Abstract
Shiga toxin-producing E. coli (STEC) is a food-borne pathogen that causes serious illness, including hemolytic-uremic syndrome (HUS). STEC colonizes the lower intestine and produces Shiga toxins (Stxs). Stxs appear to translocate across intestinal epithelia and affect sensitive endothelial cell beds at various sites. We have previously shown that Stxs cross polarized intestinal epithelial cells (IECs) via a transcellular route and remain biologically active. Since acute inflammatory infiltration of the gut and fecal leukocytes is seen in many STEC-infected patients and since polymorphonuclear leukocyte (PMN) transmigration across polarized IECs diminishes the IEC barrier function in vitro, we hypothesized that PMN transmigration may enhance Stx movement across IECs. We found that basolateral-to-apical transmigration of neutrophils significantly increased the movement of Stx1 and Stx2 across polarized T84 IECs in the opposite direction. The amount of Stx crossing the T84 barrier was proportional to the degree of neutrophil transmigration, and the increase in Stx translocation appears to be due to increases in paracellular permeability caused by migrating PMNs. STEC clinical isolates applied apically induced PMN transmigration across and interleukin-8 (IL-8) secretion from T84 cells. Of the 10 STEC strains tested, three STEC strains lacking eae and espB (eae- and espB-negative STEC strains) induced significantly more neutrophil transmigration and significantly greater IL-8 secretion than eae- and espB-positive STEC or enteropathogenic E. coli. This study suggests that STEC interaction with intestinal epithelia induces neutrophil recruitment to the intestinal lumen, resulting in neutrophil extravasation across IECs, and that during this process Stxs may pass in greater amounts into underlying tissues, thereby increasing the risk of HUS.
Multiple types of Escherichia coli which cause enteric disease in humans have been identified, including enteroaggregative, enteropathogenic (EPEC), enterotoxigenic, enteroinvasive, and Shiga-toxin producing E. coli (STEC) (35). Of all these pathogenic E. coli, STEC causes the greatest degree of morbidity. It is the leading cause of acute renal failure in children in the United States (17, 40, 41) and is a major food-borne pathogen in the developed world (35, 40). STEC encompasses a diverse group of E. coli strains defined by their possession of the stx1 or stx2 gene or both (51). Most pathogenic strains of STEC contain the locus for enterocyte effacement (LEE) pathogenicity island and a large 93-kb plasmid (22, 35, 40). Genes within LEE code for the ability of E. coli to attach and efface epithelial cells (35). The presence of LEE is, however, not a prerequisite for the development of STEC-associated disease. STEC strains lacking LEE (LEE-negative strains) as well as LEE-positive STEC strains have been associated with outbreaks and sporadic disease (5, 21, 39, 40).
STEC is usually acquired by consuming contaminated food or water or by direct transmission from an infected individual (40). Following ingestion the organisms colonize the lower parts of the gastrointestinal tract and then produce Shiga toxin 1 (Stx1) and/or Shiga toxin 2 (Stx2), after which a spectrum of clinical outcomes, both locally and systemically, may occur. Patients develop intestinal symptoms ranging from mild diarrhea to severe hemorrhagic colitis (40). Most individuals infected with STEC will recover from the infection without further complication. However, 5 to 10% of patients, primarily children and the elderly, may go on to develop systemic complications such as hemolytic-uremic syndrome (HUS), characterized by acute renal failure, thrombocytopenia, and hemolytic anemia (17, 40, 41).
The precise sequence of events leading to hemorrhagic colitis and HUS is unclear, other than the fact that the presence of Stx is a key factor (35, 40). STEC is believed not to be invasive and is thought to be restricted to the lumen of the gut. Stxs are produced in the gut and can be recovered in the stools of infected patients (4, 40; D. W. K. Acheson, S. De Breucker, A. Donohue-Rolfe, K. Kozak, A. Yi, and G. T. Keusch, Abstr. 94th Gen. Meet. Am. Soc. Microbiol. 1994, abstr. B-62, p. 39, 1994). It is thought that Stx produced within the intestinal tract is able to cross the epithelial barrier in the intestine and enter the bloodstream, targeting the endothelium of susceptible tissues, resulting in intestinal as well as systemic dysfunction (3, 40). We have previously shown that Stx1 and Stx2 are capable of crossing polarized intestinal epithelial cell (IEC) monolayers by what appears to be a transcellular process and that both Stx1 and Stx2 remain biologically active following translocation (4, 20). It is likely that the amount of toxin that gains access to the systemic circulation is one of the most critical factors in determining who will develop HUS and other systemic complications.
Human infection with STEC results in a complex sequence of cellular interactions, among which neutrophil infiltration of the intestinal epithelium is thought to occur. A variety of enteric pathogens including Salmonella spp., Shigella spp., and EPEC are known to induce neutrophil transmigration across polarized IECs (29, 31, 43). Induction of chemokines such as interleukin-8 (IL-8) may be important in this process (10, 11, 29, 30, 44). Fecal leukocytes were seen more frequently in STEC-infected patients than in patients infected with Salmonella, Campylobacter, or Shigella spp. (47). In addition, histological studies of gastrointestinal tissue of patients infected with STEC show neutrophil infiltration in the lamina propria and crypts (18, 23, 24, 42). High IL-8 levels have been noted in HUS patients, and it was suggested that IL-8 expression was, at least in part, responsible for neutrophil recruitment in the disease (13, 14, 15, 32, 50). Rabbit models of STEC infection have implicated intestinal neutrophils as contributing to pathogenesis (12, 46). Others have shown that neutrophil transmigration across polarized IEC monolayers diminishes barrier function, resulting in increased permeability to paracellular markers (33, 34).
In this study we investigated the effect of neutrophil transmigration across IECs on the apical-to-basolateral movement of Shiga toxins. In addition we explored the role of the bacteria per se in the induction of polymorphonuclear leukocyte (PMN) transmigration by screening the ability of a diverse group of STEC clinical isolates to induce neutrophil transmigration across, and/or stimulate the production of neutrophil chemokine IL-8 from, IECs.
MATERIALS AND METHODS
Tissue culture.
T84 IECs were a generous gift from Cynthia Sears (Johns Hopkins University, Baltimore, Md.). Cells were grown at 37°C at 5% CO2 in a 1:1 (vol/vol) mixture of Dulbecco modified Eagle medium (D-MEM; high glucose) and Ham's F-12 with 15 mM HEPES supplemented with 10% heat-inactivated fetal bovine serum and 100 U of penicillin G and 100 μg of streptomycin sulfate (Gibco/BRL)/ml. Cells from passages 36 to 70 were used.
Inverted monolayers of T84 cells were constructed as previously described (37). Briefly, polycarbonate filter inserts (0.33 cm2, 5-μm-pore-size Transwell membranes; Costar, Cambridge, Mass.) were placed upside down in 12-well tissue culture plates, precoated with 5 μg of rat tail collagen/ml, and seeded with T84 cells, which were allowed to attach overnight. The next day, the Transwells were flipped into their upright position in a 24-well plate. Cells were fed every 2 days and developed stable electrical resistance by 9 days postseeding.
Electrical resistance measurements across T84 monolayers were taken before each experiment with a Millicell-ERS (Millipore Corporation, Bedford, Mass.) to ensure that the monolayers demonstrated intact tight junctions and thus formed a functional barrier. Monolayers were used 9 to 13 days postseeding, when resistance measurements ranged from 2,000 to 4,000 Ω cm2.
Isolation of neutrophils.
Neutrophils were isolated from healthy human volunteers as previously described (37). Briefly, whole blood mixed with heparin sodium (20 U/ml of blood) diluted in Hank's balanced salt solution without Ca2+ and Mg2+ (HBSS w/o) was layered onto a Ficoll-Hypaque mixture and centrifuged at 250 × g for 40 min at 20°C. The pellet containing red blood cells and neutrophils was resuspended in HBSS w/o. Neutrophils were separated from red blood cells by dextran sedimentation followed by cold hypotonic lysis.
Movement of Stx during neutrophil transmigration.
Prior to the addition of neutrophils, inverted T84 monolayers were washed twice in HBSS and placed in serum-, antibiotic-, and phenol red-free D-MEM–F-12 for approximately 1 h. Purified Stx1 or Stx2 (1, 2, 8) was diluted to a concentration of 1 μg/ml in serum-, antibiotic-, and phenol red-free D-MEM–F-12, and 1.2 ml was added to the apical (bottom) chamber of the Transwell. Paracellular marker [3H]inulin (New England Nuclear, Boston, Mass.) was also added to the apical side at 1.25 μCi/ml. In certain experiments, fluorescein isothiocyanate (FITC)-dextrans of various sizes (Sigma Chemical Co., St. Louis, Mo.) were added at 1 mg/ml to the apical chamber. In the basolateral (top) chamber 200 μl of a suspension of 107 neutrophils/ml in serum-, antibiotic-, and phenol red-free D-MEM–F-12 was added. Neutrophil chemoattractant formyl-Met-Leu-Phe (fMLP) (1 μM) was added to the apical chambers of certain Transwells. The Transwells were then placed at 37°C in a 5% CO2 atmosphere for 4 h. Neutrophils transmigrating across the monolayers into the apical chamber were quantified using a myeloperoxidase assay (37). Following transmigration, the basolateral medium was filtered through a 0.2-μm-pore-size filter. Stx1 and Stx2 were quantified using a monoclonal antibody capture enzyme-linked immunosorbent assay (9). The amount of [3H]inulin was determined by counting 10 μl of sample in a scintillation counter. FITC-dextrans were quantified as units of fluorescence using the F-max fluorescence microplate reader (Molecular Devices, Sunnyvale, Calif.). The amounts of toxin, [3H]inulin, and FITC-dextrans in the starting material as well as in the medium recovered in the basolateral chamber were measured. Reported values are amounts translocated as percentages of the amounts added.
E. coli strains.
All bacterial strains used in this study are listed in Table 1. 2348/69 is a well-characterized pathogenic EPEC strain (35). STEC strains 86.24 and 933 are well-characterized pathogenic O157:H7 STEC strains (35). All other strains were obtained from patients enrolled in a multisite STEC prevalence study (D. W. K. Acheson, T. Ngo, and R. Chitrakar, Abstr. 4th Int. Symp. Workshop Shiga Toxin [Verocytotoxin]-Producing Escherichia coli Infect., abstr. 225, p. 107, 2000). Clinical details on individual patients were unavailable.
TABLE 1.
E. coli strains
| E. coli type | Strain | Serotype | Multiplex PCR resulta for:
|
PMN transmigrationb (104) | IL-8 secretionc (pg/ml) | % Adhesiond | FASe result | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| hlyA | eae | stx1 | stx2 | espB | |||||||
| NPECf | K-12 DH5α | − | − | − | − | − | 0.2 (0.1) | 35.7 (4.8) | 0.3 (0.1) | − | |
| EPEC | 2348/69 | O55 | − | + | − | − | + | 21.0 (3.7) | 64.7 (7.8) | 24.2 (21.3) | + |
| STEC | 86.24 | O157:H7 | + | + | − | + | + | 7.3 (1.3) | 61.8 (6.3) | 2.5 (0.2) | − |
| STEC | 933 | O157:H7 | + | + | + | + | + | 1.7 (0.3) | 66.7 (19.5) | 1.4 (0.2) | − |
| STEC | I-361 | O157:H7 | + | + | − | + | + | 7.5 (0.7) | 35.0 (6.4) | 2.5 (0.5) | − |
| STEC | 9-23 | O26 | + | + | + | − | + | 12.0 (2.4) | 72.7 (21.6) | 1.3 (0.1) | − |
| STEC | 9-54 | O111 | + | + | + | − | + | 1.2 (0.2) | 41.4 (3.9) | 1.8 (0.3) | − |
| STEC | 20 | OX3:H− | − | − | − | + | − | 32.8 (3.0)∗ | 129.2 (7.3)† | 1.5 (0.2) | − |
| STEC | 25 | OX25:H− | + | − | − | + | − | 38.0 (3.2)∗ | 298.2 (12.1)† | 3.0 (0.2) | − |
| STEC | 44 | O8w:H− | + | − | + | + | − | 39.7 (7.1)∗ | 154.0 (11.0)† | 1.9 (0.2) | − |
| STEC | 47 | O38:H− | − | − | + | + | − | 6.5 (1.6) | 194.1 (7.3)† | 2.0 (0.3) | − |
| STEC | 57 | O153:HNM | + | − | − | + | − | 15.7 (4.7)∗∗ | 38.0 (7.1) | 2.6 (0.4) | − |
Presence (+) or absence (−) of eae and espB was determined by both PCR and Southern blotting as described in Materials and Methods.
Values are numbers of neutrophils that transmigrated. Data points represent the means (standard errors of the means [SEM]) for a compilation of at least two experiments performed in triplicate (n = 6 for each strain). Strains 86.24 and 25 were included in every experiment (n = 20 for these strains). Positive and negative controls were also included in every experiment (n = 20). The positive control consisted of uninfected monolayers with an added fMLP gradient; PMN transmigration was 29.2 × 104 neutrophils with an SEM of 103 × 104 neutrophils. Negative controls were uninfected monolayers, for which there was an undetectable number of transmigrated neutrophils. ∗ and ∗∗, significantly different from 86.24 PMN transmigration (P < 0.0001 and P = 0.02, respectively).
Values are amounts of IL-8 collected in supernatants after exposure to E. coli as described in Materials and Methods. Data points represents the means (SEM) for a compilation of three experiments performed in triplicate (n = 9 for each strain). 86.24 was included in every experiment (n = 18). The amount of IL-8 secreted by uninfected cells (n = 18) in these experiments was 33.1 (1.9) pg/ml. †, Significantly different from 86.24 IL-8 induction (P < 0.0001).
Numbers of recovered CFU associated with the monolayer as a percentage of the number of E. coli added to the monolayer. Data points represent the means (SEM) of a compilation of at least three experiments performed in triplicate (n = 9 for each strain). DH5α, EPEC, strain 86.24, and strain 25 were included in every experiment (n = 20 for these strains).
FAS was described in Materials and Methods. +, A/E lesions present; −, A/E lesions absent.
NPEC, nonpathogenic E. coli.
PCR.
A multiplex PCR assay was used to detect the presence of genes stx1, stx2, eae, and hlyA as previously described with minor modifications to the PCR buffer (20 mM Tris-HCl, 50 mM KCl, 2.5 mM MgCl2, 0.1 mM deoxynucleoside triphosphates) (38). The presence of espB was determined by PCR (forward primer, 5′ CCGTATTGTTAGTGGTCGAG 3′; reverse primer, 5′ GATATCATCCTGCGCTCTGC 3′).
Southern blotting for eae and espB.
The presence or absence of eae and espB was confirmed by Southern blotting using standard methods. The probes were generated from the eae and the espB PCR products of the STEC 933 strain and the EPEC 2348/69 strain, respectively.
Neutrophil transmigration induced by E. coli.
Neutrophil transmigration experiments were similar to those previously described (43) except for some modifications. T84 cells were grown on inverted Transwells to stable high electrical resistance, washed twice with HBSS, and resuspended in serum-, antibiotic-, and phenol red-free D-MEM–F-12 for 1 to 2 h before addition of E. coli. Overnight cultures of E. coli grown in Luria-Bertani broth were diluted 1:125 in serum-, antibiotic-, and phenol red-free D-MEM–F-12 and grown for 2 to 2.5 h at 37°C to an optical density of 0.5 to 0.8 at 600 nm. E. coli strains were centrifuged at 11,000 × g for 5 min, washed twice in HBSS, and adjusted to a concentration of approximately 4 × 109 E. coli CFU/ml in serum-, antibiotic-, and phenol red-free D-MEM–F-12 as confirmed by CFU plate counting. Transwells containing confluent inverted T84 monolayers were flipped into 12-well plates and infected on the apical surface with 50 μl of the solution of 4 × 109 E. coli CFU/ml. E. coli attachment was allowed to proceed for 1 h at 37°C in 5% CO2. The monolayers were then washed twice in HBSS, turned back to their upright position, and placed in 24-well plates. One hundred microliters of a 107-PMN/ml solution was then added to the basolateral chamber and 600 μl of serum-, antibiotic-, and phenol red-free D-MEM–F-12 was added to the apical chamber. After 2 h, the number of PMNs which had fully transmigrated to the apical chamber was quantified.
Adhesion of E. coli to T84 cells and induction of IL-8 secretion.
T84 cells were seeded on collagen-coated 24-well plates at a density of approximately 105 cells/ml and allowed to grow for 5 to 6 days until fully confluent. T84 cells were then washed twice with HBSS followed by addition of serum-, antibiotic-, and phenol red-free D-MEM–F-12 for 1 to 2 h prior to addition of E. coli. E. coli was grown as described above and adjusted to a final concentration of 2 × 108 E. coli CFU/ml. The E. coli suspension (0.5 ml) was added to each well of the 24-well plate. Certain wells were incubated with media alone as a negative control. After 1 h, monolayers were washed three times with HBSS and resuspended in serum-, antibiotic-, and phenol red-free D-MEM–F-12 for an additional 2 h. Supernatants were then collected for IL-8 measurement using a commercially available enzyme immunoassay (Endogen, Woburn, Mass.). Following removal of the supernatants, cell monolayers were washed three times with HBSS and resuspended in 0.5% Triton X-100 for T84 cell solubilization. Solubilized cells were diluted and plated on Luria-Bertani agar plates for CFU determination.
FAS assay.
The fluorescence actin staining (FAS) assay was performed as described by Knutton et al. (25). Briefly, T84 cells were seeded on collagen-coated eight-well chambered slides and grown for 2 days. T84 cells were prewashed, and E. coli strains were prepared as described above to a concentration of 5 × 108 CFU/ml. E. coli strains (200 μl) were placed on the T84 cells for 1 h at 37°C in 5% CO2. T84 cells were then washed three times with HBSS prior to addition of serum-, antibiotic-, and phenol red-free D-MEM–F-12. Cells were incubated for a further 2 h at 37°C in 5% CO2. T84 cells were then washed three times with HBSS and fixed for 15 min with 4% paraformaldehyde at room temperature (RT), washed twice with phosphate-buffered saline (PBS), and incubated at RT for 20 min in 50 mM NH4Cl. Cells were blocked and permeabilized in PBS with 5% bovine serum albumin and 0.1% Triton X-100 for 30 min at RT. Cells were then treated for 30 min at RT with 1 U of Texas red-phalloidin (Molecular Probes, Portland, Oreg.)/ml, washed three times with PBS, and then viewed under a fluorescence microscope and interpreted as described previously (25).
Statistics.
Statistics were performed with the Instat statistics program. Unpaired Student's t tests were used to compare differences in sets of data. P values <0.05 were considered significant. Data are presented as either means ± standard deviations (SD) from a representative experiment performed in triplicate or as means and standard errors of the means from a compilation of several experiments.
RESULTS
Effect of PMN transmigration on Stx translocation.
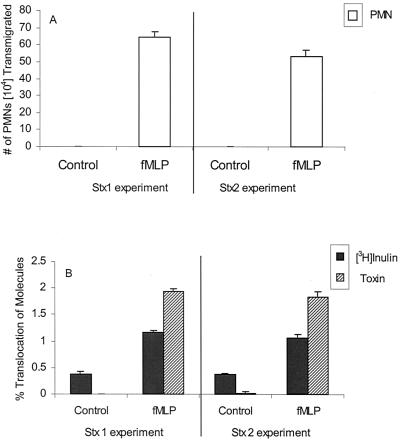
T84 cells were grown on inverted permeable Transwell filters until they formed functional polarized barriers (as measured by electrical resistance). A chemotactic gradient was then established by placing fMLP on the apical surface of the IEC monolayers and neutrophils on the basolateral surface. As shown in Fig. 1A, when no fMLP gradient was imposed, undetectable numbers of neutrophils cross the monolayer. However, when a 1-μM fMLP gradient was imposed, 15 to 30% of neutrophils added to the basolateral compartment migrated through the IEC monolayer into the apical chamber (Fig. 1A). During the process of neutrophil transmigration, the electrical resistance across the IEC monolayer drops significantly but returns to baseline approximately 12 to 16 h after removal of neutrophils (data not shown) (36). We placed the paracellular marker [3H]inulin (3 kDa) with Stx1 or Stx2 (72 kDa) in the apical compartment in order to measure the amount of movement of these molecules to the basolateral compartment in the presence or absence of neutrophil migration. Stx1 and Stx2 added to the apical chamber of T84 monolayers did not affect resistance measurements, nor did the toxins alone induce neutrophils to transmigrate (data not shown). We found that there was a highly significant (P < 0.0001) increase in apical-to-basolateral translocation of both Stx1 and Stx2 in the presence of transmigrating neutrophils. Mean percent translocations of 0.001% for Stx1 and 0.02% for Stx2 were observed in the absence of fMLP, rising to mean percent translocations of 1.9% for Stx1 and 1.8% for Stx2 in the presence of fMLP when neutrophils were stimulated to transmigrate in a basolateral-to-apical direction (Fig. 1B). In addition, there was a highly significant (P < 0.0001) increase in the movement of paracellular marker [3H]inulin during neutrophil transmigration from 0.4% without fMLP to 1.2% with fMLP for experiments with Stx1 and from 0.4% without fMLP to 1.1% with fMLP in Stx2 experiments (Fig. 1B). We also examined whether fMLP added to the apical surface of T84 monolayers in the absence of neutrophils or neutrophils added to the basolateral surface of T84 monolayers in the absence of fMLP had any effect on the movement of [3H]inulin, Stx1, or Stx2. We found that there were no significant differences between the translocation of [3H]inulin, Stx1, or Stx2 in the presence of fMLP or neutrophils alone and the translocation of these molecules across T84 monolayers in their absence (data not shown).
FIG. 1.
Effect of PMN transmigration on the translocation of [3H]inulin, Stx1, and Stx2. (A) Numbers of PMNs that transmigrated to the apical chamber with and without 1 μM fMLP added to the apical chamber. (B) Percentages of translocation of [3H]inulin, Stx1, and Stx2 from the apical to the basolateral chamber with (fMLP) and without (control) neutrophil transmigration. Data are the means ± SD of triplicate samples. One representative experiment of three performed is presented.
fMLP is known to activate PMNs (6). To determine if fMLP-mediated neutrophil activation was a factor in Stx movement, neutrophil transmigration was prevented by adding an equal concentration of fMLP to both the apical and basolateral sides of the IECs. This maneuver abolishes the chemokine gradient, and neutrophil transmigration does not occur; however, neutrophils are exposed to fMLP (34). As described above, an fMLP gradient placed in the apical chamber induced a significant amount of PMNs to transmigrate and this was accompanied by a highly significant increase in the translocation of [3H]inulin, Stx1, and Stx2. When fMLP was added to both the apical and basolateral chambers, PMN transmigration was undetectable (data not shown). The observation of loss of PMN movement coincided with the observation that there was no significant difference between the translocation of [3H]inulin, Stx1, or Stx2 in controls with no fMLP and that when fMLP was added to both apical and basolateral surfaces (data not shown). These data indicate that the increase in Stx translocation is not due to fMLP-induced release of neutrophil factors acting on IECs but suggest that the physical process of neutrophil migration results in greater Stx translocation.
Effect of increasing numbers of transmigrating PMNs on Stx translocation.
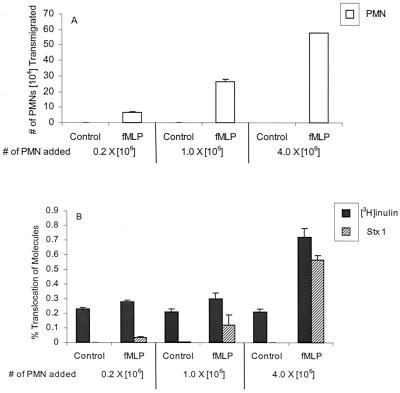
To determine if the amount of Stx translocation correlated with the number of neutrophils migrating across the IECs, we added increasing numbers of neutrophils (0.2 × 106, 1 × 106, and 4 × 106) to the basolateral chamber (34). Neutrophils were added in separate wells to the basolateral chamber, with and without fMLP added to the apical chamber. As before, undetectable amounts of PMNs moved in the absence of fMLP (Fig. 2A). In the presence of fMLP, the number of PMNs migrating increased from 6.8 × 104 to 57.9 × 104 as the number of PMNs added increased (Fig. 2A). There was also a positive correlation between the number of neutrophils migrating and the amount of Stx1 translocating. In response to increasing numbers of PMNs transmigrating, 0.04, 0.1, and 0.6% of Stx1 translocated (Fig. 2B), with significantly more Stx1 translocating in the presence of 4 × 106 PMNs than in the presence of 1 × 106 PMNs (P = 0.001).
FIG. 2.
Effect of increasing numbers of PMNs on Stx translocation. PMNs (0.2 × 106, 1.0 × 106, and 4 × 106) were added to the basolateral chamber. (A) Numbers of PMNs transmigrated to the apical chamber with or without 1 μM fMLP added to the apical chamber. (B) Percentages of translocation of [3H]inulin, Stx1, and Stx2 from the apical to the basolateral chamber with (fMLP) and without (control) neutrophil transmigration. Data are the means ± SD of triplicate samples. One representative experiment of three performed is presented. The difference in PMN transmigration and translocation of [3H]inulin, Stx1, and Stx2 under conditions with and without an fMLP gradient are highly significant (P < 0.0001). The difference in the amounts of Stx translocating during fMLP-induced PMN transmigration when 1 × 106 versus 4 × 106 PMNs are added is significant (P = 0.001).
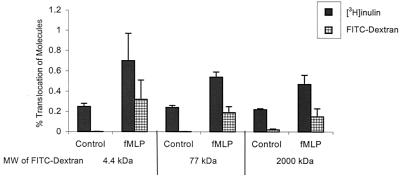
The increase of Stx1 and Stx2 movement across the T84 monolayer may be specific to Stxs or may represent a nonspecific increase of all molecules. The increase in [3H]inulin movement described above suggested the latter; however, [3H]inulin (3 kDa) is significantly smaller than Stxs (∼72 kDa). Thus we wished to investigate molecules with a size similar to that of Stxs. We measured the movement of FITC-dextrans of various sizes (4.4, 77, and 2,000 kDa). Movement of all FITC-dextrans was significantly increased by fMLP-induced neutrophil transmigration (P < 0.05) (Fig. 3). The number of PMNs transmigrating in response to an fMLP gradient was the same for all three dextrans (data not shown). We have also observed an increase in movement of horseradish peroxidase (44 kDa) during fMLP-induced neutrophil transmigration (data not shown).
FIG. 3.
Effect of PMN transmigration on the translocation of FITC-dextrans of various sizes. FITC-dextrans of 4.4, 77, and 2,000 kDa were added with [3H]inulin to the apical chamber, and the amount translocated was measured in the basolateral chamber with and without fMLP-induced PMN transmigration. Data are the means ± SD of triplicate samples. One representative experiment of three performed is presented. The differences in PMN transmigration and translocation of [3H]inulin and FITC-dextrans of all sizes under conditions with and without an fMLP gradient are significant (P < 0.05).
Effect of STEC on PMN transmigration.
The next issue we addressed was whether STEC infecting the apical surface of IECs induced neutrophils to transmigrate from the basolateral to the apical side. Our approach was to select a group of STEC clinical isolates with a diverse range of virulence traits and investigate which STEC strains induce neutrophil transmigration (Table 1). We utilized a multiplex PCR assay to screen for the presence of stx1, stx2, the eae gene (which serves as a marker for the LEE pathogenicity island), and hlyA (serving as a marker for the 93-kb plasmid) (38). E. coli DH5α was used as a nonpathogenic negative-control strain, and EPEC strain 2348/69 was used as a positive control (43).
The ability of STEC to induce neutrophil transmigration was investigated using inverted T84 monolayers infected on the apical surface with the various E. coli strains for 1 h, followed by addition of 106 neutrophils to the basolateral surface. Neutrophils crossing the monolayer and reaching the apical chamber were quantified. All strains induced significantly (P < 0.0001) more PMN transmigration than E. coli DH5α (Table 1). The EPEC strain induced 21.0 × 104 neutrophils to transmigrate, which is comparable to numbers previously reported (43). PMN transmigration in response to the various STEC strains was significantly different. Three strains in particular induced significantly more PMN transmigration than all other STEC strains tested as well as the EPEC strain (Table 1). Interestingly, these three strains were all eae and espB negative.
We also investigated whether the STEC clinical isolates induced IL-8 secretion from T84 cells grown to confluence on 24-well plates. Infection with E. coli DH5α strain (35.7 pg/ml) did not increase IL-8 secretion above the amount secreted by uninfected T84 cells (33.1 pg/ml). However, there were minor but significant increases in IL-8 production upon infection with EPEC (P = 0.0004) and one of the eae-positive STEC strains (strain 86.24) (P = 0.0004) compared to the control (Table 1). Among the eae-negative strains tested, four of five strains induced at least a fourfold increase in IL-8 secretion, a highly significant (P < 0.0001) difference compared to the control, DH5α, EPEC, and all eae-positive STEC strains (Table 1).
Overall, there was a correlation between induction of PMN transmigration and induction of IL-8 secretion by the various STEC strains (P = 0.04). However, there were two outliers in the data. eae-negative strain 57 induced a relatively large number of neutrophils to transmigrate compared with eae-positive STEC strains but did not induce IL-8. Conversely, eae-negative strain 47 induced a relatively low number of neutrophils to transmigrate but induced a highly significant sixfold increase in IL-8 secretion compared with eae-positive STEC strains.
To determine whether the large differences in ability to induce neutrophil transmigration or to promote IL-8 secretion are related to adhesion of E. coli to T84 cells, we performed adhesion assays with the various STEC strains. There were only minor differences in adhesion between the 10 STEC strains, with no correlation to either neutrophil transmigration or IL-8 production. All STEC strains tested were approximately 10-fold less efficient at adhesion to T84 cells than the EPEC eae-positive strain. Li et al. reported STEC strains which, despite containing the LEE pathogenicity island, were not capable of forming attaching and effacing (A/E) lesions on T84 cells (28). In support of this observation, none of the STEC strains, whether they were eae positive or not, formed A/E lesions whereas EPEC was capable of forming A/E lesions (Table 1).
In EPEC, the espB gene is thought to be involved in induction of both PMN transmigration and IL-8 secretion (43, 44). espB is present on the LEE pathogenicity islands of EPEC and STEC, encoding a secreted protein important in signal transduction leading to A/E lesions (35). We hypothesized that the eae-positive STEC strains would contain the espB gene and found this to be true (Table 1), while all the eae-negative STEC strains tested were espB negative. These results support the hypothesis that these eae-negative STEC strains lack the LEE pathogenicity island and suggest that these strains must contain another factor(s) which promotes neutrophil transmigration and/or IL-8 secretion.
STEC-induced PMN transmigration increased Stx translocation.
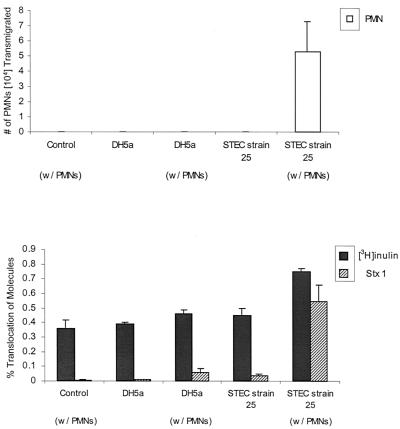
We have demonstrated that STEC induces PMN transmigration, and PMN transmigration leads to increases in Stx translocation. However, whether neutrophil migration induced by live STEC, rather than fMLP, leads to an increase in the amount of [3H]inulin and Stx1 translocation across IECs remains to be elucidated. STEC does not produce enough Stx in the 3-h time frame of the assay to allow quantitation of Stx translocation. Therefore, we added 1 μg of Stx1/ml to the E. coli-infected apical surface at the same time that the neutrophils were added to the basolateral surface. By using STEC strain 25, which produces only Stx2, and using an Stx1-specific enzyme-linked immunosorbent assay for detection of translocated Stx1, any contribution of Stx from the STEC strain in this assay can be eliminated. We measured the amount of Stx1 and [3H]inulin that translocated across uninfected monolayers and monolayers apically infected with E. coli strain DH5α or STEC strain 25 in the presence and absence of neutrophils added to the basolateral surface. When neutrophils were added to E. coli DH5α-infected monolayers, neutrophil transmigration was undetectable (Fig. 4A) and only small increases in [3H]inulin and Stx1 translocation were observed (Fig. 4B). However, PMNs introduced on the basolateral side of STEC strain 25-infected monolayers moved across in large numbers (Fig. 4A), accompanied by a significant (P < 0.005) increase in movement of both [3H]inulin and Stx1 (Fig. 4B).
FIG. 4.
Effect of PMN transmigration induced by STEC on the translocation of Stx1. In this experiment, Transwells were infected with either DH5α or STEC strain 25 as described in Materials and Methods. PMNs (2 × 106) were added to the basolateral chamber after infection to certain wells (w/PMNs). Stx1 (1 μg/ml) and [3H]inulin (1.25 μCi/ml) were added to the apical chamber in all wells. Control, uninfected Transwell with PMNs added. Data are means ± SD of triplicate samples. The difference in translocation of Stx1 and [3H]inulin in STEC-infected Transwells with and without PMNs is significant (P < 0.005).
DISCUSSION
Stx absorption from the gut lumen into the systemic circulation is believed to be a critical step in the pathogenesis of the systemic complications following STEC infections (4, 40). STEC is generally believed to be noninvasive and thus is thought not to penetrate intestinal epithelial tissue, but rather to produce Stxs in the intestinal lumen (40; Acheson et al., Abstr. 94th Gen. Meet. Am. Soc. Microbiol. 1994). IECs form an important barrier between the luminal space and underlying tissue compartments. Large molecules, such as proteins, are prevented from paracellular passage across polarized intestinal epithelial barriers by tight junctions (36). However, proteins can move across this barrier in two ways. First, they can be taken up by transcellular processes which are active and which can be either specific (receptor-mediated endocytosis) or nonspecific (pinocytosis) (7). Second, they can move across the epithelium by a paracellular route in situations where tight-junction permeability is altered. Permeability of tight junctions can be modified by infection, bacterial toxins, cytokines, hormones, drugs, and immune cells (36, 45).
We have previously shown that biologically active Stx1 and Stx2 are able to cross from the apical side to the basolateral side of HCT-8, T84, and CaCo2A IEC lines without destroying the cells or altering the tight junctions (4, 20). Data presented in this study demonstrate that neutrophil transmigration in a basolateral-to-apical direction dramatically enhances the translocation of Stx1 and Stx2 in an apical-to-basolateral direction across polarized IECs.
Neutrophil movement into the intestinal lumen is known to occur as an innate response to several enteric pathogens such as Salmonella, Shigella, and Campylobacter spp. (47). The high frequency of fecal leukocytes suggests that this response occurs frequently during STEC infection as well (47). This process involves diapedesis of neutrophils between IECs, which transiently decreases barrier function, resulting in enhanced paracellular permeability (34, 37). It has been further speculated that this process may lead to luminal threats such as toxins gaining access to underlying tissue (37). In this report, increases in movement of Stxs across the IEC barrier, in conjunction with neutrophil transmigration, are accompanied by significant increases in the movement of paracellular marker [3H]inulin. The increase in Stx movement is not due to the presence of neutrophils per se and/or neutrophil chemoattractant fMLP but requires the movement of the neutrophils across the monolayer, and the amount of Stx movement is correlated with the number of neutrophils transmigrating. Furthermore, increases in the translocation of FITC-dextrans of a wide range of sizes, which are considered to be restricted to the paracellular space (27), indicate paracellular breaches due to neutrophil transmigration that are capable of allowing increased movement of large molecules. Our data are consistent with previous data demonstrating that neutrophil migration can impair tight junctions leading to increases in paracellular permeability (34, 37). Therefore we believe that the large increase in Stx translocation described in this report is due to increases in paracellular permeability resulting from neutrophil transmigration, although we cannot exclude the possibility that there may be an increase in transcellular Stx movement during the process of neutrophil transmigration.
A recent study has reported that neutrophils bind Stx1 and suggested that neutrophil binding of Stx1 in the intestine may be a mechanism for Stx1 delivery to kidney endothelial beds (48). This study could imply that neutrophil transmigration across the epithelium might be protective with respect to Stx translocation to underlying tissue due to the ability of the neutrophils to bind Stx1. In our study we did not investigate toxin binding to transmigrating neutrophils. Despite this, our results clearly demonstrate that neutrophil transmigration enhances Stx translocation at the concentration of Stx and the numbers of neutrophils that were employed in our study.
Others have shown that EPEC induces PMN transmigration across and IL-8 secretion from T84 IECs (43, 44). We confirmed these findings and have found that STEC also induces neutrophil transmigration and IL-8 secretion, a finding which is consistent with histopathogical studies of STEC-infected patients (18, 23, 24, 42). Interestingly, the ability to induce neutrophil transmigration varies considerably among different STEC strains. Our observations that some eae- and espB-negative STEC strains cause more PMN transmigration than eae- and espB-positive or LEE-positive STEC and EPEC strains are unexpected and suggest that LEE-negative STEC strains have as yet unidentified virulence factors distinct from LEE.
It is unclear from our studies what bacterial factors in STEC are responsible for promoting neutrophil transmigration. STEC infection resulting in serious disease is more often associated with LEE-positive STEC (40); thus it was surprising to observe that LEE-negative strains induced significantly more neutrophils to transmigrate. However, several eae-negative STEC strains have been associated with HUS (5, 39, 40). The presence of the LEE pathogenicity island within the STEC strains did not appear to have any affect on the ability of these strains to adhere to T84 cells. The LEE-positive STEC strains were 10-fold less efficient at adherence to T84 cells than the LEE-positive EPEC strain. Consistent with previous reports of STEC interaction with T84 cells (28), none of the LEE-positive STEC strains studied were capable of forming A/E lesions on T84 cells. It is unknown why STEC strains positive for LEE genes are unable to form A/E lesions on T84 cells yet are able to form A/E lesions on other cell lines (35). In this study, it is difficult to determine the contribution of A/E lesion formation to STEC-induced neutrophil transmigration.
The production of IL-8 by epithelial cells in response to bacterial infection is believed to be involved in the recruitment of neutrophils from the endothelium to the intestine (30). Several bacterial pathogens have been shown to induce IL-8 production upon attachment to or invasion of IECs (11, 44). Among the various LEE-positive STEC strains tested, there were either no or only minor increases in IL-8 production upon infection of T84 cells compared with that for uninfected cells. However, four out of the five LEE-negative strains induced a highly significant increase in IL-8 production. The differences in IL-8 production could not be attributed to differences in adhesion. Lipopolysaccharide (LPS) has been reported to induce IL-8 secretion from epithelial cells (19) but is unlikely to play a role in these studies because T84 cells have been reported to be unresponsive to high doses of LPS (10).
We have previously shown that purified Stx1 and Stx2 induce IL-8 secretion from HCT-8 IECs (49). This response requires at least 10 ng of Stx1 or -2/ml, and significant increases in IL-8 secretion are not observed until at least 8 h after toxin exposure. In the IL-8 assays described in this study, supernatants were examined for IL-8 after only 3 h of T84 cell exposure to STEC and the amount of Stx1 and/or -2 produced by each strain in this time frame is less than 10 ng/ml (data not shown). Therefore, the IL-8 responses due to the various STEC strains described in this study are most likely not due to Stxs. We investigated whether T84 IECs were capable of producing IL-8 in response to a 3-h exposure of Stx1 or -2 (1 μg/ml) and found no significant difference in IL-8 secretion between T84 IECs and control monolayers (data not shown).
A question raised by these studies is what bacterial factor or factors in the LEE-negative strains are responsible for induction of neutrophil transmigration across, and the secretion of IL-8 from, T84 cells. Our data indicate that the two may be correlated; however, in at least one LEE-negative strain we saw a relatively large PMN transmigration and no IL-8 production, whereas in another LEE-negative strain there was low PMN transmigration but high IL-8 production. These discordant strains suggest that the same bacterial factor may not be responsible for both activities. A recent report has identified SipA as a factor produced by Salmonella that induces neutrophil transmigration across T84 cells (26). However, SipA was shown not to be responsible for the ability of Salmonella to induce IL-8 secretion from T84 cells; instead, other Salmonella factors, such as flagellin, were implicated in the IL-8 response (16). Further studies to elucidate the factors from LEE-negative STEC important for both neutrophil transmigration across and induction of IL-8 secretion from IECs are under way. These factors may represent important virulence factors in LEE-negative STEC strains.
In summary we have shown that the process of neutrophil transmigration enhances the translocation of Stx1 and Stx2, most likely by opening a nonspecific paracellular pathway across a polarized monolayer of T84 IECs. The amount of Stx translocating correlates with the number of neutrophils transmigrating. These data lead us to speculate that the degree of inflammation in the intestine may positively correlate with the amount of Stx gaining access to underlying tissue. Furthermore, STEC induces neutrophils to transmigrate across the IEC barrier, a process that results in an increase in Stx movement to the basolateral surface, indicating that the bacteria per se may be contributing to the toxin uptake process. These studies have provided a framework for future research to facilitate our understanding of how Stxs gain access to underlying tissue in vivo. A better understanding of Stx penetration of the intestinal barrier may lead to the development of therapies geared to diminishing Stx uptake into the systemic circulation, thereby reducing the chances of HUS following STEC infection
ACKNOWLEDGMENTS
Research support for this study included the following grants from the National Institutes of Health, Bethesda, Md.: HL-55660, A1-16242, and A1-39067, as well as P 30 DK-34928 for the Center for Gastroenterology Research on Absorptive and Secretory Processes. C.M.T. was supported by A1-01715 and the Charles H. Hood Foundation.
We thank Anne V. Kane for critical comments as well as for providing purified Stx1 and Stx2. We also thank Jennifer Ritchie for critical comments.
REFERENCES
- 1.Acheson D W K, Keusch G T. The family of Shiga toxins. In: Alouf J E, Freer J H, editors. The comprehensive sourcebook of bacterial protein toxins. London, United Kingdom: Academic Press Ltd.; 1999. pp. 229–242. [Google Scholar]
- 2.Acheson D W K, Jacewicz M, Kane A V, Donohue-Rolfe A, Keusch G T. One step high yield affinity purification of Shiga-like toxin II variants and quantitation using enzyme linked immunosorbent assays. Microb Pathog. 1993;14:57–66. doi: 10.1006/mpat.1993.1006. [DOI] [PubMed] [Google Scholar]
- 3.Acheson D W K, Lincicome L L, Jacewicz M S, Keusch G T. Shiga toxin interaction with intestinal epithelial cells. In: Kaper J B, O'Brien A D, editors. Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. Washington, D.C.: American Society for Microbiology; 1998. pp. 140–147. [Google Scholar]
- 4.Acheson D W K, Moore R, De Breucker S, Lincicome L, Jacewicz M, Skutelsky E, Keusch G T. Translocation of Shiga toxin across polarized intestinal cells in tissue culture. Infect Immun. 1996;64:3294–3300. doi: 10.1128/iai.64.8.3294-3300.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonnet R, Souweine B, Gauthier G, Rich C, Livrelli V, Sirot J, Joly B, Forestier C. Non-O157:H7 Stx2-producing Escherichia coli strains associated with sporadic cases of hemolytic-uremic syndrome in adults. J Clin Microbiol. 1998;36:1777–1780. doi: 10.1128/jcm.36.6.1777-1780.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borregaard N, Cowland B. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. 1997;89:3503–3521. [PubMed] [Google Scholar]
- 7.Deffback M E, Bryan C J, Roy C M. Protein movement across cultured guinea pig trachea: specificity and effect of transcytosis inhibitors. Am J Physiol. 1996;271:L744–L752. doi: 10.1152/ajplung.1996.271.5.L744. [DOI] [PubMed] [Google Scholar]
- 8.Donohue-Rolfe A, Acheson D W K, Kane A V, Keusch G T. Purification of Shiga and Shiga-like toxins I and II by receptor analog affinity chromatography with immobilized P1 glycoprotein and the production of cross-reactive monoclonal antibodies. Infect Immun. 1989;57:3888–3893. doi: 10.1128/iai.57.12.3888-3893.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donohue-Rolfe A, Kelley M A, Bennish M, Keusch G T. Enzyme-linked immunosorbent assay for shigella toxin. J Clin Microbiol. 1986;24:65–68. doi: 10.1128/jcm.24.1.65-68.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eckmann L, Jung H C, Schurer-Maly C, Panja A, Morzycka-Wroblewska E, Kagnoff M F. Differential cytokine expression by human intestinal epithelial cell lines: regulated expression of interleukin 8. Gastroenterology. 1993;105:1689–1697. doi: 10.1016/0016-5085(93)91064-o. [DOI] [PubMed] [Google Scholar]
- 11.Eckmann L, Kagnoff M F, Fierer J. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect Immun. 1993;61:4569–4574. doi: 10.1128/iai.61.11.4569-4574.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elliott E, Li Z, Bell C, Stiel D, Buret A, Wallace J, Brzuszczak I, O'Loughlin E. Modulation of host response to Escherichia coli O157:H7 infection by anti-CD18 antibody in rabbits. Gastroenterology. 1994;106:1554–1561. doi: 10.1016/0016-5085(94)90410-3. [DOI] [PubMed] [Google Scholar]
- 13.Fitzpatrick M M, Shah V, Filler G, Dillon M J, Barratt T M. Neutrophil activation in the haemolytic uraemic syndrome: free and complexed elastase in plasma. Pediatr Nephrol. 1992;6:50–53. doi: 10.1007/BF00856833. [DOI] [PubMed] [Google Scholar]
- 14.Fitzpatrick M M, Shah V, Trompeter R S, Dillon M J, Barratt T M. Interleukin-8 and polymorphoneutrophil leucocyte activation in hemolytic uremic syndrome of childhood. Kidney Int. 1992;42:951–956. doi: 10.1038/ki.1992.372. [DOI] [PubMed] [Google Scholar]
- 15.Forsyth K D, Simpson A C, Fitzpatrick M M, Barratt T M, Levinsky R J. Neutrophil-mediated endothelial injury in haemolytic uraemic syndrome. Lancet. 1989;ii:411–414. doi: 10.1016/s0140-6736(89)90591-6. [DOI] [PubMed] [Google Scholar]
- 16.Gewirtz A T, Simon P O, Jr, Schmitt C K, Taylor L J, Hagedorn C H, O'Brien A D, Neish A S, Madara J L. Salmonella typhimurium translocates flagellin across intestinal epithelia, inducing a proinflammatory response. J Clin Investig. 2001;107:99–109. doi: 10.1172/JCI10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffin P M. Escherichia coli O157:H7 and other enterohemorrhagic Escherichia coli. In: Blaser M J, Smith P D, Ravdin J L, Greenberg H B, Guerrant R L, editors. Infections of the gastrointestinal tract. New York, N.Y: Raven Press, Ltd.; 1995. pp. 739–761. [Google Scholar]
- 18.Griffin P M, Olmstead L C, Petras R E. Escherichia coli O157:H7-associated colitis. A clinical and histological study of 11 cases. Gastroenterology. 1990;99:142–149. doi: 10.1016/0016-5085(90)91241-w. [DOI] [PubMed] [Google Scholar]
- 19.Henderson B, Poole S, Wilson M. Bacterial modulins: a novel class of virulence factors which cause host tissue pathology by inducing cytokine synthesis. Microbiol Rev. 1996;60:316–341. doi: 10.1128/mr.60.2.316-341.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hurley B P, Jacewicz M, Thorpe C M, Lincicome L L, King A J, Keusch G T, Acheson D W K. Shiga toxins 1 and 2 translocate differently across polarized intestinal epithelial cells. Infect Immun. 1999;67:6670–6677. doi: 10.1128/iai.67.12.6670-6677.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaper J B. Enterohemorrhagic Escherichia coli. Curr Opin Microbiol. 1999;1:103–108. doi: 10.1016/s1369-5274(98)80149-5. [DOI] [PubMed] [Google Scholar]
- 22.Karch H, Schmidt H, Brunder W. Plasmid-encoded determinates of Escherichia coli O157:H7. In: Kaper J B, O'Brien A D, editors. Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. Washington, D.C.: American Society for Microbiology; 1998. pp. 183–194. [Google Scholar]
- 23.Kelly J, Oryshak A, Wenetsek M, Grabiec J, Handy S. The colonic pathology of Escherichia coli O157:H7 infection. Am J Surg Pathol. 1990;14:87–92. doi: 10.1097/00000478-199001000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Kelly J K, Pai C H, Jadusingh I H, Macinnis M L, Shaffer E A, Hershfield N B. The histopathology of rectosigmoid biopsies from adults with bloody diarrhea due to verotoxin-producing Escherichia coli. Am J Clin Pathol. 1987;88:78–82. doi: 10.1093/ajcp/88.1.78. [DOI] [PubMed] [Google Scholar]
- 25.Knutton S, Baldwin T, Williams P H, McNeish A S. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 1989;57:1290–1298. doi: 10.1128/iai.57.4.1290-1298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee C A, Silva M, Siber A M, Kelly A J, Galyov E, McCormick B A. A secreted salmonella protein induces a proinflammatory response in epithelial cells, which promotes neutrophil migration. Proc Natl Acad Sci USA. 2000;97:12283–12288. doi: 10.1073/pnas.97.22.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leonard M, Creed E, Brayden D, Baird A W. Evaluation of the Caco-2 monolayer as a model epithelium for iontophoretic transport. Pharm Res. 2000;17(10):1181–1188. doi: 10.1023/a:1026454427621. [DOI] [PubMed] [Google Scholar]
- 28.Li Z, Elliott E, Payne J, Isaacs J, Gunning P, O'Loughlin E V. Shiga toxin-producing Escherichia coli can impair T84 cell structure and function without inducing attaching/effacing lesions. Infect Immun. 1999;67:5938–5945. doi: 10.1128/iai.67.11.5938-5945.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCormick B A, Colgan S P, Delp-Archer C, Miller S I, Madara J L. Salmonella typhimurium attachment to human intestinal epithelial monolayers: transcellular signalling to subepithelial neutrophils. J Cell Biol. 1993;123:895–907. doi: 10.1083/jcb.123.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCormick B A, Hofman P M, Kim J, Carnes D K, Miller S I, Madara J L. Surface attachment of Salmonella typhimurium to intestinal epithelia imprints the subepithelial matrix with gradients chemotactic for neutrophils. J Cell Biol. 1995;131:1599–1608. doi: 10.1083/jcb.131.6.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCormick B A, Siber A M, Maurelli A T. Requirement of the Shigella flexneri virulence plasmid in the ability to induce trafficking of neutrophils across polarized monolayers of the intestinal epithelium. Infect Immun. 1998;66:4237–4243. doi: 10.1128/iai.66.9.4237-4243.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milford D V, Staten J, MacGreggor I, Dawes J, Taylor C M, Hill F G. Prognostic markers in diarrhoea-associated haemolytic-uraemic syndrome: initial neutrophil count, human neutrophil elastase and von Willebrand factor antigen. Nephrol Dial Transplant. 1991;6:232–237. doi: 10.1093/ndt/6.4.232. [DOI] [PubMed] [Google Scholar]
- 33.Milks L C, Conyers G P, Cramer E B. The effect of neutrophil migration on epithelial permeability. J Cell Biol. 1986;103:2729–2738. doi: 10.1083/jcb.103.6.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nash S, Stafford J, Madara M L. Effects of polymorphonuclear leukocyte transmigration on the barrier function of cultured intestinal epithelial monolayers. J Clin Investig. 1987;80:1104–1113. doi: 10.1172/JCI113167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nataro J P, Kaper J B. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nusrat A, Turner J R, Madara J L. Regulation of tight junctions by extracellular stimuli: nutrients, cytokines, and immune cells. Am J Physiol. 2000;279:G851–G857. doi: 10.1152/ajpgi.2000.279.5.G851. [DOI] [PubMed] [Google Scholar]
- 37.Parkos C A, Delp C, Arnaout M A, Madara J L. Neutrophil migration across a cultured intestinal epithelium. Dependence on a CD11b/CD18-mediated event and enhanced efficiency in a physiological direction. J Clin Investig. 1991;88:1605–1612. doi: 10.1172/JCI115473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paton A W, Paton J C. Detection and characterization of Shiga toxigenic Escherichia coli by using multiplex PCR assays for stx1, stx2, eaeA, enterohemorrhagic E. coli hlyA, rfbO111, and rfbO157. J Clin Microbiol. 1998;36:598–602. doi: 10.1128/jcm.36.2.598-602.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paton A W, Paton J C. Direct detection of Shiga toxigenic Escherichia coli strains belonging to serogroups O111, O157, and O113 by multiplex PCR. J Clin Microbiol. 1999;37:3362–3365. doi: 10.1128/jcm.37.10.3362-3365.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paton J C, Paton A W. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin Microbiol Rev. 1998;11:450–479. doi: 10.1128/cmr.11.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pickering L K, Obrig T G, Stapleton F B. Hemolytic-uremic syndrome and enterohemorrhagic Escherichia coli. Pediatr Infect Dis J. 1994;13:459–476. doi: 10.1097/00006454-199406000-00001. [DOI] [PubMed] [Google Scholar]
- 42.Richardson S E, Karmali M A, Becker L E, Smith C R. The histopathology of the hemolytic uremic syndrome associated with verocytotoxin-producing Escherichia coli infections. Hum Pathol. 1988;19:1102–1108. doi: 10.1016/s0046-8177(88)80093-5. [DOI] [PubMed] [Google Scholar]
- 43.Savkovic S D, Koutsouris A, Hecht G. Attachment of a noninvasive enteric pathogen, enteropathogenic Escherichia coli, to cultured human intestinal epithelial monolayers induces transmigration of neutrophils. Infect Immun. 1996;64:4480–4487. doi: 10.1128/iai.64.11.4480-4487.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Savkovic S D, Koutsouris A, Hecht G. Activation of NF-kappaB in intestinal epithelial cells by enteropathogenic Escherichia coli. Am J Physiol. 1997;273:C1160–C1167. doi: 10.1152/ajpcell.1997.273.4.C1160. [DOI] [PubMed] [Google Scholar]
- 45.Sears C L. Molecular physiology and pathophysiology of tight junctions. V. Assault of the tight junction by enteric pathogens. Am J Physiol. 2000;279:G1129–G1134. doi: 10.1152/ajpgi.2000.279.6.G1129. [DOI] [PubMed] [Google Scholar]
- 46.Sjorgren R, Neill R, Rachmilewitz D, Fritz D, Newland J, Sharpnack D, Colleton C, Fondacaro J, Gemski P, Boedeker E. Role of Shiga-like toxin I in bacterial enteritis: comparison between isogenic Escherichia coli strains induced in rabbits. Gastroenterology. 1994;106:306–317. doi: 10.1016/0016-5085(94)90587-8. [DOI] [PubMed] [Google Scholar]
- 47.Slutsker L, Ries A A, Greene K D, Wells J G, Jutwagner L, Griffin P M. Escherichia coli O157:H7 diarrhea in the United States: clinical and epidemiologic features. Ann Intern Med. 1997;126:505–513. doi: 10.7326/0003-4819-126-7-199704010-00002. [DOI] [PubMed] [Google Scholar]
- 48.Te Loo D M, Monnens L A, van der Velden T J, Vermeer M A, Preyers F, Demacker P N, van den Heuvel L P, van Hinsbergh V W. Binding and transfer of verocytotoxin by polymorphonuclear leukocytes in hemolytic uremic syndrome. Blood. 2000;95:3396–3402. [PubMed] [Google Scholar]
- 49.Thorpe C M, Hurley B P, Lincicome L L, Jacewicz M S, Keusch G T, Acheson D W K. Shiga toxins stimulate secretion of interleukin-8 from intestinal epithelial cells. Infect Immun. 1999;67:5985–5993. doi: 10.1128/iai.67.11.5985-5993.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walters M D, Matthei I U, Kay R, Dillon M J, Barratt T M. The polymorphonuclear leucocyte count in childhood haemolytic uraemic syndrome. Pediatr Nephrol. 1989;3:130–134. doi: 10.1007/BF00852893. [DOI] [PubMed] [Google Scholar]
- 51.Whittam T S. Evolution of Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. In: Kaper J B, O'Brien A D, editors. Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. Washington D.C.: American Society for Microbiology; 1998. pp. 195–212. [Google Scholar]