaVac.
Effect of vaccine type, including heterologous regimen, on nAb and T cell response
Next, we assessed the effect of vaccine type on the nAb and T cell response (Fig. 3). To avoid bias due to the number of vaccine doses, we compared the immune response between patients with the same number of vaccine doses and with the same infection status. Among non-infected patients, the 3-4-dose-CoronaVac group had significantly lower nAb titer against ancestral, BA.2 and BA.5 strains than the 3-4-dose BNT162b2 group. The 3-4-dose-CoronaVac group also had significantly lower nAb titer against ancestral and BA.2 strain those the 3-4-dose-heterologous group. Notably, there was no statistically significant difference in the nAb titers between the 3–4 dose-BNT162 group than the 3-4-dose-heterologous group. The 2-dose BNT162b2 group also had higher nAb titer against all 3 strains for the non-infected group and against ancestral strain for the infected group, but was only statistically significant for the nAb titer against the ancestral strain. There was no statistically significant difference in the T cell response between the different vaccine types.
Fig. 3.
Comparison of neutralization antibody titer and T cell response among patients with different vaccine regimens; stratified by infection status and number of vaccine doses. A–D) Non-infected patients; E–H) infected patients. Horizontal bars represent geometric mean titer, and the error bars indicate the geometric mean standard deviation. Horizontal dotted line indicates the limit of detection for neutralizing antibody. Mann Whitney U test was used for the comparison between the 2-dose-BNT162b2 and 2-dose-CoronaVac groups. For non-infected individuals, one-way ANOVA with Dunn's multiple comparisons test was performed for the comparison of the groups with 3–4 doses of vaccines. The number of patients in each group and the interval between vaccine doses are shown in Supplementary Table S2.
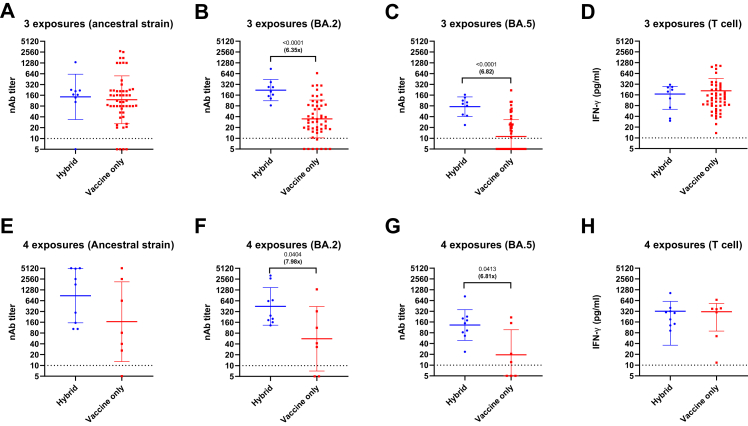
Effect of hybrid immunity on antibody and T cell response
Next, we determined the effect of hybrid immunity (vaccination plus infection) on nAb and T cell response. Here, we defined one exposure to SARS-CoV-2 spike protein as either one episode of infection or one dose of vaccination. For example, a patient who have received 2 vaccine doses and one episode of infection would be considered to have 3 exposures. Sixty two patients had 3 exposures and 16 patients had 4 exposures. Among patients with the same number of exposures, patients with hybrid immunity had statistically significantly higher nAb titers against BA.2 (3 exposures: P < 0.001; 4 exposures: P = 0.0404; Mann Whitney U test) and BA.5 (3 exposures: P < 0.0001; 4 exposures: P = 0.0413; Mann Whitney U test) than those with vaccination only (Fig. 4). However, hybrid immunity was not significantly associated with higher nAb titers against ancestral strain or T cell response.
Fig. 4.
Comparison of neutralization antibody titer and T cell response between patients with hybrid immunity (vaccination plus infection) and those with vaccine-only immunity, stratified according to number of exposures to SARS-CoV-2 spike protein. One dose of COVID-19 vaccine and an episode of SARS-CoV-2 infection is considered as one exposure. A–D) 3 exposures; E–H) 4 exposures. Horizontal bars represent the geometric mean neutralizing antibody titer, and the error bars indicate the geometric mean standard deviation. Horizontal dotted line indicates the limit of detection for neutralizing antibody. P values were calculated using Mann Whitney U test. Fold-difference between the neutralization titer against different virus strains are shown in bold. The number of patients in each group is shown in Supplementary Table S2.
Effect of booster vaccine doses and vaccine type on nAb and T cell response after adjustment for comorbidities
Since comorbidities have been shown to affect nAb response, we used multivariable regression analysis to control for these factors (Table 2). For the analysis comparing BNT162b2 and CoronaVac recipients, we only included non-infected patients who have received 3 doses of vaccines and excluded patients with heterologous boosters (n = 48). BNT162b2 remained to be an independent factor associated with higher nAb titers against ancestral strain (P < 0.001), BA.2 (P = 0.002) and BA.5 (P = 0.018), For the analysis comparing individuals who have received 3 or 4 doses of vaccines with those who have received 2 doses, we only included non-infected patients who have received BNT162b2 only (n = 45). Having received 3 or 4 doses of vaccines remained to be an independent factor associated with higher nAb titers against ancestral strain (P = 0.001), BA.2 (P < 0.001) and BA.5 (P = 0.013).
Table 2.
Univariable and multivariable regression analysis assessing the association of the BNT162b2 with higher neutralizing antibody titers against ancestral strain, Omicron BA.2 and BA.5.
| Model | Univariable regression analysisa |
||||
|---|---|---|---|---|---|
| Standardized coefficient | 95% confidence interval |
P value | |||
| Lower bound | Upper bound | ||||
| BNT162b2 vs CoronaVacc | |||||
| Ancestral strain | 0.519 | 0.372 | 1.083 | <0.001 | |
| BA.2 | 0.344 | 0.070 | 0.674 | 0.017 | |
| BA.5 | 0.276 | −0.009 | 0.518 | 0.058 | |
| 3 or 4 doses vs 2 dosesd | |||||
| Ancestral strain | 0.373 | 0.137 | 1.029 | 0.012 | |
| BA.2 | 0.397 | 0.154 | 0.911 | 0.007 | |
| BA.5 | 0.201 | −0.116 | 0.578 | 0.186 | |
|
Multivariable regression analysisa,b |
|||||
|---|---|---|---|---|---|
| Standardized coefficient |
95% confidence interval |
P value | |||
| Lower bound | Upper bound | ||||
| BNT162b2 vs CoronaVacc | |||||
| Ancestral strain | Backward | 0.581 | 0.495 | 1.133 | <0.001 |
| BA.2 | Backward | 0.427 | 0.181 | 0.742 | 0.002 |
| BA.5 | Backward | 0.341 | 0.057 | 0.572 | 0.018 |
| 3 or 4 doses vs 2 dosesd | |||||
| Ancestral strain | Backward | 0.409 | 0.219 | 1.062 | 0.001 |
| BA.2 | Backward | 0.637 | 0.493 | 1.215 | <0.001 |
| BA.5 | Backward | 0.413 | 0.107 | 0.845 | 0.013 |
Log-transformed nAb titer was used for the analysis.
Multivariable regression model adjusted for age (Age 80 years or above), sex, hypertension, diabetes mellitus, heart disease, malignancy, other endocrine disease, lung, liver disease, neurological disease, kidney disease, autoimmune disease, solid organ transplant, haematological disease, and immunosuppressive drugs. Backward selection was used as a covariant elimination strategy.
Only included the 48 study participants who were non-infected, received 3 doses of vaccines and received only one type of vaccine. Number of patients: n = 16 for CoronaVac and n = 32 for BNT162b2.
Only included the 45 study participants who were non-infected, received at least 2 doses of vaccines, and received BNT162b2 only. Number of patients: n = 11 for 2 doses and n = 34 for 3 or 4 doses.
Discussion
Among older adults with comorbidities, we found that booster doses (3–4 doses) could significantly improve the nAb titers against the currently circulating Omicron BA.2 and BA.5 sublineages. Patients primed with CoronaVac and boosted with BNT162b2 (heterologous booster) had high nAb titers at a level comparable to those with homologous BNT162b2 booster. Previously-infected patients (those with hybrid immunity) induced higher nAb titers than those with vaccine-only induced immunity against Omicron sublineages. T cell response was not significantly affected by booster doses, vaccine types and previous infection. As both nAb and T cells play important roles in protection from severe disease,26,27 our results may predict the effectiveness of COVID-19 vaccines.
The improvement in nAb titers by booster doses was observed for both non-infected and infected individuals. Our data support the recommendation for older adults to receive booster doses of vaccines which may reduce the risk of symptomatic infection.17 However, there was no significant improvement in T cell immunity after booster doses for previously infected individuals. As T cell immunity correlates with the protection from severe disease,28 booster doses may not provide additional benefit of the protection against severe disease among previously infected individuals.
Previous studies showed that CoronaVac had poorer immunogenicity than BNT162b2,10 and a single BNT162b2 booster could significantly improve nAb titers.23 In the current study, we found that among non-infected patients with 3–4 doses of vaccines, those with heterologous prime-boost regimen (primed with CoronaVac, then boosted with BNT162b2) had comparable nAbs than those with 3–4 doses of BNT162b2. Therefore, non-infected patients who have been primed with inactivated whole virion vaccine may benefit from heterologous booster with mRNA vaccine.
We did not observe a significant difference in the T cell response after booster doses, except for a significant increase in T cell response after boosting with CoronaVac. Previous study suggest that the CD8+ T cell response after booster doses was transient and lasted for less than 2 months.29 Since the blood specimens were collected at a median of over 120 days after the booster dose in our study, we would have missed the phase with the transient increase in T cell response. Since T cell is crucial for limiting disease severity, future research on vaccine boosters should explore strategies which can improve T cell response.30
Prior studies showed that BA.5, which has become the dominant SARS-CoV-2 lineage circulating worldwide since July 2022, was less susceptible to nAb elicited from prior infection or vaccination.13,15,31 Our results concur with previous findings, with BA.5 being 2.61-fold less susceptible than BA.2 for non-infected individuals. In our study, the serum specimens were collected 4 months of the peak of the 2022 Omicron-dominant fifth wave. Infection during the Omicron BA.2-dominant period have likely boosted the nAb titer against BA.5, but there was still a 3.46-fold difference between in the GMT against BA.2 and BA.5 for individuals infected during the BA.2-dominant period. The sublineage BA.5 differs from BA.2 in having the L452R and F486V mutation in the spike protein, and studies have shown that the spike mutations L452R and F486V confer immune escape.15,32 Our results have significant implication for second generation Omicron vaccines. Second generation vaccines based on Omicron BA.1 or BA.2 may induce only modest increase in nAb titers against BA.5. With waning of immunity, the effectiveness may not be as potent as the first generation vaccine against the ancestral strain.
There are several limitations in this study. First, we cannot completely rule out the possibility that some patients classified as non-infected may have prior SARS-CoV-2 infection. Previous study showed that anti-N IgG wanes rapidly,25 and anti-N IgG may not be elicited among vaccinated patients.33 The misclassification of previously infected patients as non-infected patients would have led to a falsely higher antibody titers among the non-infected group with the same number of vaccine doses. Second, all previously infected patients in this study confirmed by RT-PCR or rapid antigen test were infected during the Omicron BA.2 surge in Hong Kong between February and May 2022. Due to the stringent control measures in Hong Kong, <0.2% of the population were infected before 2022. Hence, we were not able to assess the immune response after infection with ancestral strain or pre-Omicron variants. Third, this study was not designed to assess the durability of booster doses. A previous study showed that the durability of BNT162b2 booster among individuals primed with CoronaVac were shorter for older adults aged 80 year or above.34 Fourth, our analysis is limited by the small sample size. Therefore, this study does not have sufficient statistical power to determine the difference between some of the groups, especially among the infected patients. Fifth, this is a single center study. A multicenter study with patients of different background will make the results more generalizable. Furthermore, some analyses were not preplanned because some information was not available before the study was started. For example, in the multivariable regression analysis, we decided to analyze the effect of vaccine type and vaccine doses separately only after we collected the information about the vaccine types and vaccine doses our participants have received.
The rapid surge of severe cases requiring hospitalization, especially apparent during the emergence of novel variants,35 overwhelms the healthcare system. In order to formulate preparedness plans and vaccination policies for older adults, it is important to have an accurate and objective assessment for protective immunity among that population, especially against novel variants. This study included a geriatric cohort with the distribution of comorbidities that are seen among the geriatric population in Hong Kong, and our data would be relevant for this population. Continual surveillance of immune protection against emerging variants are required to better monitor the situation, which will help guide allocation of healthcare resources. Although booster doses or BNT16b2 were associated with better neutralizing antibody response, there is a lack of improvement in T cell immunity after boosting with BNT162b2. Further research is required for strategies to improve vaccine-induced T cell immunity.
Contributors
CHYF, IFNH, JKYY and KKWT contributed to the conception and design of the study. CHYF, XZ, LLC, RWSP, BPCC and YZ contributed to data acquisition. CHYF, CKHW, and KKWT contributed to data analysis. CHYF, KHC, KYY, IFNH, JKYY and KKWT contributed to data interpretation. KYY and KKWT had roles in funding acquisition. CHYF and KKWT have verified the underlying data. All authors reviewed and edited the manuscript. CHYF, JKYY and KKWT had full access to the data in the study, and have shared the responsibility for the decision to submit for publication. All authors read and approved the final version of the manuscript.
Data sharing statement
Anonymised participant data can be made available upon reasonable request from the corresponding author.
Declaration of interests
KYY, IFHN and KKWT report collaboration with Sinovac, Sinopharm and Wantai BioPharm. IFNH received payment from M.S.D. for speaking at the COVID-19 Regional Expert Input Forum 2021; is on the advisory board of Pfizer on COVID-19 management in 2022; was on the advisory board of Gilead on evolving treatment landscape in COVID-19 in 2021 and also on the advisory board of AstraZeneca and Fosun on the prevention of COVID-19 infection in 2022. Other authors declare no competing interests.
Acknowledgements
This work was partly supported by the Health and Medical Research Fund, the Food and Health Bureau, the Government of the Hong Kong Special Administrative Region(COVID1903010 [Project 1]), Consultancy Service for Enhancing Laboratory Surveillance of Emerging Infectious Diseases and Research Capability on Antimicrobial Resistance for Department of Health of the Hong Kong; the Theme-Based Research Scheme [T11/707/15] of the Research Grants Council, Hong Kong Special Administrative Region; Emerging Collaborative Project of Guangzhou Laboratory [EKPG22-01]; and Emergency COVID-19 Project [2021YFC0866100], Major Projects on Public Security, National Key Research and Development Program. We acknowledge funding received from private donors including the Shaw Foundation Hong Kong, Richard Yu and Carol Yu, May Tam Mak Mei Yin, Michael Seak-Kan Tong, Respiratory Viral Reuter Foundation Limited, Hui Ming, Hui Hoy and Chow Sin Lan Charity Fund Limited, Chan Yin Chuen Memorial Charitable Foundation, and Marina Man-Wai Lee.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2023.104446.
Contributor Information
Jacqueline Kwan Yuk Yuen, Email: jkyuen@hku.hk.
Kelvin Kai-Wang To, Email: kelvinto@hku.hk.
Appendix A. Supplementary data
References
- 1.COVID-19 Excess Mortality Collaborators Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020-21. Lancet. 2022;399:1513–1536. doi: 10.1016/S0140-6736(21)02796-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prendki V., Tiseo G., Falcone M., ESCMID Study Group for Infections in the Elderly (ESGIE) Caring for older adults during the COVID-19 pandemic. Clin Microbiol Infect. 2022;28:785–791. doi: 10.1016/j.cmi.2022.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen L.L., Abdullah S.M.U., Chan W.M., et al. Contribution of low population immunity to the severe Omicron BA.2 outbreak in Hong Kong. Nat Commun. 2022;13:3618. doi: 10.1038/s41467-022-31395-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang F., Hammel I.S., Andrew M.K., Ruiz J.G. COVID-19 mRNA vaccine effectiveness against hospitalisation and death in veterans according to frailty status during the SARS-CoV-2 delta (B.1.617.2) variant surge in the USA: a retrospective cohort study. Lancet Healthy Longev. 2022;3:e589–e598. doi: 10.1016/S2666-7568(22)00166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McMenamin M.E., Nealon J., Lin Y., et al. Vaccine effectiveness of one, two, and three doses of BNT162b2 and CoronaVac against COVID-19 in Hong Kong: a population-based observational study. Lancet Infect Dis. 2022;22:1435–1443. doi: 10.1016/S1473-3099(22)00345-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu L., To K.K., Chan K.H., et al. High neutralizing antibody titer in intensive care unit patients with COVID-19. Emerg Microbes Infect. 2020;9:1664–1670. doi: 10.1080/22221751.2020.1791738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei J., Stoesser N., Matthews P.C., et al. Antibody responses to SARS-CoV-2 vaccines in 45,965 adults from the general population of the United Kingdom. Nat Microbiol. 2021;6:1140–1149. doi: 10.1038/s41564-021-00947-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tartof S.Y., Slezak J.M., Fischer H., et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398:1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sogaard O.S., Reekie J., Johansen I.S., et al. Characteristics associated with serological COVID-19 vaccine response and durability in an older population with significant comorbidity: the Danish Nationwide ENFORCE Study. Clin Microbiol Infect. 2022;28:1126–1133. doi: 10.1016/j.cmi.2022.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu L., Mok B.W.Y., Chen L.L., et al. Neutralization of severe acute respiratory syndrome coronavirus 2 Omicron variant by sera from BNT162b2 or CoronaVac vaccine recipients. Clin Infect Dis. 2022;75:e822–e826. doi: 10.1093/cid/ciab1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L.L., Chu A.W., Zhang R.R., Hung I.F., To K.K. Serum neutralisation of the SARS-CoV-2 omicron sublineage BA.2. Lancet Microbe. 2022;3:e404. doi: 10.1016/S2666-5247(22)00060-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen L.L., Chua G.T., Lu L., et al. Omicron variant susceptibility to neutralizing antibodies induced in children by natural SARS-CoV-2 infection or COVID-19 vaccine. Emerg Microbes Infect. 2022;11:543–547. doi: 10.1080/22221751.2022.2035195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowen J.E., Addetia A., Dang H.V., et al. Omicron spike function and neutralizing activity elicited by a comprehensive panel of vaccines. Science. 2022;377:890–894. doi: 10.1126/science.abq0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X., Chen L.L., Abdullah S.M., et al. Immune imprinting with ancestral SARS-CoV-2 strain induced potent neutralizing activity against Omicron sublineages BA.2, BA.2.12.1 and BA.4/5. 2022. https://ssrn.com/abstract=4206824 Available at: SSRN:
- 15.Cao Y., Yisimayi A., Jian F., et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature. 2022;608:593–602. doi: 10.1038/s41586-022-04980-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Q., Guo Y., Iketani S., et al. Antibody evasion by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4 and BA.5. Nature. 2022;608:603–608. doi: 10.1038/s41586-022-05053-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization Good practice statement on the use of second booster doses for COVID-19 vaccines, Dated 18 August 2022. 2022. https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE-good-practice-statement-second-booster Available at:
- 18.Jara A., Undurraga E.A., Zubizarreta J.R., et al. Effectiveness of homologous and heterologous booster doses for an inactivated SARS-CoV-2 vaccine: a large-scale prospective cohort study. Lancet Glob Health. 2022;10:e798–e806. doi: 10.1016/S2214-109X(22)00112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grewal R., Kitchen S.A., Nguyen L., et al. Effectiveness of a fourth dose of covid-19 mRNA vaccine against the omicron variant among long term care residents in Ontario, Canada: test negative design study. BMJ. 2022;378 doi: 10.1136/bmj-2022-071502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arbel R., Sergienko R., Friger M., et al. Effectiveness of a second BNT162b2 booster vaccine against hospitalization and death from COVID-19 in adults aged over 60 years. Nat Med. 2022;28:1486–1490. doi: 10.1038/s41591-022-01832-0. [DOI] [PubMed] [Google Scholar]
- 21.Tan C.Y., Chiew C.J., Lee V.J., Ong B., Lye D.C., Tan K.B. Effectiveness of a fourth dose of COVID-19 mRNA vaccine against Omicron variant among elderly people in Singapore. Ann Intern Med. 2022;175:1622–1623. doi: 10.7326/M22-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munro A.P.S., Feng S., Janani L., et al. Safety, immunogenicity, and reactogenicity of BNT162b2 and mRNA-1273 COVID-19 vaccines given as fourth-dose boosters following two doses of ChAdOx1 nCoV-19 or BNT162b2 and a third dose of BNT162b2 (COV-BOOST): a multicentre, blinded, phase 2, randomised trial. Lancet Infect Dis. 2022;22:1131–1141. doi: 10.1016/S1473-3099(22)00271-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perez-Then E., Lucas C., Monteiro V.S., et al. Neutralizing antibodies against the SARS-CoV-2 Delta and Omicron variants following heterologous CoronaVac plus BNT162b2 booster vaccination. Nat Med. 2022;28:481–485. doi: 10.1038/s41591-022-01705-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Low E.V., Tok P.S.K., Husin M., et al. Assessment of heterologous and homologous boosting with inactivated COVID-19 vaccine at 3 Months compared with homologous boosting of BNT162b2 at 6 months. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.26046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu L., Chen L.L., Zhang R.R., et al. Boosting of serum neutralizing activity against the Omicron variant among recovered COVID-19 patients by BNT162b2 and CoronaVac vaccines. eBioMedicine. 2022;79 doi: 10.1016/j.ebiom.2022.103986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khoury D.S., Cromer D., Reynaldi A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 27.McMahan K., Yu J., Mercado N.B., et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature. 2021;590:630–634. doi: 10.1038/s41586-020-03041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kent S.J., Khoury D.S., Reynaldi A., et al. Disentangling the relative importance of T cell responses in COVID-19: leading actors or supporting cast? Nat Rev Immunol. 2022;22:387–397. doi: 10.1038/s41577-022-00716-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reinscheid M., Luxenburger H., Karl V., et al. COVID-19 mRNA booster vaccine induces transient CD8+ T effector cell responses while conserving the memory pool for subsequent reactivation. Nat Commun. 2022;13:4631. doi: 10.1038/s41467-022-32324-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barouch D.H. Covid-19 vaccines - immunity, variants, boosters. N Engl J Med. 2022;387:1011–1020. doi: 10.1056/NEJMra2206573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tuekprakhon A., Nutalai R., Dijokaite-Guraliuc A., et al. Antibody escape of SARS-CoV-2 Omicron BA.4 and BA.5 from vaccine and BA.1 serum. Cell. 2022;185:2422–2433.e13. doi: 10.1016/j.cell.2022.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCallum M., Walls A.C., Sprouse K.R., et al. Molecular basis of immune evasion by the Delta and Kappa SARS-CoV-2 variants. Science. 2021;374:1621–1626. doi: 10.1126/science.abl8506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Follmann D., Janes H.E., Buhule O.D., et al. Antinucleocapsid antibodies after SARS-CoV-2 infection in the blinded phase of the randomized, placebo-controlled mRNA-1273 COVID-19 vaccine efficacy clinical trial. Ann Intern Med. 2022;175:1258–1265. doi: 10.7326/M22-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cerqueira-Silva T., de Araujo Oliveira V., Paixao E.S., et al. Duration of protection of CoronaVac plus heterologous BNT162b2 booster in the Omicron period in Brazil. Nat Commun. 2022;13:4154. doi: 10.1038/s41467-022-31839-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng V.C.C., Ip J.D., Chu A.W.H., et al. Rapid spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron subvariant BA.2 in a single-source community outbreak. Clin Infect Dis. 2022;75:e44–e49. doi: 10.1093/cid/ciac203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.