Abstract
The systemic and respiratory clinical manifestations of coronavirus disease 2019 (COVID‐19) include fever, coughing, sneezing, sore throat, rhinitis, dyspnea, chest pain, malaise, fatigue, anorexia and headache. Moreover, cutaneous manifestations have been reported in 0.2% to 20.4% of cases. Early diagnosis of COVID‐19 leads to a better prognosis; knowledge of its cutaneous manifestations is one way that may help fulfil this goal. In this review, PubMed and Medline were searched with the terms “dermatology”, “skin” and “cutaneous”, each in combination with “SARS‐CoV‐2” or “COVID‐19”. All articles, including original articles, case reports, case series and review articles published from the emergence of the disease to the time of submission, were included. In this comprehensive narrative review, we tried to provide an analysis of the cutaneous manifestations associated with COVID‐19, including maculopapular rash, urticaria, Chilblain‐like, vesicular lesions, livedo reticularis and petechiae in asymptomatic/symptomatic COVID‐19 patients that might be the first complication of infection after respiratory symptoms. Immune dysregulation, cytokine storms, side effects of antiviral drugs, environmental conditions and high‐dose intravenous immunoglobulin (IVIG) therapy might be involved in the pathogenesis of the cutaneous manifestations in COVID‐19 patients. Therefore, knowledge of cutaneous COVID‐19 manifestations might be vital in achieving a quick diagnosis in some COVID‐19 patients, which would help control the pandemic. Further research is very much warranted to clarify this issue.
Keywords: chilblain‐like, COVID‐19, cutaneous manifestations, livedo reticularis, maculopapular rash, petechiae, SARS‐CoV‐2, urticaria, vesicular lesions
1. INTRODUCTION
As of early November 2021 until the writing of this manuscript, more than 6 500 000 deaths out of 581 million confirmed cases of coronavirus disease 2019 (COVID‐19) have been reported worldwide. In March 2020, the World Health Organization declared the disease a pandemic considering its high infection rate, high mortality rate and asymptomatic transmission among the elderly and immunocompromised individuals. 1
The agent responsible for COVID‐19 is severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Belonging to the Coronaviridae family, 2 this betacoronavirus is believed to have been transmitted from bats to humans at a food market in Wuhan, China; nonetheless, the key mode of spread among humans is through respiratory droplets and aerosols, 3 , 4 with oral‐faecal transmission also being documented. 5 , 6
Infection of SARS‐CoV‐2 occurs through angiotensin‐converting enzyme 2 (ACE‐2) receptors. 3 , 7 , 8 These receptors are found on numerous mucosal sites, including gastrointestinal epithelium, the endothelium of dermal blood vessels, 9 , 10 and epithelial cells in eccrine glands – potentially explaining the disease's cutaneous manifestations. 11
In an analysis of 31 different human tissues, Li et al. showed that the small intestine, heart, thyroid, kidneys and adipose tissues had the highest levels of ACE2 expression. In contrast, medium levels of ACE2 expression were seen in the blood cells, spleen, bone marrow, brain, blood vessel walls, lungs, colon, liver, bladder and adrenal glands, while the muscles had the least amount of ACE2 expression. 12 Xue et al. analysed the general domain dataset (GEPIA2 and ARCHS4) to detect whether the skin is a potential target for SARS‐CoV‐2 infection according to ACE2 mRNA expression and ACE2‐positive cell composition in the cutaneous system. Among the various cellular components in skin tissues, the majority of cells (97.37%) are keratinocytes, with sweat gland cells coming next at 2.63%. Extensive expression of ACE2 receptors in keratinocytes suggests that SARS‐CoV‐2 can infect tissues other than those of the pulmonary system, giving rise to extrapulmonary manifestations. 12 , 13
Initially, the manifestations of Wuhan COVID‐19 were reported to resemble other viral respiratory diseases and included dry cough, anorexia, headache and fever. 14 Later, it became apparent that SARS‐CoV‐2 might lead to subclinical infection or clinical disease (COVID‐19). 15 In those with COVID‐19, signs and symptoms include cough, fever, dyspnea, anosmia, ageusia, diarrhoea and even cutaneous rashes and lesions. 14 , 16 Although cutaneous manifestations are limited in paucisymptomatic or asymptomatic patients, they may help in diagnosing and controlling the disease, especially in areas where diagnostic kits are not readily available. 17 In asymptomatic patients, cutaneous features may be the first sign of SARS‐CoV‐2 infection. 11
The cutaneous manifestations of COVID‐19 may be because of direct viral effects, though the secondary immune response is also a possible suspect. Most histopathological data have been attained from small case series, although skin lesion biopsies in children have rarely been described. 18
This review article aimed to collect data on the diverse range of cutaneous manifestations reported in COVID‐19 patients. Furthermore, we attempted to classify the manifestations into six major groups: maculopapular rashes, urticaria, Chilblain‐like lesions, vesicular lesions, livedo reticularis and petechiae. In addition, we discussed the features of each of these groups in depth.
2. CUTANEOUS MANIFESTATIONS
2.1. Maculopapular rashes
Maculopapular rashes are the predominant skin manifestations of COVID‐19. 19 These lesions mainly occur either because of viral infections or as side effects of the administered drugs. 20 , 21
In one study, among 375 COVID‐19 patients with cutaneous lesions, roughly 47% (176/375) had maculopapular lesions. 22 The prevalence of these lesions ranges from 5% to 70%, with most localised lesions being seen on the trunk. Middle‐aged and elderly individuals account for most cases, 22 , 23 , 24 , 25 though young people are also affected. 26
Although most studies indicate that maculopapular lesions appear after systemic manifestations in COVID‐19 patients, 23 , 24 , 25 , 27 one study noted the simultaneous onset of skin and systemic manifestations in a large Spanish population. 22 The mean duration of skin exanthemas in COVID‐19 patients ranges from 8.6 to 11.6 days. 22 , 23 , 24 In rashes that emerge early on, epidermal spongiosis is seen along with lymphocytic and eosinophilic perivascular infiltration of the dermis. 25 In general, this type of rash is more common in the active stage of the disease. 19 In contrast, lesions that emerge later on show infiltration of lymphocytes and histiocytes in between collagen fibres; such lesions lack mucin deposits. 28 A histological study by Reymundo et al. also confirmed a mild degree of superficial perivascular infiltration with lymphocytes (Figure 1A). 24 In one case series, over half of the patients with maculopapular lesions experienced pruritus. In that study, these lesions were linked with increased COVID‐19 severity in light of a 2% mortality rate. 22 In a study in China, out of 67 neonates born from women with COVID‐19 during pregnancy, two developed skin rashes. 29
FIGURE 1.
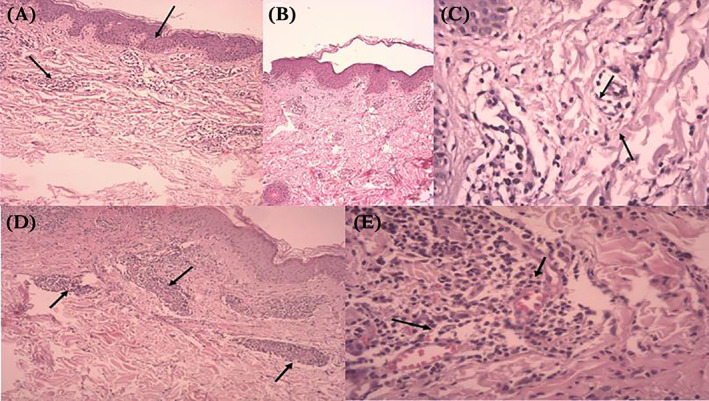
Histopathology of the main cutaneous patterns in COVID‐19 patients. (A) Maculopapular rash, Skin biopsy showed epidermal spongiosis(blue arrow), lymphocytic exocytosis, dermal infiltrate of eosinophils, lymphocytes and histocytosis (black arrow). (B) and (C) Urticaria, Skin biopsy showed superficial dermal edema, dilatation of small blood vessels with few eosinophils infiltration (blue arrow). (D) and (E) Vasculitis, Skin biopsy showed inflammatory infiltrate in wall of dermal vessels (blue arrow), red blood cell extravasation (red arrow), viriable fibrinoid necrosis of vessel walls.
Several theories have been proposed to explain the pathogenesis of maculopapular rashes. Galván Casas et al. described them as useless in diagnosing COVID‐19 given that they may arise as reactions to drugs. This may also explain the link of these rashes with severe disease, in which case drug therapy is more intense. 22 Skin side effects, including maculopapular and morbilliform rashes, are known to occur because of drugs administered to COVID‐19 patients, including ribavirin, colchicine, intravenous immunoglobulin (IVIG), lopinavir, ritonavir and other antiretroviral agents. 30 However, the drawback to this theory is that it does not explain the maculopapular lesions seen in patients who were not on therapy with any drugs. 24 Alternatively, Herrero‐Moyano et al. suggested that the cytokine storm resulting from immune hyperactivity is responsible for late‐onset maculopapular lesions. 23 Other notable hypotheses are summarised in Table 1. In most cases, topical corticosteroids are sufficient for treating these maculopapular lesions; however, systemic corticosteroids may be prescribed in more severe and extensive cases. 31
TABLE 1.
Potential causes of different cutaneous lesions
| Cutaneous lesion | Potential causes |
|---|---|
| Maculopapular rashes | Hyperactive immune system (cytokine storm); antiviral drug use (lopinavir, ritonavir, colchicine, IVIG); viral infections (measles, rubella); viral exanthema; scarlet fever; medication reaction; secondary syphilis; leukaemia; graft‐versus‐host disease |
| Urticaria | Parasitic, bacterial, or viral infections; IgE‐mediated allergic reactions; a side effect of antiviral drugs (lopinavir, ritonavir, colchicine, IVIG); cytokine storm; allergic reaction; anaphylaxis; angioedema; autoimmune disease (eg, systemic lupus erythematosus); hypereosinophilia; chronic urticaria; malignancy |
| Vesicles | Autoimmunity; heat; dermatitis (contact or infectious); immune system hyperactivity (cytokine storm); drugs; viral infections (varicella‐zoster or herpes simplex); Rhus dermatitis (eg, poison ivy, poison oak, or poison sumac); pemphigoid |
| Chilblain‐likes | Hematologic disease; neoangiogenesis; autoimmune disease; viral infection; vessel thrombosis; environmental conditions (humid environment or cold temperature); vasculitis; Raynaud's phenomenon; systemic lupus erythematosus; systemic sclerosis; Berger's disease |
| Petechiae/purpura | Immune system hyperactivity (cytokine storm); complement component disorders; thrombocytopenia; systemic lupus erythematosus; leukaemia; disseminated intravascular coagulation; hemolytic uremic syndrome; thrombotic thrombocytopenic purpura; vasculitis; vitamin C deficiency |
| Livedo racemosa | Diminished blood flow; disseminated intravascular coagulation; Macro thromboses; diminished deoxygenated haemoglobin; antiphospholipid antibody syndrome; Sneddon syndrome; cryoglobulinemia; multiple myeloma; disseminated intravascular coagulation; hemolytic uremic syndrome; deep venous thrombosis; systemic lupus erythematosus; rheumatoid arthritis; polyarteritis nodosa; Sjogren's syndrome; multiple sclerosis; Parkinson's disease; cancer (eg, renal cell cancer, breast cancer, lymphoma, or leukaemia) |
2.2. Urticaria
The second most prevalent skin rash in COVID‐19 patients is urticarial. 19 Similar to maculopapular eruptions, urticaria have been described in several COVID‐19 case series. These erythematous lesions manifest in the form of hives and angioedema accompanied by intense pruritus. 32
In COVID‐19 patients, urticaria is considered as one of the most frequent cutaneous manifestations. However, these lesions were fairly common prior to the COVID‐19 pandemic as they can occur because of common stimuli (viral/bacterial/parasitic infections and environmental allergens) and IgE‐mediated allergic reactions to drugs, foods, or insects. 33 A study in Spain reported that most cases were recorded during the active phase of SARS‐CoV‐2 infection. 19 In smaller case series, the prevalence of these lesions has varied from 7% to 40%. Although urticarial lesions seem most common in middle‐aged COVID‐19 patients, 22 , 26 a French study on 26 patients reported a median age of just 3 years old. 34
In terms of distribution, many reports describe urticaria on the trunk or limbs of COVID‐19 patients. 22 , 26 , 35 In some cases, the rash was distributed to the face or generalised across the entire body. 26 , 36 Some reports indicate that urticarial lesions appear prior to systematic manifestations, 22 , 37 though others suggest a simultaneous onset as well as a seven‐day duration. 22 In the study by Galván Casas et al., urticarial lesions were associated with more severe COVID‐19, and pruritus was reported in 92% of patients. 22 Although few histopathological studies have been reported concerning urticarial rashes, one study on a female COVID‐19 patient 32 years of age showed perivascular lymphocyte infiltration with scarce eosinophils and superficial dermal edema. 38
Drugs like lopinavir/ritonavir, hydroxychloroquine, chloroquine, corticosteroids, nitazoxanide, baricitinib, IVIG and checkpoint inhibitors are known to cause urticarial. 30 Therefore, in many cases, urticaria might be the side effect of different drugs used to treat COVID‐19 patients. 22 Another possible aetiology is immune hyperactivity, where a cytokine storm may affect the skin and give rise to urticarial. 39 Last, direct viral activity should not be overlooked as viral infections have been a primary cause of urticaria in the past. 22 Other notable hypotheses about the cause of urticarial lesions are presented in Table 1.
For treating urticaria, Shansal et al. suggested that controlling the overactive immune system in COVID‐19 patients using low‐dose corticosteroids and non‐sedating antihistamines might be useful. 40 As a result of the extreme etiological variation, it appears that urticarial lesions cannot be used as markers that accurately diagnose COVID‐19 (Figure 1B and 1C). 22
3. CHILBLAIN LIKE LESIONS
Chilblain‐like lesions (also called pernio) result from an inflammatory disorder localised to the skin, where discoloration and swelling of the limbs occur following exposure to humid environments or cold temperatures. The temporal association with viral symptoms, coupled with the increased incidence of Chilblain‐like lesions, has led to the colloquialization “COVID toes”. 1 This term is used to describe erythematous or violaceous eruptions affecting the toes of COVID‐19 patients, 1 where Chilblain‐like lesions involve the toes/heels of these patients more commonly than the fingers (97.5% versus 2.5%, respectively). 19 Although Chilblain‐like lesions are associated with autoimmune conditions like lupus, they can also occur idiopathically. 41 A retrospective study by Cappel et al. reported a potential association of these lesions with hematologic diseases and hyperviscosity. The rash follows an acral distribution, usually affecting the fingers or toes. 41 Skin lesions in COVID‐19 patients can manifest as nodules, erythematous‐violaceous papules, or macules. 1 Although the pathophysiology behind the development of Chilblain‐likes is not clear, one theory relates them to inflammation and hypoxemia occurring because of cold‐induced vasospasm and vasoconstriction. Other theories implicate autoantibodies or hyperviscosity‐induced endothelial damage. 41
Chilblain‐like lesions are the most well‐known skin manifestations of COVID‐19, partly because of the numerous studies published concerning them. 1 The rate of prevalence varies across the different studies. In a multinational case series of 505 COVID‐19 patients with cutaneous manifestations, Chilblain‐like lesions occurred in 63% of cases. 42 Other studies declared prevalence rates of between 14.3% and 72%. 22 , 26 , 42 , 43 , 44 Adolescents and young adults seem to be affected most by these lesions. 22 , 26 , 45 , 46 , 47 , 48 , 49 , 50 , 51
The onset of Chilblain‐like lesions is usually after systemic COVID‐19 symptoms, and the lesions last for an average of 1 or 2 weeks. 42 In a multinational study, out of 318 cases, most (n = 80) patients with temporal data developed Chilblain‐like lesions after the onset of general COVID‐19 symptoms. 42 In addition, a Spanish study showed that 42 out of 71 patients developed Chilblain‐like skin lesions after the onset of general COVID‐19 symptoms. 22 Interestingly, Chilblain‐likes are seen in half of all cases after complete patient recovery and in approximately 10% of asymptomatic SARS‐CoV‐2 carriers (RT‐PCR positive). 19 In one case series, Chilblain‐like‐like lesions were the only symptom in 55% of patients who manifested cutaneous lesions. 52
Despite the high‐frequency rate, recent investigations failed to link chillblain‐like eruptions with COVID‐19, suggesting that these lesions might not be helpful diagnosing this infectious disease. 53 , 54 Other dermatological symptoms that may accompany Chilblain‐like‐like lesions are pain and pruritus. 22 , 42 , 43 , 51 A large number of COVID‐19 patients who develop Chilblain‐likes are young, healthy adults with only mild disease. 42 The mechanism occurrence of these lesions is not fully understood and is unrelated to cold exposure. Bouaziz et al. proposed that the pathophysiology behind Chilblain‐like could involve immune dysregulation, vasculitis, vessel thrombosis, or neoangiogensis. 55 The researchers believed that the post‐viral or antiviral immune response is responsible, although confounding factors could not be overlooked. 55 This kind of rash is often self‐limited, and standard treatment is not yet available. 31
4. VESICULAR LESIONS
Fluid‐filled sacs situated beneath the epidermis are called vesicular lesions. Commonly referred to as blisters, these lesions are often cluster‐shaped and less than 1 cm in diameter. The most common causes are autoimmunity, heat, medications and contact or infectious dermatitis (bacterial or viral). 1
Viruses like varicella‐zoster, herpes simplex, coxsackievirus and echovirus can cause the formation of vesicular lesions. 19 , 55 These lesions are quite uncommon in COVID‐19 patients relative to those listed in the previous sections. 1 Vesicles appear in about 3.77% to 15% of patients, most of whom are middle‐aged individuals. 2 , 22 , 26 , 56 , 57 The trunk is the predominant site, though the involvement of the extremities is not rare. 2 , 20 , 52 , 53
In a prospective study conducted by Fernandez‐Nieto et al., among 22 patients with vesicular lesions, 25% had a localised pattern of monophyletic lesions, and 75% had a polymorphic pattern of lesions on the trunk. Other studies have identified single‐shaped vesicles located on the trunk. 22 , 36 The timing of the vesicular eruptions in relation to COVID‐19 symptoms has varied in the literature. Although a study from Spain showed a simultaneous pattern, 22 two studies from Italy and China indicated that vesicles appeared subsequent to general COVID‐19 manifestations. 2 , 56 , 57 Vesicular rashes reported from Spain often appeared in the active phase of the disease. 19 Nonetheless, several cases have been recorded where vesicles emerged ahead of the usual disease symptoms. 57
In the study from Spain, a mean duration of just over 8 days was recorded for vesicular rashes. 22 In one Italian study, the average duration was 8 days, while another report found it to be 10 days in patients with moderate COVID‐19. 56 , 57 Generally, these lesions are believed to be linked with moderate COVID‐19 severity. 22 , 57
The pathophysiological mechanism behind vesicular lesions remains to be clarified. Criado et al. noted that vesicular eruptions could result from a cytokine storm caused by an overactive immune system. The same study also hypothesized direct SARS‐CoV‐2 activity against cutaneous endothelial vessels as the cause of vesicles. 39 Etiologically, unlike maculopapular lesions and urticaria, COVID‐19‐associated vesicular lesions are not associated with antiviral drugs or other COVID‐19 treatments. 56 Hence, they have been described as specific skin manifestations of COVID‐19 that are potentially helpful in achieving a diagnosis (Figure 1E and 1F). 1 This kind of skin lesion is often self‐limited, and standard treatment is not yet available. 31
5. PETECHIAE
Petechiae are small, non‐spreading, non‐blanching spots with a diameter of 2 mm or less. 58 If the diameter of these subdermal haemorrhages exceeds the mentioned limit, the lesions are labelled as purpura. Pathophysiological causes include thrombocytopenia, platelet disorders, coagulation disorders and compromised vascular integrity. 58 Some viruses, including enterovirus, parvovirus B19 and dengue virus, are linked with petechiae. 58 It is one of the less frequently described cutaneous manifestations of COVID‐19; only 3% of 277 patients with patterned skin lesions had petechiae in one retrospective French study. 1 In early studies, petechiae were rarely reported in association with COVID‐19, with only two cases (0.4%), 59 , 60 one of which was initially misdiagnosed as dengue and was mistreated. 19
Petechial lesions might be present on the acral area of the body or on the limbs. 1 One study showed that in most cases, the petechial‐purpuric rash had an acral distribution. 36 The onset of petechiae/purpura lesions was subsequent to general COVID‐19 manifestations, as seen in two prior case series. 28 , 61 Interestingly, De Giorgi et al. linked diffuse petechiae and generalised palpable purpura with increased COVID‐19 severity. 62 In line with this finding, a Spanish case series found that palpable purpura lesions, present in 4 of 34 cases (11.8%), appeared predominantly in middle‐aged patients recovering from severe COVID‐19. Notably, two of the four patients also had atypical polymorphic papulovesicular lesions, and another of these patients also had urticarial. 25 In a report on five COVID‐19 patients who suffered from severe respiratory failure, three had a purpuric skin rash. 63
Pauciinflammatory thrombogenic vasculopathy is the proposed pathogenesis for petechiae/purpura skin lesions. A study by Megro et al. demonstrated the widespread deposition of C5b‐8 and C4d complement components in the dermal microvasculature of both non‐lesioned and lesioned skin by immunohistochemistry (IHC). SARS‐CoV‐2 spike glycoproteins could sometimes be seen colocalized with those complement components. 61 Drugs used in the treatment of COVID‐19 may also be implicated in the development of these lesions. 1 The use of high‐dose IVIG can induce petechiae as a side effect; purpura might be an adverse effect of camostat mesylate as a possible anti‐COVID‐19 agent. 30 Last, like some other viruses, SARS‐CoV‐2 might cause petechiae. 1 Topical corticosteroids are usually sufficient for the treatment of maculopapular rashes; however, systemic corticosteroids might be prescribed in more severe and extensive cases. 31
6. LIVEDO RETICULARIS
The term livedo reticularis refers to reticular, reddish‐blue to purple, mottled skin discolorations. 1 It can be transient or persistent and affects both genders almost equally, with lesions generally being asymptomatic. 19 This dermatosis occurs as a result of spasms of blood vessels, which diminish the delivery of oxygen to the skin. Benign livedoid eruptions that occur idiopathically or as a result of physiological conditions (eg, cutis marmorata) are named livedo reticularis, but those that develop as a result of pathological conditions are termed livedo racemose. 63 livedo racemose has a more extensive surface distribution, as well as an interrupted and irregular shape relative to livedo reticularis. 63 Livedoid eruptions represent one of the least encountered cutaneous manifestations reported during the COVID‐19 pandemic. In a study of 375 confirmed COVID‐19 patients with cutaneous manifestations, only 6% had livedoid eruptions. 22
In COVID‐19 patients, livedoid lesions have been documented on the trunk, forearms (flexor surface) and dorsal aspect of the hand and foot. 22 , 55 , 64 Temporarily, the lesions appeared simultaneously with other COVID‐19 symptoms, mostly affecting elderly patients with more severe disease. The mean duration of livedoid lesions was almost 9.4 days, and the mortality rate among patients with livedoid lesions was 10% ‐ higher than all other skin presentations. 22
The exact pathogenesis of livedoid lesions in COVID‐19 patients is not understood. However, one theory suggests that the hypercoagulable state seen in COVID‐19 may be implicated. In one retrospective investigation on 183 COVID‐19 patients, those who fell victim to the disease had higher serum concentrations of D‐dimer and fibrin degradation products as well as a longer prothrombin time. This suggests that those with severe COVID‐19, associated with livedoid lesions, have a higher risk of coagulation abnormalities. 65 Manulo et al. hypothesized that disseminated intravascular coagulation (DIC) and macrothromboses might be involved in the development of livedo reticularis in more severe cases of infection; in less severe cases of COVID‐19, microthrombi that form as a result of inflammatory cytokines or ACE2 entry into cells may be implicated. 66 This kind of rash is often self‐limited, and standard treatment is yet to be available. 31
7. CONCLUSION
Although the main clinical presentation of COVID‐19 patients is respiratory, other clinical manifestations such as cutaneous lesions might be present. Cutaneous features include maculopapular rashes, urticaria, Chilblain‐like lesions, vesicular lesions, livedo reticularis and petechiae, all of which can emerge as the sign of SARS‐CoV‐2 infection in asymptomatic/symptomatic COVID‐19 patients. The lesions might be manifested at the beginning of the disease, after antibiotic treatment, or after other clinical manifestations have subsided. Therefore, knowledge of cutaneous COVID‐19 manifestations might be vital in achieving a quick diagnosis in some COVID‐19 patients, which would help control the pandemic. Amidst a limited amount of data, it appears that the largest body organ may provide vital clues concerning the diagnosis and prognosis of COVID‐19. Further research is very much warranted as a comprehensive understanding of these cutaneous manifestations is yet to be achieved.
FUNDING INFORMATION
This research received no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
ACKNOWLEDGEMENTS
The authors thank Research Consultation Center (RCC) for improving the article's writing.
Arefinia N, Ghoreshi Za, Alipour AH, et al. A comprehensive narrative review of the cutaneous manifestations associated with COVID‐19. Int Wound J. 2023;20(3):871‐879. doi: 10.1111/iwj.13933
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in [repository name] at [URL/DOI], reference number [reference number]. These data were derived from the following resources available in the public domain: [list resources and URLs].
REFERENCES
- 1. Singh H, Kaur H, Singh K, Sen CK. Cutaneous manifestations of COVID‐19: A systematic review. Adv Wound Care. 2021;10(2):51‐80. doi: 10.1089/wound.2020.1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marzano AV, Cassano N, Genovese G, Moltrasio C, Vena GA. Cutaneous manifestations in patients with COVID‐19: a preliminary review of an emerging issue. Br J Dermatol. 2020;183(3):431‐442. doi: 10.1111/bjd.19264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mustafa NM, Selim AL. Characterisation of COVID‐19 pandemic in Paediatric age group: A systematic review and meta‐analysis. J Clin Virol. 2020;128:104395. doi: 10.1016/j.jcv.2020.104395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xiao F, Sun J, Xu Y, et al. Infectious SARS‐CoV‐2 in feces of patient with severe COVID‐19. Emerg Infect Dis J. 2020;26(8):1920. doi: 10.3201/eid2608.200681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pirofski L, Casadevall A. Pathogenesis of COVID‐19 from the perspective of the damage‐response framework. MBio. 2020;11(4):e01175‐e01120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abdel‐Mannan O, Eyre M, Löbel U, et al. Neurologic and radiographic findings associated with COVID‐19 infection in children. JAMA Neurol. 2020;77(11):1440‐1445. doi: 10.1001/jamaneurol.2020.2687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Health Policy Team . COVID‐19 ‐ guidance for management of children admitted to hospital | RCPCH. Rcpch 2020. 2020. Accessed October 10, 2022. https://www.rcpch.ac.uk/resources/covid‐19‐guidance‐management‐children‐admitted‐hospital#investigations [Google Scholar]
- 9. Jiang L, Tang K, Levin M, et al. COVID‐19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis. 2020;20(11):e276‐e288. doi: 10.1016/S1473-3099(20)30651-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dhochak N, Singhal T, Kabra SK, Lodha R. Pathophysiology of COVID‐19: why children fare better than adults? Indian J Pediatr. 2020;87(7):537‐546. doi: 10.1007/s12098-020-03322-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lavery MJ, Bouvier CA, Thompson B. Cutaneous manifestations of COVID‐19 in children (and adults): A virus that does not discriminate. Clin Dermatol. 2021;39(2):323‐328. doi: 10.1016/j.clindermatol.2020.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li MY, Li L, Zhang Y, Wang XS. Expression of the SARS‐CoV‐2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9(1):45. doi: 10.1186/s40249-020-00662-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xue X, Mi Z, Wang Z, Pang Z, Liu H, Zhang F. High expression of ACE2 on keratinocytes reveals skin as a potential target for SARS‐CoV‐2. J Invest Dermatol. 2021;141(1):206‐209.e1. doi: 10.1016/j.jid.2020.05.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID‐19) outbreak. J Autoimmun. 2020;109:102433. doi: 10.1016/j.jaut.2020.102433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang X, Chen L, Wei J, Zhou J, Cao Y, Wang G. Asymptomatic subclinical cases of coronavirus disease 2019 without viral transmission in three independent families. Infect Drug Resist. 2020;13:3267‐3271. doi: 10.2147/IDR.S261304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lechien JR, Chiesa‐Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild‐to‐moderate forms of the coronavirus disease (COVID‐19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277(8):2251‐2261. doi: 10.1007/s00405-020-05965-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lancet T. COVID‐19: learning from experience. Lancet. 2020;395(10229):1011. doi: 10.1016/S0140-6736(20)30686-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hoang A, Chorath K, Moreira A, et al. COVID‐19 in 7780 pediatric patients: A systematic review. EClinicalMedicine. 2020;24:100433. doi: 10.1016/j.eclinm.2020.100433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rahimi H, Tehranchinia Z. A comprehensive review of cutaneous manifestations associated with COVID‐19. Biomed Res Int. 2020;2020:1‐8. doi: 10.1155/2020/1236520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ely JW, Seabury SM. The generalized rash: Part I. Differential diagnosis. Am Fam Physician. 2010;81(6):726‐734. [PubMed] [Google Scholar]
- 21. Ely JW, Seabury SM. The generalized rash: Part II. Diagnostic approach. Am Fam Physician. 2010;81(6):735‐739. [PubMed] [Google Scholar]
- 22. Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID‐19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183(1):71‐77. doi: 10.1111/bjd.19163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Herrero‐Moyano M, Capusan TM, Andreu‐Barasoain M, et al. A clinicopathological study of eight patients with COVID‐19 pneumonia and a late‐onset exanthema. J Eur Acad Dermatology Venereol. 2020;34(9):e460‐e464. doi: 10.1111/jdv.16631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reymundo A, Fernáldez‐Bernáldez A, Reolid A, et al. Clinical and histological characterization of late appearance maculopapular eruptions in association with the coronavirus disease 2019. A case series of seven patients. J Eur Acad Dermatol Venereol. 2020;34(12):e755‐e757. doi: 10.1111/jdv.16707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rubio‐Muniz CA, Puerta‐Peña M, Falkenhain‐López D, et al. The broad spectrum of dermatological manifestations in COVID‐19: clinical and histopathological features learned from a series of 34 casesfile:///D:/Dr.nasir/article/corona virus/review/درماتولوژیک/3.Pdf. J Eur Acad Dermatology Venereol. 2020;34(10):e574‐e576. doi: 10.1111/jdv.16734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. de Masson A, Bouaziz JD, Sulimovic L, et al. Chilblains is a common cutaneous finding during the COVID‐19 pandemic: A retrospective nationwide study from France. J Am Acad Dermatol. 2020;83(2):667‐670. doi: 10.1016/j.jaad.2020.04.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bursal Duramaz B, Yozgat CY, Yozgat Y, Turel O. Appearance of skin rash in pediatric patients with COVID‐19: three case presentations. Dermatol Ther. 2020;33(4):e13594. doi: 10.1111/dth.13594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rubio‐Muniz CA, Puerta‐Peña M, Falkenhain‐López D, et al. The broad spectrum of dermatological manifestations in COVID‐19: clinical and histopathological features learned from a series of 34 cases. J Eur Acad Dermatology Venereol. 2020;34(10):e574‐e576. doi: 10.1111/jdv.16734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zimmermann P, Curtis N. COVID‐19 in children, pregnancy and neonates: A review of epidemiologic and clinical features. Pediatr Infect Dis J. 2020;39(6):469‐477. doi: 10.1097/INF.0000000000002700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Türsen Ü, Türsen B, Lotti T. Cutaneous sıde‐effects of the potential COVID‐19 drugs. Dermatol Ther. 2020;33(4):e13476. doi: 10.1111/dth.13476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Genovese G, Moltrasio C, Berti E, Marzano AV. Skin manifestations associated with COVID‐19: current knowledge and future perspectives. Dermatology. 2021;237(1):1‐12. doi: 10.1159/000512932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Diotallevi F, Campanati A, Bianchelli T, et al. Skin involvement in SARS‐CoV‐2 infection: case series. J Med Virol. 2020;92(11):2332‐2334. doi: 10.1002/jmv.26012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Radonjic‐Hoesli S, Hofmeier KS, Micaletto S, Schmid‐Grendelmeier P, Bircher A, Simon D. Urticaria and angioedema: an update on classification and pathogenesis. Clin Rev Allergy Immunol. 2018;54(1):88‐101. doi: 10.1007/s12016-017-8628-1 [DOI] [PubMed] [Google Scholar]
- 34. Since January 2020 Elsevier has created a COVID‐19 resource centre with free information in English and Mandarin on the novel coronavirus COVID‐ 19. The COVID‐19 resource centre is hosted on Elsevier Connect, The company's public news and information. 2020 (January).
- 35. Dalal A, Jakhar D, Agarwal V, Beniwal R. Dermatological findings in SARS‐CoV‐2 positive patients: an observational study from North India. Dermatol Ther. 2020;33(6):e13849. doi: 10.1111/dth.13849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Askin O, Altunkalem RN, Altinisik DD, Uzuncakmak TK, Tursen U, Kutlubay Z. Cutaneous manifestations in hospitalized patients diagnosed as COVID‐19. Dermatol Ther. 2020;33(6):e13896. doi: 10.1111/dth.13896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Henry D, Ackerman M, Sancelme E, Finon A, Esteve E. Urticarial eruption in COVID‐19 infection. J Eur Acad Dermatol Venereol. 2020;34(6):e244‐e245. doi: 10.1111/jdv.16472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fernandez‐Nieto D, Ortega‐Quijano D, Segurado‐Miravalles G, Pindado‐Ortega C, Prieto‐Barrios M, Jimenez‐Cauhe J. Comment on: cutaneous manifestations in COVID‐19: a first perspective. Safety concerns of clinical images and skin biopsies. J Eur Acad Dermatol Venereol. 2020;34(6):e252‐e254. doi: 10.1111/jdv.16470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Criado PR, Abdalla BMZ, de Assis IC, van Blarcum de Graaff Mello C, Caputo GC, Vieira IC. Are the cutaneous manifestations during or due to SARS‐CoV‐2 infection/COVID‐19 frequent or not? Revision of possible pathophysiologic mechanisms. Inflamm Res. 2020;69(8):745‐756. doi: 10.1007/s00011-020-01370-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shanshal M. Low‐ dose systemic steroids, an emerging therapeutic option for COVID‐19 related urticaria. J Dermatolog Treat. 2020;33:1‐2. doi: 10.1080/09546634.2020.1795062 [DOI] [PubMed] [Google Scholar]
- 41. Cappel JA, Wetter DA. Clinical characteristics, etiologic associations, laboratory findings, treatment, and proposal of diagnostic criteria of pernio (chilblains) in a series of 104 patients at Mayo Clinic, 2000 to 2011. Mayo Clin Proc. 2014;89(2):207‐215. doi: 10.1016/j.mayocp.2013.09.020 [DOI] [PubMed] [Google Scholar]
- 42. Freeman EE, McMahon DE, Lipoff JB, et al. Pernio‐like skin lesions associated with COVID‐19: A case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83(2):486‐492. doi: 10.1016/j.jaad.2020.05.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Docampo‐Simón A, Sánchez‐Pujol MJ, Juan‐Carpena G, et al. Are chilblain‐like acral skin lesions really indicative of COVID‐19? A prospective study and literature review. J Eur Acad Dermatol Venereol. 2020;34(9):e445‐e447. doi: 10.1111/jdv.16665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Duong TA, Velter C, Rybojad M, et al. Did Whatsapp(®) reveal a new cutaneous COVID‐19 manifestation? J Eur Acad Dermatol Venereol. 2020;34(8):e348‐e350. doi: 10.1111/jdv.16534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Colonna C, Genovese G, Monzani NA, et al. Outbreak of chilblain‐like acral lesions in children in the metropolitan area of Milan, Italy, during the COVID‐19 pandemic. J Am Acad Dermatol. 2020;83(3):965‐969. doi: 10.1016/j.jaad.2020.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. El Hachem M, Diociaiuti A, Concato C, et al. A clinical, histopathological and laboratory study of 19 consecutive Italian paediatric patients with chilblain‐like lesions: lights and shadows on the relationship with COVID‐19 infection. J Eur Acad Dermatol Venereol. 2020;34(11):2620‐2629. doi: 10.1111/jdv.16682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fernandez‐Nieto D, Jimenez‐Cauhe J, Suarez‐Valle A, et al. Characterization of acute acral skin lesions in nonhospitalized patients: A case series of 132 patients during the COVID‐19 outbreak. J Am Acad Dermatol. 2020;83(1):e61‐e63. doi: 10.1016/j.jaad.2020.04.093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gaspari V, Neri I, Misciali C, Patrizi A. COVID‐19: how it can look on the skin. Clinical and pathological features in 20 COVID‐19 patients observed in Bologna, North‐Eastern Italy. J Eur Acad Dermatol Venereol. 2020;34(10):e552‐e553. doi: 10.1111/jdv.16693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Garcia‐Lara G, Linares‐González L, Ródenas‐Herranz T, Ruiz‐Villaverde R. Chilblain‐like lesions in pediatrics dermatological outpatients during the COVID‐19 outbreak. Dermatol Ther. 2020;33(5):e13516. doi: 10.1111/dth.13516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wollina U, Karadağ AS, Rowland‐Payne C, Chiriac A, Lotti T. Cutaneous signs in COVID‐19 patients: A review. Dermatol Ther. 2020;33(5):e13549. doi: 10.1111/dth.13549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Andina D, Noguera‐Morel L, Bascuas‐Arribas M, et al. Chilblains in children in the setting of COVID‐19 pandemic. Pediatr Dermatol. 2020;37(3):406‐411. doi: 10.1111/pde.14215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Freeman EE, Mcmahon DE, Lipoff JB, Rosenbach M. Since January 2020 Elsevier has created a COVID‐19 resource centre with free information in English and Mandarin on the novel coronavirus COVID‐ company's public news and information website. Elsevier hereby grants permission to make all its COVID‐19‐r. 2020 (January).
- 53. Le Cleach L, Dousset L, Assier H, et al. Most chilblains observed during the COVID‐19 outbreak occur in patients who are negative for COVID‐19 on polymerase chain reaction and serology testing. Br J Dermatol. 2020;183(5):866‐874. doi: 10.1111/bjd.19377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Caselli D, Chironna M, Loconsole D, et al. No evidence of SARS‐CoV‐2 infection by polymerase chain reaction or serology in children with pseudo‐chilblain. Br J Dermatol. 2020;183(4):784‐785. doi: 10.1111/bjd.19349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bouaziz JD, Duong TA, Jachiet M, et al. Vascular skin symptoms in COVID‐19: a French observational study. J Eur Acad Dermatol Venereol. 2020;34(9):e451‐e452. doi: 10.1111/jdv.16544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Marzano AV, Genovese G, Fabbrocini G, et al. Varicella‐like exanthem as a specific COVID‐19‐associated skin manifestation: multicenter case series of 22 patients. J Am Acad Dermatol. 2020;83(1):280‐285. doi: 10.1016/j.jaad.2020.04.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fernandez‐Nieto D, Ortega‐Quijano D, Jimenez‐Cauhe J, et al. Clinical and histological characterization of vesicular COVID‐19 rashes: a prospective study in a tertiary care hospital. Clin Exp Dermatol. 2020;45(7):872‐875. doi: 10.1111/ced.14277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Caputo V, Schroeder J, Rongioletti F. A generalized purpuric eruption with histopathologic features of leucocytoclastic vasculitis in a patient severely ill with COVID‐19. J Eur Acad Dermatol Venereol. 2020;34(10):e579‐e581. doi: 10.1111/jdv.16737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Joob B, Wiwanitkit V. COVID‐19 can present with a rash and be mistaken for dengue. J Am Acad Dermatol. 2020;82(5):e177. doi: 10.1016/j.jaad.2020.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ahouach B, Harent S, Ullmer A, et al. Cutaneous lesions in a patient with COVID‐19: are they related? Br J Dermatol. 2020;183(2):e31. doi: 10.1111/bjd.19168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID‐19 infection: A report of five cases. Transl Res. 2020;220:1‐13. doi: 10.1016/j.trsl.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. De Giorgi V, Recalcati S, Jia Z, et al. Cutaneous manifestations related to coronavirus disease 2019 (COVID‐19): A prospective study from China and Italy. J Am Acad Dermatol. 2020;83(2):674‐675. doi: 10.1016/j.jaad.2020.05.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sajjan VV, Lunge S, Swamy MB, Pandit AM. Livedo reticularis: A review of the literature. Indian Dermatol Online J. 2015;6(5):315‐321. doi: 10.4103/2229-5178.164493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cordoro KM, Reynolds SD, Wattier R, McCalmont TH. Clustered cases of acral perniosis: clinical features, histopathology, and relationship to COVID‐19. Pediatr Dermatol. 2020;37(3):419‐423. doi: 10.1111/pde.14227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844‐847. doi: 10.1111/jth.14768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Manalo IF, Smith MK, Cheeley J, Jacobs R. Reply to: “reply: A dermatologic manifestation of COVID‐19: transient livedo reticularis”. J Am Acad Dermatol. 2020;83(2):e157. doi: 10.1016/j.jaad.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available in [repository name] at [URL/DOI], reference number [reference number]. These data were derived from the following resources available in the public domain: [list resources and URLs].


