Abstract
Exposure of humans to Shiga toxins (Stxs) is a risk factor for hemolytic-uremic syndrome (HUS). Because Stx-producing Escherichia coli (STEC) is a noninvasive enteric pathogen, the extent to which Stxs can cross the host intestinal epithelium may affect the risk of developing HUS. We have previously shown that Stxs can induce and superinduce IL-8 mRNA and protein in intestinal epithelial cells (IECs) in vitro via a ribotoxic stress response. We used cytokine expression arrays to determine the effect of Stx1 on various C-X-C chemokine genes in IECs. We observed that Stx1 induces multiple C-X-C chemokines at the mRNA level, including interleukin-8 (IL-8), GRO-α, GRO-β, GRO-γ, and ENA-78. Like that of IL-8, GRO-α and ENA-78 mRNAs are both induced and superinduced by Stx1. Furthermore, Stx1 induces both IL-8 and GRO-α protein in a dose-response fashion, despite an overall inhibition in host cell protein synthesis. Stx1 treatment stabilizes both IL-8 and GRO-α mRNA. We conclude that Stxs are able to increase mRNA and protein levels of multiple C-X-C chemokines in IECs, with increased mRNA stability at least one mechanism involved. We hypothesize that ribotoxic stress is a pathway by which Stxs can alter host signal transduction in IECs, resulting in the production of multiple chemokine mRNAs, leading to increased expression of specific proteins. Taken together, these data suggest that exposing IECs to Stxs may stimulate a proinflammatory response, resulting in influx of acute inflammatory cells and thus contributing to the intestinal tissue damage seen in STEC infection.
Exposure of humans to Shiga toxins (Stxs) made by Stx-producing Escherichia coli (STEC) is a risk factor for the development of hemolytic-uremic syndrome (HUS). HUS is a systemic disease characterized by thrombotic microangiopathy in affected organs, including the kidney, brain, and gastrointestinal tract. It is believed that HUS is caused by the effects of Stxs on the microvascular endothelium and other cellular components of affected tissues. Because STEC is noninvasive, the extent to which Stxs can cross the host intestinal epithelium may contribute significantly to the risk of HUS. While some of the STEC surface and secreted proteins that interact with host intestinal epithelial cells (IECs) have been described, the STEC-IEC interaction is by no means fully understood (23, 28). We are interested primarily in the factors that promote the systemic absorption of Stx from the gut.
Recently, based on histological and clinical reports concerning STEC-infected patients (12, 17, 29, 32), it has been appreciated that an inflammatory response occurs frequently during STEC infection, resulting in neutrophils in the intestinal lumen. The role of Stxs in inducing a host inflammatory response at the intestinal epithelium has not been clearly defined. However, there are both clinical data and in vitro studies implicating Stx in the stimulation of the host inflammatory response. Proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin-8 (IL-8) have been detected systemically in some patients with diarrhea-associated HUS (15, 37). In vitro, Stx1 induced the expression of TNF-α, IL-1β, and IL-6 from murine peritoneal macrophages (35) and IL-1β, TNF-α, IL-6, and IL-8 from human monocytes (38). In animal models, some early studies of Stx effects in adult rabbit intestinal loops failed to demonstrate that Stxs promoted an inflammatory response (16, 18). However, in an infant rabbit model of STEC infection, Stx2 produced histologic changes in the mid- to distal colon similar to those produced by E. coli O157:H7 infection, including lamina propria neutrophil infiltration with occasional crypt abscesses (25). More recently, in a rabbit model of STEC-induced hemorrhagic colitis, animals infected with a rabbit enteropathogenic E. coli strain engineered to produce Stx1 (RDEC H19A) developed more-severe intestinal inflammation than rabbits infected with the non-Stx1-producing parent RDEC-1 strain (3). Differences observed included increased fluid secretion, increased histopathological inflammatory changes, and elevated mucosal IL-1β levels in the rabbits infected with the Stx1-producing strain compared to those in rabbits infected with the non-Stx1-producing parent strain, suggesting that Stx1 itself, either directly or indirectly, has a significant role in promoting inflammation.
Neutrophil transmigration across the polarized intestinal epithelium with resultant accumulation in the gastrointestinal lumen occurs in many inflammatory diseases of the gastrointestinal tract, including STEC infection (32). The migration process results in transient epithelial barrier disruption with subsequent leakage of luminal contents into the systemic circulation (22, 27). An in vitro model of neutrophil movement across the intestinal epithelium has been developed, and the process is associated with increases in paracellular permeability (26). Using this model, we have demonstrated that apical-to-basolateral movement of Stx1 and Stx2 increases during the process of neutrophil migration from the basolateral surface to the apical chamber (12a).
Human IECs are the first line of defense following host intestinal colonization with enteric pathogens. It has previously been shown that bacterial invasion of human IECs in vitro results in the coordinated upregulation of a number of proinflammatory genes, especially those of the C-X-C chemokine family including the IL-8, GRO-α, and ENA-78 genes (42). The C-X-C chemokines, such as IL-8, are involved in the chemoattraction and activation of neutrophils. The production of IL-8 by epithelial cells in response to bacterial infection is believed to be involved in the recruitment of neutrophils from the endothelium to the intestine (21). A similar response can be seen if IECs are treated with proinflammatory cytokines such as IL-1 and TNF-α, which may be produced by intestinal mucosa leukocytes such as lamina propria macrophages. These data suggest that an important role of the IEC may be to provide chemoattractant signals for polymorphonuclear leukocyte migration in response to enteric pathogens. Furthermore, IEC-produced chemokines are thought to be important mediators of intercellular communication between the epithelium and immune and inflammatory cells in the adjacent and underlying mucosa (reviewed in reference 7).
Stxs are heterodimers consisting of one enzymatically active A subunit and a complex of five B subunits. The A subunit has been shown to be a single-site RNA N-glycosidase for the 28S rRNA of the mammalian ribosome (8). Recent in vitro studies from our laboratory suggest that Stxs have other properties besides interruption of mRNA translation and may affect the expression of certain host primary-response genes, in part via the mitogen/stress-activated protein kinase pathway(s) such as the p38 pathway (36). We have previously shown that Stxs can induce and superinduce IL-8 mRNA and protein in IECs via a ribotoxic stress response (36). As part of this ribotoxic stress response, host signal transduction is altered by Stxs with upregulation of the p38 mitogen-activated protein kinase pathway and induction of transcriptional activator genes c-fos and c-jun. The ribotoxic stress response is a generalized response, and we hypothesized that Stxs might influence other primary-response gene mRNAs in IECs. Furthermore, we hypothesized that induction of these mRNAs might occur through a common pathway.
In the present study we investigated the effect of Stx1 on the expression of various C-X-C chemokines and found a wide-ranging positive response. We also determined that Stx1 caused enhanced mRNA stability for specific C-X-C chemokine transcripts. These data indicate that Stxs have effects on cells that could contribute to pathogenesis beyond the conventional protein synthesis-inhibitory and cytotoxicity mechanisms associated with these toxins.
MATERIALS AND METHODS
Materials and solutions.
Cell culture media and additives were purchased from GibcoBRL-Life Technologies (Grand Island, N.Y.). Stxs were prepared as described previously (6). Stx1 and Stx2 stocks were made by diluting lyophilized Stxs in cell culture medium at 100 μg/ml. To heat-inactivate the toxins, an aliquot of active reconstituted toxin was set aside and a paired sample was inactivated by boiling it in a water bath for 8 h. Boiled toxin was checked for biological activity and did not inhibit [3H]leucine incorporation in Vero cells (14). Cytokine expression arrays, expression array reagents, and GRO-α enzyme-linked immunosorbent assay (ELISA) kits were obtained from R&D Systems (Minneapolis, Minn.). IL-8 ELISA kits, TNF-α, and IL-1β were purchased from Endogen (Woburn, Mass,). Stock solutions of TNF-α and IL-1β were made at concentrations of 10 and 5 μg/ml, respectively, and frozen at −80°C until use. Actinomycin stock solution was freshly prepared on the day of the experiment by dissolving actinomycin D (Calbiochem, La Jolla, Calif.) in distilled water at a final concentration of 250 μg/ml. [3H]leucine, [α-33P]dCTP, and [α-32P]dCTP were purchased from New England Nuclear (Boston, Mass.). QIAshredder cell homogenization spin columns and RNeasy kits were obtained from Qiagen Inc. (Santa Clarita, Calif.). Northern blotting gel preparation and transfer reagents and nylon membranes were obtained from Ambion, Inc. (Austin, Tex.). Lysis buffer for making cell lysates consisted of a pH 7.8 to 8.0 buffered solution of 75 mM potassium phosphate, 55 mM Tris, 2.25 mM MgCl2, 750 μM dithiothreitol, 0.5 μg of antipain/ml, 0.5 μg of pepstatin A/ml, and 12.5 μg of leupeptin/ml to which 1% Triton X-100 had been added. Protease inhibitors were obtained from Sigma Chemicals (St. Louis, Mo.).
Inhibition of protein synthesis.
The effect of Stxs on protein synthesis was determined by measuring [3H]leucine incorporation as previously described (14). Briefly, following the various cell treatments, cell supernatants were removed and cells were washed and then incubated for 60 min in leucine-free medium to which 1 mCi of [3H]leucine/100 ml was added. Incorporation of the label into trichloroacetic acid-precipitable material was then measured.
Cytokine expression array preparation.
HCT-8 cells were obtained from the American Type Culture Collection and cultured as previously described (36). For the arrays, total RNA was isolated, RNA concentration was assessed by measuring optical density at 260 and 280 nm (OD260/280), and cytokine expression arrays and array reagents were used according to the manufacturer's instructions. Briefly, to make cDNA probes for the cytokine expression arrays, 2 μg of total RNA from cell preparations was annealed to cytokine-specific primers and radiolabeled cDNA was synthesized using avian myeloblastosis virus reverse transcriptase in the presence of [α-33P]dCTP. Incorporation of the radiolabel was determined. Paired expression arrays were probed with radiolabeled cDNA from either Stx1-treated cells or control cells (treated with heat-inactivated Stx1). Total counts per minute were adjusted so that similar amounts of radiolabeled cDNA were added to all arrays. Paired arrays were washed and exposed to film. Autoradiographs were scanned using an Agfa II scanner. The intensity of signal corrected for background was determined for each gene on both Stx1-treated and control arrays using QuantityOne software (Bio-Rad, Hercules, Calif.). Comparison of signal intensity between Stx1-treated cells and control cells is expressed as the quotient of corrected intensity of signal from the Stx1-treated cell arrays and control cell array. Results are expressed as a ratio between expression of the mRNA of interest in Stx1-treated cells and that in control cells.
Northern blotting.
For Northern blotting, 10 to 30 μg of total RNA was separated on glyoxal agarose gels and transferred to nylon membranes according to the manufacturer's instructions. IL-8 and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) probes were used as previously described (36). The GRO-α template was made as a 250-bp fragment by PCR as previously described (33). The ENA-78 template was made as a 189-bp fragment as previously described (42). The GRO-α and ENA-78 PCR products were then inserted into a plasmid vector by TA cloning according to the manufacturer's instructions (Invitrogen, Carlsbad, Calif.), and insertion was verified by sequencing. The fragments were released by EcoRI digestion, and DNA probes were synthesized by random priming and labeled with [α-32P]dCTP. Blots were hybridized overnight at 65°C, washed, and detected as previously described using phosphate-based hybridization and wash solutions (4).
mRNA stability.
We initially determined a transcription-inhibitory concentration of actinomycin D for HCT-8 cells by measuring inhibition of protein synthesis in HCT-8 cells after a 6-h exposure to various doses of actinomycin D as follows. Cells were plated in 96-well plates as previously described (36). The following day, dilutions of actinomycin D from 0 to 100 μg/ml were made in cell culture media and added to triplicate wells. After 6 h of incubation, supernatants were removed and protein synthesis was determined by measuring [3H]leucine incorporation as described above. At 2.5 μg of actinomycin D/ml, [3H]leucine incorporation was not affected, but at 5 and 10 μg of actinomycin D/ml [3H]leucine incorporation was diminished to 71 and 57% of control levels, respectively. Above 10 μg/ml, increasing doses of actinomycin D had no further effect on [3H]leucine incorporation. We chose 10 μg of actinomycin D/ml as the lowest dose most likely to block new gene transcription in the time frame we wanted to investigate (0 to 6 h).
HCT-8 cells were plated in six-well plates and allowed to grow overnight, by which time they were approximately 90 to 100% confluent. Stx1 (1 μg/ml), TNF-α (10 ng/ml), or IL-1β (5 ng/ml) was then added by diluting stock solutions of these agents directly into cell culture media on the cells. After 5 h, RNA was harvested from a set of cells treated with each stimulus. The time of this initial harvest was designated time zero. Actinomycin D stock solution was then added directly to the remainder of the cells to a final concentration of 10 μg/ml. Total RNA was harvested as described above at various times after addition of actinomycin D. Northern blots were prepared and probed as described above.
Determination of GRO-α and IL-8 protein by ELISA.
For protein experiments, HCT-8 cells were plated in 96-well plates and grown overnight to approximately 90 to 100% confluence. Duplicate plates were prepared. Cells were exposed to Stx 1 in a range of concentrations from 100 ng/ml to 100 μg/ml, to heat-inactivated Stx 1 in the same dose range, or to TNF-α at 10 ng/ml. Cell culture supernatants were collected from one plate after 18 h, and IL-8 and GRO-α protein concentrations were determined by ELISA according to the manufacturer's instructions. There was no cross-reactivity of IL-8 and GRO-α in these assay kits (data not shown). Cells on this plate were used to prepare lysates to determine intracellular IL-8 and GRO-α. HCT-8 cell lysates were prepared by exposing cells to a buffered 1% Triton X-100 solution with protease inhibitors as described above. Cells were incubated at 4°C with lysis buffer for 10 min, and then lysates were diluted as necessary and IL-8 and GRO-α ELISAs were performed on simultaneous samples. To have sufficient material to perform both ELISAs on the same sample, duplicate wells were pooled for a total of 2 × 105 plated cells contributing to chemokine synthesis. Pooled wells were prepared for each supernatant and lysate sample at each concentration of Stx1 in triplicate. A second plate, exposed to Stxs in parallel, was used to determine the effect of Stx 1 on overall protein synthesis by measuring [3H]leucine incorporation as described above.
RESULTS
Stx1 affects multiple C-X-C chemokine mRNAs as assessed by cytokine arrays.
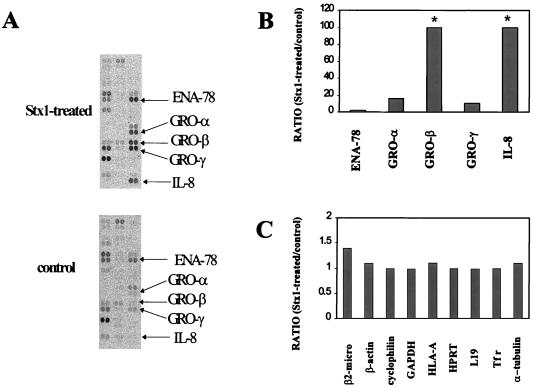
We used commercially available cytokine expression arrays to screen for cytokine and cytokine-related genes induced by Stx treatment of HCT-8 cells. We have previously demonstrated that inhibition of protein synthesis occurs by 2 h following Stx exposure and reaches a relatively steady state by 4 h postexposure (36). Since we are interested in the role of the ribotoxic stress response in inducing other C-X-C chemokines, we selected this time point for study using expression arrays. Figure 1A, top, shows C-X-C chemokine mRNA expression in HCT-8 cells treated with 1 μg of Stx1/ml for 4 h. Figure 1A, bottom, shows C-X-C chemokine mRNA expression in HCT-8 cells treated with heat-inactivated Stx1 (1 μg/ml) for 4 h (control). Duplicate spots are shown for each C-X-C chemokine. Results are expressed in Fig. 1B and C as a ratio between expression of the mRNA of interest in Stx1-treated cells and that in control cells. At this dose of Stx1 (1 μg/ml) the highest C-X-C chemokine inductions were observed for IL-8 and GRO-β (≥100-fold), modest inductions were observed for GRO-α and GRO-γ (16.1- and 9.9-fold, respectively), and a minimal induction was observed for ENA-78 (2.2-fold). Housekeeping gene mRNAs (Fig. 1C) show that the Stx1-treated and control array were similarly probed; housekeeping gene mRNA ratios ranged from 1.0 to 1.4.
FIG. 1.
Effect of Stx1 on the accumulation of various chemokine mRNAs. HCT-8 cells were treated for 4 h with either Stx1 (1 μg/ml) or heat-inactivated Stx1 (controls; 1 μg/ml). Total cellular RNA was isolated, and then radiolabeled cDNA was prepared from total cellular RNA using reverse transcriptase and cytokine-specific primers. Paired expression arrays were probed with radiolabeled cDNA from either Stx1-treated cells or control cells. Paired arrays were washed and exposed to film. Autoradiographs were scanned using an Agfa II scanner. (A) Pertinent sections from autoradiographs. Intensity of signal corrected for background was determined for each labeled chemokine gene on both Stx1-treated and control arrays using QuantityOne software (Bio-Rad). Results (B and C) are expressed as ratios between expression of the chemokine (B) or housekeeping mRNA (C) of interest in Stx1-treated and control cells. Abbreviations: β-2 micro, beta 2-microglobulin; HLA-A, major histocompatibility complex class I lymphocyte antigen (HLA-A 0201); HPRT, hypoxanthine phosphoribosyltransferase; L19, ribosomal protein L19; Tfr, transferrin receptor. Asterisk, ratio that is ≥100.
Stx1 induces and superinduces both GRO-α and ENA-78 mRNA.
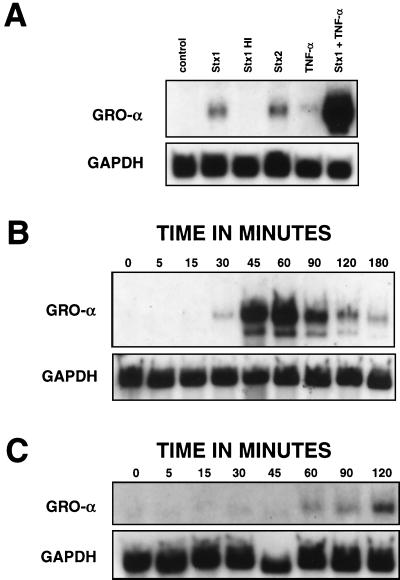
To determine the effects of Stxs on GRO-α, cells were treated with Stx1, Stx2, heat-inactivated Stx1, TNF-α (positive control), or both Stx1 and TNF-α for 5 h. (For this experiment, Stx1 and Stx2 were used at a concentration of 1 μg/ml.) Then, total RNA was isolated and a Northern blot was made from these samples and probed for GRO-α mRNA (Fig. 2A). After 5 h, treatment of cells with Stx1 or Stx2 resulted in accumulation of GRO-α mRNA. As expected, TNF-α treatment of HCT-8 cells resulted in modest expression of GRO-α mRNA after 5 h. When RNA was isolated at various times following TNF-α exposure, GRO-α mRNA began to accumulate by 30 min, was induced to high levels by 45 to 60 min of treatment, and diminished rapidly to very low levels by 3 h of treatment (Fig. 2B). In contrast, when cells were exposed to Stx1, GRO-α mRNA did not appear until approximately 1 h, was present at 2 h, and was still present at 5 h (Fig. 2A and C). Despite these differences in timing of induction, Stx1 can clearly superinduce GRO-α mRNA, as demonstrated in Fig. 2A, where simultaneous treatment of cells with Stx1 and TNF-α resulted in massive amounts of mRNA at 5 h compared to treatment with either substance alone at 5 h.
FIG. 2.
Stx1 induces and superinduces GRO-α and is a slow inducer of GRO-α mRNA compared to TNF-α. Shown are Northern blots from HCT-8 cells following various treatments. (A) HCT-8 cells were treated with Stx1, Stx2, heat-inactivated Stx1, TNF-α, or both Stx1 and TNF-α for 5 h. Stxs were used at 1 μg/ml; TNF-α was used at 5 ng/ml. Total RNA was isolated, and a Northern blot was made from these samples and probed for GRO-α mRNA as well as GAPDH mRNA as a housekeeping gene. (B) HCT-8 cells were treated with TNF-α at 5 ng/ml. Total RNA was harvested at various times following addition of TNF-α as indicated. A Northern blot was made from these samples and probed as for panel A. (C) HCT-8 cells were treated with Stx1 at 1 μg/ml. Total RNA was harvested at various times following addition of Stx1 as indicated. A Northern blot was made from these samples and probed as for panel A.
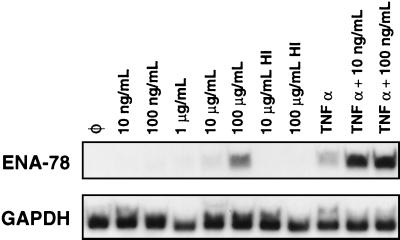
We then focused our attention on ENA-78 mRNA. As shown in Fig. 1, a very small induction of ENA-78 mRNA was demonstrated in the cells exposed to 1 μg of Stx1/ml for 4 h compared to control levels. Assessment of ENA-78 mRNA after longer incubations with this dose of Stx1, up to 24 h, did not show increased ENA-78 (data not shown). We then exposed HCT-8 cells to increasing doses of Stx1. The dose-response curve of Stx1-induced ENA-78 mRNA is shown in Fig. 3. Minimal increases in ENA-78 mRNA can be detected in HCT-8 cells exposed to Stx1 at a dose of 1 μg/ml, consistent with the cytokine expression array data presented in Fig. 1. However, Stx1 doses of 100 μg/ml resulted in significant inductions of ENA-78 mRNA. Similar doses of heat-inactivated Stx1 did not induce ENA-78. Interestingly, although low doses of Stx1 (10 and 100 ng/ml) are not inducers of ENA-78 mRNA, these same low doses of Stx1 caused marked superinduction of ENA-78 when used concomitantly with TNF-α (Fig. 3). We have observed similar results for GRO-α and IL-8 superinduction (data not shown). Furthermore, similar doses of heat-inactivated Stx1 did not superinduce ENA-78 (data not shown).
FIG. 3.
Stx1 can superinduce ENA-78 mRNA at doses lower than those required for induction. Shown is a Northern blot of HCT-8 cells following various treatments probed for ENA-78 mRNA. HCT-8 cells were exposed for 5 h to doses of Stx1 ranging from 10 ng/ml to 100 μg/ml, heat-inactivated Stx1 at 10 or 100 μg/ml, TNF-α, or TNF-α and Stx1 at 10 or 100 ng/ml. Total RNA was harvested, and a Northern blot was made from these samples and probed for ENA-78 mRNA and GAPDH mRNA as a housekeeping gene. HI, heat inactivated; Ø, no additives.
Stx1 stimulates IL-8 and GRO-α protein synthesis at different doses.
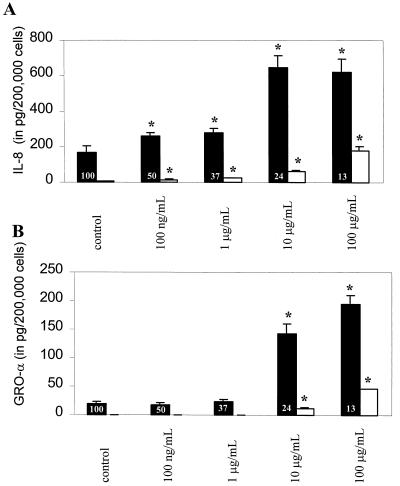
Cytokine expression arrays and Northern blots are useful tools to assess changes in cytokine gene mRNA in response to a stimulus of interest but may not reflect actual synthesis of protein, especially in the presence of protein synthesis inhibitors such as Stxs. For this reason we then assessed whether the GRO-α protein, like IL-8, was expressed in response to Stx1 treatment. After exposing HCT-8 cells to Stx1 in various concentrations for approximately 18 h, IL-8 and GRO-α protein concentrations in the cell supernatants and cell lysates were determined by ELISA. The dose responses of IL-8 and the GRO-α protein are clearly different as shown in Fig. 4. In the experiment shown, unstimulated HCT-8 cells secreted 170.1 ± 31.8 pg of IL-8 per 2 × 105 cells in cell supernatants and 5.6 ± 2.1 pg of IL-8 per 2 × 105 cells in cell lysates (Fig. 4A). Increasing concentrations of Stx1 resulted in increasing amounts of IL-8 secreted into the supernatants as well as increasing amounts of IL-8 present in cell lysates, despite an overall decrease in cell protein synthesis. These data are consistent with our previously published data on IL-8 secretion in HCT-8 cells (36), although the present study reveals the presence of the IL-8 protein in both supernatants and lysates, confirming that Stx1 induces synthesis of new IL-8 proteins. IL-8 protein expression is significantly higher than control at all doses of Stx1 used, from 100 ng/ml to 100 μg/ml. We have previously shown that the IL-8 protein response is absent if Stx1 is inactivated by boiling (36).
FIG. 4.
Stx1 induces synthesis of IL-8 and GRO-α protein in HCT-8 cells. (A) IL-8 protein was measured by ELISA in both supernatants (black bars) and lysates (white bars) of Stx1-treated HCT-8 cells. (B) GRO-α protein was measured by ELISA in both supernatants (black bars) and lysates (white bars) of Stx1-treated HCT-8 cells. Separate measurements were done in 6 to 12 wells for both IL-8 and GRO-α supernatants and lysates. The data are mean amounts of protein per 2 × 105 cells plated and standard deviations from 6 to 12 wells per condition from a single representative experiment. Overall protein synthesis as measured at the end of the Stx1 incubation is shown as a percentage of control levels, and the average of triplicate wells is inset at the base of each black bar for each dose of Stx1. Asterisk, P value <0.05 compared with control.
While Stx1 also increased GRO-α protein synthesis, the pattern was different (Fig. 4B). Unstimulated HCT-8 cells secreted 19.3 ± 3.4 pg of GRO-α per 2 × 105 cells into cell supernatants and 0.1 ± 0.2 pg of GRO-α per 2 × 105 cells into cell lysates. Although Stx1 also increased GRO-α protein expression in a dose-dependent way, the differences between levels of GRO-α protein produced by unstimulated cells and those in either supernatants or lysates produced by stimulated cells were not significant until cells were exposed to 10 μg of Stx1/ml, a dose 100-fold higher than that observed for IL-8. As was seen for IL-8, this response is absent if Stx1 is inactivated by boiling (data not shown). These data have been confirmed in separate experiments.
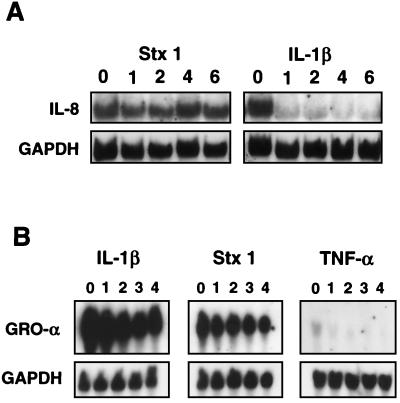
Stx1 stabilizes IL-8 and GRO-α mRNA.
We next determined if mRNA stability contributes to the increase in C-X-C chemokine mRNA seen with Stx treatment. We used actinomycin D, which binds to double-stranded DNA and prevents transcription, to determine the degradation kinetics of Stx-induced IL-8 and GRO-α mRNA. We compared the stability of IL-8 and GRO-α mRNAs following Stx treatment of HCT-8 cells with the stability of mRNAs obtained following treatment with either TNF-α or IL-1β, known inducers of IL-8 and GRO-α. Figure 5A shows IL-8 mRNA following treatment with Stx1 or IL-1β for 5 h prior to the addition of actinomycin D (time zero). At time zero, actinomycin D was added directly to the media, and RNA was obtained at various intervals up to 6 h.
FIG. 5.
Stx1 stabilizes IL-8 mRNA, whereas IL-1β does not, and the two stabilize GRO-α mRNA similarly. After 5 h of exposure to Stx1, TNF-α, or IL-1β, total RNA was harvested. The time of this initial harvest was time zero. Actinomycin D was then added directly to culture media for a final concentration of 10 μg/ml. Total RNA was harvested at various times (hours) after addition of actinomycin D as shown above each lane. Northern blots were prepared from these samples. (A) Northern blot probed for IL-8 mRNA and GAPDH mRNA as a housekeeping gene; (B) Northern blot probed for GRO-α mRNA and GAPDH mRNA as a housekeeping gene.
In IL-1β-treated cells, IL-8 mRNA is present at time zero and diminishes rapidly over the next 1 to 2 h after actinomycin D treatment. In contrast, the IL-8 mRNA detected following Stx1 treatment persists until the end of the experiment at 6 h. Control samples showed that actinomycin D treatment alone does not induce IL-8 mRNA (data not shown). TNF-α also increases IL-8 mRNA (36), but the mRNA is not stabilized and diminishes significantly over the next hour (data not shown).
Similarly, in TNF-α-treated cells, GRO-α mRNA is present at time zero and diminishes rapidly over the next 1 to 2 h (Fig. 5B). In contrast, the GRO-α mRNA present following Stx1 treatment persists until the end of the experiment at 4 h. IL-1β was used as a positive control for GRO-α stability, as it has been shown to stabilize GRO-α mRNA in fibroblasts (33, 34). We have found the same in HCT-8 cells (Fig. 5B).
DISCUSSION
In 1988, Stxs were reported to be irreversible inhibitors of mammalian protein synthesis based on their activity as single-site RNA N-glycosidases for the 28S rRNA of the mammalian ribosome (8). For many years, it was assumed that Stxs' only relevant biological activity was the destruction of cells through inhibition of protein synthesis. Then, in 1996 we showed that Stx treatment of T-84 cells, an IEC line with crypt-like characteristics, leads to an increase in expression of chemokine IL-8 (1), an observation that was somewhat paradoxical in view of the known biological actions of the toxin. We have further established that Stxs are capable of inducing and superinducing expression of IL-8 in HCT-8 cells at both the protein and mRNA levels (36). Stx-induced IL-8 expression appears to be related to protein synthesis inhibition and can be reproduced in this cell line by other protein synthesis inhibitors that act on the ribosome, such as anisomycin and ricin. This phenomenon is similar to the ribotoxic stress response, which has been described for other protein synthesis inhibitors acting via sequence-specific damage to the 28S rRNA, whereby host mitogen-activated protein kinases are activated and c-fos and c-jun mRNA is induced (13). Yamasaki et al. have recently reported that, in IEC line CaCo-2, Stx1 and Stx2 could induce IL-8 expression at both the mRNA and protein levels. Furthermore, a mutant Stx1 with alterations in glutamate 167 and arginine 170 (residues located in the ribosome binding N-glycosidase region of the active site) did not induce IL-8 (41). These data support the hypothesis that ribosomal intoxication with Stxs is required for induction of IL-8.
Genes whose mRNA induction is refractory to translational blockade are called primary-response genes. This family includes many genes involved in regulation of the inflammatory response, such as genes encoding growth factors, cytokines, adhesion molecules, and transcriptional activators. Associating Stx induction of IL-8 expression with a ribotoxic stress response in IECs allowed us to hypothesize that this type of generalized alteration in cell signal transduction by Stxs may involve multiple host inflammatory-response genes in the intestinal epithelium, not just the IL-8 gene. In the present study, we used commercially available cytokine expression arrays to screen for Stx1-induced changes in cytokine gene mRNAs occurring simultaneously with IL-8 mRNA induction. We demonstrate that multiple members of the C-X-C chemokine family are induced in response to Stx1, including GRO-α, GRO-β, GRO-γ, and ENA-78. Although it may be an interesting question with respect to STEC pathogenesis, the importance of the relative levels of induction of the various C-X-C chemokines by Stx1 was not a focus of this study, although these differences may be interesting to study in future work. One caveat to the use of gene expression arrays is that mRNA expression does not necessarily imply protein expression, especially in the setting of a protein synthesis inhibitor. Nevertheless, we have also shown that, as with IL-8, Stx1 does in fact induce the GRO-α protein.
Many primary-response genes, in addition to not requiring protein synthesis for induction, are inducible by translational inhibitors such as cycloheximide; they also exhibit massively elevated mRNA levels when exposed to an agonist in the presence of translational blockade, a phenomenon called superinduction (5, 19, 20). We demonstrate that GRO-α mRNA, like IL-8 mRNA, is induced and superinduced in response to Stx1 treatment. Superinduction of GRO-α in response to concomitant treatment with Stx1 and TNF-α is shown. How protein synthesis inhibitors cause induction and superinduction of primary-response genes is unknown, but several mechanisms have been proposed (9, 10, 11). In one recent study, cycloheximide was shown to increase IL-8 mRNA in lung epithelial cells by modifying both transcriptional activator activity and mRNA stability (30). We present here evidence of increased mRNA stability in Stx1-induced GRO-α.
While TNF-α-induced GRO-α mRNA peaks at approximately 45 to 60 min and then diminishes by 2 to 3 h after exposure, Stx1-induced GRO-α mRNA steadily accumulates beginning 60 min after exposure. We further show that both GRO-α and IL-8 mRNA stability is increased in response to Stx1 compared to their mRNA stability in response to other proinflammatory cytokines. Taken together, these data suggest that Stx1 may induce the stabilization of both IL-8 and GRO-α mRNA and that mRNA stabilization accounts, at least in part, for the superinduction of GRO-α mRNA in response to combined treatment with Stx1 and TNF-α after 5 h.
It should be noted that these data on Stx1-induced mRNA stabilization do not preclude transcriptional activation as a possible comechanism for induction or superinduction of either IL-8 or GRO-α mRNA. While there is no direct evidence that transcriptional activation occurs in response to Stxs, in a differentiated monocytic cell line Stx1 induction of TNF-α on both the mRNA and protein levels has been linked to AP-1 and NF-κB translocation to the nucleus (31). However, another group has demonstrated that preproendothelin-1 mRNA, the precursor mRNA of the endothelin-1 primary-response gene, accumulates in an endothelial cell line in response to Stx1 and Stx2 treatment in cells through enhanced mRNA stability (2). In their study, nuclear run-on data suggested that transcriptional activation did not occur in this cell line in response to Stxs. If transcriptional activation is involved in induction of C-X-C chemokines in IECs in response to Stxs, this response may prove to be a complex one. Although NF-κB appears to be an important regulatory element for IL-8, GRO-α, GRO-β, GRO-γ, and ENA-78, for both IL-8 and GRO-α a number of other transcriptional activators are required for maximal expression (24, 39, 40).
Interestingly, ENA-78 mRNA is not induced by Stx1 doses likely to be found in the gastrointestinal tract during STEC infection (estimated to be in the range of nanograms per milliliter to micrograms per milliliter based on ELISA of stool Stx in human infection [unpublished observations]). Although induction does not occur until very high doses of Stx1 are used, ENA-78 can be superinduced at much lower doses of Stx1 in the presence of proinflammatory cytokine TNF-α. It has been proposed that Stxs may locally induce TNF-α secretion from resident macrophage-like cells (35). Therefore, during STEC infection proinflammatory cytokines such as TNF-α may also be expressed locally from gastrointestinal lamina propria macrophages or systemically. In this setting, even if IECs are exposed to doses of Stx in the gut lumen that are too low for simple induction, Stxs may increase certain C-X-C gene mRNA levels in IECs via superinduction effects with proinflammatory cytokines such as TNF-α.
As shown by the lysate data, unstimulated HCT-8 cells do not store large amounts of either IL-8 or the GRO-α protein. In HCT-8 cells, inhibition of protein synthesis occurs after 4 h of exposure to Stxs and precedes IL-8 synthesis. Therefore, Stx1 treatment results in the synthesis of new IL-8 proteins, and this is true for the GRO-α protein as well. Paradoxically, as Stx dose increases, production of IL-8 and the GRO-α protein also increases despite the fact that the host cell's overall mRNA translation is clearly diminished, as demonstrated by incorporation of radiolabeled leucine into trichloroacetic acid-precipitable material. These data suggest that preferential translation of certain mRNAs may occur as a result of Stx1 treatment, although the mechanisms underlying translational initiation in the setting of Stx treatment are as yet unknown.
It has been shown that the intestinal epithelium responds to bacterial invasion or cytokine stimulation by upregulating the expression of a program of neutrophil chemotactic genes, resulting in rapid induction of GRO-α, GRO-γ, and IL-8, followed by a slower, more sustained induction of ENA-78 (42). Our data are consistent with the hypothesis that Stx1 upregulates a program of neutrophil chemotactic genes in HCT-8 cells. It is possible that Stx is responsible for stimulating the host inflammatory response in vivo that allows a noninvasive enteric infection to be associated with gastrointestinal pathology usually seen only with invasive organisms. Furthermore, Stx-induced chemokines synthesized in the intestinal mucosa and submucosa may allow enhanced and sustained recruitment of neutrophils, with subsequent compromise of the intestinal barrier, which we have demonstrated leads to increased Stx uptake in vitro.
ACKNOWLEDGMENTS
Research support for this study included the following grants from the National Institutes of Health, Bethesda, Md.: A1-39067 (D.W.K.A.), A1-01715 (C.M.T.), and P30DK-34928 for the Center for Gastroenterology Research on Absorptive and Secretory Processes. C.M.T. is also supported by the Charles H. Hood Foundation.
REFERENCES
- 1.Acheson D W K. Effect of Shiga toxins on cytokine production from intestinal epithelial cells. In: Keusch G T, Kawakami M, editors. Cytokines, cholera, and the gut. Amsterdam, The Netherlands: IOS Press; 1996. pp. 67–73. [Google Scholar]
- 2.Bitzan M M, Wang Y, Lin J, Marsden P A. Verotoxin and ricin have novel effects of preproendothelin-1 expression but fail to modify nitric oxide synthase (ecNOS) expression and NO production in vascular endothelium. J Clin Investig. 1998;101:372–382. doi: 10.1172/JCI522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blake D C I, Russell R G, Santini E, Bowen T, Boedeker E C. Pro-inflammatory mucosal cytokine responses to Shiga-like toxin-1 (SLT-1) In: Keusch G T, Kawakami M, editors. Cytokines, cholera, and the gut. Amsterdam, The Netherlands: IOS Press; 1996. pp. 75–82. [Google Scholar]
- 4.Church G M, Gilbert W. Genomic sequencing. Proc Natl Acad Sci USA. 1984;81:1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cochran B H, Refuel A C, Stiles C D. Molecular cloning of gene sequences regulated by platelet derived growth factor. Cell. 1983;33:939–947. doi: 10.1016/0092-8674(83)90037-5. [DOI] [PubMed] [Google Scholar]
- 6.Donohue-Rolfe A, Jacewicz M, Keusch G T. Isolation and characterization of functional Shiga toxin subunits and renatured holotoxin. Mol Microbiol. 1989;3:1231–1236. doi: 10.1111/j.1365-2958.1989.tb00273.x. [DOI] [PubMed] [Google Scholar]
- 7.Dwinell M B, Eckmann L, Leopard J D, Varki N M, Kagnoff M F. Chemokine receptor expression by human intestinal epithelial cells. Gastroenterology. 1999;117:359–367. doi: 10.1053/gast.1999.0029900359. [DOI] [PubMed] [Google Scholar]
- 8.Endo Y, Tsurugi K, Yatsudo T, Takeda Y, Ogasawara T, Igarashi K. Site of action of a verotoxin (VT2) from E. coli O157:H7 and of Shiga toxin on eukaryotic ribosomes: RNA N-glycosidase activity of the toxin. Eur J Biochem. 1988;171:45–50. doi: 10.1111/j.1432-1033.1988.tb13756.x. [DOI] [PubMed] [Google Scholar]
- 9.Faggioli L, Constanzo C, Merola M, Furia A, Palmieri M. Protein synthesis inhibitors cycloheximide and anisomycin induce interleukin-6 gene expression and activate transcription factor NF-κB. Biochem Biophys Res Commun. 1997;233:507–513. doi: 10.1006/bbrc.1997.6489. [DOI] [PubMed] [Google Scholar]
- 10.Fort P, Rech J, Vie A, Piechaczyk M, Bonnieu A, Jeanteur P, Blanchard J-M. Regulation of c-fos gene expression in hamster fibroblasts: initiation and elongation of transcription and mRNA degradation. Nucleic Acids Res. 1987;15:5657–5667. doi: 10.1093/nar/15.14.5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenberg M E, Hermanowski A L, Ziff E B. Effect of protein synthesis inhibitors on growth factor activation of c-fos, c-myc, and actin gene transcription. Mol Cell Biol. 1986;6:1050–1057. doi: 10.1128/mcb.6.4.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffin P M, Olmstead L C, Petras R E. Escherichia coli O157:H7-associated colitis. Gastroenterology. 1990;99:142–149. doi: 10.1016/0016-5085(90)91241-w. [DOI] [PubMed] [Google Scholar]
- 12a.Hurley B P, Thorpe C M, Acheson D W K. Shiga toxin translocation across intestinal epithelial cells is enhanced by neutrophil transmigration. Infect Immun. 2001;69:6148–6155. doi: 10.1128/IAI.69.10.6148-6155.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iordanov M, Pribnow M D, Magun J J, Dinh T-H, Pearson J A, Chen S L-Y, Magun B E. Ribotoxic stress response: activation of the stress-activated protein kinase JNK1 by inhibitors of the peptidyl transferase reaction and by sequence-specific damage to the α-sarcin loop in the 28S rRNA. Mol Cell Biol. 1997;17:3373–3381. doi: 10.1128/mcb.17.6.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacewicz M, Feldman H A, Donohue-Rolfe A, Balasubramanian K A, Keusch G T. Pathogenesis of Shigella diarrhea. XIV. Analysis of Shiga toxin receptors on cloned HeLa cells. J Infect Dis. 1989;159:881–889. doi: 10.1093/infdis/159.5.881. [DOI] [PubMed] [Google Scholar]
- 15.Karpman D, Andreasson A, Thysell H, Kaplan B S, Svanborg C. Cytokines in childhood hemolytic uremic syndrome and thrombotic thrombocytopenia purpura. Pediatr Nephrol. 1995;9:694–699. doi: 10.1007/BF00868714. [DOI] [PubMed] [Google Scholar]
- 16.Keenan K P, Sharpnack D D, Collins H, Formal S B, O'Brien A D. Morphologic evaluation of the effects of Shiga toxin and E. coli Shiga-like toxin on the rabbit intestine. Am J Pathol. 1986;125:69–80. [PMC free article] [PubMed] [Google Scholar]
- 17.Kelly J K, Pai C H, Jadusingh I H, Macinnes M L, Shaffer E A, Hershfield N B. The histopathology of rectosigmoid biopsies from adults with bloody diarrhea due to verotoxin-producing Escherichia coli. Am J Clin Pathol. 1987;88:78–82. doi: 10.1093/ajcp/88.1.78. [DOI] [PubMed] [Google Scholar]
- 18.Keusch G T, Grady G F, Takeuchi A, Sprinz H. The pathogenesis of Shigella diarrhea. II. Enterotoxin-induced acute enteritis in the rabbit ileum. J Infect Dis. 1972;126:92–95. doi: 10.1093/infdis/126.1.92. [DOI] [PubMed] [Google Scholar]
- 19.Lau L, Nathans D. Expression of a set of growth-regulated immediate early genes in BALB/c 3T3 cells: coordinate regulation with c-fos or c-myc. Proc Natl Acad Sci USA. 1987;84:1182–1186. doi: 10.1073/pnas.84.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahadevan L C, Edwards D R. Signalling and superinduction. Nature (London) 1991;349:747–748. doi: 10.1038/349747c0. [DOI] [PubMed] [Google Scholar]
- 21.McCormick B A, Hofman P M, Kim J, Carnes D K, Miller S I, Madara J L. Surface attachment of Salmonella typhimurium to intestinal epithelia imprints the subepithelial matrix with gradients chemotactic for neutrophils. J Cell Biol. 1995;131:1599–1608. doi: 10.1083/jcb.131.6.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nash S, Stafford J, Madara J L. Effects of polymorphonuclear leukocyte transmigration on barrier function of cultured intestinal epithelial monolayers. J Clin Investig. 1987;10:1104–1113. doi: 10.1172/JCI113167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nataro J P, Kaper J B. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okamoto S, Mukaida N, Yasumoto K, Horiguchi H, Matsushima K. Molecular mechanism of interleukin-8 gene expression. In: Lindley I J D, et al., editors. The chemokines. New York, N.Y: Plenum Press; 1993. pp. 87–97. [DOI] [PubMed] [Google Scholar]
- 25.Pai C H, Kelly J K, Meyers G L. Experimental infection of infant rabbits with verotoxin-producing Escherichia coli. Infect Immun. 1986;51:16–23. doi: 10.1128/iai.51.1.16-23.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parkos C A, Delp C, Arnaout M A, Madara J L. Neutrophil migration across a cultured intestinal epithelium. Dependence on a CD11b/CD18 mediated event and enhanced efficiency in a physiological direction. J Clin Investig. 1991;88:1605–1612. doi: 10.1172/JCI115473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parsons P E, Sugahara K, Cott G R, Mason R J, Henson P M. The effect of neutrophil migration and prolonged neutrophil contact on epithelial permeability. Am J Pathol. 1987;129:302–312. [PMC free article] [PubMed] [Google Scholar]
- 28.Paton J C, Paton A W. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin Microbiol Rev. 1998;11:450–479. doi: 10.1128/cmr.11.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richardson S E, Karmali M A, Becker L E, Smith C R. The histopathology of the hemolytic uremic syndrome associated with verocytotoxin-producing Escherichia coli infections. Hum Pathol. 1998;19:1102–1108. doi: 10.1016/s0046-8177(88)80093-5. [DOI] [PubMed] [Google Scholar]
- 30.Roger T, Out T, Mukaida N, Matsushima K, Jansen H, Lutter R. Enhanced AP-1 and NF-kB activities and stability of interleukin 8 (IL-8) transcripts are implicated in IL-8 mRNA superinduction in lung epithelial H292 cells. Biochem J. 1998;330:429–435. doi: 10.1042/bj3300429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakiri R, Ramegowda B, Tesh V L. Shiga toxin type 1 activates tumor necrosis factor-α gene transcription and nuclear translocation of the transcriptional activators nuclear factor-κB and activator protein-1. Blood. 1998;92:558–566. [PubMed] [Google Scholar]
- 32.Slutsker L, Ries A A, Greene K D, Wells J G, Hutwagner L, Griffin P M. Escherichia coli O157:H7 diarrhea in the United States: clinical and epidemiologic features. Ann Intern Med. 1997;126:505–513. doi: 10.7326/0003-4819-126-7-199704010-00002. [DOI] [PubMed] [Google Scholar]
- 33.Stoeckle M Y. Post-transcriptional regulation of groα, β, γ, and IL-8 mRNAs by IL-1β. Nucleic Acids Res. 1991;19:917–920. doi: 10.1093/nar/19.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stoeckle M Y. Removal of a 3′ non-coding sequence is an initial step in the degradation of groα mRNA and is regulated by interleukin-1. Nucleic Acids Res. 1992;20:1123–1127. doi: 10.1093/nar/20.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tesh V L, Ramegowda B, Samuel J E. Purified Shiga-like toxins induce expression of proinflammatory cytokines from murine peritoneal macrophages. Infect Immun. 1994;62:5085–5094. doi: 10.1128/iai.62.11.5085-5094.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thorpe C M, Hurley B P, Lincicome L L, Jacewicz M S, Keusch G T, Acheson D W K. Shiga toxins stimulate secretion of interleukin-8 from intestinal epithelial cells. Infect Immun. 1999;67:5985–5993. doi: 10.1128/iai.67.11.5985-5993.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Setten P, van Hinsbergh V, van den Heuvel L, Preyers F, Dijkman H, Assmann K, van der Velden T, Monnens L. Monocyte chemoattractant protein-1 and interleukin-8 levels in urine and serum of patients with hemolytic uremic syndrome. Pediatr Res. 1998;43:759–767. doi: 10.1203/00006450-199806000-00008. [DOI] [PubMed] [Google Scholar]
- 38.van Setten P A, Monnens L A H, Verstraten R G G, van den Heuvel L P W J, Hinsbergh V W M. Effects of verocytotoxin-1 on nonadherent human monocytes: binding characteristics, protein synthesis, and induction of cytokine release. Blood. 1996;88:174–183. [PubMed] [Google Scholar]
- 39.Wood L D, Farmer A A, Richmond A. HMGI(Y) and Sp1 in addition to NF-κB regulate transcription of the MGSA/GROα gene. Nucleic Acids Res. 1995;23:4210–4219. doi: 10.1093/nar/23.20.4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wood L D, Richmond A. Constitutive and cytokine-induced expression of the melanoma growth stimulatory activity/GROα requires both NF-κB and novel constitutive factors. J Biol Chem. 1995;270:30619–30626. doi: 10.1074/jbc.270.51.30619. [DOI] [PubMed] [Google Scholar]
- 41.Yamasaki C, Natori Y, Zheng X-T, Ohmura M, Yamasaki S, Takeda Y, Natori Y. Induction of cytokines in a human colonic epithelial cell line by Shiga toxin 1 (Stx1) and Stx2 but not by a non-toxic mutant Stx1 which lacks N-glycosidase activity. FEBS Lett. 1999;442:231–234. doi: 10.1016/s0014-5793(98)01667-6. [DOI] [PubMed] [Google Scholar]
- 42.Yang S K, Eckmann L, Panja A, Kagnoff M F. Differential and regulated expression of C-X-C, C-C, and C-chemokines by human colon epithelial cells. Gastroenterology. 1997;113:1214–1223. doi: 10.1053/gast.1997.v113.pm9322516. [DOI] [PubMed] [Google Scholar]