Abstract
Background
Gravity‐dependent positioning therapy is an established concept in the treatment of severe acute respiratory distress syndrome and improves oxygenation in spontaneously breathing patients with hypoxemic acute respiratory failure. In patients with coronavirus disease 2019, this therapy seems to be less effective. Electrical impedance tomography as a point‐of‐care functional imaging modality for visualizing regional ventilation can possibly help identify patients who might benefit from positioning therapy and guide those maneuvers in real‐time. Therefore, in this prospective observational study, we aimed to discover typical patterns in response to positioning maneuvers.
Methods
Distribution of ventilation in 10 healthy volunteers and in 12 patients with hypoxemic respiratory failure due to coronavirus disease 2019 was measured in supine, left, and right lateral positions using electrical impedance tomography.
Results
In this study, patients with coronavirus disease 2019 showed a variety of ventilation patterns, which were not predictable, whereas all but one healthy volunteer showed a typical and expected gravity‐dependent distribution of ventilation with the body positions.
Conclusion
Distribution of ventilation and response to lateral positioning is variable and thus unpredictable in spontaneously breathing patients with coronavirus disease 2019. Electrical impedance tomography might add useful information on the immediate reaction to postural maneuvers and should be elucidated further in clinical studies. Therefore, we suggest a customized individualized positioning therapy guided by electrical impedance tomography.
Keywords: ARDS, COVID‐19, EIT, electrical impedance tomography, postural therapy, regional ventilation
Editorial Comment.
Electrical impedance tomography may have screening or diagnostic value as a bedside imaging modality for guidance in the treatment of major atelectasis in critically ill cases. This study applies this imaging modality and different positioning in both a health and a COVID cohort, to describe patterns of regional ventilation. The findings show that atelectasis patterns are not highly consistent or predictable, particularly in the COVID respiratory insufficiency group.
1. INTRODUCTION
Lateral and prone positioning in mechanically ventilated patients has become the standard of care for patients with acute respiratory distress syndrome (ARDS) to improve oxygenation, ventilation, and outcome. 1 The physiological rationale behind those positioning maneuvers in typical ARDS is to reduce ventilation/perfusion mismatching, hypoxemia, and shunting. 2 This is due to three mechanisms: (1) the re‐aeration of pulmonary units may lead to a more homogeneous distribution of ventilation in lateral and prone compared to supine position, (2) lateral and prone positioning may result in a net gain in functional lung volume as more lung tissue remains open in the now non‐dependent zones than collapses in the dependent regions due to hydrostatic pressure and (3) perfusion distribution remains similar compared to supine position. 3 Data suggest that prone positioning also improves oxygenation in spontaneously breathing non‐intubated patients with acute hypoxemic respiratory failure. 4
However, in many patients with coronavirus disease 2019 (COVID‐19), lateral and prone positioning seems less effective, although it increases blood oxygenation rapidly, its effect after re‐supination is maintained in only half of the patients. 5 However, sustained oxygenation improvement is associated with liberation from mechanical ventilation and lower mortality in critically ill COVID‐19 patients. 6 Furthermore, the impact on intubation rates and mortality is uncertain. 7 , 8 , 9 , 10 One reason for these findings might be a difference in the morphological distribution of the pathologies. In ARDS, a typical gravity‐dependent layered appearance can be seen in CT scans, whereas in many patients COVID‐19 presents like viral pneumonia with an inhomogeneous distribution of the injuries resulting in a “crazy‐paving radio‐morphological pattern” on CT scans. 11 Froelich and co‐workers demonstrated in their case series that placing those parts of the lung(s) that appear predominantly affected by the disease on chest CT scans on top can improve oxygenation and reduce discomfort. 12 The optimization of the matching of ventilation and perfusion is obvious, but one cannot draw inferences about the underlying mechanism as CT is not available at the bedside and is prohibitive for routine use due to radiation exposure. 10
Electrical impedance tomography (EIT) is a point‐of‐care, radiation‐free imaging modality that can visualize the regional distribution of ventilation within the lungs in real‐time. 13 Therefore, it might enable the clinician to better identify patients potentially benefitting from positioning maneuvers as well as to implement and optimize such interventions. The ability of EIT to reliably depict the distribution of ventilation in health and disease has been validated in previous studies. 14
Accordingly, the aim of this study was to describe the patterns of ventilation distribution and the impact of lateral positioning in spontaneously breathing patients with COVID‐19 and to compare these findings with those detected in healthy volunteers.
2. METHODS
This prospective observational study was performed in cooperation between the Departments of Anesthesiology and Intensive Care Medicine of the Klinikum Osnabrück GmbH and of the University Medical Center of Rostock between April and November 2020. Ethical approval was provided by the ethical board of the medical faculty of the University of Rostock on April 3, 2020 (A2020‐0072) and confirmed by the ethics committee of the chamber of physicians of Lower Saxony on April 6, 2020. The study has been performed according to the Declaration of Helsinki. In addition, the study was registered at the German Clinical Trials Register (DRKS00021276; https://www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00021276). Written informed consent was obtained from all participants before enrolment. Novel EIT images showing dynamic relative regional strain in health and disease have been published recently for the same subjects as included in this study. 15
2.1. Study subjects
Patients with confirmed Severe Acute Respiratory Syndrome Corona Virus 2 (SARS‐CoV‐2) infection suffering from hypoxemic respiratory failure, requiring oxygen insufflation therapy and showing elevated work of breathing (respiratory rate ≥ 30 breaths per minute) were included in the study. Healthy volunteers with no known lung disease served as controls.
2.2. EIT measurements and analysis
Details of EIT technology and clinical applications have been described in detail elsewhere. 13 Data were recorded at a sampling rate of 48 Hz by the BB2 EIT monitor and textile EIT belts of appropriate size (Sentec‐AG, EIT‐branch, Landquart, Switzerland). Patient‐specific ventilation images of breathing‐induced impedance changes were reconstructed from measured voltages using the manufacturer's imaging algorithm. The primary step was a visual inspection of the ventilation images in combination with an analysis of the distribution to global ventilation of each lung. As we suspected inhomogeneity due to COVID‐19 could be a factor responsible for the different behavior, the Global Inhomogeneity Index (GII) was calculated as described by Zhao. 16 Also, the Center of Ventilation (CoV) as introduced by Frerichs et al. 17 and modified by Bleul et al. 18 finally implemented in the Swisstom algorithm 19 was calculated using ibeX software version 1.5 (Swisstom AG, Switzerland) for the ventrodorsal direction (CoVVD); in addition, an analogous approach was pursued for the right‐left direction 18 (CoVRL) to quantify the topographical changes of ventilation distribution expected to occur in the lateral direction between the dependent and non‐dependent lung due to the lateral positions.
2.3. Study protocol
All subjects were breathing spontaneously with no mechanical ventilatory support. Oxygen was administered via nasal cannula or face mask according to pulse oximetry and capillary blood gas analysis. Examination started in supine position to gather baseline values and continued with left and finally right lateral position. Subjects were asked to turn themselves into their respective position and 1 min after a comfortable and stable position had been reached, EIT data of at least 10 consecutive breaths were recorded.
2.4. Statistical analysis
Statistical analysis was performed using SigmaPlot 13.0 (Systat Software, San José, CA). Due to the explorative and descriptive nature of this study, power analysis for sample size determination was not feasible. Results are presented as median (interquartile range) and/or absolute (relative) frequencies. For the positioning maneuvers, an increase in relative ventilation to at least the 1.1‐fold of baseline or a decline to the 0.9‐fold or less of baseline was considered relevant, reasoning that any change >10% might be of clinical relevance and not just due to the known inaccuracies of the EIT measurements, especially after the contact between the skin and the electrodes has been challenged by the lateral positioning maneuvers. 20 , 21 Mann–Whitney‐Rank‐Sum‐Test was used to test for differences between volunteers and patients and Friedman Repeated Measures Analysis of Variance (RM ANOVA) on ranks was performed to test for differences between the positions within each group with a Tukey Test for all pairwise multiple comparison procedures. An alpha level of <0.05 was considered statistically significant.
3. RESULTS
3.1. Volunteers' and patients' characteristics
Twelve COVID‐19 patients and 10 volunteers without known severe lung disease were included in this study between April and November 2020. Demographics, blood gas analysis, and oxygen flows are given in Table 1.
TABLE 1.
Demographic data/volunteers' and patients' characteristics
| Volunteers (n = 10) | Patients with COVID‐19 (n = 12) | p | |
|---|---|---|---|
| Age (years) | 30 (26–35) | 59 (43–72) | .016* |
| Gender (male/female) | 6/4 | 8/4 | 1.000 |
| Height (m) | 1.77 (1.72–1.85) | 1.77 (1.68–1.83) | .643 |
| Weight (kg) | 70 (65–81) | 88 (81–98) | .044* |
| O2 applied (l/min) | n.a. | 2 (2–4) | n.a. |
| sO2 (%) | n.a. | 94 (92–96) | n.a. |
| pO2 capillary (kPa) | n.a. | 8.8 (8.0–11.5) | n.a. |
| pCO2 capillary (kPa) | n.a. | 4.0 (3.9–4.7) | n.a. |
| Ph | n.a. | 7.48 (7.44–7.48) | n.a. |
Note: Data are presented as median (interquartile range); categorical data are presented as frequencies; p < .05 was considered as statistically significant (*); O2: oxygen; l/min: liters per minute; data from capillary blood gas analysis: sO2: oxygen saturation; pO2: partial pressure of oxygen; pCO2: partial pressure of carbon dioxide. m: metres; kg: kilograms; kPa: kilo‐Pascal n.a.: data not available/not applicable.
Complete data sets of EIT with sufficient quality could be obtained in 9 of the 10 healthy volunteers and in 11 of the 12 patients. In the other cases, EIT data of the right lateral position could not be obtained due to technical reasons, however, those in supine and left lateral positions were analyzed.
3.2. Examples of regional ventilation under different body positions
Representative EIT images of healthy volunteers (upper row) and two COVID‐19 patients (middle and lower row) in supine, right, and left lateral positions are depicted in Figure 1. In the representative healthy volunteer, starting from an evenly distributed ventilation, the physiological shift of ventilation towards the dependent lung regions in each body position could be recognized. In the two examples of COVID‐19 patients, EIT images are clearly different: in patient 1, the right lung was predominantly ventilated irrespective of the position; in patient 2, a shift of ventilation could be identified between the three positions, but not in the physiologically expected way.
FIGURE 1.
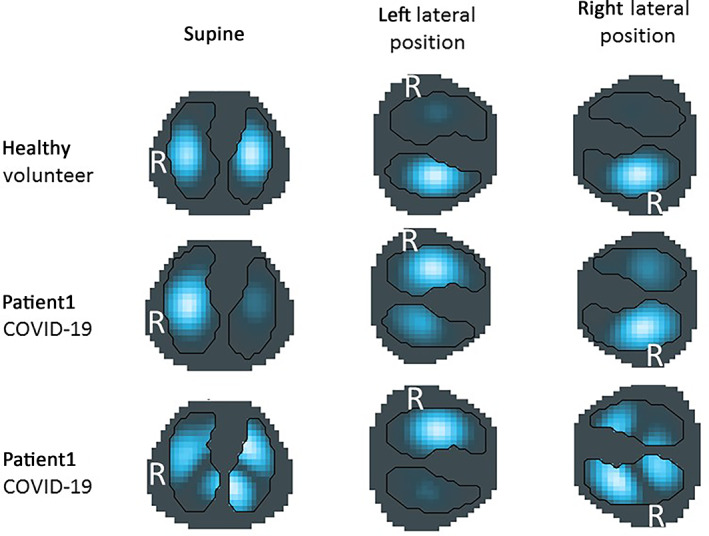
Examples of EIT images in supine, left, and right lateral positions. Assumed contours of the right and left lung are outlined by black lines. R = right hemithorax. Lighter blue to white colors represent higher ventilation‐induced regional impedance changes corresponding to more regional ventilation.
3.3. Distribution of ventilation for the groups of healthy volunteers and COVID‐19 patients
Figure 2 presents a summary of the division of total tidal ventilation between the hemithoraces for all three body positions in healthy volunteers and COVID‐19 patients. In healthy volunteers our findings reflected the physiological response to changes in body position in spontaneously breathing subjects: in supine position, ventilation was distributed homogeneously in both lungs with a tendency towards right‐sided dominance. After lateral positioning to either side, ventilation increased in the lower, dependent lung areas with a significant difference between the right and left lateral positions (#; p = .013). Also, in COVID‐19 patients, dominance of the right side could be recognized in supine position. However, neither after rotation to the right nor to the left side, pooled data show any significant changes in the division of ventilation within both hemithoraces compared to the other positions (p = .307). Left lateral position resulted in significant differences between COVID‐19 patients and healthy volunteers (*; p = .031).
FIGURE 2.
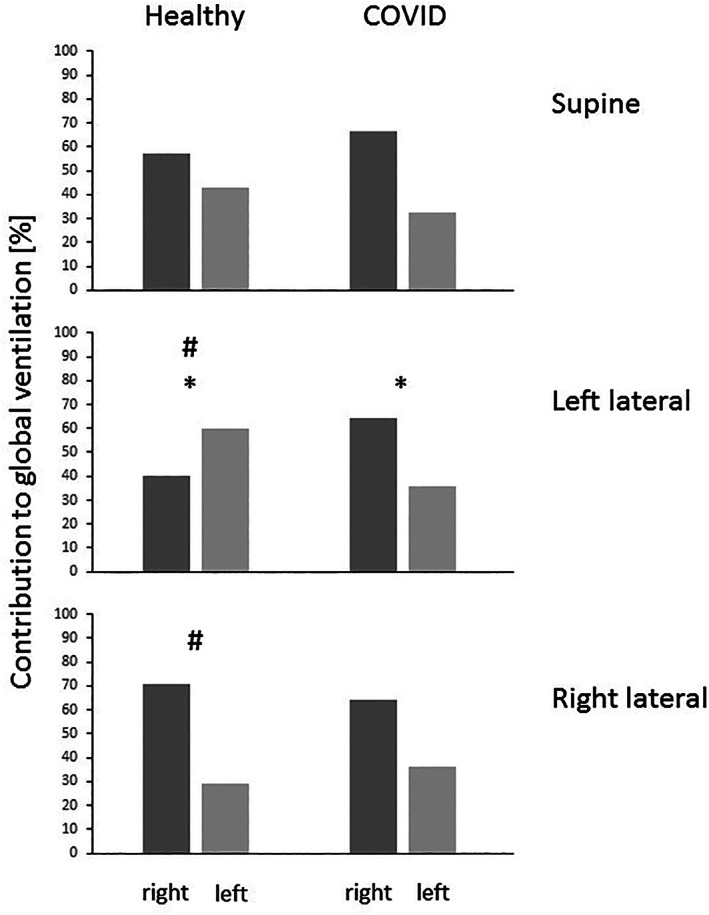
Relative contribution of right and left lung in supine, left and right lateral position summarized for all healthy and sick lungs. Columns show median relative ventilation. In the healthy lungs, ventilation increased in the respective dependent lung whereas right‐sided dominance persisted during the positioning maneuvers in COVID‐19 patients. (#) significant difference between the respective body position in healthy volunteers, (*) significant difference between the healthy volunteers and the COVID‐19 patients in the left lateral position
The pooled GII is presented in Table 2. In neither position, a significant difference between the groups was found. In the healthy volunteers, lateral positioning increased the median GII markedly but not significantly (p = .069), whereas the median GII remained almost constant in the COVID‐19 patients (p = .739).
TABLE 2.
Global inhomogeneity index
| Volunteers (n = 10) | COVID‐19 (n = 12) | p (inter‐group) | |
|---|---|---|---|
| Supine | 0.50 (0.48–0.54) | 0.55 (0.41–0.88) | 0.741 |
| Left lateral | 0.61 (0.47–0.76) | 0.53 (0.48–0.63) | 0.488 |
| Right lateral | 0.72 (0.49–0.89) | 0.56 (0.46–0.86) | 0.542 |
| P (intra‐group) | 0.069 | 0.739 |
Note: Data are presented as median (interquartile range). p < .05 was considered statistically significant.
Table 3 shows the pooled data of CoV for the different body positions. In the healthy volunteers, after lateral positioning to either side, the CoVRL moved towards the respective dependent lung by a marked but not significant distance compared to supine position. In contrast, CoVVD remained almost constant. In the COVID‐19 patients, rotation to the left lateral position moved the pooled CoVRL towards the non‐dependent lung, which resulted in a significant difference between the two groups (p = .027). In right lateral position, the change of the pooled CoVRL was minimal. CoVVD in COVID‐19 patients showed a shift towards the dorsal regions in the lateral positions compared to supine, but the differences did not reach significance (p = .078).
TABLE 3.
Center of ventilation (pooled data)
| Volunteers (n = 10) | COVID‐19 (n = 12) | p (RL) | p (VD) | |||
|---|---|---|---|---|---|---|
| CoV_RL (%) | CoV_VD (%) | CoV_RL (%) | CoV_VD (%) | |||
| Supine | 48 (38–56) | 57 (53–58) | 44 (35–53) | 54 (52–62) | .575 | .869 |
| Left lateral | 62* (47–70) | 56 (53–57) | 38* (34–53) | 58 (53–64) | .027* | .448 |
| Right lateral | 35 (27–52) | 58 (55–61) | 44 (37–56) | 62 (57–66) | .287 | .129 |
Note: Two coordinate Center of Ventilation (CoV): RL = right to left ratio, with 0% being the most lateral right lung and 100% the most lateral left lung; VD = ventral to dorsal, with 0% being the most ventral and 100% the most dorsal point of the lungs. Data are presented as median (interquartile range) in %. p < .05 was considered statistically significant (*).
3.4. Subject‐specific distribution of ventilation
The shift of ventilation from one hemithorax to the other during the positioning maneuvers is shown for each individual in Figure 3. Gravity dependency is highlighted by the columns facing downward. Again, COVID‐19 patients did not show the rather uniform pattern found in the healthy controls.
FIGURE 3.
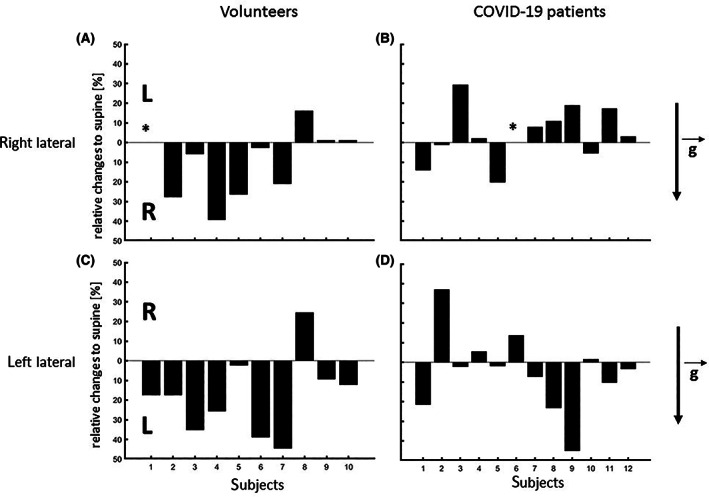
Changes in ventilation compared to supine position for individual subjects. The value of 0 marks the respective baseline in the supine position and columns indicate the absolute gain or loss of ventilation after each lateral positioning maneuver within the gravity field. = gravity vector. The respective dependent lung is always located below the 0‐line. For Figure 3A,B representing right lateral position, columns facing downwards depict a gain towards the dependent right lung and for Figures 3C,D representing left lateral position, columns facing downwards depict a gain towards the dependent left lung. * missing measurement due to poor electrode skin contact
In the healthy controls, rotation to the right side led to a shift (defined as a relative change of more than 10%) of the CoVRL towards the dependent right lung in six individuals, in another two no significant changes were recognized, and in one (volunteer 8) a displacement towards the contralateral left side could be seen. Rotating the body to the left side led to a shift of CoVRL towards the dependent left lung in six individuals, no significant change in three, and a shift towards the non‐dependent lung in one (volunteer 8).
In the COVID‐19 patients, right lateral positioning led to a marked movement of the CoVRL towards the dependent right lung in six patients. In one patient (patient 7) there was no relevant change, and in four patients, there was an obvious, but not significant shift toward the non‐dependent left lung. In the left lateral position, only three patients demonstrated a relocation of the CoVRL towards the dependent left lung. In this position, five individuals presented with less than 10% change and another four with a change to the non‐dependent contralateral right side.
Lateral positioning led to an increase in the median GII not only in the group but also in the majority of the healthy volunteers (12/19 maneuvers). In the COVID‐19 patients, lateral positioning resulted in an increase in 9/23 maneuvers (39%), but GII decreased by 48% (11/23) and did not change in 13% (3/23) of the cases.
Comparing the ventilation distribution of the individuals with the GII, patients with evenly distributed ventilation had a lower GII than the ones with one‐sided dominancy, but the GII was not significantly different (0.40 vs. 0.63; p = .107).
3.5. Effects of lateral position on the most affected lung
Aggregated data of the COVID‐19 patients suggest a similar distribution of ventilation in supine position to the volunteers. The patients, however, presented a greater range of ventilation division between the two hemithoraces: the right lung contributed 36 to 87% to global ventilation in the patients while these values were between 43 and 87% in the healthy volunteers. Correspondingly, the CoVRL for the patients presents with a wider range (45.6% between maximum and minimum, vs. 25.3% for the healthy volunteers). Five patients (3, 7, 8, 11, and 12) presented with a right‐sided dominance of ventilation whereas four subjects (2, 5, 6, and 10) had a left‐sided dominance (Figure 4).
FIGURE 4.
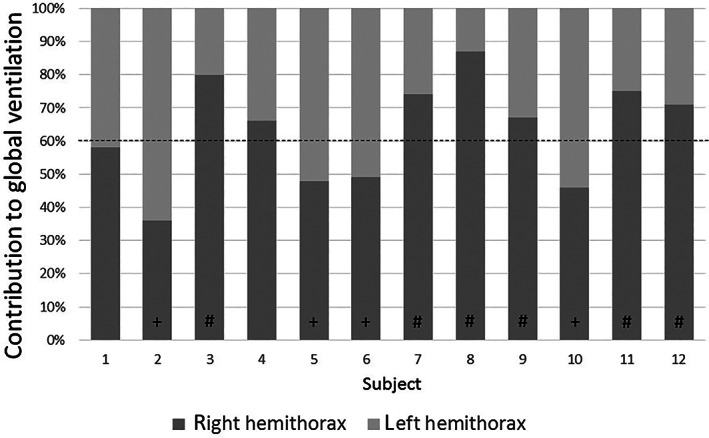
Relative contribution of right and left lung in supine position for individual COVID‐19 patients. The broken line marks the physiological division of ventilation in healthy subjects as previously described, with a right‐sided dominancy. (+) marks patients with a left‐sided dominancy, suggesting right‐sided affection. (#) marks patients with right‐sided dominancy greater than 10% of physiological range, suggesting left‐sided affection
Table 4 presents their reactions to lateral positioning: placing the less ventilated, that is, presumably the more affected lung, downwards, increased ventilation in the dependent lung in 7/9 (78%) cases, placing it up (i.e., placing the better‐ventilated lung downwards), lead to an increase in ventilation of the non‐dependent lung in 8/10 (80%) cases, whereas it did not change in two cases. No deterioration of the less ventilated side was observed. In contrast, for the two patients with equally distributed ventilation in supine position, ventilation increased in the respective dependent lung in one patient whereas it decreased in the dependent lung in left lateral position and underwent no changes for right lateral position. No connections to the respective GII were found.
TABLE 4.
Reactions to the lateral position of COVID patients with respect to the predominantly ventilated lung
| Predominantly ventilated lung down | Less ventilated lung down | ||||
|---|---|---|---|---|---|
| Pat. | Predominantly ventilated lung | Ventilation in dependent/“better” lung | Ventilation in non‐dependent/“more affected” lung | Ventilation in dependent/“more affected” lung | Ventilation in non‐dependent/“better” lung |
| 02 | Left | − | + | 0 | 0 |
| 03 | Right | − | + | + | − |
| 05 | Left | 0 | 0 | + | − |
| 06 | left | − | + | n.a. | n.a. |
| 07 | Right | − | + | + | − |
| 08 | Right | − | + | + | − |
| 09 | Right | − | + | + | − |
| 10 | Left | − | + | 0 | 0 |
| 11 | Right | − | + | + | − |
| 12 | Right | 0 | 0 | + | − |
Note: Reactions to lateral position are defined as follows: +: more than 10% relative increase compared to supine position; −: more than 10% relative decrease compared to supine position; 0: less than 10% relative increase/decrease compared to supine position—with relative increase/decrease being from for example, 60:40 to greater than 66:34 or to less than 54:46. n.a.: data not available.
4. DISCUSSION
In this prospective observational EIT study, we found significant differences in the pattern of ventilation distribution and in the reaction to lateral positioning between healthy volunteers and patients with COVID‐19.
With the exception of one subject, volunteers showed equally distributed ventilation between both lungs in the supine position and responded in a predictable gravity‐dependent manner to lateral positioning showing dominance of ventilation in the dependent lung. In COVID‐19 patients, ventilation was distributed more inhomogeneously already in supine position, and the physiological increase in ventilation of the dependent lung by respective lateral positioning could only be observed in about 40% (10/23) of the measurements. However, lateral positioning to either side led to an increase of ventilation in the previously less ventilated presumably sicker side irrespective of gravity in 15/19 (79%) of the cases; no deterioration with lateral positioning was seen. If ventilation was distributed evenly between the two lungs, reactions to lateral positioning were not predictable.
The visual difference (cf. Figure 1) was not associated with marked differences in the GII; for both groups, the GII in supine position was close to the GII reference previously described. 22 CoVRL changed accordingly with the distribution to global ventilation.
Gravity‐dependent increases in ventilation of the dependent lung during spontaneous breathing have been described before. 23 , 24 , 25 As the most gravity‐dependent alveoli have a lower volume at end‐expiration due to superimposed hydrostatic pressure, a given increase in transpulmonary pressure causes a larger increase in volume in those alveoli compared to the ones in the upper parts of the lungs, which are already more distended. 25 In addition, under spontaneous breathing with normal intra‐abdominal pressure, there are two possible mechanisms that allow the actively contracting diaphragm to exert more force in the dependent regions, irrespective of the body position. First, as the abdominal contents tend to push the dependent parts of the diaphragm more cephalad than the non‐dependent ones, the dependent parts of the diaphragm have a smaller radius of curvature (i.e., are more domed) and can, therefore, according to the Laplace relationship, generate greater traction. Second, with the dependent diaphragmatic muscles being more stretched they can develop more force due to the length/tension relationship. 26 , 27 , 28 EIT findings of the here investigated pulmonary healthy volunteers illustrate these gravity‐dependent changes in ventilation and even allow their quantification. This was nearly uniform in all subjects but one. This volunteer was a particularly young (28 years), slim (58 kg), and short (162 cm) female with a body mass index of 22.1 kg/m2, which was identical to the volunteers' average of 22.3 kg/m2. Since no diagnosis of lung or abdominal disease could be found also in retrospect, no exclusion criteria were met and thus her data remained included in the analysis. We cannot explain the opposing findings.
In ARDS areas of relatively normal lung parenchyma are juxtaposed to areas of overdistension as well as dense consolidation and atelectasis resulting in reduced compliance of the respiratory system. 29 In consequence, postural therapy, especially prone positioning, has become the standard of care to improve gas exchange as this can reduce ventilation/perfusion mismatching and shunting. 2 , 3 EIT has been shown helpful in diagnosing, decision making for therapies, and optimizing mechanical ventilation settings in ARDS. 30 Although COVID‐19 typically presents as acute respiratory failure, the early stages of COVID‐19 are different from “typical” ARDS: patients present with marked hypoxemia, which is likely due to a loss of regulation of perfusion causing intrapulmonary right to left shunt. 31 , 32 , 33 Moreover, mechanical properties of the lung, especially compliance, can be relatively normal. 34 Also, radiographic images of COVID‐19 look different from those of ARDS. 35 , 36 , 37 They present various patterns, especially in the early stages showing only a few atelectasis, which might explain the high compliance, the low recruitability, and the lack of (sustained) response to positioning therapy. 33
In our cohort of COVID‐19 patients, lateral positioning to either side led to increased ventilation in the hemithorax that was less ventilated in supine position but this increase in ventilation was not gravity‐dependent. Therefore, we cannot provide a morphological explanation for the findings by Froelich and coworkers, 12 as our findings suggest an improvement in ventilation irrespective of the position of the presumably more affected parts of the lung in the gravity field. We could neither find any association with the other parameters collected nor could we find an explanation for this obvious positioning‐dependent but not gravity‐dependent reaction.
As the effects of lateral positioning seem to depend on the distribution of ventilation in the starting position and were not to be foreseen in this cohort of spontaneously breathing COVID‐19 patients and as described before, 5 , 7 , 38 , 39 continuous monitoring of therapy should be sought. Computed tomography (CT) has proven to be effective in directing ventilation in ARDS and in detecting complications. 40 Apart from exposure to radiation, transportation of patients to CT examination is risky. 41 Especially with SARS‐CoV‐2 being highly infectious and causing life‐threatening disease, such intrahospital transports are particularly challenging. Therefore, point‐of‐care monitoring could be advantageous for patient and staff safety. In contrast to CT, EIT is a non‐invasive radiation‐free bedside imaging tool that provides functional images in real‐time. Therefore, EIT could be useful for monitoring and guiding positioning therapy in COVID‐19 pneumonia, in particular for the identification of affected areas and the monitoring of the responses to postural maneuvers. Furthermore, EIT can demonstrate whether dyspnea‐ or saturation‐optimized positions correlate with the particular topography of lung lesions as suggested by Froelich and collegues. 12
4.1. Limitations
Due to the small sample size results need to be interpreted with caution. As the morphological and functional pattern of COVID‐19 changes over time, we cannot rule out that in our patients, differences in ventilation distribution at baseline (supine position) and in the behavior to postural maneuvers might at least in part be due to different stages of the disease at inclusion. However, this once again underlines the need for repetitive analysis, or even better, the continuous monitoring of the individual's response to therapy.
We describe the effect of lateral positioning, and not of proning, which is much more commonly used in clinical practice. The reasons for that approach are twofold: (1) lateral positioning is known to have a greater impact on the matching of ventilation and perfusion as the gravity vector is running through the major axis of the ellipsoid thoracic cross‐section and (2) lateral position was assumed to be less cumbersome and more tolerable for these spontaneously breathing COVID‐19 patients with significant impairment of oxygenation and ventilation. However, data from volunteers and patients in the prone position should be assessed in future studies to confirm our findings.
The studied patients were significantly older than the healthy control subjects. After the first two decades of life, aging is associated with a progressive decrease in lung performance due to changes in the chest wall, respiratory muscle function, and lung parenchyma. These changes result, amongst others, in increased work of breathing, premature closing of dependent airways, and decreased elastic recoil of the lung. 42 Considering the response to positioning maneuvers, it has been shown that the effects on the distribution of ventilation were less or even absent in elderly humans. 23 Nevertheless, some impact of positioning could be observed in each one of the COVID‐19 patients, but the responses were less uniform than in the healthy controls, which could not be attributed to physiological aging alone. Thus, it can only be speculated that the effects of lateral or even prone positioning might have been more pronounced in younger COVID‐19 patients. Also, COVID‐19 patients in our study were significantly heavier than the healthy control subjects at a comparable height. Although obesity is known to change pulmonary function and mechanics as well as perfusion, studies suggest that heterogeneity of ventilation is normal or close to normal, even in extreme obesity. 43 Furthermore, Froese and Bryan found in their investigation that doming and stretching of the dependent diaphragm and the respective behavior during lateral position and spontaneous breathing were not influenced by BMI, at least not with moderately elevated BMI as seen in their studied subjects (22.9–29.7 kg/m2). 28 One explanation might be that higher BMI will result in a larger pressure gradient between the thoracic and abdominal compartment and therefore require higher force to move the abdominal components caudally during inspiration, but the aforementioned doming and stretching of the diaphragm that contribute to the higher efficiency are also intensified. Therefore, one can assume that inhomogeneities in ventilation and reactions to positioning maneuvers in our patients, in whom BMI was also only slightly elevated (28.1 kg/m2), were caused by COVID‐19.
The duration of the positioning maneuvers was rather short; measurements of all three positions were performed within 30 min. There is the risk that the final effects of gravity on recruitment and adaptation of regional transpulmonary pressure gradients in an inhomogeneously injured lung might have been missed due to the short measurement period. Usually positioning therapy is used for several hours and it has been shown that physiologic effects can increase for long periods (i.e., 24 h and more in some patients) with wide variability, even intra‐individually, 44 which is supposedly not only due to recruitment but to a combination of different mechanisms. Assuming that long‐term effects are based on these short‐term effects, we solely wanted to investigate gravity‐induced changes of ventilation, which we expected to be in place after a rather short time span in these patients with early stages of COVID‐19 which are said to have few(er) atelectasis, high compliance, and low recruitability. 33 But unquestionably, further EIT data on potential long term‐effects are desirable.
COVID‐19 patients had higher respiratory rates (RR > 30 as an inclusion criterion) and presumably also higher tidal volumes and therefore higher minute ventilation than the quietly breathing healthy volunteers. This respiratory distress might have contributed to differences between the two groups, but, as they were part or the result of the disease, we did not try to match them with the controls by forcing the volunteers to take over a higher respiratory rate or minute ventilation. Nevertheless, as elevated work of breathing was one inclusion criterion for the COVID‐19 patients, this fact alone does not explain the inhomogeneity within the group and the non‐uniform reactions to the positioning maneuvers.
The order of the positioning was not randomized. Therefore, we cannot rule out that some of the observed changes would have been different if positioning maneuvers had been performed in a different sequence, especially with the observation of increased ventilation in the previously less ventilated side. Nevertheless, healthy volunteers reacted in a uniform, fast and predictable manner while COVID‐19 patients did not. Furthermore, as many patients had heterogeneous distribution, to begin with, starting the maneuvers with the same side without knowing the pathology, contributed to some form of non‐planned randomization.
Finally, this study was meant to be observatory and descriptive in order to contribute to a better understanding of the pathophysiology. Postural maneuvers were meant to exert gravitational impact on the lungs irrespective of potential therapeutic effects. 12 , 45 Therefore, neither subjective (e.g., evaluation of dyspnea) nor objective measures (e.g., pulse oximetry, respiratory rate, blood gas samples) of clinical improvement have been assessed during the postural maneuvers.
5. CONCLUSION
In conclusion, the distribution of ventilation and the response to lateral positioning is variable within the group of COVID‐19 patients and different than in healthy volunteers. The effect of postural maneuvers is not predictable and is not necessarily influenced by gravity. EIT can visualize these immediate effects and assist in implementing customized and individualized positioning therapy. Further studies on the clinical relevance should be performed, looking at a gas exchange and clinical outcomes.
FUNDING INFORMATION
The study equipment was supported by internal resources of the Department of Anaesthesiology and Intensive Care of University Medical Center Rostock.
CONFLICT OF INTEREST
All authors declare that they have no competing interests.
ACKNOWLEDGMENT
The authors would like to thank all volunteers and patients as well as the staff of the wards that supported the team with measurements and data collection. Open Access funding enabled and organized by Projekt DEAL.
Zitzmann A, Pulletz S, Gonzales‐Rios P, et al. Regional ventilation in spontaneously breathing COVID‐19 patients during postural maneuvers assessed by electrical impedance tomography. Acta Anaesthesiol Scand. 2023;67(2):185‐194. doi: 10.1111/aas.14161
REFERENCES
- 1. Guérin C, Reignier J, Richard J‐C, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159‐2168. [DOI] [PubMed] [Google Scholar]
- 2. Henderson WR, Griesdale DEG, Dominelli P, Ronco JJ. Does prone positioning improve oxygenation and reduce mortality in patients with acute respiratory distress syndrome? Can Respir J. 2014;21:213‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guérin C, Albert RK, Beitler J, et al. Prone position in ARDS patients: why, when, how and for whom. Intensive Care Med. 2020;46:2385‐2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Scaravilli V, Grasselli G, Castagna L, et al. Prone positioning improves oxygenation in spontaneously breathing nonintubated patients with hypoxemic acute respiratory failure: a retrospective study. J Crit Care. 2015;30:1390‐1394. [DOI] [PubMed] [Google Scholar]
- 5. Coppo A, Bellani G, Winterton D, et al. Feasibility and physiological effects of prone positioning in non‐intubated patients with acute respiratory failure due to COVID‐19 (PRON‐COVID): a prospective cohort study. Lancet Respir Med. 2020;8:765‐774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Scaramuzzo G, Gamberini L, Tonetti T, et al. Sustained oxygenation improvement after first prone positioning is associated with liberation from mechanical ventilation and mortality in critically ill COVID‐19 patients: a cohort study. Ann Intensive Care. 2021;11:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ferrando C, Mellado‐Artigas R, Gea A, et al. Awake prone positioning does not reduce the risk of intubation in COVID‐19 treated with high‐flow nasal oxygen therapy: a multicenter, adjusted cohort study. Crit Care. 2020;24:597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kaur R, Vines DL, Mirza S, et al. Early versus late awake prone positioning in non‐intubated patients with COVID‐19. Crit Care. 2021;25:340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Perez‐Nieto OR, Escarraman‐Martinez D, Guerrero‐Gutierrez MA, et al. Awake prone positioning and oxygen therapy in patients with COVID‐19: the APRONOX study. Eur Respir J. 2022;59:2100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ehrmann S, Li J, Ibarra‐Estrada M, et al. Awake prone positioning for COVID‐19 acute hypoxaemic respiratory failure: a randomised, controlled, multinational, open‐label meta‐trial. Lancet Respir Med. 2021;9:1387‐1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Wever W, Meersschaert J, Coolen J, Verbeken E, Verschakelen JA. The crazy‐paving pattern: a radiological‐pathological correlation. Insights Imaging. 2011;2:117‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Froelich S, Mandonnet E, Julla J‐B, et al. Towards individualised and optimalised positioning of non‐ventilated COVID‐19 patients: putting the affected parts of the lung(s) on top? Diabetes Metab. 2021;47:101167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frerichs I, Amato MBP, van Kaam AH, et al. Chest electrical impedance tomography examination, data analysis, terminology, clinical use and recommendations: consensus statement of the TRanslational EIT developmeNt stuDy group. Thorax. 2017;72:83‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bachmann MC, Morais C, Bugedo G, et al. Electrical impedance tomography in acute respiratory distress syndrome. Crit Care. 2018;22:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pulletz S, Krukewitt L, Gonzales‐Rios P, et al. Dynamic relative regional strain visualized by electrical impedance tomography in patients suffering from COVID‐19. J Clin Monit Comput. 2021;36:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhao Z, Möller K, Steinmann D, Frerichs I, Guttmann J. Evaluation of an electrical impedance tomography‐based global inhomogeneity index for pulmonary ventilation distribution. Intensive Care Med. 2009;35:1900‐1906. [DOI] [PubMed] [Google Scholar]
- 17. Frerichs I, Hahn G, Golisch W, Kurpitz M, Burchardi H, Hellige G. Monitoring perioperative changes in distribution of pulmonary ventilation by functional electrical impedance tomography. Acta Anaesthesiol Scand. 1998;42:721‐726. [DOI] [PubMed] [Google Scholar]
- 18. Bleul U, Wey C, Meira C, Waldmann A, Mosing M. Assessment of postnatal pulmonary adaption in bovine neonates using electric impedance tomography (EIT). Animals. 2021;11:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Waldmann A, Brunner JX, Böhm S. EIT Analysis Details: Lung Stretch and Silent Spaces Analysis: Unpublished. 2015.
- 20. Sophocleous L, Frerichs I, Miedema M, et al. Clinical performance of a novel textile interface for neonatal chest electrical impedance tomography. Physiol Meas. 2018;39:44004. [DOI] [PubMed] [Google Scholar]
- 21. Waldmann AD, Wodack KH, März A, et al. Performance of novel patient Interface for electrical impedance tomography applications. J Med Biol Eng. 2017;37:561‐566. [Google Scholar]
- 22. Yang L, Dai M, Cao X, et al. Regional ventilation distribution in healthy lungs: can reference values be established for electrical impedance tomography parameters? Ann Transl Med. 2021;9:789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Frerichs I, Braun P, Dudykevych T, Hahn G, Genée D, Hellige G. Distribution of ventilation in young and elderly adults determined by electrical impedance tomography. Respir Physiol Neurobiol. 2004;143:63‐75. [DOI] [PubMed] [Google Scholar]
- 24. Kaneko K, Milic‐Emili J, Dolovich MB, Dawson A, Bates DV. Regional distribution of ventilation and perfusion as a function of body position. J Appl Physiol. 1966;21:767‐777. [DOI] [PubMed] [Google Scholar]
- 25. Frerichs I, Hahn G, Hellige G. Gravity‐dependent phenomena in lung ventilation determined by functional EIT. Physiol Meas. 1996;17(Suppl 4A):A149‐A157. [DOI] [PubMed] [Google Scholar]
- 26. Froese AB. Gravity, the belly, and the diaphragm: you can't ignore physics. Anesthesiology. 2006;104:193‐196. [DOI] [PubMed] [Google Scholar]
- 27. Levy JH. Anesthesia for thoracic surgery. Chest. 1988;94:A‐23. [Google Scholar]
- 28. Froese AB, Bryan AC. Effects of anesthesia and paralysis on diaphragmatic mechanics in man. Anesthesiology. 1974;41:242‐255. [DOI] [PubMed] [Google Scholar]
- 29. Amato MBP, Meade MO, Slutsky AS, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372:747‐755. [DOI] [PubMed] [Google Scholar]
- 30. Zhao Z, Lee L‐C, Chang M‐Y, et al. The incidence and interpretation of large differences in EIT‐based measures for PEEP titration in ARDS patients. J Clin Monit Comput. 2020;34:1005‐1013. [DOI] [PubMed] [Google Scholar]
- 31. Mahjoub Y, Rodenstein DO, Jounieaux V. Severe Covid‐19 disease: rather AVDS than ARDS? Crit Care. 2020;24:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chiumello D, Busana M, Coppola S, et al. Physiological and quantitative CT‐scan characterization of COVID‐19 and typical ARDS: a matched cohort study. Intensive Care Med. 2020;46:2187‐2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gattinoni L, Chiumello D, Caironi P, et al. COVID‐19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46:1099‐1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li X, Ma X. Acute respiratory failure in COVID‐19: is it “typical” ARDS? Crit Care. 2020;24:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cozzi D, Albanesi M, Cavigli E, et al. Chest X‐ray in new coronavirus disease 2019 (COVID‐19) infection: findings and correlation with clinical outcome. Radiol Med. 2020;125:730‐737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Robba C, Battaglini D, Ball L, et al. Distinct phenotypes require distinct respiratory management strategies in severe COVID‐19. Respir Physiol Neurobiol. 2020;279:103455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang C, Shi B, Wei C, Ding H, Gu J, Dong J. Initial CT features and dynamic evolution of early‐stage patients with COVID‐19. Radiol Infect Dis. 2020;7:195‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Koeckerling D, Barker J, Mudalige NL, et al. Awake prone positioning in COVID‐19. Thorax. 2020;75:833‐834. [DOI] [PubMed] [Google Scholar]
- 39. Tomasino S, Sassanelli R, Marescalco C, Meroi F, Vetrugno L, Bove T. Electrical impedance tomography and prone position during ventilation in COVID‐19 pneumonia: case reports and a brief literature review. Semin Cardiothorac Vasc Anesth. 2020;24:287‐292. [DOI] [PubMed] [Google Scholar]
- 40. Sheard S, Rao P, Devaraj A. Imaging of acute respiratory distress syndrome. Respir Care. 2012;57:607‐612. [DOI] [PubMed] [Google Scholar]
- 41. Beckmann U, Gillies DM, Berenholtz SM, Wu AW, Pronovost P. Incidents relating to the intra‐hospital transfer of critically ill patients. An analysis of the reports submitted to the Australian incident monitoring study in intensive care. Intensive Care Med. 2004;30:1579‐1585. [DOI] [PubMed] [Google Scholar]
- 42. Janssens JP, Pache JC, Nicod LP. Physiological changes in respiratory function associated with ageing. Eur Respir J. 1999;13:197‐205. [DOI] [PubMed] [Google Scholar]
- 43. Salome CM, King GG, Berend N. Physiology of obesity and effects on lung function. J Appl Physiol. 2010;108:206‐211. [DOI] [PubMed] [Google Scholar]
- 44. Jochmans S, Mazerand S, Chelly J, et al. Duration of prone position sessions: a prospective cohort study. Ann Intensive Care. 2020;10:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Roldán R, Rodriguez S, Barriga F, et al. Sequential lateral positioning as a new lung recruitment maneuver: an exploratory study in early mechanically ventilated Covid‐19 ARDS patients. Ann Intensive Care. 2022;12:13. [DOI] [PMC free article] [PubMed] [Google Scholar]


