Dear Editor,
As mass immunization against the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is being performed with vaccines on various platforms, a growing number of skin reactions have been attributed to these vaccines. Besides the most commonly reported cutaneous adverse effects, several cases of immune‐mediated reactions like immunobullous dermatoses are being reported following the Coronavirus disease 2019 (COVID‐19) vaccination. 1 Here, we describe two cases who developed purpuric dermatosis and lymphocytic vasculopathy following the administration of COVID‐19 vaccines.
A 53‐year‐old female with a medical history of treated breast cancer 8 years ago, presented with erythematous swelling of bilateral lower legs 9 days after receiving the first dose of COVID‐19 vaccine, BBIBP‐CorV‐2 (Figure 1A, left). She was febrile, and her physical examination was significant for deep‐seated, firm erythematous nodules, and plaques on the calves. Her laboratory exams showed elevated erythrocyte sedimentation rate (ESR), and C‐reactive protein (CRP) with mild elevation of D‐dimer. Color doppler sonography ruled out deep vein thrombosis. A skin biopsy was performed which revealed lymphocytic vasculopathy characterized by mild perivascular lymphocytic infiltrate with endothelial swelling, and extravasation of erythrocytes in the superficial, and the mid dermis. There was no evidence of intravascular thrombosis (Figure 1A, right). Subsequently, she was started on topical corticosteroids to which she showed marked improvement within 2 weeks.
FIGURE 1.
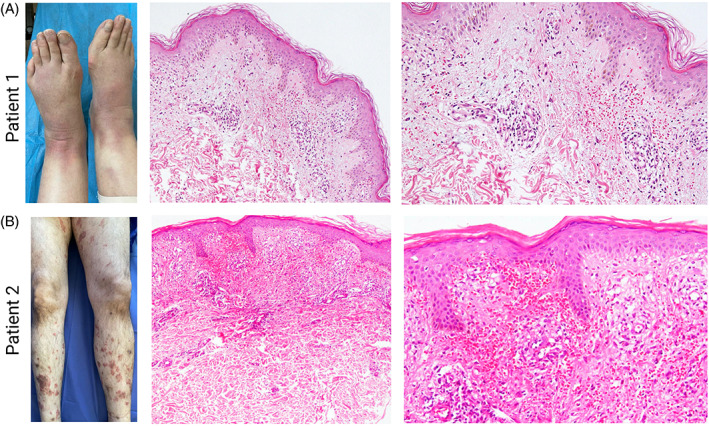
Purpuric dermatosis and lymphocytic vasculopathy after SARS‐CoV‐2 vaccination in Patients 1–2 (A) (left) In Patient 1, there are deep‐seated erythematous nodules and plaques with histopathology (right) of mild perivascular lymphocytic infiltration with extravasation of erythrocytes in the superficial and mid‐dermis with no evidence of intravascular thrombosis (B) In Patient 2 (left) non‐blanchable purpuric plaques were noted with histopathology of (right) moderate superficial perivascular lymphocytic infiltration with microhemorrhage, consistent with lymphocytic vasculopathy
A 50‐year‐old male presented with non‐blanchable purpuric papules and plaques on his legs and buttocks 2 months after receiving the BBIBP‐CorV‐2 COVID‐19 vaccine (Figure 1B, left). He had no known medical history and did not recall taking any medication recently. His skin biopsies demonstrated moderately dense infiltration of lymphocytes, and histiocytes around superficial blood vessels with severe extravasation of erythrocytes, and endothelial swelling with no sign of intravascular thrombosis (Figure 1B, right). Other laboratory findings were insignificant. Afterward, he was prescribed topical corticosteroids to which he responded notably.
Acrally distributed purpuric lesions can be related to a variety of vascular pathologies including vasculitis, and vasculopathies. Vasculitis is an inflammatory process that can affect blood vessels of any size leading to hemorrhagic, and ischemic events. The most common type of vasculitis is leukocytoclastic vasculitis (LCCV), which is histologically defined by intramural inflammation, swelling of endothelium, pyknosis, and karyorrhexis of nuclei, hemorrhage, and fibrinoid necrosis of vessel walls. 2 The term vasculopathy however is reserved for noninflammatory lesions due to either immune complex deposition or intravascular thrombosis. 3 Cases of vasculitis have been reported in association with both COVID‐19 infection, and COVID‐19 vaccines, 4 but cases of cutaneous vasculopathies are relatively rare. Falkenhain‐Lopez et al first reported a case of widespread purpura annularis telangiectodes shortly after receiving mRNA SARS‐CoV‐2 vaccine, which histologically demonstrated dermal perivascular lymphocytic infiltration with no evidence of fibrinoid necrosis, and fibrin deposition. 5 Atak et al reported a case of pigmented purpuric dermatosis following administration of BNT162B2 mRNA COVID‐19 vaccine with histologic features of epidermal spongiosis, and lymphocytic exocytosis with dermal perivascular lymphocytic infiltration, endothelial swelling, and red blood cell extravasation. 6
It is suggested that the underlying mechanism is related to endothelial damage by the virus, and the inflammatory infiltrates. 7 Expression of Angiotensin‐Converting enzyme 2 (ACE2) on endothelial cells may be indicative of their susceptibility to the SARS‐CoV‐2, and their eventual destruction. 8 Recognition of viral pathogen associated molecular patterns such as S protein by the endothelial innate immune receptors could also contribute to vasculopathy in Covid‐19 patients. 9 In whole‐virion inactivated vaccines of Sars‐CoV‐2, T‐helper 1‐based response may lead to an inflammatory reaction in vessel walls. 10 Molecular mimicry and cross‐reactivity between COVID‐19 and self‐antigens like actin, and nuclear antigens (NA), could facilitate the autoimmune reactions. 11 Although it is yet indefinite whether COVID‐19 vaccines can induce vasculopathy in recipients, the temporal relation of these reactions with COVID‐19 vaccination can be indicative of a causative relationship.
AUTHOR CONTRIBUTIONS
Zahra Saffarian, Alireza Hadizadeh, and Hassan Vahidnezhad contributed to developing the research idea and composing and revising the manuscript. Alireza Ghanadan, Hassan Vahidnezhad, and Rana Samii contributed to compiling and revising the manuscript. All authors read and confirmed the final draft.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
This study was approved by the research and ethics committee of the Tehran University of Medical Sciences. The patients and their families have given their informed consent to publish this case.
INFORMED CONSENT
Written informed consent was obtained from the patients for the publication of this case series and any accompanying images. A copy of the written consent is available.
ACKNOWLEDGMENTS
The authors thank the patients for their contribution to this research project.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1. Saffarian Z, Samii R, Ghanadan A, Vahidnezhad H. De novo severe pemphigus vulgaris following SARS‐CoV‐2 vaccination with BBIBP‐CorV. Dermatol Ther. 2022;35(6):e15448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jorizzo JL. Classification of vasculitis. J Invest Dermatol. 1993;100(1):106s‐110s. [DOI] [PubMed] [Google Scholar]
- 3. Seshan S. Lupus vasculopathy and Vasculitis: what is the difference and when do they occur? Pathol Case Rev. 2007;12:214‐221. [Google Scholar]
- 4. Jamshidi P, Hajikhani B, Mirsaeidi M, Vahidnezhad H, Dadashi M, Nasiri MJ. Skin manifestations in COVID‐19 patients: are they indicators for disease severity? A systematic review. Front Med (Lausanne). 2021;8:634208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Falkenhain‐López D, Gutiérrez‐Collar C, Arroyo‐Andrés J, Gallego‐Gutiérrez I, Rodríguez‐Peralto JL, Sánchez‐Velázquez A. Widespread purpura annularis telangiectodes following mRNA SARS‐CoV‐2 vaccine. J Eur Acad Dermatol Venereol. 2021;35(11):e719‐e721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Atak MF, Farabi B, Kalelioglu MB, Rao BK. Pigmented purpuric dermatosis after BNT162B2 mRNA COVID‐19 vaccine administration. J Cosmet Dermatol. 2022;21(2):435‐437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet (London, England). 2020;395(10234):1417‐1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Becker RC. COVID‐19 update: Covid‐19‐associated coagulopathy. J Thromb Thrombolysis. 2020;50(1):54‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Flaumenhaft R, Enjyoji K, Schmaier AA. Vasculopathy in COVID‐19. Blood. 2022;140(3):222‐235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ella R, Vadrevu KM, Jogdand H, et al. Safety and immunogenicity of an inactivated SARS‐CoV‐2 vaccine, BBV152: a double‐blind, randomised, phase 1 trial. Lancet Infect Dis. 2021;21(5):637‐646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vojdani A, Kharrazian D. Potential antigenic cross‐reactivity between SARS‐CoV‐2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol (Orlando, Fla). 2020;217:108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.


