Abstract
Background
Corticosteroids improve outcomes in patients with severe COVID‐19. In the COVID STEROID 2 randomised clinical trial, we found high probabilities of benefit with dexamethasone 12 versus 6 mg daily. While no statistically significant heterogeneity in treatment effects (HTE) was found in the conventional, dichotomous subgroup analyses, these analyses have limitations, and HTE could still exist.
Methods
We assessed whether HTE was present for days alive without life support and mortality at Day 90 in the trial according to baseline age, weight, number of comorbidities, category of respiratory failure (type of respiratory support system and oxygen requirements) and predicted risk of mortality using an internal prediction model. We used flexible models for continuous variables and logistic regressions for categorical variables without dichotomisation of the baseline variables of interest. HTE was assessed both visually and with p and S values from likelihood ratio tests.
Results
There was no strong evidence for substantial HTE on either outcome according to any of the baseline variables assessed with all p values >.37 (and all S values <1.43) in the planned analyses and no convincingly strong visual indications of HTE.
Conclusions
We found no strong evidence for HTE with 12 versus 6 mg dexamethasone daily on days alive without life support or mortality at Day 90 in patients with COVID‐19 and severe hypoxaemia, although these results cannot rule out HTE either.
Keywords: corticosteroids, COVID‐19, critical illness, days alive without life support, hypoxaemia, mortality
Editorial Comment.
In this post hoc explorative sub‐study of the COVID STEROID 2 trial, no strong evidence for substantial heterogeneity in treatment effects was found. The authors included the S‐value for the interpretation of probabilities, which may be a more understandable measurement compared to the standard p‐value, both for clinicians and researchers.
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) may cause critical illness and high mortality rates due to severe pulmonary inflammation and hypoxaemia. 1 Anti‐inflammatory treatment, including corticosteroids, reduces mortality and is recommended for patients with severe and critical COVID‐19. 2 , 3
In the COVID STEROID 2 randomised controlled trial, we assessed 12 versus 6 mg of dexamethasone for patients with COVID‐19 and severe hypoxaemia and found high probabilities of benefit from the higher dose for all outcomes assessed up until Day 90; long‐term outcomes were similarly mostly compatible with benefit, albeit not reaching the thresholds for statistical significance. 4 , 5 , 6
Despite probable overall benefits with a higher dose, heterogeneous treatment effects (HTE) 7 , 8 , 9 in different patient groups may be present, as has been suggested in previous critical care trials. 9 , 10 , 11 , 12 In the conventional, dichotomous subgroup analyses, no statistically significant HTE was found for the primary outcome (days alive without life support at Day 28) according to several baseline characteristics (including age and weight dichotomised at 70 years and 80 kg, respectively). 4 , 13 However, conventional subgroup analyses are at risk of type 2 errors as trials are generally only powered for the primary analysis, 8 , 9 dichotomisation of continuous variables further decreases power, 14 and focus on individual variables may not correspond well with the clinical reality, where risk and treatment decisions are affected by the combinations of multiple factors. 8 , 9
In this post hoc exploratory sub‐study of the COVID STEROID 2 trial, we aimed to assess whether HTE with two different doses of dexamethasone was present for the number of days alive without life support and mortality at Day 90 according to four baseline characteristics (age, weight, category of respiratory failure and number of comorbidities) and the predicted risk of 90‐day mortality, all assessed without dichotomisation of the variables included or the conclusions. 15
2. METHODS
These post hoc exploratory analyses of HTE in the COVID STEROID 2 trial were conducted according to a statistical analysis plan, which was written after the pre‐planned analyses of the trial were reported, 4 , 5 , 6 but before any of the analyses reported in this manuscript were conducted. 15 This manuscript was prepared according to the Strengthening the Reporting of Observational Studies in Epidemiology (STRO) checklist 16 (supplement).
2.1. The COVID STEROID 2 trial
The COVID STEROID 2 trial was an investigator‐initiated, international, parallel‐group, stratified, blinded (including patients, clinicians, investigators and outcome assessors) randomised clinical trial, approved by the regulatory authorities and ethics committees in all participating countries. 4 , 17 One thousand adult patients hospitalised with COVID‐19 and severe hypoxaemia (≥10 L oxygen/min, use of non‐invasive ventilation [NIV], continuous use of continuous positive airway pressure [CPAP] or invasive mechanical ventilation) were enrolled at 31 sites in 26 hospitals in Denmark, India, Sweden and Switzerland between 27 August 2020 and 20 May 2021. 4 Patients were primarily excluded due to previous use of systemic corticosteroids for COVID‐19 in doses >6 mg for ≥5 days, unobtainable consent, and use of higher‐dose steroids for other indications than COVID‐19. 4 , 17 Patients were randomised 1:1 to dexamethasone 12 or 6 mg intravenously once daily for up to 10 days. Additional details are provided in the primary protocol and trial report. 4 , 17
2.2. Outcomes and patients assessed
In this sub‐study, we assessed the following two outcomes at 90 days:
Days alive without life support (including invasive mechanical ventilation, circulatory support and kidney replacement therapy; the actual number of days was used without assigning dead patients the worst possible value).
Mortality.
Of note, the primary outcome in the COVID STEROID 2 trial was days alive without life support after 28 days of follow‐up for logistical/ethical reasons due to the urgency of the pandemic. 4 , 17
Both outcomes were assessed in the complete intention‐to‐treat (ITT) population (n = 982 after exclusion of patients without consent for the use of their data 4 ); no formal sample size calculation was conducted for this post hoc study.
2.3. Statistical analyses
2.3.1. Descriptive data
We present descriptive data for all baseline and outcome variables assessed in this study in both treatment groups with continuous variables presented as medians with interquartile ranges (IQRs) and full ranges, and binary and categorical variables presented as numbers with percentages.
2.3.2. Heterogeneity in treatment effects
We assessed HTE using frequentist analyses without adjustment according to the following four baseline characteristics:
Age (years)
Weight (kg)
- Category of respiratory failure on a 1–6‐point scale defined as follows:
- Open system, low oxygen flow (oxygen flow rate ≤ median oxygen flow rate in all patients on open systems).
- Open system, high oxygen flow (oxygen flow rate > median oxygen flow rate).
- NIV/CPAP, low fraction of inspired oxygen (FiO2; FiO2 ≤ median FiO2 in all patients on closed systems).
- NIV/CPAP, high FiO2 (FiO2 > median FiO2).
- Invasive mechanical ventilation, low FiO2 (defined as above).
- Invasive mechanical ventilation, high FiO2 (defined as above).
Number of comorbidities (diabetes mellitus, ischemic heart disease or heart failure, chronic obstructive pulmonary disease or immunosuppression within 3 months prior to randomisation) on a 1–4‐point scale with patients with three or four comorbidities analysed in the same category as only one patient had all four comorbidities.
In addition, we assessed HTE according to the baseline predicted risk of 90‐day mortality using an internal prediction model developed in the control group (6 mg) as described below.
2.3.3. Analytical strategy
We assessed HTE on the continuous scale for age, weight and predicted mortality risk using generalised additive models (linear/logistic regressions, respectively, for the two outcomes) with cubic regression splines, fixed degrees of freedom and five knots at the 5, 27.5, 50, 77.5 and 95 percentiles. 15 , 18 Primary models included treatment and a smooth‐by‐treatment allocation separately for each characteristic assessed with likelihood ratio tests used to assess the treatment‐by‐baseline variable‐interaction by comparing the full models to models only including treatment and a smooth transformation of the variable of interest not stratified by treatment allocation.
HTE according to the category of respiratory failure and the number of comorbidities was assessed by conventional linear/logistic regression models, including treatment, the baseline variable of interest (as a categorical variable) and an interaction term; likelihood ratio tests were used to assess interactions similarly as for the generalised additive models. Of note, this was not specified in the statistical analysis plan, 15 but necessary as generalised additive models could not be used due to a few distinct values for these two baseline variables.
Results are presented graphically using predicted mean outcome values with 95% confidence intervals (CIs) in each treatment group according to values of the baseline variable in question, supplemented with plots illustrating the absolute differences between groups with 95% CIs.
The p values from the likelihood ratio tests are presented; results were not dichotomised according to significance thresholds but interpreted as continuous measures of evidence, with results interpreted cautiously and only as hypothesis generating due to their post hoc nature. 15 To supplement the interpretation, we converted p values to S values (S‐value = −log2[p‐value]). 19 In brief, S values measure how ‘surprising’ the observed results are assuming that there is truly no difference on an interpretable scale; S values thus correspond to the chance of getting all heads in S consecutive fair coin tosses. 19
2.3.4. Internal prediction model
We developed an internal prediction model 15 , 20 for 90‐day mortality using the control group only. The following baseline variables were entered into a logistic regression model: age (years), weight (kg), type of respiratory support (open system [reference], NIV/CPAP, or invasive mechanical ventilation), diabetes mellitus, ischaemic heart disease or heart failure, chronic obstructive pulmonary disease, immunosuppression within 3 months prior to randomisation, baseline lactate concentration (mmol/L), use of vasopressors or inotropes at baseline and limitations in care (e.g., cardio‐pulmonary resuscitation, life support) at baseline.
Continuous variables were modelled using multivariable fractional polynomials 21 with the best‐fitting second‐degree fractional polynomial transformation of each continuous variable used. Apparent internal performance was assessed using the area under the receiver operating characteristics curve (AUROC; assessing discrimination, i.e., the chance that a patient with the event in question has a higher predicted risk than one without, with 0.5 being equal to chance and 1.0 corresponding to perfect discrimination); and calibration was assessed using calibration plots (with predicted/observed mortality presented in tenths, using a loess smoother and a linear regression on the predicted/observed values). 22 We present predicted risks in both treatment groups and the full resulting model.
2.3.5. Missing data
The amounts of missing data for the outcome variables and most baseline variables assessed were negligible except for lactate levels; 4 in total, 1.4%–1.5% of the ITT population had missing data for the outcomes assessed, 1.3% of the ITT population had missing FiO2‐data and 10.8% had missing lactate values. All analyses except the prediction model‐based analyses were thus conducted using complete cases only, while the prediction model‐based analyses were conducted using multiply imputed datasets. 23 We generated 25 imputed datasets separately in each group using the predictive mean matching and logistic regression methods, including all variables mentioned above and the country of enrollment. 15 , 24 Knot positions and optimal fractional polynomial transformations were calculated using a single imputed dataset (with prediction imputation not accounting for between‐imputation uncertainty and without imputation of missing outcome data), followed by fitting all final models on the 25 multiply imputed datasets. Predicted values were combined using Rubin's rules; p values from the likelihood ratio test for the model comparisons were pooled after transformation to the Z‐scale followed by back‐transformation. 25 For all plots, predicted values were calculated with 95% CIs for 100 distinct values equally spaced between the minimum/maximum values displayed.
2.3.6. Software
Analyses were conducted using R (R Core Team, R Foundation for Statistical Computing, Vienna, Austria) v. 4.1.0 with the mgcv, mice, mfp and Tidyverse packages.
2.3.7. Additional analyses added during peer review
During the peer review process, additional descriptive baseline data and analyses not outlined in the statistical analysis plan 15 were added. These were analyses on the continuous scale according to PaO2/FiO2‐ratios in patients on closed systems only and according to PaO2/oxygen flow‐ratios in patients on open systems only. These analyses were conducted in complete cases only.
3. RESULTS
Descriptive baseline and outcome data for the 982 patients in the ITT population are presented in Table 1. Treatment groups were largely similar, although the number of comorbidities was slightly lower in the 12‐mg group, mostly due to the lower presence of diabetes. As previously reported, 4 the 12‐mg group had a higher median number of days alive without life support and lower mortality at day 90, although smaller effects in the opposite directions could not be excluded.
TABLE 1.
Descriptive baseline and outcome data
| Variable | 12 mg (n = 497) | 6 mg (n = 485) |
|---|---|---|
| Baseline variables | ||
| Country of inclusion | ||
| Denmark | 251 (50.5%) | 234 (48.2%) |
| India | 182 (36.6%) | 187 (38.6%) |
| Sweden | 40 (8.0%) | 39 (8.0%) |
| Switzerland | 24 (4.8%) | 25 (5.2%) |
| Age (years) | 65 (56–74) [22–88] | 64 (54–72) [22–90] |
| Weight (kg) | 80 (68–96) [45–198] | 80 (68–95) [42–164] |
| Type of respiratory support | ||
| Open system | 272 (54.7%) | 258 (53.2%) |
| NIV/CPAP | 118 (23.7%) | 128 (26.4%) |
| Invasive mechanical ventilation | 107 (21.5%) | 99 (20.4%) |
| Respiratory failure category (presented as numerical values) a | 2 (1–4) [1–6] | 2 (1–4) [1–6] |
| Respiratory failure category a | ||
| 1: Open system, low flow b | 141 (28.7%) | 126 (26.4%) |
| 2: Open system, high flow b | 131 (26.6%) | 132 (27.7%) |
| 3: NIV/CPAP, low FiO2 c | 69 (14.0%) | 73 (15.3%) |
| 4: NIV/CPAP, high FiO2 c | 45 (9.1%) | 47 (9.9%) |
| 5: Invasive mechanical ventilation, low FiO2 c | 64 (13.0%) | 51 (10.7%) |
| 6: Invasive mechanical ventilation, high FiO2 c | 42 (8.5%) | 48 (10.1%) |
| Oxygen flow rate (L/min, in patients on open system only) | 22 (15–40) [10–61] | 24 (15–40) [10–70] |
| FiO2 (%, in patients on closed system only) a | 60 (50–75) [25–100] | 60 (45–80) [25–100] |
| Number of comorbidities | 0 (0–1) [0–3] | 1 (0–1) [0–4] |
| Number of comorbidities (categorical) | ||
| 0 | 270 (54.3%) | 240 (49.5%) |
| 1 | 164 (33.0%) | 174 (35.9%) |
| 2 | 54 (10.9%) | 57 (11.8%) |
| 3 | 9 (1.8%) | 13 (2.7%) |
| 4 | 0 (0.0%) | 1 (0.2%) |
| Diabetes mellitus | 135 (27.2%) | 163 (33.6%) |
| Ischemic heart disease or heart failure | 67 (13.5%) | 69 (14.2%) |
| Chronic obstructive pulmonary disease | 57 (11.5%) | 56 (11.5%) |
| Immunosuppression within 3 months prior to randomisation | 40 (8.0%) | 43 (8.9%) |
| Lactate (mmol/L) d | 1.6 (1.1–2.3) [0.3–16.7] | 1.7 (1.2–2.3) [0.2–13.8] |
| Use of vasopressors or inotropes | 81 (16.3%) | 68 (14.0%) |
| Limitations in care (cardio‐pulmonary resuscitation or life‐support) | 30 (6.0%) | 25 (5.2%) |
| Predictions | ||
| Predicted risk of mortality at day 90 e (%) | 35.5 (22.2–52.9) [2.6–99.7] | 34.6 (22.6–49.4) [3.1–98.3] |
| Outcome variables | ||
| Days alive without life support at day 90 f | 84 (9–90) [0–90] | 80 (6–90) [0–90] |
| Mortality at day 90 g | 157 (32.0%) | 180 (37.7%) |
Note: Descriptive baseline, outcome and predicted data according to the internal prediction model for all variables assessed. Some baseline and the outcome data have been presented previously elsewhere. 4 Numeric data are presented as medians (interquartile ranges) [full ranges], whereas binary/categorical data are presented as numbers (%).
Abbreviations: CPAP, continuous positive airway pressure; NIV, non‐invasive ventilation.
A total of 13 patients on closed systems (1.3% of the full intention‐to‐treat population; 5 patients in the 12‐mg group and 8 patients in the 6‐mg group) could not be classified due to missing FiO2 values.
Low flow includes patients with oxygen flow values ≤ the median value in all patients on open systems (22 L/min), whereas high flow includes patients with values > this value.
Low FiO2 includes patients with values ≤ the median value in all patients on closed systems (60%), whereas high FiO2 includes patients with values > this value.
Lactate values were missing in 106 patients (10.8% of the intention‐to‐treat population; 57 patients in the 12‐mg group and 49 patients in the 6‐mg group).
Calculated using the stacked multiply imputed datasets; the mean predicted risk was 38.5% in the 12‐mg group and 37.6% in the 6‐mg group.
Days alive without life support at Day 90 were missing in 15 patients (1.5% of the intention‐to‐treat population; 8 patients in the 12 mg group and 7 patients in the 6 mg group).
Mortality at Day 90 values were missing in 14 patients (1.4% of the intention‐to‐treat population; 7 patients in the 12‐mg group and 7 patients in the 6‐mg group).
HTE according to treatment allocation and simple baseline characteristics.
Figures 1 and 2 (and Figures S1 and S2) present the expected mean number of days alive without life support and mortality at Day 90, respectively, according to treatment allocation and baseline characteristics. While outcomes appeared better with 12 mg dexamethasone in general, 4 , 5 there was no strong evidence of HTE according to any of the baseline characteristics on either outcome, with substantial overlap and parallelism between curves and all p values >.37 corresponding to all S values being <1.43. Visually and numerically, point estimates favoured 12 mg in patients weighing more and 6 mg in patients weighing less, but substantial uncertainty remains. Similarly, treatment effects appeared to be reversed (possibly favouring the lower dose) or neutral compared to the overall findings for patients on closed systems (NIV/CPAP or invasive mechanical ventilation) with the highest FiO2 values; as for weight, there was substantial overlap and uncertainty remains.
FIGURE 1.
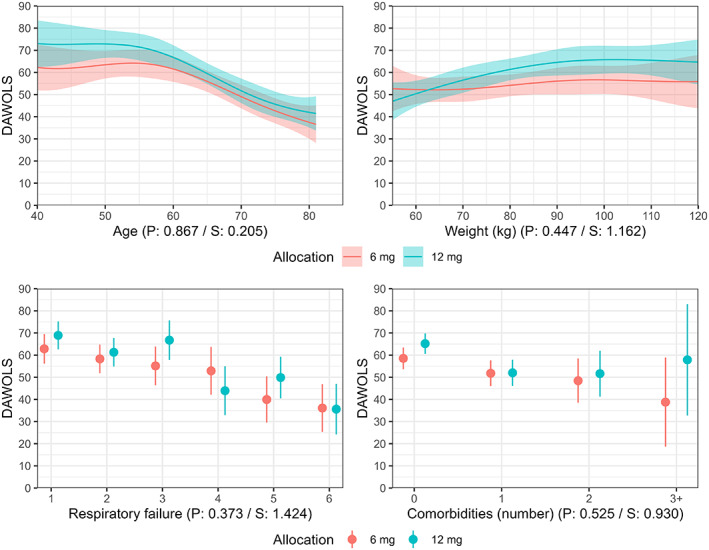
Days alive without life support at Day 90 according to treatment allocation and baseline characteristics. Expected mean number of days alive without life support (DAWOLS) with 95% confidence intervals according to four baseline variables (as described in methods section) according to the model fit. Predicted values and 95% confidence intervals are truncated at the lowest/highest possible values (0/90 days). The p and S values from the likelihood ratio tests assessing evidence in favour of heterogeneous treatment effects are displayed below each plot. For the continuous variables, predictions are only displayed for the central 90% of values in the data due to the large uncertainty at the extreme values with limited data. Figure S1 displays predicted values across all observed values in the datasets as specified in the statistical analysis plan. 15
FIGURE 2.
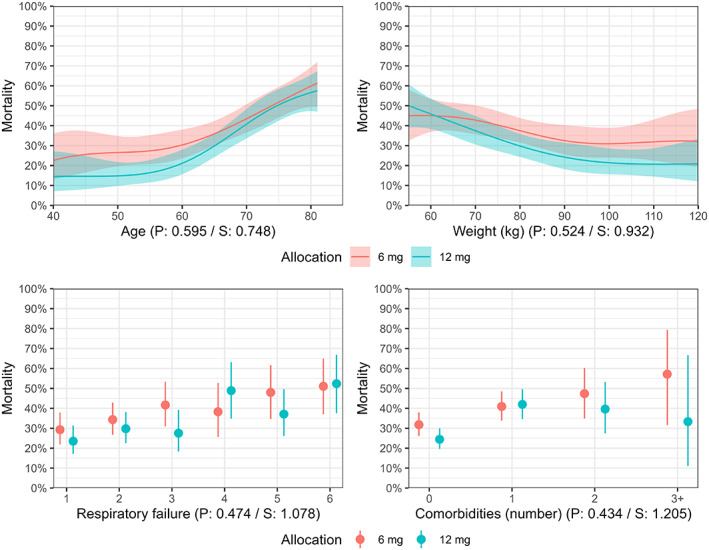
Mortality Day 90 according to treatment allocation and baseline characteristics. Expected mortality rates at Day 90 with 95% confidence intervals according to four baseline variables (as described in the methods section) according to the model fit. The p and S values from the likelihood ratio tests assessing evidence in favour of heterogeneous treatment effects are displayed below each plot. For the continuous variables, predictions are only displayed for the central 90% of values in the data due to the large uncertainty at the extreme values with limited data. Figure S2 displays predicted values across all observed values in the datasets as specified in the statistical analysis plan. 15
3.1. Internal prediction model and HTE
The performance of the internal prediction model (full model presented in the supplement) was adequate regarding both discrimination (AUROC 0.73, 95% CI 0.68–0.77) and calibration (Figure S3).
The median predicted risks of mortality were 35.5% (12 mg) versus 34.6% (6 mg) with mean predicted probabilities of 38.5% (12 mg) versus 37.6% (6 mg), respectively, while actual mortality rates were 32.0% (12 mg) versus 37.7% (6 mg) (Table 1).
The expected outcomes according to predicted mortality risk for both days alive without life support and mortality at Day 90 are presented in Figure 3 (and Figure S4). As for the simple baseline characteristics, there was no strong evidence for HTE with largely parallel and overlapping curves and p values of .50 for days alive without life support (S‐value 0.99) and .42 for mortality (S‐value 1.24).
FIGURE 3.
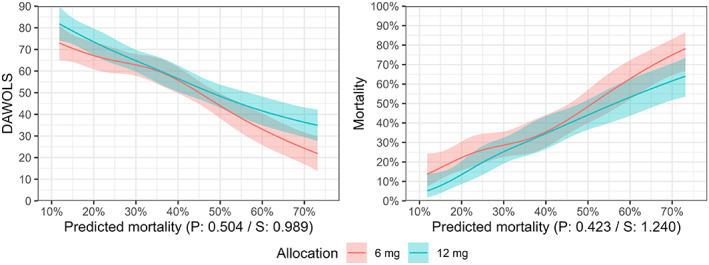
Days alive without life support and mortality Day 90 according to treatment allocation and predicted mortality risk. Expected mean number of days alive without life support (DAWOLS) and risk of mortality at Day 90 with 95% confidence intervals according to the predicted risks of mortality at Day 90 using the internal prediction model. For DAWOLS, predicted values and 95% confidence intervals are truncated at the lowest/highest possible values (0/90 days). p and S values from the likelihood ratio tests assessing evidence in favour of heterogeneous treatment effects are displayed below each plot. For these continuous variables, predictions are only displayed for the central 90% of values in the data due to the large uncertainty at the extreme values with limited data. Figure S4 displays predicted values across all observed values in the datasets as specified in the statistical analysis plan. 15
3.2. Treatment effect differences
Estimated treatment effects for both outcomes according to the variables assessed are presented in Figure 4 (and Figure S5), which shows the same patterns as Figures 1, 2, 3.
FIGURE 4.
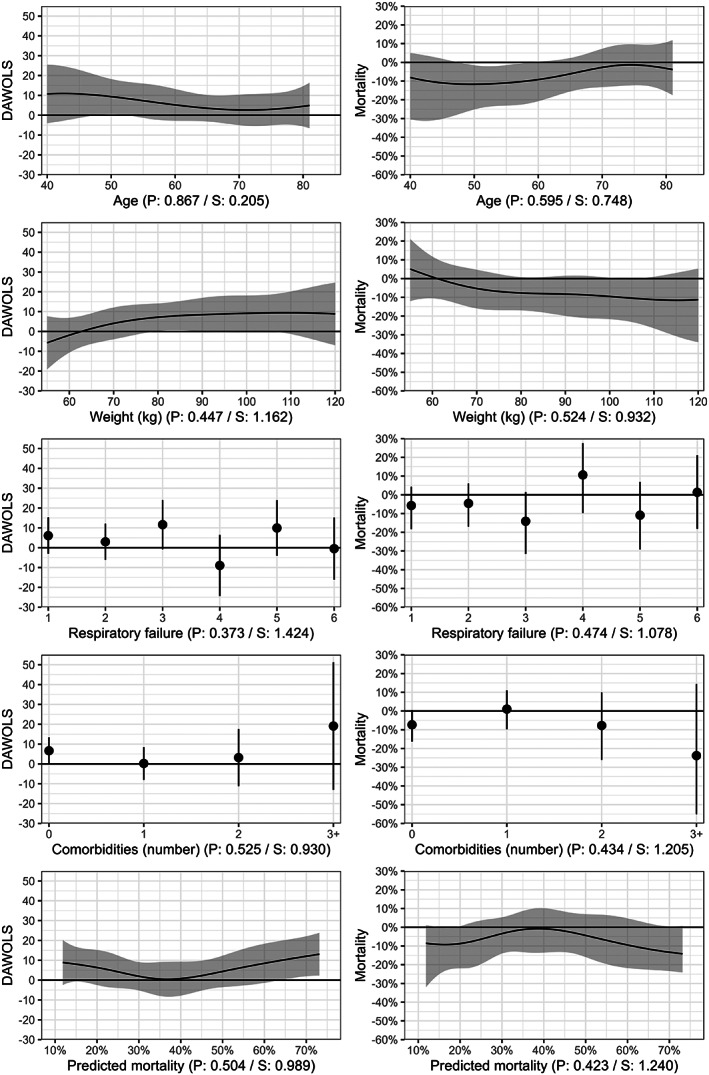
Between‐group differences in outcomes according to various baseline characteristics. Differences in days alive without life support (DAWOLS) and mortality at Day 90 with 95% confidence intervals according to the variables assessed at baseline (including predicted risks of mortality at Day 90 using the internal prediction model) with 95% confidence intervals. Values are presented as the treatment effects of 12‐mg dexamethasone, that is, positive differences indicate higher values in the 12‐mg group and vice versa. For both outcomes, predicted values and 95% confidence intervals are truncated at the lowest/highest possible values (0/90 days and 0/100%, respectively). The p and S values from the likelihood ratio tests assessing evidence in favour of heterogeneous treatment effects are displayed below each plot. For the continuous variables, predicted differences are only displayed for the central 90% of values in the data due to the large uncertainty at the extreme values with limited data. Figure S5 displays predicted differences across all observed values in the datasets as specified in the statistical analysis plan. 15
3.3. Additional analyses added during peer review
Additional descriptive data and results from analyses added during peer review are presented in the supplement (Table S1 and Figures S6–S7); in brief, there was no strong evidence of HTE, although analyses according to PaO2/FiO2‐ratios in those on closed systems suggested benefit with 12 mg in most patients, while 6 mg seemed preferable in those with lowest PaO2/FiO2‐ratios despite substantial uncertainty.
4. DISCUSSION
In this post hoc exploratory sub‐study of the COVID STEROID 2 trial, we found no strong evidence for substantial HTE with higher (12 mg) versus lower (6 mg) doses of dexamethasone on days alive without life support or mortality at Day 90 in patients with COVID‐19 and severe hypoxaemia. All S values were <1.43 meaning that if there are truly no differences, then observing these results is less surprising than obtaining two heads in a row using a fair coin. While these results provide no strong evidence for HTE, they cannot firmly rule it out either.
We previously hypothesised that higher doses of dexamethasone may be more beneficial in younger patients, 17 but these results provide no meaningful support for this hypothesis. Others have hypothesised that relatively higher doses may be required in obese patients to avoid underdosing; 26 while these results do not provide any strong evidence for that hypothesis either, point estimates did point in that direction. Interestingly, in a previous prospective meta‐analysis assessing the effects of systemic corticosteroids, the effects of steroids on mortality seemed to be higher in patients not on invasive mechanical ventilation than in those mechanically ventilated, although the former group only included 144 patients. 2 We found no firm evidence of HTE according to our categorical scale of respiratory failure; however, some numerical differences in treatment effects were found in patients on closed systems (NIV/CPAP or invasive mechanical ventilation), with possibly reversed or neutral treatment effects in the groups with FiO2 above the median value. Similarly, results from the analysis added during peer review assessing HTE according to PaO2/FiO2‐ratios in those on closed systems were mostly compatible with reversed treatment effects (i.e., preferring 6 mg) in patients with the lowest PaO2/FiO2‐ratios. A similar signal was not found in those on open systems according to PaO2/oxygen flow‐ratios. In keeping with the aforementioned prospective meta‐analysis, 2 these results could suggest that increased immunosuppression (i.e., higher doses of dexamethasone) provide additional benefits early in the disease course, while this may not be the case later when the disease has progressed. Due to the high uncertainty and post hoc nature of this exploratory study, all these findings should be interpreted very cautiously and need confirmation in subsequent studies.
Assessing HTE according to illness severity defined as the risk of poor outcomes has been recommended, 8 , 27 and patients at higher risk of a poor outcome may be hypothesised to have larger beneficial effects of the treatment. We assessed HTE both according to the cumulated number of comorbidities and using a risk modelling approach using an internal prediction model, 8 , 27 but found no firm evidence of HTE according to these variables. Thus, it seems that the treatment effects are relatively similar independent of comorbidity burdens and risk of a poor outcome, at least in patients with COVID‐19 and severe hypoxaemia.
4.1. Strengths and limitations
This study comes with several strengths, including the overall strengths of the COVID STEROID 2 trial, that is, a relatively large, international pragmatic trial with blinding and limited missing data. 4 In addition, the strengths of this study include the analysis plan, which was written and made publicly available before the analyses were conducted. 4 , 15 Further, we conducted analyses of HTE without dichotomisation of variables (and concomitant loss of information) 14 and with interpretation of the evidence on the continuous scale without dichotomisation according to p‐value thresholds. 28 Finally, we assessed HTE according to multiple relevant baseline variables, including the overall risk of a poor outcome (as recommended), and the cumulated comorbidity burden, which may better reflect clinical reality than assessing HTE according to individual variables. 8 , 9
The study also has limitations, including those general to the COVID STEROID 2 trial, that is, the evolving pandemic and changes in care during and after the trial (i.e., recommendations in favour of interleukin‐6 receptor antagonists introduced after randomisation concluded 3 ), and limited power for some analyses. 4 Moreover, this was a post hoc exploratory study, and despite public registration of the statistical analysis plan prior to the conduct of the analyses, this was done after the primary trial results were known. Consequently, these results should be interpreted cautiously and as hypothesis generating only. Second, to simplify the analyses, we did not adjust for the stratification variables; however, as the results were similar for both outcomes assessed here in the primary adjusted and unadjusted analyses, 4 this is unlikely to have had any substantial influence on our results. Third, comorbidities were selected according to availability and prevalence in the trial and weighted equally in the analyses according to the number of comorbidities, although some may increase the risk of poor outcomes more than others. Yet, this limitation is mitigated by their inclusion in the internal prediction model. Fourth, our categorisation of respiratory failure is somewhat arbitrary, data‐driven, and specific to this study. The PaO2/FiO2‐ratio might have been a better measure of respiratory failure, but unfortunately, data were not available to calculate this for patients on open systems, 4 , 17 which was the case for slightly more than 50% of the included patients at baseline. However, an additional analysis according to PaO2/FiO2‐ratios in those on closed systems added during peer review seemed to support the results from the planned analysis of respiratory failure categories. Fifth, we used an internal prediction model as data for external, previously developed prediction models were not registered in the trial. The internal prediction model was developed in the control group only as we knew that mortality rates at Day 90 seemed to differ between groups. 4 , 5 , 15 This approach may come with a risk of potentially exaggerating interactions; 27 however, as no strong evidence for HTE according to predicted mortality risks was found, this was not an issue here. Finally, while we found no firm evidence of HTE according to the variables assessed, we cannot exclude that it exists and was merely not found due to limited power.
5. CONCLUSIONS
In conclusion, we found no convincingly strong evidence for substantial HTE with higher (12 mg) versus lower (6 mg) doses of dexamethasone on days alive without life support or mortality at Day 90 in patients with COVID‐19 and severe hypoxaemia according to age, weight, number of comorbidities, category of respiratory failure or predicted risks of mortality.
AUTHOR CONTRIBUTIONS
This exploratory, post hoc study was conceived and planned by Anders Granholm, Marie Warrer Munch and Anders Perner. Anders Granholm conducted all analyses presented in this manuscript and wrote the first draft, which was critically revised by all authors. Marie Warrer Munch was the coordinating investigator of the COVID STEROID 2 trial, and Anders Perner was the trial sponsor. All authors contributed to the design and/or conduct of the trial. Detailed author contributions for the full COVID STEROID 2 trial were presented in the primary trial report. 4
FUNDING INFORMATION
The COVID STEROID 2 trial was funded by Novo Nordisk Foundation and the Research Council of Rigshospitalet. The funders had no role in the design, conduct, analyses or reporting of the trial or this secondary study.
CONFLICT OF INTEREST
Anders Granholm, Marie Warrer Munch, Morten Hylander Møller and Anders Perner are affiliated with the Department of Intensive Care at Rigshospitalet—Copenhagen University Hospital, which has received funding for other projects from the Novo Nordisk Foundation, Sygeforsikringen ‘danmark’, Pfizer and Fresenius Kabi, and conducts contract research for AM‐Pharma. Vivekanand Jha has received grant funding from GSK, Baxter Healthcare and Biocon and has received honoraria from Bayer, AstraZeneca, Boeringer Ingelheim, NephroPlus and Zydus Cadilla, under the policy of all honoraria being paid to the organisation. Stephan M. Jakob reports that the Department of Intensive Care Medicine, University Hospital Bern, has or has had in the past, research and development/consulting contracts with Edwards Lifesciences Services GmbH, Phagenesis Limited, Nestlé and Cytel Inc. The money was paid into a departmental fund, Dr Jakob did not receive any financial gain. The Department of Intensive Care Medicine, University Hospital Bern has received in the past unrestricted educational grants from the following organisations for organising bi‐annual postgraduate courses in the fields of critical care ultrasound, management of ECMO and mechanical ventilation: Pierre Fabre Pharma AG (formerly known as RobaPharm), Pfizer AG, Bard Medica S.A., Abbott AG, Anandic Medical Systems, PanGas AG Healthcare, Orion Pharma, Bracco, Edwards Lifesciences AG, Hamilton Medical AG, Fresenius Kabi (Schweiz) AG, Getinge Group Maquet AG, Dräger Schweiz AG and Teleflex Medical GmbH.
Supporting information
Appendix S1: Supporting Information
ACKNOWLEDGMENTS
We thank everyone involved in the COVID STEROID 2 4 and the first COVID STEROID trial. 29
Granholm A, Munch MW, Andersen‐Ranberg N, et al. Heterogeneous treatment effects of dexamethasone 12 mg versus 6 mg in patients with COVID‐19 and severe hypoxaemia—Post hoc exploratory analyses of the COVID STEROID 2 trial. Acta Anaesthesiol Scand. 2023;67(2):195‐205. doi: 10.1111/aas.14167
Funding information Novo Nordisk Fonden; Research Council of Rigshospitalet
REFERENCES
- 1. Haase N, Plovsing R, Christensen S, et al. Characteristics, interventions and longer‐term outcomes of COVID‐19 ICU patients in Denmark—a nationwide, observational study. Acta Anaesthesiol Scand. 2021;65:68‐75. [DOI] [PubMed] [Google Scholar]
- 2. WHO Rapid Evidence Appraisal for COVID‐19 Therapies (REACT) Working Group , Sterne JAC, Murthy S, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID‐19: a meta‐analysis. JAMA. 2020;324:1330‐1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Agarwal A, Rochwerg B, Lamontagne F, et al. A living WHO guideline on drugs for covid‐19 [tenth version/ninth update, 22 April 2022]. BMJ. 2020;370:m3379. [DOI] [PubMed] [Google Scholar]
- 4. COVID STEROID 2 Trial Group . Effect of 12 mg vs 6 mg of dexamethasone on the number of days alive without life support in adults with COVID‐19 and severe hypoxemia. JAMA. 2021;326:1807‐1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Granholm A, Munch MW, Myatra SN, et al. Dexamethasone 12 mg versus 6 mg for patients with COVID‐19 and severe hypoxaemia: a pre‐planned, secondary Bayesian analysis of the COVID STEROID 2 trial. Intensive Care Med. 2022;48:45‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Granholm A, Kjær MN, Munch MW, et al. Long‐term outcomes of dexamethasone 12 mg versus 6 mg in patients with COVID‐19 and severe hypoxaemia. Intensive Care Med. 2022;48:580‐589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Iwashyna TJ, Burke JF, Sussman JB, Prescott HC, Hayward RA, Angus DC. Implications of heterogeneity of treatment effect for reporting and analysis of randomized trials in critical care. Am J Respir Crit Care Med. 2015;192:1045‐1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kent DM, Steyerberg E, van Klaveren D. Personalized evidence based medicine: predictive approaches to heterogeneous treatment effects. BMJ. 2018;363:k4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Granholm A, Alhazzani W, Derde LPG, et al. Randomised clinical trials in critical care: past, present and future. Intensive Care Med. 2022;48:164‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Granholm A, Marker S, Krag M, et al. Heterogeneity of treatment effect of prophylactic pantoprazole in adult ICU patients: a post hoc analysis of the SUP‐ICU trial. Intensive Care Med. 2020;46:717‐726. [DOI] [PubMed] [Google Scholar]
- 11. Zampieri FG, Costa EL, Iwashyna TJ, et al. Heterogeneous effects of alveolar recruitment in acute respiratory distress syndrome: a machine learning reanalysis of the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial. Br J Anaesth. 2019;123:88‐95. [DOI] [PubMed] [Google Scholar]
- 12. The PEPTIC Investigators for the Australian and New Zealand Intensive Care Society Clinical Trials Group, Alberta Health Services Critical Care Strategic Clinical Network, The Irish Critical Care Trials Group et al. Effect of stress ulcer prophylaxis with proton pump inhibitors vs histamine‐2 receptor blockers on In‐hospital mortality among ICU patients receiving invasive mechanical ventilation the PEPTIC randomized clinical trial. JAMA. 2020;323:616‐626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Munch MW, Granholm A, Perner A. Dexamethasone and number of days alive without life support in adults with COVID‐19 and severe hypoxemia—reply. JAMA. 2022;327:683. [DOI] [PubMed] [Google Scholar]
- 14. Altman DG, Royston P. The cost of dichotomising continuous variables. BMJ. 2006;332:1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Granholm A, Munch MW, Perner A. COVID STEROID 2 trial: outline for a secondary post‐hoc study assessing heterogeneous treatment effects on the continuous scale. OSF Registries 2022. 10.17605/OSF.IO/523KH.
- 16. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344‐349. [DOI] [PubMed] [Google Scholar]
- 17. Munch MW, Granholm A, Myatra SN, et al. Higher vs. lower doses of dexamethasone in patients with COVID‐19 and severe hypoxia (COVID STEROID 2) trial: protocol and statistical analysis plan. Acta Anaesthesiol Scand. 2021;65:834‐845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. Springer International Publishing AG; 2015. [Google Scholar]
- 19. Rafi Z, Greenland S. Semantic and cognitive tools to aid statistical science: replace confidence and significance by compatibility and surprise. BMC Med Res Methodol. 2020;20:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kent DM, van Klaveren D, Paulus JK, et al. The Predictive Approaches to Treatment effect Heterogeneity (PATH) statement: explanation and elaboration. Ann Intern Med. 2020;172:W1‐W25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sauerbrei W, Perperoglou A, Schmid M, et al. State of the art in selection of variables and functional forms in multivariable analysis—outstanding issues. Diagnostic Progn Res. 2020;4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Labarère J, Bertrand R, Fine MJ. How to derive and validate clinical prediction models for use in intensive care medicine. Intensive Care Med. 2014;40:513‐527. [DOI] [PubMed] [Google Scholar]
- 23. Vesin A, Azoulay E, Ruckly S, et al. Reporting and handling missing values in clinical studies in intensive care units. Intensive Care Med. 2013;39:1396‐1404. [DOI] [PubMed] [Google Scholar]
- 24. van Buuren S, Groothuis‐Oudshoorn K. Mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45:67. doi: 10.18637/jss.v045.i03 [DOI] [Google Scholar]
- 25. Marshall A, Altman DG, Holder RL, Royston P. Combining estimates of interest in prognostic modelling studies after multiple imputation: current practice and guidelines. BMC Med Res Methodol. 2009;9:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Abouir K, Calmy A, Lorenzini KRI. Dexamethasone and number of days alive without life support in adults with COVID‐19 and severe hypoxemia. JAMA. 2022;327:682‐683. [DOI] [PubMed] [Google Scholar]
- 27. Kent DM, Paulus JK, van Klaveren D, et al. The Predictive Approaches to Treatment effect Heterogeneity (PATH) statement. Ann Intern Med. 2020;172:35‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Amrhein V, Greenland S, McShane B. Scientists rise up against statistical significance. Nature. 2019;567:305‐307. [DOI] [PubMed] [Google Scholar]
- 29. Munch MW, Meyhoff TS, Helleberg M, et al. Low‐dose hydrocortisone in patients with COVID‐19 and severe hypoxia: the COVID STEROID randomised, placebo‐controlled trial. Acta Anaesthesiol Scand. 2021;65:1421‐1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information


