Abstract
Main aim of this systematic review is to quantify the risk and identify predictors of clinical evolution of SARS‐CoV‐2 in hematological patients compared to different control populations. Two independent reviewers screened the literature assessing clinical outcomes of SARS‐CoV‐2 infection in adult patients with active hematological malignancies published up to June 2021. Primary outcome was COVID‐19 related mortality, secondary outcomes were hospital and intensive‐care admission, mechanical ventilation (MV), and thromboembolic events. Variables related to study setting, baseline patients' demographic, comorbidities, underlying hematological disease, ongoing chemotherapy, COVID‐19 presentation, and treatments were extracted. A total of 67 studies including 10,061 hematological patients and 111,143 controls were included. Most of the studies were retrospective cohorts (51 studies, 76%) and only 19 (13%) provided data for a control group. A significant increased risk of clinical progression in the hematological population compared to the controls was found in terms of COVID‐19 related mortality (OR, 2.12; 95% CI, 1.77–2.54), hospitalization (OR, 1.98; 95% CI, 1.15–3.43), intensive‐care admission (OR, 1.77; 95% CI, 1.38–2.26), and MV (OR, 2.17; 95% CI, 1.71–2.75). The risk remained significantly higher in the subgroup analysis comparing hematological patients versus solid cancer. Meta‐regression analysis of uncontrolled studies showed that older age, male sex, and hypertension were significantly related to worse clinical outcomes of COVID‐19 in hematological population. Older age and hypertension were found to be associated also to thromboembolic events. In conclusion, hematological patients have a higher risk of COVID‐19 clinical progression compared to both the general population and to patients with solid cancer.
Keywords: COVID‐19, determinants, hematological malignancies, mortality, severity
1. INTRODUCTION
Two years after the start of the COVID‐19 pandemic in March 2020, SARS‐CoV‐2 has caused over 250 million confirmed cases and more than 5 million deaths. 1 So far, several predictors of clinical evolution have been identified and widely used to identify patients at risk of worse outcomes and prioritize the access to preventive and therapeutic resources. 2 , 3 , 4 , 5 Among those risk factors, age, and chronic health conditions (diabetes, hypertension, cardiac disease, chronic lung disease, cerebrovascular disease, dementia, mental disorders, chronic kidney disease, immunosuppression, obesity, and cancer) have been reported with strong associations since the early phases of the pandemic. 6
SARS‐CoV‐2 causes severe lymphocyte T depletion, and at the same time works as a trigger to both the innate and the adaptive immune response, leading to an excessive and prolonged cytokine/chemokine response associated with critical and fatal COVID‐19. Therefore, several therapeutic options have shown their efficacy in preventing disease progression thanks to their ability of inhibiting the inflammatory response. 7 , 8 , 9 , 10 , 11
Based on this rationale, some authors hypothesized that patients with hematologic malignancies might be at lower risk of severe COVID‐19 due to an attenuated inflammatory response and concomitant immunosuppressive therapies. 12 On the other hand, several studies have reported an actual risk of COVID‐19 related serious events in patients with hematological malignancy compared to COVID‐19 patients without cancer. 13 , 14 As of today, though, precise mechanisms triggering disease progression remain largely unknown. Moreover, patients with hematological malignancies represent a highly heterogeneous population with several kind of disease‐ or therapy‐induced immunosuppression, hence requiring a more customized clinical approach for COVID‐19 management and treatment. 15
Main aim of this systematic review is to summarize existing evidence on clinical evolution of SARS‐CoV‐2 in hematological patients compared to non‐hematological and identify major risk factors for disease progression.
2. METHODS
This systematic review was conducted according to the PRISMA 2020 guidelines. The protocol was registered on the PROSPERO database on 25 August 2021 (CRD42021262398).
2.1. Literature search
A literature search was performed using PubMed and LOVE Database (Epistemonikos Foundation) with pre‐defined COVID‐19 filters using the following search strategy: (hematol* OR haematol*) AND (cancer OR malignancy OR neoplasm OR lymphoma OR leukemia OR transplant). The search was run on 30 June 2021.
2.2. Exclusion criteria
Studies focusing on pediatric populations, studies including patients in clinical remission (i.e., not undergoing treatment in the last 6 months), small case series (less than 10 patients), and non‐English studies were excluded.
2.3. Inclusion criteria
All studies assessing clinical outcomes of adult inpatients and outpatients with active hematological malignancies and SARS‐CoV‐2 infection were included. Studies with mixed patients' populations were included only if the outcome was specified for the hematological population subgroup. When subgroup analysis for hematological population was not performed, at least 80% of the included population needed to have an active hematological disease for the study to be included. Both controlled and uncontrolled studies were included. In case of case‐controlled studies, to be included in the analysis, the two populations needed to be comparable for at least two among the following relevant variables: age, sex, comorbidities, COVID‐19 baseline severity. No restriction was applied on publication date, and both peer‐reviewed and pre‐prints were included. References of all the included studies were also inspected, abstract or full text of potentially eligible articles were retrieved and examined applying the same inclusion criteria.
2.4. Data extraction
The following predictors were included: (1) baseline conditions (i.e., age, comorbidities type of hematological malignancy, ongoing chemotherapy), (2) COVID‐19 related baseline variables (prevalence of symptoms at enrollment, prevalence of confirmed pneumonia, oxygen need at baseline, need for hospitalization or intensive care unit (ICU) admission at baseline), (3) treatment related variables (modification of chemotherapy, COVID‐19 administered treatments).
Primary outcome was COVID‐19 related mortality at any time point. Secondary outcomes were hospital admission, ICU admission, mechanical ventilation (MV), oxygen requirement, clinical recovery (as opposite of clinical failure, both as defined by the study), bacterial or fungal superinfection, thromboembolic events, asymptomatic disease only, hematological progression, length of hospital/ICU stay.
The inclusion and exclusion process, the data extraction and the quality assessment were carried out by four reviewers working in two blinded pairs. The inclusion and exclusion process was performed with the support of the Rayyan software, data extraction was conducted using a pre‐defined Excel database. Any disagreement was solved via discussion within the pair or with the involvement of a fifth reviewer.
All studies fulfilling the inclusion criteria were assessed for risk of bias via an adapted version of the Newcastle‐Ottawa scale (NOS) 16 for cohort and case‐control studies (details of the adapted NOS score are available in the Supplementary Material).
2.5. Statistical analysis
Individual studies offering a comparison group were included in a first meta‐analysis, where the excess risk of clinical development of the hematological population compared with the non‐hematological controls was computed as pooled odds ratios with 95% CI. All studies (controlled and non‐controlled) contributed to a second meta‐analysis model, where the occurrence of the included clinical outcomes in the hematological population was computed as a pooled prevalence with 95% CI.
All meta‐analyses were conducted using a random effects model, assuming a priori significant heterogeneity resulting from diverse study populations and different models for adjusted analyses. Heterogeneity was assessed using a chi square test of heterogeneity and the I‐squared measure of inconsistency. Subgroup analysis with computation of the between‐study variance was performed for the main categorical variables (i.e., study setting, main hematological disease included, study design, and type of population enrolled). Mixed effect univariate meta‐regression was conducted using the unrestricted maximum likelihood method to assess the impact of the following continuous variables: patients' mean age, prevalence of female, prevalence of different race, comorbidities, proportion of patients undergoing chemotherapy, COVID‐19 baseline characteristics, and COVID‐19 treatments.
2.6. Ethics approval
Since the study was designed as a systematic review and meta‐analysis of published studies, ethical approval was not deemed necessary.
3. RESULTS
From 1871 identified references, a total of 67 studies accounting for 10,061 hematological patients and 111,143 controls were included in the systematic review (Table S3 summarizes the included studies and reported outcomes). 13 , 15 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 A PRISMA flow‐chart detailing the inclusion and exclusion process is available in the Supplementary Material (Figure S1).
Sample size ranged from 12 to 1389 patients per study (median 51; interquartile range (IQR), 22–164); median study duration was 66 days (IQR, 45–118). Most of the studies were cohort studies with retrospective data collection in 51 studies (76%) and prospective in 8 (12%). Whereas a control population of non‐hematological patients was available in 19 (13%) studies, only eight (12%) were designed as case‐control studies. Most of the studies were conducted in the USA, Europe (mostly in Italy, France, Spain, Germany, United Kingdom, Belgium), and China.
Forty studies (59%) enrolled only hospitalized patients, while the remaining 27 included a mixed population of both in‐ and outpatients. Thirty‐eight studies (57%) described a mixed population of hematological patients. Four studies (6%) focused on non‐Hodgkin and Hodgkin lymphoma patients and two (3%) studies on central nervous system lymphoma. Five (7%) studies included only patients with multiple myeloma, eight studies (11%) included stem cell transplant (SCT) recipients, four (6%) studies focused on patients with chronic lymphocytic leukemia and one study on chronic myeloid leukemia. Two studies included patients with myeloproliferative neoplasm and one myelodysplastic syndrome. Only one study reported data on patients with acute leukemia.
Only 30 (43%) studies reported specifically on systemic anticancer chemotherapy and 35 (52%) studies reported information regarding specific COVID‐19 treatment.
Study quality was considered low in 19 studies (28%; NOS score <5), 39 studies (58%) were considered as medium quality (NOS score between 6 and 7), and 9 studies (14%) were considered high quality (NOS score >8). Newcastle‐Ottawa scale domains where a lower quality was found were representativeness of the cohort, selection of control and comparability (Figure 1).
FIGURE 1.
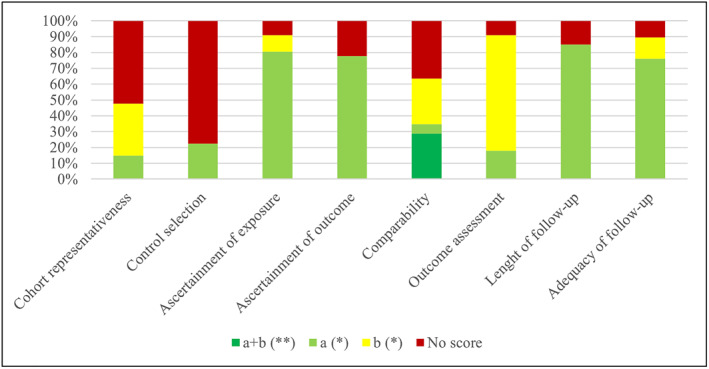
NOS score 16
3.1. COVID‐19 related mortality
Fourteen studies provided adequately matched data for COVID‐19 related mortality in the hematological population compared with controls. The overall analysis showed a significant increased mortality for hematological patients (OR, 2.12; 95% CI, 1.77–2.54; p < 0.001). The pooled OR remained significant also when grouping the studies according to the type of control included (general population in 9 studies and other immunodeficiencies, mainly solid tumors, in 5 studies, see Figure 2). A moderate level heterogeneity (I 2, 50.3%) was measured with the meta‐analysis, less heterogeneous results were found by grouping the studies by design and setting (Table 1 describes the subgroups analysis of COVID‐19 related mortality).
FIGURE 2.
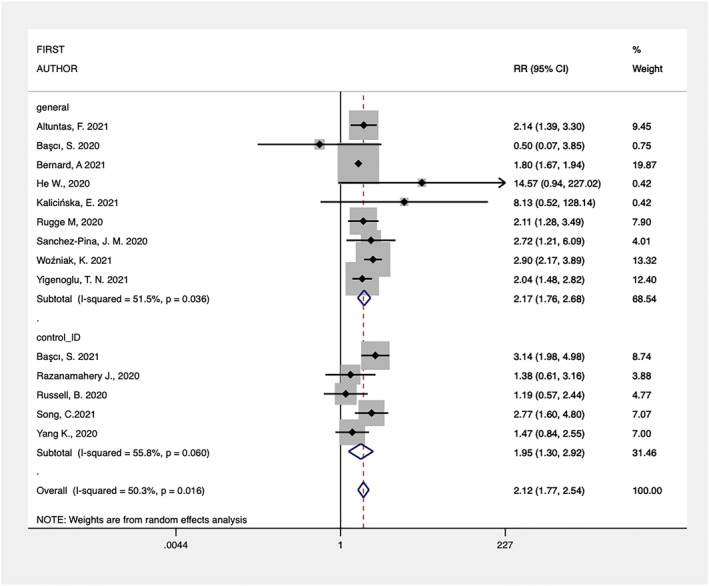
Forest‐plot of controlled studies assessing COVID‐19 related mortality grouped by type of control (general population or other immunosuppressive conditions)
TABLE 1.
Subgroup analysis of COVID‐19 related mortality in controlled and uncontrolled studies
| Studies with control | Uncontrolled studies | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Subgroup | N | Pooled OR | 95% CI | I 2 (%) | N | Pooled prevalence | 95% CI | I 2 (%) |
| Setting | Hospitalized | 9 | 1.91 | 1.5–2.3 | 18 | 44 | 0.34 | 0.29–0.39 | 91 |
| Mix | 5 | 2.32 | 1.7–3.0 | 48 | 21 | 0.24 | 0.20–0.28 | 89 | |
| Outpatients | ‐ | ‐ | ‐ | ‐ | 2 | 0.18 | 0.03–0.32 | 0 | |
| Study design a | Cohort retrospective | 8 | 1.80 | 1.7–1.9 | 0 | 38 | 0.33 | 0.26–0.39 | 97 |
| Cohort prospective | ‐ | ‐ | ‐ | ‐ | 2 | 0.36 | 0.28–0.44 | 0 | |
| Case‐control | 6 | 2.58 | 1.9–3.4 | 32 | 6 | 0.29 | 0.16–0.42 | 78 | |
| Hematological malignancy a | Hematopoietic cell transplantation | ‐ | ‐ | ‐ | ‐ | 7 | 0.21 | 0.07–0.36 | 79 |
| Mix | 13 | 2.14 | 1.8–2.6 | 51 | 31 | 0.36 | 0.31–0.41 | 88 | |
| Leukemia | 1 | 0.50 | 0.1–3.8 | 0 | 4 | 0.23 | 0.12–0.34 | 82 | |
| Myeloma | ‐ | ‐ | ‐ | ‐ | 5 | 0.26 | 0.01–0.51 | 98 | |
| Myeloproliferative neoplasm | ‐ | ‐ | ‐ | ‐ | 2 | 0.48 | 0.37–0.58 | 0 | |
| Lymphoma | ‐ | ‐ | ‐ | ‐ | 5 | 0.30 | 0.25–0.34 | 8 | |
Only studies reporting hospitalized patients were included in this analysis.
Meta‐regression analysis of continuous variables showed a significant inverse correlation between the effect size and mean age of the patients enrolled (OR, 0.77; 95% CI, 0.60–0.98; p = 0.04), implying that older patients had less impact of the hematological disease on the outcome. No other significant correlation was found for the other continuous variables (prevalence of female sex, diabetes, hypertension, chronic obstructive pulmonary disease, cardiovascular disease, and chemotherapy related variables).
The overall analysis of uncontrolled studies (66 studies) showed a pooled prevalence of COVID‐19 related mortality of 30% (95% CI, 26%–34%) with a very high heterogeneity (I 2, 96%). As expected, a lower mortality was found in studies enrolling only outpatients or in studies with a mixed setting at enrollment (both in‐ and outpatients). For this reason, subgroup analysis and meta‐regression to assess heterogeneity were conducted including studies where at least 80% of the population was hospitalized (46 studies). In the subgroups analysis on hospitalized patients, lower mortality rates were found in SCT patients. All subgroups showed a remarkable heterogeneity except for studies including patients with lymphoma.
The meta‐regression analysis found that age was directly associated with increased mortality (p = 0.001; Adj R 2 46%; Figure 3). No other variables were found to be significantly related to COVID‐19 related mortality (details in supplementary Table S1).
FIGURE 3.
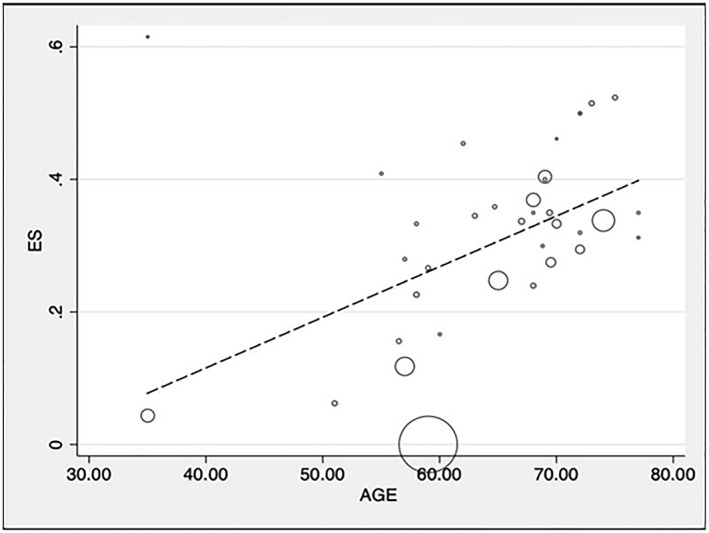
Meta‐regression of COVID‐19 related mortality and mean age in cohort studies
3.2. Hospital admission
Controlled studies assessing hospital admission showed an increased odds of hospitalization in patients with hematological malignancies compared to either the general population or to patients with other immunodeficiencies (pooled OR 1.98; 95% CI, 1.15–3.43; I 2 94%; p = 0.014).
The overall analysis of 11 uncontrolled studies showed an overall hospital admission rate of 56% (95% CI, 43%–69%) with a very high heterogeneity (I 2, 99%). No significant reduction in heterogeneity was seen by grouping the studies according to setting and hematological population. Meta‐regression analysis found a positive association between hospital admission and diabetes, hypertension, chronic pulmonary disease, and cardiovascular disease (details in supplementary Tables S1 and S2).
3.3. Intensive care unit admission
The meta‐analysis of controlled studies showed that hematological patients compared to general population and patients with other immunodeficiencies have a significant higher risk of being admitted to ICU (9 studies included, overall OR, 1.77; 95% CI, 1.38–2.26; p = 0.04; I 2, 52.8%). The overall analysis of 31 studies not displaying a control group showed a pooled prevalence of ICU admission of 18% (95% CI, 15%–21%) with a high heterogeneity (I 2, 89%). No significant variations in the effect size were detected in the subgroup analysis by study design, hematological disease or study setting. The only factor associated with ICU admission rates was sex, with studies displaying higher percentages of females enrolled showing a decreased rate of ICU admission (p = 0.01; adjusted R 2: 24.8%) (details in supplementary Tables S1 and S2).
3.4. Mechanical ventilation
Only five studies displayed rates of hematological patients undergoing mechanical ventilation (MV) compared to controls. When considering both control groups (general population and patients with other immunocompromizing conditions), hematological patients showed higher odds of undergoing MV with a low heterogeneity among included studies (pooled OR, 2.17; 95% CI, 1.71–2.75; p = 0.005; I 2, 6.2%).
A total of 16 studies reported on the rate of hematological patients with COVID‐19 that were mechanically ventilated. The overall pooled prevalence was 16% (95% CI, 13%–19%) with an overall heterogeneity of 55% and no significant reduction detected via subgroup analysis. None of the continuous variables tested with the meta‐regression analysis resulted significatively associated with MV (details in supplementary Tables S1 and S2).
3.5. Thromboembolic events
No studies with a control group reported on this outcome. A total of nine uncontrolled studies analyzed the occurrence of thromboembolic events in the hematological population with a pooled prevalence of 9% (95% CI, 3%–9%; I 2, 72%). The main reported events were deep venous thrombosis, pulmonary embolism and arterial thrombosis. Age and hypertension were found to be positively correlated with thromboembolic events in the meta‐regression analysis (details in supplementary Tables S1 and S2).
3.6. Other outcomes
Among other outcomes included in the systematic review (oxygen requirement, clinical recovery, superinfection, asymptomatic disease only, hematological progression and length of hospitalization) no meta‐analysis was undertaken due to the low number of studies reporting data.
4. DISCUSSION
During the two years following the start of the pandemic, two other systematic reviews assessing the clinical outcome of hematological patients with COVID‐19 have been published. 14 , 82 Those papers included a total of 3377 and 2316 patients respectively, and the search was conducted up to August 2020 for the first one and May 2020 for the second. Both publications reported data on overall mortality and ICU admission, and their findings are in line with our results.
Our updated analysis included studies published from the beginning of the SARS‐CoV‐2 pandemic up to June 2021. Overall, in our study the pooled prevalence of COVID‐19 related mortality in hematological patients was 30% (95% CI, 26%–34%) with a very high heterogeneity among studies (I 2, 96%). Similarly, according to Vijenthira et al. 14 adult patients with hematologic malignancy and COVID‐19 had a 34% risk of death, whereas Venkatesulu et al. 82 found that hematological malignancies patients had an all‐cause in‐hospital mortality rate of 33%.
Our data confirmed previous results on the ICU admission rate, which was 18% (95% CI, 15%–21%) in our analysis compared to a 21% risk (95% CI, 16%–27%) found by Vijenthira et al. 14 Very high heterogeneity was detected in both meta‐analyses. As for MV, our findings showed a pooled prevalence of 16% (95% CI, 13%–19%; I2, 55%), whereas Vijenthira et al. measured a pooled risk of 17% (95% CI, 13%–21%; I 2, 63%). 14
We did not find any other systematic review reporting on less common outcomes such as thromboembolic events, bacterial superinfection, clinical recovery, length of hospitalization or delay of systemic anticancer chemotherapy.
The availability of a significant number of studies including a control group of COVID‐19 patients without hematological malignancies allowed us to compute the excess risk of worse outcome in this specific patients' population. All outcomes (mortality, hospital admission, ICU admission and MV) were significantly worse in hematological patients when compared to the general population, and to patients with other immunocompromizing conditions (mostly solid organ cancer).
These results do not support the initial belief that immunocompromized patients might less frequently experience worse clinical outcomes compared to the general population, and provide evidence that adequate preventive policy targeting fragile populations is essential.
We used subgroup analysis and meta‐regression to test whether some of the included clinical variables could predict clinical evolution in hematological patients. Among those, we found a positive correlation between age and COVID‐19 related mortality (p = 0.001; Adj R 2 41% coefficient 0.007), hypertension (p = 0.03; coefficient 0.001) and thromboembolic events (p = 0.003; coefficient 0.001). Once results are quantitatively summarized, one of the added values of meta‐analysis is the ability to detect and try to assess between‐study variance. In both our analyses (controlled and uncontrolled studies), a high heterogeneity was detected, suggesting remarkable differences among included studies. Meta‐regression of continuous variables did not show remarkable reduction in heterogeneity, whereas a reduced heterogeneity was measured when grouping studies by design, and in some specific type of hematological patients' population. However, as expected when including observational studies, a significant proportion of the between‐study variance could not be explained by any of the included variables.
Our work has some limitations, mostly related to the study design and the underreporting of possibly relevant outcome predictors. Most of the studies had a retrospective design and only 14 studies included an adequate control group for the mortality analysis. Some key examples of incomplete reporting of relevant variables are the type of hematological malignancies (unspecified in more than half of the studies), the setting (mixed in almost one‐third of the studies), or the type and the percentage of patients undergoing systemic chemotherapy for which details were rarely provided. Also, inclusion of relevant COVID‐19 related variables (from disease severity to specific pharmacological interventions) was highly heterogeneous among studies.
Among laboratory abnormalities, mostly lymphopenia, mild thrombocytopenia and elevated D‐dimer values are reported among patients with COVID‐19. 83 More severe abnormalities have been often associated with more severe infection; D‐dimer and, to a lesser extent, lymphopenia seem to have the largest prognostic associations. 84 , 85 As for our selected studies, laboratory findings were seldom reported, and hard to harmonize. Thus, no estimation of their predictive value in the hematological population could be done.
Most of the studies were rated as having a medium quality, and “selection” and “comparability” were the domains with the lowest scores and at higher risk of introducing bias in the analysis.
Compared to other systematic reviews, this work constitutes a relevant update of the evidence on the topic and summarizes quite many studies including a relevant sample size. One of the strengths of our systematic review is that it provides wide and comprehensive analysis of different variables that could potentially predict clinical evolution of COVID‐19 in hematological patients. However, it must be noted that the systematic search dates back to June 2021 and all the included studies were conducted before mass vaccination was available.
In conclusion, this systematic review quantifies the higher risk of death, hospitalization, ICU admission and MV in hematological patients with COVID‐19 compared to the general population and to patients with other immunodeficiencies. In line with available data on the general population, older age, male sex, and hypertension are significantly related to worse clinical outcomes of COVID‐19 in hematological population.
Large cohort studies, such as the recent collaborative effort of the ITA‐HEMA‐COV (the ITAlian HEMatology Alliance on Covid‐19) on SARS‐CoV‐2 infection in patients with lymphoma, 86 are necessary to better analyze the role of relevant clinical determinants related to the hematological disease (i.e., type of disease, role of chemotherapy). Additionally, a further update of our data collection is needed to measure the effect of mass vaccination and use of recently approved drugs in immunocompromized patients.
AUTHOR CONTRIBUTIONS
All authors contributed to the population, intervention, control, outcome selection and the protocol draft. Elisa Razzaboni, Anna Maria Azzini, Mariana Nunes Pinho Guedes, Maria Elena De Rui performed the literature search and the data extraction. Elena Carrara performed the analysis and drafted manuscript. All authors have reviewed and approved the manuscript.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/hon.3084.
Supporting information
Supporting Information S1
ACKNOWLEDGMENTS
We acknowledge writing assistance by Ruth Joanna Davis. The contribution of Eduardo Reyna‐Villasmil, Natascia Caroccia, and Francesca Fanì as part of the working group are also acknowledged. This work has been financed by the ORCHESTRA project under the European Union's Horizon 2020 research and innovation program Grant Agreement No. 101016167. The views expressed in this publication are the sole responsibility of the author and the Commission is not responsible for any use that may be made of the information it contains.
Carrara E, Razzaboni E, Azzini AM, et al. Predictors of clinical evolution of SARS‐CoV‐2 infection in hematological patients: a systematic review and meta‐analysis. Hematol Oncol. 2022;1‐10. 10.1002/hon.3084
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. World Health Organization Coronavirus (COVID‐19) Dashboard ; 2021.
- 2. Khan MMA, Khan MN, Mustagir MG, Rana J, Islam MS, Kabir MI. Effects of underlying morbidities on the occurrence of deaths in COVID‐19 patients: a systematic review and meta‐analysis. J Glob Health. 2020;10(2):020503. 10.7189/jogh.10.020503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Treskova‐Schwarzbach M, Haas L, Reda S, et al. Pre‐existing health conditions and severe COVID‐19 outcomes: an umbrella review approach and meta‐analysis of global evidence. BMC Med. 2021;19(1):212. 10.1186/s12916-021-02058-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Noh EB, Nam HK, Lee H. Which group should be vaccinated first?: a systematic review. Infect Chemother. 2021;53(2):261‐270. 10.3947/ic.2021.0029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wingert A, Pillay J, Gates M, et al. Risk factors for severity of COVID‐19: a rapid review to inform vaccine prioritisation in Canada. BMJ Open. 2021;11(5):e044684. 10.1136/bmjopen-2020-044684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934‐943. 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rochwerg B, Agarwal A, Siemieniuk RA, et al. A living WHO guideline on drugs for covid‐19. Br Med J. 2020;370:m3379. [DOI] [PubMed] [Google Scholar]
- 8. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033‐1034. 10.1016/s0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marconi VC, Ramanan AV, de Bono S, et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID‐19 (COV‐BARRIER): a randomised, double‐blind, parallel‐group, placebo‐controlled phase 3 trial. Lancet Respir Med. 2021;9(12):1407‐1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ghosn L, Chaimani A, Evrenoglou T, et al. Interleukin‐6 blocking agents for treating COVID‐19: a living systematic review. Cochrane Database Syst Rev. 2021;3(3):Cd013881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sterne JAC, Murthy S, Diaz JV, et al. Association between administration of systemic corticosteroids and mortality among critically Ill patients with COVID‐19: a meta‐analysis. JAMA. 2020;324(13):1330‐1341. 10.1001/jama.2020.17023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thibaud S, Tremblay D, Bhalla S, Zimmerman B, Sigel K, Gabrilove J. Protective role of Bruton tyrosine kinase inhibitors in patients with chronic lymphocytic leukaemia and COVID‐19. Br J Haematol. 2020;190(2):e73‐e76. 10.1111/bjh.16863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yigenoglu TN, Ata N, Altuntas F, Korkmaz S, Turgut B. The outcome of COVID‐19 in patients with hematological malignancy. J Med Virol. 2021;93(2):1099‐1104. 10.1002/jmv.26607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vijenthira A, Gong IY, Fox TA, et al. Outcomes of patients with hematologic malignancies and COVID‐19: a systematic review and meta‐analysis of 3377 patients. Blood. 2020;136(25):2881‐2892. 10.1182/blood.2020008824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kalicińska E, Szymczak D, Andrasiak I, et al. Lymphocyte subsets in haematological patients with COVID‐19: multicentre prospective study. Transl Oncol. 2021;14(1):100943. 10.1016/j.tranon.2020.100943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wells GSB, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. 2013.
- 17. Survival factors ID'd for patients with blood cancer + COVID‐19. Cancer Discov. 2021;11(2):214. [DOI] [PubMed] [Google Scholar]
- 18. Altuntas F, Ata N, Yigenoglu TN, et al. COVID‐19 in hematopoietic cell transplant recipients. Bone Marrow Transpl. 2021;56(4):952‐955. 10.1038/s41409-020-01084-x [DOI] [PubMed] [Google Scholar]
- 19. Aries JA, Davies JK, Auer RL, et al. Clinical outcome of coronavirus disease 2019 in haemato‐oncology patients. Br J Haematol. 2020;190(2):e64‐e67. 10.1111/bjh.16852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Barbui T, De Stefano V, Alvarez‐Larran A, et al. Among classic myeloproliferative neoplasms, essential thrombocythemia is associated with the greatest risk of venous thromboembolism during COVID‐19. Blood Cancer J. 2021;11(2):21. 10.1038/s41408-021-00417-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barbui T, Vannucchi AM, Alvarez‐Larran A, et al. High mortality rate in COVID‐19 patients with myeloproliferative neoplasms after abrupt withdrawal of ruxolitinib. Leukemia. 2021;35(2):485‐493. 10.1038/s41375-020-01107-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Başcı S, Ata N, Altuntaş F, et al. Outcome of COVID‐19 in patients with chronic myeloid leukemia receiving tyrosine kinase inhibitors. J Oncol Pharm Pract. 2020;26(7):1676‐1682. 10.1177/1078155220953198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Başcı S, Ata N, Altuntaş F, et al. Patients with hematologic cancers are more vulnerable to COVID‐19 compared to patients with solid cancers. Intern Emerg Med. 2021;17:1‐5. 10.1007/s11739-021-02784-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bernard A, Cottenet J, Bonniaud P, et al. Comparison of cancer patients to non‐cancer patients among COVID‐19 inpatients at a national level. Cancers. 2021;13(6):1436. 10.3390/cancers13061436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Biernat MM, Kolasińska A, Kwiatkowski J, et al. Early administration of convalescent plasma improves survival in patients with hematological malignancies and COVID‐19. Viruses. 2021;13(3):436. 10.3390/v13030436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bird PW, Badhwar V, Kennedy B, Ladani S, Tang JW. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) seroconversion in hematology‐oncology patients. J Med Virol. 2021;93(7):4585‐4591. 10.1002/jmv.26886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Booth S, Willan J, Wong H, et al. Regional outcomes of severe acute respiratory syndrome coronavirus 2 infection in hospitalised patients with haematological malignancy. Eur J Haematol. 2020;105(4):476‐483. 10.1111/ejh.13469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Borah P, Mirgh S, Sharma SK, et al. Effect of age, comorbidity and remission status on outcome of COVID‐19 in patients with hematological malignancies. Blood Cells Mol Dis. 2021;87:102525. 10.1016/j.bcmd.2020.102525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chari A, Samur MK, Martinez‐Lopez J, et al. Clinical features associated with COVID‐19 outcome in multiple myeloma: first results from the International Myeloma Society data set. Blood. 2020;136(26):3033‐3040. 10.1182/blood.2020008150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Coll E, Fernández‐Ruiz M, Sánchez‐Álvarez JE, et al. COVID‐19 in transplant recipients: the Spanish experience. Am J Transpl. 2021;21(5):1825‐1837. 10.1111/ajt.16369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cook G, John Ashcroft A, Pratt G, et al. Real‐world assessment of the clinical impact of symptomatic infection with severe acute respiratory syndrome coronavirus (COVID‐19 disease) in patients with multiple myeloma receiving systemic anti‐cancer therapy. Br J Haematol. 2020;190:e83‐e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. de Joode K, Dumoulin DW, Tol J, et al. Dutch Oncology COVID‐19 consortium: outcome of COVID‐19 in patients with cancer in a nationwide cohort study. Eur J Cancer. 2020;141:171‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. de Melo AC, Thuler LCS, da Silva JL, et al. Cancer inpatients with COVID‐19: a report from the Brazilian national cancer Institute. PLoS One. 2020;15(10):e0241261. 10.1371/journal.pone.0241261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dufour I, Raedemaeker J, Andreozzi F, et al. COVID‐19, impact on myeloma patients. Ann Hematol. 2020;99(8):1947‐1949. 10.1007/s00277-020-04147-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. El Fakih R, Haroon A, Alfraih F, et al. Clinical course and outcomes of COVID‐19 in hematopoietic cell transplant patients, a regional report from the Middle East. Bone Marrow Transpl. 2021;56(9):2144‐2151. 10.1038/s41409-021-01312-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Engelhardt M, Shoumariyeh K, Rösner A, et al. Clinical characteristics and outcome of multiple myeloma patients with concomitant COVID‐19 at Comprehensive Cancer Centers in Germany. Haematologica. 2020;105(12):2872‐2878. 10.3324/haematol.2020.262758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fattizzo B, Giannotta JA, Sciumè M, et al. Reply to “COVID‐19 in persons with haematological cancers”: a focus on myeloid neoplasms and risk factors for mortality. Leukemia. 2020;34(7):1957‐1960. 10.1038/s41375-020-0877-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ferreri AJM, Steffanoni S, Calimeri T, et al. SARS‐COV‐2 infection in 50 patients with primary CNS lymphoma: presentation, effects on tumor treatment and outcome in a series of the international PCNSL collaborative group. Hematol Oncol. 2021;39(S2). 10.1002/hon.68_2880 [DOI] [Google Scholar]
- 39. Fox TA, Troy‐Barnes E, Kirkwood AA, et al. Clinical outcomes and risk factors for severe COVID‐19 in patients with haematological disorders receiving chemo‐ or immunotherapy. Br J Haematol. 2020;191(2):194‐206. 10.1111/bjh.17027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. García‐Sancho AM, Izuzquiza M, Bastos‐Oreiro M, et al. Outcomes of patients with lymphoma and COVID‐19: an observational cohort study from geltamo Spanish group. Hematol Oncol. 2021;39(S2). 10.1002/hon.200_2880 [DOI] [Google Scholar]
- 41. Garnett C, Foldes D, Bailey C, et al. Outcome of hospitalized patients with hematological malignancies and COVID‐19 infection in a large urban healthcare trust in the United Kingdom. Leuk Lymphoma. 2021;62(2):469‐472. 10.1080/10428194.2020.1838506 [DOI] [PubMed] [Google Scholar]
- 42. Ghandili S, Pfefferle S, Roedl K, et al. Challenges in treatment of patients with acute leukemia and COVID‐19: a series of 12 patients. Blood Adv. 2020;4(23):5936‐5941. 10.1182/bloodadvances.2020002543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. He W, Chen L, Yuan G, et al. COVID‐19 in persons with haematological cancers. Leukemia. 2020;34(6):1637‐1645. 10.1038/s41375-020-0836-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Höllein A, Bojko P, Schulz S, et al. Characteristics and outcomes of patients with cancer and COVID‐19: results from a cohort study. Acta Oncol. 2021;60(1):24‐27. 10.1080/0284186x.2020.1863464 [DOI] [PubMed] [Google Scholar]
- 45. Hultcrantz M, Richter J, Rosenbaum C, et al. COVID‐19 infections and outcomes in patients with multiple myeloma in New York City: a cohort study from five academic centers. medRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Infante MS, González‐Gascón YMI, Muñoz‐Novas C, et al. COVID‐19 in patients with hematological malignancies: a retrospective case series. Int J Lab Hematol. 2020;42(6):e256‐e259. 10.1111/ijlh.13301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jeyaraman P, Agrawal N, Bhargava R, et al. Convalescent plasma therapy for severe Covid‐19 in patients with hematological malignancies. Transfus Apher Sci. 2021;60(3):103075. 10.1016/j.transci.2021.103075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kořen J, Steinerová K, Janíková A, et al. Multicenter retrospective analysis of risk factors for mortality of COVID‐19 infection in patients with lymphoma. Hematol Oncol. 2021;39(S2). 10.1002/hon.197_2880 [DOI] [Google Scholar]
- 49. Lamure S, Duléry R, Di Blasi R, et al. Determinants of outcome in Covid‐19 hospitalized patients with lymphoma: a retrospective multicentric cohort study. EClinicalMedicine. 2020;27:100549. 10.1016/j.eclinm.2020.100549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lattenist R, Yildiz H, De Greef J, Bailly S, Yombi JC. COVID‐19 in adult patients with hematological disease: analysis of clinical characteristics and outcomes. Indian J Hematol Blood Transfus. 2020;37(1):1‐5. 10.1007/s12288-020-01318-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Laurenge A, Ursu R, Houillier C, et al. SARS‐CoV‐2 infection in patients with primary central nervous system lymphoma. J Neurol. 2021;268(9):3072‐3080. 10.1007/s00415-020-10311-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ljungman P, de la Camara R, Mikulska M, et al. COVID‐19 and stem cell transplantation; results from an EBMT and GETH multicenter prospective survey. Leukemia. 2021;35(10):2885‐2894. 10.1038/s41375-021-01302-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Malard F, Genthon A, Brissot E, et al. COVID‐19 outcomes in patients with hematologic disease. Bone Marrow Transpl. 2020;55(11):2180‐2184. 10.1038/s41409-020-0931-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Martín‐Moro F, Marquet J, Piris M, et al. Survival study of hospitalised patients with concurrent COVID‐19 and haematological malignancies. Br J Haematol. 2020;190:e16‐e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mato AR, Roeker LE, Lamanna N, et al. Outcomes of COVID‐19 in patients with CLL: a multicenter international experience. Blood. 2020;136(10):1134‐1143. 10.1182/blood.2020006965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mehta V, Goel S, Kabarriti R, et al. Case fatality rate of cancer patients with COVID‐19 in a New York hospital system. Cancer Discov. 2020;10(7):935‐941. 10.1158/2159-8290.cd-20-0516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mossuto S, Attardi E, Alesiani F, et al. SARS‐CoV‐2 in myelodysplastic syndromes: a snapshot from early Italian experience. Hemasphere. 2020;4(5):e483. 10.1097/hs9.0000000000000483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Muntañola A, Villacampa G, Hernández‐Rivas JÁ, et al. Clinical characteristics and outcome of SARS‐CoV‐2 infection in admitted patients with chronic lymphocytic leukemia from a single European country. Exp Hematol Oncol. 2020;9(1):37. 10.1186/s40164-020-00195-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Niu A, Ning B, Socola F, et al. High mortality with High false negative rate: COVID‐19 infection in patients with hematologic malignancies. Leuk Res. 2021;106:106582. 10.1016/j.leukres.2021.106582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Passamonti F, Cattaneo C, Arcaini L, et al. Clinical characteristics and risk factors associated with COVID‐19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol. 2020;7(10):e737‐e745. 10.1016/s2352-3026(20)30251-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pinato DJ, Scotti L, Gennari A, et al. Determinants of enhanced vulnerability to coronavirus disease 2019 in UK patients with cancer: a European study. Eur J Cancer. 2021;150:190‐202. 10.1016/j.ejca.2021.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pinato DJ, Zambelli A, Aguilar‐Company J, et al. Clinical portrait of the SARS‐CoV‐2 epidemic in European cancer patients. Cancer Discov. 2020;10(10):1465‐1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ramachandran P, Kathirvelu B, Chakraborti A, et al. COVID‐19 in cancer patients from New York city: a comparative single center retrospective analysis. Cancer Control. 2020;27(1):1073274820960457. 10.1177/1073274820960457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Razanamahery J, Soumagne T, Humbert S, et al. Does type of immunosupression influence the course of Covid‐19 infection? J Infect. 2020;81(2):e132‐e135. 10.1016/j.jinf.2020.05.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Roeker LE, Knorr DA, Pessin MS, et al. Anti‐SARS‐CoV‐2 antibody response in patients with chronic lymphocytic leukemia. Leukemia. 2020;34(11):3047‐3049. 10.1038/s41375-020-01030-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rugge M, Zorzi M, Guzzinati S. SARS‐CoV‐2 infection in the Italian Veneto region: adverse outcomes in patients with cancer. Nature Cancer. 2020;1(8):784‐788. 10.1038/s43018-020-0104-9 [DOI] [PubMed] [Google Scholar]
- 67. Russell B, Moss C, Papa S, et al. Factors affecting COVID‐19 outcomes in cancer patients: a first report from guy's cancer center in London. Front Oncol. 2020;10:1279. 10.3389/fonc.2020.01279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Russo D, Polverelli N, Malagola M, et al. Changes in stem cell transplant activity and procedures during SARS‐CoV2 pandemic in Italy: an Italian bone marrow transplant group (GITMO) nationwide analysis (TransCOVID‐19 survey). Bone Marrow Transpl. 2021;56(9):2272‐2275. 10.1038/s41409-021-01287-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Salisbury RA, Curto‐Garcia N, O'Sullivan J, et al. Results of a national UK physician reported survey of COVID‐19 infection in patients with a myeloproliferative neoplasm. Leukemia. 2021;35(8):2424‐2430. 10.1038/s41375-021-01143-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sanchez‐Pina JM, Rodríguez Rodriguez M, Castro Quismondo N, et al. Clinical course and risk factors for mortality from COVID‐19 in patients with haematological malignancies. Eur J Haematol. 2020;105(5):597‐607. 10.1111/ejh.13493 [DOI] [PubMed] [Google Scholar]
- 71. Scarfò L, Chatzikonstantinou T, Rigolin GM, et al. COVID‐19 severity and mortality in patients with chronic lymphocytic leukemia: a joint study by ERIC, the European Research Initiative on CLL, and CLL Campus. Leukemia. 2020;34(9):2354‐2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Shah V, Ko Ko T, Zuckerman M, et al. Poor outcome and prolonged persistence of SARS‐CoV‐2 RNA in COVID‐19 patients with haematological malignancies; King's College Hospital experience. Br J Haematol. 2020;190(5):e279‐e282. 10.1111/bjh.16935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Singh SRK, Thanikachalam K, Jabbour‐Aida H, Poisson LM, Khan G. COVID‐19 and cancer: lessons learnt from a Michigan hotspot. Cancers (Basel). 2020;12(9):2377. 10.3390/cancers12092377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Song C, Dong Z, Gong H, et al. An online tool for predicting the prognosis of cancer patients with SARS‐CoV‐2 infection: a multi‐center study. J Cancer Res Clin Oncol. 2021;147(4):1247‐1257. 10.1007/s00432-020-03420-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Thompson MA, Henderson JP, Shah PK, et al. Association of convalescent plasma therapy with survival in patients with hematologic cancers and COVID‐19. JAMA Oncol. 2021;7(8):1167‐1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Varma A, Kosuri S, Ustun C, et al. COVID‐19 infection in hematopoietic cell transplantation: age, time from transplant and steroids matter. Leukemia. 2020;34(10):2809‐2812. 10.1038/s41375-020-01019-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wang B, Van Oekelen O, Mouhieddine TH, et al. A tertiary center experience of multiple myeloma patients with COVID‐19: lessons learned and the path forward. J Hematol Oncol. 2020;13(1):94. 10.1186/s13045-020-00934-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wang Q, Berger NA, Xu R. When hematologic malignancies meet COVID‐19 in the United States: infections, death and disparities. Blood Rev. 2021;47:100775. 10.1016/j.blre.2020.100775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Worel N, Shaw BE, Aljurf M, et al. Changes in hematopoietic cell transplantation practices in response to COVID‐19: a survey from the worldwide network for blood & marrow transplantation. Transplant Cell Ther. 2021;27(3):270‐276. 10.1016/j.jtct.2020.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Xhaard A, Xhaard C, D'Aveni M, et al. Risk factors for a severe form of COVID‐19 after allogeneic haematopoietic stem cell transplantation: a Société Francophone de Greffe de Moelle et de Thérapie cellulaire (SFGM‐TC) multicentre cohort study. Br J Haematol. 2021;192:e121‐e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Yang K, Sheng Y, Huang C, et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID‐19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21(7):904‐913. 10.1016/s1470-2045(20)30310-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Venkatesulu BP, Chandrasekar VT, Girdhar P, et al. A systematic review and meta‐analysis of cancer patients affected by a novel coronavirus. JNCI Cancer Spectr. 2021;5(2): pkaa102. 10.1093/jncics/pkaa102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. 10.1016/s0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. 10.1056/nejmoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wu Z, McGoogan JM. Characteristics of and Important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239‐1242. 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 86. Visco C, Marcheselli L, Mina R, et al. A prognostic model for patients with lymphoma and COVID‐19: a multicentre cohort study. Blood Advances. 2021:2021005691. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


