Abstract
In March 2022, the Omicron variant of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) surged during the Coronavirus Disease 2019 (COVID‐19) pandemic in Shanghai, but over 90% of patients were mild. This study included 1139 COVID‐19 patients mildly infected with the Omicron variant of SARS‐CoV‐2 in Shanghai from May 1 to 10, 2022, aiming to clarify the demographic characteristics and clinical symptoms of patients with mild Omicron infection. The clinical phenotypes of Omicron infection were identified by model‐based cluster analysis to explore the features of different clusters. The median age of the patients was 41.0 years [IQR: 31.0–52.0 years] and 73.0% were male. The top three clinical manifestations are cough (57.5%), expectoration (48.3%), and nasal congestion and runny nose (43.4%). The prevalence of nasal congestion and runny nose varied significantly across the doses of vaccinations, with 23.1% in the unvaccinated population and 30%, 45.9%, and 44.3% in the 1‐dose, 2‐dose and 3‐dose vaccinated populations, respectively. In addition, there were significant differences for fever (23.1%, 26.0%, 28.6%, 18.4%), head and body heaviness (15.4%, 14.0%, 26.7%, 22.4%), and loss of appetite (25.6%, 30.0%, 33.6%, 27.7%). The unvaccinated population had a lower incidence of symptoms than the vaccinated population. Cluster analysis revealed that all four clusters had multisystemic symptoms and were dominated by both general and respiratory symptoms. The more severe the degree of the symptoms was, the higher the prevalence of multisystemic symptoms will be. The Omicron variant produced a lower incidence of symptoms in mildly infected patients than previous SARS‐CoV‐2 variants, but the clinical symptoms caused by the Omicron variant are more complex, so that it needs to be differentiated from influenza.
Keywords: clinical characteristics, COVID‐19, mild infection, Omicron variant
1. INTRODUCTION
Coronavirus Disease 2019 (COVID‐19) is a highly contagious respiratory disease caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), which has rapidly swept around the world in the past 3 years. As of June 6, 2022, over 529 million infections and over six million deaths have been reported globally. 1 SARS‐COV‐2 continues to mutate rapidly, with Alpha, Beta, Delta, Lambda, Omicron, and other variants appearing successively. The Omicron variant, that was originally reported in South Africa in November 2021, has emerged as a virulent variant of concern, 2 becoming the dominant variant in the fourth wave of the global COVID‐19 pandemic. It has since infected an estimated 300 million people worldwide in just a few months. 1 , 3 In China, the Omicron variant was first discovered in November 2021 and has spread diffusely since February 2022. 4 , 5 By June 6, 2022, more than 600 000 infections had been found cumulatively in Shanghai. 6
The Omicron variant differs markedly from previous major variants in that it can recognize host cells, elicit an immune response quickly, and is associated with increased infectivity as well as a greater immune escape. 7 , 8 However, although Omicron is 60% more infectious than the original variant and is considered to be the most infectious variant known, its clinical symptoms are relatively mild. 9 , 10 From March 1 to April 18, 2022, 397 933 cases of SARS‐CoV‐2 infection were reported in Shanghai, of which only 21 were severe. 11 In fact, in patients with a mild infection, previous studies still observed some atypical multisystem symptoms besides respiratory and systemic symptoms, such as dyssomnia, hypomnesia, and depression. 12 Therefore, it is particularly important to study the clinical symptoms in patients with mild Omicron infections since many people think of the Omicron infection as a “big flu.” To date, six studies have reported the clinical symptoms of COVID‐19 cases caused by the Omicron variant, but most of them had relatively small sample sizes or listed few types of symptoms. 3 , 5 , 9 , 13 , 14 , 15 In addition, most of the studies were reported during the early stages of the Omicron outbreak. However, most importantly, the reported studies did not describe the clinical symptoms in terms of symptom levels, which can provide a better understanding of the difference between the same symptoms in Omicron and other SARS‐CoV‐2 variants or other diseases. Hence, the clinical symptoms of mild COVID‐19 infection caused by the SARS‐CoV‐2 Omicron variant remain unclear.
This study investigated the mild cases of Omicron variant infection in Shanghai. We described their clinical characteristics, identified the clinical phenotypes of Omicron infection features using cluster analysis, explored the different characteristics among different clusters, and provided a reference basis for the prevention and treatment of the Omicron variant infection.
2. MATERIALS AND METHODS
2.1. Study objects
The study participants were patients with mild COVID‐19 admitted to the mobile cabin hospital in Tianhua Road, Jinshan district, between May 1 and 10, 2022. The diagnostic and classification criteria of COVID‐19 cases were based on the “Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 9)” (Table 1). This study was approved by the Ethics Committee of Longhua Hospital (Ethical Approval No. 2022LCSY020), and written informed consent was obtained from all patients.
Table 1.
Classification criteria of COVID‐19 cases
| Classification | Criteria |
|---|---|
| Mild | Mild clinical symptoms; no sign of pneumonia on imaging |
| Moderate | Fever and respiratory symptoms with radiological findings of pneumonia |
| Severe | 1. Respiratory distress (≥30 breaths/min) |
| 2. Oxygen saturation ≤93% at rest | |
| 3. PaO2/FiO2 ≤ 300 mmHg. In high‐altitude areas (>1000 m), PaO2/FiO2 shall be corrected as: PaO2/FiO2 × (atmospheric pressure [mmHg]/760) | |
| Cases with chest imaging that shows obvious lesion progression within 24–48 h >50% shall be managed as severe cases. | |
| Fatal | 1. Respiratory failure and requiring mechanical ventilation |
| 2. Shock | |
| 3. With other organ failure that requires ICU care |
Abbreviations: FiO2, fractional inspired oxygen; ICU, intensive care unit; PaO2, arterial oxygen partial pressure.
2.2. Data collection
Demographic characteristics and clinical symptoms were collected respectively through the patients' medical records and a questionnaire using an online crowdsourcing platform (Wenjuanxing, a platform providing functions equivalent to Amazon Mechanical Turk). The demographic characteristics included age, sex, marital status, occupation, ethnicity, history of hypertension and diabetes, allergies, smoking history, and vaccinations. Clinical symptoms included fever, chills, sweating, coughing, expectoration, hemoptysis, sore throat, loss of taste and smell, thirst, bitterness in the mouth, chest tightness, chest pain, nasal congestion, runny nose, shortness of breath, fatigue, depression, headache, head and body heaviness, soreness in the limbs, lack of appetite, nausea and vomiting, abdominal distension, diarrhea, constipation, hypomnesia, and dyssomnia. All symptoms were divided into four levels of severity (Supporting Information: Table 1). If any data were missing from the record or the record was unclear, we re‐obtained the data through direct communication with the treating physician from the mobile cabin hospital in Tianhua Road, Jinshan district, within 24 h of the record being taken. All data were checked by two physicians (M. W. and Y. T.) who were in charge of treating the patients.
2.3. Statistical methods
Baseline descriptions of demographic and clinical characteristics are presented as counts and percentages for categorical variables and as medians with first and third quartiles for continuous variables. The differences in demographic and clinical characteristics by vaccination dose were tested using Fisher's exact test for categorical variables and the Kruskal–Wallis test for continuous variables. Model‐based clustering was used to identify the clinical features of patients. The integrated completed likelihood criterion was used to estimate the number of clusters. To identify demographic features associated with cluster membership, Fisher's exact test for categorical variables and the Kruskal–Wallis test for continuous variables were performed accordingly.
All statistical analyses were performed using R 4.1.3, and model‐based clustering was performed using the VarSelLCM R package. Results with a p‐value <0.05 were considered to be statistically significant.
3. RESULTS
3.1. Demographic characteristics of cases
The demographic characteristics of the different vaccination‐dose populations were significantly different, except for those with comorbidities and allergies. In the vaccinated population, age gradually increased with the number of vaccination doses the participant had taken; however, all ages were lower than that in the unvaccinated population. This suggests that advanced age is predominant in the unvaccinated population. Moreover, more men than women had infections (Table 2).
Table 2.
Demographic characteristics of 1139 infections with mild COVID‐19
| Variable | Doses of vaccinations | p | ||||
|---|---|---|---|---|---|---|
| 0 (n = 39) | 1 (n = 50) | 2 (n = 318) | 3 (n = 732) | Total (n = 1139) | ||
| Age (year), median (IQR) | 51.0 (30.5–8.0) | 32.0 (29.0–39.0) | 35.0 (28.0–47.0) | 45.0 (33.0–53.0) | 41.0 (31.0–52.0) | <0.001 |
| Gender‐no. (%) | ||||||
| Male | 19 (48.7) | 41 (82.0) | 234 (73.6) | 537 (73.4) | 831 (73.0) | 0.004 |
| Female | 20 (51.3) | 9 (18.0) | 84 (26.4) | 195 (26.6) | 308 (27.0) | |
| Marriage‐no. (%) | ||||||
| Divorced/widowed | 1 (2.6) | 2 (4.0) | 8 (2.5) | 12 (1.6) | 23 (2.0) | <0.001 |
| Unmarried | 14 (35.9) | 30 (60.0) | 133 (42.0) | 218 (29.9) | 395 (34.8) | |
| Married | 24 (61.5) | 18 (36.0) | 176 (55.5) | 500 (68.5) | 718 (63.2) | |
| Occupation‐no. (%) | ||||||
| Employee | 17 (43.6) | 23 (46.0) | 157 (49.5) | 394 (54.0) | 591 (52.0) | <0.001 |
| Former | 2 (5.1) | 4 (8.0) | 10 (3.2) | 55 (7.5) | 71 (6.2) | |
| Others | 12 (30.8) | 22 (44.0) | 135 (42.6) | 261 (35.8) | 430 (37.9) | |
| Retiree/unemployed | 8 (20.5) | 1 (2.0) | 12 (3.8) | 19 (2.6) | 40 (3.5) | |
| Student | 0 (0.0) | 0 (0.0) | 3 (0.9) | 1 (0.1) | 4 (0.4) | |
| Ethnicity‐no. (%) | ||||||
| Han | 39 (100.0) | 43 (86.0) | 309 (97.5) | 709 (97.1) | 1100 (96.8) | 0.003 |
| Others | 0 (0.0) | 7 (14.0) | 8 (2.5) | 21 (2.9) | 36 (3.2) | |
| Comorbidity‐no. (%) | ||||||
| Hypertension | 5 (12.8) | 2 (4.0) | 18 (5.7) | 37 (5.1) | 62 (5.5) | 0.208 |
| Diabetes | 0 (0.0) | 0 (0.0) | 7 (2.2) | 10 (1.4) | 17 (1.5) | 0.472 |
| Personal history‐no. (%) | ||||||
| Allergy | 4 (10.3) | 3 (6.0) | 8 (2.5) | 24 (3.3) | 39 (3.4) | 0.062 |
| Smoking | 9 (23.1) | 21 (42.0) | 100 (31.4) | 176 (24.0) | 306 (26.9) | 0.006 |
3.2. Clinical characteristics of cases
Overall, the 10 most frequently reported symptoms were coughing (57.5%), expectoration (48.3%), nasal congestion and runny nose (43.4%), thirst (43.2%), sweating (36.1%), a sore throat (35.9%), bitterness in the mouth (34.6%), fatigue (34.2%), limb soreness (32.7%), and dyssomnia (31.5%). The majority of the symptoms were levels 0 and 1, but the percentage of levels 2 and 3 for cough and sputum production was higher than 10%. For the unvaccinated population, the five most frequently reported symptoms were coughing (53.8%), expectoration (38.5%), fatigue (35.9%), soreness in the limbs (35.9%), and thirst (33.3%); while for the vaccinated population, the five most frequently reported symptoms were coughing (57.6%), expectoration (48.6%), nasal congestion and runny nose (44.1%), thirst (43.5%), and a sore throat (36.5%). Symptom incidence in the vaccinated population was similar to that of the total population. The unvaccinated population had a lower incidence of symptoms, and high‐frequency symptoms included both respiratory and systemic symptoms (Table 3, Supporting Information: Table 2).
Table 3.
The main clinical symptoms characteristics of 1139 infections with mild COVID‐19
| Variable | Doses of vaccinations | p | ||||
|---|---|---|---|---|---|---|
| 0 (n = 39) | 1 (n = 50) | 2 (n = 318) | 3 (n = 732) | Total (n = 1139) | ||
| Fever‐no. (%) | ||||||
| 0 | 30 (76.9) | 37 (74.0) | 227 (71.4) | 597 (81.6) | 891 (78.2) | 0.003 |
| 1 | 7 (17.9) | 10 (20.0) | 70 (22.0) | 115 (15.7) | 202 (17.7) | |
| 2 | 1 (2.6) | 2 (4.0) | 20 (6.3) | 18 (2.5) | 41 (3.6) | |
| 3 | 1 (2.6) | 1 (2.0) | 1 (0.3) | 2 (0.3) | 5 (0.4) | |
| Nasal congestion and runny nose‐no. (%) | ||||||
| 0 | 30 (76.9) | 35 (70.0) | 172 (54.1) | 408 (55.7) | 645 (56.6) | 0.010 |
| 1 | 6 (15.4) | 13 (26.0) | 124 (39.0) | 294 (40.2) | 437 (38.4) | |
| 2 | 3 (7.7) | 1 (2.0) | 21 (6.6) | 26 (3.6) | 51 (4.5) | |
| 3 | 0 (0.0) | 1 (2.0) | 1 (0.3) | 4 (0.5) | 6 (0.5) | |
| Head and body heaviness‐no. (%) | ||||||
| 0 | 33 (84.6) | 43 (86.0) | 233 (73.3) | 568 (77.6) | 877 (77.0) | 0.009 |
| 1 | 4 (10.3) | 7 (14.0) | 74 (23.3) | 143 (19.5) | 228 (20.0) | |
| 2 | 1 (2.6) | 0 (0.0) | 10 (3.1) | 21 (2.9) | 32 (2.8) | |
| 3 | 1 (2.6) | 0 (0.0) | 1 (0.3) | 0 (0.0) | 2 (0.2) | |
| Lack of appetite‐no. (%) | ||||||
| 0 | 29 (74.4) | 35 (70.0) | 211 (66.4) | 529 (72.3) | 804 (70.6) | 0.001 |
| 1 | 7 (17.9) | 10 (20.0) | 81 (25.5) | 170 (23.2) | 268 (23.5) | |
| 2 | 3 (7.7) | 3 (6.0) | 25 (7.9) | 32 (4.4) | 63 (5.5) | |
| 3 | 0 (0.0) | 2 (4.0) | 1 (0.3) | 1 (0.1) | 4 (0.4) | |
3.3. Four phenotypes from cluster analysis
Four clusters were identified using model‐based clustering, and the details of the distribution in the four clusters can be found in Supporting Information: Table 3. The difference of distribution of all symptoms in the four clusters was significant (p < 0.05), indicating that clustering results were reliable. The characteristics of the four clusters were described according to the degree and classification of symptoms (Figure 1).
Figure 1.
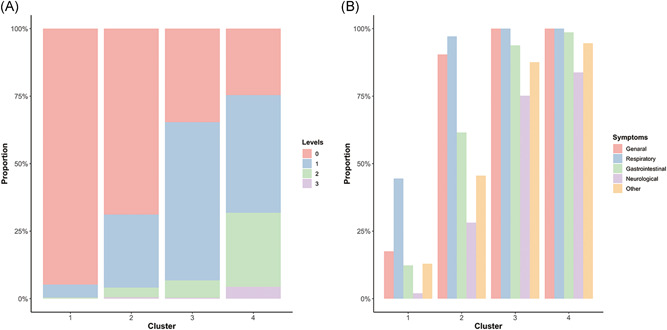
Distribution of symptom levels 0–3 and systematic classification in each cluster. Comparison of symptom levels and systematic classification in different clusters. The percentage of symptom level (A) and systematic classification in the clusters (B) are shown for all four clusters. Clusters represent the results obtained from the cluster analysis of the clinical symptom information in 1139 patients, and each cluster had similar clinical symptom characteristics. Level represents the degree of clinical symptoms, level 0 is asymptomatic and level 3 is the highest degree of symptoms. The system represents the organism system to which the symptoms belong.
In general, the severity of patients' symptoms increased sequentially from Clusters 1 through 4. Level 0 patients had the highest proportion in Cluster 1 and gradually decreased in the other clusters. In Clusters 2 and 3, the symptom severity gradually increased as levels 1 and 2 patients increased. In Cluster 4, levels 2 and 3 increased significantly, presenting with the most serious symptoms (Figure 1A).
The clinical symptoms were divided into five categories: general, respiratory, gastrointestinal, neurological, and other symptoms. In Cluster 1, the proportion of each systemic symptom was significantly lower than that in the other clusters. Compared with Cluster 1, general, respiratory, and gastrointestinal symptoms increased by 72.9%, 52.6%, and 49.2%, respectively, in Cluster 2. All patients in Clusters 3 and 4 had general and respiratory symptoms, and the incidence of other systemic symptoms exceeded 70% (Figure 1B). Combining the above results, we found that multisystem symptoms can occur in different clusters. General and respiratory symptoms were the predominant symptoms. Simultaneously, the more severe the symptoms, the higher is the incidence of multisystem symptoms.
3.4. Demographic characteristics and vaccination status in different clusters
The demographic characteristics and vaccination status of the four clusters are shown in Table 4, in which age and allergy history were significantly different between the different clusters (p < 0.05). Age tended to increase across the four clusters, and there were fewer people with an allergy history in Clusters 1, 2, and 3, and more people with an allergy history in Cluster 4. However, no statistical significance was found for other variables.
Table 4.
Demographic characteristics and vaccination status in different clusters
| Variable | Clusters | p | ||||
|---|---|---|---|---|---|---|
| 1 (n = 501) | 2 (n = 419) | 3 (n = 145) | 4 (n = 74) | Total (n = 1139) | ||
| Age (year), median (IQR) | 45.0 (31.0–53.2) | 39.0 (31.0–50.0) | 39.0 (31.0–49.0) | 45.0 (33.0–51.0) | 41.0 (31.0–52.0) | 0.003 |
| Gender‐no. (%) | ||||||
| Male | 375 (74.9) | 308 (73.5) | 98 (67.6) | 50 (67.6) | 831 (73.0) | 0.242 |
| Female | 126 (25.1) | 111 (26.5) | 47 (32.4) | 24 (32.4) | 308 (27.0) | |
| Marriage‐no. (%) | ||||||
| Divorced/widowed | 7 (1.4) | 10 (2.4) | 6 (4.2) | 0 (0.0) | 23 (2.0) | 0.083 |
| Unmarried | 178 (35.6) | 156 (37.3) | 40 (27.8) | 21 (28.4) | 395 (34.8) | |
| Married | 315 (63.0) | 252 (60.3) | 98 (68.1) | 53 (71.6) | 718 (63.2) | |
| Occupation‐no. (%) | ||||||
| Employee | 267 (53.4) | 209 (50.0) | 76 (52.8) | 39 (52.7) | 591 (52.0) | 0.373 |
| Former | 30 (6.0) | 27 (6.5) | 9 (6.2) | 5 (6.8) | 71 (6.2) | |
| Others | 179 (35.8) | 173 (41.4) | 52 (36.1) | 26 (35.1) | 430 (37.9) | |
| Retiree/unemployed | 23 (4.6) | 7 (1.7) | 6 (4.2) | 4 (5.4) | 40 (3.5) | |
| Student | 1 (0.2) | 2 (0.5) | 1 (0.7) | 0 (0.0) | 4 (0.4) | |
| Ethnicity‐no. (%) | ||||||
| Han | 484 (96.8) | 402 (96.2) | 142 (98.6) | 72 (97.3) | 1100 (96.8) | 0.571 |
| Others | 16 (3.2) | 16 (3.8) | 2 (1.4) | 2 (2.7) | 36 (3.2) | |
| Comorbidity‐no. (%) | ||||||
| Hypertension | 28 (5.6) | 23 (5.5) | 5 (3.5) | 6 (8.1) | 62 (5.5) | 0.543 |
| Diabetes | 7 (1.4) | 5 (1.2) | 5 (3.5) | 0 (0.0) | 17 (1.5) | 0.210 |
| Personal history‐no. (%) | ||||||
| Allergy | 7 (1.4) | 11 (2.6) | 9 (6.2) | 12 (16.2) | 39 (3.4) | <0.001 |
| Smoking | 127 (25.3) | 126 (30.1) | 39 (26.9) | 14 (18.9) | 306 (26.9) | 0.169 |
| Doses of vaccinations‐no. (%) | ||||||
| 0 | 18 (3.6) | 17 (4.1) | 3 (2.1) | 1 (1.4) | 39 (3.4) | 0.473 |
| 1 | 28 (5.6) | 15 (3.6) | 4 (2.8) | 3 (4.1) | 50 (4.4) | |
| 2 | 125 (25.0) | 129 (30.8) | 41 (28.3) | 23 (31.1) | 318 (27.9) | |
| 3 | 330 (65.9) | 258 (61.6) | 97 (66.9) | 47 (63.5) | 732 (64.3) | |
4. DISCUSSION
Based on a large sample investigation, we described the clinical symptom characteristics of patients with mild COVID‐19 infection from the Omicron outbreak in Shanghai. Four clusters based on clinical symptoms were determined to depict the characteristics of different clinical phenotypes and to analyze the difference between Omicron infection and general influenza and other SARS‐CoV‐2 variant infections.
Compared with other SARS‐CoV‐2 variants, patients with mild Omicron infection had a much lower incidence of symptoms and extent of disease. In previous studies, the predominant clinical symptoms of 99 patients infected with the original variant of SARS‐CoV‐2 in Wuhan (2020) were fever (83%), cough (82%), and shortness of breath (31%) 16 ; while, in 419 patients infected with the Delta variant in Guangzhou (2021), China, fever (75%), cough (74%), expectoration (43%), and fatigue (25%) were predominant. 17 These values were significantly higher than the results of our study. There are three possible reasons for this discrepancy. First, the vaccination rates were high by the time the Omicron outbreak occurred. Since the COVID‐19 outbreak, vaccination has been gradually implemented globally to achieve herd immunity. 18 In our study, vaccination rates of up to 96.68% were achieved, with 92.19% of the patients receiving more than two doses. Second, there were differences in the mutation sites of the Omicron variants. According to the results of viral gene sequencing at the Shanghai Center for Disease Control and Prevention on April 26, 2022, Omicron BA.2 was the main variant responsible for the outbreak. 19 Thirty‐one mutations were found in the S protein of Omicron BA.2, including 10 characteristic mutations, whereas the highly pathogenic Delta variant had only four mutations in the S protein. 10 , 20 Third, this study was conducted on mild cases to better characterize the predominant type of Omicron infections. The differences in the study populations may account for the lower incidence of symptoms in Omicron than in other variants.
By clustering the clinical phenotypes of patients with mild Omicron infection, we found that four clinical phenotypes correlated with the severity of symptoms and symptom classification, with more severe patients having more complex clinical symptoms. Due to its mild and influenza‐like symptoms and low mortality rate, Omicron has often been misperceived as the “big flu,” leading to a lack of attention and consequently a rapid spread in a short period of time. In the early stages of the infection, both influenza and Omicron infection may experience generalized and respiratory symptoms such as fever, fatigue, sore throat, and shortness of breath, but the Omicron variant may also cause other systemic symptoms such as nausea and vomiting, loss of taste and smell, hypomnesia, and dyssomnia. In our study, all five systemic symptoms were present in different clinical phenotypes, and their incidence increased with the severity of the symptoms. Because of the high expression of angiotensin‐converting enzyme 2 (ACE2) receptors in multiple tissues, such as the alveolar cells, esophageal and gastrointestinal epithelial cells, and hepatobiliary cells, Omicron causes complex disease symptoms and invades more body tissues. 21 Moreover, the long‐term quality of life, exercise capacity, and mental health status of patients discharged from the hospital with Omicron infection are relatively poor. 22 , 23 Therefore, there is a significant difference between Omicron and influenza in the complexity of infection symptoms and disease sequelae. Such differences warrant more attention in the prevention and control of Omicron infection.
The demographic characteristics of the identified clusters differed significantly in terms of age and allergy history. Age is associated with immunocompetence, and thus affects symptom severity. The aging immune system reduces the ability of an organism to guard against infection and leads to an increase in the intensity and duration of the inflammatory response, making the elderly vulnerable to inflammatory diseases; thus, immune escape may be increased in the advanced population. 24 Other demographic characteristics including sex, comorbidities, and vaccination status did not differ significantly in different clusters, probably because we mainly observed mild patients and could not represent the whole population. Moreover, Omicron can increase the current immune escape capacity by 27‐fold, which is 17‐fold more than the Delta variant. 25 Yamasoba et al. 26 experimentally found that the Omicron subvariant BA.2 was highly resistant to antisera triggered by mRNA‐1273 and ChAdOx1 vaccines, with 18‐fold and 24‐fold lower neutralizing antibody capacities than the original variant, respectively. This prominent immune escape ability may explain why we found no significant differences in the distribution of vaccination status across clusters.
In this study, we did not observe an obvious relationship between vaccination and symptom presentation. There are three possible reasons for this finding. First, there were only 39 (3.4%) unvaccinated individuals in our study. This large population difference may be the reason why we did not observe the effect of vaccination on patients' clinical symptoms. Second, the timing of the vaccination was not recorded in the present study. Owing to the apparently time‐sensitive nature of COVID‐19 vaccines, the longer the vaccination time, the lower the immune effect it produces, resulting in compromised vaccine protection. 27 Third, the majority of study participants were healthy, working‐class people, which may have influenced the study results. More than 50% of the population (n = 1139) were employed, and less than 40 individuals were over 60 years of age. This means our study population tended to be younger and in a better physical condition, ultimately presenting with a lower incidence of symptoms.
In conclusion, we found that the incidence of symptoms was lower in patients with mild Omicron infection than in those with previous SARS‐CoV‐2 variants. High vaccination rates and viral variations are the possible reasons for milder symptoms of infection. A clustering analysis of symptoms in patients with mild infection demonstrated that the clinical symptoms caused by Omicron are more complex and need to be distinguished from influenza.
AUTHOR CONTRIBUTIONS
Miao Wang, Zhijie Zhang, and Guojiang Mei conceived the idea and designed the study. Miao Wang and Yu Tian collected the data, and Zengliang Wang contributed to the data processing and table/figure preparation. Zengliang Wang, Zhidong Liu, and Ke Li contributed to the statistical analysis. Zhidong Liu, Zengliang Wang, and Ke Li drafted the manuscript. Jie Hong and Jin Shi were involved in the manuscript revision. Zhijie Zhang and Guojiang Mei revised the final manuscript. All authors approved the manuscript submission.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
This study was supported by the Science and Exploration Plan of Shanghai Traditional Chinese Medicine [2022YJ‐07]; the National Natural Science Foundation of China [81973102]; the Shanghai New Three‐year Action Plan for Public Health [GWV‐10.1‐XK16].
Wang M, Liu Z, Wang Z, et al. Clinical characteristics of 1139 mild cases of the SARS‐CoV‐2 Omicron variant infected patients in Shanghai. J Med Virol. 2022;95:e28224. 10.1002/jmv.28224
Miao Wang, Zhidong Liu, Zengliang Wang, and Ke Li contributed equally to this study.
Contributor Information
Zhijie Zhang, Email: zhj_zhang@fudan.edu.cn.
Guojiang Mei, Email: mgj_761014@sina.com.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article.
REFERENCES
- 1. WHO . Coronavirus disease (COVID‐19) pandemic. 2022. Accessed June 6, 2022. https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- 2. Arora S, Grover V, Saluja P, et al. Literature review of Omicron: a grim reality amidst COVID‐19. Microorganisms. 2022;10(2):451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maslo C, Friedland R, Toubkin M, Laubscher A, Akaloo T, Kama B. Characteristics and outcomes of hospitalized patients in South Africa during the COVID‐19 Omicron wave compared with previous waves. JAMA. 2022;327(6):583‐584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gu H, Krishnan P, Ng DYM, et al. Probable transmission of SARS‐CoV‐2 Omicron variant in quarantine hotel, Hong Kong, China, November 2021. Emerging Infect Dis. 2022;28(2):460‐462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang J, Chen N, Zhao D, Zhang J, Hu Z, Tao Z. Clinical characteristics of COVID‐19 patients infected by the Omicron variant of SARS‐CoV‐2. Front Med. 2022;9:912367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. China TCPsGotPsRo . Update on the Coronavirus Disease (COVID‐19) outbreak as of June 6, 2022. Accessed June 6, 2022. http://www.gov.cn/xinwen/2022-06/07/content_5694407.html
- 7. Cao Y, Wang J, Jian F, et al. Omicron escapes the majority of existing SARS‐CoV‐2 neutralizing antibodies. Nature. 2022;602(7898):657‐663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li M, Lou F, Fan H. SARS‐CoV‐2 variant Omicron: currently the most complete “escapee” from neutralization by antibodies and vaccines. Signal Transduct Target Ther. 2022;7(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meo SA, Meo AS, Al‐Jassir FF, Klonoff DC. Omicron SARS‐CoV‐2 new variant: global prevalence and biological and clinical characteristics. Eur Rev Med Pharmacol Sci. 2021;25(24):8012‐8018. [DOI] [PubMed] [Google Scholar]
- 10. Tian D, Sun Y, Xu H, Ye Q. The emergence and epidemic characteristics of the highly mutated SARS‐CoV‐2 Omicron variant. J Med Virol. 2022;94(6):2376‐2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xian LF, Lin JS, Yu SC, Zhao Y, Zhao P, Cao GW. Epidemiological characteristics analysis of spring outbreak of novel coronavirus infection in Shanghai in 2022. Shanghai Prev Med. 2022; 34(4):294‐299. [Google Scholar]
- 12. Boyton RJ, Altmann DM. The immunology of asymptomatic SARS‐CoV‐2 infection: what are the key questions? Nat Rev Immunol. 2021;21(12):762‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim MK, Lee B, Choi YY, et al. Clinical characteristics of 40 patients infected with the SARS‐CoV‐2 Omicron variant in Korea. J Korean Med Sci. 2022;37(3):e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deng H, Lin H, Mai Y, Liu H, Chen W. Clinical features and predictive factors related to liver injury in SARS‐CoV‐2 Delta and Omicron variant‐infected patients. Eur J Gastroenterol Hepatol. 2022;34:933‐939. [DOI] [PubMed] [Google Scholar]
- 15. Team CC‐R . SARS‐CoV‐2 B.1.1.529 (Omicron) variant—United States, December 1–8, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(50):1731‐1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang Y, Chen R, Hu F, et al. Transmission, viral kinetics and clinical characteristics of the emergent SARS‐CoV‐2 Delta VOC in Guangzhou, China. EClinicalMedicine. 2021;40:101129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang X, Powell CA. How to translate the knowledge of COVID‐19 into the prevention of Omicron variants. Clin Transl Med. 2021;11(12):e680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Government Smps . Shanghai Coronavirus Disease (COVID‐19) Epidemic Prevention and Control Press Conference April 26, 2022. 2022. Accessed June 6, 2022. http://news.cyol.com/gb/articles/2022-04/26/content_Z6vm7f2qe.html
- 20. Harvey WT, Carabelli AM, Jackson B, et al. SARS‐CoV‐2 variants, spike mutations and immune escape. Nat Rev Microbiol. 2021;19(7):409‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single‐cell RNA‐seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019‐nCoV infection. Front Med. 2020;14(2):185‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yuan B, Li W, Liu H, et al. Correlation between immune response and self‐reported depression during convalescence from COVID‐19. Brain Behav Immun. 2020;88:39‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang LX, Li X, Gu XY, et al. Health outcomes in people 2 years after surviving hospitalisation with COVID‐19: a longitudinal cohort study. Lancet Respir Med. 2022;10(9):863‐876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen Y, Klein SL, Garibaldi BT, et al. Aging in COVID‐19: vulnerability, immunity and intervention. Ageing Res Rev. 2021;65:101205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. GOV.UK . SARS‐CoV‐2 variants of concern and variants under investigation in England [EB/OL]. 2022. Accessed on March 16, 2022. https://assets.publishing.service.gov.uk
- 26. Yamasoba D, Kimura I, Nasser H, et al. Virological characteristics of SARS‐CoV‐2 BA.2 spike. Cell. 2022; 185(12):2103‐2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fiolet T, Kherabi Y, MacDonald CJ, Ghosn J, Peiffer‐Smadja N. Comparing COVID‐19 vaccines for their characteristics, efficacy and effectiveness against SARS‐CoV‐2 and variants of concern: a narrative review. Clin Microbiol Infect. 2022;28(2):202‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
Data sharing is not applicable to this article.


