Abstract
Bacterial DNA and its synthetic immunostimulatory oligodeoxynucleotide analogs (ISS-ODN) activate innate immunity and promote Th1 and cytotoxic T-lymphocyte immune responses. Based on these activities, we investigated whether ISS-ODN could modify the course of Mycobacterium avium infection. M. avium growth in vitro was significantly inhibited by ISS-ODN treatment of human and mouse macrophages, and M. avium growth in vivo was similarly inhibited in C57BL/6 mice treated with ISS-ODN. This protective effect of ISS-ODN was largely independent of tumor necrosis factor alpha (TNF-α), interleukin 12 (IL-12), nitric oxide, NADPH oxidase, alpha/beta interferon (IFN-α/β), and IFN-γ. In contrast, we found that the induction of indoleamine 2,3-dioxygenase (IDO) was required for the antimycobacterial effect of ISS-ODN. To evaluate the potential for synergism between ISS-ODN and other antimycobacterial agents, treatment with a combination of ISS-ODN and clarithromycin (CLA) was tested in vitro and in vivo. ISS-ODN significantly enhanced the therapeutic effect of CLA in both human and mouse macrophages and in C57BL/6 mice. This study newly identifies IDO as being involved in the antimicrobial activity of ISS-ODN and suggests the usefulness of ISS-ODN when used in combination with conventional chemotherapy for microbial infections.
Mycobacterium avium infection is a common opportunistic infection in patients with AIDS (22), while M. avium rarely causes disease in immunocompetent individuals. In AIDS, disseminated M. avium infection occurs only after severe depletion of CD4+ T cells (10) and is a major cause of morbidity and mortality. Although intensive antiretroviral therapy prevents the onset of M. avium infection to some extent (3), M. avium infection in AIDS is extremely difficult to treat when encountered because it responds poorly to available antimycobacterial therapies (33).
M. avium predominantly infects and multiplies within macrophages (14). This organism is known to attach to and enter macrophages with the help of complement, transferrin, and integrin receptors expressed on the surface of macrophages (6, 42). Macrophages secrete several cytokines in response to infection with this organism, including tumor necrosis factor alpha (TNF-α), interleukin 1β (IL-1β), IL-6, granulocyte-macrophage colony-stimulating factor (GM-CSF), and granulocyte colony-stimulating factor (G-CSF) (17, 38).
Immunostimulatory DNA sequences (ISS) were initially discovered in the mycobacterial genome as short DNA sequences that selectively enhanced NK cell activity (53). In addition, bacterial DNA or oligodeoxynucleotides containing ISS (ISS-ODN) activate antigen-presenting cells, such as dendritic cells and macrophages. ISS-ODN activation leads to the up-regulation of costimulatory receptors (32), the release of IFN-α/β, TNF-α, IL-12, and IL-18 (25, 28, 44, 47, 48, 55), and the priming of Th1 responses and cytotoxic T-lymphocyte (CTL) responses to exogenous antigens (9, 30). This immune profile has been used to generate cellularly mediated immunity to coinjected antigens in the development of novel vaccine strategies (9, 29, 44).
Recent studies have also demonstrated the ability of ISS-ODN to facilitate control of the intracellular pathogens Listeria monocytogenes, Leishmania major, and Francisella tularensis (24, 27, 51). This effect is generally attributed to the activation of innate immunity, which is crucial for both the initial control of L. monocytogenes and L. major and the long-term control from subsequent induction of Th1 and CTL responses.
Here, we examine whether ISS-ODN can modify the course of M. avium infection. Our studies demonstrate that ISS-ODN is able to restrict the growth of M. avium via the induction of indoleamine 2,3-dioxygenase (IDO), revealing a novel facet of ISS-ODN-induced activation of innate immunity. As a therapeutic adjunct, ISS-ODN significantly enhances the ability of the conventional antimycobacterial agent clarithromycin (CLA) to treat M. avium infection in mouse and human macrophages as well as in a mouse model of M. avium infection.
MATERIALS AND METHODS
Mice.
Female C57BL/6 mice (7 to 8 weeks old) and 129/SvEv mice were purchased from The Jackson Laboratory (Bar Harbor, Maine) and Taconic Laboratories, Germantown, New York, respectively. The inducible nitric oxide synthetase (iNOS−/−) IL-12 p40−/−, TNF-α−/−, and NADPH oxidase (gp91 phox−/−) knockout mice are on the C57BL/6 background and were purchased from The Jackson Laboratory. IFN-α/β receptor (IFN-α/βR−/−) and IFN-γ receptor (IFN-γR−/−) (129/EvSv background) knockout mice were obtained from B & K Universal, Ltd. (East Yorkshire, United Kingdom).
Reagents and cytokines.
Endotoxin-free (<1 ng of DNA per mg) phosphorothioate single-stranded oligodeoxynucleotides were obtained from Trilink Biotechnologies, San Diego, Calif. The sequence of the ISS-ODN was 5′-TGACTGTGAACGTTCGAGATGA-3′. The sequence of the mutated-ODN (M-ODN) was 5′-TGACTGTGAAGGTTAGAGATGA-3′ (underlining indicates immunostimulatory sequences [ISS]). We used 10 μg of ISS-ODN or M-ODN per ml unless otherwise noted. l-Tryptophan (l-Try) was obtained from Gibco BRL (Grand Island, N.Y.) and Dulbecco's modified Eagle's medium (DMEM) was supplemented with l-Try for a final concentration of 66 μg/ml. 1-Methyl-dl-tryptophan (M-Try) was purchased from Aldrich Chemicals (Milwaukee, Wis.). Anti-CD3, anti-CD28, anti-CD4, and anti-CD8 monoclonal antibodies as well as monensin and murine recombinant IFN-γ were purchased from BD Pharmingen (San Diego, Calif.). Abbott Laboratories (Abbott Park, Ill.) kindly provided CLA.
Culture of M. avium and CFU assay.
M. avium strain I13 isolated from an AIDS patient at University of California, San Diego (34), was used in all experiments. Bacteria were cultured as described previously (21). The numbers of CFU in a sample were determined by colony counting as described previously (39).
Preparation of murine macrophages and isolation of human monocytes.
Murine bone marrow-derived macrophages (mBMDMs) were prepared from mouse bone marrow using L-cell conditioned medium, as described previously (32). Human monocytes were isolated from normal human buffy coats obtained from the San Diego Blood Bank by Ficoll-Hypaque and Percoll gradient centrifugation (21) and then cultured in Teflon beakers for 7 days to yield mature human monocyte-derived macrophages (hMDMs).
M. avium infection of macrophages in vitro.
To study the protective effect of ISS-ODN treatment before infection, 5 × 104 mBMDMs or hMDMs were treated with ISS-ODN for 3 days before infection. Macrophages treated with M-ODN or with medium alone served as controls. After 3 days, the cells were infected for 2 h with M. avium at a macrophage/bacterium ratio of 2:1 for mBMDMs or 1:10 for hMDMs and subsequently cultured in fresh medium without antibiotics. To examine attachment and/or invasion of M. avium, the macrophages were lysed immediately after washing, and the number of bacteria was enumerated by the CFU assay. Intracellular growth of M. avium was determined on days 1, 3, and 7 after infection. To account for all of the mycobacteria in each well, corresponding lysates were combined with the culture media, and then the number of CFU was determined. To examine the efficacy of antimycobacterial treatments, CFU recovered from cells treated with medium alone was considered as 100% growth.
To determine whether treatment with ISS-ODN after infection alters M. avium growth, adherent cells were first infected with M. avium for 2 h and then cultured with fresh medium containing ISS-ODN. Infected cells treated with M-ODN or medium alone served as controls. At day 7, intracellular growth of M. avium was assessed by the CFU assay.
M. avium infection of mice in vivo.
To study the effect of treatment with ISS-ODN before infection, mice were injected intradermally (i.d.)with ISS-ODN (50 or 100 μg/mouse) 3 days before infection, while control mice received phosphate-buffered saline (PBS). In the preliminary experiments, the numbers of CFU in the spleen and lungs were similar in the M-ODN-treated mice and control (PBS treated) mice 4 weeks after infection, but were significantly higher than those in the ISS-ODN-treated mice (by 2 logs in spleen and 1 log in lungs, respectively). Therefore, treatment with M-ODN was excluded in this set of experiments. All mice were infected intravenously (i.v.) with M. avium (107/mouse). At 2, 4, and 6 weeks after infection, the mice were sacrificed, and the spleen, liver, and lungs from each mouse were collected and weighed. A section of each organ was homogenized with 0.25% sodium dodecyl sulfate (SDS) in PBS. The number of CFU in the tissue homogenates was determined by the CFU assay, and the results were expressed as CFU per organ.
To study the effect of ISS-ODN when combined with CLA in vivo, 25 mice were injected with M. avium (107/mouse). One week after infection, treatment with either ISS-ODN or M-ODN and CLA was initiated. Mice were divided into five treatment groups (n = 5 per group): group 1, no treatment; group 2, ISS-ODN alone; group 3, CLA alone; group 4, CLA and ISS-ODN; and group 5, CLA and M-ODN. CLA (200 mg/kg of body weight) was administered intraperitoneally three times a week for 4 weeks as described previously (13), and bacterial growth in the spleen, liver, and lungs was determined.
Detection of intracellular IFN-γ by FACS and IFN-γ secretion by ELISA.
Mice were injected i.d. with ISS-ODN (50 μg/mouse). The control mice received M-ODN (50 μg/mouse) or PBS. All mice were infected with 107 M. avium cells. Three weeks after infection, the mice were sacrificed, and splenocytes from mice receiving the same treatment were pooled. Intracellular cytokine staining was performed with the Cytofix/Cytoperm kit (BD Pharmingen) according to the manufacturer's instructions. Briefly, the splenocytes were stimulated with anti-CD3 and anti-CD28 activating antibodies in the presence of monensin to allow intracellular IFN-γ to accumulate for 6 h. Next, the cells were stained for surface CD4 and CD8 and fixed, and the plasma membranes were permeabilized, allowing for intracellular staining with anti-IFN-γ. The cells were analyzed by fluorescence-activated cell sorting (FACS) with a FACSCalibur flow cytometer (Becton Dickinson). To study IFN-γ secretion, splenocytes were incubated with anti-CD3 and anti-CD-28 antibodies or M. avium sonicates (20) as antigens in vitro for 24 h, and then the supernatant was assayed for IFN-γ secretion by enzyme-linked immunosorbent assay (ELISA) as previously described (32).
RNA extraction, RT-PCR, and IDO activity assay.
mBMDMs (2 × 106) were treated with ISS-ODN or M-ODN. After 3 days, the cells were infected with M. avium for 2 h. At 2, 24, and 48 h after infection, the M. avium-infected macrophages were lysed, and total RNA was isolated by using the Trizol reagent (Gibco BRL). The induction of IDO gene transcription was measured by semiquantitative reverse transcription-PCR (RT-PCR). First-strand cDNA preparation and PCR amplification were carried out with the SuperScript Pre-amplification System (Gibco BRL) and AdvanTaq Plus DNA polymerase (Clontech, San Francisco, Calif.), respectively. PCR products were visualized by electrophoresis on 2% agarose gels. The primer sequences used were as follows: IDO, 5′-TTATGCAGACTGTGTCCTGGCAAA-3′ and 5′-TTTCCAGCCAGACAGATATATGCG–3′; and glucose-3-phosphate dehydrogenase (G3PDH), 5′-ACCACAGTCCATGCCATCAC-3′ and 5′-TCCACCACCCTGTTGCTGTA-3′.
Histological examination.
Tissue sections of the spleen collected from the experimental and control mice were fixed overnight in 10% buffered formalin at room temperature and then embedded in paraffin. The paraffin-embedded tissue was further sectioned (5-μm thickness) and stained with hematoxylin-eosin. The sections were observed under an Olympus microscope. At least three sections of each organ from each of the experimental and control mice were evaluated.
Statistical analysis.
Results are expressed as means ± standard deviations (SD), and statistical differences were determined with the nonpaired Student's t test (two-tailed distribution). A P value below 0.05 is considered to be statistically significant.
RESULTS
Treatment with ISS-ODN inhibits the growth of M. avium in vitro.
M. avium is able to infect and replicate in macrophages (14). We performed the following studies to address whether ISS-ODN-induced activation of macrophages in vitro inhibits intracellular growth of M. avium.
(i) Treatment prior to infection.
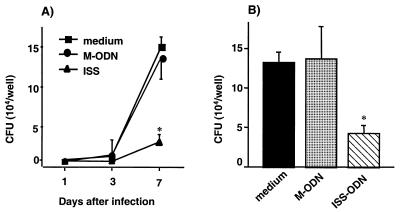
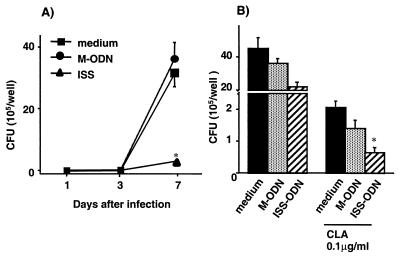
To examine whether treatment with ISS-ODN can stimulate macrophages to inhibit the growth of M. avium, mBMDMs were first treated with ISS-ODN or M-ODN (3, 10, and 30 μg/ml) for 72 h and then infected with M. avium. The number of CFU was counted on days 1, 3, and 7 postinfection (Fig. 1A). By day 7, treatment with ISS-ODN inhibited intracellular growth of M. avium in mBMDMs by 80% (P < 0.001). No significant difference in M. avium growth was seen among the different concentrations of ISS-ODN tested. Since viability of macrophages can affect M. avium growth, we assessed the viability of macrophages by trypan blue exclusion. At day 7 after infection, mBMDMs treated with ISS-ODN, M-ODN, or medium alone were all >90% viable. The experiments were terminated at day 7 after infection, since the viability of mBMDMs began to decline from this point on.
FIG. 1.
Antimycobacterial effects of ISS-ODN in vitro. (A) mBMDMs were treated with ISS-ODN or M-ODN for 3 days prior to M. avium infection, and intracellular growth of M. avium was assessed by the CFU assay on days 1, 3, and 7 after infection. (B) mBMDMs were treated with ISS-ODN or M-ODN immediately after infection, and M. avium growth was assessed by the CFU assay 7 days postinfection. Each condition was tested in triplicate, and the results are expressed as mean ± SD CFU per well. The results shown are representative of three experiments. ∗, P < 0.01 compared to CFU recovered from cells treated with M-ODN or medium alone.
ISS-ODN activates macrophages and induces the expression of surface adhesion molecules such as ICAM-1 (32). These molecules may affect the attachment of M. avium or its invasion into mBMDMs. In order to determine whether the ability of ISS-ODN to inhibit M. avium growth is due to alterations in M. avium invasion, the ability of ISS-ODN to influence the number of bacteria that attached to and invaded mBMDMs after incubation with M. avium was examined. The number of CFU recovered immediately from cells treated with ISS-ODN before infection was not significantly different from the number of CFU recovered from cells treated with M-ODN or with medium alone (data not shown).
(ii) Treatment after infection.
To evaluate the therapeutic antimycobacterial effect of ISS-ODN, infected mBMDMs were treated with ISS-ODN for 7 days after infection, starting 2 h after the time of infection. Treatment with a single dose of ISS-ODN significantly decreased the intracellular growth of M. avium in mBMDMs by 68% (P < 0.05), compared to the number of CFU in infected cells treated with M-ODN or medium alone (Fig. 1B).
ISS-ODN transiently inhibits the growth of M. avium in vivo.
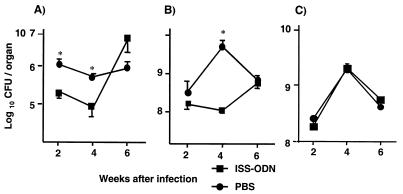
To determine whether ISS-ODN can enhance resistance to M. avium infection in vivo, mice were treated with ISS-ODN and infected i.v. 3 days later with 107 organisms per mouse. In a preliminary experiment, bacterial growth in spleen and lung from mice treated with 50 μg of ISS-ODN was similar to bacterial growth in mice treated with 100 μg of ISS-ODN 4 weeks after infection (data not shown). Therefore, we used 50 μg of ISS-ODN per mouse for the in vivo experiment. At 2, 4, and 6 weeks after infection, the number of CFU was determined in the spleen, lungs, and liver. Based on the preliminary experiments, which demonstrated no differences in colony counts among the ISS-ODN, M-ODN, or control mice at 6 versus 8 weeks postinfection, all future experiments were terminated at 6 weeks postinfection.
At week 2, the lungs of M. avium-infected mice treated with ISS-ODN prior to infection contained a significantly lower number of viable bacteria compared to control PBS-treated mice (P < 0.05) (Fig. 2A). In addition, mice treated with ISS-ODN prior to M. avium infection had nearly 2 logs fewer bacteria in the spleen at 4 weeks (P < 0.05) compared to the PBS-treated mice (Fig. 2B). By 6 weeks, however, the numbers of splenic CFU were similar in control and ISS-ODN groups. There was no significant difference in the mycobacterial loads in the liver of mice treated with ISS-ODN prior to infection compared to those of PBS-treated mice at any of these time points (Fig. 2C). Thus, a single injection of ISS-ODN significantly reduced the mycobacterial growth in the spleen and lungs, but not in the liver of M. avium-infected mice. This protective effect was transient and was most apparent at 2 and 4 weeks after a single administration of ISS-ODN.
FIG. 2.
Antimycobacterial effects of ISS-ODN in vivo. Mice (n = 5 per group) were injected with ISS-ODN or PBS i.d. 3 days before infection with 107 M. avium. M. avium growth in the lungs (A), spleen (B), and liver (C) of the animals was assessed by the CFU assay at 2, 4, and 6 weeks after infection. The results shown are mean ± SD CFU per organ. ∗, P < 0.05 compared to CFU in the organs of control mice that received PBS instead of ISS-ODN.
Although treatment with ISS-ODN after infection inhibited M. avium growth in vitro, there was no difference in the mycobacterial counts in spleen and liver at any time point observed when we used ISS-ODN 1 week after infection in vivo. Overall, ISS-ODN significantly reduces the growth of M. avium when ISS-ODN was used in a preventive mode, but not when used in a therapeutic mode.
ISS-ODN protection in vivo is not mediated through augmentation of the T-cell response.
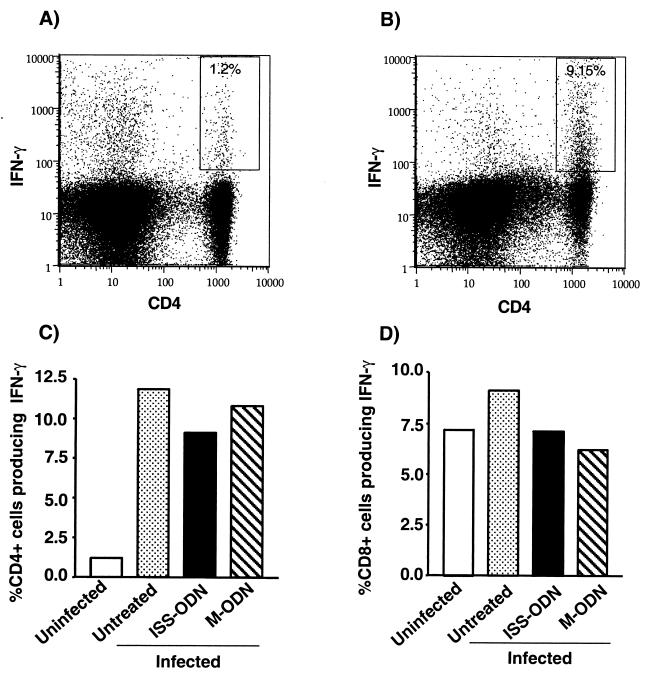
The observation that ISS-ODN protects isolated macrophages in vitro (Fig. 1) and that the protective effect observed in ISS-ODN-treated mice is transient (Fig. 2) suggests a T-cell-independent mechanism of protection via innate immunity. To further investigate the potential role of adaptive immunity in this model of ISS-ODN-mediated protection against M. avium, mice were treated with ISS-ODN or M-ODN (50 μg/mouse) and infected with 107 organisms/mouse, and then their T-cell response was evaluated. The mice were sacrificed at 3 weeks postinfection, and the spenocytes were restimulated with anti-CD3 and anti-CD28 antibodies or M. avium sonicates to amplify the response from preexisting memory and activated T cells. T cells were then examined for their production of IFN-γ. FACS-based intracellular cytokine staining was used to determine the frequencies of IFN-γ-producing CD4+ (Fig. 3A, B, and C) and CD8+ (Fig. 3B) T cells, while the total quantity of IFN-γ secreted by splenocytes was determined by ELISA (data not shown). There was a dramatic increase in the IFN-γ response of the CD4+ T cells in the infected versus uninfected animals, demonstrating that the observed Th1 response is infection specific. However, treatment of M. avium-infected mice with ISS-ODN did not further increase the frequency of IFN-γ-positive CD4+ or CD8+ T cells. In addition, treatment with ISS-ODN did not enhance the IFN-γ secretion by splenocytes, which were restimulated by anti-CD3 and anti-CD28 antibodies or M. avium sonicates, compared to M-ODN treatment (data not shown). These data, combined with the observation that ISS-ODN protects isolated macrophages in vitro (Fig. 1) and that the protective effect observed in ISS-ODN-treated mice is transient (Fig. 2), suggest that it is unlikely T-cell-dependent immunity plays a role in the antimycobacterial effect of ISS-ODN during early stages of infection.
FIG. 3.
ISS-ODN treatment of infected mice does not augment the anti-M. avium T-cell response. At 3 weeks postinfection, the IFN-γ+, CD4+, and CD8+ T-cell responses of mice treated with either ISS-ODN or M-ODN prior to infection were determined. Splenocytes were pooled within groups for the intracellular cyotkine assays. Splenocytes were restimulated in vitro with anti-CD3 and anti-CD28 activating antibodies, and the percentages of intracellular IFN-γ-producing CD4+ cells (A, B, and C) and CD8+ cells (D) were determined. Percentages of CD4+ IFN-γ+ cells within the total CD4+ T-cell population are shown in fluorocytometric plots of splenocytes recovered from uninfected mice (A) and M. avium-infected mice treated with ISS-ODN (B).
ISS-ODN inhibition of M. avium growth in macrophages is independent of iNOS, NAPDH oxidase, IL-12, TNF-α, IFN-α/β, and IFN-γ.
To further investigate the mechanisms of the antimycobacterial effects of ISS-ODN, mice with targeted disruptions of genes known to play roles in M. avium infection were used. Oxygen radicals generated by NADPH oxidase and induction of nitric oxide (NO) by iNOS result in antimicrobial activity against many microorganisms (16, 35). IL-12, TNF-α, and IFN-γ play important roles in the clearance of M. avium (2, 13, 26). Furthermore, macrophages produce IL-12, TNF-α, and IFN-α/β in response to ISS-ODN treatment (25, 44). To study the role of these molecules in the antimycobacterial effect of ISS-ODN, mBMDMs from NADPH oxidase−/−, iNOS−/−, TNF-α−/−, IL-12 p40−/−, IFN-α/βR−/−, and IFN-γR−/− mice were treated with ISS-ODN for 3 days and then infected with M. avium. ISS-ODN inhibited the intracellular growth of M. avium in mBMDMs from these knockout mice by 60 to 85% (P < 0.05), similar to wild-type mice (Table 1). These results indicate that these gene products (e.g., nitrogen intermediates, oxygen radicals, TNF-α, etc.) are not central to the antimycobacterial effect induced by ISS-ODN in vitro.
TABLE 1.
Effect of ISS on M. avium growth in mBMDMs from mice with targeted disruption of genes known to play a protective role against M. avium infection
| Mouse | Strain | % of CFUa
|
||
|---|---|---|---|---|
| Untreated (104 CFU) | M-ODN | ISS-ODNb | ||
| Wild type | C57BL/6 | 100 (8.3) | 117.2 ± 18.5 | 18.2 ± 7.3 |
| Wild type | 129S6/SvEV | 100 (15.0) | 110.0 ± 15.9 | 17.0 ± 4.0 |
| iNOS−/− | C57BL/6 | 100 (25.0) | 105.0 ± 13.0 | 37.3 ± 4.6 |
| NADPH oxidase−/− (gp91 phox−/−) | C57BL/6 | 100 (14.3) | 107.0 ± 15.5 | 11.4 ± 1.5 |
| TNF-α−/− | C57BL/6 | 100 (33.3) | 120.0 ± 20.0 | 24.0 ± 6.0 |
| IL-12 p40−/− | C57BL/6 | 100 (22.7) | 117.6 ± 9.2 | 26.4 ± 10.1 |
| IFN-αR−/− | 129S6/SvEv | 100 (14.5) | 106.9 ± 11.9 | 10.9 ± 1.7 |
| IFN-γR−/− | 129S6/SvEv | 100 (9.2) | 108.1 ± 10.8 | 7.6 ± 1.3 |
mBMDMs were treated with M-ODN or ISS-ODN (10 μg/ml) for 72 h prior to infection and then infected with M. avium mBMDMs treated with medium alone served as the control. On day 7, M. avium growth was assessed by CFU assay. The average numbers of CFU recovered in the wells of untreated BMDMs are indicated in parentheses. For comparison, results are presented as percent CFU compared to mBMDMs treated with medium alone, rather than absolute CFU, in order to normalize for strain variation (i.e., C57BL/6 versus 129S6/SvEv). The number of CFU of cells treated with medium alone was considered 100%. Mean ± SD from three independent experiments are shown.
P < 0.05 compared to mBMDMs treated with medium alone.
Induction of IDO contributes to the antimycobacterial activity of ISS-ODN.
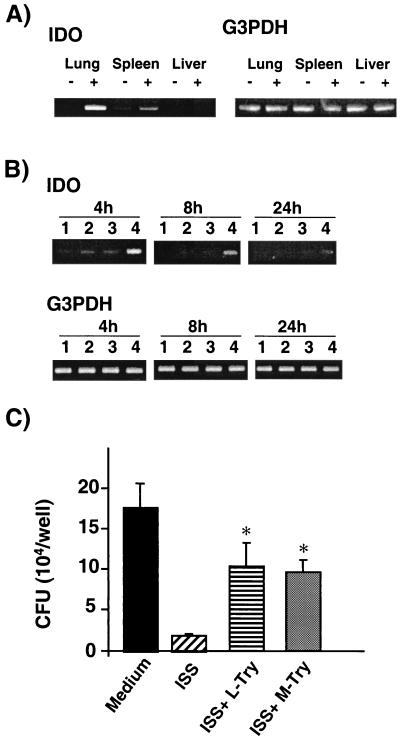
Macrophages orchestrate the activation of innate immunity to directly attack an invading pathogen by secreting a battery of cytokines, expressing costimulatory molecules, and generating free radicals. However, macrophages also employ effector mechanisms to deny the pathogens required substrates for their growth. One such example is the depletion of tryptophan due to the induction of IDO. IDO is the rate-limiting enzyme in the catabolism of tryptophan. Induction of this enzyme limits the availability of this important amino acid to invading pathogens (11). To study the potential role of IDO in the antimycobacterial effect of ISS-ODN, we assessed (i) the induction of IDO activity as measured by semiquantitative RT-PCR in vivo and in vitro and (ii) the abrogation of the antimycobacterial effect of ISS-ODN by addition of excess l-Try or by using a competitive inhibitor for IDO, M-Try.
When mice were injected i.v. with 50 μg of ISS-ODN, IDO gene induction was observed in the lungs and spleen after 16 h, but not in the liver (Fig. 4A). Injection of M-ODN did not result in any detectable induction of IDO (data not shown). For in vitro studies, mBMDMs were treated with ISS-ODN for 3 days prior to M. avium infection. Cells were then lysed at 4, 8, and 24 h after infection, total RNA was extracted, and semiquantitative RT-PCR was performed. We found that optimal induction of IDO gene transcription required both treatments with ISS-ODN and M. avium infection (Fig. 4B).
FIG. 4.
IDO is involved in the antimycobacterial effect of ISS-ODN. (A) ISS-ODN induced IDO gene expression in the lungs and spleen, but not liver. Uninfected mice were injected with 50 μg of ISS-ODN, and the lungs and spleen were collected 16 h after injection. Total RNA was isolated, and semiquantitative RT-PCR was performed to assess the induction of IDO gene expression. −, injected with saline; +, injected with ISS-ODN. (B) M. avium infection with ISS-ODN treatment of mBMDMs induces IDO gene expression. mBMDMs were treated with ISS-ODN for 3 days prior to infection with M. avium. Total RNA was evaluated by semiquantitative RT-PCR to assess the induction of IDO gene expression at 4, 8, and 24 h postinfection. Lanes: 1, medium alone; 2, ISS-ODN treatment alone; 3, M. avium infection alone; 4, ISS-ODN treatment and M. avium infection. (C) Addition of excess l-Try or a competitive IDO inhibitor, M-Try, partially inhibited the antimycobacterial effect of ISS-ODN. mBMDMs were treated with ISS-ODN for 3 days in the presence of excess l-Try (final concentration of 66 μg/ml) or M-Try (125 μM) and subsequently infected with M. avium for 7 days in the presence of l-Try or M-Try. Intracellular growth of M. avium was assessed by the CFU assay. Each condition was tested in triplicate, and the results are expressed as mean ± SD CFU per well. The results shown are representative of three experiments. ∗, P < 0.01 compared to CFU recovered from cells treated with ISS-ODN.
In order to further investigate the role of IDO in the inhibition of M. avium growth, mBMDMs were cultured with the IDO inhibitor M-Try (125 μM) or with excess l-Try (final concentration, 66 μg/ml) as previously described (23, 36). Addition of l-Try or M-Try alone at these concentrations did not alter the viability of mBMDMs or M. avium growth in mBMDMs (data not shown). However, when the M. avium-infected cells were cultured with l-Try or M-Try-supplemented medium, fourfold reductions in the antimycobacterial ability of ISS-ODN treatment were observed (P < 0.05) (Fig. 4C). Taken together, these data suggest that IDO plays a role in the observed antimycobacterial properties of ISS-ODN.
ISS-ODN is an adjunct to antimycobacterial therapy with CLA.
Treatment of M. avium infection by conventional chemotherapy is difficult and requires prolonged administration (8, 33). In in vivo experiments, ISS-ODN did not affect the colony counts when administered in an established infection. Therefore, we hypothesized that coadministration of ISS-ODN along with a chemotherapeutic agent such as CLA may further enhance mycobacterial clearance.
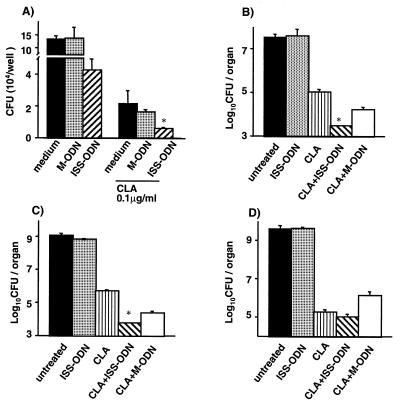
mBMDMs were first infected with M. avium and then treated with CLA (0.1 μg/ml) in the presence or absence of ISS-ODN (10 μg/ml) or M-ODN (10 μg/ml), and M. avium growth in vitro was determined 7 days after infection. ISS-ODN and CLA (0.1 μg/ml), when used individually, reduced bacterial growth in mBMDMs by 68 and 84%, respectively (P < 0.01; Fig. 5A). When ISS-ODN was used together with CLA, bacterial counts were further reduced (95%, P < 0.01) compared to medium alone (Fig. 5A).
FIG. 5.
ISS-ODN enhances the antimycobacterial effect of CLA. (A) Enhancement of the antimycobacterial effect of CLA by ISS-ODN in mBMDMs in vitro. Infected macrophages were treated with ISS-ODN or M-ODN in the presence or absence of CLA (0.1 μg/ml), and the intracellular growth of M. avium was assessed by the CFU assay on day 7 after infection. Each condition was tested in triplicate, and the results are expressed as mean ± SD CFU per well. The results shown are representative of three experiments. ∗, P < 0.01 compared to CFU recovered from cells treated with medium alone. (B, C, and D) Enhancement of the antimycobacterial effect of CLA by ISS-ODN in mBMDMs in vivo. Mice were infected with 107 M. avium organisms and then treated with ISS-ODN alone, CLA alone, CLA and ISS-ODN, and CLA and M-ODN (n = 5 per group) 1 week later. Mice received CLA (200 mg/kg of body weight, three times a week for 4 weeks) and ISS-ODN or M-ODN (50 μg/mouse, with the first and the seventh administrations of CLA). M. avium growth in the lungs (B), spleen (C), and liver (D) of the animals was determined by the CFU assay at 5 weeks after infection. The data shown are representative of one of two experiments performed with similar results. The results shown are mean ± SD CFU per organ. ∗, P < 0.05 compared to CFU in the organs of control mice that received CLA only or a combination of CLA and M-ODN.
C57BL/6 mice were infected intravenously with M. avium (107 CFU) and treated with a combination of ISS-ODN (50 μg/mouse) and/or CLA (200 mg/kg of body weight) 1 week after infection. Mice were sacrificed 5 weeks after infection, and the number of CFU was counted in the spleen, liver, and lungs. Figure 5 shows representative data of one of two experiments performed. Treatment with CLA alone decreased bacterial growth in the lungs (1.5-log reduction, Fig. 5B), spleen (3.5-log reduction, Fig. 5C), and liver (4-log reduction, Fig. 5D) In this therapeutic model, ISS-ODN alone did not inhibit the growth of M. avium. However, when ISS-ODN was combined with CLA, there was a further reduction of bacterial counts, particularly in the lungs (3-log reduction, P < 0.01, Fig. 5B to D) and in the spleen (2-log reduction, P < 0.01, Fig. 5B). Despite the fact that treatment with ISS-ODN postinfection did not affect the course of the infection in vivo, these findings suggest that ISS-ODN can enhance the therapeutic efficacy of CLA in the setting of established M. avium infection. Tissue sections from spleen fixed and stained with hematoxylin-eosin showed that the white pulp from M. avium-infected mice treated with PBS was replaced with granulomas. The spleen sections from infected mice treated with CLA showed fewer granulomas with smaller sizes compared to the infected mice treated with PBS. The sections from infected mice treated with ISS-ODN alone or CLA plus ISS-ODN showed even smaller granulomas in the white pulp and increased mononuclear cells in the red pulp compared to those in the infected mice treated with PBS or CLA alone, respectively.
ISS-ODN inhibits M. avium growth in human macrophages in vitro.
To study the relevance of the antimycobacterial effects of ISS-ODN in humans, hMDMs were treated with ISS-ODN or M-ODN (3, 10, and 30 μg/ml) for 3 days and then infected with M. avium. There was maximal inhibition of M. avium growth at 3 μg of ISS-ODN per ml (Fig. 6A) with no further increase in inhibition at the higher concentrations (data not shown). At 7 days postinfection, treatment with ISS-ODN inhibited intracellular growth of M. avium by 91% (Fig. 6A, P < 0.001). No changes in cell viability were observed in the various groups. To study the therapeutic effects of ISS-ODN on established M. avium infection, infected hMDMs were treated with ISS-ODN (10 μg/ml) for 7 days. Treatment with ISS-ODN significantly decreased the intracellular growth of M. avium in hMDMs by 53% (P < 0.05) (Fig. 6B). When infected cells were treated with ISS-ODN together with CLA (0.1 μg/ml), M. avium growth was further inhibited up to 99% (P < 0.01), compared to medium alone (Fig. 6B). These findings indicate that ISS-ODN can provide a significant antimycobacterial effect in human macrophages as well as in the mouse model.
FIG. 6.
ISS-ODN has antimycobacterial properties in human macrophages. (A) hMDMs were treated with ISS-ODN or M-ODN for 3 days prior to M. avium infection, and intracellular growth of M. avium by the CFU assay was assessed on days 1, 3, and 7 after infection. (B) hMDMs were treated with ISS-ODN or M-ODN immediately after infection, and M. avium growth was assessed by the CFU assay 7 days postinfection. Each condition was tested in triplicate, and the results are expressed as mean ± SD CFU per well. The results shown are representative of three experiments. ∗, P < 0.01 compared to CFU recovered from cells treated with M-ODN or medium alone.
DISCUSSION
This study demonstrates that ISS-ODN augments host resistance against M. avium infection and that the induction of IDO plays an important role in this effect. Furthermore, ISS-ODN synergizes with the chemotherapeutic drug CLA to further promote mycobacterial clearance. These data support recent reports showing that administration of ISS-ODN provides protection against the intracellular pathogens Listeria monocytogenes (27), Leishmania major (51), and Francisella tularensis (15). Studies with L. monocytogenes showed that injection of mice with Escherichia coli DNA or ISS-ODN resulted in a much lower bacterial burden in the spleen and liver (27). Similarly, ISS-ODN conferred a protective immunity against primary infection and resistance to secondary infection with L. major (51). This antimicrobial activity was attributed mainly to ISS-ODN-induced release of cytokines such as IL-12 and to the subsequent induction of a protective Th1 and/or CTL response. Indeed, administration of exogenous IL-12 or IFN-γ, which are induced by ISS-ODN, increases protection against L. major (51).
Our study suggests that ISS-ODN-induced resistance to M. avium infection does not act via augmentation of the adaptive T-cell response. First, the in vitro studies showed that treatment with ISS-ODN enhanced the ability of macrophages to resist M. avium growth despite the absence of T cells (Fig. 1). Second, ISS-ODN-induced resistance to M. avium in vivo decayed by 6 weeks, at which point ISS-ODN-treated mice had the same mycobacterial burden as control, PBS-treated mice (Fig. 2). The induction of a protective antimycobacterial T-cell response would be expected to provide a long-lasting antimycobacterial effect, rather than the transient protective effect observed in our experiments. Third, our data showed that T cells from ISS-treated, M. avium-infected mice did not display any increased infection-specific IFN-γ response over untreated mice upon restimulation in vitro (Fig. 3), indicating that ISS-ODN treatment of M. avium-infected mice does not enhance the anti-M. avium T-cell response in these animals. This finding is supported by the observation by Doherty and Sher (12) that the absence of cell-mediated immunity affects pathogen growth only in the chronic stage of M. avium infection in T-cell-deficient mice (BL/scid).
To further investigate the possible factors for the antimycobacterial effects of ISS-ODN, we used mice with targeted disruptions of genes known to play protective roles against M. avium infection in other systems (Table 1). ISS-ODN induces reactive oxygen species in murine B cells and monocytes (54). However, oxygen radicals and NO did not appear to play a role in the observed ISS-ODN-mediated growth inhibition of M. avium in mBMDMs. These findings are consistent with previous reports in which certain strains of M. avium are resistant to the bactericidal effect of NO or other oxygen radicals (1, 5), despite the fact that reactive radicals show antimicrobial activity against many microorganisms (16, 35). In addition, ISS-ODN-induced cytokines such as TNF-α, IL-12, and IFNs did not play significant roles in the ISS-ODN-mediated antimycobacterial effect.
The reduced M. avium growth in the lung and spleen but the unaffected M. avium growth in the liver (Fig. 2) led us to search for an organ-specific inducible factor with antimicrobial effects. IDO is such a factor. IDO is induced by IFNs (18), and induction of this enzyme is required to prevent rejection of the allogeneic fetus by maternal T cells (37). IDO also inhibits the growth of a variety of intracellular organisms, such as Toxoplasma gondi (41), Plasmodium berghe (in a murine model of malaria [46]), Chlamydia psittaci (7), and Chlamydia trachomatis (4), by breaking down l-Try, which is required for their growth, to l-kynurenine. Furthermore, in vivo ISS-ODN administration induced organ-specific IDO gene expression. As shown in Fig. 4, the induction of IDO was observed in the spleen and lungs, but not in the liver of ISS-ODN-injected mice, which agrees with the observed pattern of M. avium inhibition in vivo (Fig. 2). Although mycobacteria are able to synthesize all amino acids required for their growth, including tryptophan (43), the inhibition of M. avium growth is likely due to the combined result of reduced tryptophan availability and increased levels of the toxic metabolites l-kynurenine, anthranilic acid, quinolinic acid, and picolinic acid (40). Indeed, as shown in Fig. 4, the depletion of tryptophan is not the sole mechanism responsible for the antimycobacterial effect of ISS-ODN, since repletion of l-Try or addition of an IDO inhibitor, M-Try, did not fully restore M. avium growth. Another mechanism by which ISS-ODN inhibits M. avium growth may be the enhancement of CD40 expression by ISS-ODN (32), which could result in enhanced CD40-CD40 ligand binding. We have previously shown that CD40-CD40 ligand signaling results in the inhibition of the intracellular growth of M. avium (21). Further study is necessary to determine whether other factors or mechanisms are involved in the antimycobacterial effect of ISS-ODN in vivo.
ISS-ODN was initially identified in mycobacterial genomes (50), and it is well known that mycobacterial DNA is immunostimulatory. This raises a question: why is it that the M. avium genomic DNA contained within infected cells such as macrophages does not facilitate the clearance or inhibit the growth of this intracellular pathogen? A potential explanation for this paradox may lie in the recent observation that agents such as chloroquine (31), which inhibits the acidification of lysosomes, neutralize the immunostimulatory effects of ISS-ODN. Interestingly, mycobacteria also inhibit the generation of low pH in the lysosomes (49). Therefore, neutralization of the acidic environment of the lysosome may protect M. avium both directly, by avoiding digestion, and indirectly, by blocking the detection of its own immunostimulatory genomic DNA by the host cell.
The antimycobacterial effect of ISS-ODN in vivo on established M. avium infection is less than its effect when used prior to infection, while ISS-ODN significantly inhibits M. avium growth in vitro (Fig. 1B). In vivo, M. avium delivered i.v. can infect different macrophage populations, such as splenic macrophages, alveolar macrophages, and peritoneal macrophages. Peripheral macrophage populations have been shown to exhibit distinct functional phenotypes, including cytokine production, response to immunomodulatory stimuli, and clearance of pathogens (reviewed in reference 45). Gangadharam and Pratt reported that alveolar macrophages and peritoneal macrophages have the distinct ability to ingest and control the multiplication of Mycobacterium cells (19). Alveolar macrophages are known to release more nitrate than peritoneal macrophages when exposed to LPS and IFN-γ in C3H/HeJ mice (52). Indeed, in our preliminary study, alveolar macrophages, splenic macrophages, and BMDMs show unique individual patterns of cytokine secretion induced by ISS-ODN (data not shown). It is likely that various macrophage populations behave differently in response to ISS-ODN and M. avium infection.
Although treatment of macrophages with ISS-ODN inhibited M. avium growth in vitro, in vivo administration of ISS-ODN alone to mice with an established M. avium infection had no protective effect (Fig. 5B). However, when ISS-ODN was used together with CLA, there was a synergistic anti-M. avium therapeutic effect in mouse and human macrophages in vitro. This synergism might be due to more effective protein synthesis inhibition. CLA inhibits protein synthesis by binding to the 50S ribosome, while IDO induced by ISS-ODN interferes with protein synthesis by depletion of tryptophan. In in vivo experiments, ISS-ODN plus CLA showed a significant though modest synergistic therapeutic effect. Additional work is required with different doses and timing of ISS-ODN administration in order to optimize combined therapy as an alternate regimen in the treatment of established M. avium infection.
In summary, this study demonstrates that administration of ISS-ODN enhances resistance against M. avium infection through the induction of IDO. ISS-ODN itself provides limited protection against M. avium. However, this effect can be amplified upon codelivery with an antimycobacterial drug. The combined administration of ISS-ODN with other antibiotics or other antimicrobial agents provides a novel therapeutic strategy for microbial infections and warrants further investigation.
ACKNOWLEDGMENTS
Funds from the National Institutes of Health (grants AI40682 and AI47078 to E.R., AI35258 and HL57911 to R.S.K., and AR44850 for D.A.C) and from Dynavax Technologies Corporation and Sequella Global TB Foundation grant VIP 012 supported this research.
We thank Sunil J. Sahdeo for excellent technical assistance. We also thank Lucinda Beck for helpful editorial assistance.
REFERENCES
- 1.Appelberg R, Orme I M. Effector mechanisms involved in cytokine-mediated bacteriostasis of Mycobacterium avium infections in murine macrophages. Immunology. 1993;80:352–359. [PMC free article] [PubMed] [Google Scholar]
- 2.Appelberg R, Sarmento A, Castro A G. Tumour necrosis factor-alpha (TNF-alpha) in the host resistance to mycobacteria of distinct virulence. Clin Exp Immunol. 1995;101:308–313. doi: 10.1111/j.1365-2249.1995.tb08356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Autran B, Carcelain G, Li T S, Blanc C, Mathez D, Tubiana R, Katlama C, Debrae P, Leibowitch J. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–116. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 4.Beatty W L, Belanger T A, Desai A A, Morrison R P, Byrne G I. Tryptophan depletion as a mechanism of gamma interferon-mediated chlamydial persistence. Infect Immun. 1994;62:3705–3711. doi: 10.1128/iai.62.9.3705-3711.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bermudez L E. Differential mechanisms of intracellular killing of Mycobacterium avium and Listeria monocytogenes by activated human and murine macrophages. The role of nitric oxide. Clin Exp Immunol. 1993;91:277–281. doi: 10.1111/j.1365-2249.1993.tb05895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bermudez L E, Young L S, Enkel H. Interaction of Mycobacterium avium complex with human macrophages: roles of membrane receptors and serum proteins. Infect Immun. 1991;59:1697–1702. doi: 10.1128/iai.59.5.1697-1702.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlin J M, Borden E C, Byrne G I. Interferon-induced indoleamine 2:3-dioxygenase activity inhibits Chlamydia psittaci replication in human macrophages. J Interferon Res. 1989;9:329–337. doi: 10.1089/jir.1989.9.329. [DOI] [PubMed] [Google Scholar]
- 8.Chin D P, Reingold A L, Stone E N, Vittinghoff E, Horsburgh C R, Jr, Simon E M, Yajko D M, Hadley W K, Ostroff S M, Hopewell P C. The impact of Mycobacterium avium complex bacteremia and its treatment on survival of AIDS patients—a prospective study. J Infect Dis. 1994;170:578–584. doi: 10.1093/infdis/170.3.578. [DOI] [PubMed] [Google Scholar]
- 9.Cho H J, Takabayashi K, Cheng P M, Nguyen M D, Corr M, Tuck S, Raz E. Immunostimulatory DNA-based vaccines induce cytotoxic lymphocyte activity by a T-helper cell-independent mechanism. Nat Biotechnol. 2000;18:509–514. doi: 10.1038/75365. [DOI] [PubMed] [Google Scholar]
- 10.Crowe S M, Carlin J B, Stewart K I, Lucas C R, Hoy J F. Predictive value of CD4 lymphocyte numbers for the development of opportunistic infections and malignancies in HIV-infected persons. J Acquir Immune Defic Syndr. 1991;4:770–776. [PubMed] [Google Scholar]
- 11.Daubener W, MacKenzie C R. IFN-gamma activated indoleamine 2:3-dioxygenase activity in human cells is an antiparasitic and an antibacterial effector mechanism. Adv Exp Med Biol. 1999;467:517–524. doi: 10.1007/978-1-4615-4709-9_64. [DOI] [PubMed] [Google Scholar]
- 12.Doherty T M, Sher A. Defects in cell-mediated immunity affect chronic, but not innate, resistance of mice to Mycobacterium avium infection. J Immunol. 1997;158:4822–4831. [PubMed] [Google Scholar]
- 13.Doherty T M, Sher A. IL-12 promotes drug-induced clearance of Mycobacterium avium infection in mice. J Immunol. 1998;160:5428–5435. [PubMed] [Google Scholar]
- 14.Edwards D, Kirkpatrick C H. The immunology of mycobacterial diseases. Am Rev Respir Dis. 1986;134:1062–1071. doi: 10.1164/arrd.1986.134.5.1062. [DOI] [PubMed] [Google Scholar]
- 15.Elkins K L, Rhinehart-Jones T R, Stibitz S, Conover J S, Klinman D M. Bacterial DNA containing CpG motifs stimulates lymphocyte-dependent protection of mice against lethal infection with intracellular bacteria. J Immunol. 1999;162:2291–2298. [PubMed] [Google Scholar]
- 16.Fang F C. Perspectives series: host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J Clin Investig. 1997;99:2818–2825. doi: 10.1172/JCI119473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fattorini L, Xiao Y, Li B, Santoro C, Ippoliti F, Orefici G. Induction of IL-1 beta, IL-6, TNF-alpha, GM-CSF and G-CSF in human macrophages by smooth transparent and smooth opaque colonial variants of Mycobacterium avium. J Med Microbiol. 1994;40:129–133. doi: 10.1099/00222615-40-2-129. [DOI] [PubMed] [Google Scholar]
- 18.Feng G S, Dai W, Gupta S L, Werner-Felmayer G, Wachter H, Takikawa O, Taylor M W. Analysis of interferon-gamma resistant mutants that are possibly defective in their signaling mechanism. Mol Gen Genet. 1991;230:91–96. doi: 10.1007/BF00290655. [DOI] [PubMed] [Google Scholar]
- 19.Gangadharam P R, Pratt P F. In vitro response of murine alveolar and peritoneal macrophages to Mycobacterium intracellulare. Am Rev Respir Dis. 1983;128:1044–1047. doi: 10.1164/arrd.1983.128.6.1044. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi T, Catanzaro A, Rao S P. Apoptosis of human monocytes and macrophages by Mycobacterium avium sonicate. Infect Immun. 1997;65:5262–5271. doi: 10.1128/iai.65.12.5262-5271.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayashi T, Rao S P, Meylan P R, Kornbluth R S, Catanzaro A. Role of CD40 ligand in Mycobacterium avium infection. Infect Immun. 1999;67:3558–3565. doi: 10.1128/iai.67.7.3558-3565.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horsburgh C R., Jr Mycobacterium avium complex infection in the acquired immunodeficiency syndrome. N Engl J Med. 1991;324:1332–1338. doi: 10.1056/NEJM199105093241906. [DOI] [PubMed] [Google Scholar]
- 23.Hwu P, Du M X, Lapointe R, Do M, Taylor M W, Young H A. Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J Immunol. 2000;164:3596–3599. doi: 10.4049/jimmunol.164.7.3596. [DOI] [PubMed] [Google Scholar]
- 24.Klinman D M, Conover J, Coban C. Repeated administration of synthetic oligodeoxynucleotides expressing CpG motifs provides long-term protection against bacterial infection. Infect Immun. 1999;67:5658–5663. doi: 10.1128/iai.67.11.5658-5663.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klinman D M, Yi A K, Beaucage S L, Conover J, Krieg A M. CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc Natl Acad Sci USA. 1996;93:2879–2883. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobayashi K, Kasama T, Yamazaki J, Hosaka M, Katsura T, Mochizuki T, Soejima K, Nakamura R M. Protection of mice from Mycobacterium avium infection by recombinant interleukin-12. Antimicrob Agents Chemother. 1995;39:1369–1371. doi: 10.1128/aac.39.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krieg A M, Love-Homan L, Yi A K, Harty J T. CpG DNA induces sustained IL-12 expression in vivo and resistance to Listeria monocytogenes challenge. J Immunol. 1998;161:2428–2434. [PubMed] [Google Scholar]
- 28.Krieg A M, Yi A K, Matson S, Waldschmidt T J, Bishop G A, Teasdale R, Koretzky G A, Klinman D M. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 29.Krieg A M, Yi A K, Schorr J, Davis H L. The role of CpG dinucleotides in DNA vaccines. Trends Microbiol. 1998;6:23–27. doi: 10.1016/S0966-842X(97)01145-1. [DOI] [PubMed] [Google Scholar]
- 30.Lipford G B, Bauer M, Blank C, Reiter R, Wagner H, Heeg K. CpG-containing synthetic oligonucleotides promote B and cytotoxic T cell responses to protein antigen: a new class of vaccine adjuvants. Eur J Immunol. 1997;27:2340–2344. doi: 10.1002/eji.1830270931. [DOI] [PubMed] [Google Scholar]
- 31.Macfarlane D E, Manzel L. Antagonism of immunostimulatory CpG-oligodeoxynucleotides by quinacrine, chloroquine, and structurally related compounds. J Immunol. 1998;160:1122–1131. [PubMed] [Google Scholar]
- 32.Martin-Orozco E, Kobayashi H, Van Uden J, Nguyen M D, Kornbluth R S, Raz E. Enhancement of antigen-presenting cell surface molecules involved in cognate interactions by immunostimulatory DNA sequences. Int Immunol. 1999;11:1111–1118. doi: 10.1093/intimm/11.7.1111. [DOI] [PubMed] [Google Scholar]
- 33.Masur H. Recommendations on prophylaxis and therapy for disseminated Mycobacterium avium complex disease in patients infected with the human immunodeficiency virus. Public Health Service Task Force on Prophylaxis and Therapy for Mycobacterium avium Complex. N Engl J Med. 1993;329:898–904. doi: 10.1056/NEJM199309163291228. [DOI] [PubMed] [Google Scholar]
- 34.Meylan P R, Richman D D, Kornbluth R S. Characterization and growth in human macrophages of Mycobacterium avium complex strains isolated from the blood of patients with acquired immunodeficiency syndrome. Infect Immun. 1990;58:2564–2568. doi: 10.1128/iai.58.8.2564-2568.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller R A, Britigan B E. Role of oxidants in microbial pathophysiology. Clin Microbiol Rev. 1997;10:1–18. doi: 10.1128/cmr.10.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Munn D H, Shafizadeh E, Attwood J T, Bondarev I, Pashine A, Mellor A L. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189:1363–1372. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Munn D H, Zhou M, Attwood J T, Bondarev I, Conway S J, Marshall B, Brown C, Mellor A L. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 38.Newman G W, Gan H X, McCarthy P L, Jr, Remold H G. Survival of human macrophages infected with Mycobacterium avium intracellulare correlates with increased production of tumor necrosis factor-alpha and IL-6. J Immunol. 1991;147:3942–3948. [PubMed] [Google Scholar]
- 39.Ogata K, Linzer B A, Zuberi R I, Ganz T, Lehrer R I, Catanzaro A. Activity of defensins from human neutrophilic granulocytes against Mycobacterium avium-Mycobacterium intracellulare. Infect Immun. 1992;60:4720–4725. doi: 10.1128/iai.60.11.4720-4725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pais T F, Appelberg R. Macrophage control of mycobacterial growth induced by picolinic acid is dependent on host cell apoptosis. J Immunol. 2000;164:389–397. doi: 10.4049/jimmunol.164.1.389. [DOI] [PubMed] [Google Scholar]
- 41.Pfefferkorn E R, Guyre P M. Inhibition of growth of Toxoplasma gondii in cultured fibroblasts by human recombinant gamma interferon. Infect Immun. 1984;44:211–216. doi: 10.1128/iai.44.2.211-216.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rao S P, Ogata K, Catanzaro A. Mycobacterium avium-M. intracellulare binds to the integrin receptor αvβ3 on human monocytes and monocyte-derived macrophages. Infect Immun. 1993;61:663–670. doi: 10.1128/iai.61.2.663-670.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ratledge C. Nutrition, growth and metabolism. In: Ratledge C, Stanford J, editors. The biology of the mycobacteria. Vol. 1. London, United Kingdom: Academic Press; 1982. pp. 183–271. [Google Scholar]
- 44.Roman M, Martin-Orozco E, Goodman J S, Nguyen M D, Sato Y, Ronaghy A, Kornbluth R S, Richman D D, Carson D A, Raz E. Immunostimulatory DNA sequences function as T helper-1-promoting adjuvants. Nat Med. 1997;3:849–854. doi: 10.1038/nm0897-849. [DOI] [PubMed] [Google Scholar]
- 45.Rutherford M S, Witsell A, Schook L B. Mechanisms generating functionally heterogeneous macrophages: chaos revisited. J Leukoc Biol. 1993;53:602–618. doi: 10.1002/jlb.53.5.602. [DOI] [PubMed] [Google Scholar]
- 46.Sanni L A, Thomas S R, Tattam B N, Moore D E, Chaudhri G, Stocker R, Hunt N H. Dramatic changes in oxidative tryptophan metabolism along the kynurenine pathway in experimental cerebral and noncerebral malaria. Am J Pathol. 1998;152:611–619. [PMC free article] [PubMed] [Google Scholar]
- 47.Sparwasser T, Koch E S, Vabulas R M, Heeg K, Lipford G B, Ellwart J W, Wagner H. Bacterial DNA and immunostimulatory CpG oligonucleotides trigger maturation and activation of murine dendritic cells. Eur J Immunol. 1998;28:2045–2054. doi: 10.1002/(SICI)1521-4141(199806)28:06<2045::AID-IMMU2045>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 48.Sparwasser T, Miethke T, Lipford G, Erdmann A, Hacker H, Heeg K, Wagner H. Macrophages sense pathogens via DNA motifs: induction of tumor necrosis factor-alpha-mediated shock. Eur J Immunol. 1997;27:1671–1679. doi: 10.1002/eji.1830270712. [DOI] [PubMed] [Google Scholar]
- 49.Sturgill-Koszycki S, Schlesinger P H, Chakraborty P, Haddix P L, Collins H L, Fok A K, Allen R D, Gluck S L, Heuser J, Russell D G. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science. 1994;263:678–681. doi: 10.1126/science.8303277. [DOI] [PubMed] [Google Scholar]
- 50.Tokunaga T, Yano O, Kuramoto E, Kimura Y, Yamamoto T, Kataoka T, Yamamoto S. Synthetic oligonucleotides with particular base sequences from the cDNA encoding proteins of Mycobacterium bovis BCG induce interferons and activate natural killer cells. Microbiol Immunol. 1992;36:55–66. doi: 10.1111/j.1348-0421.1992.tb01642.x. [DOI] [PubMed] [Google Scholar]
- 51.Walker P S, Scharton-Kersten T, Krieg A M, Love-Homan L, Rowton E D, Udey M C, Vogel J C. Immunostimulatory oligodeoxynucleotides promote protective immunity and provide systemic therapy for leishmaniasis via IL-12- and IFN-gamma-dependent mechanisms. Proc Natl Acad Sci USA. 1999;96:6970–6975. doi: 10.1073/pnas.96.12.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang M J, Jeng K C, Shih P C. Differential expression of inducible nitric oxide synthase gene by alveolar and peritoneal macrophages in lipopolysaccharide-hyporesponsive C3H/HeJ mice. Immunology. 1999;98:497–503. doi: 10.1046/j.1365-2567.1999.00908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamamoto S, Yamamoto T, Shimada S, Kuramoto E, Yano O, Kataoka T, Tokunaga T. DNA from bacteria, but not from vertebrates, induces interferons, activates natural killer cells and inhibits tumor growth. Microbiol Immunol. 1992;36:983–997. doi: 10.1111/j.1348-0421.1992.tb02102.x. [DOI] [PubMed] [Google Scholar]
- 54.Yi A K, Klinman D M, Martin T L, Matson S, Krieg A M. Rapid immune activation by CpG motifs in bacterial DNA. Systemic induction of IL-6 transcription through an antioxidant-sensitive pathway. J Immunol. 1996;157:5394–5402. [PubMed] [Google Scholar]
- 55.Zimmermann S, Egeter O, Hausmann S, Lipford G B, Rocken M, Wagner H, Heeg K. CpG oligodeoxynucleotides trigger protective and curative Th1 responses in lethal murine leishmaniasis. J Immunol. 1998;160:3627–3630. [PubMed] [Google Scholar]