Abstract
We assessed the humoral and cellular response to the fourth BNT162b2 mRNA COVID‐19 vaccine dose in patients with CLL. A total of 67 patients with CLL and 85 age matched controls tested for serologic response and pseudo‐neutralization assay. We also tested the functional T‐cell response by interferon gamma (IFNγ) to spike protein in 26 patients. Two weeks after the fourth vaccine antibody serologic response was evident in 37 (55.2%) patients with CLL, 20 /22 (91%) of treatment naïve, and 9/32 (28%) patients with ongoing therapy, compared with 100% serologic response in age matched controls. The antibody titer increased by 10‐fold in patients with CLL, however, still 88‐folds lower than age matched controls. Predictors of better chances of post fourth vaccination serologic response were previous positive serologies after second, third, and pre‐fourth vaccination, neutralizing assay, and treatment naïve patients. T‐cell response improved from 42.3% before the fourth vaccine to 84.6% 2 weeks afterwards. During the time period of 3 months after the fourth vaccination, 14 patients (21%) developed COVID‐19 infection, all recovered uneventfully. Our data demonstrate that fourth SARS‐CoV‐2 vaccination improves serologic response in patients with CLL to a lesser extent than healthy controls and induces functional T‐cell response.
Keywords: BNT162b2 mRNA, booster, chronic lymphocytic leukemia, CLL, COVID‐19, COVID‐19 vaccine, immune response
Novelty Statements.
-
What is the NEW aspect of your work?
This is the first report of cellular and humoral response to the fourth BNT162b2 mRNA COVID‐19 vaccine dose in patients with chronic lymphocytic leukemia. Anti‐spike antibody correlation with pseudo‐typed virus neutralizing titers, comparison of the serologic response with age matched control and short‐term outcome are also presented.
-
What is the CENTRAL finding of your work?
Serologic response after the fourth vaccine dose was evident in 55% of patients with CLL and 100% in age matched controls. Titers increased by 10‐fold in patients with CLL, still 88‐fold lower than in the controls. Predictors of better serologic response were positive serologies after previous vaccinations, neutralizing assay, and treatment naïve patients. T‐cell response was evident in 84.6% of patients, twice as high as before the fourth vaccine dose.
-
What is (or could be) the SPECIFIC clinical relevance of your work?
The fourth BNT162b2 mRNA COVID‐19 vaccine dose has a clear positive impact on the serologic response as well as functional T‐cell response in patients with CLL. Yet, anti‐spike titers are much reduced compared to age matched controls.
1. INTRODUCTION
Since the beginning of the COVID‐19 pandemic, multiple waves have led to more than six million deaths globally. Particularly at risk are the elderly and individuals with comorbidities. 1 The rapid development of mRNA vaccination and its worldwide deployment proved to be a successful factor in reducing the disease burden and infectivity. 2 , 3 , 4 However, the decline in the vaccination's protection over time and the emergence of resistant variants required a booster dose to restore serologic response. 5 , 6 Patients with chronic lymphocytic leukemia (CLL) are in a high risk of severe disease and mortality due to impaired cellular and humoral response related to the disease and its therapy. 7 In effect, these patients' antibody response rate is reduced post SARS‐CoV‐2 infection and in response to COVID‐19 vaccination. 8 , 9
In November 2021, the Omicron (B.1.1.529) SARS‐CoV‐2 variant emerged, causing a steep increase in community infection. This variant is reported to have various mutations in the receptor binding domain (RBD) that leads to breakthrough infection in fully vaccinated people. Although the Omicron variant was found to have lower risk for a severe disease, its high transmissibility rate has led to a high cumulative death. 10 A second booster vaccine was found to be immunogenic, safe, and efficacious primarily against symptomatic disease. 11 , 12 , 13 In January 2022, the Israeli Ministry of Health approved the fourth Pfizer and Moderna mRNA vaccines for the elderly, health care workers, and caregivers of individuals who belong to high‐risk groups. The FDA approval followed in March 2022. Considering the progression of events depicted above, we decided to evaluate the serologic and cellular response to a fourth BNT162b2 mRNA COVID‐19 vaccine in patients with CLL. This evaluation can broaden our ongoing understanding of repeated booster and assist in better modeling the treatment for this group of high‐risk patients.
2. METHODS
This prospective study investigates the efficacy of fourth BNT162b2 mRNA COVID‐19 vaccine in patients with CLL/SLL, who are followed up at the Chaim Sheba Medical Centre. The study was approved by the Institutional Review Board. All subjects provided informed consent and were vaccinated through a national Israeli vaccination program of administering a fourth vaccine dose to individuals aged 60 years and older and immunocompromised subjects. Eligibility criteria for the study included diagnosis of CLL/SLL per the guidelines of the International Workshop on Chronic Lymphocytic Leukemia for ages 18 years or older. 14 Elderly individuals in long term care facilities and community residences served as a control group and signed informed consent. Blood serum samples of patients with CLL/SLL and control subjects were collected before and 2 weeks after the administration of the fourth vaccine. The primary end point was the proportion increase of anti‐SARS‐CoV‐2 S antibodies, neutralizing antibodies and T‐cell response. Other endpoints included safety, factors affecting response, short term COVID19 infection rate and outcome.
2.1. Serological response assay
Serum samples were evaluated for immunoglobulin G (IgG), aimed at the SARS‐CoV‐2 S protein receptor–binding domain (RBD), using the commercial automatic chemiluminescent microparticle immunoassay SARS‐CoV‐2 IgG II Quant (Abbott Laboratories, Abbott Park, Illinois), according to the manufacturer's instructions. Antibody levels were measured in binding antibody units (BAU) as per the World Health Organization standard measurements. This assay considered positive concentration of 21.4 BAU/ml and higher.
2.2. Neutralization antibody assay
A surrogate viral assay was used to test antiviral humoral response based on a highly infectious recombinant vesicular stomatitis virus (VSV) bearing the SARS‐CoV‐2 spike glycoprotein S. This recombinant virus, rVSV‐SARS‐CoV‐2 or SARS‐CoV‐2 pseudo‐virus (psSARS‐2), closely resembles SARS‐CoV‐2 in its entry related properties. A psSARS‐2 neutralization assay was performed as previously described, to detect SARS‐CoV‐2 neutralizing antibodies. 15 Sera not capable of reducing viral replication by 50% at a 1 in 8 dilution or below were considered non‐neutralizing.
2.3. T‐cell response analysis
Functional T‐cell response to BNT162b2 mRNA COVID‐19 vaccine was measured in peripheral blood collected before the vaccination and 2 weeks after, from treatment naïve and patients with ongoing Bruton's Tyrosine Kinase inhibitor (BTKi) therapy. Interferon gamma (IFNγ) response to spike protein measured in an enzyme‐linked immunospot and a whole blood‐based interferon gamma release assay (IGRA). Whole blood was collected into sodium heparin vacutainer tubes and processed within 4 h from draw. For the evaluation of SARS‐CoV‐2 Spike‐specific T‐cell responses, heparinized whole‐blood samples were stimulated ex‐vivo with Spike protein (SARS‐CoV‐2 IGRA stimulation tube set, EuroImmun, Germany), in strict adherence to the manufacture protocol. Plasma was collected after 24 h of stimulation, and secreted IFNγ was quantified (ELISA DuoSet, R&D Systems, Minneapolis, Minnesota, USA). Results are presented as the difference between IFNγ levels in response to Spike versus background response to no antigen control. Values above 50 pg/ml of Spike‐specific response were considered positive.
On the day of the serologic test, all subjects were queried and filled in a questionnaire about local or systemic adverse events that occurred after the fourth vaccine dose. Relevant data were extracted from the medical records and included demographic characteristics, complete blood count, Binet stage, serum immunoglobulin levels, mutational status of the immunoglobulin heavy chain variable (IGHV) gene and analysis of genomic aberrations by fluorescent in situ hybridization (FISH), and TP53 mutations whenever available.
2.4. Statistical analysis
All statistical analysis and visualization were done using R statistical computing software (version 3.6.3). Non‐normal variables were described as Median with Inter‐Quartile Range (IQR). Pearson's Chi‐square test was used for testing association between two large‐sample categorical variables, and Fisher's exact test was used for testing association between small‐sample categorical variables. Continuous variables were tested for normality using QQ‐plots and Shapiro–Wilk's tests. Some continuous variables were log‐10 scaled prior to analysis to fit normality. Correlations between normally distributed variables were calculated using Pearson's product–moment method, and correlation significance was calculated using F‐test. Paired t‐tests were used to compare two measures from the same patient. Univariate and multivariate binary logistic regression models were fitted to determine the influence of both categorical and continuous variables upon patients' immune response. Variables which were significantly associated with response at a significance level p < .1 in the univariate models were included in the multivariate analysis.
3. RESULTS
3.1. Patient characteristics
From January to April 2022, this prospective study involved 67 patients with CLL/SLL who are treated in Chaim Sheba Medical Centre and 85 age matched control subjects from home care facilities in Israel. Patients' baseline demographic and disease characteristics are summarized in Table 1. The median age of patients with CLL was 71.5 years (IQR, 64.9–75.8 years), and 72.3 (IQR, 67.2–75.8) for healthy control subjects. The proportion of males in the CLL group was 70.1% (N = 41) and 33% (N = 28) in the control group. Twenty‐two patients with CLL (32.8%) were treatment naïve, 32 (47.7%) were on active therapy, 10 (14.7%) were previously treated, in clinical complete remission (CR) or partial remission (PR), and 3 patients (4.5%) were experiencing disease relapse after being previously treated. The median time from CLL diagnosis to vaccination was 7.7 years (IQR, 4.7–10.6). The median time from the third vaccine dose to the fourth vaccine dose was 175 days (IQR, 174–175 days), with median time of 11.67 months (IQR, 11.53–11.73 months) from the second vaccine dose. The time from the fourth vaccine dose to serology and cellular testing was 14 days.
TABLE 1.
Patient baseline characteristics
| Parameter | Patients with CLL (n = 67) |
|---|---|
| Age, median (IQR), y | 71.46 [64.90, 75.82] |
| Male sex, N (%) | 47 (70.1) |
| Time since CLL diagnosis, median (IQR), y | 10 (7.9, 14.1) |
| Time since 3rd booster (IQR), days | 175 [174, 175] |
| Binet stage, a N (%) | |
| A | 27 (40.3) |
| B | 3 (4.5) |
| C | 3 (4.5) |
| R‐CIRS | |
| <6 | 49 (73.1) |
| ≥6 | 18 (26.9) |
| IGHV mutational status, N (%) | |
| Mutated | 13 (19.4) |
| Unmutated | 22 (32.8) |
| Unknown | 32 (47.8) |
| FISH, N (%) | |
| Normal | 12 (17.9) |
| del(13q) | 16 (23.9) |
| Trisomy 12 | 11 (16.4) |
| del(11q) | 9 (13.4) |
| del(17p) and/or TP53mut | 8 (12.0) |
| Disease/treatment status, N (%) | |
| Treatment‐naive | 22 (32.8) |
| On‐therapy | 32 (47.8) |
| Off‐therapy in remission | 10 (14.9) |
| Off‐therapy in relapse | 3 (4.5) |
| Laboratory parameters, median (IQR) | |
| Absolute lymphocyte count, (103/L) | 4.88 [1.78, 13.77] |
| IgG, mg/dL | 681 [541, 966] |
| IgM, mg/dL | 24.8 [18.8, 42.8] |
| IgA, mg/dL | 68.10 [46.80, 130.00] |
| Ongoing treatment, N (%) | |
| BTKi | 22 (32.8) |
| Venetoclax | 10 (14.9) |
| Time since last anti‐CD20 antibody N (%) | |
| <12 mo | 6 (17.6) |
| ≥12 mo | 28 (82.4) |
Abbreviation: FISH, fluorescence in situ hybridization.
Treatment‐naive patients and patients in relapse.
3.2. Anti‐spike antibody response in patients with CLL compared to controls
Two weeks after the fourth vaccine, IgG antibodies were detected in 37 out of 67 CLL patients (55.2%), in comparison with 33 patients (49.3%) before the fourth vaccine. Although only 4 patients seroconverted, the antibody titer increased by 10‐fold, from 4.3 (IQR, 0.1–117.65 BAU/ml) before to 41.3 (IQR, 0.4–1185 BAU/ml) after the fourth vaccination. Healthy control subjects had 91.5% (77/85) positive serologic response prior to the fourth vaccination and 100% positive serologic response 2 weeks after the fourth vaccination with a 6‐fold increase in titers from 608.5 (IQR, 301.8–1205.6 BAU/ml) to 3642.3 (IQR, 2025.4–7356.9 BAU/ml). In comparison, control subjects had 141‐fold higher titer than CLL patients before the vaccine and 88‐fold higher titer than CLL patients 2 weeks after the fourth vaccine (Figure 1). Among treatment naïve patients with CLL, 20 of 22 (91%) had positive serologic response with antibody titer increase of median 112.5 (IQR 8.9–436.2) to 794.1 (IQR 71.9–3474.1), compared to 9/32 (28.1%) of patients of ongoing therapy. Seven of 22 (31.8%) BTKi treated patients responded, and 2/10 (20%) BCL2i treated patients responded. Only 1 of 6 patients (16.6%) treated with anti CD20 during the last 12 months had positive serologic response with antibody titer of 15.5 and 14.8 BAU/ml before and after the fourth vaccine, respectively.
FIGURE 1.
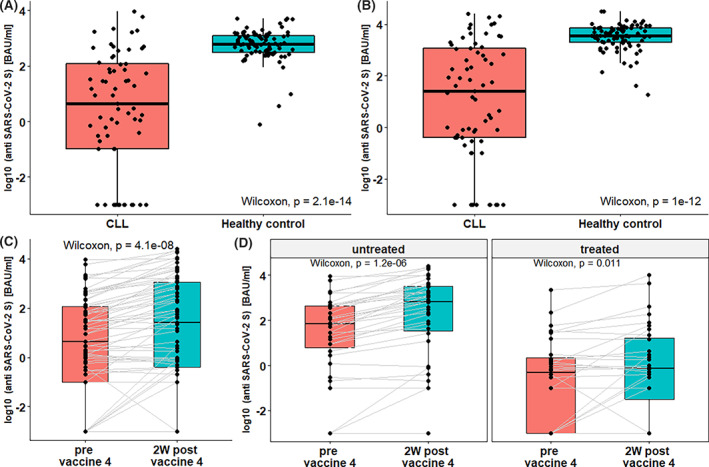
Anti‐SARS CoV‐2 spike antibody response in patients with CLL and healthy controls before and after the fourth vaccine. Boxplots comparing antibody response rate in patients with CLL (n = 67) and healthy controls (HC, n = 85) prior the fourth vaccination (A) and 2 weeks after (B). Wilcoxon tests found significant lower titer among patients with CLL for both comparisons (p < .001), with 141‐fold lower titers prior‐fourth vaccination and 88‐fold lower titers post‐ fourth vaccination. (C) Antibody titers rise among all patients, with CLL (n = 67) post‐fourth vaccination (Wilcoxon, p < .001). (D) Serologic titer before and after vaccination for untreated (N = 34, Wilcoxon p < .001) and treated (N = 33, p = .011) patients
3.3. Neutralization assay and anti‐IgG antibody levels
Anti‐RBD antibody levels linearly correlated with neutralizing antibodies titers (log transformed, R p = 0.7, p < .001), both pre‐ and post‐fourth vaccine. Neutralization titers were lower in patients with CLL in comparison with healthy controls, and lower in treated patients in comparison with treatment naïve patients (Figure 2).
FIGURE 2.
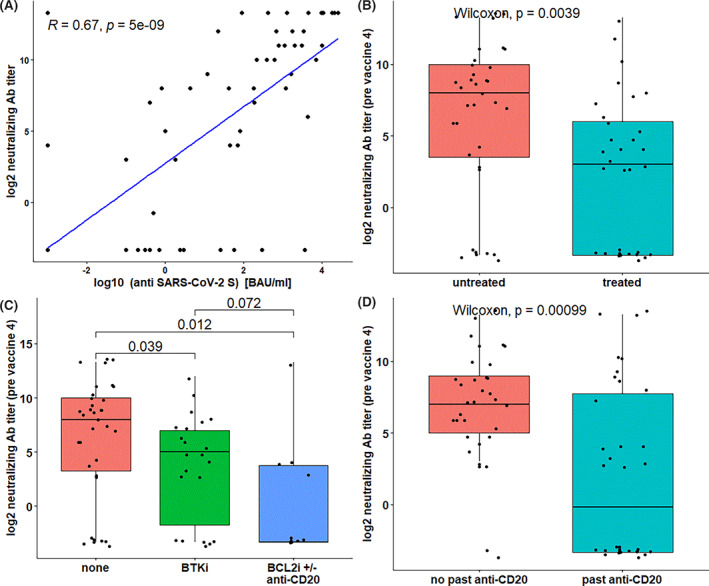
Pseudo‐typed virus neutralization assay and the anti‐spike antibody levels. A scatter plot of anti‐spike protein antibody titers vs. anti‐SARS CoV‐2 neutralizing antibody titers (A) reveal strong correlation between the results of two assays (Peasron's product–moment R = .67, F‐test p < .001). Boxplots shows significantly higher neutralizing antibody titers prior‐fourth vaccine (B) among untreated patients (n = 34) compared with treated patients (n = 33, Wilcoxon p = .004), (C) compared with BTKi‐treated (n = 22, p = .039) and BCL2i ± anti‐CD20‐treated patients (n = 10, p = .012), and compared with (D) patients treated with anti‐CD20 therapy (n = 34) and untreated (n = 33, p < .001)
3.4. SARS‐CoV2 spike specific T‐cell response
Functional T‐cell response after ex‐vivo stimulation with Spike protein assay was performed in 26 patients from our cohort, on blood samples drawn right before the fourth vaccination and 2 weeks afterward. Fourteen of the patients were treatment naïve and 12 were treated with BTKi. Pre‐vaccination, 11 of 26 (42.3%) patients had positive T‐cell response, compared with 22 (84.6%) responders 2 weeks after vaccination. When adjusted for treatment, 4 of 10 (40%) patients treated with BTKi and 7 of 16 (43.8%) treatment naïve patients had positive T‐cell response before vaccination. Two weeks post vaccination, 8 of 10 (80%) treated with BTKi and 14 of 16 (87.5%) treatment naïve patients tested positive. Fisher's exact test found significant association between therapy when the fourth vaccine was given and T‐cell response, both for pre‐vaccination (p = .021) and post vaccination (p = .033) results (Figure 3).
FIGURE 3.
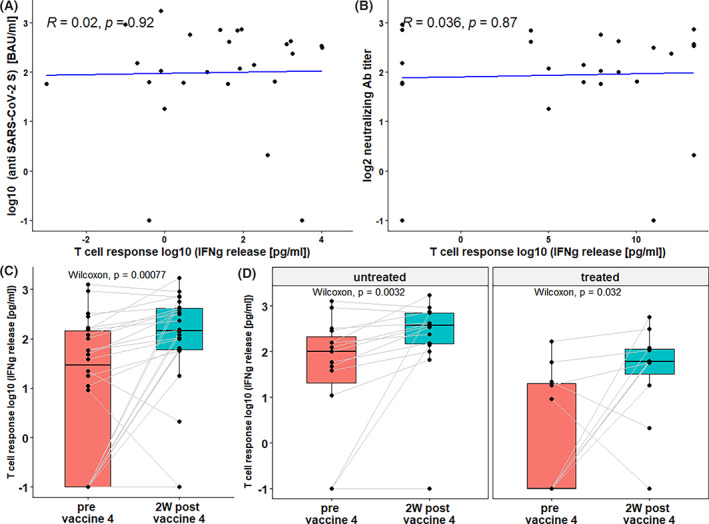
Humoral and T‐cell immune response after fourth BNT162b2 mRNA COVID‐19 vaccine. Spike specific T‐cell response was determined using IFNγ‐release assay in (n = 26) patients with CLL. Scatter plots of T‐cell response vs. anti‐SARS CoV‐2 Spike protein antibody titers (A) and anti‐SARS CoV‐2 neutralizing antibody titers (B) reveals no significant correlation between T‐cell response and neither antibodies (Peasron's product–moment R = .02, .036; F‐test p = .92, .87, respectively). (C) Boxplots show significant rise in T‐cell response after fourth vaccine (Wilcoxon p < .001). T‐cell response was significantly elevated in untreated patients (n = 14, p = .003) and BTKi‐treated patients (n = 12, p = .03)
3.5. Correlation between anti RBD, neutralization assay and T‐cell response
No significant correlation was found between spike‐specific T‐cell count and standard IgG titers or neutralizing antibody titer, both for all patients and when adjusting for BTKi therapy. Eight of 12 patients (67%) with negative T‐cell response before the fourth vaccination had positive neutralizing antibodies, with median level of 512 (range 16–512). Among 22 of 26 patients (86%) with positive T‐cell response after the fourth vaccination, 17 patients (77%) had neutralizing antibodies, with median level of 512 (range 16–10 000), and 2 of the 3 patients (67%) with negative T‐cell response had neutralizing antibodies (Figure 3).
3.6. Predictors of serologic response to fourth vaccine
In a univariate analysis conducted to identify baseline covariates associated with serologic response to the fourth vaccination, we found that prior positive serology after the second (OR = 1.55, [CI:1.1–2.18], p = .015) or third (OR = 1.99, [CI:1.48–2.68], p < .001) vaccination, and pre‐fourth vaccination positive serology (OR = 2.4, [CI:2.14–2.69], p < .001) or neutralizing antibodies titer (OR = 2.04, CI = [1.65–2.51], p < .001), were associated with better chances of post‐fourth vaccination serologic response. Additional significant predictors of serologic outcome were treatment naïve patients (OR = 1.69, [CI:1.29–2.21], p < .001), years since diagnosis (OR = 0.51, [CI: .35–.74], p < .001) and increase in platelet count (OR = 2.37, [CI: 1.04–5.38], p = .04). In contrast, pre‐vaccination anti‐CD20 therapy (OR = 0.6, [CI: 0.49–0.74], p < .001), current BTKi therapy (OR = 0.68, [CI: 0.54–0.87], p = .003), current BCL2i therapy (OR = 0.56, [CI: 0.4–0.76], p < .001), and any active therapy on the fourth vaccination day (OR = .63, CI = [.48–.81], p < .001) were associated with failure to achieve positive serologic response. No significant association was found between serologic response and patients' age, gender, absolute lymphocyte count, hemoglobin, and blood immunoglobulin (IgG, IgA, IgM) titers. Notably, pre‐ and post‐fourth T‐cell response was not significantly associated with serologic response (Table 2).
TABLE 2.
Univariate analysis for serologic response to fourth vaccine
| Positive | Negative | Total | p | OR | 95% CI | |
|---|---|---|---|---|---|---|
| Age at vaccination 4 | 67 | |||||
| ≤65 y | 11 | 6 | 17 | Reference | ||
| >65 y | 26 | 24 | 50 | .37 | 0.88 | [0.67–1.16] |
| Gender | 67 | |||||
| Male | 26 | 21 | 47 | Reference | ||
| Female | 11 | 9 | 20 | .98 | 1 | [0.77–1.3] |
| Years since diagnosis | 67 | <.001 | 0.95 | [0.93–0.98] | ||
| Past anti‐CD20 Tx | 67 | |||||
| No | 28 | 5 | 33 | Reference | ||
| Yes | 9 | 25 | 34 | <.001 | 0.56 | [0.46–0.68] |
| Tx at vaccine 4 | 66 | |||||
| Untreated | 27 | 7 | 34 | Reference | ||
| BTKi | 8 | 14 | 22 | <.001 | 0.65 | [0.51–0.82] |
| BCL2i | 2 | 8 | 10 | <.001 | 0.55 | [0.4–0.75] |
| Active Tx at vaccine 4 | ||||||
| No | 28 | 7 | 34 | Reference | ||
| Yes | 9 | 23 | 32 | <.001 | 0.6 | [0.48–0.73] |
| Hemoglobin (mg/dL) | 67 | .72 | 1.01 | [0.96–1.07] | ||
| Platelets (K/μL) | 67 | .028 | 1.0026 | [1.0037–1.0014] | ||
| WBC (K/μL) | 67 | .746 | 1.05 | [0.78–1.42] | ||
| ALC | 67 | .31 | 1.11 | [0.91–1.34] | ||
| ANC | 67 | .74 | 1.1 | [0.62–1.98] | ||
| IgG | 67 | |||||
| >median | 19 | 14 | 33 | Reference | ||
| <median | 18 | 16 | 34 | .7 | 0.95 | [0.75–1.22] |
| IgA | 67 | |||||
| >median | 18 | 15 | 33 | Reference | ||
| <median | 19 | 15 | 34 | .9 | 1.01 | [0.8–1.29] |
| IgM | 67 | |||||
| >median | 20 | 13 | 33 | Reference | ||
| <median | 17 | 17 | 34 | .4 | 0.9 | [0.71–1.14] |
| Vaccine 2 seropositivity | 53 | |||||
| Negative | 19 | 25 | 44 | Reference | ||
| Positive | 8 | 1 | 9 | .011 | 1.58 | [1.12–2.22] |
| Vaccine 3 seropositivity | 67 | |||||
| Negative | 1 | 9 | 10 | Reference | ||
| Indeterminate | 3 | 11 | 14 | .51 | 1.12 | [0.8–1.57] |
| Positive | 26 | 7 | 33 | <.001 | 1 99 | [1.48–2.67] |
| Pre vaccine 4 seropositivity | 67 | |||||
| Negative | 4 | 30 | 34 | Reference | ||
| Positive | 33 | 0 | 33 | <.001 | 2.42 | [2.16–2.7] |
3.7. Adverse events
Within 2 weeks after the fourth vaccination, 52 of 64 patients (81.3%) reported adverse events. The most common adverse event was local pain in the injection site, which was reported by 44 patients (68.8%). Overall, 32 patients (50%) reported at least one systemic adverse event, and all were mild. The most frequently reported systemic reactions were weakness or fatigue (n = 22, 34.4%), myalgia (n = 11, 17.2%), headache (n = 9, 14.1%), runny nose (n = 7, 10.9%) and night sweats (n = 6, 9.4%). Only 4 patients reported fever or shivering (6.25%). Other less common adverse events reported in a few patients were rash, itching, increase in lymphadenopathy and sore throat.
3.8. COVID‐19 infection during follow‐up
During a time period of 3 months post‐fourth vaccination, 14 of all 67 patients (21%) developed COVID‐19 infection. Eleven of them (77%) were treated with anti‐viral therapy (8 paxlovid, 3 molnupiravir) post infection. All except one had non‐severe disease and recovered uneventfully. One patient was hospitalized due to severe disease and recovered uneventfully. The infected patients' serologic titer and neutralizing antibodies levels were 0.8 (IQR: 0.5–35.25) and 8 (IQR: 0–16), respectively, compared with 80.55 (IQR: .725–1536) and 256 (IQR: 0–4096) in the non‐infected patients. Five of 6 tested patients had positive T‐cell response post vaccination. Thirteen of 14 COVID‐19 infected patients were males.
In a univariate model fitting, lower COVID‐19 infection risk was significantly associated with age > 65, female gender, second vaccine titer, and pre‐ and post‐fourth vaccine titer. Post‐third vaccination titer showed a tendency to significance (p = .06). CLL treatment at fourth vaccine showed closely significant association with an infection risk (p = .06). In a multi‐variate model combining post‐fourth vaccine antibody titer, CLL treatment at fourth vaccine, age group and gender, only age > 65, gender and post‐fourth vaccine antibody titer were found as significant predictors of infection.
4. DISCUSSION
Patients with CLL are among the most vulnerable to COVID19 complications. Therefore, protecting them from SARS‐CoV‐2 infection continues to be a major challenge. Since the third vaccine efficacy decreases over time, we decided to prospectively evaluate the humoral and cellular response of CLL patients to a second booster and compare their serologic response with that of age matched individuals.
The global immune response after standard two‐dose BNT162b2 mRNA vaccination in patients with CLL is in the range of 40%–66%, with up to 50%–73% positive antibody response rate in treatment naïve, less than 30% in patients who had been previously treated with BTKi and BCL2i, and less than 10% after anti CD20 antibodies. 9 , 16 Although antibody titers significantly decline overtime, a quarter of patients with negative serologic response responded to a booster dose given 6 months after the second vaccine. 17 , 18 , 19 In our study, the positive serologic response 6 months after the third vaccine was 49.3%, with only marginal increase after a fourth dose to 55%, while in age matched controls positive serologic response increased from 91% to 100%. As expected, the response is more robust in treatment naïve patients not compromised by the effect of therapy, with 91% serologic response in treatment naïve compared with only 28.1% in patients previously treated. Importantly, the rise in RBD‐specific antibody levels increased by 10‐fold following the second booster. Nevertheless, antibody titers were 88‐fold higher in age matched controls, in comparison with patients with CLL. By comparison with our results, Parry et al. have recently showed an improved response rate to a third vaccine dose with positive serologic response in 80% of patients with CLL. However, after a fourth vaccination dose serologic response was positive in 77% of patients showing no further increase in seroconversion rate. 20
We found that treatment naïve patients and more recent diagnosis of CLL were more likely to produce serologic response. Consistent with previous publications, after second and third vaccination we also found markedly impaired vaccine response with all forms of therapy including ongoing BTK or BCL2 inhibitors at time of vaccination or in patients with prior anti‐CD20 mAb. 9 , 16 , 19 , 21 , 22 Our results indicate that failure to respond to two vaccination doses and low titers prior‐fourth vaccine are associated with low response rates to second booster and with low levels of anti‐S. Unlike reports of previous vaccinations, we did not find significant association between serologic response and patients' age, absolute lymphocyte count, and blood immunoglobulin levels. 9 , 16 , 19 , 23
Although known to be a risk factor for COVID‐19 infection, age > 65 was associated with lower infection risk in our cohort. This finding may be explained by more precautious behavior and lower environmental exposure among this age group.
Higher levels of immune markers, including binding and neutralizing antibodies, correlate with risk reduction of symptomatic disease and severe infection; however, there is no single threshold value indicative of absolute protection. 24
The SARS‐CoV‐2‐specific T‐cells are associated with accelerated viral clearance and protection from severe COVID‐19. 25 , 26 However, the exact contribution of the cellular immune response in protection of SARS‐CoV2 from infection, transmission, and clinical outcome is largely unknown. Much less is known regarding the vaccine impact on the cellular response. 27 , 28 , 29 Patients with a greater number of CD8 T‐cells had improved survival in mixed hematological malignancies. 30 Previously tested small cohorts demonstrated SARS CoV‐2 specific T‐cell immunity in about 80% of CLL convalescents and vaccinated patients who were usually, but not always, paralleled by seroconversion. 21 , 23 , 31 In our study, T‐cell response improved from 42.3% before the vaccine to 84.6% after 2 weeks. While more treatment naïve patients had T‐cell response (87.5%) compared with patients treated with BTKi (80%), both groups had a 2‐folds increase in the number of responders after the booster. Although positive T‐cell response in 5 of 6 patients who acquired COVID19 infection indicated no protection from future infection, their good outcome could be related to preserved T‐cell function. The relation between T‐cell response and outcome of COVID19 infection in patients with CLL needs to be further investigated in more patients. While humoral and cellular responses are generally coordinated in intact immune response, we found no association between anti‐RBD serology and neutralizing activity to SARS‐CoV‐2 specific T‐cell response.
In 3 months follow up, fifth of patients with CLL acquired COVID19 infection. Despite differences in exact timing, exposure risk behavior, age and comorbidity, this was not different from the findings of a study performed in health care workers in our center. 11 Our study was too small to assess vaccine efficacy, and the good outcome compared with earlier pandemic reports of 11%–35% mortality could be attributed to different virulence of variants, patients' heterogeneity and antiviral therapy promptly initiated in most of our patients. 8 , 31 , 32 , 33 , 34
The strength of our study is the comprehensive evaluation of serologic response, neutralization assay, and assessment of T‐cell response after a second booster in patients with CLL, given the uncertainty about cellular immune response and its protective role against SARS‐CoV2.
Furthermore, we used seroneutralization assay in comparison with controls of the same age and reported on the clinical outcome of patients with CLL who were infected with SARS‐CoV‐2. However, we have not tested specific neutralizing assay against the prevalent and resistant Omicron variant. In addition, since all infected patients recovered uneventfully and most of them received antiviral therapy with acquisition of first symptoms, we cannot indicate a surrogate of vaccine efficacy or whether certain antibody titers confer protective immunity. Still, our findings suggest that a second booster is safe and increases protection against SARS‐CoV‐2 infection in patients with CLL.
While our study clearly demonstrates reduced immune response to BNT162b2 fourth vaccine in comparison with healthy subjects, it also demonstrates that the second booster has a clear positive impact on the serologic as well as cellular response in patients with CLL.
AUTHOR CONTRIBUTIONS
Ohad Benjamini: designed, organized contributed patients' data and wrote the manuscript. Rotem Gershon: performed all statistical analysis. Erez Bar‐Haim, Hila Cohen, Noam Erez: performed T cell response assessment. Yaniv Lustig, Meirav Kedmi, Eelena Ribakovsky, Abraham Kneller, Ram Doolman, Tammy Hod, Itzhak Levi: contributed patients' data. Galia Rahav, Abraham Avigdor: contributed patients' data and wrote the manuscript.
CONFLICT OF INTEREST
No conflict of interest to declare.
ACKNOWLEDGMENTS
We thank the study coordinators, with special thanks to Mrs. Halperin Rivka.
Benjamini O, Gershon R, Bar‐Haim E, et al. Cellular and humoral response to the fourth BNT162b2 mRNA COVID‐19 vaccine dose in patients with CLL. Eur J Haematol. 2023;110(1):99‐108. doi: 10.1111/ejh.13878
Galia Rahav and Abraham Avigdor have equally contributed to the manuscript.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. https://coronavirus.jhu.edu/map.html. 2022.
- 2. Barda N, Dagan N, Balicer RD. BNT162b2 mRNA COVID‐19 vaccine in a nationwide mass vaccination setting. Reply. N Engl J Med. 2021;384(20):1970. [DOI] [PubMed] [Google Scholar]
- 3. Regev‐Yochay G, Amit S, Bergwerk M, et al. Decreased infectivity following BNT162b2 vaccination: a prospective cohort study in Israel. Lancet Reg Health Eur. 2021;7:100150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID‐19 vaccine. N Engl J Med. 2020;383(27):2603‐2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bar‐On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against COVID‐19 in Israel. N Engl J Med. 2021;385(15):1393‐1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 COVID‐19 vaccine over 6 months. N Engl J Med. 2021;385(24):e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Forconi F, Moss P. Perturbation of the normal immune system in patients with CLL. Blood. 2015;126(5):573‐581. [DOI] [PubMed] [Google Scholar]
- 8. Chatzikonstantinou T, Kapetanakis A, Scarfò L, et al. COVID‐19 severity and mortality in patients with CLL: an update of the international ERIC and campus CLL study. Leukemia. 2021;35(12):3444‐3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Benjamini O, Rokach L, Itchaki G, et al. Safety and efficacy of the BNT162b mRNA COVID‐19 vaccine in patients with chronic lymphocytic leukemia. Haematologica. 2022;107(3):625‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iuliano AD, Brunkard JM, Boehmer TK, et al. Trends in disease severity and health care utilization during the early omicron variant period compared with previous SARS‐CoV‐2 high transmission periods – United States, December 2020–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(4):146‐152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Regev‐Yochay G, Gonen T, Gilboa M, et al. Efficacy of a fourth dose of COVID‐19 mRNA vaccine against omicron. N Engl J Med. 2022;386:1377‐1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Magen O, Waxman JG, Makov‐Assif M, et al. Fourth dose of BNT162b2 mRNA COVID‐19 vaccine in a nationwide setting. N Engl J Med. 2022;386:1603‐1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bar‐On YM, Goldberg Y, Mandel M, et al. Protection by a fourth dose of BNT162b2 against omicron in Israel. N Engl J Med. 2022;386:1712‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hallek M, Cheson BD, Catovsky D, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131(25):2745‐2760. [DOI] [PubMed] [Google Scholar]
- 15. Lustig Y, Sapir E, Regev‐Yochay G, et al. BNT162b2 COVID‐19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single‐centre, longitudinal cohort study in health‐care workers. Lancet Respir Med. 2021;9(9):999‐1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Herishanu Y, Avivi I, Aharon A, et al. Efficacy of the BNT162b2 mRNA COVID‐19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137(23):3165‐3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tadmor T, Benjamini O, Braester A, Rahav G, Rokach L. Antibody persistence 100 days following the second dose of BNT162b mRNA COVID‐19 vaccine in patients with chronic lymphocytic leukemia. Leukemia. 2021;35(9):2727‐2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Herishanu Y, Avivi I, Levi S, et al. Six‐month antibody persistence after BNT162b2 mRNA COVID‐19 vaccination in patients with chronic lymphocytic leukemia. Blood Adv. 2022;6(1):148‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Herishanu Y, Rahav G, Levi S, et al. Efficacy of a third BNT162b2 mRNA COVID‐19 vaccine dose in patients with CLL who failed standard 2‐dose vaccination. Blood. 2022;139(5):678‐685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Parry H, Bruton R, Roberts T, et al. COVID‐19 vaccines elicit robust cellular immunity and clinical protection in chronic lymphocytic leukemia. Cancer Cell. 2022;40(6):584‐586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shen Y, Freeman JA, Holland J, et al. COVID‐19 vaccine failure in chronic lymphocytic leukaemia and monoclonal B‐lymphocytosis; humoural and cellular immunity. Br J Haematol. 2022;197(1):41‐51. [DOI] [PubMed] [Google Scholar]
- 22. Roeker LE, Knorr DA, Pessin MS, et al. Anti‐SARS‐CoV‐2 antibody response in patients with chronic lymphocytic leukemia. Leukemia. 2020;34(11):3047‐3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Haydu JE, Maron JS, Redd RA, et al. Humoral and cellular immunogenicity of SARS‐CoV‐2 vaccines in chronic lymphocytic leukemia: a prospective cohort study. Blood Adv. 2022;6(6):1671‐1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Feng S, Phillips DJ, White T, et al. Correlates of protection against symptomatic and asymptomatic SARS‐CoV‐2 infection. Nat Med. 2021;27(11):2032‐2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sette A, Crotty S. Adaptive immunity to SARS‐CoV‐2 and COVID‐19. Cell. 2021;184(4):861‐880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bertoletti A, le Bert N, Qui M, Tan AT. SARS‐CoV‐2‐specific T cells in infection and vaccination. Cell Mol Immunol. 2021;18(10):2307‐2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sekine T, Perez‐Potti A, Rivera‐Ballesteros O, et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID‐19. Cell. 2020;183(1):158‐168 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goel RR, Painter MM, Apostolidis SA, et al. mRNA vaccines induce durable immune memory to SARS‐CoV‐2 and variants of concern. Science. 2021; 374(6572):abm0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cohen H, Rotem S, Elia U, et al. T cell response following anti‐COVID‐19 BNT162b2 vaccination is maintained against the SARS‐CoV‐2 omicron B.1.1.529 variant of concern. Viruses. 2022;14(2):347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bange EM, Han NA, Wileyto P, et al. CD8(+) T cells contribute to survival in patients with COVID‐19 and hematologic cancer. Nat Med. 2021;27(7):1280‐1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Blixt L, Bogdanovic G, Buggert M, et al. COVID‐19 in patients with chronic lymphocytic leukemia: clinical outcome and B‐ and T‐cell immunity during 13 months in consecutive patients. Leukemia. 2022;36(2):476‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cuneo A, Rigolin GM, Coscia M, et al. Management of chronic lymphocytic leukemia in Italy during a one year of the COVID‐19 pandemic and at the start of the vaccination program. A campus CLL report. Hematol Oncol. 2021;39(4):570‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mato AR, Roeker LE, Lamanna N, et al. Outcomes of COVID‐19 in patients with CLL: a multicenter international experience. Blood. 2020;136(10):1134‐1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Roeker LE, Eyre TA, Thompson MC, et al. COVID‐19 in patients with CLL: improved survival outcomes and update on management strategies. Blood. 2021;138(18):1768‐1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


