Summary
Background
Evidence supports prophylactic use of olanzapine for the treatment of chemotherapy-induced nausea and vomiting (CINV). However, most studies to date have focused on patients with single-day highly emetogenic chemotherapy (HEC). Currently, administration of antiemetic therapies for nausea and vomiting induced by multiday chemotherapy regimens remains a challenge. In this study, we evaluated the efficacy of olanzapine combined with triple antiemetic therapy for the prevention of CINV in patients receiving multiday chemotherapy.
Methods
We performed a randomized, double-blind, placebo-controlled phase 3 trial in 22 hospitals. Eligible patients were between 18 and 75 years old, were diagnosed with malignant solid tumors, and they had an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2. All the study participants were scheduled to be treated with chemotherapy regimens containing 3-day cisplatin (3-day total dose ≥75 mg/m2). Randomization was computer generated and stratified by gender and chemotherapy treatment history. Allocation was done via an interactive web response system. Enrolled patients were randomly assigned 1:1 to receive either 5 mg olanzapine or placebo orally before bedtime for 5 days combined with intravenous fosaprepitant (150 mg) 1 h before the administration of cisplatin on day 1, ondansetron hydrochloride intravenously, and dexamethasone orally 30 min before cisplatin from days 1 to 3. Dexamethasone was also administered at the same time on days 4 and 5. The primary endpoint was the proportion of subjects with complete response (no vomiting and no rescue therapy) within the overall phase (days 1–8) after starting chemotherapy. Baseline plasma concentrations of P-substance and 5-HT were measured for exploratory analysis. This study was registered at ClinicalTrials.gov, number NCT04536558.
Findings
Between December 2020 and September 2021, 349 patients with malignant solid tumors were enrolled in the study, with 175 participants randomly assigned to receive olanzapine and 174 participants assigned to receive placebo. The proportion of patients who achieved a complete response in the overall phase was significantly higher in the olanzapine group than in the placebo group (69% vs. 58%, P = 0.031). A complete response benefit was observed in the olanzapine group versus the placebo group in almost all the subgroups. Four factors were considered significantly associated with complete response in multivariable analysis: treatment group, gender, baseline plasma concentration of 5-HT, and prior radiotherapy. All the reported adverse events associated with olanzapine administration were grades 1 and 2.
Interpretation
Olanzapine (5 mg) combined with fosaprepitant, ondansetron, and dexamethasone was better than triple antiemetic therapy alone for patients receiving multiday chemotherapy regimens. Based on these results, the four-drug combination should be recommended as the best antiemetic regimen given to patients receiving multiday cisplatin-based chemotherapy and baseline plasma concentration of 5-HT may be used to identify individuals who are prone to CINV. However, all these findings need to be further validated in future studies.
Funding
Jiangsu Hansoh Pharmaceutical Group Co., Ltd. provided research grant and study drugs for this investigator-initiated study.
Keywords: Olanzapine, Chemotherapy-induced nausea and vomiting, CINV, Multiday chemotherapy, Clinical trial
Research in context.
Evidence before this study
We searched PubMed on June 1, 2022, using the terms “Chemotherapy-induced nausea and vomiting”, “CINV”, “multiday chemotherapy”, “multiple-day chemotherapy”, “highly emetogenic chemotherapy”, “HEC” and “olanzapine”, selecting relevant clinical trials published in English between January 1, 2010 and June 1, 2022. For antiemetic prophylaxis of multiday HEC, current guidelines recommend a three-drug regimen (NK1 receptor antagonist, 5-HT3 receptor antagonist, and dexamethasone) based on a small phase 3 randomized clinical trial, several single-arm, non-randomized studies, and data from a meta-analysis. Moreover, it is unclear whether olanzapine can further improve the antiemetic effects of the three-drug regimen currently prescribed to patients receiving multiday HEC.
Added value of this study
To our knowledge, this study is the first randomized, double-blind, placebo-controlled, phase 3 trial investigating the efficacy and safety of preventive olanzapine combined with triple antiemetic therapy for multiday cisplatin-based chemotherapy in patients with solid tumors. Our results showed the proportion of patients who achieved a complete response in the overall phase was significantly higher in the olanzapine group than in the placebo group. The no nausea rate was also significantly improved in the olanzapine group during the overall phase.
Implications of all the available evidence
The study suggested that olanzapine in combination with fosaprepitant, ondansetron, and dexamethasone was better than the use of triple antiemetic therapy alone in preventing CINV in patients receiving multiday cisplatin-based chemotherapy. Based on the result obtained here, the four-drug treatment strategy should be recommended as the best antiemetic regimen for multiday cisplatin-treated patients with solid tumors.
Introduction
Multiday chemotherapy remains the mainstay treatment strategy for many cancers.1, 2, 3 Compared to single-day cisplatin treatment, multiday cisplatin treatment can provide comparable efficacy with less toxicity and can avoid the need for time-consuming hydration procedures.4 Currently, multiday cisplatin treatment is widely used in patients with germ cell tumors,5,6 small cell lung cancer (SCLC),7,8 and other solid tumors.3,4,9,10
Chemotherapy-induced nausea and vomiting (CINV) is a common and distressing toxicity associated with cancer chemotherapy that deteriorates patients’ quality of life (QOL), impairs medication compliance, and decreases the efficacy of therapy.11 Over the past few decades, compelling advances have been achieved for the treatment of CINV. However, antiemetic therapy for nausea and vomiting caused by multiday chemotherapy regimens remains a challenge owing to continuous daily emetic stimuli, especially since the onset of acute and delayed CINV may overlap after the initial day of chemotherapy through to the last day of treatment.12,13 Ultimately, it is often difficult to determine an appropriate antiemetic regimen for affected patients.
Recently, two randomized phase 3 studies confirmed the benefits of olanzapine in the prevention of CINV.14,15 The four-drug combination of a neurokinin 1 (NK1) receptor antagonist, a serotonin (5-HT3) receptor antagonist, dexamethasone, and olanzapine is currently recommended for adult patients treated with highly emetogenic chemotherapy (HEC) per the American Society of Clinical Oncology (ASCO) antiemetic guidelines.16 The National Comprehensive Cancer Network (NCCN) and the Multinational Association of Supportive Care in Cancer (MASCC)/the European Society for Medical Oncology (ESMO) also recommend the four-drug regimen as a treatment option for HEC patients.17,18 However, these guidelines mostly refer to patients with solid tumors receiving single-day HEC. Prophylaxis of CINV associated with multiday chemotherapy is relatively under-represented in the literature. Current guidelines recommend a three-drug regimen (NK1 receptor antagonist, 5-HT3 receptor antagonist, and dexamethasone) for antiemetic prophylaxis of multiday HEC, which is based on a small phase 3 randomized clinical trial, several single-arm, non-randomized studies, and data from a meta-analysis.18, 19, 20, 21, 22, 23 Moreover, it is unclear whether olanzapine can further improve the antiemetic effects of the three-drug regimen currently prescribed to patients receiving multiday HEC.
This randomized, double-blind, placebo-controlled, phase 3 trial was conducted to determine whether the addition of olanzapine to current triple antiemetic therapy regimens (fosaprepitant, ondansetron, and dexamethasone) could better prevent CINV in patients receiving multiday cisplatin-based chemotherapy.
Methods
Study design and patients
This multi-center, randomized, double-blind, placebo-controlled, phase 3 trial was performed in 22 hospitals in China between December 2020 and September 2021 after approval of each site's institutional review board.
The study included patients with malignant solid tumors who were scheduled to be treated with chemotherapy regimens containing 3-day cisplatin (3-day total dose ≥75 mg/m2). Additionally, eligible patients had to be between 18 and 75 years old, have an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2, a life expectancy ≥3 months, and be able to read, understand, and complete study questionnaires and diaries, including a visual analog scale (VAS).
Patients were excluded for any one of the following criteria: the presence of cognitive impairment (e.g., dementia or severe learning difficulties), were taking illicit drugs including medicinal marijuana or with a long-time problematic pattern of alcohol use leading to clinically significant cognitive impairment, were scheduled to receive stem cell therapy during the cisplatin-based chemotherapy, planned to be treated with a chemotherapy regimen including standard paclitaxel (using castor oil as the solvent) requiring pretreatment with more than 6 mg of dexamethasone or moderately to highly emetogenic chemotherapeutic drugs within 6 days before or after cisplatin infusion. Patients were also excluded due to abnormal laboratory parameter values including an absolute neutrophil count (ANC) <1.5 × 109, a white blood cell count <3 × 109/L, a platelet count <100 × 109/L, AST level (aspartate aminotransferase) >2.5 × upper limit of normal, ALT level (alanine aminotransferase) >2.5 × upper limit of normal, bilirubin >1.5 × upper limit of normal, or creatinine >1.5 × upper limit of normal. The full inclusion and exclusion criteria can be found in the protocol (Supplemental Material p10)
The study protocol was approved by the ethics committee of Sun Yat-sen University Cancer Center (approved number: B2020-250-01) and each participating institution. The trial was performed in accordance with the Good Clinical Practice (GCP) guidelines. All of the patients provided written informed consent before treatment initiation.
Randomization and masking
Enrolled patients were randomly assigned 1:1 to receive either 5 mg olanzapine or matching placebo orally in combination with fosaprepitant, ondansetron, and dexamethasone based on a computer-generated randomization sequence using Proc Plan procedure of SAS software (version 9.4.). Randomization was balanced by using randomly permuted blocks with size of four and was stratified according to gender and previous chemotherapy history. Allocation was done via an interactive web-response system (IWRS). An investigator at each hospital site registered patients via the IWRS and assigned them to a treatment group.
All of the patients, investigators and medical personnel involved in the study were masked to group allocation. An olanzapine simulation agent was used as a placebo. Olanzapine and the matching placebo (provided by Jiangsu Hansoh Pharmaceutical Group Co., Ltd.) were identical in packaging and appearance to preserve the masking.
Procedures
Patients received either olanzapine (5 mg) or placebo orally before bedtime from days 1 to 5. All of the patients received fosaprepitant (150 mg) intravenously approximately 1 h before cisplatin treatment on day 1, and ondansetron hydrochloride (8 mg) intravenously and dexamethasone (6 mg) orally 30 min before cisplatin administration on days 1–3. Dexamethasone (6 mg) was also administered at the same time on days 4 and 5 (Supplemental Table S1).
Assessments of treatment efficacy, tolerability, and safety variables were performed after the start of chemotherapy. Episodes of nausea and vomiting or rescue therapy were recorded in a patient diary on days 1–8. The degree of nausea was evaluated daily using the VAS scale for nausea. Safety evaluations including recording and assessing all vital signs (temperature, respiration, heart rate, and blood pressure), performing physical examinations, tracking adverse events, serious adverse events, electrocardiogram (ECG) results, and laboratory tests (hematology, blood chemistry, and urinalysis) were done on day 1 and during the follow-up visits (on day 9 and day 21 ± 5 days) according to the protocol (Supplemental Material p9)
Blood samples were collected to detect baseline plasma concentrations of substance P and 5-HT before treatment with the consent of the patients. Blood samples were managed with Watson LIMS software (Thermo Fisher Scientific, Version 7.6, Thermo Fisher, USA) and stored at ≤−65 °C until assayed. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) used to detect plasma concentrations of 5-HT, and Analyst software (Version 1.7.1, SCIEX, USA) used for data acquisition. Watson LIMS was used to calculate and summarize standard curves, quality controls (QC), and sample concentration data. The levels of circulating substance P were measured with commercially available enzyme-linked immunosorbent assay (ELISA) kits (Shanghai Enzyme-linked Biotechnology Co., Ltd.) following the manufacturers’ protocols. Samples were assayed in triplicate, and the mean value was used as the final concentration.
Outcomes
The primary endpoint of the study was the proportion of subjects with complete response (no vomiting and no rescue therapy administered) within the overall phase (days 1–8) after starting chemotherapy. Secondary endpoints included: 1) complete response rate in the acute phase (days 1–3) and delayed phase (days 4–8); 2) no significant nausea (maximum of nausea VAS <25 mm), no nausea (maximum of nausea VAS <5 mm),24,25 and complete control rate (no vomiting, no rescue therapy and no slight nausea) in acute phase, delayed phase and overall phase; 3) time to treatment failure (based on time to onset of first vomiting experience or time to rescue therapy, whichever occurred first); 4) proportion of subjects receiving rescue therapy; 5) change of Functional Living Index-Emesis (FLIE) scores and Hospital Anxiety and Depression Scale (HAD) scores before and after treatment; and 6) subgroup analysis and risk factors of complete response in patients with baseline plasma concentrations of substance P and 5-HT.
Statistical analysis
All of the statistical analyses were performed in the safety set (SS; all of the patients who received at least one dose of study treatment), the full analysis set (FAS; all of the SS patients who had ≥1 efficacy assessment), and the per protocol set (PPS; all of the FAS patients who had no protocol violations that directly affected the primary endpoint). The two-sided test was used for all statistical tests, the P-value of less than 0.05 was considered statistically significant for the tested difference. A superiority test was used to assess efficacy and sample size was estimated based on the primary endpoint. Assuming the complete response rate during the overall observation phase (days 1–8) was 65% and 80% in the placebo group and the olanzapine group respectively,26 a significance level of 5% in a two-sided test, and a detection power of 85%, the sample size was calculated to be 352 patients (176 per group, given the actual possible dropout calculated as 10%).
We performed chi-square tests to compare the proportion of patients with complete response between treatment groups in the overall phase, and to determine the inter-group differences of several secondary endpoints: complete response in acute phase and delayed phase, no significant nausea, no nausea, complete control rate in the acute phase, delayed phase, and the overall phase. The 95% confidence intervals (CIs) were calculated for the proportion of patients achieving a complete response and its inter-group differences using the Wald test. To analyze the variance of other secondary endpoints, a t-test, non-parametric test, or chi-square test was used depending on the dataset. Subgroup analyses of the primary endpoint were conducted to assess the homogeneity of the treatment effect across key demographic and baseline characteristics. Logistic regression models were used to assess the impact of risk factors on complete response rates.
All of the analyses were conducted using SAS software (version 9.4.).
This trial is registered with ClinicalTrials.gov, NCT04536558.
Role of the funding source
The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of this paper. The corresponding author has full access to all of the data in the study and is responsible for the decision to submit the paper for publication.
Results
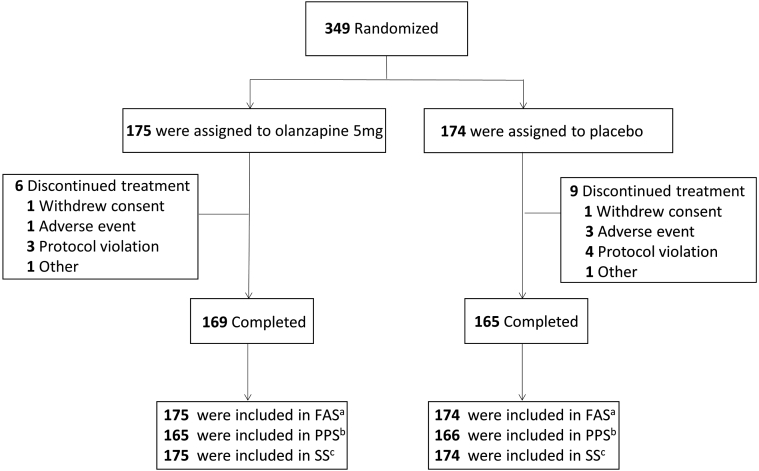
Between December 2020 and September 2021, 349 patients with malignant solid tumors were enrolled in the study, with 175 randomly assigned to receive olanzapine and 174 assigned to receive placebo. Three hundred and forty-nine patients were included in the SS and FAS, and 331 patients in the PPS (Fig. 1). Demographic and clinical characteristics are presented in Table 1 for the FAS. No obvious differences in the baseline demographic or medical characteristics were observed between the olanzapine and placebo groups (Table 1).
Fig. 1.
Study flow chart. aFull analysis set (FAS) comprised all the SS patients who had ≥1 efficacy assessment; bPer protocol set (PPS) comprised all the FAS patients who had no protocol violations that directly affected the primary endpoint; cSafety set (SS) comprised all the patients who received at least one dose of study treatment.
Table 1.
Baseline characteristics (FAS).
| Variable | Olanzapine group (n = 175) | Placebo group (n = 174) |
|---|---|---|
| Age, years | ||
| Median (range) | 60 (20–73) | 58 (20–77) |
| <65 | 117 (67%) | 127 (73%) |
| ≥65 | 58 (33%) | 47 (27%) |
| Gender | ||
| Male | 137 (78%) | 134 (77%) |
| Female | 38 (22%) | 40 (23%) |
| ECOG PS | ||
| 0 | 56 (32%) | 58 (33%) |
| 1 | 117 (67%) | 109 (63%) |
| 2 | 2 (1%) | 6 (3%) |
| Unknown | 0 | 1 (1%) |
| Cancer type | ||
| Lung | 126 (72%) | 126 (72%) |
| Head and neck | 24 (14%) | 21 (12%) |
| Other | 25 (14%) | 27 (16%) |
| Prior chemotherapy | ||
| Yes | 38 (22%) | 38 (22%) |
| No | 137 (78%) | 136 (78%) |
| Prior radiotherapy | ||
| Yes | 14 (8%) | 22 (13%) |
| No | 161 (92%) | 152 (87%) |
| Prior antiemetic therapy | ||
| Yes | 13 (7%) | 14 (8%) |
| No | 162 (93%) | 160 (92%) |
Data are expressed as the median (inter-quartile range) or number (%).
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; FAS, full analysis set.
Efficacy
In olanzapine group, 157 patients had complete diary (d1–8) and 18 had partial diary information; in placebo group, 158 patients had complete diary (d1–8) and 16 had partial diary information. Patients with missing data of primary endpoint were imputed as without complete response. For primary endpoints, the proportion of patients who achieved a complete response in the overall phase was significantly higher in the olanzapine group than in the placebo group 69% (121/175) vs. 58% (101/174), the between-group difference was 11% (95% CI, 0.67–21.20), P = 0.031 (Table 2 and Supplemental Table S2). In the PPS, a higher complete response rate was also observed in the olanzapine group (Supplemental Table S2). In sum, data from this study met the predefined primary endpoint.
Table 2.
Proportion of patients achieving complete response and no nausea.
| Olanzapine group n (%) | Placebo group n (%) | P valuea | |
|---|---|---|---|
| Complete response (overall phase) | |||
| n | 175b | 174b | |
| Yes | 121 (69%) | 101 (58%) | 0.031 |
| No | 54 (31%) | 73 (42%) | |
| Complete response (acute phase) | |||
| n | 166 | 168 | |
| Yes | 142 (86%) | 138 (82%) | 0.40 |
| No | 24 (14%) | 30 (18%) | |
| Complete response (delayed phase) | |||
| n | 164 | 165 | |
| Yes | 124 (76%) | 106 (64%) | 0.025 |
| No | 40 (24%) | 59 (36%) | |
| No nausea (overall phase) | |||
| n | 165 | 168 | |
| Yes | 118 (72%) | 100 (60%) | 0.021 |
| No | 47 (28%) | 68 (40%) | |
| No nausea (acute phase) | |||
| n | 165 | 168 | |
| Yes | 142 (86%) | 133 (79%) | 0.097 |
| No | 23 (14%) | 35 (21%) | |
| No nausea (delayed phase) | |||
| n | 163 | 164 | |
| Yes | 119 (73%) | 100 (61%) | 0.020 |
| No | 44 (27%) | 64 (39%) |
Complete response (no vomiting and no rescue therapy), no nausea (maximum of nausea VAS <5 mm); acute phase (Days 1–3 after starting chemotherapy), delayed phase (Days 4–8 after starting chemotherapy), overall phase (Days 1–8 after starting chemotherapy). Bold values indicate statistical significance (P < 0.05).
Abbreviation: n, number of patients.
P values were calculated with the use of the chi-square test.
Patients with missing data of primary endpoint (complete response in overall phase) were imputed as without complete response.
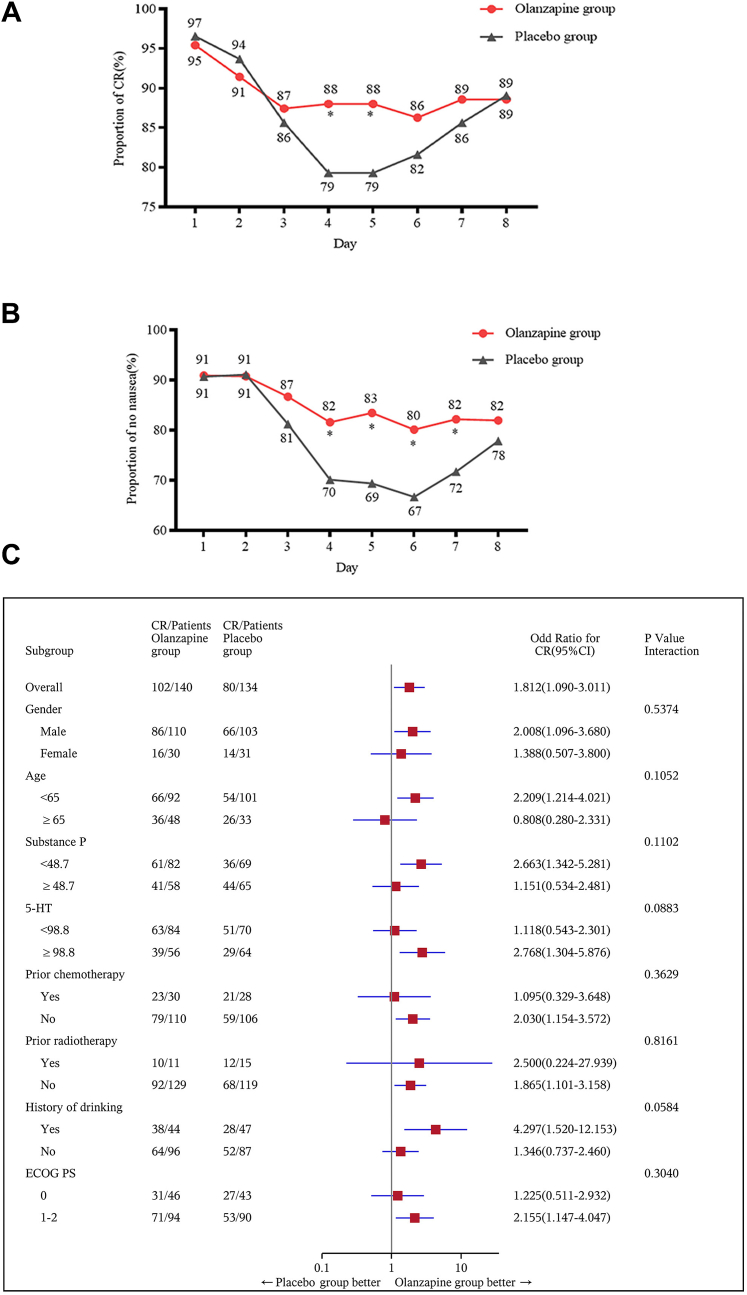
For secondary endpoints, the complete response rate was also significantly increased in patients receiving olanzapine during the delayed phase (76% vs. 64%, P = 0.025). More patients in the olanzapine group had no nausea compared to those in the placebo group in the overall phase (72% vs. 60%, P = 0.021) and the delayed phase (73% vs. 61%, P = 0.020). However, in the acute phase, the proportion of patients with a complete response (86% vs. 82% P = 0.40) and no nausea (86% vs. 79% P = 0.097) was not significantly different between the two groups (Table 2). Changes in the proportion of patients achieving a complete response and no nausea (every 24 h) are presented in Fig. 2. Backed with a statistical test, we found that the olanzapine group demonstrated an improved complete response on day 4 (88% vs. 79%, P = 0.028) and day 5 (88% vs. 79%, P = 0.028) compared to the placebo group (Fig. 2A). Further, the no nausea rate was higher from days 4 to 7 in the olanzapine group (Day 4, 82% vs. 70%, P = 0.020; Day 5, 83% vs. 69%, P = 0.0036; Day 6, 80% vs. 67%, P = 0.0076; Day 7, 82% vs. 72%, P = 0.033, Fig. 2B).
Fig. 2.
(A) Proportion of patients achieving complete response from days 1 to 8 (every 24 h); (B) Proportion of patients experiencing no nausea from days 1 to 8 (every 24 h); (C) Subgroup analysis of complete response during the overall phase in a subset of patients with baseline blood samples (n = 274). ECOG PS, Eastern Cooperative Oncology Group performance status; substance P (pg/mL), 5-HT (ng/mL); CI, confidence interval; n, number of patients. ∗, P < 0.05, calculated with the chi-square test.
Regarding other secondary endpoints, we did not observe any significant differences in the incidence rate of no significant nausea or complete control between the olanzapine and placebo groups (Supplemental Table S3). The proportion of subjects receiving rescue therapy (5% vs. 8%), median time to treatment failure (62.8 h vs. 64.2 h), and changes in FLIE and HAD scores were also similar between both groups.
Subgroup analysis
Baseline blood samples from 274 patients (140 samples from the olanzapine group and 134 samples from the placebo group) were collected and used for testing plasma concentrations of substance P and 5-HT. Baseline clinical characteristics including age, gender, ECOG PS, cancer type, and prior treatment were well balanced between these 274 patients and similar to the FAS (Supplemental Table S4). Based on these results, we assumed that the exploratory analysis of the 274-patient cohort could represent the FAS in this study.
Complete response and no nausea benefit were observed in the olanzapine group versus the placebo group in almost all of the subgroups (Fig. 2C and Supplemental Figure S1). We investigated the relationship between plasma 5-HT and substance P and explored the effect of plasma concentrations of these proteins on complete response. The mean plasma concentrations of substance P and 5-HT were 46 ± 32 pg/mL and 110 ± 119 ng/mL, respectively. There was no correlation observed between plasma concentrations of substance P and 5-HT (R2 = 0.042, P = 0.2861; Supplemental Figure S2). A receiver operator characteristic (ROC) curve was used to determine the best cutoff value of substance P and 5-HT according to the primary endpoint (complete response during the overall phase). The cutoff value of substance P and 5-HT were 48.7 pg/mL and 98.8 ng/mL respectively and patients were divided into different subgroup according to base line concentrations of substance P and 5-HT. Subgroup analysis demonstrated that patients with high or low baseline concentrations of substance P and 5-HT could benefit from the olanzapine-containing treatment regimen (Fig. 2C and Supplemental Figure S1).
Risk factors associated with our primary endpoint complete response in the overall phase were also investigated. In addition to the clinical related factors, baseline plasma concentrations of 5-HT and substance P were included in logistic regression analysis. In univariate analyses, five factors were significantly associated with complete response: treatment group, gender, age, baseline plasma concentration of 5-HT, and prior radiotherapy. Based on multivariable analysis, treatment group, gender, baseline plasma concentration of 5-HT, and prior radiotherapy were confirmed to be related to complete response. Higher concentrations of 5-HT were associated with a higher risk of CINV (Supplemental Table S5).
Toxicity
Of the 349 patients in the SS, 74% (257/349 patients) experienced ≥ grade 1 adverse events, with no significant difference observed between the olanzapine group and placebo group (69% vs. 78%, P = 0.056). The proportion of patients who reported insomnia was significantly lower in the olanzapine group than in the placebo group (1% vs. 6%, P = 0.020), while the incidence of dry mouth was higher in the olanzapine group (6% vs. 1%, P = 0.035) (Table 3). All of the treatment-related adverse events were grades 1 and 2, as shown in Table 3. There were seven grade 3 and three grade 4 adverse events in the olanzapine group, while six grade 3 and four grade 4 adverse events were reported in the placebo group. There were no grade 5 adverse events reported in the study. The grade 3 or 4 adverse events included myelosuppression, transaminase elevation, hyponatremia, hypokalemia, and diarrhea, with none attributed to olanzapine treatment (Table 3). Other common adverse events were also presented in Table 3.
Table 3.
Summary of adverse events in the safety set.
| Olanzapine group (n = 175) |
Placebo group (n = 174) |
P valuea | |||
|---|---|---|---|---|---|
| Grade 1–2 | Grade 3–4 | Grade 1–2 | Grade 3–4 | ||
| All adverse events | 111 (63%) | 10 (6%) | 126 (72%) | 10 (6%) | 0.15 |
| Common adverse eventsb | |||||
| Treatment-related | |||||
| Insomnia | 2 (1%) | 0 | 10 (6%) | 0 | 0.020 |
| Constipation | 35 (20%) | 0 | 31 (18%) | 0 | 0.68 |
| Hiccups | 14 (8%) | 0 | 13 (7%) | 0 | 1.00 |
| Dry mouth | 10 (6%) | 0 | 2 (1%) | 0 | 0.035 |
| Somnolence | 1 (0·6%) | 0 | 2 (1%) | 0 | 0.62 |
| Dizziness | 15 (9%) | 0 | 14 (8%) | 0 | 1.00 |
| Appetite loss | 25 (14%) | 0 | 32 (18%) | 0 | 0.31 |
| Hematological | |||||
| Leukopenia | 17 (10%) | 2 (1%) | 18 (10%) | 2 (1%) | 0.95 |
| Neutropenia | 14 (8%) | 1 (0.6%) | 15 (7%) | 2 (1%) | 0.83 |
| Anemia | 25 (14%) | 0 | 28 (16%) | 0 | 0.66 |
| Thrombocytopenia | 11 (6%) | 2 (1%) | 10 (6%) | 0 | 0.54 |
| Nonhematological | |||||
| Fatigue | 25 (14%) | 0 | 27 (16%) | 0 | 0.77 |
| Nephrotoxicity | 6 (3%) | 0 | 4 (2%) | 0 | 0.75 |
| Hepatoxicity | 12 (7%) | 1 (0.6%) | 10 (6%) | 1 (0.6%) | 0.91 |
| Diarrhea | 9 (5%) | 1 (0.6%) | 10 (6%) | 3 (2%) | 0.62 |
| Hyponatremia | 7 (4%) | 2 (1%) | 4 (2%) | 1 (0.6%) | 0.59 |
| Hypokalemia | 4 (2%) | 1 (0.6%) | 5 (3%) | 1 (0.6%) | 0.88 |
| Hypoalbuminemia | 6 (3%) | 0 | 4 (2%) | 0 | 0.75 |
| Alopecia | 9 (5%) | 0 | 10 (6%) | 0 | 0.82 |
| Myalgia | 5 (3%) | 0 | 4 (2%) | 0 | 1.00 |
| Pruritus | 3 (2%) | 0 | 2 (1%) | 0 | 1.00 |
Abbreviation: n, number of patients.
Bold values indicate statistical significance (P < 0.05).
P values were calculated with the Fisher's exact test or the chi-square test.
Adverse events with incidence rate ≥1% in at least one treatment arm.
Discussion
To our knowledge, this study is the first randomized, double-blind, placebo-controlled, phase 3 trial investigating the efficacy and safety of preventive olanzapine combined with triple antiemetic therapy for multiday cisplatin-based chemotherapy in patients with solid tumors. We found that the complete response-rate during the overall phase was significantly increased in patients who received olanzapine compared with those who received the placebo (69% vs. 58%, P = 0.031). The no nausea rate was also significantly improved in the olanzapine group than the placebo group during the overall phase (72% vs. 60%, P = 0.021). Our study provides new evidence supporting combined olanzapine and triple antiemetic therapy for the prevention of CINV, and it should be considered as the best antiemetic regimen for patients receiving multiday chemotherapy treatments.
In our study, we found no significant improvement in the complete response rate and no nausea rate during the acute phase. These results were consistent with the results of another study evaluating olanzapine in patients with multiday non-cisplatin HEC and hematopoietic cell transplantation regimens in the hematological malignancy setting, where complete response was significantly better in the overall phase and delayed phase only.27 Previous studies have demonstrated that during the acute phase, few patients experienced severe CINV with multiday chemotherapy. This is different from single-day HEC regimens,28 thus indicating that triple antiemetic therapy may be enough to induce beneficial results during the acute phase for patients with multiday treatments. As shown in Fig. 2 of our study, the complete response rate in the placebo group during the days 1 and 2 was 97% and 95%, suggesting that there is little room for further improvement. On the contrary, for patients receiving single-day HEC in another phase 3 trial, the complete response rate in the placebo group was 89% and 84% during the first two days, which was much lower than that in our study and olanzapine improved the complete response rate to 95%.15 These data may explain why in two phase 3 studies of patients receiving single-day HEC, an improved complete response rate was observed in the olanzapine group during the acute phase, which was inconsistent with the results of our study.14,15
The most severe CINV typically occurs during the delayed phase for patients receiving multiday chemotherapy, when a complex overlap of acute and delayed emesis is likely present.28 We also observed a peak incidence of CINV on days 4–6, with the four-agent regimen containing olanzapine greatly improving the complete response on days 4 and 5, and the no-nausea rate from days 4 to 7, indicating an improved CINV during the delayed phase (Fig. 2). On day 8, since the emetogenic effect of the drug (DDP) decreased and incidence of CINV reduced, the difference in the antiemetic efficacy may not be so obvious between the two groups that may be why the curves merged together at that time in Fig. 2. A meta-analysis also demonstrated that olanzapine-containing prophylaxis regimens were superior to other anti-emetic regimens in terms of complete response in both the delayed and overall phases, but not in the acute phase for patients with single-day HEC.29 Recently, two randomized controlled trials conducted by the same research team focused on the antiemetic therapy for patients with multiday chemotherapy. Although from a single center with small sample size, the two trials also demonstrated olanzapine played an important role in preventing nausea and vomiting induced by multiday cisplatin-based chemotherapy.30,31
CINV is complicated by patient-related risk factors, such as age, gender, alcohol consumption history, and a history of motion sickness.32,33 Further, a limited number of biomarkers associated with CINV have been investigated previously.34 In the current study, we explored the correlation between substance P and 5-HT, and their relationship with the prognosis of CINV in a subset of patients with baseline blood samples. Baseline plasma concentration of 5-HT was found to be another risk factor associated with complete response in addition to clinical risk factors. Specifically, higher concentration was associated with higher risk of CINV, which may be used clinically to identify individuals who are prone to CINV. These data should be further explored and validated in future studies.
Generally, olanzapine was well tolerated with no increase in adverse effects. Further, not one patient discontinued olanzapine due to toxicity. The incidence of somnolence, the most common adverse event associated with olanzapine,14,15,35 was very low since olanzapine was taken before bedtime in our study.
One limitation of this study was that only patients treated exclusively with a regimen containing multiday cisplatin were enrolled, thus limiting the scope of the data obtained. The appropriate antiemetic regimen for patients with other multiday chemotherapy regimens should be investigated in future clinical trials. Second, we used intravenous NK1 receptor antagonist (fosaprepitant) and 5-HT3 receptor antagonist (ondansetron) in our study. The efficacy and compliance of the four-drug regimen with orally given NK1 receptor antagonist and 5-HT3 receptor antagonist (like NEPA) still needed to be validated.25,36 Third, only substance P and 5-HT were included in our biomarker analysis. Dopamine as a biomarker for the treatment of olanzapine would be useful and deserve further investigation.
In conclusion, our study is the first to demonstrate that olanzapine in combination with fosaprepitant, ondansetron, and dexamethasone was better than the use of triple antiemetic therapy alone in preventing CINV in patients receiving multiday cisplatin-based chemotherapy. Based on the result obtained here, the four-drug treatment strategy should be recommended as the best antiemetic regimen for multiday cisplatin-treated patients with solid tumors.
Contributors
LZ, YZ, YY, and YH conceived and designed this study. YZ, YY, FG, CH, DZ, FG, CH, DZ, ML, ZY, JZ, JM, YL, JZ, CW, JH, YZ, YH, and LZ enrolled patients and collected the data. All authors participated in data interpretation. The manuscript was drafted by YZ, YY, and YH and was reviewed or revised by all authors. The final version was approved to be submitted by all authors. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. YZ, YY, FG, CH, DZ, and LZ have accessed and verified the underlying data. LZ supervised the study.
Data sharing statement
Research data can be requested from the corresponding author on reasonable request. Any request should be sent to the corresponding author, along with a detailed description of your research protocol. The corresponding author and Sun Yat-sen University Cancer Centre will evaluate the reasonability of the request for our data and reserve the right to decide whether to share the data or not. Once approved, identified participant data will be made available.
Declaration of interests
LZ has received research support from Hengrui, BeiGene, Xiansheng, Eli Lilly, Novartis, Roche, Hansoh and Bristol-Myers Squibb Pharma and consulting for MSD, Beigene and Xiansheng Pharma. All other authors declare no competing interests.
Acknowledgments
We thank all the patients, their families, and the institutions for supporting this study. We thank Jiangsu Hansoh Pharmaceutical Group Co., Ltd. for providing research grant and study drugs. We thank Ashermed Medical Technology (Shanghai) Co., Ltd. for the help in the statistical analysis and interpretation. We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2022.101771.
Contributor Information
Yan Huang, Email: huangyan@sysucc.org.cn.
Li Zhang, Email: zhangli@sysucc.org.cn.
Appendix A. Supplementary data
References
- 1.Patel M.P., Woodring S., Randazzo D.M., et al. Randomized open-label phase II trial of 5-day aprepitant plus ondansetron compared to ondansetron alone in the prevention of chemotherapy-induced nausea-vomiting (CINV) in glioma patients receiving adjuvant temozolomide. Support Care Cancer. 2020;28(5):2229–2238. doi: 10.1007/s00520-019-05039-x. [DOI] [PubMed] [Google Scholar]
- 2.Abdel-Malek R., Abbas N., Shohdy K.S., et al. Addition of 3-day aprepitant to ondansetron and dexamethasone for prophylaxis of chemotherapy-induced nausea and vomiting among patients with diffuse large B cell lymphoma receiving 5-day cisplatin-based chemotherapy. J Egypt Natl Canc Inst. 2017;29(3):155–158. doi: 10.1016/j.jnci.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Kamiya T., Sakurai M., Kikuchi T., et al. Efficacy of ondansetron against emesis induced by a multiple-day cisplatin-based chemotherapy regimen for malignant lymphoma. Hematology. 2021;26(1):945–949. doi: 10.1080/16078454.2021.2001150. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J., Lin M., Jin Y., et al. Cisplatin given at three divided doses for three consecutive days in metastatic breast cancer: an alternative schedule for one full dose with comparable efficacy but less CINV and hypomagnesaemia. Breast Cancer Res Treat. 2020;182(3):719–726. doi: 10.1007/s10549-020-05730-2. [DOI] [PubMed] [Google Scholar]
- 5.Einhorn L.H. Curing metastatic testicular cancer. Proc Natl Acad Sci U S A. 2002;99(7):4592–4595. doi: 10.1073/pnas.072067999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kondagunta G.V., Motzer R.J. Chemotherapy for advanced germ cell tumors. J Clin Oncol. 2006;24(35):5493–5502. doi: 10.1200/JCO.2006.08.7882. [DOI] [PubMed] [Google Scholar]
- 7.Evans W.K., Shepherd F.A., Feld R., Osoba D., Dang P., Deboer G. VP-16 and cisplatin as first-line therapy for small-cell lung cancer. J Clin Oncol. 1985;3(11):1471–1477. doi: 10.1200/JCO.1985.3.11.1471. [DOI] [PubMed] [Google Scholar]
- 8.Faivre-Finn C., Snee M., Ashcroft L., et al. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomised, superiority trial. Lancet Oncol. 2017;18(8):1116–1125. doi: 10.1016/S1470-2045(17)30318-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim J.H., Lee D.H., Shin H.C., et al. A phase II study with gemcitabine and split-dose cisplatin in patients with advanced non-small cell lung cancer. Lung Cancer. 2006;54(1):57–62. doi: 10.1016/j.lungcan.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 10.Funai K., Takamochi K., Itaya T., et al. Feasibility study of adjuvant chemotherapy with gemcitabine and split-dose cisplatin for completely resected non-small-cell lung cancer. Lung Cancer. 2010;68(1):78–83. doi: 10.1016/j.lungcan.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 11.Bloechl-Daum B., Deuson R.R., Mavros P., Hansen M., Herrstedt J. Delayed nausea and vomiting continue to reduce patients' quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol. 2006;24(27):4472–4478. doi: 10.1200/JCO.2006.05.6382. [DOI] [PubMed] [Google Scholar]
- 12.Gupta K., Walton R., Kataria S.P. Chemotherapy-induced nausea and vomiting: pathogenesis, recommendations, and new trends. Cancer Treat Res Commun. 2021;26 doi: 10.1016/j.ctarc.2020.100278. [DOI] [PubMed] [Google Scholar]
- 13.Roila F., Hesketh P., Herrstedt J. Prevention of chemotherapy- and radiotherapy-induced emesis: results of the 2004 Perugia International Antiemetic Consensus Conference. Ann Oncol. 2006;17(1):20–28. doi: 10.1093/annonc/mdj078. [DOI] [PubMed] [Google Scholar]
- 14.Navari R.M., Qin R., Ruddy K.J., et al. Olanzapine for the prevention of chemotherapy-induced nausea and vomiting. N Engl J Med. 2016;375(2):134–142. doi: 10.1056/NEJMoa1515725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hashimoto H., Abe M., Tokuyama O., et al. Olanzapine 5 mg plus standard antiemetic therapy for the prevention of chemotherapy-induced nausea and vomiting (J-FORCE): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21(2):242–249. doi: 10.1016/S1470-2045(19)30678-3. [DOI] [PubMed] [Google Scholar]
- 16.Hesketh P.J., Kris M.G., Basch E., et al. Antiemetics: American society of clinical oncology clinical practice guideline update. J Clin Oncol. 2017;35(28):3240–3261. doi: 10.1200/JCO.2017.74.4789. [DOI] [PubMed] [Google Scholar]
- 17.Herrstedt J. The latest consensus on antiemetics. Curr Opin Oncol. 2018;30(4):233–239. doi: 10.1097/CCO.0000000000000450. [DOI] [PubMed] [Google Scholar]
- 18.NCCN . National Comprehensive Cancer Network; 2022. NCCN clinical practice guidelines in oncology version 1.Antiemesis. [Google Scholar]
- 19.Albany C., Brames M.J., Fausel C., Johnson C.S., Picus J., Einhorn L.H. Randomized, double-blind, placebo-controlled, phase III cross-over study evaluating the oral neurokinin-1 antagonist aprepitant in combination with a 5HT3 receptor antagonist and dexamethasone in patients with germ cell tumors receiving 5-day cisplatin combination chemotherapy regimens: a hoosier oncology group study. J Clin Oncol. 2012;30(32):3998–4003. doi: 10.1200/JCO.2011.39.5558. [DOI] [PubMed] [Google Scholar]
- 20.Jordan K., Kinitz I., Voigt W., Behlendorf T., Wolf H.H., Schmoll H.J. Safety and efficacy of a triple antiemetic combination with the NK-1 antagonist aprepitant in highly and moderately emetogenic multiple-day chemotherapy. Eur J Cancer. 2009;45(7):1184–1187. doi: 10.1016/j.ejca.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 21.Olver I.N., Grimison P., Chatfield M., et al. Results of a 7-day aprepitant schedule for the prevention of nausea and vomiting in 5-day cisplatin-based germ cell tumor chemotherapy. Support Care Cancer. 2013;21(6):1561–1568. doi: 10.1007/s00520-012-1696-0. [DOI] [PubMed] [Google Scholar]
- 22.Hamada S., Hinotsu S., Kawai K., et al. Antiemetic efficacy and safety of a combination of palonosetron, aprepitant, and dexamethasone in patients with testicular germ cell tumor receiving 5-day cisplatin-based combination chemotherapy. Support Care Cancer. 2014;22(8):2161–2166. doi: 10.1007/s00520-014-2182-7. [DOI] [PubMed] [Google Scholar]
- 23.Jordan K., Warr D.G., Hinke A., Sun L., Hesketh P.J. Defining the efficacy of neurokinin-1 receptor antagonists in controlling chemotherapy-induced nausea and vomiting in different emetogenic settings-a meta-analysis. Support Care Cancer. 2016;24(5):1941–1954. doi: 10.1007/s00520-015-2990-4. [DOI] [PubMed] [Google Scholar]
- 24.Rapoport B.L., Chasen M.R., Gridelli C., et al. Safety and efficacy of rolapitant for prevention of chemotherapy-induced nausea and vomiting after administration of cisplatin-based highly emetogenic chemotherapy in patients with cancer: two randomised, active-controlled, double-blind, phase 3 trials. Lancet Oncol. 2015;16(9):1079–1089. doi: 10.1016/S1470-2045(15)00035-2. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L., Lu S., Feng J., et al. A randomized phase III study evaluating the efficacy of single-dose NEPA, a fixed antiemetic combination of netupitant and palonosetron, versus an aprepitant regimen for prevention of chemotherapy-induced nausea and vomiting (CINV) in patients receiving highly emetogenic chemotherapy (HEC) Ann Oncol. 2018;29(2):452–458. doi: 10.1093/annonc/mdx698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ioroi T., Furukawa J., Kume M., et al. Phase II study of palonosetron, aprepitant and dexamethasone to prevent nausea and vomiting induced by multiple-day emetogenic chemotherapy. Support Care Cancer. 2018;26(5):1419–1423. doi: 10.1007/s00520-017-3967-2. [DOI] [PubMed] [Google Scholar]
- 27.Clemmons A.B., Orr J., Andrick B., Gandhi A., Sportes C., DeRemer D. Randomized, placebo-controlled, phase III trial of fosaprepitant, ondansetron, dexamethasone (FOND) versus FOND plus olanzapine (FOND-O) for the prevention of chemotherapy-induced nausea and vomiting in patients with hematologic malignancies receiving highly emetogenic chemotherapy and hematopoietic cell transplantation regimens: the FOND-O trial. Biol Blood Marrow Transplant. 2018;24(10):2065–2071. doi: 10.1016/j.bbmt.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Einhorn L.H., Grunberg S.M., Rapoport B., Rittenberg C., Feyer P. Antiemetic therapy for multiple-day chemotherapy and additional topics consisting of rescue antiemetics and high-dose chemotherapy with stem cell transplant: review and consensus statement. Support Care Cancer. 2011;19(Suppl 1):S1–S4. doi: 10.1007/s00520-010-0920-z. [DOI] [PubMed] [Google Scholar]
- 29.Yoodee J., Permsuwan U., Nimworapan M. Efficacy and safety of olanzapine for the prevention of chemotherapy-induced nausea and vomiting: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2017;112:113–125. doi: 10.1016/j.critrevonc.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 30.Gao J., Zhao J., Jiang C., et al. Olanzapine (5 mg) plus standard triple antiemetic therapy for the prevention of multiple-day cisplatin hemotherapy-induced nausea and vomiting: a prospective randomized controlled study. Support Care Cancer. 2022;30(7):6225–6232. doi: 10.1007/s00520-022-07067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu G., Jin Y., Jiang Y., et al. A comparison of the efficacy of 5 mg olanzapine and aprepitant in the prevention of multiple-day cisplatin chemotherapy-induced nausea and vomiting. Int J Clin Pract. 2022;2022 doi: 10.1155/2022/5954379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dranitsaris G., Molassiotis A., Clemons M., et al. The development of a prediction tool to identify cancer patients at high risk for chemotherapy-induced nausea and vomiting. Ann Oncol. 2017;28(6):1260–1267. doi: 10.1093/annonc/mdx100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu Z., Liang W., Yang Y., et al. Personalized estimate of chemotherapy-induced nausea and vomiting: development and external validation of a nomogram in cancer patients receiving highly/moderately emetogenic chemotherapy. Medicine. 2016;95(2) doi: 10.1097/MD.0000000000002476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park H.S., Won H.S., An H.J., et al. Elevated serum substance P level as a predictive marker for moderately emetogenic chemotherapy-induced nausea and vomiting: a prospective cohort study. Cancer Med. 2021;10(3):1057–1065. doi: 10.1002/cam4.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yanai T., Iwasa S., Hashimoto H., et al. A double-blind randomized phase II dose-finding study of olanzapine 10 mg or 5 mg for the prophylaxis of emesis induced by highly emetogenic cisplatin-based chemotherapy. Int J Clin Oncol. 2018;23(2):382–388. doi: 10.1007/s10147-017-1200-4. [DOI] [PubMed] [Google Scholar]
- 36.Chang J., Chen G., Wang D., et al. Efficacy of NEPA, a fixed antiemetic combination of netupitant and palonosetron, vs a 3-day aprepitant regimen for prevention of chemotherapy-induced nausea and vomiting (CINV) in Chinese patients receiving highly emetogenic chemotherapy (HEC) in a randomized phase 3 study. Cancer Med. 2020;9(14):5134–5142. doi: 10.1002/cam4.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.