Abstract
Background
The impact of the COVID‐19 pandemic on palliative care intervention (PCIs) in patients with do‐not‐resuscitate (DNR) status remains uncertain.
Methods
Case–control study of patients with DNR order with RT‐PCR confirmed SARS‐COV2 infection (cases), and those with DNR order but without SARS‐COV2 infection (controls). The primary outcome measures included timing and delivery of PCIs, and secondary measures included pre‐admission characteristics and in‐hospital death.
Results
The ethnicity distribution was comparable between 69 cases and 138 controls, including Black/African Americans (61% vs. 44%), Latino/Hispanics (16% vs. 26%) and White (9% vs. 20%) (trend‐p = .54). Cases were employed more (17% vs. 6%, adjusted‐p = .012), less frail (fit 47% vs. 21%; mildly frail 22% vs. 36%; frail 31% vs. 43%, trend‐p = .018) and had fewer comorbidities than controls. Cases had higher chances of intensive care unit admission (HR 1.76 [95% CI: 1.03–3.02]) and intubation (53% vs. 30%, p = .002), lower chances to be seen by palliative care team (HR .46 [.30–.70]) and a longer time to palliative care visit than controls (β per ln‐day .67 [.00–1.34]). In the setting of no‐visiting hospitals policy, we did not find significant increase in utilisation of video conferencing (22% vs. 13%) and religious services (12% vs. 12%) both in case and in controls.
Conclusion
Do‐not‐resuscitate patients with COVID‐19 had better general health and higher employment status than ‘typical’ DNR patients, but lower chances to be seen by the palliative care team. This study raises a question of the applicability of the current palliative care model in addressing the needs of DNR patients with COVID‐19 during the pandemic.
Keywords: COVID‐19, do not resuscitate, palliative care, SARS‐COV2 infection, underrepresented minorities
Do‐not‐resuscitate patients with COVID‐19 had better general health and higher employment status than ‘typical’ DNR patients, but lower chances to be seen by the palliative care team. This study raises a question of the applicability of the current palliative care model in addressing the needs of DNR patients with COVID‐19 during the pandemic.
1. INTRODUCTION
The COVID‐19 pandemic has had an unprecedented impact on the healthcare system and lives of patients and their caregivers. As of January 2022, COVID‐19 resulted in >350 million cases and >5.5 million deaths worldwide, and in the United States >70 million cases and >860,000 deaths. 1 Given high case fatality and prevalence of severe acute respiratory syndrome coronavirus 2 (SARS‐COV2) infection requiring intensive care unit admissions, many patients and their families have been burdened with a sudden bearing of bad news, ‘no‐visiting’ hospitals' policy, and expedited encounter of end‐of‐life decisions. 2 , 3
Do‐not‐resuscitate (DNR) status has imposed another ethical challenge in a severe acute illness such as SARS‐COV2 infection. DNR status can be obtained as advanced directives or direct communication with the patients and or their family members. In extreme cases, a DNR status can be acquired as a unilateral decision when life‐sustaining treatments, such as intubation and cardiopulmonary resuscitation (CPR), are considered ineffective. 4 The COVID‐19 pandemic has driven a massive surge in hospitalised patients with high mortality forcing to adopt unilateral DNR status or even ‘blanket’ DNR status for patients with SARS‐COV2 infection, which has inflicted significant ethical dilemma in the medical community. 4 , 5 , 6
Physicians have been extremely challenged to provide adequate palliative care interventions (PCIs) amidst the COVID‐19 pandemic due to the high volume and acuity of patients with SARS‐COV2 infection bounded by limited resources and rationing of care. 7 More than ever, it became critically important to ensure that life‐sustaining treatments are aligned with the patient's values and goals to avoid unnecessary invasive therapy in seriously ill or frail patients, but also not to restrict their use in those who would benefit from them.
Hence, the uncertainty regarding the impact of the COVID‐19 pandemic on the acquisition of DNR status, and PCIs is at least twofold. First, it remains unclear whether patients with SARS‐COV2 infection who acquired DNR status differed from the ‘typical’ DNR population without SARS‐COV2 infection. Second, whether discrepancies have occurred in the timing and delivery of PCIs between these two groups. For this purpose, this study aimed at comparing pre‐admission characteristics and multiple aspects of delivery of PCIs between DNR patients with and those without SARS‐COV2 infection.
2. METHODS
This is a retrospective case–control study of patients admitted between 08/01/2019 and 10/01/2020 to University Hospital (UH) in Newark, New Jersey, an academic not‐for‐profit safety‐net hospital. Most of the patient population served by UH are underrepresented minorities, such as Black/African Americans and Latino/Hispanics. This study was approved by the institutional review board and conducted under the Health and Insurance Portability and Accountability Act (HIPAA) to protect personal information. Reporting of the study conforms to broad EQUATOR guidelines. 8
2.1. Participants
The source population consisted of all patients (n = 1370) 18 years or older who acquired DNR status on their latest admission during the study period. The case group included all patients with SARS‐COV2 infection confirmed by reverse transcription‐polymerase chain reaction (RT‐PCR) assays (n = 69). The controls were chosen by a computer‐generated random sampling procedure in a 1:2 ratio using all patients with DNR status but without SARS‐COV2 infection (n = 1301) based on their admission time (prepandemic: n = 69, pandemic: n = 69) (Figure 1). We decided to include both control groups in analyses and adjusted for admission time, showing the effect of SARS‐COV infection regardless of whether control was admitted during or outside the pandemic period.
FIGURE 1.
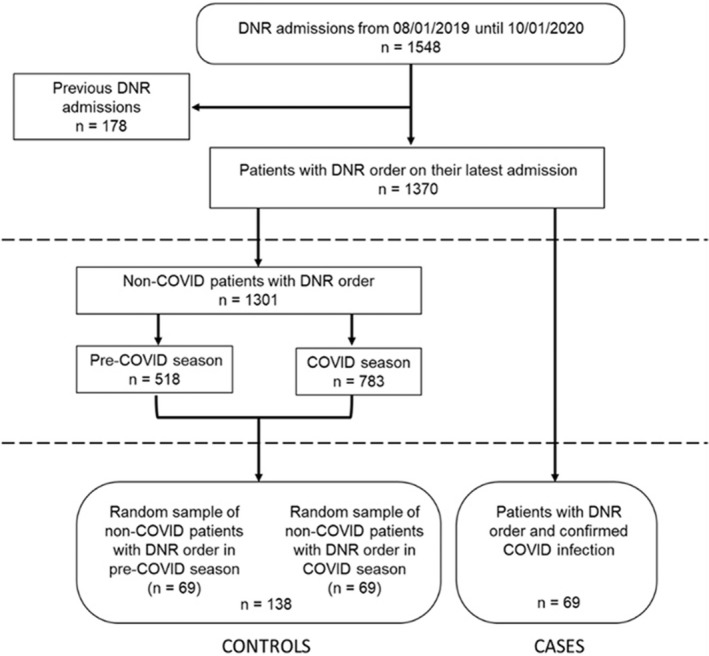
Flow chart of the study population. All patients (n = 1370) 18 years or older who acquired DNR status on their latest admission were included. Cases were all patients with SARS‐COV2 infection confirmed by RT‐PCR (n = 69), and controls were a random sample of all patients with DNR status but without SARS‐COV2 infection in 1:2 ratio (total n = 138: prepandemic 69, pandemic 69). These two subgroups of controls were chosen to test the effect of the SARS‐COV2 infection on DNR status and palliative care interventions by controlling for the ‘pandemic’ factor (i.e. availability and quality of care in the setting of limited resources). DNR, do‐not‐resuscitate
2.2. Data collection
Patients' pre‐admission characteristics included age, sex, body mass index, ethnicity, marital status, employment status, health insurance, the living situation before admission, presence of Practitioners Orders for Life‐Sustaining Treatment (POLST) or advanced directives (AD) forms, oxygen requirements before admission, and comorbidities including hypertension, diabetes, coronary artery disease, peripheral artery disease, heart failure, chronic kidney disease, stroke, dementia, active cancer, chronic obstructive pulmonary disease, liver disease and human immunodeficiency virus status. We also collected information on immunosuppression (if a patient was on the immunosuppressive agent or had CD4 count <500 cell/mm3 in the past 6 months), opioid use disorder, drug and alcohol use disorders, and the total number of admissions in the year preceding the index admission.
We calculated clinical frailty score (CFS) and performance status (PS) for each patient using the information provided in the initial Emergency Department physician's note and history and physical examination note from the admitting team. The CFS bases the frailty assessment on how a patient functioned before hospital admission. 9 The CFS is an ordinal hierarchical scale that numerically ranks frailty on the scale from 1 to 9 with a score of 1 being very fit, 2 well, 3 managing well, 4 vulnerable, 5 mildly frail, 6 moderately frail, 7 severely frail, 8 very severely frail and 9 terminally ill. 10 Recently, the CFS has been validated as a prediction tool for in‐hospital mortality in patients with SARS‐COV2 infection. 11 The PS is another ordinal hierarchical scale that numerically ranks patient's daily living abilities on the scale from 1 to 3 with a score of 1 being fully active or capable of self‐care, 2 limited self‐care, and the need for subacute rehabilitation or nursing home, and 3 no self‐care or bedbound.
The primary outcome measures included timing and delivery of PCIs, and secondary measures included pre‐admission characteristics and in‐hospital mortality. Multiple aspects of the hospital course of DNR patients with and without COVID‐19 were analysed, including patient's decision‐making capacity (DMC) on admission and at the time when DNR order was acquired, code status on admission, days from admission until DNR order, who determined DNR status (i.e. patient, family member, partner, spouse, physician or unknown), patient's location when DNR order was placed (ED, floor or ICU), admission to intensive care unit (ICU), days from admission to ICU, days in ICU, requirements for mechanical ventilation (MV), days on MV, consult to palliative care (PC) team, days from admission to PC consult, utilisation of assisted family video conferencing and religious services and in‐hospital deaths. The loss of DMC before the change of DNR status was derived by subtraction of the DMC variable when the DNR order was acquired from the DMC variable on admission.
2.3. Statistical analysis
The categorical variables are presented as percentages, and continuous variables due to uniformity as median and interquartile range. The CFS score was transformed into three categories (fit 1–3, mildly frail 4–5, and frail 6–9) as previously described. 11 The PS score was dichotomized into fully active or capable of self‐care and limited activity. Variables ‘Days from admission to DNR’, ‘Days from admission to ICU’, ‘Days in ICU’, ‘Days on mechanical ventilation’ and ‘Days from admission to palliative care consult’ were ln‐transformed for further analyses. The distributions of continuous variables were examined for normality by visual histogram inspection, calculating the skewness coefficient, and using the Kolmogorov–Smirnov test of normality. If the normality assumption was met, statistically significant differences were evaluated using the Student's t‐test. However, if the normality assumption was unmet, a Mann–Whitney U test was performed; the χ 2 test tested categorical variables.
We performed binary logistic regression to test whether a difference in pre‐admission characteristics between DNR patients with and without COVID‐19 remain robust after controlling for a patient's admission time, showing the effect of COVID‐19 regardless of the time when control was admitted. Multiplicative interaction between admission time and SARS‐COV2 infection was also explored. We performed Cox regression analysis to test the impact of COVID‐19 on time to palliative care consult and time to admission to ICU. Furthermore, we performed Cox regression analysis to test the effect of COVID‐19 on time to death during hospitalisation. To control for potential confounders, we adjusted the analysis using a patient's admission time and the propensity score made of age, sex, and all covariates from Table 1 showing a crude p‐value ≤ .10 including BMI, ethnicity, employment status, hypertension, diabetes, chronic kidney disease, active cancer, liver disease, number of comorbidities, immunosuppression, opioid use disorder and clinical frailty score. We also provided Kaplan–Meier estimate curves for these outcomes stratified by case and control groups.
TABLE 1.
Pre‐admission characteristics of the study population
| Controls with DNR order (n = 138) | COVID‐19 cases with DNR order (n = 69) | p‐Value | Adj. p‐Value | |
|---|---|---|---|---|
| Age, years median (IQR) | 69 (60–79) | 69 (62–80) | .82 | .99 |
| Male sex, n (%) | 70 (51) | 38 (55) | .56 | .86 |
| BMI, kg/m2 median (IQR) | 26.0 (23.5–29.7) | 28.1 (24.9–33.9) | .006 * | .001 * |
| <18.5 | 11 (8) | 2 (3) | ||
| 18.5–24.9 | 43 (31) | 15 (22) | ||
| 25.0–29.9 | 53 (38) | 25 (36) | ||
| ≥30.0 | 31 (23) | 27 (39) | ||
| Ethnicity/race, n (%) | .56* | .54* | ||
| Black | 60 (44) | 42 (61) | .018 | .30 |
| Hispanic/Latino | 36 (26) | 11 (16) | ||
| White | 27 (20) | 6 (9) | ||
| Other | 15 (11) | 10 (14) | ||
| Marital status, n (%) | .99 | .76 | ||
| Married | 36 (26) | 18 (26) | ||
| Single | 63 (46) | 33 (48) | ||
| Divorced/separated | 13 (9) | 6 (9) | ||
| Widowed | 23 (17) | 11 (16) | ||
| Unknown | 3 (2) | 1 (1) | ||
| Employment status, n (%) | ||||
| Employed | 7 (6) | 12 (17) | .012 | .020 |
| Unemployed | 83 (60) | 34 (50) | ||
| Retired | 46 (34) | 23 (33) | ||
| Insurance status, n (%) | ||||
| No insurance or charity care | 18 (13) | 9 (13) | .99 | .81 |
| Medicare | 68 (49) | 30 (44) | ||
| Medicaid | 29 (21) | 15 (22) | ||
| Other | 23 (17) | 15 (22) | ||
| Living situation, n (%) | ||||
| Home | 112 (83) | 51 (74) | .23 | .22 |
| SAR | 6 (5) | 2 (3) | ||
| Long‐term care facility/nursing home | 15 (11) | 15 (22) | ||
| Hospice | 1 (1) | 1 (1) | ||
| Comorbidities, n (%) | ||||
| Hypertension | 93 (69) | 55 (80) | .12 | .39 |
| Diabetes | 52 (39) | 35 (51) | .10 | .20 |
| Coronary artery disease | 22 (17) | 8 (12) | .35 | .15 |
| Peripheral artery disease | 6 (5) | 2 (3) | .58 | .97 |
| Heart failure | 26 (20) | 11 (16) | .56 | .62 |
| Chronic kidney disease | 40 (30) | 11 (16) | .03 | .017 |
| Stroke | 21 (16) | 11 (16) | .96 | .87 |
| Dementia | 18 (13) | 11 (16) | .62 | .97 |
| Active cancer | 25 (19) | 4 (6) | .01 | .025 |
| COPD | 23 (17) | 14 (21) | .55 | .66 |
| Liver disease | 30 (22) | 6 (9) | .02 | .14 |
| HIV | 8 (6) | 3 (4) | .86 | .33 |
| No. of comorbidities, median (IQR) | 4 (2–5) | 3 (2–4) | .67 | .38 |
| Need for O2 pre‐admission, n (%) | 12 (9) | 7 (10) | .73 | .55 |
| Immunosuppression, n (%) | 15 (11) | 3 (4) | .11 | .08 |
| Opioid use disorder, n (%) | 20 (15) | 0 (0) | na | na |
| Drug abuse, n (%) | 17 (12) | 3 (4) | .15 | .43 |
| Alcohol abuse, n (%) | 16 (12) | 1 (1) | .010 | .20 |
| No. of admissions in the past year, median (IQR) | 1 (0–3) | 1 (0–2) | .10 | .10 |
| Clinical frailty score, n (%) | .002 | .018 | ||
| Fit 1–3 | 27 (21) | 32 (47) | ||
| Mildly frail 4–5 | 48 (36) | 15 (22) | ||
| Frail 6–9 | 57 (43) | 21 (31) | ||
| Performance status, n (%) | ||||
| Fully active/capable of self‐care | 61 (44) | 43 (62) | .015 | .12 |
| POLST/AD prior to admission, n (%) | 20 (14) | 5 (7) | .14 | .18 |
Note: The adj. p‐value shows a statistically significant difference between patients with and without COVID‐19 regardless of when a control was admitted. Bold p‐value signifies p < .05.
Abbreviations: AD, advanced directives; Adj., adjusted; BMI, body mass index; DNR, do‐not‐resuscitate; No., number; O2, oxygen; POLST, practitioners orders for life‐sustaining treatment; SAR, subacute rehabilitation facility.
p‐Value for trend.
Differences between values were considered to be statistically significant at p < .05. All analyses were performed with a complete dataset using the spss 28.0 software package for Microsoft Windows.
3. RESULTS
Table 1 shows the pre‐admission characteristics of 207 investigated patients. The mean age of patients with and without COVID‐19 was 69 (IQR: 60–80) years with a similar male‐to‐woman ratio (men: cases 55%, controls 51%, adjusted p = .86), but higher BMI class in patients with COVID‐19 than in patients without COVID‐19 (28.1 kg/m2 [24.9–33.9] vs. 26.0 [23.5–29.7], adjusted p = .001). The composition of ethnicity was comparable between patients with and without SARS‐COV2 infection, including Black/African Americans (61% vs. 44%), Latino/Hispanics (16% vs. 26%) and White (9% vs. 20%) (adjusted p‐value for trend = .54). The subgroup analysis showed more Black/African Americans in patients with COVID‐19 than patients without COVID‐19 (61% vs. 44%, crude p = .018) but lost statistical significance after adjusting for admission time.
Notably, the percentage of employed participants was more than two times higher in patients with COVID‐19 than those without COVID‐19 regardless of when control was admitted (cases 17% vs. controls 6%, adjusted p = .020). Similarly, clinical frailty score demonstrated that patients with COVID‐19 were less frail before the admission than those without COVID‐19 (fit [score 1–3] cases 47% vs. controls 21%; mildly frail 4 , 5 22% vs. 36%; frail 6 , 7 , 8 , 9 31% vs. 43%, adjusted p‐Value for trend = .020). These results were supported by analysis of comorbidities showing fewer comorbidities among patients with COVID‐19, including chronic kidney disease, active cancer, liver disease and opioid use disorder (Table 1). No significant interaction of COVID‐19 and the patient's admission time on pre‐admission characteristics between the two groups were noted.
Figure 2 describes hospital course of DNR patients with COVID‐19 and those without COVID‐19. Both patients with and without COVID‐19 had similar DMC with predominantly full code on admission (DMC: 54% vs. 51%, adjusted p = .86; Full Code: 81% vs. 72%, adjusted p = .17). Despite more patients with COVID‐19 lost their DMC by the time DNR status was acquired (23% vs. 12%, p = .044), this difference lost statistical significance after adjusting for the admission time (p = .19). Time from admission until DNR order was acquired in patients with COVID‐19 lagged on average of 2 days behind patients without COVID‐19 regardless of when control was admitted (median: 3 days [IQR: 1–9] vs. 1 day [0–5], p = .0017). Similar results were noted regarding the information on who determined the DNR status, as well as the patient's location at the time when DNR was acquired.
FIGURE 2.
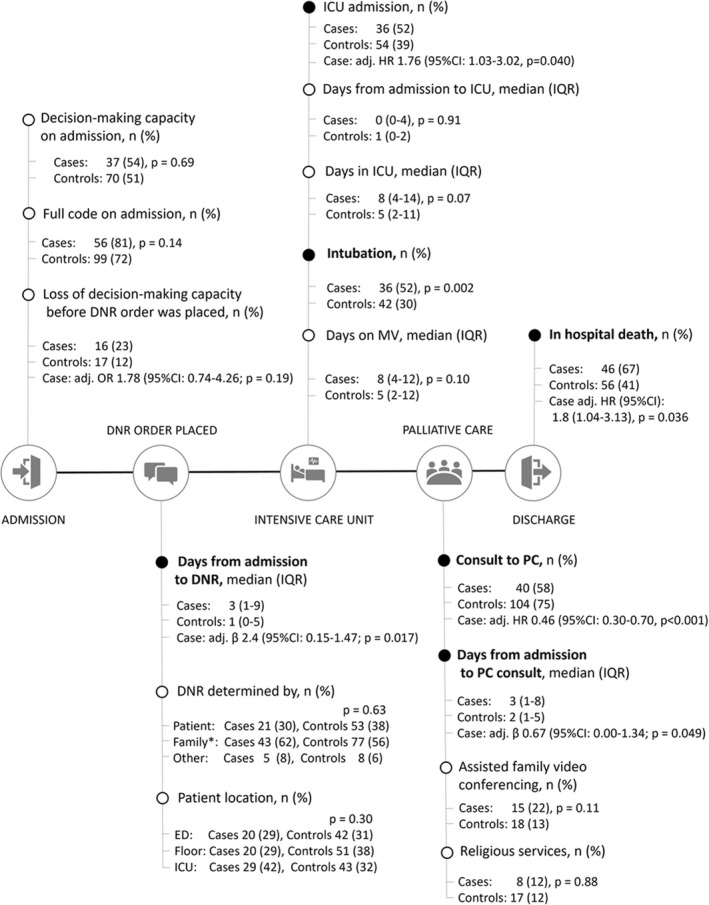
Hospital course of DNR patients with COVID‐19 and those without COVID‐19. For each feature, the figure displays the absolute number and percentage among patients with and without COVID‐19 with a corresponding p‐value for the difference. The effect was expressed as either odds ratio (OR), hazard ratio (HR), or beta coefficient with 95% confidence interval (CI) adjusted for admission time, showing the effect of COVID‐19 regardless of when a control was admitted. The ‘day‐’variables were presented per one ln‐day. Adj., adjusted; DNR, do‐not‐resuscitate; ICU, intensive care unit; PC, palliative care. A black dot indicates statistically significant results (p < .05).
Patients with COVID were more likely to be admitted to ICU (HR 1.76 [95% CI: 1.03–3.02], p = .040). They also had higher percentage of intubation (52% vs. 30%, p = .002) (Figure S1). However, they had .46 times (95% CI: .30–.70, p < .001) lower chances of having a palliative care consult than those without COVID‐19 (Figures 2 and 3), and longer time to initial visit by palliative care team (β per ln‐day .67 [.00–1.340], p = .049). Notably, both patients with and without COVID‐19 did not show increase in utilisation of assisted family video conferencing (22% vs. 13%, adjusted p = .67) and religious services (12% vs. 12%, adjusted p = .57) despite no‐visiting hospital policy.
FIGURE 3.
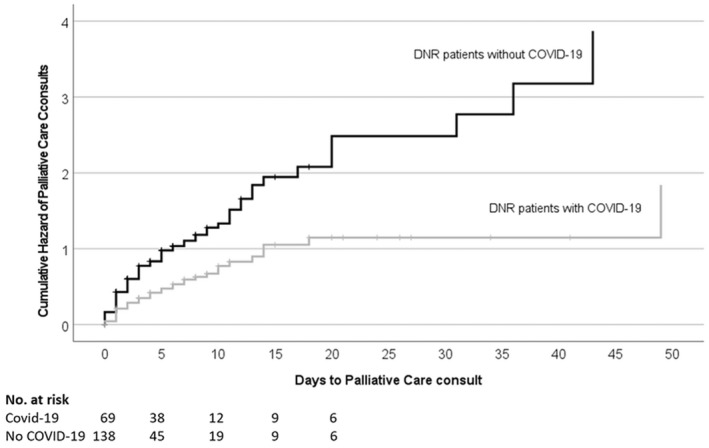
Kaplan–Meier estimates of the time to palliative care consult during hospitalisation.
The Cox regression analysis showed that the chances of in‐hospital death among patients with COVID‐19 were 1.8 times higher than in those without COVID‐19 after controlling for multiple confounders (95% CI: 1.04–3.11, p = .036) (Figure S2). This analysis was adjusted for admission time and propensity score, which included age, sex, BMI, ethnicity, employment status, hypertension, diabetes, chronic kidney disease, active cancer, liver disease, number of comorbidities, immunosuppression, opioid use disorder and clinical frailty score.
4. DISCUSSION
The relevance of this study for a medical and general public audience is threefold. First, our results demonstrate the extent of pre‐admission differences between patients with and without COVID‐19 who acquired DNR status during hospitalisation. Second, we identified several intervening points that may be used to improve the current palliative care system in COVID‐19 or similar future pandemics. Third, these results are derived from the cohort of underrepresented minorities as the most vulnerable population affected by the COVID‐19 pandemic.
Our findings add strength to previously reported data on Black/African Americans predominance among patients with COVID‐19 who acquired DNR status during hospitalisation. These findings could not be explained by different health insurance status, marital status, or comorbidities and are comparable with the results published by Moriyama et al. 12 in their cohort of palliative care patients across three New York hospitals. Unfortunately, it remains unclear whether a predominance of Black/African Americans with COVID‐19 who acquired DNR status were also influenced by factors such as the patient's preference, clinician‐dependent factors or institutional biases. 7 , 13
The results showed in this manuscript extend the current knowledge 12 , 14 by showing that among DNR patients, those who had COVID‐19 also had better general health than ‘typical’ DNR patients without COVID‐19 regardless of whether ‘typical’ DNR patients were sampled inside or outside the pandemic period. This interpretation is supported by fewer comorbidities, higher employment status, and better CFS score on admission—a recently validated prognostic marker of in‐hospital mortality in the European cohort of patients with COVID‐19. 11 While frail patients with multiple chronic comorbidities have higher morbidity and mortality, physicians are experienced in treating acute exacerbation of chronic conditions; therefore, they have more predictable clinical course and less urgency for goals of care discussion. At the time of this study sample, COVID‐19 has had high morbidity and mortality with little known effective treatment. Thus, physicians have heightened sense for goals of care discussion leading to more DNR orders. Our study illustrates a rare time where one diagnosis alone, COVID‐19, has far more outcome predictive value than baseline frailty and comorbidities combined.
Furthermore, this study raises a question of the applicability of the current palliative care model, which was previously developed to address the needs of patients with chronic illnesses, in addressing the needs of DNR patients with COVID‐19. This question is essential considering that patients' dependents (e.g. children, spouse and family members) are affected both by a sudden loss of their loved ones and direct economic support. It is also feasible to think that the patients with COVID‐19 and their families were less likely prepared for goals of care discussion compared with the control group who suffered from more chronic, terminal illnesses, and therefore likely had more immediate needs for palliative care consult in hospital. Yet, future research is necessary to explore this issue in depth.
A unique strength of our study was the analysis of timing and delivery of PCIs during hospitalisation. Despite heightened awareness of COVID‐19 leads to poor outcomes, more DNR orders were placed only after patients with COVID‐19 had become intubated. On average, they lagged 2 days behind controls for the acquisition of DNR status and had a 54% lower chance to be seen by the palliative care team. This information is important given that timely goals of care discussion could have helped avoid unwanted intubations. Specifically for hospitalised patients, a focal discussion regarding the inappropriate or unwanted use of invasive interventions helps both patients and their family members with suffering and the rationing of care resources in severely limited settings. Finally, additional training of healthcare providers on effective delivery of high‐quality goals of care discussion would be beneficial in critical situations with limited human resources, such as the COVID‐19 pandemic.
This study also indicates that there might be an underutilisation of assisted family video conferencing and religious services. These results might be biased to some extent by ununiform reporting in patient charts. Nonetheless, the findings should be considered seriously given the likelihood of passing away without anyone present at the bedside and its burden to the patients and their family members during the COVID‐19 pandemic. 15 Further improvements are, therefore, required on an organisational level including, for example, a built‐in video call system to facilitate communication among patients, family members and healthcare providers. This approach would facilitate a team‐based delivery of more dedicated PCIs when physical human‐to‐human interaction is limited. To this end, some authors have also suggested developing decision algorithms for rationing care, training on effective symptoms management, and exploring alternative delivery methods of palliative care services such as telemedicine. 16 Our study supports such concept and adds new research evidence to help its implementation in clinical practice.
The study findings should be interpreted in the context of their limitations. The first limitation was retrospective data collection, which inherently carries the risk of bias. However, we chose a pragmatic study design as we have been limited by the acuity of SARS‐COV2 infection and extremely demanding period for healthcare professionals. This also limited the availability of potentially important information, such as the specific reasons for acquiring DNR status. Nonetheless, we tried to restrict the bias by selecting all patients with DNR status and not only those seen by the palliative care team. As our data have shown, many patients with COVID‐19 who acquired DNR status were not seen by the palliative care team. Furthermore, data from cases and controls were collected in the same way using the standardised criteria for chart review and randomly distributed among data collectors to restrict information bias. Thus, even if information bias existed, it would dilute the effect towards zero or no effect. Finally, we have restricted participants to the initial COVID‐19 surge, limiting the sample size. However, we wanted to ensure that both study groups were exposed to the same PCIs during the study period.
5. CONCLUSION
Do‐not‐resuscitate patients with COVID‐19 had better general health and higher employment status than ‘typical’ DNR patients, but lower chances to be seen by the palliative care team. This study raises a question of the applicability of the current palliative care model in addressing the needs of DNR patients with COVID‐19 during the pandemic.
CONFLICT OF INTEREST
Authors report no conflict of interest.
Supporting information
Appendix S1
Brankovic M, Jeon H, Markovic N, et al. Palliative care of COVID‐19 patients with do‐not‐resuscitate status in underrepresented minorities. Eur J Clin Invest. 2023;53:e13889. doi: 10.1111/eci.13889
REFERENCES
- 1. John Hopkins Coronavirus Resource Center . Accessed August 25, 2020. https://coronavirus.jhu.edu/map.html
- 2. Schloesser K, Simon ST, Pauli B, et al. “Saying goodbye all alone with no close support was difficult” – dying during the COVID‐19 pandemic: an online survey among bereaved relatives about end‐of‐life care for patients with or without SARS‐CoV2 infection. BMC Health Serv Res. 2021;21(1):998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vahidy FS, Drews AL, Masud FN, et al. Characteristics and outcomes of COVID‐19 patients during initial peak and resurgence in the Houston Metropolitan Area. JAMA. 2020;324(10):998‐1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Curtis JR, Kross EK, Stapleton RD. The importance of addressing advance care planning and decisions about do‐not‐resuscitate orders during novel coronavirus 2019 (COVID‐19). JAMA. 2020;323(18):1771‐1772. [DOI] [PubMed] [Google Scholar]
- 5. Dyer C. Some care home residents may have died because of blanket DNR orders, says regulator. BMJ. 2020;371:m4733. [DOI] [PubMed] [Google Scholar]
- 6. Dyer C. Covid‐19: campaigner calls for national guidance to stop DNR orders being made without discussion with patients and families. BMJ. 2020;369:m1856. [DOI] [PubMed] [Google Scholar]
- 7. Sultan H, Mansour R, Shamieh O, Al‐Tabba A, Al‐Hussaini M. DNR and COVID‐19: the ethical dilemma and suggested solutions. Front Public Health. 2021;9:560405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Simera I, Moher D, Hoey J, Schulz KF, Altman DG. A catalogue of reporting guidelines for health research. Eur J Clin Invest. 2010;40(1):35‐53. [DOI] [PubMed] [Google Scholar]
- 9. Jørgensen R, Brabrand M. Screening of the frail patient in the emergency department: a systematic review. Eur J Intern Med. 2017;45:71‐73. [DOI] [PubMed] [Google Scholar]
- 10. Darvall JN, Bellomo R, Paul E, et al. Frailty in very old critically ill patients in Australia and New Zealand: a population‐based cohort study. Med J Aust. 2019;211(7):318‐323. [DOI] [PubMed] [Google Scholar]
- 11. Sablerolles RSG, Lafeber M, van Kempen JAL, et al. Association between clinical frailty scale score and hospital mortality in adult patients with COVID‐19 (COMET): an international, multicentre, retrospective, observational cohort study. Lancet Healthy Longev. 2021;2(3):e163‐e170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moriyama D, Scherer JS, Sullivan R, Lowy J, Berger JT. The impact of COVID‐19 surge on clinical palliative care: a descriptive study from a New York hospital system. J Pain Symptom Manage. 2021;61(3):e1‐e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johnson T, Walton S Jr, Levine S, Fister E, Baron A, O'Mahony S. Racial and ethnic disparity in palliative care and hospice use. Am J Manag Care. 2020;26(2):e36‐e40. [DOI] [PubMed] [Google Scholar]
- 14. Ouellet JA, Prsic EH, Spear RA, et al. An observational case series of targeted virtual geriatric medicine and palliative care consults for hospitalized older adults with covid‐19. Ann Palliat Med. 2021;10(6):6297‐6306. [DOI] [PubMed] [Google Scholar]
- 15. Strang P, Bergström J, Martinsson L, Lundström S. Dying from COVID‐19: loneliness, end‐of‐life discussions, and support for patients and their families in nursing homes and hospitals. A National Register Study. J Pain Symptom Manage. 2020;60(4):e2‐e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fadul N, Elsayem AF, Bruera E. Integration of palliative care into COVID‐19 pandemic planning. BMJ Support Palliat Care. 2021;11(1):40‐44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


