Summary
Background
Obesity increases the severity of coronavirus disease 2019 illness in adults. The role of obesity in short‐term complications and post‐acute sequelae in children is not well defined.
Objective
To evaluate the relationship between obesity and short‐term complications and post‐acute sequelae of SARS‐CoV‐2 infection in hospitalized paediatric patients.
Methods
An observational study was conducted in three tertiary hospitals, including paediatric hospitalized patients with a confirmatory SARS‐CoV‐2 RT‐PCR from March 2020 to December 2021. Obesity was defined according to WHO 2006 (0–2 years) and CDC 2000 (2–20 years) growth references. Short‐term outcomes were intensive care unit admission, ventilatory support, superinfections, acute kidney injury, and mortality. Neurological, respiratory, and cardiological symptoms and/or delayed or long‐term complications beyond 4 weeks from the onset of symptoms were considered as post‐acute sequalae. Adjusted linear, logistic regression and generalized estimating equations models were performed.
Results
A total of 216 individuals were included, and 67 (31.02%) of them had obesity. Obesity was associated with intensive care unit admission (aOR = 5.63, CI95% 2.90–10.94), oxygen requirement (aOR = 2.77, CI95% 1.36–5.63), non‐invasive ventilatory support (aOR = 6.81, CI95% 2.11–22.04), overall superinfections (aOR = 3.02 CI95% 1.45–6.31), and suspected bacterial pneumonia (aOR = 3.00 CI95% 1.44–6.23). For post‐acute sequalae, obesity was associated with dyspnea (aOR = 9.91 CI95% 1.92–51.10) and muscle weakness (aOR = 20.04 CI95% 2.50–160.65).
Conclusions
In paediatric hospitalized patients with COVID‐19, severe short‐term outcomes and post‐acute sequelae are associated with obesity. Recognizing obesity as a key comorbidity is essential to develop targeted strategies for prevention of COVID‐19 complications in children.
Keywords: adolescent, child, COVID‐19, intensive care units, obesity, post‐acute COVID‐19 syndrome
Abbreviations
- aOR
adjusted Odds Ratio
- BMI
body mass index
- CDC
centers for disease control and prevention
- CI95%
confidence interval 95%
- COVID‐19
Coronavirus disease 2019
- CRP
C‐reactive protein
- ECLS
extracorporeal life support
- ICU
intensive care unit
- IQR
interquartile range
- LOS
length of hospital stay
- MIS‐C
multisystem inflammatory syndrome
- NIMV
non‐invasive mechanical ventilation
- OR
odds ratio
- PASC
post‐acute sequelae of sars‐Cov‐2 infection
- RT‐PCR
reverse‐transcription polymerase chain reaction
- SARS‐CoV‐2
severe acute respiratory syndrome Coronavirus 2
- VOC
variant of concern
- WFL
weight for length index
- WHO
world health organization
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) affects both adults and paediatric patients, although with a lower hospitalization rate in the paediatric population. 1 Nevertheless, intensive care unit (ICU) admission in paediatric patients can reach up to 22.9%, with a reported mortality of 3.6%. 2 Therefore, early identification of predictors of unfavourable prognosis in children and adolescents with COVID‐19 is crucial.
Over the past decades, obesity rates have increased worldwide. According to the Organization for Economic Cooperation and Development, Mexico, Chile, and the United States are the countries with the highest rates of obesity in the adult population. 3 Furthermore, Chilean rates of childhood obesity have doubled in the last four decades, reaching alarming levels of 23.5% and 25.4%, in 2019 and 2020, respectively. 4 In addition, obesity in the paediatric population in general is a relevant risk factor for severe viral infections, and has also been identified as one of the underlying conditions related to poorer outcomes in COVID‐19. 5 , 6 A report on children with obesity has analysed short‐term complications as a composite endpoint, which includes various outcomes: positive pressure ventilation, vasoactive/inotropic drugs, pulmonary vasodilators, extracorporeal life support (ECLS), or renal replacement therapy, indicating that these composite outcomes were associated with obesity and increased severity. 6 However, interpreting the results of composite endpoints can be confusing because the effect of obesity on each component of the composite endpoint might be different. Moreover, if the frequency of occurrence of the components is not similar, the composite outcome will be mainly influenced by the predominant event. 7 Therefore, a more detailed analysis might be helpful to elucidate the role played by obesity in severe disease progression.
Patients with severe COVID‐19 have an increased susceptibility to subsequent bacterial superinfection with immunoparalysis, a state of profound immunosuppression after a severe infection that has been related to critical illness and is characterized by a decreased antigen‐presenting capacity and decreased counts and function of lymphocytes and NK cells, as one of the underlying mechanisms. 8 Obesity has been associated with an impaired immune system and higher susceptibility to severe in‐hospital infections. 9 Therefore, it is relevant to address the link between obesity and superinfections (bacterial, fungal, and viral) in paediatric COVID‐19.
Prolonged symptoms have been described after COVID‐19. These symptoms are referred to as long COVID or post‐acute sequelae of SARS‐CoV‐2 infection (PASC). 10 Although PASC have been mainly reported in adults, a recent meta‐analysis including studies conducted in paediatric individuals reported a prevalence of PASC of 11% to 58%. 11 Nevertheless, the absence of laboratory confirmation of SARS‐CoV‐2 infection, assessment of the emergence of symptoms mostly after mild COVID‐19, and parent‐reported symptoms without clinical assessment are methodological limitations in some of these studies. 11 In addition, the inaccessibility of proper controls hinders the adequate differentiation between symptoms secondary to COVID‐19 from those emerging because of the impact of a global pandemic on childhood health. 11 Although obesity has been proposed as a risk factor for the emergence of PASC in adults, the role of obesity in paediatric patients remains unclear. 3 , 10 , 11 Hence, we sought to evaluate the association between obesity and severe outcomes, including short‐term complications, and PASC, in paediatric inpatients with COVID‐19.
2. METHODS
2.1. Reporting guidelines and ethics statement
This study adheres to the Strengthening The Reporting of Observational Studies in Epidemiology statement 12 (Supplemental file 1). In this study, clinical data were collected from databases, which contained anonymized records. As a result of its study design, the ethics committee waived the need to obtain informed consent from individual patients. The protocol ID: 220228004 was approved by the Health Sciences Scientific Ethics Committee at Pontificia Universidad Católica de Chile and the Ethics Committee of the Servicio de Salud Metropolitano Suroriente, Chile.
2.2. Data source, eligibility criteria, and sample size
This multicenter retrospective cohort study was conducted in the Metropolitan Region of Santiago, Chile, and included inpatients from three high‐complexity academically affiliated hospitals (Hospital Clínico Red Salud UC‐Christus, Hospital Dr. Sótero del Río, and Hospital Clínico La Florida). Data were obtained from the electronic medical record systems of each health center.
Inclusion criteria were age up to 19 years, a reverse transcription polymerase chain reaction (RT‐PCR) test positive for SARS‐CoV‐2 from a nasopharyngeal swab, and date of discharge between March 3rd, 2020, and December 31st, 2021. Incomplete admission, anthropometric assessment (including weight and height), and asymptomatic COVID‐19 were considered exclusion criteria. We used a convenience sampling approach, and hence, the number of paediatric COVID‐19 patients hospitalized in the high‐complexity hospitals during the study period, who fulfilled the inclusion and exclusion criteria, determined the sample size and it was not previously calculated.
2.3. Exposure variable and covariables
Weight (kg) and height (cm) were recovered from within the first 24 h of admission. Anthropometric assessments were performed according to World Health Organization (WHO) 2006 and Centers for Disease Control and Prevention (CDC) 2000 growth charts. 13 , 14 Following CDC and the American Academy of Paediatrics recommendations, subjects were categorized as having underweight, normal weight, overweight, or obesity. Weight for Length index (WFL) from the WHO growth charts were used for children under 2 years of age, and BMI from the CDC growth charts were used for those with 2 years of age and older. Obesity was defined as WFL or BMI at 95th percentile or greater. 15 , 16 Percentiles and Z‐scores for the WFL and BMI were obtained using the WHO Anthro software version 3.2.2 and the Baylor College of Medicine children's BMI‐percentile‐for‐age calculator, respectively. 17
Data regarding age, sex, symptoms at presentation, comorbidities, previous treatments (namely antibiotics, systemic corticosteroids, inhaled corticosteroids, and immunosuppressive drugs), vital signs, and laboratory parameters including blood count and C‐reactive protein (CRP) levels were obtained at admission. Age was used either as a continuous or categorical variable, which was classified in ranges adhering to the United States Food and Drug Administration criteria as follows: neonates (newborns up to 1 month), infants (1 month to 2 years), children (2 to 12 years), adolescents (12 to 16 years), and others (16 to 19 years). 18 Comorbidities were classified using the International Classification of Diseases, 10th revision, Clinical Modification. 19 Vital signs were interpreted according to age in agreement with the Paediatric Advanced Life Support guidelines. 20 Blood counts were interpreted in accordance with age‐defined reference intervals as previously reported. 21 Diagnosis of COVID‐19 after 48 h of hospitalization was considered as a healthcare‐associated COVID‐19. 22
Based on national sequencing data, a SARS‐CoV‐2 lineage or variant of concern (VOC) was considered predominant if over 50% of COVID‐19 tests performed nationwide in a certain period of time were positive for that lineage or VOC. 23 Accordingly, for this study three epidemiological periods were defined: (1) circulation of other lineages and Alpha (2020), (2) circulation of Gamma and Lambda (first semester 2021), and (3) circulation of Delta (second semester 2021). In addition, the hospital where each patient was treated was also considered.
2.4. Study outcomes
Short‐term outcomes were defined as COVID‐19 complications occurring during hospital stay, and included: (A) ventilatory and/or vital organ support: (1) oxygen requirement and duration, (2) high‐flow nasal cannula use and duration, (3) non‐invasive mechanical ventilation (NIMV) use and duration, (4) invasive mechanical ventilation use and duration, (5) high‐frequency oscillatory ventilation use, (6) ECLS, (7) vasoactive–inotropic support, (8) pulmonary vasodilator therapy (inhaled nitric oxide), (9) length of hospital stay (LOS), (10) admission and length of stay in ICU, and (11) in‐hospital mortality; (B) clinically‐relevant superinfections such as: (1) microbiologically‐proven superinfections (bacterial, fungal or viral) defined accordingly as a positive culture, nucleic acid amplification test, serology or immunofluorescence test after 48 h from hospital admission if clinically relevant (persistence of positive tests for the same infection were excluded), and (2) suspected bacterial pneumonia defined by the initiation of antibiotics or changes in antibiotic treatment driven by clinical deterioration (persistent fever over 48 h, increased ventilatory support and/or progressive radiological findings); (C) diagnosis of multisystem inflammatory syndrome in children (MIS‐C) according to CDC criteria. 24
PASC included the presence of palpitations, muscle weakness, or dyspnea (Borg scale ≥1 point) persisting for over 4 weeks post‐SARS‐CoV‐2 infection. 25 , 26 , 27 This data was obtained from outpatient follow‐up visits up to 6 months after COVID‐19 diagnosis, as recorded on medical records.
2.5. Statistical analysis
Categorical variables were expressed as numbers and percentages. Continuous variables were described using median and interquartile range (IQR). Wilcoxon rank‐sum test was applied in univariate analysis to analyse differences in continuous variables, and chi‐squared or Fisher's exact test were used for comparison of categorical variables. For categorical outcomes, unadjusted odds ratios (OR) with 95% confidence intervals (CI95%) through univariate logistic binary regression were also estimated. In addition, β‐coefficients for continuous variables were obtained through univariate linear regression. Subsequently, a multivariate logistic binary regression was performed for each categorical outcome, adjusting by age, sex, and appropriate covariables (including comorbidities, and previous treatments). Linear regression was conducted for each continuous outcome, adjusting by age, sex, and appropriate covariables (including comorbidities, and previous treatments).
Cluster analysis with generalized estimated equations was applied to include dependency related to the health center (considering the eventual differences in each's hospital clinical management) and to the time periods of lineage or VOC predominance. The association between obesity and categorical outcomes was analysed using generalized estimated equations with a logit‐link function and binomial distribution, obtaining adjusted odds ratios (aOR). The association between obesity and continuous outcomes was investigated through generalized estimated equations with an identity‐link function and Gaussian distribution. We performed stratified analyses to assess effect modification by age, generating two new different GEE models for each outcome significantly associated with obesity, in which we considered two groups: patients aged ≥12, and patients <12 years old, based on the previously mentioned FDA‐defined age groups.
Participants with missing data were excluded from the analysis. The number of missing values for each variable of interest is displayed in the results section.
Statistical analyses were carried out using STATA MP 15 and Graph Prism 8. A p < 0.05 was considered statistically significant.
3. RESULTS
3.1. Obesity is associated with older age, respiratory symptoms, and high CRP at admission
A total of 216 patients with COVID‐19 were included (Figure 1). The baseline characteristics of the study sample are displayed in Table 1. Male represented 53.2% (115) of the study participants and the median age was 9.3 years (IQR 0.62–15.35). Obesity was present in 31.0% (67) of patients. Individuals with obesity were significantly older than those without this comorbidity, with a median age of 13.7 (IQR 10.7–16.08) and 2.9 (IQR 0.2–14.2) years, respectively (Figure 2). In accordance, there were statistically significant differences in the proportion of obesity by age group (p < 0.001). Adolescents and others (12 years to 19 years) had a higher frequency of obesity (n = 44, 50.0%) than neonates, infants and children (newborns to 12 years, n = 23, 18.0%; p < 0.001). There were no differences in the proportion of obesity in patients with healthcare‐associated COVID‐19 (p = 0.402).
FIGURE 1.
Flowchart of included patients. From a total of 237 patients included from the three high complexity participating hospitals, 216 fulfilled inclusion criteria. Reasons for exclusion are detailed. HCUC, Hospital Clínico Red Salud UC‐Christus; HSR, Hospital Dr. Sótero del Río; HLF, Hospital de La Florida Dra. Eloísa Díaz; RT‐PCR, Reverse‐Transcription Polymerase Chain Reaction; SARS‐CoV‐2, Severe Acute Respiratory Syndrome Coronavirus 2
TABLE 1.
Baseline characteristics of inpatients infected with SARS‐CoV‐2 according to the presence of obesity
| Variables | All patients (n = 216) | Patients with obesity (n = 67) | Patients without obesity (n = 149) | p value |
|---|---|---|---|---|
| Age (years), median (IQR) | 9.18 (0.62–15.35) | 13.69 (10.63–16.08) | 2.97 (0.18–14.13) | <0.001 |
| Male, n (%) | 115 (53.24) | 37 (55.22) | 78 (52.35) | 0.695 |
| zWFL or zBMI, median (IQR) | 0.80 (−0.13–1.93) | 2.24 (2.00–2.46) | 0.31 (−0.52–0.95) | <0.001 |
| Healthcare‐associated COVID‐19, n (%) | 15 (6.94) | 3 (4.48) | 12 (8.05) | 0.402 |
| Time from symptoms onset to positive RT‐PCR (days), median (IQR) | 2 (1–4) | 3 (1–6) | 1 (0–3) | <0.001 |
| Previous treatment | ||||
| Inhaled corticosteroids, n (%) | 11 (5.09) | 2 (2.99) | 9 (6.04) | 0.502 |
| Systemic corticosteroids, n (%) | 16 (7.41) | 5 (7.46) | 11 (7.38) | 0.983 |
| Antibiotics, n (%) | 29 (13.43) | 10 (14.93) | 19 (12.75) | 0.665 |
| Immunosuppressants, n (%) | 13 (6.02) | 2 (2.99) | 11 (7.38) | 0.353 |
| Comorbidities** | ||||
| Immunosuppression (D80‐89), n (%) | 20 (9.26) | 5 (7.46) | 15 (10.07) | 0.541 |
| Oncologic (C00‐D49), n (%) | 13(6.02) | 2 (2.99) | 11 (7.38) | 0.353 |
| Respiratory (J00‐J49), n (%) | 40 (18.52) | 14 (20.90) | 26 (17.45) | 0.546 |
| Cardiovascular (I00‐I99), n (%) | 22 (10.19) | 8 (11.94) | 14 (9.40) | 0.567 |
| Neuromuscular/Neurological (GOO‐G99), n (%) | 37 (17.13) | 8 (11.94) | 29 (19.46) | 0.175 |
| Endocrine (E00‐E36), n (%) | 29 (13.43) | 11 (16.42) | 18 (12.08) | 0.387 |
| Gastrointestinal (K00‐K95), n (%) | 16 (7.41) | 3 (4.48) | 13 (8.72) | 0.401 |
| Allergy (T78), n (%) | 20 (9.26) | 5 (7.46) | 15 (10.07) | 0.541 |
Note: Bold values indicate the significant values.
Abbreviations: zBMI, z score for Body mass index (Centers for Disease Control and Prevention, 2000 reference); zWFL, z score for Weight/Length index (World Health Organization 2006 reference).
FIGURE 2.
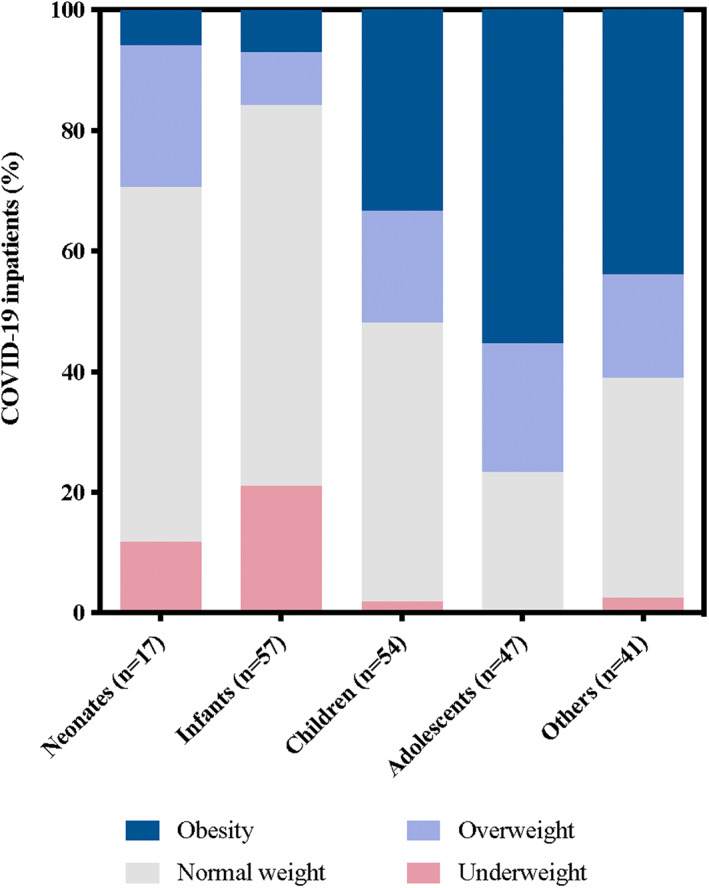
Nutritional status by age group. Nutritional diagnosis was made according to recommendations by Centers for Disease Control and Prevention and the American Academy of Paediatrics: obesity (dark blue), overweight (light blue), normal weight (grey), and low weight (red). Age groups were defined by Food and Drug Administration guidelines criteria. COVID‐19, Coronavirus Disease 2019
Longer time from symptoms onset to diagnosis was detected in SARS‐CoV‐2 patients with obesity (p < 0.001). However, this difference did not persist in the healthcare‐associated COVID‐19 subgroup (p = 0.402). Respiratory symptoms such as dyspnea (59.7% vs. 32.21%; p < 0.001), cough (70.15% vs. 49.66%; p = 0.005), and chest pain (14.93% vs. 6.04%; p = 0.033) were more frequently observed in patients with obesity. No significant differences were detected in the presence of fever at admission according to obesity (79.10% vs. 66.44%, p = 0.059). Diarrhoea (28.36% vs. 16.11%; p = 0.037), headache (41.79% vs. 14.09%, p < 0.001), ageusia (11.94% vs. 4.03%, p = 0.029), but not anosmia (11.94% vs. 6.04%, p = 0.136) were more frequently informed in patients with obesity. There were no differences in comorbidities among both groups; in particular, asthma was not more prevalent in patients with obesity (16.42% vs. 11.41%, p = 0.311). No significant differences were detected in treatments received before hospital admission, including corticosteroids.
Vital signs and laboratory findings at admission, according to the presence of obesity, are shown in Supplemental file 2. At hospital admission, patients with obesity had a significantly higher proportion of tachycardia and tachypnea. Only two patients had missing blood counts (0.92% of the study sample, one with obesity and one without obesity). A higher proportion of lymphopenia was observed in individuals with obesity, without statistical significance (p = 0.070). An increased frequency of thrombocytosis was identified in individuals without obesity. CRP level was missing in 10 participants (4.6%, nine patients without obesity and one with obesity). A significant elevation of CRP was observed in patients with obesity, and 50.00% (n = 33) of them had a CRP ≥3 mg/dl at hospital admission (p = 0.040).
3.2. Oxygen requirement, NIMV, and superinfections were associated with obesity
Obesity was associated with the following short‐term outcomes: ICU admission, oxygen requirement, and NIMV (Table 2). In addition, a non‐significant higher requirement of invasive ventilation was observed in individuals with obesity (p = 0.06). Three patients in our cohort required ECLS, all of them with obesity. According to nutritional status, there were no differences regarding the requirement of vasoactive drugs or pulmonary vasodilators. None of the patients who died had obesity. A higher frequency of acute kidney injury (p = 0.046) was observed in patients with obesity. Up to nine and six additional days of hospital stay and oxygen use, respectively, were observed in patients with obesity in the univariate analysis.
TABLE 2.
Short‐term complications and post‐acute sequela in paediatric inpatients with obesity and COVID‐19
| Outcomes | All patients (n = 216) | Patients with obesity (n = 67) | Patients without obesity (n = 149) | p value | Unadjusted OR (CI95%) or β coefficient (CI95%) |
|---|---|---|---|---|---|
| Short‐term: LOS, respiratory support, and organ support | |||||
| Hospital LOS, median (IQR), days | 7 (4–13.5) | 8 (6–21) | 6 (3–13) | 0.001 | 9.33 (−3.73–22.79) |
| ICU admission, n (%) | 91 (42.12) | 48 (71.64) | 43 (28.86) | <0.001 | 6.23 (3.15–12.48) |
| ICU LOS, median (IQR), days | 6 (4–18) | 6 (3.50–16.5) | 7 (4–18) | 0.593 | −11.37 (−29.60‐ ‐6.87) |
| Oxygen, n (%) | 104 (48.15) | 49 (73.13) | 55 (36.91) | <0.001 | 4.65 (2.37–9.32) |
| Oxygen duration, median (IQR), days | 6 (4–12.50) | 7 (5–23) | 5 (2–11) | 0.002 | 6.67 (−1.00–14.33) |
| HFNC, n (%) | 27 (12.50) | 11 (16.42) | 16 (10.74) | 0.243 | 1.63 (0.64–4.01) |
| HFNC duration, median (IQR), days | 2 (1–5) | 1 (0–5) | 2 (1–5) | 0.730 | 1.48 (−2.77–5.74) |
| NIMV, n (%) | 25 (11.57) | 20 (29.85) | 5 (3.36) | <0.001 | 12.26 (4.11–43.52) |
| NIMV duration, median (IQR), days | 4 (3–5) | 4 (3–4.5) | 4 (2–5) | 1.000 | −0.85 (−3.30–1.60) |
| IMV, n (%) | 31 (14.35) | 14 (20.90) | 17 (11.41) | 0.066 | 2.05 (0.87–4.76) |
| IMV duration, median (IQR), days | 13 (6–23) | 14.5 (6–19) | 11 (5–23) | 0.736 |
−4.44 (−21.46–12.59) |
| HFOV, n (%) | 3 (1.39) | 1 (1.49) | 2 (1.39) | 0.930 | 1.11 (0.02–21.63) |
| ECLS, n (%) | 3 (1.39) | 3 (4.48) | 0 (0) | 0.029 | NA |
| Inotropes and vasoactive use, n (%) | 22 (10.19) | 9 (13.43) | 13 (8.72) | 0.290 | 1.62 (0.58–4.36) |
| iNO use, n (%) | 4 (1.85) | 2 (2.99) | 2 (1.34) | 0.407 | 2.26 (0.16–31.69) |
| Short‐term: superinfections | |||||
| Overall superinfections a , n (%) | 58 (26.85) | 29 (43.28) | 29 (19.46) | <0.001 | 3.16 (1.60–6.22) |
| Suspected bacterial pneumonia, n (%) | 50 (23.15) | 27 (40.30) | 23 (15.44) | <0.001 | 3.70 (1.81–7.54) |
| Overall proven superinfections, n (%) | 29 (13.43) | 13 (19.40) | 16 (10.74) | 0.084 | 2.00 (0.82–4.77) |
| Bacterial proven superinfections, n (%) | 25 (11.57) | 13 (19.40) | 12 (8.05) | 0.016 | 2.75 (1.08–7.02) |
| Viral proven superinfections, n (%) | 6 (2.78) | 2 (2.99) | 4 (2.68) | 0.601 | 1.12 (0.10–8.00) |
| Fungal proven superinfections, n (%) | 8 (3.70) | 3 (4.48) | 5 (3.36) | 0.706 | 1.35 (0.20–7.17) |
| Short‐term: medical complications | |||||
| MIS‐C, n (%) | 6 (2.78) | 3 (4.48) | 3 (2.01) | 0.308 | 2.28 (0.3–17.42) |
| AKI, n (%) | 30 (13.89) | 14 (20.9) | 16 (10.74) | 0.046 | 2.20 (0.92–5.16) |
| Thrombosis, n (%) | 8 (3.70) | 5 (7.46) | 3 (2.01) | 0.111 | 3.92 (0.73–25.86) |
| In‐hospital mortality, n (%) | 7 (3.24) | 0 (0) | 7 (4.70) | 0.071 | NA |
| Post‐acute sequelae b | |||||
| Tracheostomy, n (%) | 8 (3.70) | 5 (7.46) | 3 (2.01) | 0.111 | 3.92 (0.73–25.86) |
| Dyspnea, n (%) | 12 (5.74) | 10 (14.93) | 2 (1.41) | <0.001 | 12.28 (2.47–117.20) |
| Muscle weakness, n (%) | 15 (7.18) | 14 (20.90) | 1 (0.70) | <0.001 | 35.25 (5.33–1585.31) |
| Palpitations, n (%) | 5 (2.39) | 3 (4.48) | 2 (1.41) | 0.189 | 3.28 (0.36–939.92) |
Note: For numerical predictor variables, the beta (β) coefficient represents the degree of change in the predicted value of the outcome variable for every 1‐unit of change in the predictor variable, if all the other predictor variables included in the model remain constant. For categorical predictor variables, Odds Ratio (OR) value represents a measure of association between variables and obesity. Bold values denote statistical significance (p < 0.05).
Overall superinfections included patients with proven superinfections and/or suspected bacterial pneumonia.
For the included variables on Post‐Acute Sequelae of SARS CoV‐2 infection, patients who died during hospitalization for COVID‐19 (n = 7) were excluded. None of them were within the group of patients with obesity (n = 66), therefore 142 patients without obesity were analysed at follow‐up.
Abbreviations: AKI, acute kidney injury; CI95%, Confidence Interval of 95%; ECLS, Extracorporeal Life Support; HFNC, High Flow Nasal Cannula; HFOV, High Frequency Oscillatory Ventilation; ICU, Intensive Care Unit; IMV, Invasive Mechanical Ventilation; iNO, inhaled Nitric Oxide, IQR, Interquartile Range; LOS, Length Of Stay; MIS‐C, Multisystem Inflammatory Syndrome in Children; NA, not applicable; NIMV, Non‐Invasive Mechanical Ventilation; OR, Odds Ratio.
The odds of microbiologically proven bacterial superinfections were higher in participants with obesity (OR 2.75; CI95% 1.08–7.02). Out of 25 patients with proven bacterial superinfections, 13 (52.00%) had obesity. The main bacteria found in patients with obesity were Gram‐negative bacilli and Staphylococcus aureus. There was no association between viral or fungal infection and obesity, but a higher proportion of obesity was observed in individuals with suspicion of bacterial pneumonia (OR = 3.70; CI95% 1.81–7.54). Overall, up to three times higher odds for superinfections (proven infections, bacterial superinfections, and/or suspected bacterial pneumonia) were found in patients with obesity.
Adjusting by age, sex, and other covariables it was observed that ICU admission (aOR = 4.92; CI95% 2.49–9.75), oxygen requirement (aOR = 3.22; CI95% 1.60–6.50), NIMV (aOR = 8.70; CI95% 2.98–25.40), overall superinfections (aOR = 2.89; CI95% 1.38–6.02), and suspected bacterial pneumonia (aOR = 2.92; CI95% 1.40–6.13) were associated with obesity in paediatric patients (Figure 3 and Supplemental file 3).
FIGURE 3.
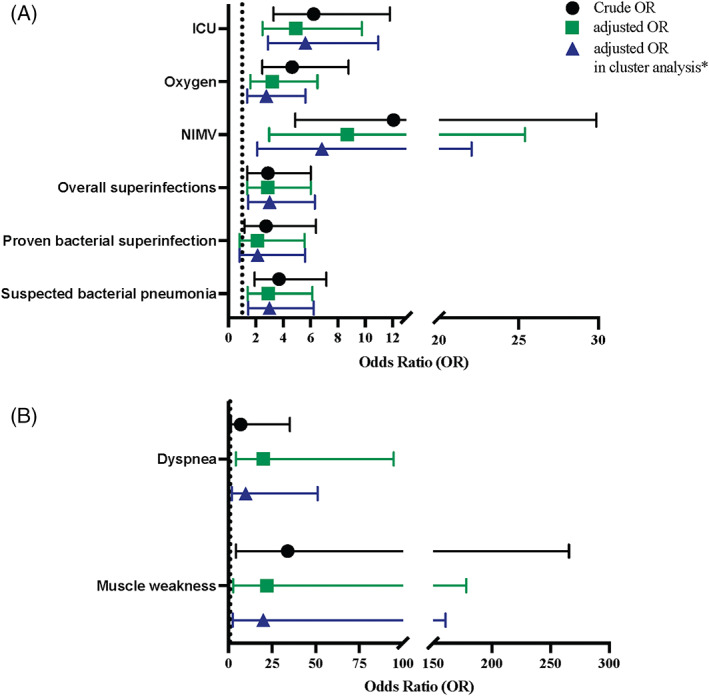
Short‐term complications and post‐acute sequelae of SARS‐CoV‐2 infection in paediatric patients with obesity. (A) Short‐term complications. Models for ICU admission were adjusted by age, sex, and presence of cardiovascular underlying disease. Models for oxygen requirement were adjusted by age, sex and presence of respiratory underlying disease. Models for NIMV were adjusted by age and sex. Models for overall superinfections were adjusted by age, sex, previous use of antibiotic, presence of immunosuppression, presence of respiratory underlying disease, presence of endocrinologic underlying disease, and healthcare‐associated COVID‐19. Models for proven bacterial superinfection were adjusted by age, sex, previous use of antibiotic, healthcare‐associated COVID‐19, presence of immunosuppression, and presence of endocrinologic underlying disease. Models for suspected bacterial pneumonia were adjusted by age, sex, previous use of antibiotic, presence of immunosuppression, presence of respiratory underlying disease, and presence of endocrinologic underlying disease. (B) Post‐acute sequelae. Models for dyspnea and muscle weakness were adjusted by age and sex. *Patients were clustered according to the center where they were admitted and the predominant lineage or the time period of SARS‐CoV‐2 variant of concern circulating in Chile. ICU, intensive care unit; NIMV, noninvasive mechanical ventilation; OR, odds ratio
The following variables had a statistically significant association with obesity when short‐term complications were analysed considering dependency given by the predominant lineage or VOC time‐period and the hospital in which each participant was treated: ICU admission (aOR = 5.63; CI95% 2.90–10.94), oxygen requirement (aOR = 2.77; CI95% 1.36–5.63), NIMV (aOR = 6.81; CI95% 2.11–22.04), overall superinfections (aOR = 3.02; CI95% 1.45–6.31), and bacterial pneumonia (aOR = 3.00; CI95% 1.44–6.23) (Figure 3 and Supplemental file 4). When comparing the crude OR, the OR adjusted by age, sex, and other covariables, and the OR adjusted including dependency, the cluster analysis yielded a slightly lower OR for NIMV in patients with obesity (Figure 3A). A higher odd for bacterial superinfection was found in association with obesity in multivariate (aOR = 2.12; CI95% 0.80–5.57) and cluster analysis (aOR = 2.13; CI95% 0.81–5.59), but it did not reach statistical significance. The same was observed for AKI (multivariate aOR = 2.03; CI95% 0.84–4.91 and cluster analysis aOR = 2.11; CI95% 0.98–4.53), when we adjusted by age, sex, and presence of immunosuppression.
In the stratified analyses, we obtained different results for each of the outcomes (Supplemental file 5). While the association of obesity with ICU admission remained significant in both age groups, the associations of obesity with oxygen requirement and NIMV were only significant for patients aged ≥12 years. Furthermore, the associations of obesity with overall superinfections and suspected pneumonia were found significant only in patients <12 years old. These results indicate that overall obesity was always associated with a poorer outcome in all age groups.
3.3. Dyspnea and muscle weakness were the most reported post‐acute sequelae in convalescent COVID‐19 children with obesity
Altogether, 28 (13.40%) surviving patients had neurological, cardiological, and respiratory complications after discharge at follow up to 6 months after symptoms onset. Dyspnea and muscle weakness were found in a high proportion in individuals with obesity (Table 2). Once adjusted by age and sex, the following variables demonstrated a strong association with obesity in cluster analysis: dyspnea (aOR = 20.04; CI95% 2.50–160.65), and muscle weakness (aOR = 9.91; CI95% 1.92–51.10) (Figure 3B).
In the stratified analyses (Supplemental file 5), we found that the association of obesity with muscle weakness remained significant in both age groups. On the other hand, the association of obesity with dyspnea was found significant only in patients under 12 years old.
4. DISCUSSION
This multicentric and longitudinal study examined the role of obesity over the clinical course of 216 paediatric inpatients with SARS‐CoV‐2 infection. Our results demonstrate higher rates of oxygen requirement, NIMV use, and ICU admission in patients with obesity. Furthermore, cluster analysis highlighted obesity as a relevant factor when assessing the risk of superinfections. In addition, patients with obesity were at increased risk of evolving with dyspnea and muscle weakness as post‐acute sequelae of COVID‐19. Our findings demonstrate the relevance of obesity in children infected with SARS‐CoV‐2.
Chile is considered as a high‐income country according to the World Bank Country Classification. 28 Recent data indicates that Chile occupies a leading position in the prevalence of paediatric obesity with one of the highest statistics worldwide. 29 A frequency of obesity of 30% was obtained in our sample of paediatric patients infected with SARS‐CoV‐2, which is in accordance with what has been previously reported in the literature. 6
As severe outcomes among paediatric patients with COVID‐19 are less common than in adults, and our understanding of how the disease behaves in childhood is more limited. 30 Valuable data has emerged from a systematic review evaluating pre‐existing factors associated with severe disease, early admission to critical care units, and death secondary to SARS‐CoV‐2 infection in hospitalized children, 30 demonstrating that obesity, either alone or with concomitant conditions, markedly increased the risk of critical care admission and mortality. 30 Despite concordance with the observation of higher ICU admission rates in the group with obesity, our results did not show an increased risk of death. This finding has been previously mentioned in the literature as “the obesity paradox”. 6 , 31 However, discrepancies between our results and this systematic review could also be explained by differences in the eligibility criteria (for instance, including patients with negative RT‐PCR), exclusion of several studies not reporting obesity, and publication bias. 30 on the other hand, our results in mortality could also be secondary to a lack of statistical power.
The observed increased use of supplemental oxygen and ventilatory support in individuals with obesity is in agreement with previous reports. 6 , 32 In other viral infections, excess body weight has been shown to reduce functional residual capacity and lung compliance due to the accumulation of adipose tissue in abdominal and visceral cavities. 33 In addition, a dysregulated immune response against viral infections is described in patients with obesity. 34 An exacerbated immune response with consequent alveolar damage and acute respiratory distress syndrome has been observed in COVID‐19 adult patients. 32 All these factors could explain why, in our sample, patients with obesity required more ventilatory support than eutrophic ones. In our cohort, paediatric patients with obesity required more oxygen, which could be secondary to alveolar damage linked to elevated leptin levels. 35
A systematic review including adult and paediatric patients reported a frequency of bacterial superinfections of 20% (CI95% 13%–28%), which is higher than what is reported in our study (11.57%). 36 Among bacterial superinfections, a Gram‐negative bacilli predominance was observed, results that are comparable with previous data. 36 We also noted the isolation of Staphylococcus aureus in bacterial superinfections, particularly in patients with obesity. This microorganism has been previously isolated in individuals with COVID‐19. 36 Additional research to elucidate the role of microbiome disturbance of the respiratory tract after SARS‐CoV‐2 infection is needed to clarify these findings, as has been studied in other viral infections. 37 After SARS‐CoV‐2 infection, carriers of certain immunophenotypes develop an increased susceptibility to subsequent bacterial superinfection, mediated by an immune system impairment referred to as immunoparalysis. 8 , 9 Decreased antigen‐presenting capacity, and reduced levels of lymphocytes and natural killer cells are among the underlying mechanisms involved. 8 In fact, excess adiposity negatively impacts immune function and host defences against infections. 9 Increased visceral adiposity induces higher levels of local and systemic inflammatory biomarkers such as CRP, 38 as observed in our study. Furthermore, deregulated cytokines and chemokines profiles (e.g., IL‐6, IP‐10, and IL‐10), might be part of the physiopathological pathway behind severe COVID‐19 outcomes. 38
In our cohort of symptomatic paediatric inpatients, dyspnea and muscle weakness were the main sequelae reported at six‐month follow up, even after adjustment by age to reduce possible reporting bias. Fatigue, respiratory symptoms, and persistent muscle pain are among most frequently reported symptoms in the limited evidence addressing paediatric PASC. 39 , 40 , 41 Nevertheless, this data comes mostly from SARS‐CoV‐2 infections of ambulatory care, with scarce data on PASC after COVID‐19 hospitalization. Symptoms grouped under the term PASC are not just a consequence of ICU stay, since in paediatric patients not requiring ICU admission, moderate and severe obesity has been associated with a greater risk of PASC. 42 Physical deconditioning and dysregulated pro‐inflammatory innate immune response observed in severe COVID‐19 are possible factors explaining emergence of PASC. 43 Nevertheless, biological mechanisms underlying paediatric PASC remain unclear to date. 11 Although visceral adiposity has been demonstrated to be a marker of severity in COVID‐19, 44 , 45 , 46 studies in children with obesity should be conducted to provide a mechanistic explanation of disease severity and PASC, and to develop targeted treatments.
We found a high difference in age medians between the groups of patients with and without obesity. In order to address this difference and evaluate a possible effect modification by age, we performed stratified analyses. Although the different results could indicate that there are different patterns of effect modification by age in the association of obesity with each outcome, these results are more likely to reflect a lower statistical power for these analyses. Moreover, these results do not agree with the hypothesis of poorer outcomes only being related to a higher age in patients with obesity.
The strengths of our study are the inclusion of detailed data on anthropometry, comorbidities, and a comprehensive record of symptoms at presentation of paediatric COVID‐19 patients, with a low proportion of missing data. In addition, analysis of clinical features during follow‐up visits allowed us to evaluate information on persistent symptoms and PASC, adding value to a topic that has been controversial in the literature. Thus, our study provides evidence that associates obesity with short‐term outcomes and the appearance of PASC, contributing with data from a Latin American population.
The main limitation of our study is its small sample size. However, reports of paediatric inpatients tend to be smaller than those conducted in adults, since manifestations of COVID‐19 have demonstrated to be milder in childhood. 30 Nonetheless, we were able to identify several significant associations between obesity, short‐term outcomes, and PASC. Additionally, we dealt with the known limitations of retrospective data gathering. Therefore, to further address the relationship between obesity and these outcomes and to assess the effect of vaccination on outcomes, more extensive prospective studies are warranted.
In conclusion, in paediatric inpatients with symptomatic COVID‐19, obesity is significantly associated with short‐term complications and PASC. Paediatric patients with obesity are at increased risk of ICU admission, oxygen requirement, NIMV use and superinfections during in‐hospital stay, and have a higher risk of evolving with persistent dyspnea and muscle weakness after COVID‐19. Elucidating the immunological and virological mechanisms that explain these findings, along with increasing vaccination efforts, may prevent COVID‐19‐related morbidity in these patients.
AUTHOR CONTRIBUTIONS
Gonzalo Valenzuela, Gonzalo Alarcón‐Andrade and Clara Schulze‐Schiapacasse conceived and designed the study, coordinated, and supervised data collection, carried out the analyses, and drafted the initial manuscript. Rocío Rodríguez, Monserrat Gutiérrez, Loreto Godoy, Pamela Céspedes, Sandra Bermudez, Javiera Aravena, Irini Nicolaides, Eliana Martínez, Constanza Gómez‐Canobbio, Macarena Jofré, Andrea Salinas, Daniela Depaoli, Carolina Loza, Andrés Muñoz and José Barriga contributed to clinical data acquisition for the study, coordinated and supervised data collection and revised the manuscript; Sandra Bermudez, Erick Salinas, Andrés Muñoz‐Marcos and Diana Manzur collected laboratory data. Tamara García‐Salum, Jorge Levican, Erick Salinas, Leonardo I. Almonacid, María José Avendaño and Catalina Pardo‐Roa reviewed and revised the manuscript. Salesa Barja conceptualized and designed the study and critically reviewed the manuscript for intellectual content. Rafael A. Medina conceptualized and designed the study and critically reviewed the manuscript for intellectual content and obtained funding for the study. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
FUNDING INFORMATION
Supported in part by the FONDECYT 1212023 grant from the Agencia Nacional de Investigación y Desarrollo (ANID) of Chile, the National Institutes of Health (NIH) funded Center of Excellence for Influenza Research and Surveillance (contract number HHSN272201400008C) and the FLUOMICS Consortium (NIH‐NIAD grant U19AI135972) to RAM, a grant from Paediatric Division of the Pontificia Universidad Católica de Chile and DIDEMUC at the Pontificia Universidad Católica de Chile to GV. NIH and the Paediatric Division and DIDEMUC from the Pontificia Universidad Católica de Chile had no role in the design or conduction of this study.
CONFLICT OF INTEREST
The authors have no relevant financial or non‐financial interests to disclose.
Supporting information
APPENDIX S1 Supporting Information
ACKNOWLEDGEMENTS
Protocols and the study set‐up used for this study were based on influenza virus studies established in part with the support of the FONDECYT 1161971, FONDECYT 1212023, FONDECYT Post‐doctorado 2019 N°3190648 and ACT 1408 grants from the Agencia Nacional de Investigación y Desarrollo (ANID) from Chile, the FLUOMICS Consortium (NIAID grant U19AI135972) funded by NIH and with funding from CRIP (Center for Research on Influenza Pathogenesis), an NIH funded Center of Excellence for Influenza Research and Surveillance (CEIRS, contract number HHSN272201400008C) to RAM.
Valenzuela G, Alarcón‐Andrade G, Schulze‐Schiapacasse C, et al. Short‐term complications and post‐acute sequelae in hospitalized paediatric patients with COVID‐19 and obesity: A multicenter cohort study. Pediatric Obesity. 2023;18(2):e12980. doi: 10.1111/ijpo.12980
Funding information Concurso Becarios Residentes 2020 ‐ Dirección de Investigación de la Escuela de Medicina y Doctorado (DIDEMUC) y Hospital Clínico de la Florida (HLF), Grant/Award Number: PB23/20; NIH NIAID, Fluomics: The Next Generation, Grant/Award Number: 1U19AI135972; NIAID NIH, Centers of Excellence for Influenza Research and Surveillance (CEIRS), Grant/Award Number: HHSN272201400008C; Agencia Nacional de Investigación y Desarrollo (ANID), FONDECYT, Grant/Award Numbers: 1162791, 1212023, 1212023
REFERENCES
- 1. Coronavirus Disease 2019 (COVID‐19)‐Associated Hospitalization Surveillance Network (COVID‐NET) | CDC. Accessed April 2, 2022. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covid-net/purpose-methods.html
- 2. Irfan O, Muttalib F, Tang K, Jiang L, Lassi ZS, Bhutta Z. Clinical characteristics, treatment and outcomes of paediatric COVID‐19: a systematic review and meta‐analysis. Arch Dis Child. 2021;106(5):440‐448. doi: 10.1136/ARCHDISCHILD-2020-321385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Health at a Glance 2021 OECD Indicators | Health at a Glance | OECD iLibrary. Accessed March 20, 2022. https://www.oecd-ilibrary.org/social-issues-migration-health/health-at-a-glance-2021_ae3016b9-en
- 4. Ministerio de Educación . Mapa Nutricional JUNAEB. Accessed March 20, 2022. https://www.junaeb.cl/mapa-nutricional
- 5. Moser JAS, Galindo‐Fraga A, Ortiz‐Hernández AA, et al. Underweight, overweight, and obesity as independent risk factors for hospitalization in adults and children from influenza and other respiratory viruses. Influenza Other Respi Viruses. 2019;13(1):3‐9. doi: 10.1111/IRV.12618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tripathi S, Christison AL, Levy E, et al. The impact of obesity on Disease severity and outcomes among hospitalized children with COVID‐19. Hosp Pediatr. 2021;11(11):e297‐e316. doi: 10.1542/HPEDS.2021-006087 [DOI] [PubMed] [Google Scholar]
- 7. Palileo‐Villanueva LM, Dans AL. Composite endpoints. J Clin Epidemiol. 2020;128:157‐158. doi: 10.1016/J.JCLINEPI.2020.07.017 [DOI] [PubMed] [Google Scholar]
- 8. Hall MW, Joshi I, Leal L, Ooi EE. Immune immunomodulation in coronavirus Disease 2019 (COVID‐19): strategic considerations for personalized therapeutic intervention. Clin Infect Dis Offic Publ Infect Dis Soc Am. 2022;74(1):144‐148. doi: 10.1093/CID/CIAA904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Milner JJ, Beck MA. Micronutrients, immunology and inflammation the impact of obesity on the immune response to infection. Proc Nutr Soc. 2012;71(2):298‐306. doi: 10.1017/S0029665112000158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rajan S, Khunti K, Alwan N, et al. In the wake of the pandemic preparing for long COVID HEALTH SYSTEMS AND POLICY ANALYSIS. 2021:1–30. Accessed March 25, 2022. http://www.euro.who.int/en/about-us/partners/ [PubMed]
- 11. Zimmermann P, Pittet L. Disease NCTPI Undefined. The challenge of studying long COVID: an updated review. 2022. Accessed April 3, 2022. europepmc.org https://europepmc.org/article/med/35213866 [DOI] [PMC free article] [PubMed]
- 12. Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4(10):1628‐1654. doi: 10.1371/JOURNAL.PMED.0040297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. WHO Child growth standards. Accessed March 25, 2022. https://www.who.int/toolkits/child-growth-standards/standards
- 14. Growth Charts ‐ Clinical Growth Charts. Accessed April 3, 2022. https://www.cdc.gov/growthcharts/clinical_charts.htm
- 15. Recommendations | Assess Growth Birth to 2 Years | WHO | Growth Chart Training | Nutrition | DNPAO | CDC. Accessed March 25, 2022. https://www.cdc.gov/nccdphp/dnpao/growthcharts/who/recommendations/index.htm
- 16. Defining Childhood Weight Status | Overweight & Obesity | CDC . Accessed April 3, 2022. https://www.cdc.gov/obesity/childhood/defining.html
- 17. Baylor Collage of Medicine . Children's BMI‐percentile‐for‐age Calculator. Accessed March 25, 2022. https://www.bcm.edu/cnrc-apps/bodycomp/bmiz2.html
- 18. Pediatric Exclusivity Study Age Group | FDA. Accessed March 25, 2022. https://www.fda.gov/drugs/data‐standards‐manual‐monographs/pediatric‐exclusivity‐study‐age‐group
- 19. ICD ‐ ICD‐10‐CM ‐ International Classification of Diseases, Tenth Revision, Clinical Modification. Accessed April 3, 2022. https://www.cdc.gov/nchs/icd/icd10cm.htm
- 20. PALS Algorithms 2021 Pediatric advanced life support. Accessed April 22, 2022. https://www.acls-pals-bls.com/algorithms/pals/
- 21. Lubin B. Reference values in infancy and childhood (appendix). In: Nathan D, Oski F, eds. Hematology of infancy and childhood. 4th ed. W. B. Saunders Company; 1994. [Google Scholar]
- 22. Meredith LW, Hamilton WL, Warne B, et al. Rapid implementation of SARS‐CoV‐2 sequencing to investigate cases of health‐care associated COVID‐19: a prospective genomic surveillance study. Lancet Inf Dis. 2020;20:1263‐1271. doi: 10.1016/S1473-3099(20)30562-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ministerio de Salud de Chile . Informe Epidemiológico N°21 Vigilancia Genómica de SARS‐CoV‐2 (COVID‐19); 2021. Accessed April 22, 2022. https://www.minsal.cl/wp-content/uploads/2022/01/Informe_Variantes-21.pdf
- 24. Information for Healthcare Providers about Multisystem Inflammatory Syndrome in Children (MIS‐C) | CDC. Accessed April 22, 2022. https://www.cdc.gov/mis/mis-c/hcp/index.html
- 25. Nalbandian A, Sehgal K, Gupta A, et al. Post‐acute COVID‐19 syndrome. Nat Med. 2021;27(4):601‐615. doi: 10.1038/s41591-021-01283-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Borg G. Psychophysical scaling with applications in physical work and the perception of exertion. Scand J Work Env Health. 1990;16(SUPPL. 1):55‐58. doi: 10.5271/sjweh.1815 [DOI] [PubMed] [Google Scholar]
- 27. Williams CA, Oades PJ. Exercise and respiratory diseases in paediatrics. 1st ed. Taylor and Francis; 2021. doi: 10.4324/9781003020462 [DOI] [Google Scholar]
- 28. World Bank Country and Lending Groups – World Bank Data Help Desk. Accessed March 26, 2022. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519‐world‐bank‐country‐and‐lending‐groups
- 29. Kain J, Leyton B, Baur L, Lira M, Corvalán C. Demographic, social and health‐related variables that predict Normal‐weight preschool children having overweight or obesity when entering primary education in Chile. Nutrients. 2019;11(6):1277. doi: 10.3390/NU11061277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Harwood R, Yan H, Da Camara NT, et al. Which children and young people are at higher risk of severe disease and death after hospitalisation with SARS‐CoV‐2 infection in children and young people: A systematic review and individual patient meta‐analysis. eClinicalMedicine. 2022;44:101287. 10.1016/J.ECLINM.2022.101287/ATTACHMENT/5575E4E1-954C-4A89-9EA4-70E293B98E55/MMC1.DOCX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Audibert G, Bannay A, Ziegler O. Obesity paradox in ICU? A topic of discussion, not a key issue! Int J Obes. 2022;46:1248‐1249. doi: 10.1038/s41366-022-01099-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van Son J, Oussaada SM, Şekercan A, et al. Overweight and obesity are associated with acute kidney injury and acute respiratory distress syndrome, but not with increased mortality in hospitalized COVID‐19 patients: a retrospective cohort study. Front Endocrinol. 2021;12:1661. doi: 10.3389/FENDO.2021.747732/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Peters U, Suratt BT, Bates JHT, Dixon AE. Beyond BMI: obesity and lung Disease. Chest. 2018;153(3):702‐709. doi: 10.1016/J.CHEST.2017.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gleeson LE, Roche HM, Sheedy FJ. Obesity, COVID‐19 and innate immunometabolism. Br J Nutr. 2021;125(6):1‐632. doi: 10.1017/S0007114520003529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Maurya R, Sebastian P, Namdeo M, Devender M, Gertler A. COVID‐19 severity in obesity: leptin and inflammatory cytokine interplay in the link between high morbidity and mortality. Front Immunol. 2021;12:2349. doi: 10.3389/FIMMU.2021.649359/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Musuuza JS, Watson L, Parmasad V, Putman‐Buehler N, Christensen L, Safdar N. Prevalence and outcomes of co‐infection and superinfection with SARS‐CoV‐2 and other pathogens: a systematic review and meta‐analysis. PLoS ONE. 2021;16(5):e0251170. doi: 10.1371/JOURNAL.PONE.0251170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kaul D, Rathnasinghe R, Ferres M, et al. Microbiome disturbance and resilience dynamics of the upper respiratory tract during influenza a virus infection. Nat Commun. 2020;11(1):2537. doi: 10.1038/S41467-020-16429-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Manolis AS, Manolis AA, Manolis TA, Apostolaki NE, Melita H. COVID‐19 infection and body weight: a deleterious liaison in a J‐curve relationship. Obes Res Clin Pract. 2021;15(6):523‐535. doi: 10.1016/J.ORCP.2021.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Radtke T, Ulyte A, Puhan MA, Kriemler S. Long‐term symptoms after SARS‐CoV‐2 infection in children and adolescents. JAMA. 2021;326(9):869‐871. doi: 10.1001/JAMA.2021.11880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Say D, Crawford N, McNab S, Wurzel D, Steer A, Tosif S. Post‐acute COVID‐19 outcomes in children with mild and asymptomatic disease. Lancet Child Adolesc Health. 2021;5(6):e22‐e23. doi: 10.1016/S2352-4642(21)00124-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Buonsenso D, Munblit D, de Rose C, et al. Preliminary evidence on long COVID in children. Acta Paediatrica (Oslo, Norway: 1992). 2021;110(7):2208‐2211. doi: 10.1111/APA.15870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Aminian A, Bena J, Pantalone KM, Burguera B. Association of obesity with postacute sequelae of COVID‐19. Diabetes Obes Metab. 2021;23(9):2183‐2188. doi: 10.1111/DOM.14454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jahn K, Sava M, Sommer G, et al. Exercise capacity impairment after COVID‐19 pneumonia is mainly caused by deconditioning. Eur Respir J. 2021;59(1):2101136. doi: 10.1183/13993003.01136-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Földi M, Farkas N, Kiss S, et al. Visceral adiposity elevates the risk of critical condition in COVID‐19: a systematic review and meta‐analysis. Obesity (Silver Spring). 2021;29(3):521‐528. doi: 10.1002/OBY.23096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Watanabe M, Caruso D, Tuccinardi D, et al. Visceral fat shows the strongest association with the need of intensive care in patients with COVID‐19. Metabolism. 2020;111:154319. doi: 10.1016/J.METABOL.2020.154319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pranata R, Lim MA, Huang I, et al. Visceral adiposity, subcutaneous adiposity, and severe coronavirus disease‐2019 (COVID‐19): systematic review and meta‐analysis. Clin Nutr ESPEN. 2021;43:163‐168. doi: 10.1016/J.CLNESP.2021.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
APPENDIX S1 Supporting Information


