Abstract
The SARS‐CoV‐2 pandemic challenges healthcare systems worldwide. Within inherited metabolic disorders (IMDs) the vulnerable subgroup of intoxication‐type IMDs such as organic acidurias (OA) and urea cycle disorders (UCD) show risk for infection‐induced morbidity and mortality. This study (observation period February 2020 to December 2021) evaluates impact on medical health care as well as disease course and outcome of SARS‐CoV‐2 infections in patients with intoxication‐type IMDs managed by participants of the European Registry and Network for intoxication type metabolic diseases Consortium (E‐IMD). Survey's respondents managing 792 patients (n = 479 pediatric; n = 313 adult) with intoxication‐type IMDs (n = 454 OA; n = 338 UCD) in 14 countries reported on 59 (OA: n = 36; UCD: n = 23), SARS‐CoV‐2 infections (7.4%). Medical services were increasingly requested (95%), mostly alleviated by remote technologies (86%). Problems with medical supply were scarce (5%). Regular follow‐up visits were reduced in 41% (range 10%–50%). Most infected individuals (49/59; 83%) showed mild clinical symptoms, while 10 patients (17%; n = 6 OA including four transplanted MMA patients; n = 4 UCD) were hospitalized (metabolic decompensation in 30%). ICU treatment was not reported. Hospitalization rate did not differ for diagnosis or age group (p = 0.778). Survival rate was 100%. Full recovery was reported for 100% in outpatient care and 90% of hospitalized individuals. SARS‐CoV‐2 impacts health care of individuals with intoxication‐type IMDs worldwide. Most infected individuals, however, showed mild symptoms and did not require hospitalization. SARS‐CoV‐2‐induced metabolic decompensations were usually mild without increased risk for ICU treatment. Overall prognosis of infected individuals is very promising and IMD‐specific or COVID‐19‐related complications have not been observed.
Keywords: coronavirus, COVID‐19, E‐IMD, IMD, intoxication‐type inherited metabolic diseases, pandemic, SARS‐CoV‐2, survey
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus type 2 (SARS‐CoV‐2) was isolated first in January 2020 and declared as pandemic by the WHO in March 2020. 1 More than 565 million patients were infected worldwide up to July 2022, (https://covid19.who.int; accessed on July 25, 2022).
SARS‐CoV‐2 is a single‐stranded RNA virus. 2 The clinical spectrum of SARS‐CoV‐2 infections varies from asymptomatic to severe respiratory infections (“COVID‐19”) and lethal courses. Frequent symptoms include fever, coughing, and respiratory distress, but also fatigue, anosmia, muscle pain, headache, weight loss, vomiting, or diarrhea. 3 Compared to adults, symptoms in children are less severe. 4 General hospitalization rate for all patients are 7.3%, but differs between adults >60 years (21.9%), newborns and infants (8.8%), and children and adolescents (0.7%–1.3%). Intensive care unit (ICU) treatment was required in 1%–5% of children and 7%–10% of adults. 5 , 6 However, children and adolescents with chronic medical conditions more frequently require inpatient and ICU treatment. 7 , 8 COVID‐19‐associated complications in this age group comprise myocarditis 9 and pediatric inflammatory multisystem syndrome (PIMS). 10 Postacute entities comprise “subacute” (4–12 weeks) and “chronic” COVID‐19 (>3 months) with variable multisystem involvement primarily described in adults, while prevalence in children is thought to be low. 11 , 12
For individuals with inherited metabolic diseases (IMDs), previous studies reported on significant impact of the COVID‐19 pandemic on medical health care in Europe 13 and other continents. 14 Disease courses of SARS‐CoV‐2 infections in patients with IMDs in general, however, were mostly mild. 15 , 16
Within IMDs, the subgroup of intoxication‐type IMDs, such as organic acidurias (OA) and urea cycle disorders (UCD), are characterized by accumulation of small metabolites, such as ammonium, organic acids and corresponding CoA esters, causing endogenous intoxication through impairment of energy metabolism and ureagenesis. 17 Acute metabolic decompensations, occurring in all age groups, are life‐threatening and precipitated by catabolic episodes such as infections. 18 , 19 International patient registries, such as European Registry and Network for intoxication type metabolic diseases (E‐IMD), have been established to investigate long‐term disease course and impact of treatment. 18 , 19 , 20
Systematic analyses of SARS‐CoV‐2 infections in individuals with intoxication‐type IMDs do not exist and it is unclear, whether SARS‐CoV‐2 infections in these patients are associated with increased risk for metabolic decompensation or long‐term complications. This study aims at evaluating the impact of the COVID‐19 pandemic on medical health care in pediatric and adult patients with intoxication‐type IMD and to characterize the disease course and outcome of SARS‐CoV‐2 infections using an international survey.
2. POPULATION AND METHODS
2.1. The European Registry and Network for intoxication type metabolic diseases
E‐IMD, initiated in 2011 as an EU‐funded activity (CHAFEA agreement no. December 1, 2010), manages a web‐based patient registry 20 (https://www.eimd-registry.org/; German Clinical Trials register: DRKS00013085) gathering comprehensive clinical data on individuals with several OA, that is, methylmalonic aciduria (MMA; OMIN #251000), propionic aciduria (OMIN #606054), isovaleric aciduria (OMIN #243500), and glutaric aciduria type 1 (OMIN #231670), as well as UCDs, that is, N‐acetylglutamate synthase (OMIN #237310), carbamylphosphate synthetase 1 (OMIN #237300), ornithine transcarbamylase (OTC; OMIN #311250), argininosuccinate synthetase (OMIN #215700), argininosuccinate lyase (OMIN #207900), arginase 1 (OMIN #207800) deficiency and hyperornithinemia–hyperammonemia–homocitrullinuria syndrome (OMIN #238970). E‐IMD is implemented as an observational, noninterventional, multicenter registry study with participation of 44 international healthcare providers in 20 countries (Figure 1; June 2022).
FIGURE 1.
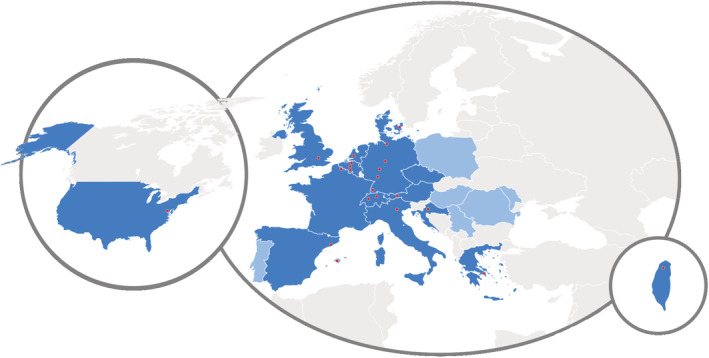
E‐IMD study centers participating the SARS‐CoV‐2 survey. Twenty‐two E‐IMD study centers (red dots in 14 countries on two continents [dark blue]) of all current E‐IMD centers (n = 44 in 20 countries [dark and light blue]) participated in the survey.
2.2. Survey organization
The online questionnaire (Table S1) was developed using LimeSurvey (https://www.limesurvey.org/de). It consisted of 17 main questions on (1) general informations on the participating metabolic center (e.g., number and diagnoses of IMD patients, adaptions in medical care during the pandemic), (2) management and care of patients with IMDs and SARS‐CoV‐2 infection (e.g., out‐ or inpatient care, requirement of ICU treatment), and (3) outcome and mortality. The survey covered a 22 months observation period (February 1, 2020–December 1, 2021), was distributed to all 44 E‐IMD study centers, followed by two reminders, and was closed for data entryon December 31, 2021. The principle investigator at the coordinating study center at the University Children's Hospital Heidelberg, Germany, was responsible for administrative management and communication with the local investigators, providing assistance to participating clinical centers in study management and record keeping.
2.3. Ethical and legal aspects
E‐IMD was first approved by the local ethics committee of Heidelberg Medical Faculty (application no. S‐525/2010), followed by approvals of the respective ethics committees of further associated and collaborating partners contributing to the registry.
2.4. Statistical analysis
All data were extracted from the questionnaires and analyzed using Microsoft Excel and R language 21 for statistical computing. For comparative analyses of OA and UCD pediatric and adult groups, we used χ 2 test and Pearson residuals to interpret differences in observed and expected frequencies.
3. RESULTS
Fifty percent (n = 22/44) of E‐IMD study centers responded (Supporting Information Material S1). The participating E‐IMD centers in 14 countries from three continents (Figure 1) manage a total of 792 patients (n = 479 pediatric; n = 313 adult) with intoxication‐type IMDs (n = 454 OA; n = 338 UCD; Figure 2). All participating centers managed both pediatric as well as adult patients.
FIGURE 2.
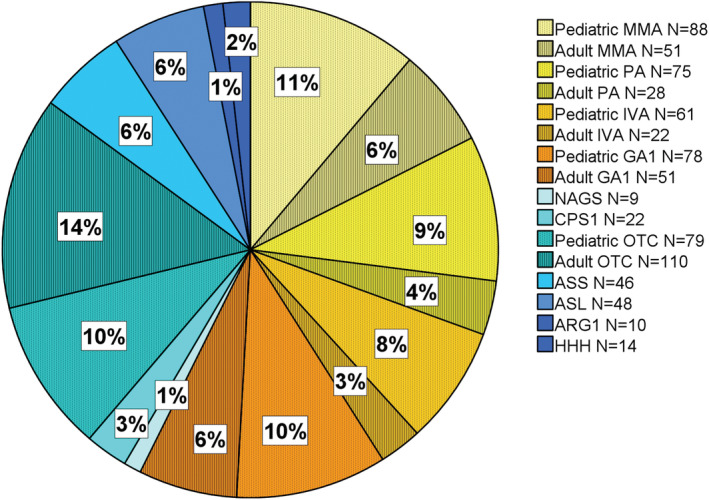
Total patients (adult and pediatric) with intoxication‐type IMDs followed by contributing E‐IMD study centers. Seven hundred ninety‐two patients (N = 478 pediatric; N = 314 adult) with (IMDs) N = 454 organic acidurias (OA; yellow‐orange); N = 338 urea cycle disorders (UCD; green blue) are followed‐up at the participating centers. All centers cared pediatric as well as adult patients.
3.1. Impact of the SARS‐CoV‐2 pandemic on management and medical health care of patients with intoxication‐type IMDs
During the observation period (February 1, 2020–December 1, 2021), 41% (n = 8/22) of the participating E‐IMD study centers provided less regular outpatient visits for patients with intoxication‐type IMDs (mean reduction rate visits of 40%, range 10%–50% [n = 5]). Almost all centers (95%; 21/22) were increasingly contacted throughout the pandemic by the patients and families with intoxication‐type IMD managed at the center asking for COVID‐19‐specific advices, including general information, additional risks, recommendations for prevention, school attendance/home office, or indication for vaccination. The vast majority of participating centers (86%; 19/22) compensated the increased consultation requests by phone/video consultations. COVID‐19‐specific recommendations, that is, in addition to the general recommendations for managing potential metabolic risk situations for intoxication‐type IMDs such as febrile infections (e.g., specific letter, webinars, tele care, information) were provided to the patients by 32% (7/22) of the study centers. Noteworthy, only 33% of centers (7/21) reported reimbursement for their additional COVID‐19‐associated services, while significant problems with medical supply (medication, prescriptions, dietary products) were reported to be rare during the study period (5%; 1/22). This included shortage of hydroxocobalamin in the United States.
3.2. Incidence, disease course, and management of SARS‐CoV‐2 infections in intoxication‐type IMDs
Within the observation period of 22 months 59 of 792 individuals (OA: n = 36 [13 pediatric; 23 adult]; UCD: n = 23 [17 pediatric, 6 adult]), that is, 7.4% of all intoxication‐type IMD patients managed at the study centers, were reported to suffer from a SARS‐CoV‐2 infection. Infection rates with respect to age and diagnosis group were analyzed and revealed significant differences (χ 2 = 36.096, df = 4; p < 0.0001). According to Pearson residuals, noninfected pediatric UCD patients were underrepresented compared to noninfected adult UCD patients and, vice versa, noninfected adult OA patients were underrepresented compared to noninfected pediatric OA patients (Figure S1). Infection rate was higher in adult OA (n = 23) compared to pediatric OA patients (n = 13), but expected frequencies did not differ between pediatric UCD (n = 17) and adult UCD patients (n = 6).
While 49 of 59 (83%) of infected individuals were treated at home showing no or only mild clinical symptoms, 17% of the SARS‐CoV‐2‐positive individuals with intoxication‐type IMD (n = 10 [7 pediatric, 3 adults]; n = 6 OA [4 pediatric; 2 adult]; and n = 4 UCD [3 pediatric, 1 adult]) had to be admitted to a hospital (Figure 3). Hospitalization rate did not differ between age (pediatric/adult) and diagnosis groups (OA/UCD; χ 2 = 0.08, df = 1; p = 0.778). Indications for hospitalizations and clinical characteristics of hospitalized patients are summarized in Table 1. Median (range) age at admission of pediatric patients was 10 (3–17), and 31 (22–32) years for adult patients. Of the 10 patients, 9 were admitted with acute manifestation of SARS‐CoV‐2 infection. Four (two pediatric, two adult) of five admitted MMA patients earlier underwent liver and/or kidney transplantation, of which three of four patients (p3, p8, p9) were admitted due to acute symptoms, while one pediatric MMA patient (p2) due to deteriorated chronic MMA‐complications, possibly triggered by immunosuppression. All 10 admitted patients were treated on normal wards for a median (range) length of seven (1–25) days. ICU treatment, invasive ventilation or extracorporeal membrane oxygenation were not required in any patient. One adult OTC patient (p10) with pneumonia and secondary pulmonary embolism, and one pediatric MMA patient (p1) with obstructive bronchitis received ventilation assistance with oxygen (Table 1). Of the 10 admitted patients, 2 received total parenteral feeding. Thirty percent (p2, p4, p5) of admitted patients showed mild to moderate laboratory signs of metabolic decompensation effectively managed by metabolic emergency treatment which was (preventively) administered to all but two (p8, p9) admitted patients. In contrast, only six (12%) of infected individuals (three pediatric OA, two adult OA, one adult UCD) managed at home received metabolic emergency treatment.
FIGURE 3.
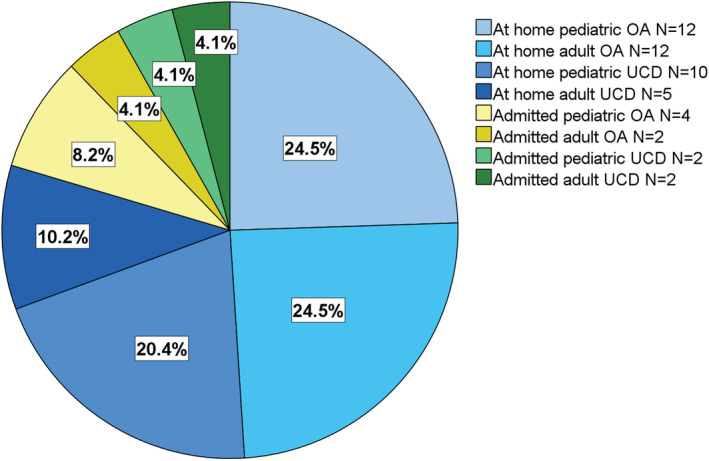
Clinical course and management of intoxication‐type IMD patients with SARS‐CoV‐2‐infection. The vast majority of SARS‐CoV‐2‐positive patients (49/59; 83%) was managed at home showing no or only mild clinical symptoms. Ten patients (17%; seven pediatric, three adults; n = 6 OA [4 pediatric patients; 2 adults] and n = 4 UCD [3 pediatric, 1 adult]) were hospitalized. Clinical characteristics of admitted patients is shown in Table 1.
TABLE 1.
Clinical characteristics of admitted patients with intoxication‐type IMDs and SARS‐CoV‐2 infection
| Pat# | Metabolic diagnosis | Age Age at admission | Length of stay (days) | Reason for admission | Fever | Treatment | Oxygen | Invasive ventilation | Metabolic decompensation | Metabolic emergency treatment | Full recovery |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | MMA | 4 years | 4 | Obstructive bronchitis | No | IV fluid | Yes | No | No | Yes (preventive) | Yes |
| 2 | MMA with liver transplantation at age 7 years (2019) | 9 years | 25 | Weight loss 20%, feeding disorder, seizure (tacrolimus‐induced?) | No | IV fluids, IV scavengers, carglumic acid, total parenteral feeding | No | No | Mild | Yes | No (mild neurologic symptoms) |
| 3 | MMA with kidney‐liver transplantation at age 13 years | 14 years | 13 | Hoarseness, fever | Yes | Observation reduction of immunosuppression | No | No | No | No | Yes |
| 4 | PA | 17 yearrs | 5 | Vomiting, reduced oral intake | Yes | IV fluids, carnitine, metronidazole, caglumic acid | No | No | Yes (NH3 143 μM), elevated lactate 4.1 mmol/L, ketone 4+ | Yes | Yes |
| 5 | OTC def | 3 years | 4 | Fever, vomiting, lethargy | No | IV fluid, IV scavengers + IV scavenger bolus | No | No | Yes (NH3 251 μM) | Yes | Yes |
| 6 | CPS1 def | 10 years | 8 | Feeding problems | No | IV fluid | No | No | No | Yes (preventive) | Yes |
| 7 | Arginase def | 16 years | 1 | Preventively | No | IV fluid, IV scavengers | No | No | No | Yes (preventive) | Yes |
| 8 | MMA with liver transplantation at age 19 years (2018) | 22 years | 6 | Vomiting, diarrhea, reduced oral intake | Yes | IV fluids, total parenteral feeding | No | No | No | No | Yes |
| 9 | MMA with kidney‐liver transplantation at age 28 years (2018) | 31 years | 8 | Mild respirator symptom, reduced oral intake | Yes | IV fluids | No | No | No | No | Yes |
| 10 | OTC def | 32 years | 6 + 4 | Pneumonia, pulmonary embolism | Yes | IV fluid, remdesevir, dexamethasone, amoxiclav, toculizumab, enoxaparin | Yes | No | No | Yes (preventive) | Yes |
| N per item or median (range) | |||||||||||
| All |
N = 6 OA N = 4 UCD |
15 (3–32) | 7 (1–25) | n.a. |
N = 5 yes N = 5 no |
n.a. |
N = 2 yes N = 8 no |
N = 10 no |
N = 3 yes N = 7 no |
N = 7 yes N = 3 no |
N = 9 yes N = 1 no |
Abbreviations: CPS1, carbamoyl‐phosphate synthetase 1; def, deficiency; IV, intravenous; MMA, methylmalonic aciduria; n.a., not applicable; OA, organic acidurias; OTC, ornithine transcarbamylase; PA, propionic aciduria; UCD, urea cycle defects.
3.3. Selected case descriptions of hospitalized patients
P1: A 4‐year‐old girl with late‐onset mut−/mut0 MMA, diagnosed symptomatically at age 11 months after severe metabolic decompensation (pH 6.9; NH3 178 μM). Genetic evaluation revealed an additional heterozygous missense variant in SPINK1, a genetic risk factor for chronic pancreatitis. The patient experienced repetitive episodes of acute pancreatitis and febrile infections frequently resulting in metabolic decompensation requiring hospitalization. She developed chronic kidney disease (CKD) III–IV and is currently under peritoneal dialysis. However, SARS‐CoV‐2‐positive obstructive bronchitis did not result in metabolic decompensation or signs of pancreatitis. After treatment with IV fluids (preventive), metabolic emergency treatment, and noninvasive oxygen administration, she fully recovered and received combined liver/kidney transplantation 3 months later.
P2: A 9‐year old individual with homozygous mut0 MMA, who underwent liver transplantation 1 year before she suffered from CKD. SARS‐CoV‐2 infection started with loss of taste and chronic diarrhea. Subsequently, she lost 10 kg (20%) of her body weight. Admission to hospital followed an epileptic seizure, probably caused by calcineurin induced neurotoxicity with high tacrolimus levels induced by a decline of kidney function due to dehydration. Tacrolimus was discontinued and switched to everolimus. A mild metabolic decompensation (Table 1) was treated with carglumic acid, IV fluids, IV scavengers, and total parenteral feeding. She did not fully recover and still shows mild neurological deficits.
P5: 3.5‐year‐old boy with genetically confirmed early‐onset OTC deficiency who suffered from a severe neonatal hyperammonemic crisis at Day 3 (NH3 5200 μM) followed by repetitive (>15) metabolic decompensations precipitated by febrile infections since then. SARS‐CoV‐2 infection started with mild coughing, repetitive vomiting, lethargy and reduced general state of health. Laboratory work‐up showed moderate hyperammonemia (NH3 251 μM), elevated plasma glutamine concentration (1440 μM), and moderate signs of hepatopathy (GOT 196 U/L [N 43], GPT 415 U/L [N 50]). Metabolic emergency treatment was started as sodium benzoate including additional bolus, followed by IV fluids with glucose, l‐arginine and accompanied by dietary emergency regimen. Glycerol phenylbutyrate was continued via gastrostomy tube. After 3 days, he showed full clinical recovery and normalization of laboratory parameters.
P10: 32‐year‐old female patient with late onset female OTC deficiency. SARS‐CoV‐2 infection started with shortness of breath and mild hypoxia (oxygen saturation 90%) 2 weeks postpartum. Ammonium level was only mildly elevated (67 μM), but she showed neutropenia and lymphopenia. She was treated with oxygen, anti‐COVID‐19 remdesivir, dexamethasone, and antibiotics due to pulmonary bacterial superinfection. Thorax CT scan showed marked multifocal consolidation and ground glass with parenchymal involvement of 30% (predominantly the right lower lobe). Brain CT was normal. She received metabolic emergency treatment and IV medication. She was discharged after 6 days, but re‐admitted 1 day later with fever, secondary deterioration and hypoxia (oxygen saturation 86%). d‐Dimer concentration was raised (11 770 μg/L, N 0‐550) and thorax CT scan confirmed pulmonary embolism with low volume thromboembolic clot in the distal arteries leading to the right lower lobe. The 4C Mortality Score and 4C Deterioration score (ISARIC 4C), used for hospitalized COVID‐19 patients, was assessed as 4–8 (intermediate risk, 9.9% inpatient mortality). Treatment consisted of oxygen, tocilizumab, and therapeutic enoxaparin followed by apixaban for 3 months. She recovered completely. Causal embolic factors (e.g., postpartum period and/or SARS‐CoV‐2 infection) could not be clearly identified.
3.4. Outcome of SARS‐CoV‐2 infections in intoxication‐type IMDs
Survival rate during the observation period for the reported SARS‐CoV‐2 infections in patients with intoxication type IMDs was 100%. All patients (100%) managed at home or the outpatient department and the vast majority of hospitalized individuals (90%) with intoxication‐type IMD and SARS‐CoV‐2 infection showed full recovery. One hospitalized pediatric MMA patient (p2) developed persistent mild neurological deficits following the acute treatment phase. Of note, this patient was admitted due to chronic MMA complications and earlier underwent liver transplantation. Outcome in the other three admitted MMA patients with immunosuppression due to transplantations was favorable all showing full recovery. No deterioration or new onset of intoxication‐type IMD‐specific disease manifestations was reported. No case of COVID‐19‐related complications such as subacute (4–12 weeks after infection) or postacute (beyond 12 weeks after the infection) manifestations (PIMS, chronic pulmonary symptoms, subacute or chronic long COVID syndrome) were reported for the observed period.
4. DISCUSSION
The main findings of this study on SARS‐CoV‐2 infections in individuals with intoxication‐type IMD in a 22 months period from 2020 to 2021 are that (1) the pandemic significantly affects but not endangers medical care of patients, (2) increased request of medical services is effectively alleviated by remote techniques, (3) disease course is mostly mild and managed without hospitalization, (4) metabolic decompensations in hospitalized patients are rare and, if present, mild to moderate (5) hospitalization rate is higher than in the general population but comparable to other reported chronic medical conditions, (6) mortality rate is not increased, and (7) overall prognosis of patients is promising without increased incidence of IMD‐specific or COVID‐19‐related complications.
4.1. Challenge of medical health care for individuals with intoxication‐type IMDs during the pandemic
Studies on the impact of COVID‐19 on medical health care of patients suffering from IMD are scarce and especially systematic analyses for individuals with intoxication‐type IMDs do not exist. Previously published data for the beginning of the pandemic analyzed patient satisfaction and management in IMDs. In the beginning of the pandemic (March–May 2020), a significant disruption of care of 50–100% was reported for Europe 13 and of 60%–80% in 16 centers in Asia, Africa, and Europe. 14 Regular follow‐up appointments in these studies were canceled (55%) or missed frequently (75%–100%) during this period 13 and the median worldwide reduction rate of medical services was reported to be 60%–80%. 14 In comparison, the lower rate of 41% of centers reducing medical services for intoxication type IMDs in our study may highlight improved compensating strategies of metabolic healthcare providers to reduce the rate of missed follow‐up investigations for this vulnerable patient group.
In contrast to other studies reporting on problems with treatment administration in IMD patients 22 or treatment discontinuation in up to 65%, 13 however, the rate of relevant problems with medical supply for intoxication‐type IMDs was comparably low in this study, demonstrating effective compensation mechanisms developed by healthcare providers and pharmacological companies enabling continuous treatment for patients with intoxication‐type IMD. Of note, most patients themselves (n = 175) stated in an online survey not to have faced significant problems in receiving special therapies (91%) but suffered from the consequences of quarantine (e.g., crowded apartments, isolation 23 ).
Telemedicine has experienced a strong upward trend since the beginning of the pandemic and people have become familiar with the technology. 24 Many centers started telemedicine appointments and used videoconferences as a replacement for face‐to‐face meetings. 13 , 25 Rates of satisfaction among patients proved to be high, with lack of laboratory tests 26 and medication being reported by patient organizations 13 as the main disadvantages. Rate of telemedicine used for IMD patients (90%) was reported to be higher compared to the general rare disease community in Europe and United States. 13 Our study confirms the high compensation rate of increasingly requested medical services using remote techniques (83% of centers) and highlights that these relevant and new digital tools for reducing physical and geographical barriers have been successfully established in health care for IMD patients by metabolic centers worldwide.
The low overall incidence of 7.4% positive SARS‐CoV‐2 infections in our study cohort may be hampered by the fact that the observation period did not cover very recent viral variants such as Omicron showing a much higher infection rate. 27 Of note, another study 16 also found an infection rate of 7% in 272 patients covering an observation period of 12 months (March 2020–2021). Furthermore, as SARS‐CoV‐2 infections showed asymptomatic to mild diseases courses in children, also underreporting has to be considered.
4.2. The disease course of SARS‐CoV‐2 infections is mostly mild in intoxication‐type IMDs
In general, pediatric patients with SARS‐CoV‐2 infections are more frequently asymptomatic compared to adults and show milder symptoms. 4 One single center study reported that disease severity of SARS‐CoV‐2 infections in 272 patients with different IMDs was comparable to the reference population in pediatric and adult patients. 16 Another study reported on 44 IMD patients with mostly mild symptoms. 22 A pan‐European survey by the European Reference Network for hereditary metabolic diseases (MetabERN; https://metab.ern-net.eu) among healthcare providers following about 26 000 metabolic patients 15 reported 452 (213 pediatric) cases of COVID‐19 in the first year of the pandemic with the majority of adult and pediatric patients being asymptomatic and 37.5% of individuals showing mild symptoms. Complementary, our study confirms that, similarly to the general population, the clinical course of SARS‐CoV‐2‐infections is mostly asymptomatic or mild also for individuals with intoxication‐type IMDs who, in more than 80% of individuals, could be managed at home and did not require hospitalization.
Overall hospitalization rate for patients with SARS‐CoV‐2‐infections in Germany was reported to be 7.3%, but differ between adults >60 years (21.9%), newborns and infants (8.8%), and children and adolescents (0.7%–1.3%). 28 The hospitalization rate of 17% found in our study are therefore higher than in the general population, and is comparable to another report on SARS‐CoV‐2‐positive patients with diabetes. 15 For IMDs, one survey‐based multicenter study found severe symptoms and hospitalization rates in 1%–25% of IMD patients reported by less than 10% of survey respondents, 15 while one single center study reported on 1 of 19 hospitalized patient. 16 Further data for hospitalization rates for patients with IMDs or intoxication‐type IMDs are not available.
ICU treatment of SARS‐CoV‐2 infections are required in 1%–5% of pediatric and 7%–10% of adults. 5 , 6 , 29 However, children and adolescents with chronic diseases are admitted to hospital and treated on ICU more frequently. Risk factors for severe disease course in children comprise age <1 month, male sex, asthma, obesity, diabetes mellitus, immunosuppression or trisomy 21. 7 , 8 Of note, our study demonstrates that intoxication‐type IMDs are not a risk factor for ICU treatment in adult or pediatric patients.
4.3. Inpatient management and risk for metabolic decompensations
Compared to patients with other IMDs, patients with intoxication‐type IMDs have an additional risk for life‐threatening acute metabolic decompensations during catabolic episodes such as intercurrent infections, that may require metabolic emergency treatment, hospitalization, or even ICU admission with potentially hemofiltration. These episodes are associated with increased morbidity and mortality. 30 , 31 , 32 , 33 For UCD patients for instance, a recent study found a mean frequency of 0.6–1.7 hyperammonemic events per years requiring hospitalization, depending on disease severity. 34 It was hypothesized that in case of SARS‐CoV‐2 infection the risk for metabolic decompensation may even be higher and the disease course may show gradual worsening, or even progressive neurological deterioration. 13 , 25 However, a systematic evaluation whether intoxication type IMDs are at increased risk for life threatening metabolic decompensation triggered by SARS‐CoV‐2 was not performed so far. Although our study cohort is too small to assess the general risk for metabolic decompensation and further data for comparison rates of emergency visits do not exist, our findings of only 30% mild to moderate metabolic decompensations in 10 admitted patients without any further complications, ICU treatment, invasive ventilation, or extracorporeal membrane oxygenation demonstrates that SARS‐CoV‐2‐related decompensations in intoxication‐type IMDs do not seem to be aggravated compared to other intercurrent infections or conditions likely to induce catabolism (such as febrile reactions to vaccinations) and are generally well manageable. In line with this, one recent review found no increased risk for metabolic decompensation in OA or UCD following vaccinations. 35 Of note, several patients in our study admitted to hospital without metabolic decompensation received preventive metabolic emergency treatment. However, more studies are necessary to evaluate the risk for metabolic decompensation in patients with intoxication‐type IMDs in case of SARS‐CoV‐2 infections.
Anosmia and gastrointestinal dysfunction like nausea, vomiting, and diarrhea that affect both, food intake and absorption frequently accompanied COVID‐19. About 15% of hospitalized patients with COVID‐19 are reported to need parenteral nutritional support, in particular those with the severe conditions, high inflammasome profile, or ICU treatment. 36 but also patients with intoxication‐type IMDs treated on normal wards as our data show (2 of 10 admitted patients). To recover, high protein content diet for about 8 weeks following discharge from hospital, is recommended for SARS‐CoV‐2‐infected patients with chronic endocrinological disorders. 37 Certainly, this is not practicable in patients with intoxication‐type IMDs treated since high protein intake in this disease group can precipitate life‐threatening metabolic decompensations.
4.4. Outcome and mortality
Mortality in SARS‐CoV‐2 has been reported to be 0.08% in children. 5 Most centers in one survey‐based study did not report on death among their patients (85% adults to 97% pediatric), 15 but in contrast, several lethal courses in pediatric and adult patients with IMDs have been published. 15 , 22 In our study, survival rate was 100%, showing that SARS‐CoV‐2‐infection does not seem to be associated with increased mortality in intoxication‐type IMDs. Although the vaccination status was not covered in our questionnaire, these data are insufficient to decide whether or not individuals with intoxication‐type IMD should be classified as a high priority group for receiving the COVID‐19 vaccination.
Furthermore, we observed full recovery in 100% of outpatients and 9 out of 10 admitted infected individuals including four admitted MMA patients treated with immunosuppression following transplantation, while only one female MMA patient with immunosuppression suffered from chronic mild neurological deficits after discharge. No cases of acute COVID‐19‐related complications like myocarditis or PIMS have been detected. This observation also confirms that chronic COVID‐19‐related complications comprising “subacute” (4–12 weeks) and “postacute” COVID‐19 (>3 months) are not increased in our cohort of pediatric or adult patients with intoxication‐type IMD.
4.5. Limitations
First, the amount of collected data is relatively small and only involves half of the E‐IMD study centers. Second, the questionnaire was only directed at healthcare professionals and not to patients, since the main study focus was to evaluate the impact on disease course and treatment decisions and the resulting effect on health outcome. As a consequence, data do not reflect the patient perspective. Third, assessment of hospitalization and severe cases may be hampered by the fact that the age group with the most severe courses in the general population, i.e. individuals aged 60 years and older and suffering from various comorbidities, is underrepresented in our cohort of individuals with intoxication‐type IMDs. Fourth, impact of vaccinations, becoming broadly accessible at the end of the observation period and to a variable degree in each country, was beyond the scope of this study, and, therfore, could not be evaluated. Fifth, SARS‐CoV‐2‐associated long‐term complications might be underreported since data entry was closed shortly after the end of the observation period.
5. CONCLUSION
In conclusion, this study demonstrates a significant impact of the COVID‐19 pandemic on medical health care of individuals with intoxication‐type IMDs worldwide with different medical services being increasingly requested by patients and families, while medical supply and management was not endangered. Notably, the clinical course of SARS‐CoV‐2‐infections in this subgroup is predominantly mild and mostly managed in the outpatient clinic. Rates of metabolic decompensations for admitted patients, mostly receiving preventively metabolic emergency treatment, was not increased and the overall prognosis for the vast majority of affected individuals is promising.
CONFLICT OF INTEREST
Rene Santer, Chris Mühlhausen, Elisenda Cortès‐Saladelafont, and Sarah C. Grünert, gave presentations during meetings organized by a pharmaceutical company. Mathias Baumgartner received research grants by a pharmaceutical company. Kimberly A. Chapman reports unpaid advocacy for two metabolic associations. Matthias Gautschi was consultant for a pharmaceutical company. Ivo Barić received support for attended meetings. Other authors declare no conflict of interest.
ETHICS STATEMENT
The Institutional Ethics Committee of the coordinating center and all contributing study sites approved the study (University Hospital Heidelberg, application no. S‐525/2010).
Supporting information
FIGURE S1. Mosaic plot of cross‐tabulation disease (UCD/OA), group (pediatric/adult) and Infection (not infected/infected). Width and height of mosaics are proportional to their cell counts with respect to grouping variable, color indicates Pearson residuals visualizing differences between observed and expected frequency: red indicates less observed cases than expected from marginal frequency, blue more cases. OA, organic acidurias; UCD, urea cycle defects
FILE S1. Supporting Information
ACKNOWLEDGMENTS
The authors thank all E‐IMD (European Registry and Network for intoxication type metabolic diseases) sites that contributed to the data used in this publication, as well as the E‐IMD registry for the support during the study period. Furthermore, we are indebted to all E‐IMD individuals and their families for their trust, patience and participation in longitudinal registry studies for many years. The E‐IMD patient registry has received funding by the European Union (E‐IMD; EAHC no 2010 1201; coordinator: Stefan Kölker), in the framework of the Health Program. After the end of the EU funding period the E‐IMD patient registry has been sustained by funding from the Kindness‐for‐Kids Foundation (Munich, Germany), the Kettering Fund, and Dietmar Hopp Foundation. Open Access funding enabled and organized by Projekt DEAL.
Mütze U, Gleich F, Barić I, et al. Impact of the SARS‐CoV‐2 pandemic on the health of individuals with intoxication‐type metabolic diseases—Data from the E‐IMD consortium. J Inherit Metab Dis. 2022;1‐12. doi: 10.1002/jimd.12572
Communicating Editor: Olaf Bodamer
Funding information European Commission
DATA AVAILABILITY STATEMENT
The datasets of this study are not publicly available due to existing data protection laws. Data ownership is incumbent upon the members of the E‐IMD consortium making data available for specific research purposes upon request.
REFERENCES
- 1. Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J Med Virol. 2020;92:401‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Viner RM, Mytton OT, Bonell C, et al. Susceptibility to SARS‐CoV‐2 infection among children and adolescents compared with adults: a systematic review and meta‐analysis. JAMA Pediatr. 2021;175:143‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liguoro I, Pilotto C, Bonanni M, et al. SARS‐COV‐2 infection in children and newborns: a systematic review. Eur J Pediatr. 2020;179:1029‐1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Viner RM, Ward JL, Hudson LD, et al. Systematic review of reviews of symptoms and signs of COVID‐19 in children and adolescents. Arch Dis Child. 2021;106:802‐807. [DOI] [PubMed] [Google Scholar]
- 7. Götzinger F, Santiago‐García B, Noguera‐Julián A, et al. COVID‐19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;4:653‐661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Graff K, Smith C, Silveira L, et al. Risk factors for severe COVID‐19 in children. Pediatr Infect Dis J. 2021;40:e137‐e145. [DOI] [PubMed] [Google Scholar]
- 9. Singer ME, Taub IB, Kaelber DC. Risk of myocarditis from COVID‐19 infection in people under age 20: a population‐based analysis. medRxiv. 2022. doi: 10.1101/2021.07.23.21260998 [DOI] [Google Scholar]
- 10. Radia T, Williams N, Agrawal P, et al. Multi‐system inflammatory syndrome in children & adolescents (MIS‐C): a systematic review of clinical features and presentation. Paediatr Respir Rev. 2021;38:51‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Molteni E, Sudre CH, Canas LS, et al. Illness duration and symptom profile in symptomatic UK school‐aged children tested for SARS‐CoV‐2. Lancet Child Adolesc Health. 2021;5:708‐718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nalbandian A, Sehgal K, Gupta A, et al. Post‐acute COVID‐19 syndrome. Nat Med. 2021;27:601‐615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lampe C, Dionisi‐Vici C, Bellettato CM, et al. The impact of COVID‐19 on rare metabolic patients and healthcare providers: results from two MetabERN surveys. Orphanet J Rare Dis. 2020;15:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Elmonem MA, Belanger‐Quintana A, Bordugo A, et al. The impact of COVID‐19 pandemic on the diagnosis and management of inborn errors of metabolism: a global perspective. Mol Genet Metab. 2020;131:285‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Paneghetti L, Bellettato CM, Sechi A, Stepien KM, Scarpa M. One year of COVID‐19: infection rates and symptoms in patients with inherited metabolic diseases followed by MetabERN. Orphanet J Rare Dis. 2022;17:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tummolo A, Paterno G, Dicintio A, Stefanizzi P, Melpignano L, Aricò M. COVID‐19 and inherited metabolic disorders: one‐year experience of a referral center. Children. 2021;8:781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tuncel AT, Boy N, Morath MA, Horster F, Mutze U, Kolker S. Organic acidurias in adults: late complications and management. J Inherit Metab Dis. 2018;41:765‐776. [DOI] [PubMed] [Google Scholar]
- 18. Kölker S, Garcia‐Cazorla A, Valayannopoulos V, et al. The phenotypic spectrum of organic acidurias and urea cycle disorders. Part 1: the initial presentation. J Inherit Metab Dis. 2015;38:1041‐1057. [DOI] [PubMed] [Google Scholar]
- 19. Kölker S, Valayannopoulos V, Burlina AB, et al. The phenotypic spectrum of organic acidurias and urea cycle disorders. Part 2: the evolving clinical phenotype. J Inherit Metab Dis. 2015;38:1059‐1074. [DOI] [PubMed] [Google Scholar]
- 20. Kölker S, Dobbelaere D, Häberle J, et al. Networking across Borders for individuals with organic acidurias and urea cycle disorders: the E‐IMD consortium. JIMD Rep. 2015;22:29‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. R_CoreTeam JhwR‐po . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2013. [Google Scholar]
- 22. Tobór‐Świętek E, Sykut‐Cegielska J, Bik‐Multanowski M, et al. COVID‐19 pandemic and patients with rare inherited metabolic disorders and rare autoinflammatory diseases‐organizational challenges from the point of view of healthcare providers. J Clin Med. 2021;10:4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oge Enver E, Hopurcuoglu D, Ahmadzada S, Zubarioglu T, Aktuglu Zeybek AC, Kiykim E. Challenges of following patients with inherited metabolic diseases during the COVID‐19 outbreak. A cross‐sectional online survey study. J Pediatr Endocrinol Metab. 2021;34:103‐107. [DOI] [PubMed] [Google Scholar]
- 24. Colbert GB, Venegas‐Vera AV, Lerma EV. Utility of telemedicine in the COVID‐19 era. Rev Cardiovasc Med. 2020;21:583‐587. [DOI] [PubMed] [Google Scholar]
- 25. Brunetti‐Pierri N, Fecarotta S, Staiano A, Strisciuglio P, Parenti G. Ensuring continuity of care for children with inherited metabolic diseases at the time of COVID‐19: the experience of a metabolic unit in Italy. Genet Med. 2020;22:1178‐1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koç Yekedüz M, Doğulu N, Sürücü Kara İ, et al. Pros and cons of telemedicine for inherited metabolic disorders in a developing country during the COVID‐19 pandemic. Telemed J E Health. 2022. doi: 10.1089/tmj.2021.0610. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 27. Ren SY, Wang WB, Gao RD, Zhou AM. Omicron variant (B.1.1.529) of SARS‐CoV‐2: mutation, infectivity, transmission, and vaccine resistance. World J Clin Cases. 2022;10:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vygen‐Bonnet S, Koch J, Bogdan C, Harder T, Heininger U, Kling K, et al. Beschlussentwurf der STIKO zur 4. Aktualisierung der COVID‐19‐Impfempfehlung und die dazugehörige wissenschaftliche Begründung. 2021.
- 29. Zepp F, Knuf MJMK. “Coronavirus disease 2019 (COVID‑19)” im Kindes‐ und Jugendalter. Impfempfehlungen in der Pädiatrie. 2021;169:1010‐1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Forny P, Hörster F, Ballhausen D, et al. Guidelines for the diagnosis and management of methylmalonic acidaemia and propionic acidaemia: first revision. J Inherit Metab Dis. 2021;44:566‐592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grünert SC, Müllerleile S, De Silva L, et al. Propionic acidemia: clinical course and outcome in 55 pediatric and adolescent patients. Orphanet J Rare Dis. 2013;8:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Posset R, Garcia‐Cazorla A, Valayannopoulos V, et al. Age at disease onset and peak ammonium level rather than interventional variables predict the neurological outcome in urea cycle disorders. J Inherit Metab Dis. 2016;39:661‐672. [DOI] [PubMed] [Google Scholar]
- 33. Summar ML, Dobbelaere D, Brusilow S, Lee B. Diagnosis, symptoms, frequency and mortality of 260 patients with urea cycle disorders from a 21‐year, multicentre study of acute hyperammonaemic episodes. Acta Paediatr. 2008;97:1420‐1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Posset R, Kölker S, Gleich F, et al. Severity‐adjusted evaluation of newborn screening on the metabolic disease course in individuals with cytosolic urea cycle disorders. Mol Genet Metab. 2020;131:390‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hady‐Cohen R, Dragoumi P, Barca D, Plecko B, Lerman‐Sagie T, Zafeiriou D. Safety and recommendations for vaccinations of children with inborn errors of metabolism. Eur J Paediatr Neurol. 2021;35:93‐99. [DOI] [PubMed] [Google Scholar]
- 36. Ramos A, Joaquin C, Ros M, et al. Impact of COVID‐19 on nutritional status during the first wave of the pandemic. Clin Nutr. 2021. doi: 10.1016/j.clnu.2021.05.001. Online ahaed of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Puig‐Domingo M, Marazuela M, Yildiz BO, Giustina A. COVID‐19 and endocrine and metabolic diseases. An updated statement from the European society of endocrinology. Endocrine. 2021;72:301‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1. Mosaic plot of cross‐tabulation disease (UCD/OA), group (pediatric/adult) and Infection (not infected/infected). Width and height of mosaics are proportional to their cell counts with respect to grouping variable, color indicates Pearson residuals visualizing differences between observed and expected frequency: red indicates less observed cases than expected from marginal frequency, blue more cases. OA, organic acidurias; UCD, urea cycle defects
FILE S1. Supporting Information
Data Availability Statement
The datasets of this study are not publicly available due to existing data protection laws. Data ownership is incumbent upon the members of the E‐IMD consortium making data available for specific research purposes upon request.


